Mechanisms for the Accumulation of Organic Matter in Sediments of the Middle Permian around Bogda Mountain, Southern Junggar Basin, NW China
Abstract
1. Introduction
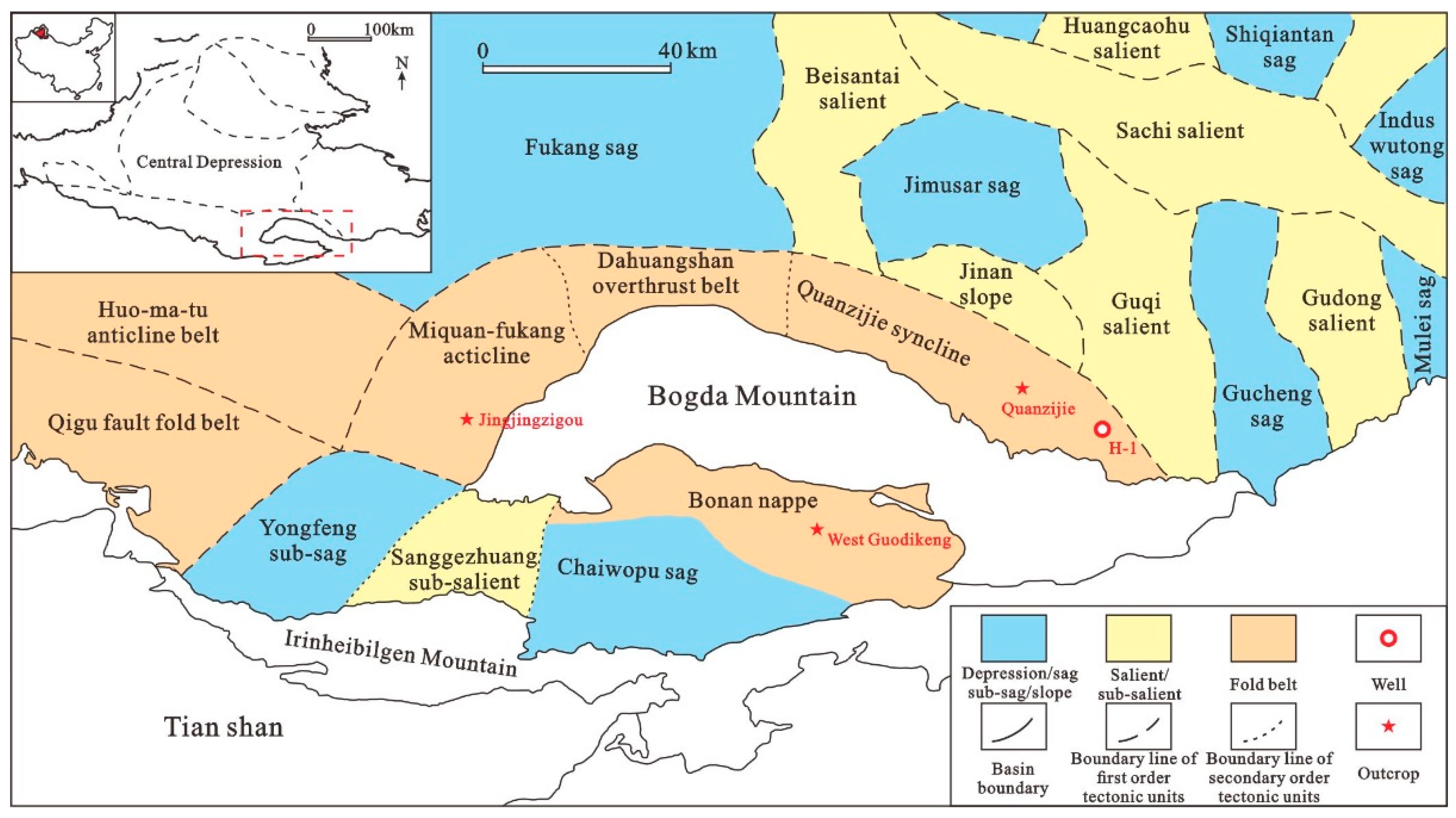
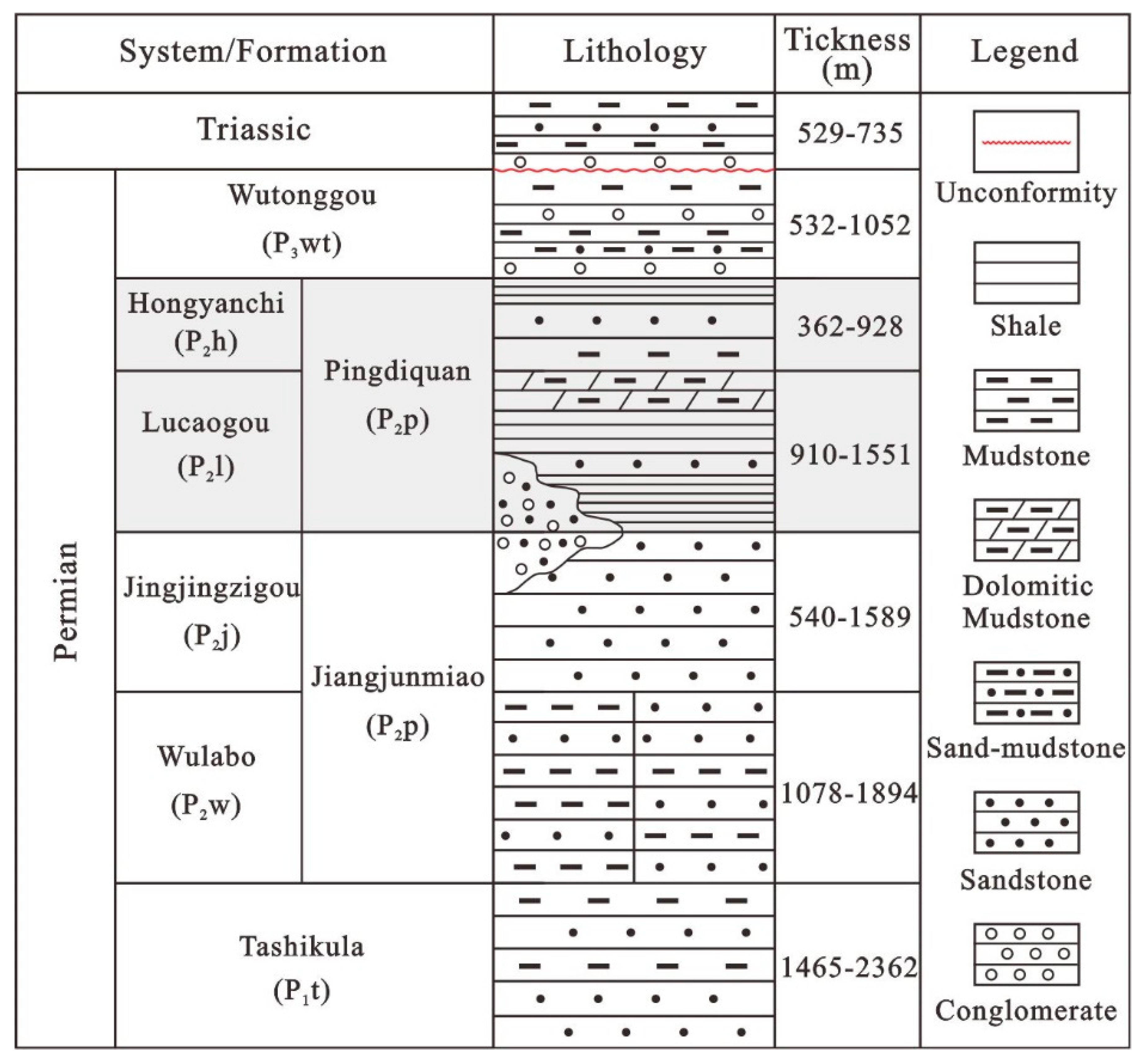
2. Geological Setting
3. Material and Methods
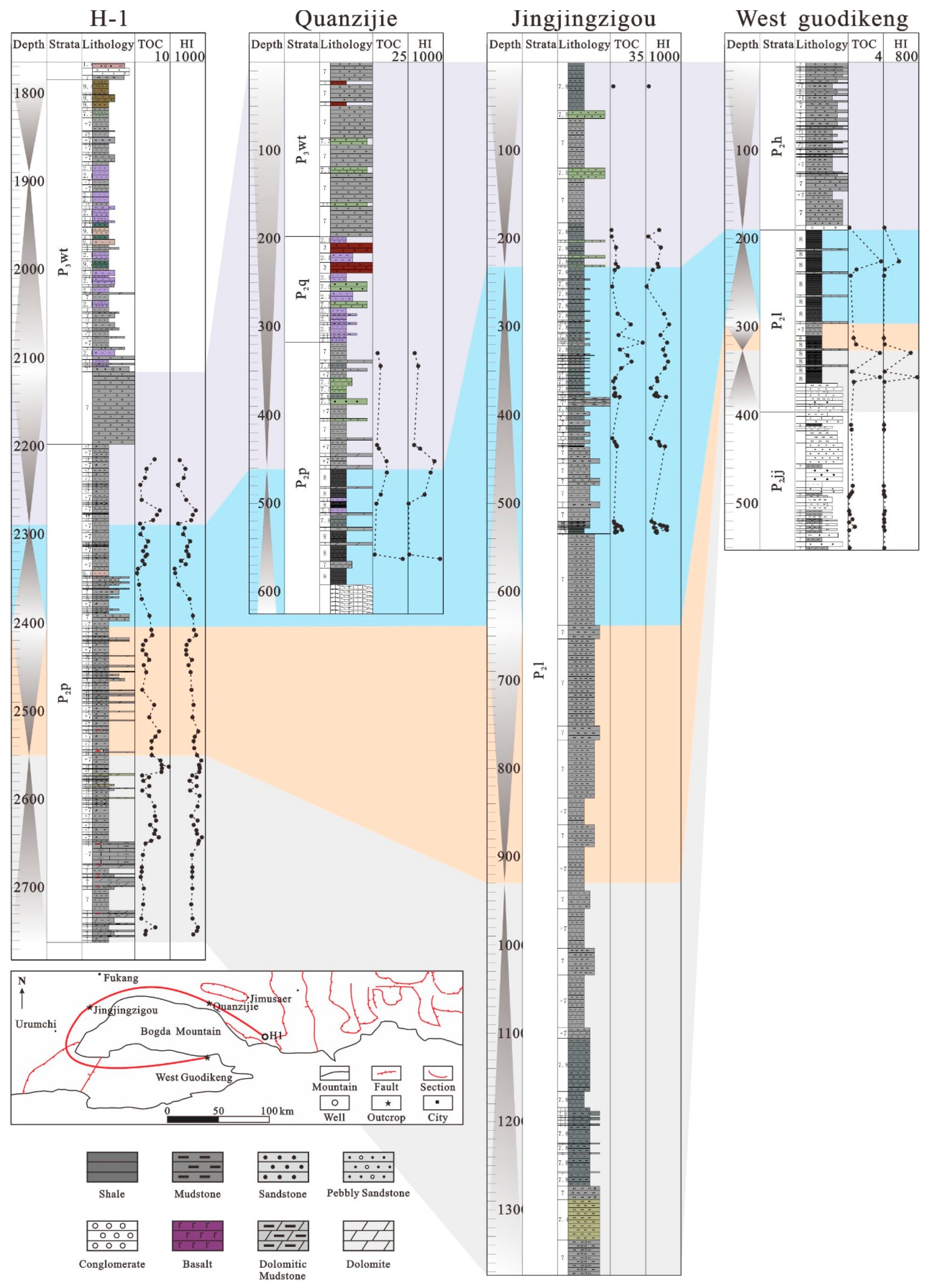
3.1. Sampling
3.2. Measurement of Total Organic Carbon and Rock-Eval Pyrolysis
3.3. Analysis of Molecular Markers
3.4. Major Oxides and Trace Elements
3.5. Organic Petrology
3.6. Stable Carbon Isotope
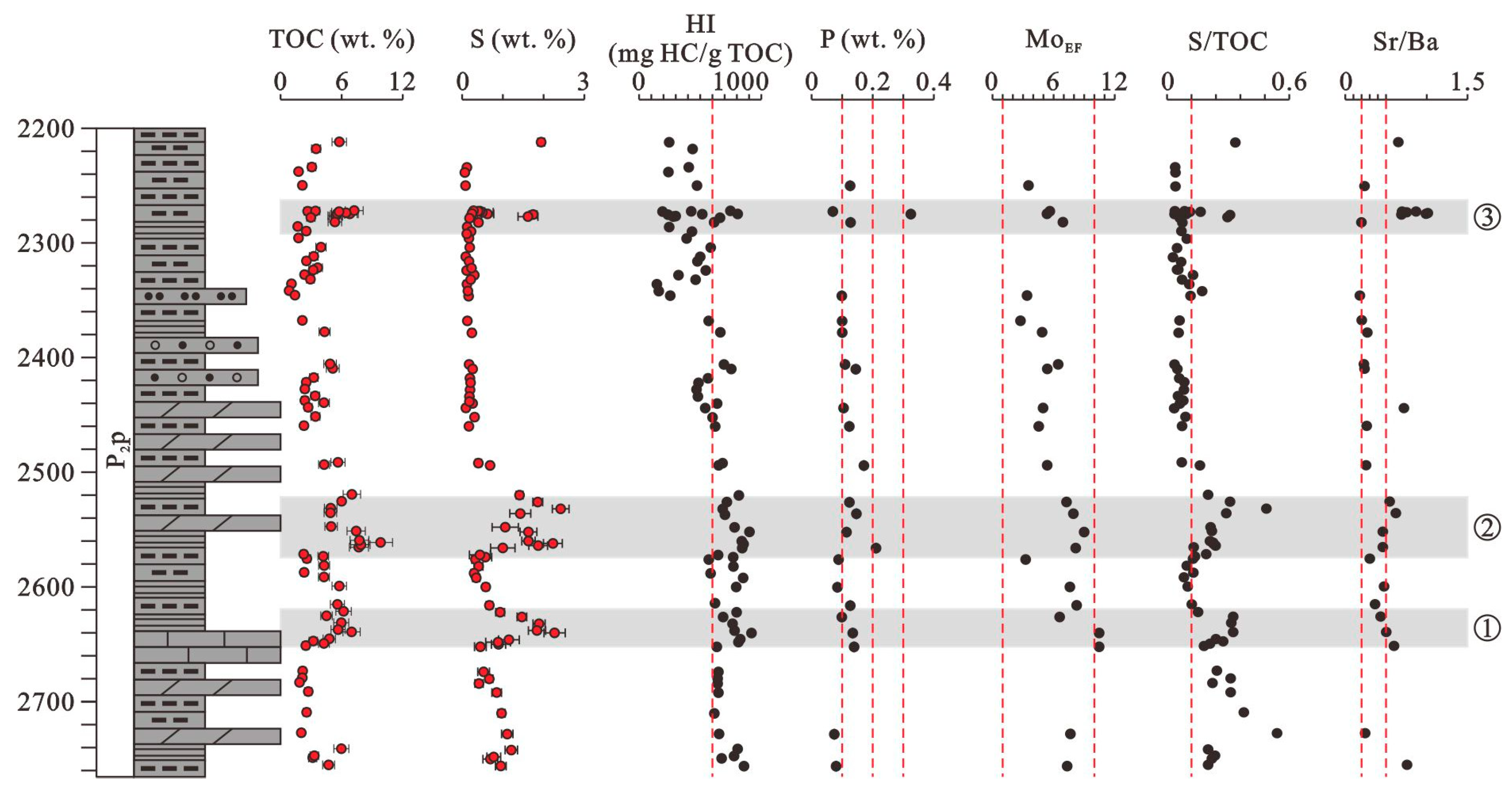
4. Results
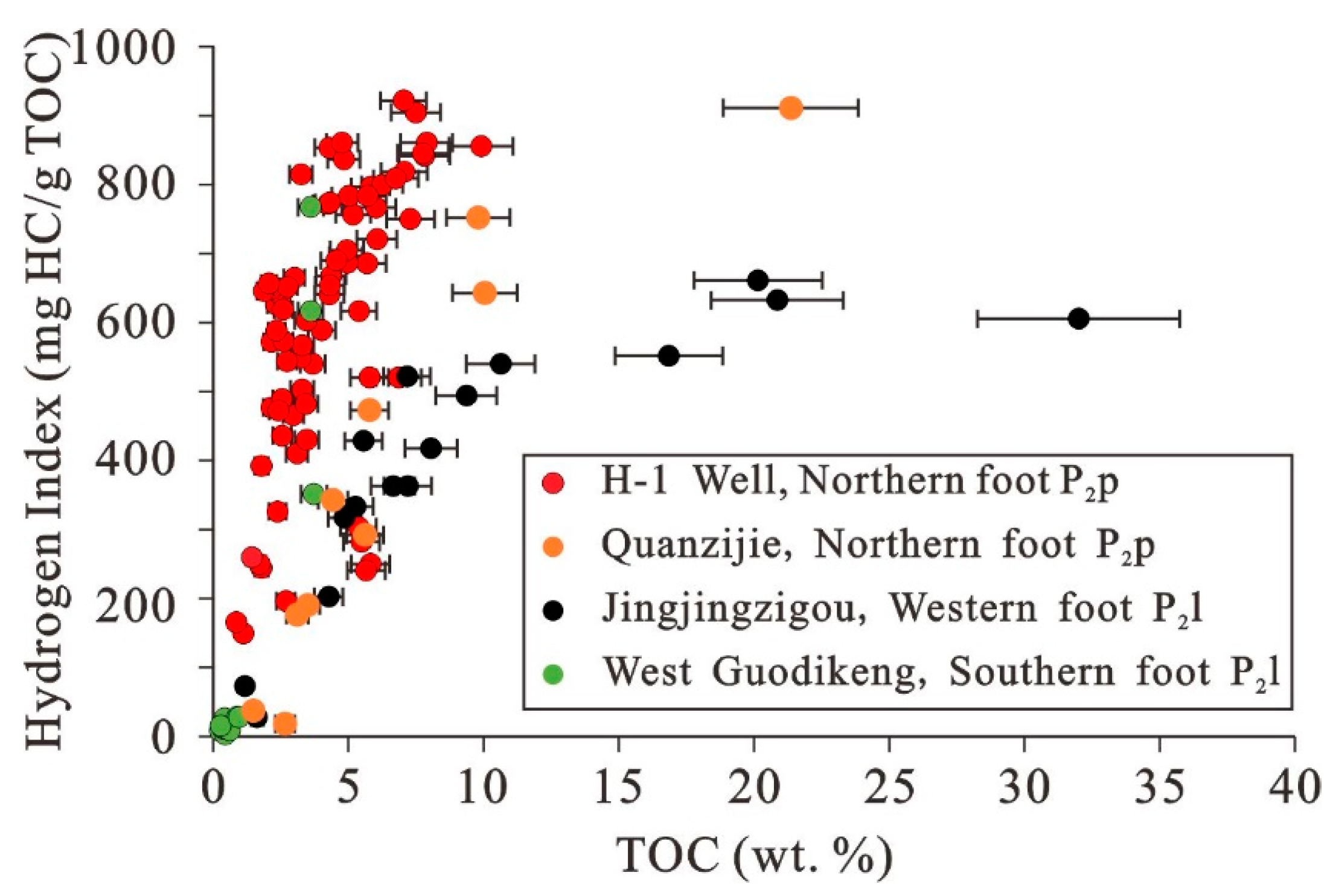
4.1. Distribution and Evaluation of Organic Rich Shales and Mudstones
4.2. Saturated Hydrocarbon Species
4.3. Major and Trace Elements
4.4. Stable Carbon Isotopes
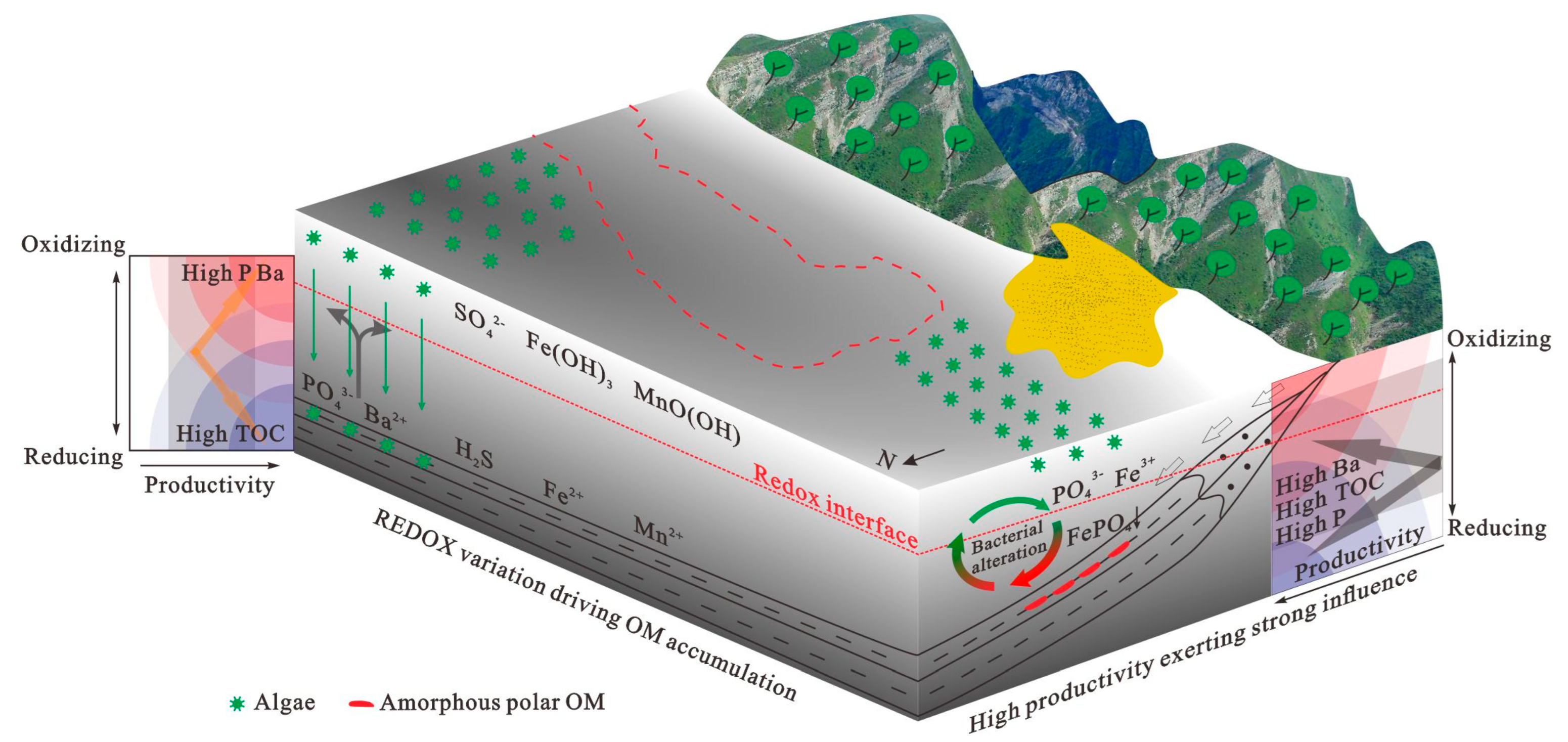
5. Discussion
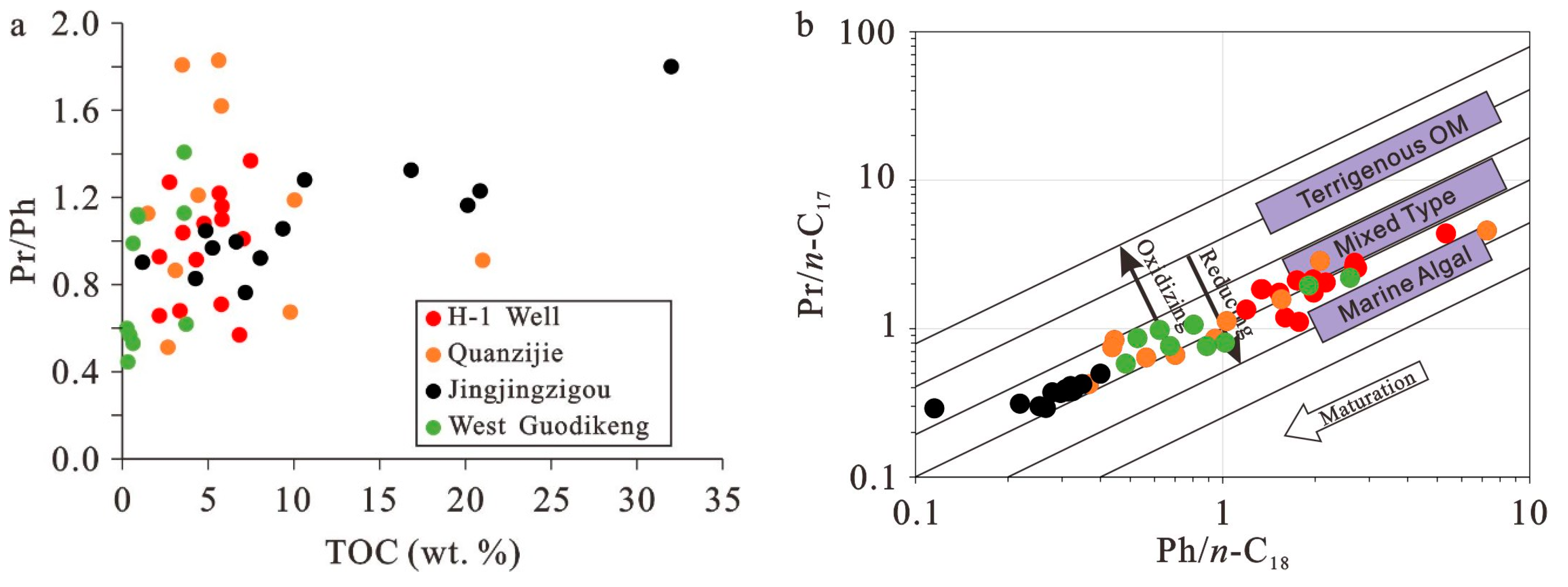
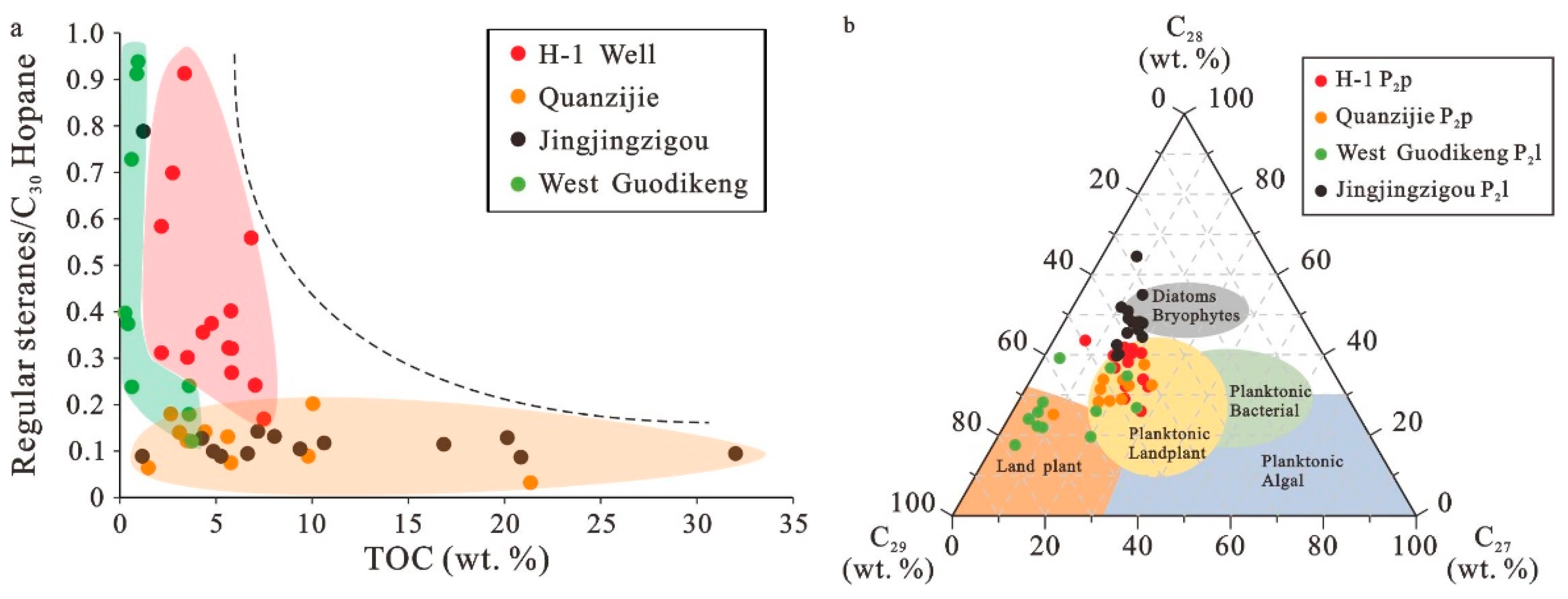
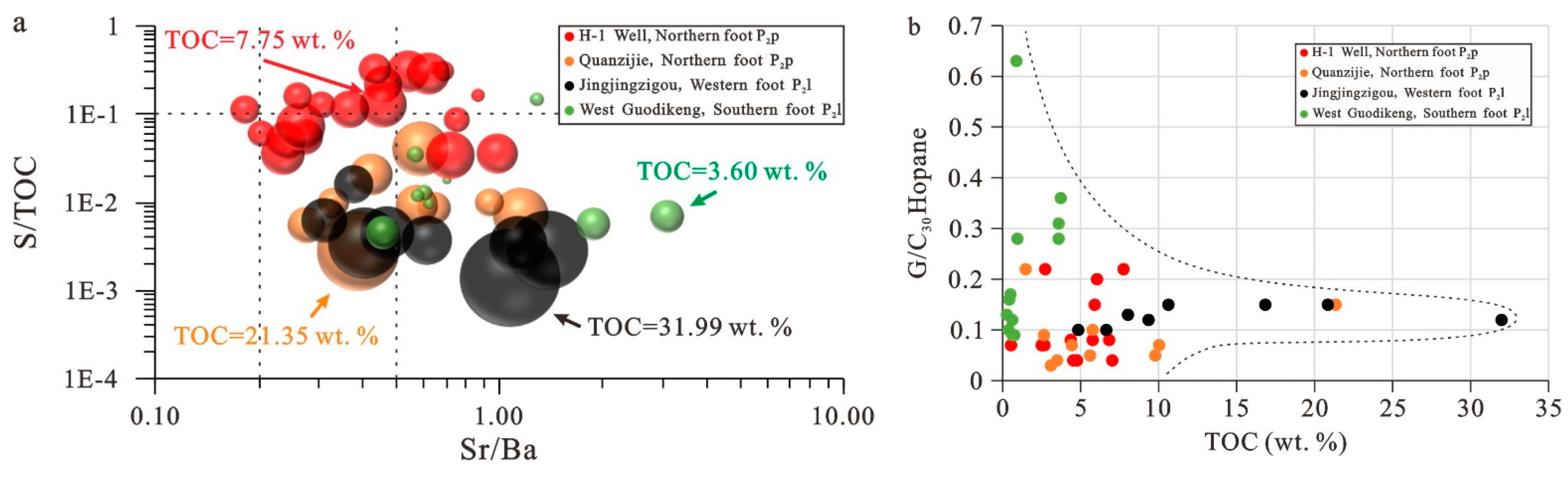
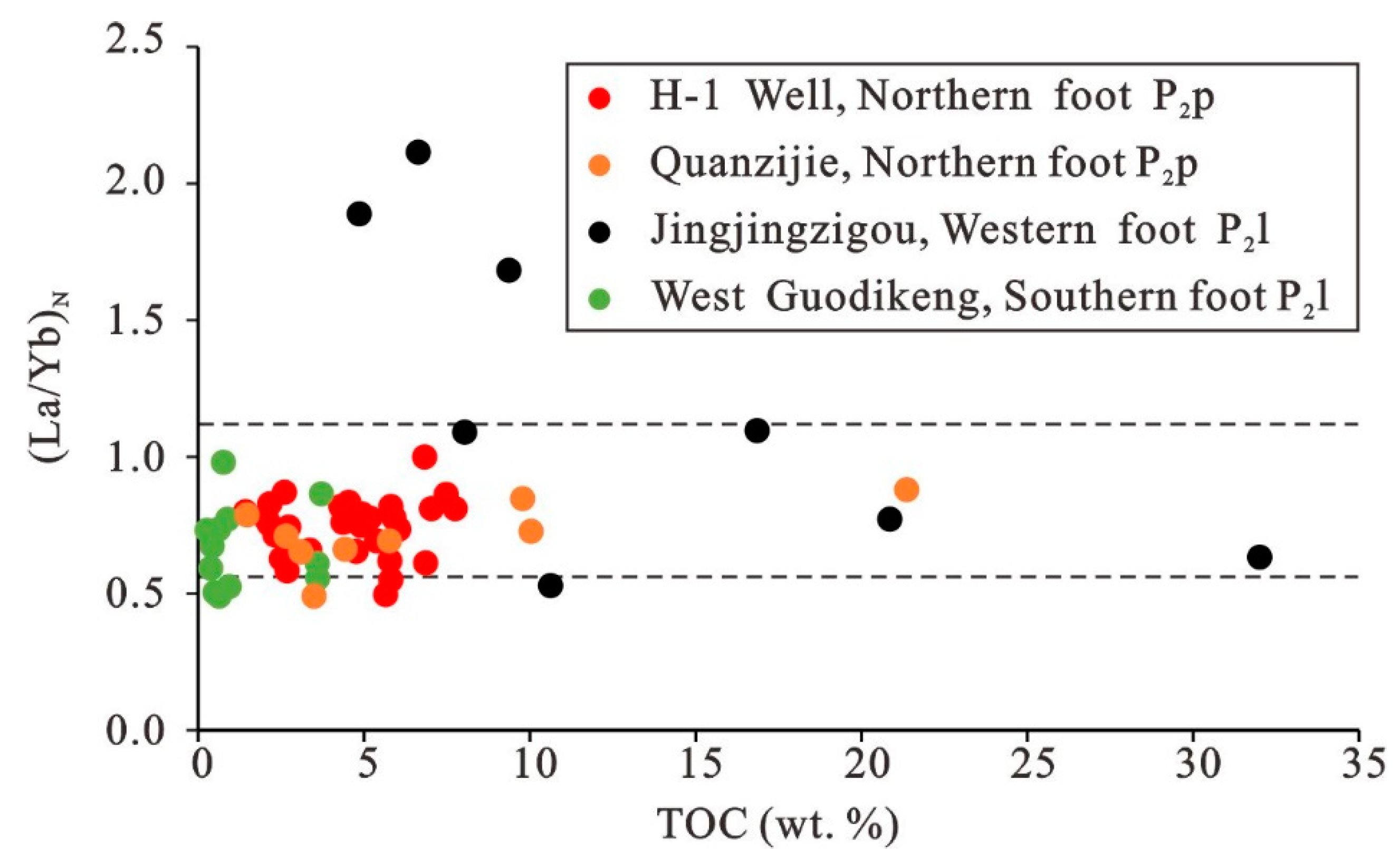
5.1. Source of Organic Matter in Shales and Mudstones
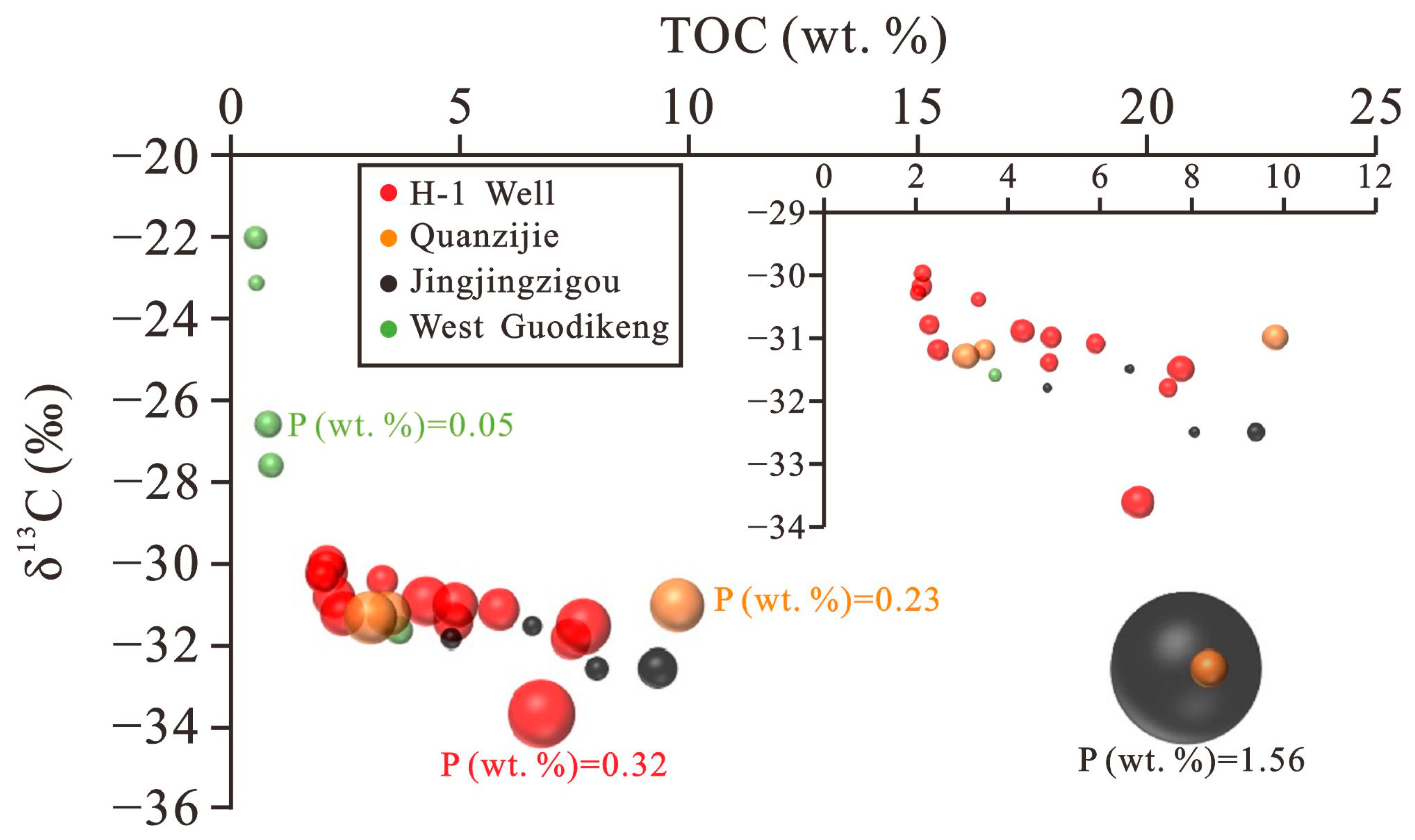
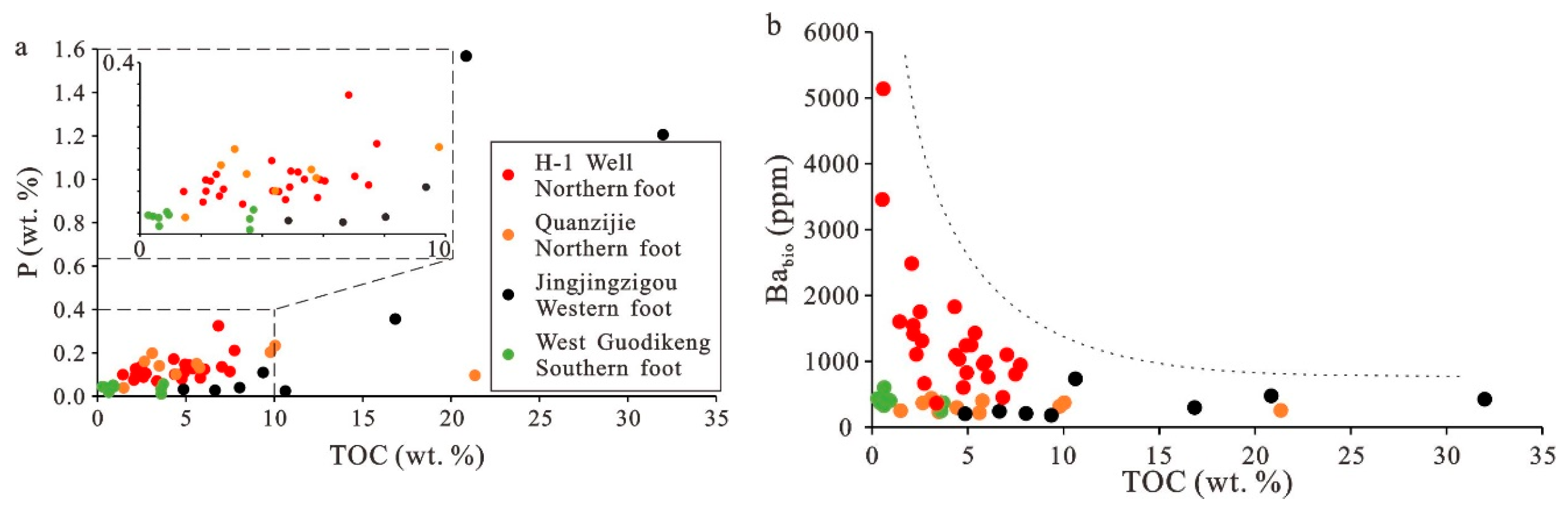
5.2. Paleoproductivity Study
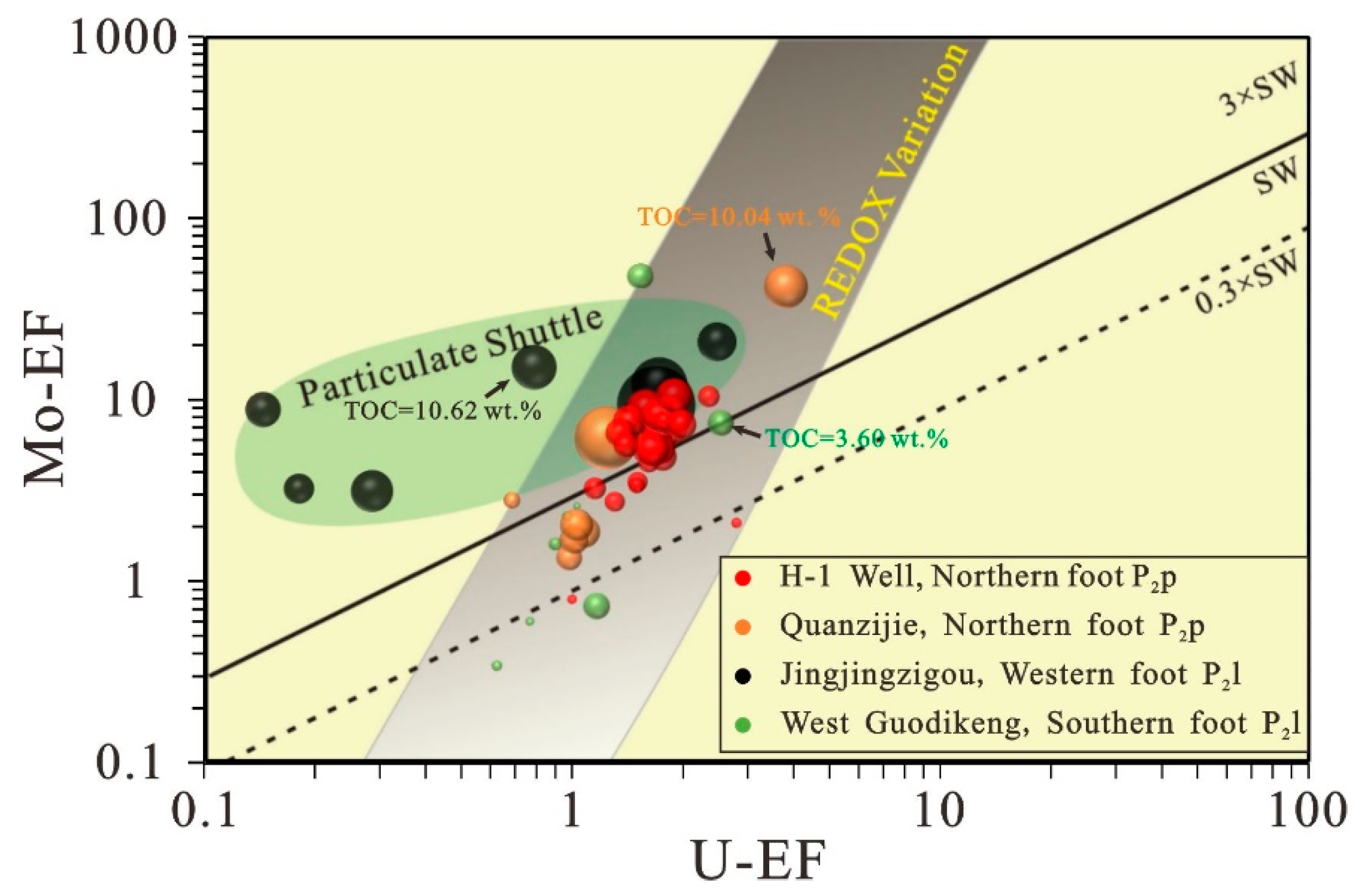
5.3. Paleoredox Conditions
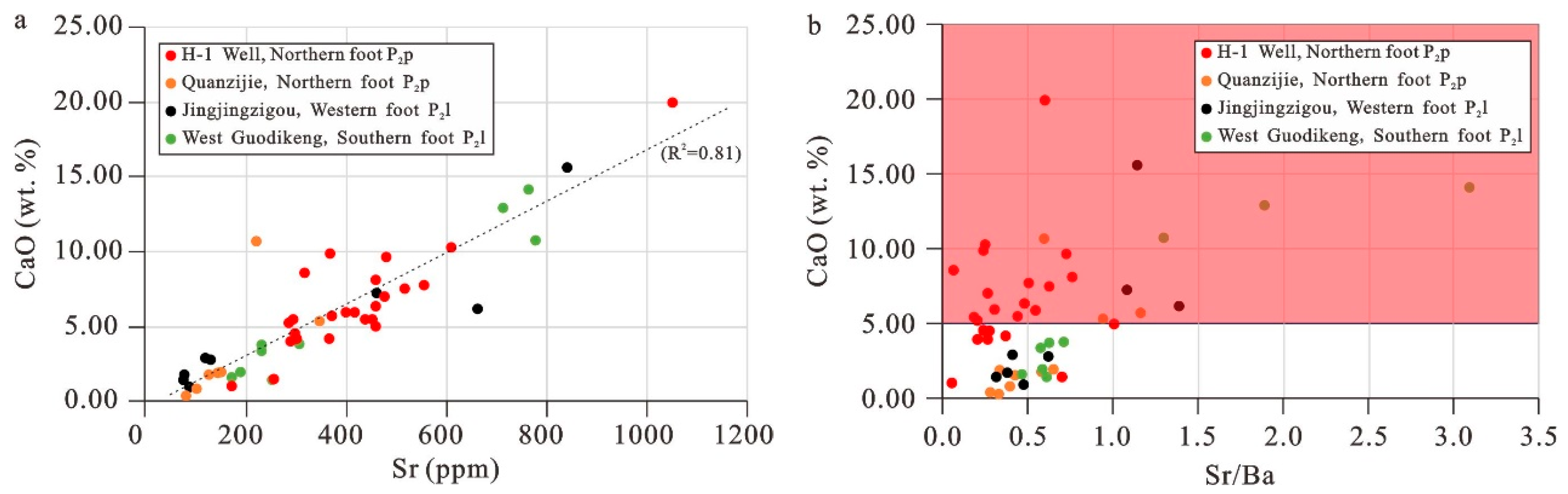
5.4. Paleosalinity
5.5. Accumulation of Organic Matter in Different Study Areas
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanizadeh, A.; Clarkson, C.; Aquino, S.; Ardakani, O.; Sanei, H. Petrophysical and geomechanical characteristics of Canadian tight oil and liquid-rich gas reservoirs: I. Pore network and permeability characterization. Fuel 2015, 153, 664–681. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar] [CrossRef]
- Luo, Q.; Gong, L.; Qu, Y.; Zhang, K.; Zhang, G.; Wang, S. The tight oil potential of the Lucaogou Formation from the southern Junggar Basin, China. Fuel 2018, 234, 858–871. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Z.; Li, H.; Zhong, N.; Xiao, L.; Jin, X.; Li, H. Mechanism for the enrichment of organic matter in the Liushagang Formation of the Weixinan Sag, Beibuwan Basin, China. Mar. Pet. Geol. 2020, 122, 104649. [Google Scholar] [CrossRef]
- Liu, B.; Bechtel, A.; Sachsenhofer, R.F.; Gross, D.; Gratzer, R.; Chen, X. Depositional environment of oil shale within the second member of Permian Lucaogou Formation in the Santanghu Basin, Northwest China. Int. J. Coal Geol. 2017, 175, 10–25. [Google Scholar] [CrossRef]
- Liu, B.; Song, Y.; Zhu, K.; Su, P.; Ye, X.; Zhao, W. Mineralogy and element geochemistry of salinized lacustrine organic-rich shale in the Middle Permian Santanghu Basin: Implications for paleoenvironment, provenance, tectonic setting and shale oil potential. Mar. Pet. Geol. 2020, 120, 104569. [Google Scholar] [CrossRef]
- Burton, Z.F.M.; Moldowan, J.M.; Magoon, L.B.; Sykes, R.; Graham, S.A. Interpretation of source rock depositional environment and age from seep oil, east coast of New Zealand. Int. J. Earth Sci. 2019, 108, 1079–1091. [Google Scholar] [CrossRef]
- Dow, W.G. Kerogen studies and geological interpretations. J. Geochem. Explor. 1977, 7, 79–99. [Google Scholar] [CrossRef]
- Hutton, A.; Kantsler, A.; Cook, A.; McKirdy, D. Organic matter in oil shales. APPEA J. 1980, 20, 44–67. [Google Scholar] [CrossRef]
- Schulz, H.-M.; Yang, S.; Schovsbo, N.H.; Rybacki, E.; Ghanizadeh, A.; Bernard, S.; Mahlstedt, N.; Krüger, M.; Amann-Hildebrandt, A.; Krooss, B.M.; et al. The Furongian to Lower Ordovician Alum Shale Formation in conventional and unconventional petroleum systems in the Baltic Basin—A review. Earth-Science Rev. 2021, 218, 103674. [Google Scholar] [CrossRef]
- Slatt, R.M. Important geological properties of unconventional resource shales. Open Geosci. 2011, 3, 435–448. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Liu, S.; Gao, G.; Jun, J.; Misch, D.; Xinsong, W.; Gang, W.; Wang, M.; Baoli, X.; Wanyun, M. Mechanism of differential enrichment of shale oils in the upper and lower members of the Lucaogou Formation in the Jimusaer Sag, Junggar Basin. Mar. Pet. Geol. 2022, 142, 105747. [Google Scholar] [CrossRef]
- Liu, B.; Bechtel, A.; Gross, D.; Fu, X.; Li, X.; Sachsenhofer, R.F. Middle Permian environmental changes and shale oil potential evidenced by high-resolution organic petrology, geochemistry and mineral composition of the sediments in the Santanghu Basin, Northwest China. Int. J. Coal Geol. 2017, 185, 119–137. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhang, J.; Gao, Y.; Zhou, X.; Sun, X.; Wen, L.; Miao, M. Sedimentary characteristics and main controlling factors of the Middle-Upper Permian and Middle-Upper Triassic around Bogda Mountain of Xinjiang, NW China. Pet. Explor. Dev. 2022, 49, 770–784. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Z. Thermal maturity of the Permian Lucaogou Formation organic-rich shale at the northern foot of Bogda Mountains, Junggar Basin (NW China): Effective assessments from organic geochemistry. Fuel 2018, 211, 278–290. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, J.; Hou, Q.; Yu, B.; Li, M.; Niu, Q. Anisotropic characteristics of low-rank coal fractures in the Fukang mining area, China. Fuel 2017, 211, 182–193. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.; Jiang, S.; Wang, Q.; Zheng, X.; Ding, X.; Zhao, Y.; Zhu, C.; Li, H. Oil content evaluation of lacustrine organic-rich shale with strong heterogeneity: A case study of the Middle Permian Lucaogou Formation in Jimusaer Sag, Junggar Basin, NW China. Fuel 2018, 221, 196–205. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Q.; Zhang, C.; Gao, Y.; Du, W.; Song, Y.; Zhang, Z.; Luo, Q.; Jiang, Z.; Huang, Z. Paleoenvironment evolution of the Permian Lucaogou Formation in the southern Junggar Basin, NW China. Palaeogeogr. Palaeoclim. Palaeoecol. 2022, 603, 111198. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Y.; Jiao, X.; Ma, L.; Zhou, D.; Li, H.; Cao, Q.; Zhao, M.; Yang, Y. Petrological and organic geochemical characteristics of the Permian Lucaogou Formation in the Jimsar Sag, Junggar Basin, NW China: Implications on the relationship between hydrocarbon accumulation and volcanic-hydrothermal activities. J. Pet. Sci. Eng. 2021, 210, 110078. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, J.; Jin, J.; Yang, G.; Xiang, B.; Zhou, N.; Ma, W.; Qiao, J.; Li, S.; Liu, S. Geochemical evaluation of produced petroleum from the Middle Permian Lucaogou reservoirs Junggar Basin and its implication for the unconventional shale oil play. J. Pet. Sci. Eng. 2022, 211, 110202. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Damsté, J.S.S.; Kenig, F.; Koopmans, M.P.; Köster, J.; Schouten, S.; Hayes, J.; de Leeuw, J.W. Evidence for gammacerane as an indicator of water column stratification. Geochim. et Cosmochim. Acta 1995, 59, 1895–1900. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Chen, J. Stable carbon isotope evidence for the origin of C28 steranes in lacustrine source rocks from the Qikou Sag, Bohai Bay Basin, Eastern China. Org. Geochem. 2020, 145, 104028. [Google Scholar] [CrossRef]
- Algeo, T.J.; Liu, J. A re-assessment of elemental proxies for paleoredox analysis. Chem. Geol. 2020, 540, 119549. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Z.; Zhao, J.; Jin, X.; Li, H.; Li, J.; Wei, X. Discussion on the applicability of Th/U ratio for evaluating the paleoredox conditions of lacustrine basins. Int. J. Coal Geol. 2021, 248, 103868. [Google Scholar] [CrossRef]
- Wei, W.; Algeo, T.J. Elemental proxies for paleosalinity analysis of ancient shales and mudrocks. Geochim. et Cosmochim. Acta 2019, 287, 341–366. [Google Scholar] [CrossRef]
- Wei, W.; Algeo, T.J.; Lu, Y.; Lu, Y.; Liu, H.; Zhang, S.; Peng, L.; Zhang, J.; Chen, L. Identifying marine incursions into the Paleogene Bohai Bay Basin lake system in northeastern China. Int. J. Coal Geol. 2018, 200, 1–17. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Z.; Li, J.; Li, K.; Chen, Y.; Guo, Z. Late Paleozoic to Jurassic tectonic evolution of the Bogda area (northwest China): Evidence from detrital zircon U–Pb geochronology. Tectonophysics 2014, 626, 144–156. [Google Scholar] [CrossRef]
- Shi, Y.; Ji, H.; Yu, J.; Xiang, P.; Yang, Z.; Liu, D. Provenance and sedimentary evolution from the Middle Permian to Early Triassic around the Bogda Mountain, NW China: A tectonic inversion responding to the consolidation of Pangea. Mar. Pet. Geol. 2020, 114, 104169. [Google Scholar] [CrossRef]
- Peters, K.; Moldowan, J.; Schoell, M.; Hempkins, W. Petroleum isotopic and biomarker composition related to source rock organic matter and depositional environment. Org. Geochem. 1986, 10, 17–27. [Google Scholar] [CrossRef]
- Peters, K.E.; Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2005; Volume 1. [Google Scholar]
- Hanson, A.D.; Ritts, B.D.; Zinniker, D.; Moldowan, J.M.; Biffi, U. Upper Oligocene lacustrine source rocks and petroleum systems of the northern Qaidam basin, northwest China. AAPG Bull. 2001, 85, 601–619. [Google Scholar]
- Burton, Z.F.M.; Moldowan, J.M.; Sykes, R.; Graham, S.A. Unraveling Petroleum Degradation, Maturity, and Mixing and Addressing Impact on Petroleum Prospectivity: Insights from Frontier Exploration Regions in New Zealand. Energy Fuels 2018, 32, 1287–1296. [Google Scholar] [CrossRef]
- Yurchenko, I.A.; Moldowan, J.M.; Peters, K.E.; Magoon, L.B.; Graham, S.A. The role of calcareous and shaly source rocks in the composition of petroleum expelled from the Triassic Shublik Formation, Alaska North Slope. Org. Geochem. 2018, 122, 52–67. [Google Scholar] [CrossRef]
- Algeo, T.J.; Li, C. Redox classification and calibration of redox thresholds in sedimentary systems. Geochim. Cosmochim. Acta 2020, 287, 8–26. [Google Scholar] [CrossRef]
- Liu, D.; Jolivet, M.; Yang, W.; Zhang, Z.; Cheng, F.; Zhu, B.; Guo, Z. Latest Paleozoic–Early Mesozoic basin–range interactions in South Tian Shan (northwest China) and their tectonic significance: Constraints from detrital zircon U–Pb ages. Tectonophysics 2013, 599, 197–213. [Google Scholar] [CrossRef]
- Fang, S.H.; Guo, Z.J.; Song, Y.; Wu, C.D.; Zhang, Z.C.; Wang, M.N.; Fan, R.D. Sedimentary facies evolution and basin pattern of the Jurassic in southern margin area of Junggar Basin. J. Palaeogeogr 2005, 7, 347–356. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. An Examination of the Geochemical Record Preserved in Sedimentary Rocks. In The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Oxford, UK, 1985; p. 312. [Google Scholar]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosystems 2001, 2, 2000GC000109. [Google Scholar] [CrossRef]
- Schoepfer, S.D.; Shen, J.; Wei, H.; Tyson, R.V.; Ingall, E.; Algeo, T.J. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity. Earth-Sci. Rev. 2014, 149, 23–52. [Google Scholar] [CrossRef]
- ASTM, D2797; Standard Practice for Preparing Coal Samples for Microscopical Analysis by Reflected Light. ASTM International: West Conshohocken, PA, USA, 2011.
- Zhang, J.; Liu, G.; Cao, Z.; Tao, S.; Felix, M.; Kong, Y.; Zhang, Y. Characteristics and formation mechanism of multi-source mixed sedimentary rocks in a saline lake, a case study of the Permian Lucaogou Formation in the Jimusaer Sag, northwest China. Mar. Pet. Geol. 2019, 102, 704–724. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, G.; Zhan, H.; Gao, J.; Zhang, J.; Li, C.; Xiang, B. Geological roles of the siltstones in tight oil play. Mar. Pet. Geol. 2017, 83, 333–344. [Google Scholar] [CrossRef]
- Moldowan, J.; Fago, F.J.; Carlson, R.M.; Young, D.C.; Duvne, G.A.; Clardy, J.; Schoell, M.; Pillinger, C.T.; Watt, D.S. Rearranged hopanes in sediments and petroleum. Geochim. et Cosmochim. Acta 1991, 55, 3333–3353. [Google Scholar] [CrossRef]
- Peters, K.E.; Peters, K.E.; Walters, C.C.; Moldowan, J.M. Biomarkers and Isotopes in Petroleum Systems and Earth History. In The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2007; Volume 2. [Google Scholar]
- Volkman, J.K. A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Xu, H.; George, S.C.; Hou, D. Algal-derived polycyclic aromatic hydrocarbons in Paleogene lacustrine sediments from the Dongying Depression, Bohai Bay Basin, China. Mar. Pet. Geol. 2019, 102, 402–425. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Meinschein, W. Sterols as ecological indicators. Geochim. Cosmochim. Acta 1979, 43, 739–745. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Seifert, W.K.; Gallegos, E.J. Relationship between petroleum composition and depositional environment of petroleum source rock. AAPG Bull. 1985, 69, 1255–1268. [Google Scholar]
- Sepúlveda, J.; Wendler, J.; Leider, A.; Kuss, H.-J.; Summons, R.E.; Hinrichs, K.-U. Molecular isotopic evidence of environmental and ecological changes across the Cenomanian–Turonian boundary in the Levant Platform of central Jordan. Org. Geochem. 2009, 40, 553–568. [Google Scholar] [CrossRef]
- Grantham, P. The occurence of unusual C27 and C29 sterane predominances in two types of Oman crude oil. Org. Geochem. 1986, 9, 1–10. [Google Scholar] [CrossRef]
- Rielley, G.; Collier, R.J.; Jones, D.M.; Eglinton, G. The biogeochemistry of Ellesmere Lake, U.K.—I: Source correlation of leaf wax inputs to the sedimentary lipid record. Org. Geochem. 1991, 17, 901–912. [Google Scholar] [CrossRef]
- Algeo, T.J.; Henderson, C.M.; Tong, J.; Feng, Q.; Yin, H.; Tyson, R.V. Plankton and productivity during the Permian–Triassic boundary crisis: An analysis of organic carbon fluxes. Glob. Planet. Chang. 2012, 105, 52–67. [Google Scholar] [CrossRef]
- Bostick, B.C.; Fendorf, S.; Helz, G.R. Differential Adsorption of Molybdate and Tetrathiomolybdate on Pyrite (FeS2). Environ. Sci. Technol. 2002, 37, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 1999, 400, 525–531. [Google Scholar] [CrossRef]
- González-Muñoz, M.T.; Martinez-Ruiz, F.; Morcillo, F.; Martin-Ramos, J.; Paytan, A. Precipitation of barite by marine bacteria: A possible mechanism for marine barite formation. Geology 2012, 40, 675–678. [Google Scholar] [CrossRef]
- Rieder, N.; Ott, H.A.; Pfundstein, P.; Schoch, R. X-ray Microanalysis of the Mineral Contents of Some Protozoa. J. Protozool. 1982, 29, 15–18. [Google Scholar] [CrossRef]
- Brooks, J.D.; Gould, K.; Smith, J.W. Isoprenoid Hydrocarbons in Coal and Petroleum. Nature 1969, 222, 257–259. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.; Baudin, F.; Riboulleau, A. Analysis of marine environmental conditions based onmolybdenum–uranium covariation—Applications to Mesozoic paleoceanography. Chem. Geol. 2012, 324, 46–58. [Google Scholar] [CrossRef]
- Algeo, T.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Ai, J.; Zhong, N.; Zhang, T.; Zhang, Y.; Wang, T.; George, S.C. Oceanic water chemistry evolution and its implications for post-glacial black shale formation: Insights from the Cryogenian Datangpo Formation, South China. Chem. Geol. 2021, 566, 120083. [Google Scholar] [CrossRef]
- Tao, S.; Wang, C.; Du, J.; Liu, L.; Chen, Z. Geochemical application of tricyclic and tetracyclic terpanes biomarkers in crude oils of NW China. Mar. Pet. Geol. 2015, 67, 460–467. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Z.; Chen, C.; Wang, T.-G.; Li, M.; Ali, S.; Lu, X.; Dai, J. Geochemistry and origins of petroleum in the Neogene reservoirs of the Baiyun Sag, Pearl River Mouth Basin. Mar. Pet. Geol. 2019, 107, 127–141. [Google Scholar] [CrossRef]
- Jaraula, C.M.B.; Siringan, F.P.; Klingel, R.; Sato, H.; Yokoyama, Y. Records and causes of Holocene salinity shifts in Laguna de Bay, Philippines. Quat. Int. 2014, 349, 207–220. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Z.; Liu, J.; Xu, N.; Li, H. The Sr/Ba ratio response to salinity in clastic sediments of the Yangtze River Delta. Chem. Geol. 2020, 559, 119923. [Google Scholar] [CrossRef]
- Ho, T.Y.; Rogers, M.A.; Drushel, H.V.; Koons, C.B. Evolution of Sulfur Compounds in Crude Oils. AAPG Bull. 1974, 58, 2338–2348. [Google Scholar]
- Jin, X.; Wu, J.; Fang, P.; Zhang, Z.; Li, M.; Zhong, N. Kinetics and fate of organosulphur compounds during the metagenesis stage of thermal maturation: Hydrous pyrolysis investigations on dibenzothiophene. Mar. Pet. Geol. 2021, 130, 105129. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Z.; Wu, J.; Zhang, C.; He, Y.; Cao, L.; Zheng, R.; Meng, W.; Xia, H. Origin and geochemical implication of relatively high abundance of 17α (H)-diahopane in Yabulai basin, northwest China. Mar. Pet. Geol. 2019, 99, 429–442. [Google Scholar] [CrossRef]
- Elderfield, H.; Greaves, M.J. The rare earth elements in seawater. Nature 1982, 296, 214–219. [Google Scholar] [CrossRef]
- Ding, X.; Liu, G.; Zha, M.; Huang, Z.; Gao, C.; Lu, X.; Sun, M.; Chen, Z.; Liuzhuang, X. Relationship between total organic carbon content and sedimentation rate in ancient lacustrine sediments, a case study of Erlian basin, northern China. J. Geochem. Explor. 2015, 149, 22–29. [Google Scholar] [CrossRef]
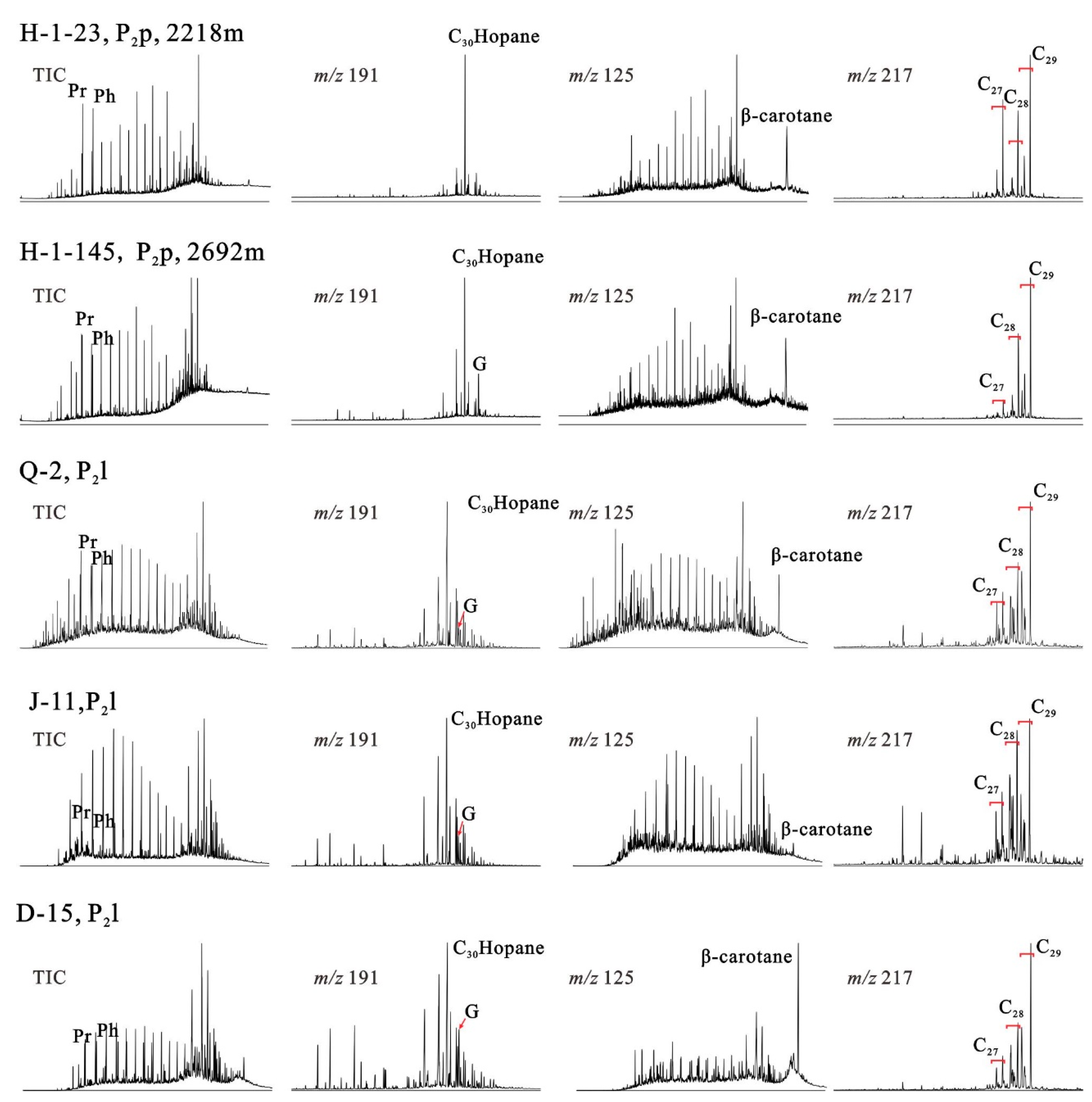



| Sample ID | H1-23 | H1-30 | H1-54 | H1-83 | H1-100 | H1-118 | H1-134 | H1-145 | H1-153 | H1-154 | H1-155 | B-1 | B-3 | B-5 | D-13 |
| Stratum | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2l |
| Well/Outcrops | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | W |
| TOC | 3.52 | 2.15 | 2.16 | 4.31 | 7.48 | 5.81 | 7.03 | 2.74 | 4.76 | 3.36 | 6.83 | 5.81 | 5.77 | 5.65 | 0.31 |
| S | 0.12 | 0.08 | 0.13 | 0.69 | 1.63 | 0.58 | 2.27 | 0.85 | 0.95 | 0.38 | 0.63 | 1.94 | 0.5 | 1.74 | 0.04 |
| HI | 410 | 477 | 572 | 654 | 905 | 797 | 922 | 652 | 861 | 430 | 809 | 250 | 521 | 240 | 13 |
| Pr/Ph | 1.04 | 0.93 | 0.66 | 0.91 | 1.37 | 1.1 | 1.01 | 1.27 | 1.08 | 0.68 | 0.57 | 1.16 | 0.71 | 1.22 | 0.45 |
| CPI | 1.47 | 1.1 | 1.49 | 1.26 | 1.2 | 1.31 | 1.23 | 1.24 | 1.25 | 1.55 | 1.5 | 1.5 | 1.44 | 1.44 | 1.07 |
| OEP | 1.61 | 1.16 | 1.63 | 1.42 | 1.3 | 1.44 | 1.31 | 1.32 | 1.33 | 1.67 | 1.61 | 1.68 | 1.5 | 1.58 | 1.13 |
| G/C30H | 0.07 | 0.08 | 0.07 | 0.08 | 0.22 | 0.2 | 0.22 | 0.42 | 0.15 | 0.04 | 0.04 | 0.07 | 0.04 | 0.08 | 0.1 |
| Steranes/C30H | 0.3 | 0.31 | 0.58 | 0.36 | 0.17 | 0.32 | 0.24 | 0.7 | 0.37 | 0.91 | 0.56 | 0.27 | 0.4 | 0.32 | 0.23 |
| β-carotane | 0.17 | 0.01 | 0.07 | 0.47 | 0.63 | 0.09 | 0.11 | 0.05 | 0.08 | 0.08 | 0.43 | 0.29 | 0.24 | 0.14 | 0.01 |
| δ13C | −0.2 | −30 | −30.9 | −31.8 | −30.4 | −33.6 | −30.9 | −33.3 | −30.9 | ||||||
| Sample ID | D-14 | D-15 | D-16 | D-17 | D-18 | D-19 | D-20 | D-21 | D-22 | J-2 | J-4 | J-6 | J-8 | J-9 | J-10 |
| Stratum | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2l |
| Well/Outcrops | W | W | W | W | W | W | W | W | W | J | J | J | J | J | J |
| TOC | 0.63 | 3.6 | 0.42 | 3.59 | 0.88 | 0.61 | 0.27 | 0.94 | 3.72 | 1.17 | 5.26 | 4.26 | 7.16 | 20.14 | 8.04 |
| S | 0.09 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.01 | 0.03 | 0.03 | 0.07 | 0.07 | 0.03 |
| HI | 22 | 767 | 26 | 617 | 28 | 8 | 15 | 28 | 351 | 73 | 333 | 202 | 522 | 661 | 417 |
| Pr/Ph | 0.99 | 1.13 | 0.57 | 1.41 | 1.12 | 0.53 | 0.6 | 1.11 | 0.62 | 0.9 | 0.97 | 0.83 | 0.76 | 1.16 | 0.92 |
| CPI | 1.22 | 1.16 | 1.12 | 1.2 | 1.25 | 1.12 | 1.06 | 1.47 | 1.38 | 1.14 | 1 | 1.1 | 1.08 | 1.06 | 1.05 |
| OEP | 1.2 | 1.2 | 1.16 | 1.13 | 1.25 | 1.09 | 0.93 | 1.33 | 1.14 | 1.13 | 0.95 | 1.09 | 1.08 | 1 | 1.02 |
| G/C30H | 0.09 | 0.31 | 0.16 | 0.28 | 0.63 | 0.12 | 0.13 | 0.28 | 0.36 | 0.13 | 0.08 | 0.06 | 0.1 | 0.13 | 0.13 |
| Steranes/C30H | 0.24 | 0.18 | 0.37 | 0.24 | 0.91 | 0.73 | 0.4 | 0.94 | 0.12 | 0.09 | 0.09 | 0.13 | 0.14 | 0.13 | 0.13 |
| β-carotane | 0.01 | 0.2 | 0.01 | 0.38 | 0.01 | 0.01 | 0.01 | 0.01 | 0.29 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 |
| δ13C | −23.2 | −26.6 | −22.1 | −27.6 | −31.6 | −32.5 | |||||||||
| Sample ID | J-11 | J-12 | J-13 | J-14 | J-15 | J-16 | J-17 | Q-1 | Q-2 | Q-3 | Q-4 | Q-5 | Q-6 | Q-7 | Q-10 |
| Stratum | P2l | P2l | P2l | P2l | P2l | P2l | P2l | P2p | P2p | P2p | P2p | P2p | P2p | P2p | P2p |
| Well/Outcrops | J | J | J | J | J | J | J | Q | Q | Q | Q | Q | Q | Q | Q |
| TOC | 31.99 | 9.36 | 16.84 | 20.85 | 10.62 | 4.85 | 6.64 | 3.49 | 5.61 | 3.1 | 4.43 | 9.79 | 10.04 | 5.77 | 21 |
| S | 0.04 | 0.04 | 0.06 | 0.06 | 0.04 | 0.08 | 0.04 | 0.03 | 0.05 | 0.03 | 0.02 | 0.06 | 0.4 | 0.12 | 0.06 |
| HI | 606 | 494 | 552 | 632 | 540 | 317 | 363 | 190 | 292 | 176 | 342 | 753 | 643 | 473 | 912 |
| Pr/Ph | 1.8 | 1.06 | 1.33 | 1.23 | 1.28 | 1.05 | 1 | 1.81 | 1.83 | 0.87 | 1.21 | 0.67 | 1.19 | 1.62 | 0.91 |
| CPI | 0.91 | 0.95 | 1.09 | 1.1 | 1.09 | 1.14 | 1.06 | 1.25 | 1.2 | 1.14 | 1.23 | 1.14 | 1.11 | 1.11 | 0.9 |
| OEP | 0.88 | 0.95 | 1.1 | 1.1 | 1.07 | 1.19 | 1.07 | 1.31 | 1.26 | 1.14 | 1.09 | 1.09 | 1.14 | 1.13 | 0.89 |
| G/C30H | 0.12 | 0.12 | 0.15 | 0.15 | 0.15 | 0.1 | 0.1 | 0.04 | 0.05 | 0.03 | 0.07 | 0.05 | 0.07 | 0.1 | 0.15 |
| Steranes/C30H | 0.09 | 0.1 | 0.11 | 0.09 | 0.12 | 0.1 | 0.09 | 0.12 | 0.13 | 0.14 | 0.14 | 0.09 | 0.2 | 0.07 | 0.03 |
| β-carotane | 0.01 | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.01 | 0.03 | 0.1 | 0.1 | 0.24 | 0.23 | 0.15 | 0.13 | 0.09 |
| δ13C | −32.5 | −32.5 | −31.8 | −31.5 | −31.2 | −31.3 | −31 | −32.5 |
| Sample ID | PAAS | H1-14 | H1-21 | H1-30 | H1-35 | H1-51 | H1-54 | H1-56 | H1-61 | H1-62 | H1-71 | H1-75 | H1-83 | H1-91 | H1-94 | H1-100 | H1-106 | H1-110 |
| Well/Outcrops | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | |
| TOC | 2.2 | 5.4 | 1.4 | 2.2 | 4.4 | 4.9 | 5.2 | 2.7 | 2.3 | 4.3 | 6.1 | 4.9 | 7.5 | 7.8 | 2.6 | |||
| SiO2 | 62.8 | 58.6 | 53.8 | 51.2 | 56.8 | 55 | 55.1 | 55.6 | 55.3 | 55.8 | 47.4 | 57.7 | 53.3 | 55.3 | 53 | 54.4 | 53.2 | 54.6 |
| Al2O3 | 18.9 | 16.9 | 11.3 | 11.1 | 10.9 | 12.5 | 12.5 | 11.1 | 11.1 | 11.3 | 9.2 | 11.2 | 10.8 | 10.9 | 11.3 | 10.3 | 10.8 | 12 |
| Fe2O3 | 7.2 | 6.9 | 6 | 4.6 | 4 | 6.1 | 5.7 | 4.2 | 4.9 | 5.1 | 4.4 | 5 | 4.5 | 4.1 | 4.1 | 3.7 | 4 | 4.5 |
| MgO | 2.2 | 1.3 | 1.8 | 2.1 | 2 | 2 | 2.7 | 2.8 | 2.7 | 2.4 | 5.2 | 2.4 | 2.4 | 1.8 | 1.9 | 2.1 | 2.1 | 2.7 |
| CaO | 1.3 | 1.1 | 8.6 | 9.9 | 5.3 | 5.5 | 4 | 4.5 | 4.6 | 4.1 | 9.7 | 4 | 7.1 | 5.9 | 7.5 | 5.7 | 5.5 | 6 |
| Na2O | 1.2 | 1 | 2.1 | 1.8 | 1.7 | 2 | 2.1 | 2 | 1.6 | 1.7 | 1.5 | 1.7 | 1.9 | 1.9 | 2.3 | 2.3 | 2.3 | 1.9 |
| K2O | 3.7 | 1.4 | 1.4 | 1.8 | 1.9 | 2 | 2.1 | 2.1 | 2.1 | 2.1 | 1.7 | 2.1 | 2.2 | 2.5 | 2.4 | 2.4 | 2.5 | 2.7 |
| MnO | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| TiO2 | 1 | 0.9 | 0.8 | 0.6 | 0.5 | 0.8 | 0.7 | 0.5 | 0.6 | 0.7 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 |
| P2O5 | 0.2 | 0.1 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | 0.5 | 0.2 |
| Sample ID | H1-118 | H1-123 | H1-128 | H1-134 | H1-140 | H1-148 | H1-153 | H1-154 | H1-155 | D-14 | D-15 | D-16 | D-17 | D-18 | D-19 | D-20 | D-21 | D-22 |
| Well/Outcrops | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | H-1 | W | W | W | W | W | W | W | W | W |
| TOC | 5.8 | 5.9 | 4.5 | 7 | 2.5 | 2.1 | 4.8 | 3.4 | 6.8 | 0.6 | 3.6 | 0.4 | 3.6 | 0.9 | 0.6 | 0.3 | 0.9 | 3.7 |
| SiO2 | 52.8 | 55.8 | 55.6 | 52.7 | 39.7 | 49.6 | 49.9 | 65 | 55 | 47.3 | 40.1 | 58.5 | 37.2 | 59.5 | 58.1 | 60.8 | 60.5 | 61.2 |
| Al2O3 | 10.1 | 11.2 | 11.6 | 9.7 | 8.5 | 10.4 | 9.3 | 10.3 | 10.2 | 13.3 | 4.2 | 13.7 | 7.2 | 14.3 | 14.7 | 13.1 | 12.1 | 12.9 |
| Fe2O3 | 4 | 4.7 | 4.8 | 3.5 | 3.7 | 4 | 3.6 | 5.8 | 4.3 | 3.6 | 4.4 | 5.7 | 4.1 | 6.1 | 7.4 | 4.3 | 4.1 | 4.1 |
| MgO | 3 | 2.1 | 2.1 | 2 | 1.9 | 3 | 4.2 | 2.3 | 2.4 | 2.3 | 8.3 | 2.4 | 8.5 | 2.8 | 3.1 | 2.5 | 2.3 | 2.3 |
| CaO | 6.4 | 4.2 | 5.5 | 7.8 | 20 | 10.3 | 8.2 | 1.5 | 5 | 10.8 | 14.2 | 3.8 | 12.9 | 1.5 | 2 | 3.8 | 3.4 | 1.7 |
| Na2O | 2.2 | 2.2 | 2 | 1.8 | 1.6 | 2.7 | 2.3 | 1.3 | 1.3 | 3.6 | 1.3 | 4.5 | 2.5 | 4 | 5.2 | 3.6 | 3.3 | 3.3 |
| K2O | 2.3 | 2.6 | 3 | 3 | 2.1 | 2.9 | 2.5 | 1.5 | 1.6 | 5.3 | 1.6 | 3.9 | 1.7 | 4.3 | 2.7 | 3.8 | 3.5 | 3.5 |
| MnO | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0 |
| TiO2 | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.5 | 0.6 | 0.2 | 0.6 | 0.3 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 |
| P2O5 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.7 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sample ID | J-10 | J-11 | J-12 | J-13 | J-14 | J-15 | J-16 | J-17 | Q-1 | Q-2 | Q-3 | Q-4 | Q-5 | Q-6 | Q-7 | Q-8 | Q-9 | Q-10 |
| Well/Outcrops | J | J | J | J | J | J | J | J | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q |
| TOC | 8 | 32 | 9.4 | 16.8 | 20.9 | 10.6 | 4.9 | 6.6 | 3.5 | 5.6 | 3.1 | 4.4 | 9.8 | 10 | 5.8 | 2.6 | 1.5 | 21.4 |
| SiO2 | 53.4 | 40 | 59 | 50.4 | 45.8 | 25.2 | 61.6 | 55.5 | 63.5 | 64.2 | 70 | 68.3 | 54.8 | 47.9 | 58.9 | 51.4 | 69.5 | 56.3 |
| Al2O3 | 2 | 6.3 | 13.8 | 9.5 | 8.1 | 5.2 | 12.3 | 13.8 | 12.4 | 12.3 | 10.2 | 13.4 | 9 | 5.3 | 11.5 | 10.9 | 14.1 | 10.1 |
| Fe2O3 | 6.5 | 3.2 | 4 | 4.7 | 3.9 | 3.1 | 5 | 6.1 | 3.2 | 2.9 | 3.4 | 1.9 | 3.3 | 4.9 | 5.1 | 5 | 2.3 | 3.1 |
| MgO | 1.5 | 1.8 | 0.4 | 1.5 | 2.9 | 11 | 1.2 | 1.4 | 1.8 | 1.7 | 0.8 | 1.3 | 2.9 | 4.6 | 2.5 | 3.8 | 0.5 | 0.4 |
| CaO | 2.8 | 7.3 | 1 | 3 | 6.2 | 15.6 | 1.8 | 1.5 | 2 | 1.8 | 1.9 | 0.5 | 5.8 | 10.7 | 1.6 | 5.4 | 0.4 | 0.9 |
| Na2O | 4.1 | 2.7 | 4 | 3.6 | 3.3 | 1.7 | 5.1 | 4.7 | 1.1 | 1.1 | 1.3 | 1.2 | 1.2 | 0.7 | 1.7 | 2.2 | 4.5 | 3.9 |
| K2O | 2.5 | 0.9 | 2.9 | 1.6 | 1.2 | 1.1 | 2 | 3.1 | 2 | 1.9 | 1.9 | 2.1 | 1.5 | 1 | 2.1 | 2.5 | 2.4 | 1.4 |
| MnO | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0 | 0 | 0.1 | 0 | 0.1 | 0.2 | 0.1 | 0.1 | 0 | 0 |
| TiO2 | 0.6 | 0.3 | 0.6 | 0.4 | 0.4 | 0.3 | 0.6 | 0.7 | 0.6 | 0.6 | 0.5 | 0.6 | 0.4 | 0.3 | 0.5 | 0.5 | 0.6 | 0.5 |
| P2O5 | 0.1 | 2.8 | 0.3 | 0.8 | 3.6 | 0.1 | 0.1 | 0.1 | 0.3 | 0.4 | 0.5 | 0.2 | 0.5 | 0.5 | 0.3 | 0.4 | 0.1 | 0.2 |
| Sample ID | H1-14 | H1-21 | H1-30 | H1-35 | H1-51 | H1-54 | H1-56 | H1-61 | H1-62 | H1-71 | H1-75 | H1-83 | H1-91 | H1-94 | H1-100 | H1-106 | H1-110 | H1-118 |
| MoEF | 0.78 | 2.05 | 3.46 | 6.76 | 3.31 | 2.68 | 4.76 | 6.31 | 5.26 | 4.85 | 4.44 | 5.25 | 7.1 | 7.77 | 8.8 | 7.99 | 3.18 | 7.43 |
| VEF | 0.83 | 1.51 | 1.13 | 1.25 | 1.1 | 1.1 | 1.15 | 1.23 | 1.19 | 1.46 | 1.19 | 1.19 | 1.2 | 1.14 | 1.23 | 1.23 | 1.07 | 1.16 |
| CuEF | 0.92 | 1.24 | 1.12 | 1.38 | 0.99 | 1.07 | 1.35 | 1.37 | 1.42 | 1.29 | 1.25 | 1.32 | 1.69 | 1.41 | 1.73 | 1.7 | 1.26 | 1.5 |
| UEF | 0.99 | 2.79 | 1.5 | 1.85 | 1.49 | 1.3 | 1.75 | 1.69 | 1.72 | 1.62 | 1.61 | 1.68 | 1.69 | 1.87 | 1.57 | 1.8 | 1.15 | 1.42 |
| BaEF | 5.96 | 13.28 | 4.06 | 3.83 | 3.72 | 3.3 | 2.86 | 3.24 | 3.22 | 2.09 | 2.88 | 4.94 | 2.04 | 2.13 | 2.27 | 2.55 | 3.19 | 2.76 |
| Sr/Ba | 0.05 | 0.06 | 0.24 | 0.2 | 0.18 | 0.2 | 0.27 | 0.24 | 0.24 | 0.72 | 0.26 | 0.26 | 0.55 | 0.62 | 0.46 | 0.46 | 0.3 | 0.48 |
| (La/Yb)N | 0.62 | 0.56 | 0.59 | 0.54 | 0.62 | 0.65 | 0.59 | 0.59 | 0.61 | 0.58 | 0.56 | 0.64 | 0.57 | 0.62 | 0.67 | 0.63 | 0.68 | 0.64 |
| Sample ID | H1-123 | H1-128 | H1-134 | H1-140 | H1-148 | H1-153 | H1-154 | H1-155 | D-14 | D-15 | D-16 | D-17 | D-18 | D-19 | D-20 | D-21 | D-22 | J-10 |
| MoEF | 8.07 | 6.44 | 10.23 | 10.24 | 7.48 | 7.16 | 5.5 | 5.23 | 2.23 | 47.25 | 0.59 | 7.3 | 1.82 | 0.33 | 2.54 | 1.57 | 0.71 | 20.54 |
| VEF | 1.38 | 1.23 | 1.3 | 1.1 | 1.09 | 1.16 | 1.36 | 1.37 | 1.47 | 3.03 | 0.72 | 1.25 | 0.79 | 0.76 | 0.6 | 0.79 | 0.68 | 2.82 |
| CuEF | 1.74 | 1.59 | 1.56 | 1.11 | 1.29 | 1.22 | 1.62 | 1.47 | 1.61 | 2.04 | 1.14 | 1.64 | 1.19 | 1.11 | 0.93 | 1.43 | 1.07 | 7.5 |
| UEF | 1.7 | 1.34 | 1.88 | 2.34 | 1.77 | 1.97 | 1.4 | 1.63 | 0.97 | 1.52 | 0.76 | 2.52 | 1 | 0.62 | 1.02 | 0.89 | 1.16 | 2.47 |
| BaEF | 2.57 | 2.59 | 3.29 | 5.98 | 6.93 | 1.89 | 1.03 | 1.29 | 1.31 | 1.69 | 0.79 | 1.54 | 0.84 | 0.65 | 0.96 | 0.97 | 0.84 | 3.08 |
| Sr/Ba | 0.37 | 0.44 | 0.5 | 0.6 | 0.25 | 0.76 | 0.7 | 1.01 | 1.29 | 3.09 | 0.63 | 1.89 | 0.61 | 0.58 | 0.71 | 0.57 | 0.46 | 0.62 |
| (La/Yb)N | 0.61 | 0.65 | 0.63 | 0.49 | 0.6 | 0.51 | 0.51 | 0.78 | 0.38 | 0.43 | 0.53 | 0.48 | 0.6 | 0.57 | 0.57 | 0.41 | 0.68 | 0.85 |
| Sample ID | J-11 | J-12 | J-13 | J-14 | J-15 | J-16 | J-17 | Q-1 | Q-2 | Q-3 | Q-4 | Q-5 | Q-6 | Q-7 | Q-8 | Q-9 | Q-10 | |
| MoEF | 9.16 | 3.08 | 11.78 | 7.19 | 14.86 | 3.17 | 8.67 | 1.32 | 1.83 | 6.7 | 1.65 | 5.92 | 41.37 | 1.99 | 6.67 | 2.74 | 6.04 | |
| VEF | 1.08 | 0.42 | 1.08 | 1.01 | 2.58 | 0.77 | 0.71 | 1.02 | 0.86 | 1.12 | 0.83 | 1.13 | 1.61 | 1.02 | 1.5 | 0.52 | 0.67 | |
| CuEF | 1.51 | 1.04 | 1.83 | 1.75 | 1.62 | 1.01 | 1.36 | 1.2 | 1.21 | 1.44 | 1.15 | 1.7 | 3.52 | 1.43 | 1.64 | 0.83 | 1.32 | |
| UEF | 1.68 | 0.28 | 1.72 | 1.62 | 0.78 | 0.18 | 0.14 | 0.98 | 1.07 | 1.29 | 1 | 1.64 | 3.8 | 1.02 | 1.85 | 0.68 | 1.23 | |
| BaEF | 1.97 | 0.39 | 0.91 | 1.72 | 4.1 | 0.48 | 0.51 | 0.54 | 0.51 | 1.25 | 0.65 | 1.04 | 2.05 | 1.02 | 0.99 | 0.52 | 0.75 | |
| Sr/Ba | 1.08 | 0.47 | 0.41 | 1.39 | 1.14 | 0.38 | 0.31 | 0.65 | 0.58 | 0.33 | 0.28 | 1.16 | 0.59 | 0.43 | 0.94 | 0.33 | 0.39 | |
| (La/Yb)N | 0.49 | 1.31 | 0.86 | 0.6 | 0.41 | 1.48 | 1.65 | 0.38 | 0.42 | 0.51 | 0.52 | 0.66 | 0.57 | 0.54 | 0.55 | 0.62 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Feng, Y.; Qiu, W.J.; Luo, X.; Wen, X.; Zhang, S.; Zhang, Z. Mechanisms for the Accumulation of Organic Matter in Sediments of the Middle Permian around Bogda Mountain, Southern Junggar Basin, NW China. Minerals 2023, 13, 332. https://doi.org/10.3390/min13030332
Jin X, Feng Y, Qiu WJ, Luo X, Wen X, Zhang S, Zhang Z. Mechanisms for the Accumulation of Organic Matter in Sediments of the Middle Permian around Bogda Mountain, Southern Junggar Basin, NW China. Minerals. 2023; 13(3):332. https://doi.org/10.3390/min13030332
Chicago/Turabian StyleJin, Xiao, Yanfang Feng, Wenhong Johnson Qiu, Xiaoling Luo, Xinyu Wen, Suowen Zhang, and Zhihuan Zhang. 2023. "Mechanisms for the Accumulation of Organic Matter in Sediments of the Middle Permian around Bogda Mountain, Southern Junggar Basin, NW China" Minerals 13, no. 3: 332. https://doi.org/10.3390/min13030332
APA StyleJin, X., Feng, Y., Qiu, W. J., Luo, X., Wen, X., Zhang, S., & Zhang, Z. (2023). Mechanisms for the Accumulation of Organic Matter in Sediments of the Middle Permian around Bogda Mountain, Southern Junggar Basin, NW China. Minerals, 13(3), 332. https://doi.org/10.3390/min13030332






