Structural and Geochemical Assessment of the Coralline Alga Tethysphytum antarcticum from Terra Nova Bay, Ross Sea, Antarctica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Characteristics and Sample Collection
2.2. Species Identification
2.2.1. Morphological Investigations
2.2.2. Phylogenetic Analysis
2.3. Calcified Thallus Structure, Mineralogy and Element Characterization
3. Results
3.1. Species Identification
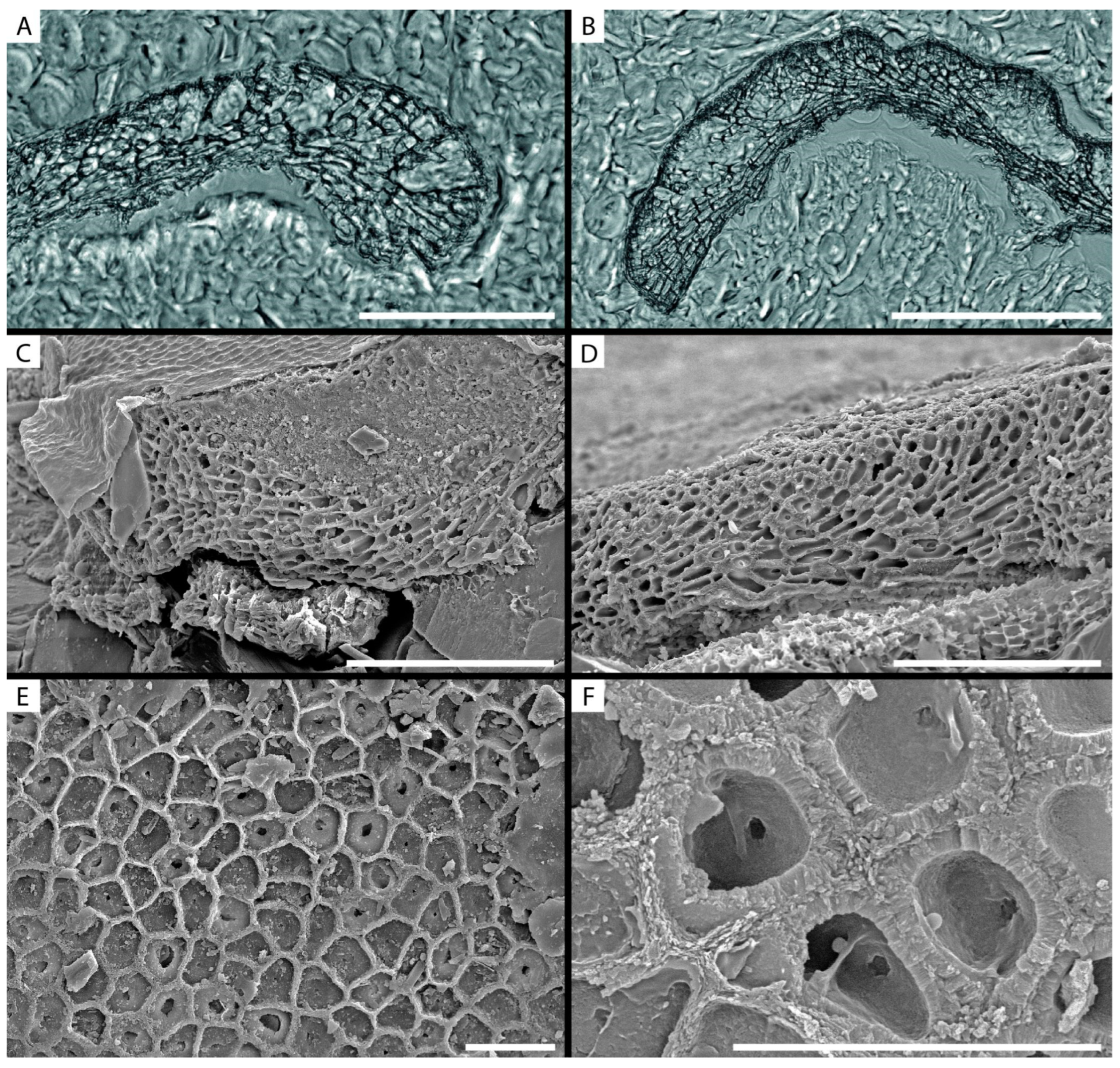
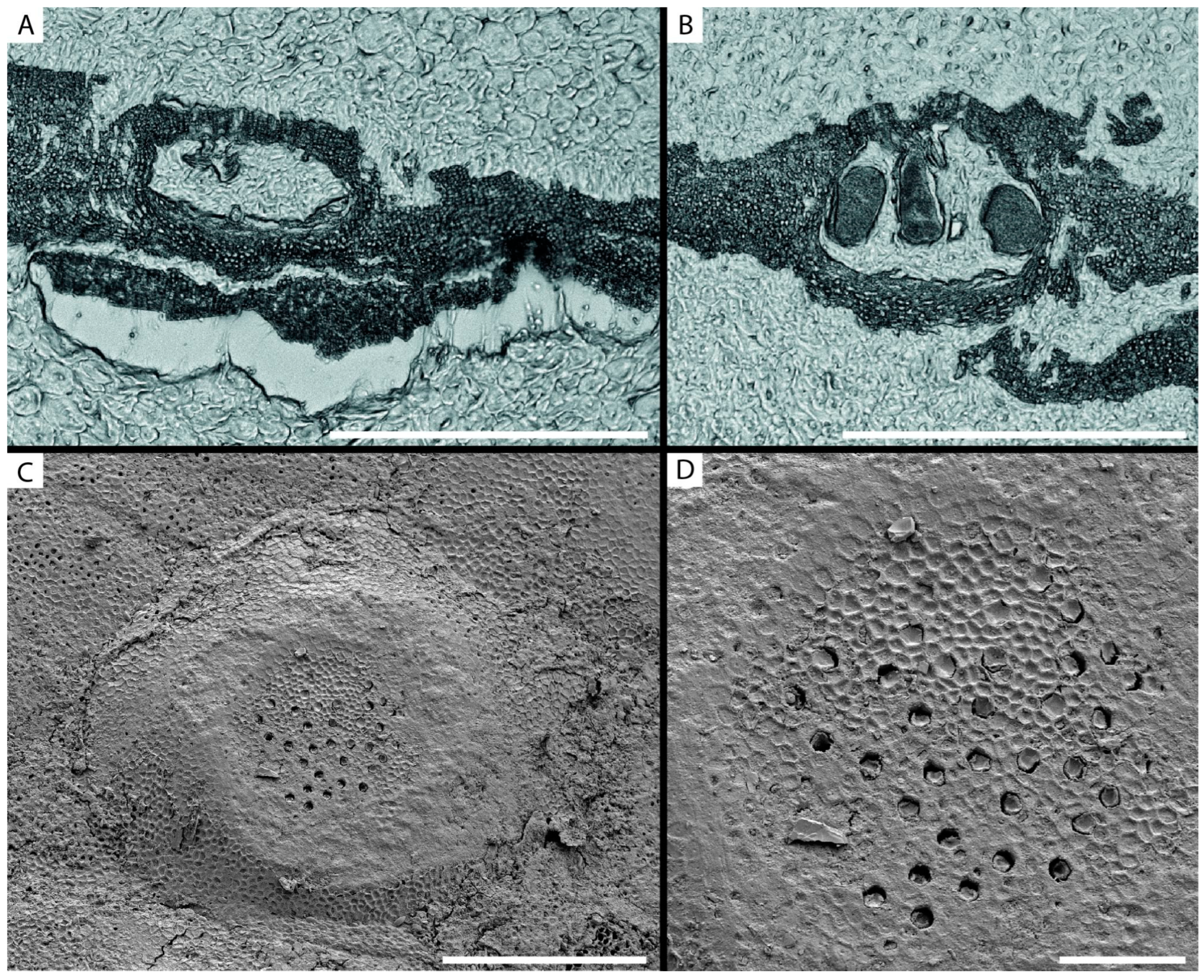
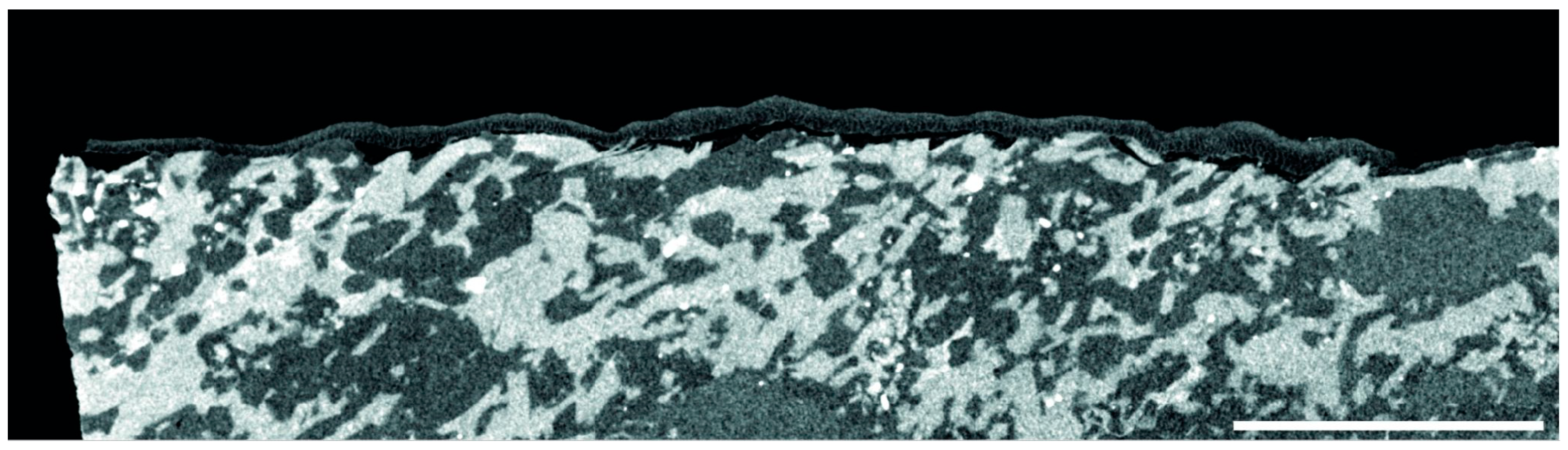
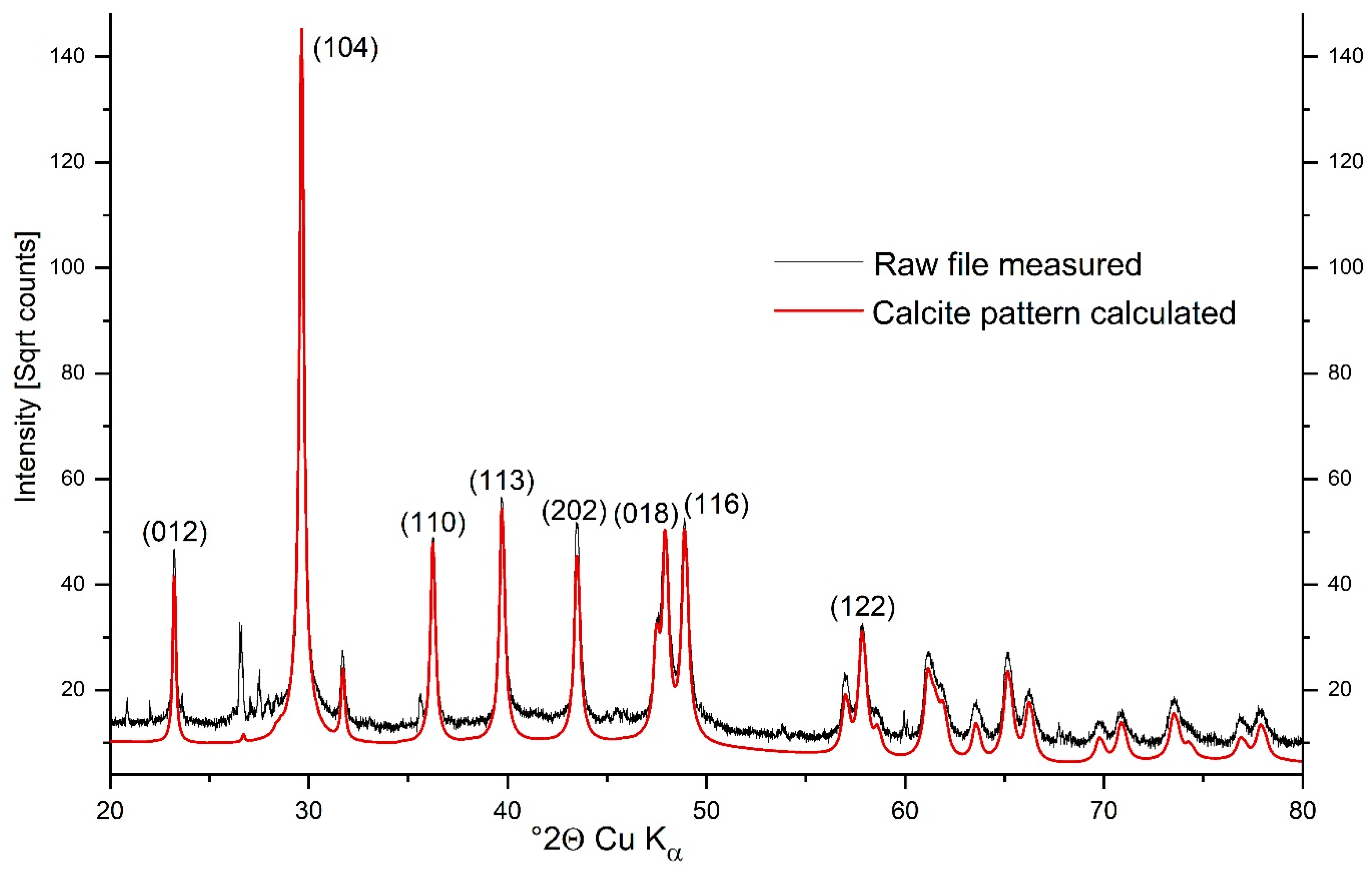
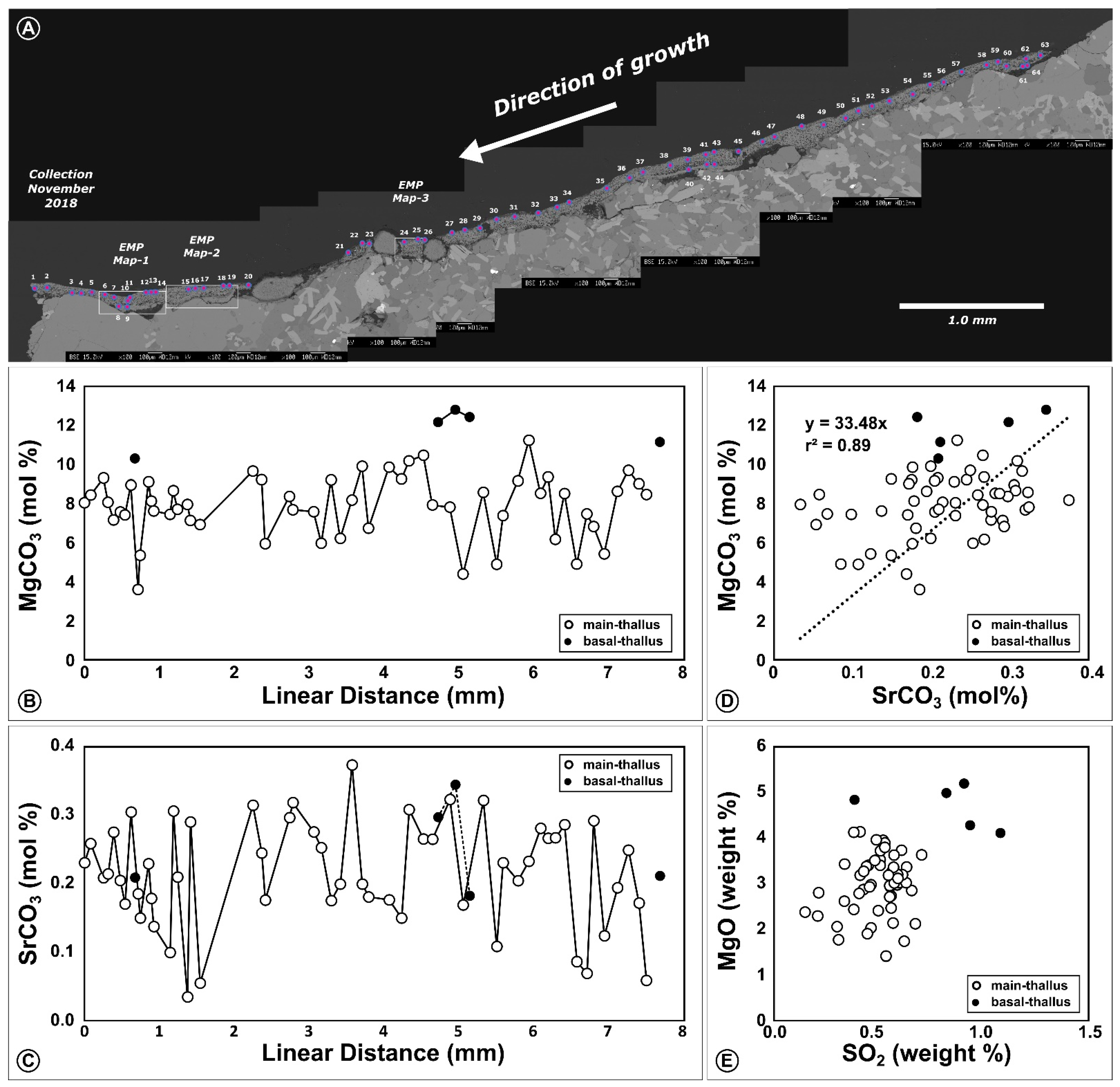
3.2. Calcified Thallus Structure, Mineralogy and Elemental Composition
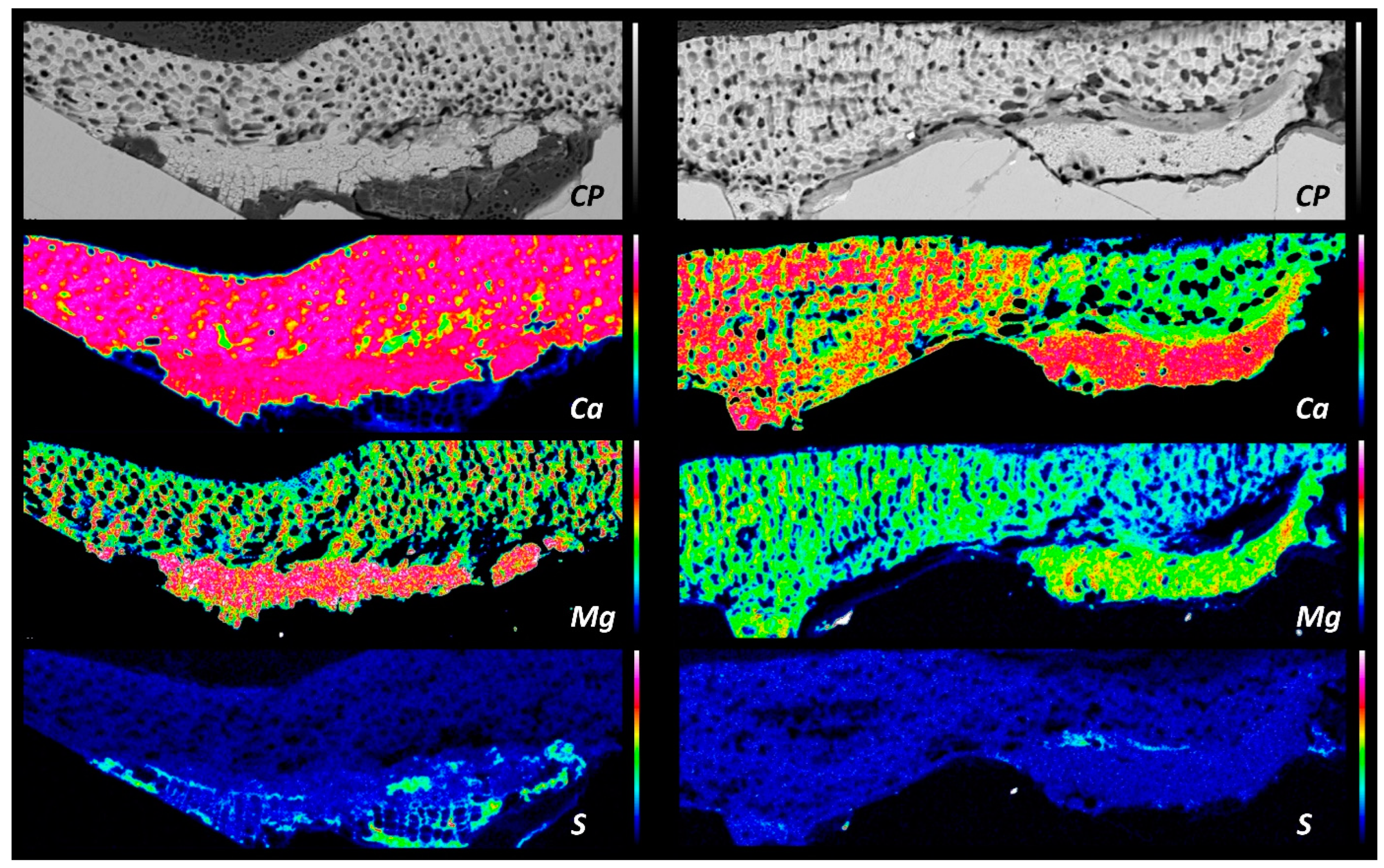
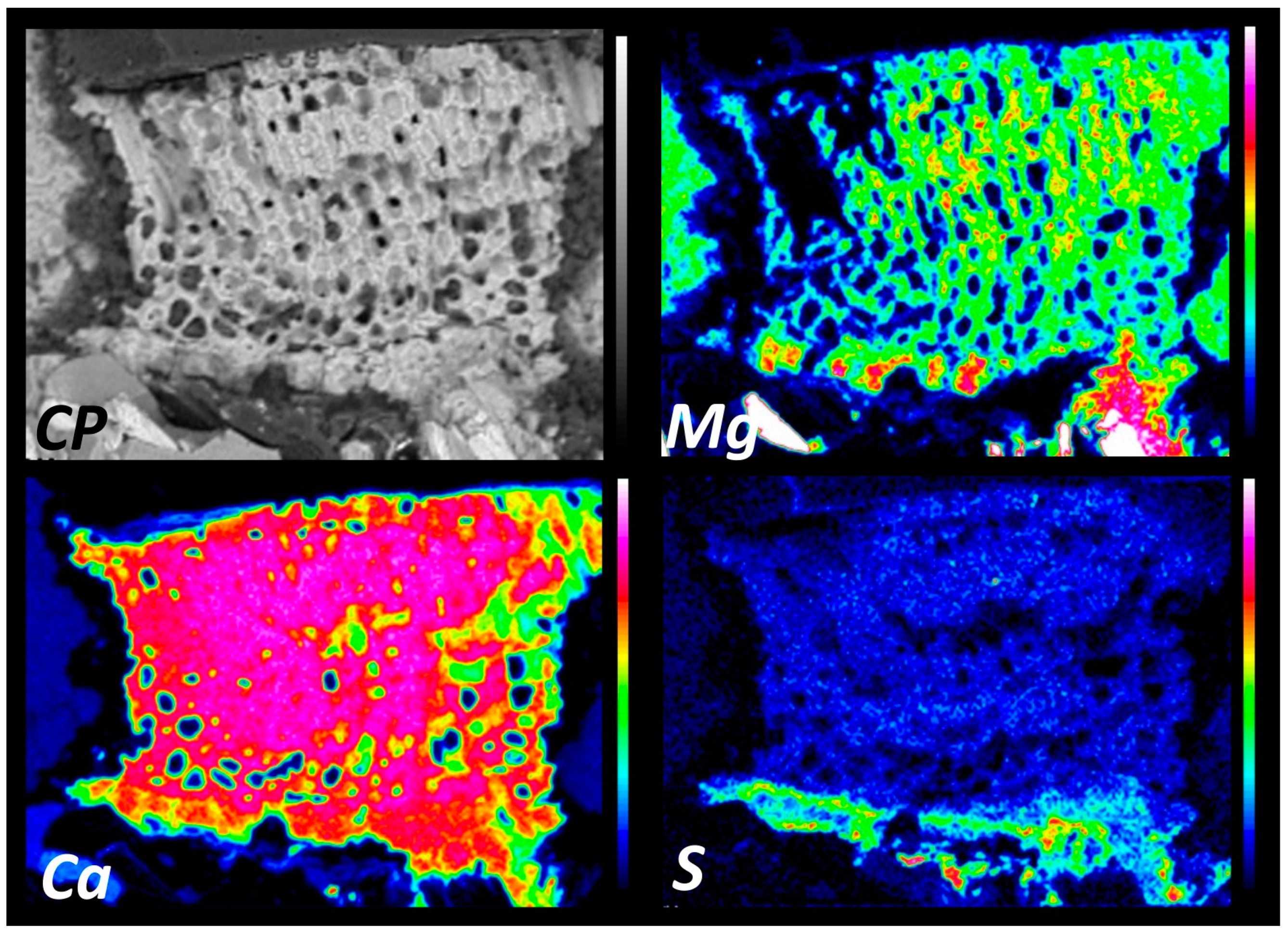
3.3. Elemental Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freiwald, A.; Henrich, R. Reefal Coralline Algal Build-Ups within the Arctic Circle: Morphology and Sedimentary Dynamics under Extreme Environmental Seasonality. Sedimentology 1994, 41, 963–984. [Google Scholar] [CrossRef]
- Foster, M.S. Rhodoliths: Between Rocks and Soft Places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith Bed Diversity in the Gulf of California: The Importance of Rhodolith Structure and Consequences of Disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Bilan, M.I.; Usov, A.I. Polysaccharides of Calcareous Algae and Their Effect on the Calcification Process. Russ. J. Bioorgan. Chem. 2001, 27, 2–16. [Google Scholar] [CrossRef]
- Pavez, J.; Silva, J.F.; Melo, F. Effects of Alginic Acid from Marine Algae on Calcium Carbonate Electrodeposited Coating. J. Cryst. Growth 2005, 282, 438–447. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Cusack, M.; Moore, P.G. Coralline Algae Are Global Palaeothermometers with Bi-Weekly Resolution. Geochim. Cosmochim. Acta 2008, 72, 771–779. [Google Scholar] [CrossRef]
- Williams, S.; Adey, W.; Halfar, J.; Kronz, A.; Gagnon, P.; Bélanger, D.; Nash, M. Effects of Light and Temperature on Mg Uptake, Growth, and Calcification in the Proxy Climate Archive Clathromorphum Compactum. Biogeosciences 2018, 15, 5745–5759. [Google Scholar] [CrossRef]
- Chave, K.E. Aspects of the Biogeochemistry of Magnesium 1. Calcareous Marine Organisms. J. Geol. 1954, 62, 266–283. [Google Scholar] [CrossRef]
- Ragazzola, F.; Kolzenburg, R.; Zekonyte, J.; Teichert, S.; Jiang, C.; Žuljević, A.; Caragnano, A.; Falace, A. Structural and Elemental Analysis of the Freshwater, Low-Mg Calcite Coralline Alga Pneophyllum Cetinaensis. Plants 2020, 9, 1089. [Google Scholar] [CrossRef]
- De Carvalho, R.T.; Rocha, G.M.; Karez, C.S.; da Gama Bahia, R.; Pereira, R.C.; Bastos, A.C.; Salgado, L.T. Global Assessment of Coralline Algae Mineralogy Points to High Vulnerability of Southwestern Atlantic Reefs and Rhodolith Beds to Ocean Acidification. Sci. Rep. 2022, 12, 9589. [Google Scholar] [CrossRef]
- Steneck, R.S. The Ecology of Coralline Algal Crusts: Convergent Patterns and Adaptative Strategies. Annu. Rev. Ecol. Syst. 1986, 17, 273–303. [Google Scholar] [CrossRef]
- Zaneveld, J.S.; Sanford, R.B. Crustose Corallinaceous Algae (Rhodophyta) of the New Zealand and United States Scientific Expedition to the Ross Sea, Balleny Islands, and Macquarie Ridge, 1965. Blumea Biodivers. Evol. Biogeogr. Plants 1980, 26, 205–231. [Google Scholar]
- Connell, S.D. The Monopolization of Understorey Habitat by Subtidal Encrusting Coralline Algae: A Test of the Combined Effects of Canopy-Mediated Light and Sedimentation. Mar. Biol. 2003, 142, 1065–1071. [Google Scholar] [CrossRef]
- Schwarz, A.-M.; Hawes, I.; Andrew, N.; Mercer, S.; Cummings, V.; Thrush, S. Primary Production Potential of Non-Geniculate Coralline Algae at Cape Evans, Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2005, 294, 131–140. [Google Scholar] [CrossRef]
- Cinelli, F.; Mendoza, M.L.; Cabioch, J. Note Sur Quelques Espèces de Corallinacées (Rhodophyta) Recoltées Dans l’Antarctique. Phycologia 1989, 28, 136–139. [Google Scholar] [CrossRef]
- Guiry, M.; Guiry AlgaeBase. World-Wide Electronic Publication. 2013. Available online: http://www.algaebase.org (accessed on 30 January 2023).
- Foslie, M. Det Kongelige Norske Videnskabers Selskabs Aarsbertning. Det. K. Nor. Vidensk. Selsk. Aarsbertning 1904, 6, 15–18. [Google Scholar]
- Miller, K.A.; Pearse, J.S. Ecological Studies of Seaweeds in McMurdo Sound, Antarctica. Am. Zool. 1991, 31, 35–48. [Google Scholar] [CrossRef]
- Sciuto, K.; Moschin, E.; Alongi, G.; Cecchetto, M.; Schiaparelli, S.; Caragnano, A.; Rindi, F.; Moro, I. Tethysphytum antarcticum Gen. et Sp. Nov. (Hapalidiales, Rhodophyta), a New Non-Geniculate Coralline Alga from Terra Nova Bay (Ross Sea, Antarctica): Morpho-Anatomical Characterization and Molecular Phylogeny. Eur. J. Phycol. 2021, 56, 416–427. [Google Scholar] [CrossRef]
- Pezzolesi, L.; Peña, V.; Le Gall, L.; Gabrielson, P.W.; Kaleb, S.; Hughey, J.R.; Rodondi, G.; Hernandez-Kantun, J.J.; Falace, A.; Basso, D.; et al. Mediterranean Lithophyllum stictiforme (Corallinales, Rhodophyta) Is a Genetically Diverse Species Complex: Implications for Species Circumscription, Biogeography and Conservation of Coralligenous Habitats. J. Phycol. 2019, 55, 473–492. [Google Scholar] [CrossRef]
- Peña, V.; Hernandez-Kantun, J.J.; Adey, W.H.; Gall, L.L. Assessment of Coralline Species Diversity in the European Coasts Supported by Sequencing of Type Material: The Case Study of Lithophyllum nitorum (Corallinales, Rhodophyta). Cryptogam. Algol. 2018, 39, 123–137. [Google Scholar] [CrossRef]
- Duquette, A.; Halanych, K.M.; Angus, R.A.; Mcclintock, J.B. Inter and Intraspecific Comparisons of the Skeletal Mg/Ca Ratios of High Latitude Antarctic Echinoderms. Antarct. Sci. 2018, 30, 160–169. [Google Scholar] [CrossRef]
- Lombardi, C.; Kuklinski, P.; Bordone, A.; Spirandelli, E.; Raiteri, G. Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea). Minerals 2021, 11, 374. [Google Scholar] [CrossRef]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The Oceanic Sink for Anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Polar zoobenthos blue carbon storage increases with sea ice losses, because across-shelf growth gains from longer algal blooms outweigh ice scour mortality in the shallows. Glob. Chang. Biol. 2017, 23, 5083–5091. [Google Scholar] [CrossRef]
- Gutt, J.; Isla, E.; Bertler, A.N.; Bodeker, G.E.; Bracegirdle, T.J.; Cavanagh, R.D.; Comiso, J.C.; Convey, P.; Cummings, V.; De Conto, R.; et al. Cross-disciplinarity in the advance of Antarctic ecosystem research. Mar. Gen. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Henley, S.F.; Schofield, O.M.; Hendry, K.R.; Schloss, I.R.; Steinberg, D.K.; Moffat, C.; Peck, L.S.; Costa, D.P.; Bakker, D.C.; Hughes, C.; et al. Variability and change in the west Antarctic Peninsula marine system: Research priorities and opportunities. Prog. Oceanogr. 2019, 173, 208–237. [Google Scholar]
- Illuminati, S.; Annibaldi, A.; Romagnoli, T.; Libani, G.; Antonucci, M.; Scarponi, G.; Totti, C.; Truzzi, C. Distribution of Cd, Pb and Cu between dissolved fraction, inorganic particulate and phytoplankton in seawater of Terra Nova Bay (Ross Sea, Antarctica) during austral summer 2011–12. Chemosphere 2017, 185, 1122–1135. [Google Scholar] [CrossRef]
- Budillon, G.; Pacciaroni, M.; Cozzi, S.; Rivaro, P.; Catalano, G.; Ianni, C.; Cantoni, C. An optimum multiparameter mixing analysis of the shelf waters in the Ross Sea. Antarct. Sci. 2003, 15, 105–118. [Google Scholar] [CrossRef]
- Nihashi, S.; Ohshima, K.I. Circumpolar Mapping of Antarctic Coastal Polynyas and Landfast Sea Ice: Relationship and Variability. J. Clim. 2015, 28, 3650–3670. [Google Scholar] [CrossRef]
- Faranda, F.M.; Guglielmo, L.; Ianora, A. The Italian oceanographic cruises in the Ross Sea (1987–1995): Strategy, general consideration description of the sampling sites. In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–14. [Google Scholar]
- Pusceddu, A.; Dell’Anno, A.; Fabiano, M. Organic matter composition in coastal sediments at Terra Nova Bay (Ross Sea) during summer 1995. Polar Biol. 2000, 23, 288–293. [Google Scholar] [CrossRef]
- Silvano, A.; Foppert, A.; Rintoul, S.R.; Holland, P.R.; Tamura, T.; Kimura, N.; Castagno, P.; Falco, P.; Budillon, G.; Haumann, F.A.; et al. Recent recovery of Antarctic BottomWater formation in the Ross Sea driven by climate anomalies. Nat. Geosci. 2020, 13, 780–786. [Google Scholar] [CrossRef]
- Caputi, S.S.; Careddu, G.; Calizza, E.; Fiorentino, F.; Maccapan, D.; Rossi, L.; Costantini, M.L. Seasonal Food Web Dynamics in the Antarctic Benthos of Tethys Bay (Ross Sea): Implications for Biodiversity Persistence Under Different Seasonal Sea-Ice Coverage. Front. Mar. Sci. 2020, 7, 594454. [Google Scholar] [CrossRef]
- Calizza, E.; Rossi, L.; Careddu, G.; Caputi, S.S.; Costantini, M.L. Species richness and vulnerability to disturbance propagation in real food webs. Sci. Rep. 2019, 9, 19331. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.F.; Stark, J.S.; Palmer, A.S.; Riddle, M.J.; Johnston, E.L. The Roles of Sea-Ice, Light and Sedimentation in Structuring Shallow Antarctic Benthic Communities. PLoS ONE 2017, 12, e0168391. [Google Scholar] [CrossRef] [PubMed]
- Pearse, J.S.; McClintock, J.B.; Bosch, I. Reproduction of Antarctic Benthic Marine Invertebrates: Tempos, Modes, and Timing. Am. Zoöl. 1991, 31, 65–80. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hackett, J.D.; Bhattacharya, D. A Single Origin of the Peridinin- and Fucoxanthin-Containing Plastids in Dinoflagellates through Tertiary Endosymbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 11724–11729. [Google Scholar] [CrossRef]
- Maneveldt, G.W.; Jeong, S.Y.; Cho, T.O.; Hughey, J.R.; Gabrielson, P.W. Reassessment of Misapplied Names, Phymatolithon Ferox and P. Repandum (Hapalidiales, Corallinophycidae, Rhodophyta) in South Africa, Based on DNA Sequencing of Type and Recently Collected Material. Phycologia 2020, 59, 449–455. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Di Vincenzo, G.; Rocchi, S. Origin and interaction of mafic and felsic magmas in an evolving late orogenic setting: The Early Paleozoic Terra Nova Intrusive Complex, Antarctica. Contrib. Mineral. Petrol. 1999, 137, 15–35. [Google Scholar] [CrossRef]
- Titschack, J.; Goetz-Neunhoeffer, F.; Neubauer, J. Magnesium Quantification in Calcites [(Ca,Mg)CO3] by Rietveld-Based XRD Analysis: Revisiting a Well-Established Method. Am. Mineral. 2011, 96, 1028–1038. [Google Scholar] [CrossRef]
- Henrich, R.; Freiwald, A.; Wehrmann, A.; Schäfer, P.; Samtleben, C.; Zankl, H. Nordic cold-water carbonates: Occurrences and controls. In Global and Regional Controls on Biogenic Sedimentation, I: Reef Evolution. Research Reports; Göttinger Arbeiten zur Geologie und Paläontologie: Göttingen, Germany, 1996. [Google Scholar]
- Halfar, J.; Zack, T.; Kronz, A.; Zachos, J.C. Growth and High-Resolution Paleoenvironmental Signals of Rhodoliths (Coralline Red Algae): A New Biogenic Archive. J. Geophys. Res. Oceans 2000, 105, 22107–22116. [Google Scholar] [CrossRef]
- Fietzke, J.; Ragazzola, F.; Halfar, J.; Dietze, H.; Foster, L.C.; Hansteen, T.H.; Eisenhauer, A.; Steneck, R.S. Century-Scale Trends and Seasonality in PH and Temperature for Shallow Zones of the Bering Sea. Proc. Natl. Acad. Sci. USA 2015, 112, 2960–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetzinger, S.; Halfar, J.; Kronz, A.; Steneck, R.S.; Adey, W.; Lebednik, P.A.; Schöne, B.R. High-Resolution Mg/Ca Ratios in a Coralline Red Alga as a Proxy for Bering Sea Temperature Variations from 1902 to 1967. Palaios 2009, 24, 406–412. [Google Scholar] [CrossRef]
- Hetzinger, S.; Halfar, J.; Zack, T.; Gamboa, G.; Jacob, D.E.; Kunz, B.E.; Kronz, A.; Adey, W.; Lebednik, P.A.; Steneck, R.S. High-Resolution Analysis of Trace Elements in Crustose Coralline Algae from the North Atlantic and North Pacific by Laser Ablation ICP-MS. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 302, 81–94. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Hoey, T.B.; Nienow, P.; Fallick, A.E.; Claverie, T. Reconstructing Greenland Ice Sheet Runoff Using Coralline Algae. Geology 2012, 40, 1095–1098. [Google Scholar] [CrossRef]
- Teichert, S.; Voigt, N.; Wisshak, M. Correction to: Do Skeletal Mg/Ca Ratios of Arctic Rhodoliths Reflect Atmospheric CO2 Concentrations? Polar Biol. 2021, 44, 1745. [Google Scholar] [CrossRef]
- Piazza, G.; Bracchi, V.A.; Langone, A.; Meroni, A.N.; Basso, D. Growth Rate Rather than Temperature Affects the B/Ca Ratio in the Calcareous Red Alga Lithothamnion corallioides. Biogeosciences 2022, 19, 1047–1065. [Google Scholar] [CrossRef]
- Gould, J.; Halfar, J.; Adey, W.; Ries, J.B. Growth as a Function of Sea Ice Cover, Light and Temperature in the Arctic/Subarctic Coralline C. Compactum: A Year-Long in Situ Experiment in the High Arctic. Front. Mar. Sci. 2022, 9, 900033. [Google Scholar] [CrossRef]
- Ragazzola, F.; Caragnano, A.; Basso, D.; Schmidt, D.N.; Fietzke, J. Establishing Temperate Crustose Early Holocene Coralline Algae as Archives for Palaeoenvironmental Reconstructions of the Shallow Water Habitats of the Mediterranean Sea. Palaeontology 2020, 63, 155–170. [Google Scholar] [CrossRef] [Green Version]


| A | T/U | C | G | |
|---|---|---|---|---|
| A | - | 5.96 | 2.52 | 9.70 |
| T/U | 4.55 | - | 21.14 | 1.97 |
| C | 3.14 | 34.51 | - | 0.46 |
| G | 12.31 | 3.28 | 0.47 | - |
| Thallus-Unit | MgO | CaO | SrO | SO2 | CO2 | Mg/Ca | MgCO3 | Sr/Ca | SrCO3 | CaCO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| (weight%) | (weight%) | (weight%) | (weight%) | (weight%) | (mol/mol) | (mol%) | (mol/mol) | (mol%) | (mol%) | |
| Main thallus | 3.207 ± 0.642 | 51.809 ± 0.771 | 0.223 ± 0.080 | 0.506 ± 0.099 | 44.255 ± 0.121 | 0.086 ± 0.018 | 7.908 ± 1.566 | 0.0023 ± 0.0009 | 0.214 ± 0.077 | 91.878 ± 1.593 |
| Basal thallus | 4.787 ± 0.382 | 49.755 ± 0.421 | 0.259 ± 0.065 | 0.259 ± 0.065 | 44.384 ± 0.142 | 0.134 ± 0.012 | 11.775 ± 0.912 | 0.0028 ± 0.0007 | 0.247 ± 0.061 | 87.977 ± 0.948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Correa, M.; Teichert, S.; Ragazzola, F.; Cazorla Vázquez, S.; Engel, F.B.; Hurle, K.; Mazzoli, C.; Kuklinski, P.; Raiteri, G.; Lombardi, C. Structural and Geochemical Assessment of the Coralline Alga Tethysphytum antarcticum from Terra Nova Bay, Ross Sea, Antarctica. Minerals 2023, 13, 215. https://doi.org/10.3390/min13020215
López Correa M, Teichert S, Ragazzola F, Cazorla Vázquez S, Engel FB, Hurle K, Mazzoli C, Kuklinski P, Raiteri G, Lombardi C. Structural and Geochemical Assessment of the Coralline Alga Tethysphytum antarcticum from Terra Nova Bay, Ross Sea, Antarctica. Minerals. 2023; 13(2):215. https://doi.org/10.3390/min13020215
Chicago/Turabian StyleLópez Correa, Matthias, Sebastian Teichert, Federica Ragazzola, Salvador Cazorla Vázquez, Felix B. Engel, Katrin Hurle, Claudio Mazzoli, Piotr Kuklinski, Giancarlo Raiteri, and Chiara Lombardi. 2023. "Structural and Geochemical Assessment of the Coralline Alga Tethysphytum antarcticum from Terra Nova Bay, Ross Sea, Antarctica" Minerals 13, no. 2: 215. https://doi.org/10.3390/min13020215
APA StyleLópez Correa, M., Teichert, S., Ragazzola, F., Cazorla Vázquez, S., Engel, F. B., Hurle, K., Mazzoli, C., Kuklinski, P., Raiteri, G., & Lombardi, C. (2023). Structural and Geochemical Assessment of the Coralline Alga Tethysphytum antarcticum from Terra Nova Bay, Ross Sea, Antarctica. Minerals, 13(2), 215. https://doi.org/10.3390/min13020215








