Provenance Analysis of Marbles by Combination of Cathodoluminescence Spectroscopy and Electron Microprobe Analyses—Methodological Comments
Abstract
:1. Introduction
2. Geological Setting, Previous Petrographic Data and Marble Employment for Decorative Purposes
2.1. Geological Setting
2.2. Mineralogical-Petrographic Description of Studied Samples
2.3. Decorative Applications of Studied Samples
3. Materials and Methods
3.1. Sampling
3.2. EMPA
3.3. CL Spectroscopy and CL Optical Microscopy
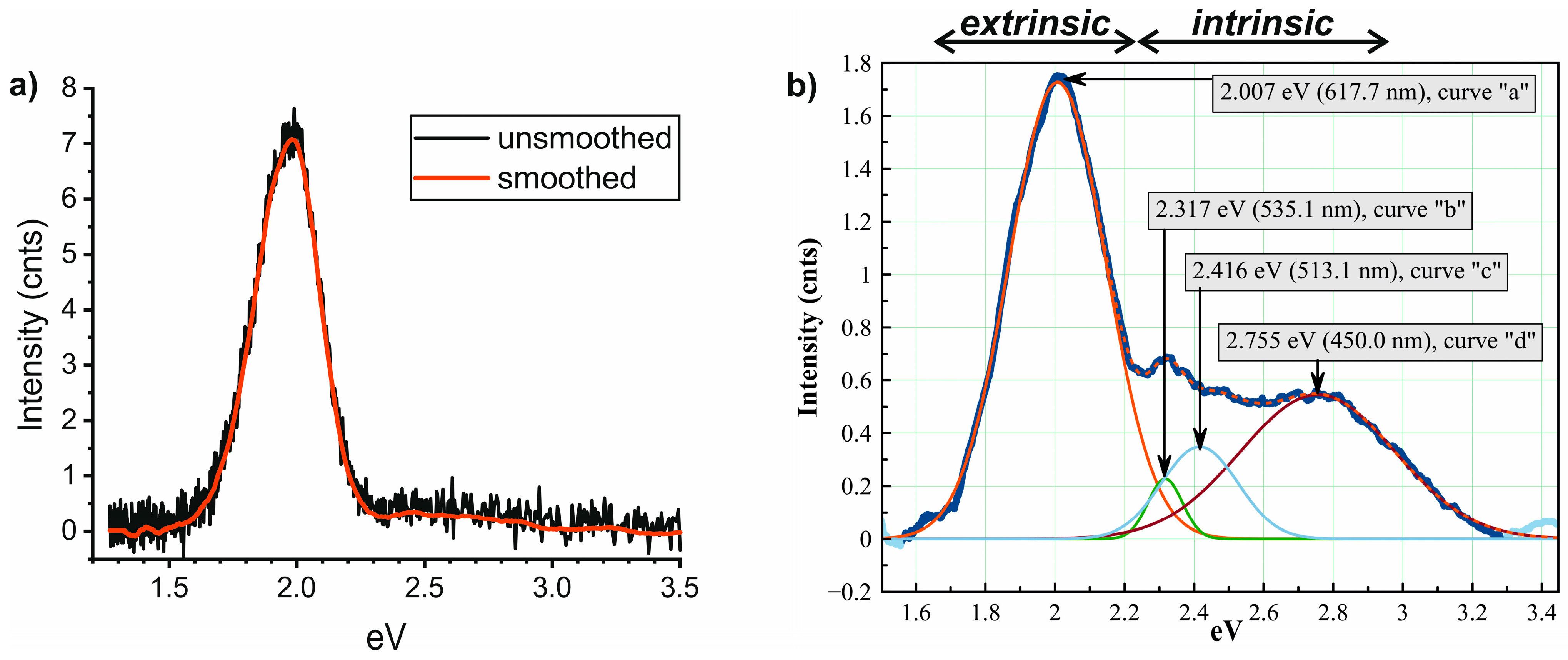
3.4. Processing of CL-Spectra
4. Results
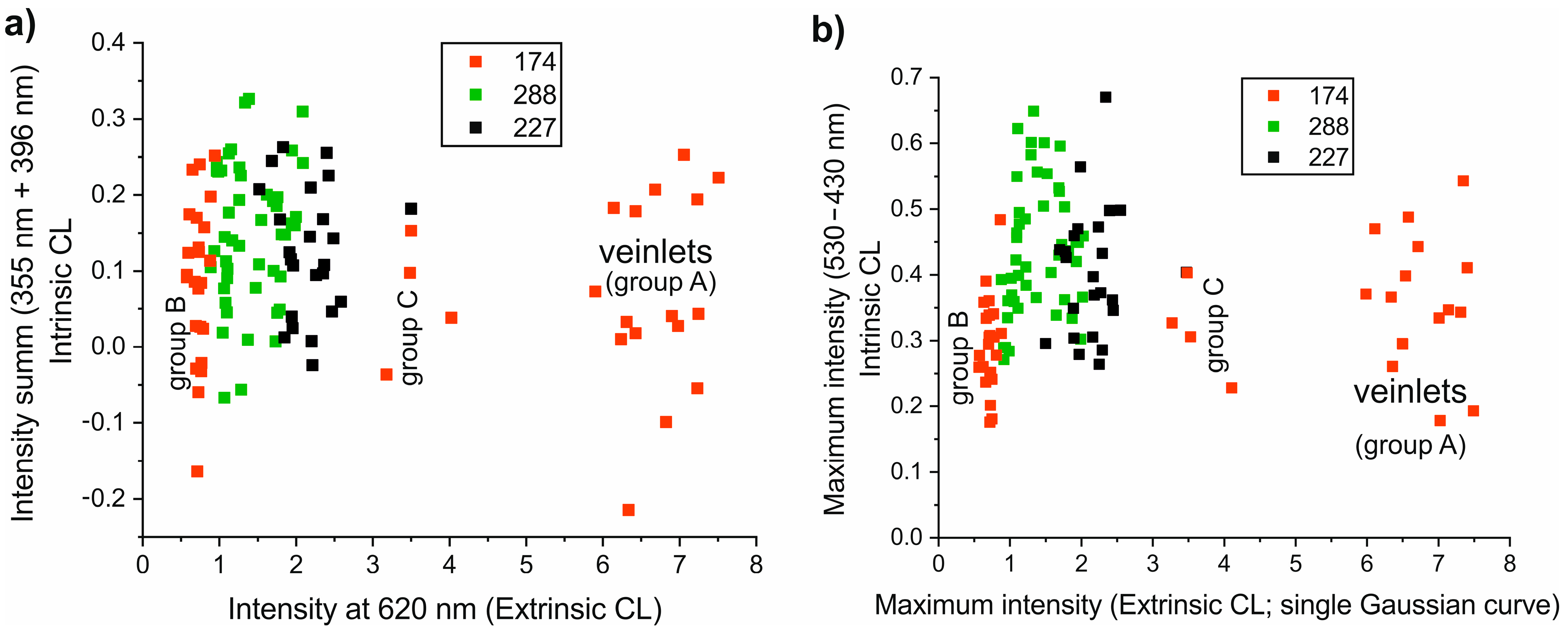
4.1. Provenance Discrimination of Samples Using Spectral Data Only
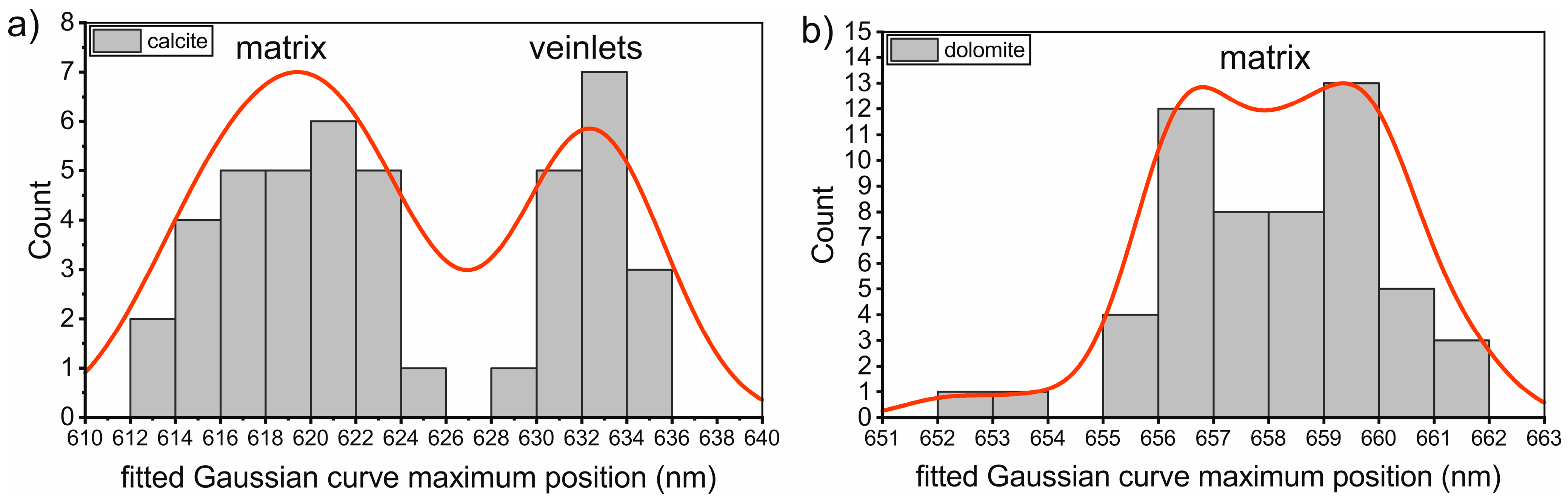
4.2. Fitting Calcite and Dolomite Spectra by a Single Gaussian Curve
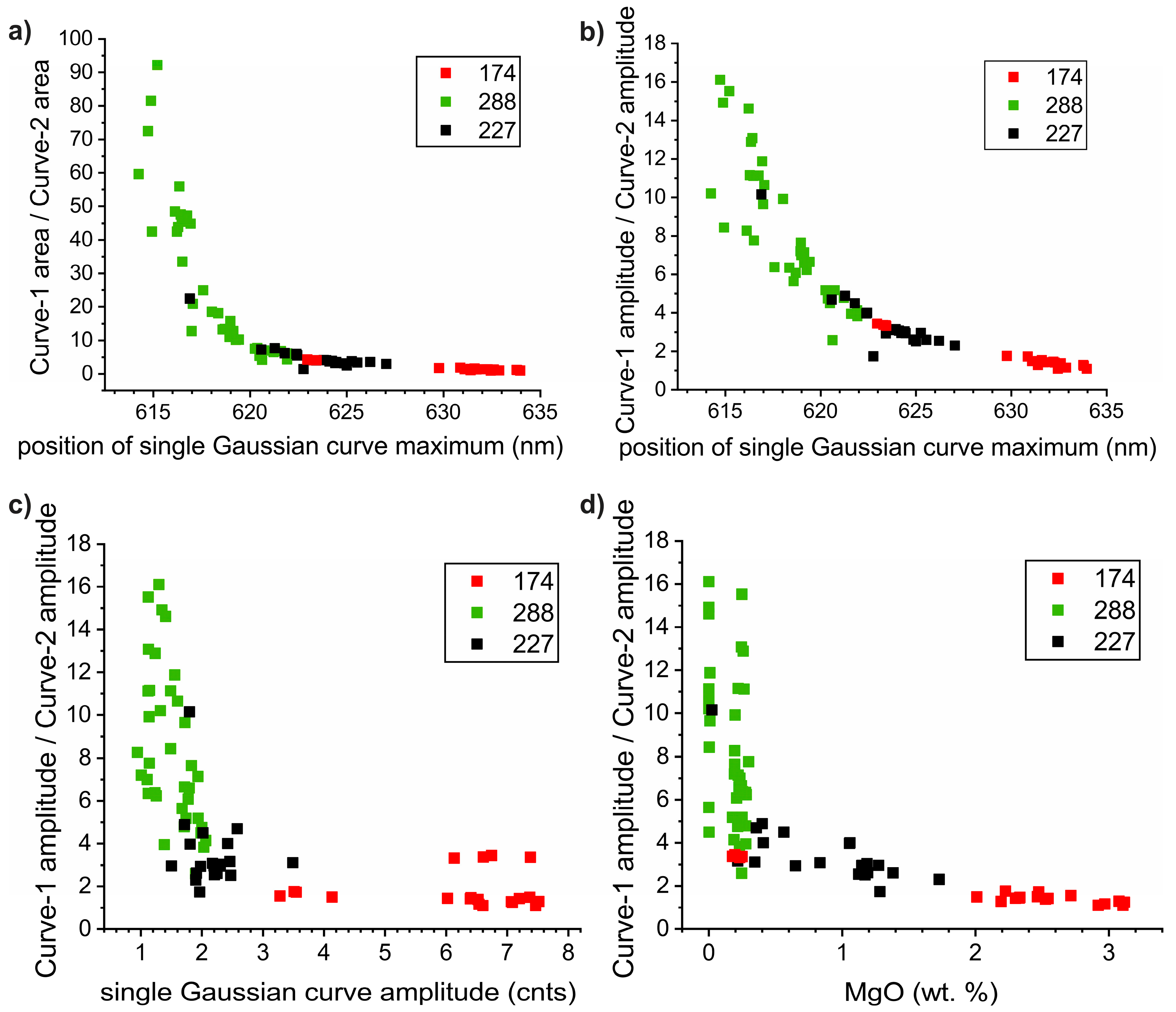
4.3. Fitting Calcite and Dolomite Spectra by Two or More Gaussian Curves
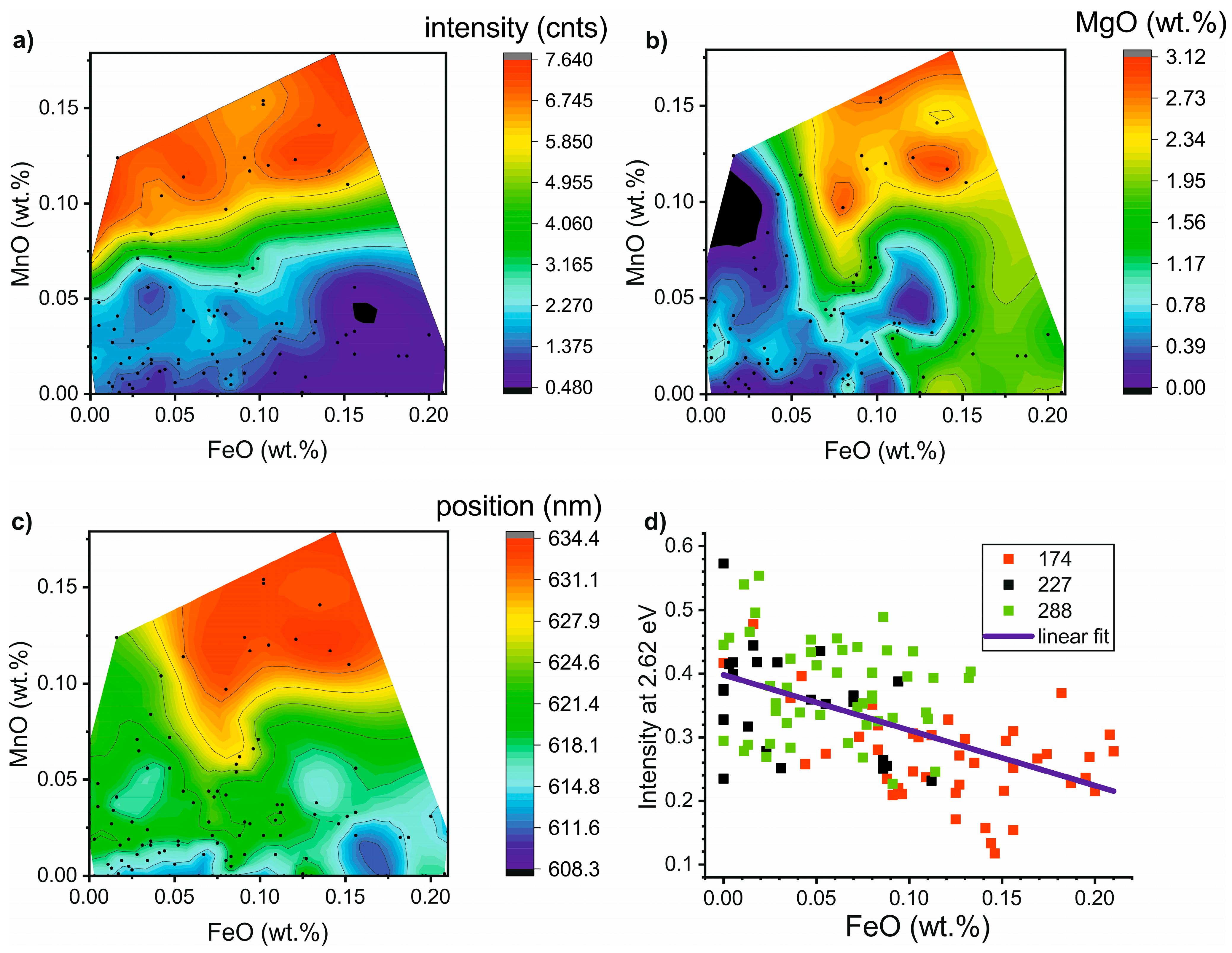
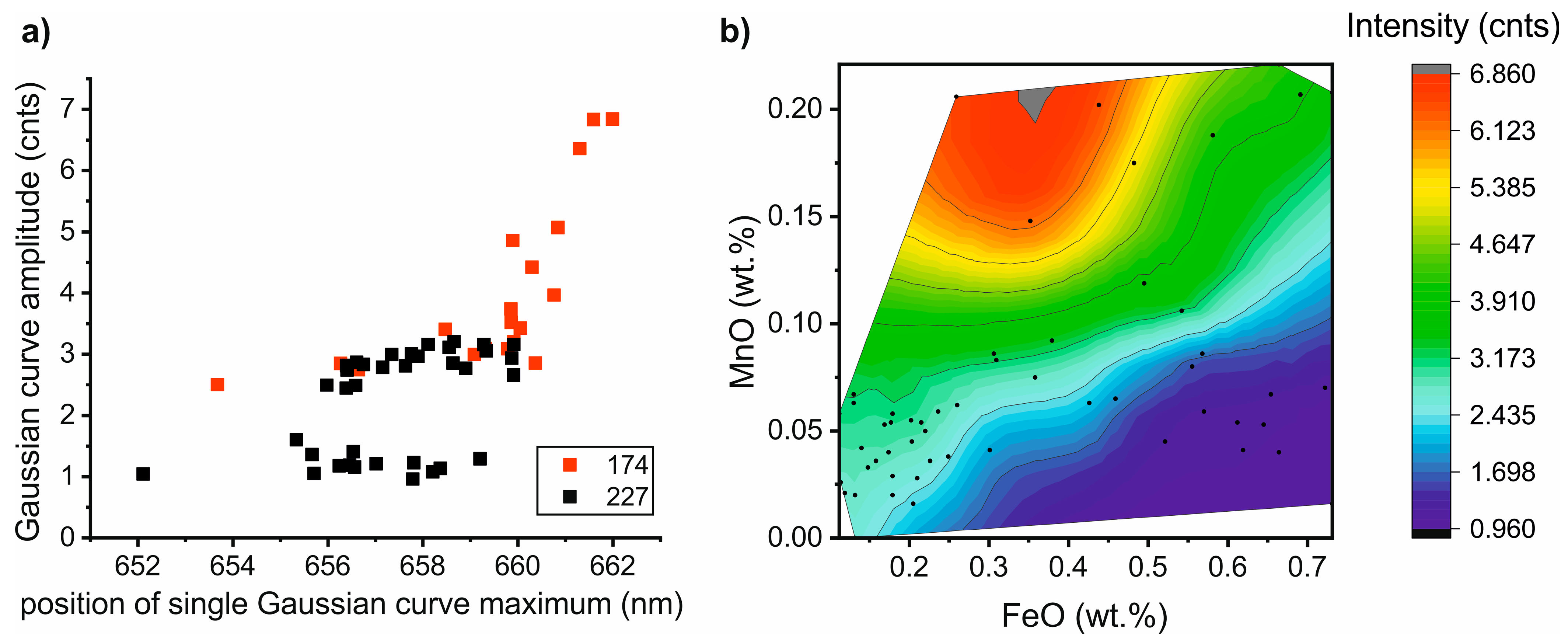
4.4. Correlation between CL-Spectra Parameters and Calcite Chemistry
4.5. Correlation between CL-Spectra Parameters and Dolomite Chemistry
5. Discussion
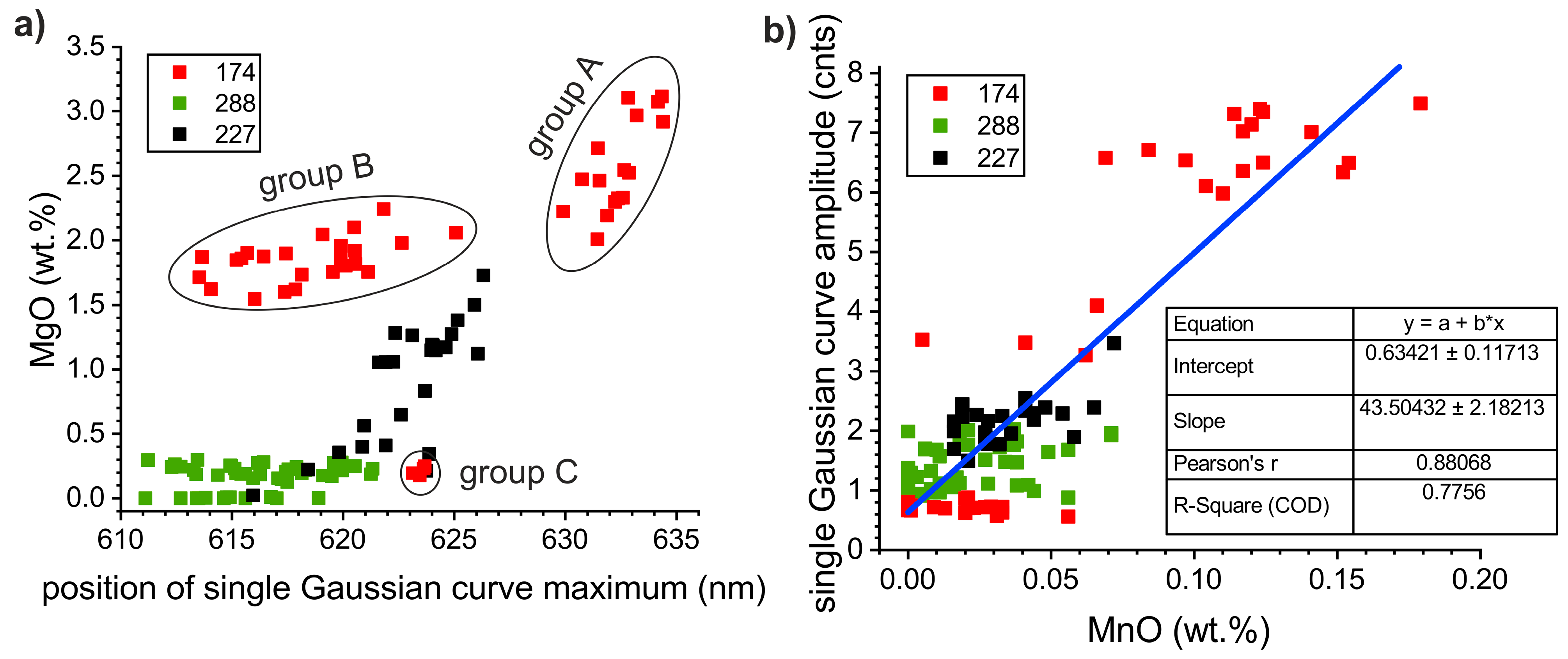
5.1. Provenance Discrimination of Studied Samples
5.2. Correlation between the Carbonate Chemistry and CL-Spectra Parameters
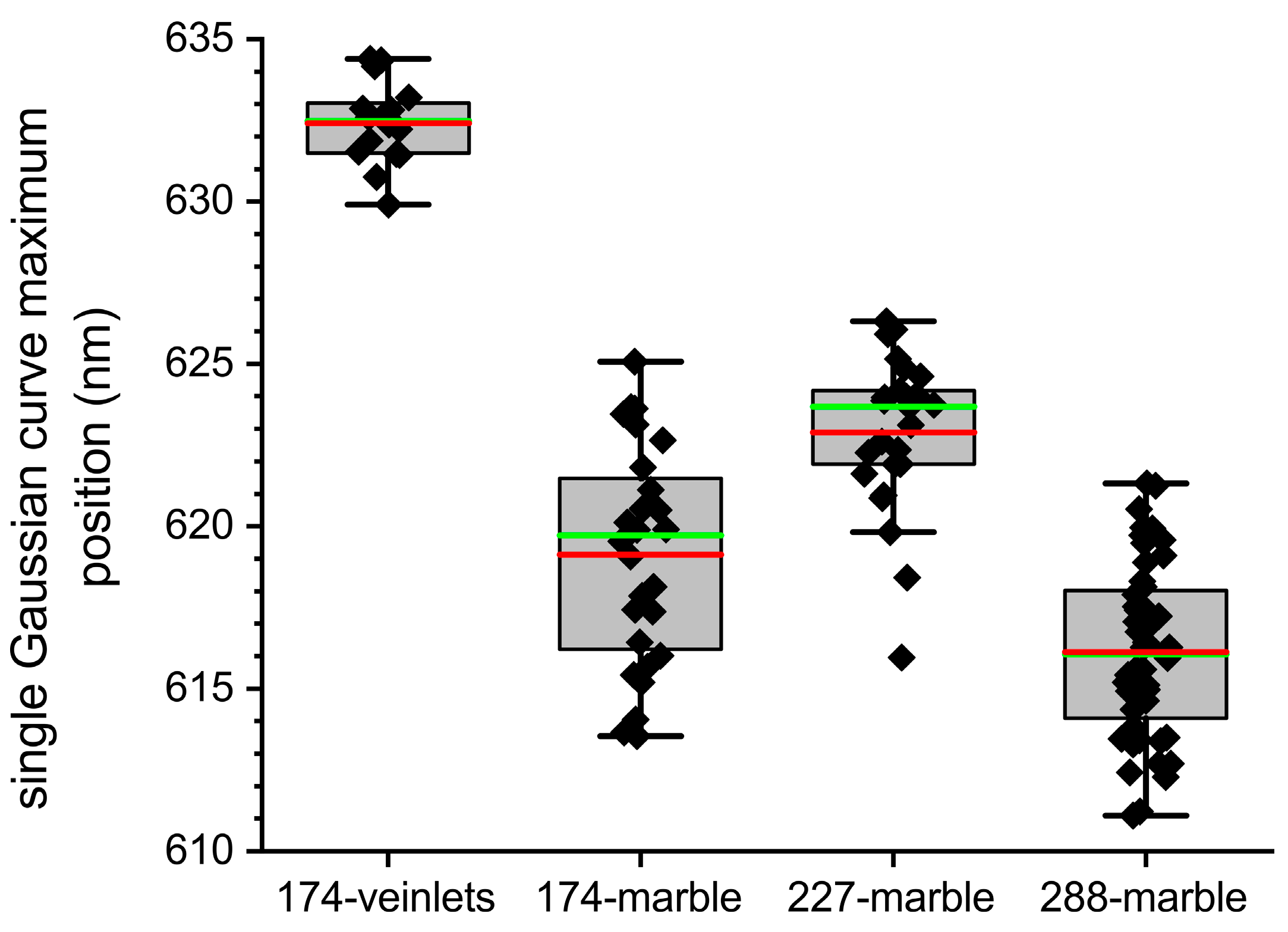
5.3. Position of the Main CL-Band of the Calcite and Dolomite Spectra
5.4. Provenance Discrimination of Studied Samples
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschall, D.J. Cathodoluminescence of Geological Materials; Unwin Hyman Ltd.: London, UK, 1988. [Google Scholar]
- Machel, H.G. Application of cathodoluminescence to carbonate diagenesis. In Cathodoluminescence in Geosciences; Pagel, M., Barbin, V., Blanc, P.H., Ohnenstetter, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 271–301. [Google Scholar]
- Habermann, D.; Neuser, R.D.; Richter, D.K. Quantitative high resolution analysis of Mn2+ in sedimentary calcite. In Cathodoluminescence in Geosciences; Pagel, M., Barbin, V., Blanc, P., Ohnenstetter, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 331–358. [Google Scholar]
- Calderón, T.; Aguilar, M.; Jaque, F.; Coy-Ill, R. Thermoluminescence from natural calcite. J. Phys. C Solid State Phys. 1984, 17, 2027–2038. [Google Scholar] [CrossRef]
- Boggs, S., Jr.; Krinsley, D. Application of Cathodoluminescence Imaging to the Study of Sedimentary Rocks; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Pagel, M.; Barbin, V.; Blanc, P.H.; Ohnenstetter, D. (Eds.) Cathodoluminescence in Geosciences; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Richter, D.; Götte, T.; Götze, J.; Neuser, R.D. Progress in application of cathodoluminescence (CL) in sedimentary petrology. Miner. Petrol. 2003, 79, 127–166. [Google Scholar] [CrossRef]
- El Ali, A.; Barbin, V.; Calas, G.; Cervelle, B.; Ramseyer, K.; Bouroulec, J. Mn2+-activated luminescence in dolomite, calcite and magnesite: Quantitative determination of manganese and site distribution by EPR and CL spectroscopy. Chem. Geol. 1993, 104, 189–202. [Google Scholar] [CrossRef]
- Gillhaus, A.; Habermann, D.; Meijer, J.; Richter, D.K. Cathodoluminescence spectroscopy and micro-PIXE: Combined high resolution Mn-analyses in dolomites—First results. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2000, 161–163, 842–845. [Google Scholar] [CrossRef]
- Gillhaus, A.; Richter, D.K.; Meijer, J.; Neuser, R.D.; Stephan, A. Quantitative high resolution cathodoluminescence spectroscopy of diagenetic and hydrothermal dolomites. Sediment. Geol. 2001, 140, 191–199. [Google Scholar] [CrossRef]
- Barbin, V.; Ramseyer, K.; Decrouez, D.; Burns, S.J.; Chamay, J.; Maier, J.L. Cathodoluminescence of white marbles: An overview. Archaeometry 1992, 34, 175–183. [Google Scholar] [CrossRef]
- Herrmann, J.J.; Barbin, V. The exportation of marble from the Aliki quarries on Thasos: Cathodoluminescence of samples from Turkey and Italy. Am. J. Archaeol. 1993, 97, 91–103. [Google Scholar] [CrossRef]
- Lapuente, M.P.; Turi, B.; Blanc, P.H. Marbles from Roman Hispania: Stable isotope and cathodoluminescence characterization. App. Geochem. 2000, 15, 1469–1493. [Google Scholar] [CrossRef]
- Habermann, D. Quantitative cathodoluminescence (CL) spectroscopy of minerals: Possibilities and limitations. Mineral. Petrol. 2002, 76, 247–259. [Google Scholar] [CrossRef]
- Götze, J. Application of Cathodoluminescence Microscopy and Spectroscopy in Geosciences. Microsc. Microanal. 2012, 18, 1270–1284. [Google Scholar] [CrossRef] [Green Version]
- Blanc, P.; Mercadal, M.P.L.; Garcia-Moreno, A.G. A new database of the quantitative cathodoluminescence of the main quarry marbles used in antiquity. Minerals 2020, 10, 381. [Google Scholar] [CrossRef]
- Mercadal, M.P.L.; Oterino, J.A.C.; Blanc, P.; Brilli, M. Louvie-Soubiron Marble: Heritage Stone in the French Pyrenean Ossau Valley-First Evidence of the Roman Trans-Pyrenean Use. Geoheritage 2021, 13, 17. [Google Scholar] [CrossRef]
- Antonelli, F.; Lazzarini, L. An updated petrographic and isotopic reference database for white marbles used in antiquity. Rend. Lincei 2015, 26, 399–413. [Google Scholar] [CrossRef]
- Prochaska, W.; Attanasio, D. The challenge of a successful discrimination of ancient marbles (part I): A databank for the marbles from Paros, Prokonnesos, Heraklea/Miletos and Thasos. J. Archaeol. Sci. Rep. 2021, 35, 102676. [Google Scholar] [CrossRef]
- Prochaska, W.; Attanasio, D. The challenge of a successful discrimination of ancient marbles (part III): A databank for Aphrodisias, Carrara, Dokimeion, Göktepe, Hymettos, Parian Lychnites and Pentelikon. J. Archaeol. Sci. Rep. 2022, 45, 103582. [Google Scholar]
- Jarč, S.; Zupančič, N. A cathodoluminescence and petrographical study of marbles from Pohorje area in Slovenia. Geochemistry 2009, 69, 75–80. [Google Scholar] [CrossRef]
- Kuchařová, A.; Přikryl, R. Mineralogical and geochemical (stable C and O isotopes) variability of marbles from the Moldanubian Zone (Bohemian Massif, Czech Republic): Implications for provenance studies. Environ Earth Sci. 2017, 76, 48. [Google Scholar] [CrossRef]
- Šťastná, A.; Přikryl, R.; Černíková, A. Comparison of quantitative petrographic, stable isotope and cathodoluminescence data for fingerprinting Czech marbles. Environ. Earth Sci. 2011, 63, 1651–1663. [Google Scholar] [CrossRef]
- Winchester, J.A.; Patočka, F.; Kachlík, V.; Melzer, M.; Nawakowski, C.; Crowley, Q.G.; Floyd, P.A. Geochemical discrimination of metasedimentary sequences in the Krkonoše-Jizera Terrane (West Sudetes, Bohemian Massif): Palaeotectonic and stratigraphic constraints. Geol. Carpathica. 2003, 54, 267–280. [Google Scholar]
- Kachlík, V. Relationship between Moldanubicum, the Kutná Hora Crystalline Unit and Bohemicum (Central Bohemia, Czech Republic): A result of the polyphase Variscan nappe tectonics. J. Czech Geol. Soc. 1999, 44, 201–291. [Google Scholar]
- Mazur, S. Geology of the Karkonosze-Izera Massif: An overview. Miner. Soc. Pol. Spec. Pap. 2002, 20, 22–34. [Google Scholar]
- Houzar, S.; Novák, M.; Doležalová, H.; Hrazdil, V.; Pfeiferová, A. Mineralogy, petrography and geology of the Nedvědice marbles, Svratka Crystalline Complex: A review. Acta Mus. Morav. Sci. Geol. 2006, LXXXXI, 3–77. (In Czech) [Google Scholar]
- Hladká, N. Mineralogical-petrographic conditions in the Vápenný Vrch near Raspenava and its wider surrounding. Sbor. Ústř. Úst. Geol. Odd. Geol. 1957, 24, 169–207. (In Czech) [Google Scholar]
- Krutský, N. From the History of stones mining and exploitation in the Northen Bohemia. Geolog. Průzkum 1993, 35, 261–264. (In Czech) [Google Scholar]
- Krutský, N. From the history of exploitation and utilization of limestones in ČSR. In History of the Exploitation of Non-Ore Resources; Kužvart, M., Ed.; NTM Prague: Prague, Czech Republic, 1986; pp. 70–79. (In Czech) [Google Scholar]
- Rybařík, V. Dimension Stones from the Czech Republic; Nadace Střední průmyslové školy kamenické a sochařské v Hořicích v Podkrkonoší: Hořice, Czech Republic, 1994; pp. 1–218. (In Czech) [Google Scholar]
- Houzar, S.; Malý, K. Marbles of the Bohemian-Moravian Highlands as a source of materials for historic buildings: An overview of localities and research methodology. Acta Rerum Nat. 2021, 26, 19–46. (In Czech) [Google Scholar]
- Microsoft Excel Software. Available online: https://www.microsoft.com/cs-cz/microsoft-365/excel (accessed on 6 January 2023).
- Origin Software. Available online: https://www.originlab.com/Origin (accessed on 6 January 2023).
- Essential FTIR Software. Available online: https://www.essentialftir.com/ (accessed on 6 January 2023).
- Magicplot Software. Available online: https://magicplot.com/ (accessed on 6 January 2023).
- MagicPlot Manual. Available online: https://magicplot.com/wiki/ (accessed on 6 January 2023).
- Šťastná, A.; Přikryl, R. Decorative marbles from the Krkonoše-Jizera Terrane (Bohemian Massif, Czech Republic): Provenance criteria. Int. J. Earth Sci. 2009, 98, 357–366. [Google Scholar] [CrossRef]
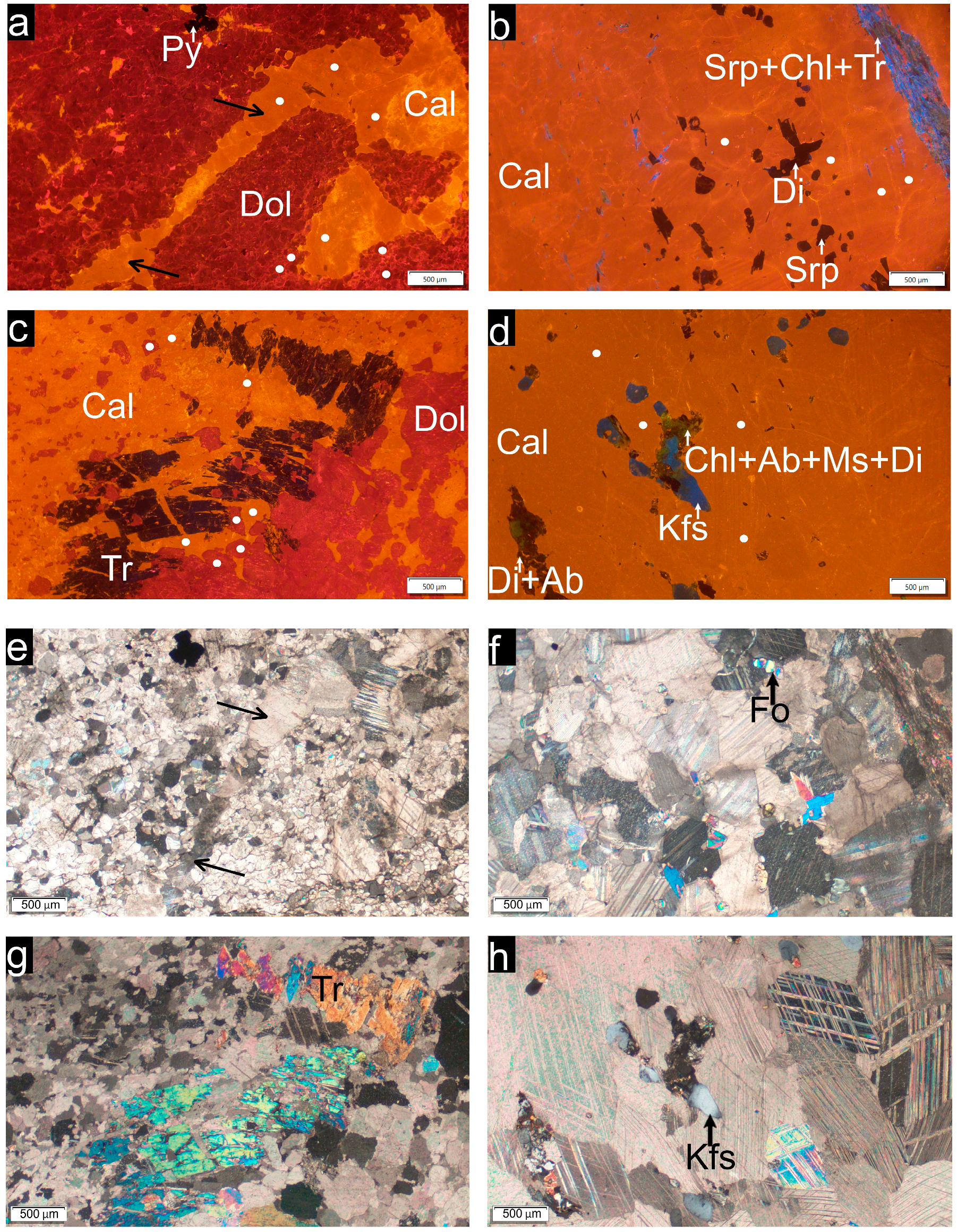


| Sample No.: Locality GPS | Geological Unit (Bohemian Massif) | Geological Description |
|---|---|---|
| 174 Raspenava 50°53′32″ N, 15°8′11″ E | Lugicum (Krkonoše-Jizera Terrane) | Palaeozoic, elongated marble lenses in mica shists to paragneisses, mesozonal peak metamorphism in amphibolite facies [26,28] |
| 227 Bohdaneč 49°46′19″ N, 15°13′13″ E | Kutná Hora Crystalline Complex | Palaeozoic, marble lenses in two-mica gneisses (Micashist Zone), sillimanite zone peak metamorphism (affected by younger retrogression) [25] |
| 288 Nedvědice 49°27′24″ N, 16°19′43″ E | Svratka Crystalline Complex | Palaeozoic, elongated marble lenses in muscovite-biotite paragneisses, polyphase amphibolite facies (T > 550 °C at P = 200 MPa, XCO2 < 0.2–0.1) [27] |
| Sample (Locality) | Group | Non-Carbonate Mineral Assemblage | ED of CG (mm) | Microfabric |
|---|---|---|---|---|
| 174 (Raspenava) | Dolomitic marble (Dol+Cal) | Tr+Srp+Chl+Py±Fo±Di | 0.20 | Polygonal fabric, HO, straight/curved GB, short irregular calcite filling (veinlets) |
| 227 (Bohdaneč) | Dolomitic marble (Dol+Cal) | Phl+Tr+Qtz ±Pl±Kfs±Chl±Tlc±Zrn±Ap ±Ti±Py±CM | 0.31 | Polygonal fabric, HO, embayed/curved/straight GB |
| 288 (Nedvědice) | Calcitic marble (Cal) | Phl+Tr+Di+Qtz ±Pl±Kfs±Chl±Wo±Zrn ±Ti±Py±CM | 0.71 | Polygonal fabric, HE, embayed GB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zachariáš, J.; Kuchařová, A.; Kotrlý, M. Provenance Analysis of Marbles by Combination of Cathodoluminescence Spectroscopy and Electron Microprobe Analyses—Methodological Comments. Minerals 2023, 13, 244. https://doi.org/10.3390/min13020244
Zachariáš J, Kuchařová A, Kotrlý M. Provenance Analysis of Marbles by Combination of Cathodoluminescence Spectroscopy and Electron Microprobe Analyses—Methodological Comments. Minerals. 2023; 13(2):244. https://doi.org/10.3390/min13020244
Chicago/Turabian StyleZachariáš, Jiří, Aneta Kuchařová, and Marek Kotrlý. 2023. "Provenance Analysis of Marbles by Combination of Cathodoluminescence Spectroscopy and Electron Microprobe Analyses—Methodological Comments" Minerals 13, no. 2: 244. https://doi.org/10.3390/min13020244
APA StyleZachariáš, J., Kuchařová, A., & Kotrlý, M. (2023). Provenance Analysis of Marbles by Combination of Cathodoluminescence Spectroscopy and Electron Microprobe Analyses—Methodological Comments. Minerals, 13(2), 244. https://doi.org/10.3390/min13020244






