Mineralogical Evidence for Hydrothermal Uranium Mineralization: Discovery and Genesis of the Uranyl Carbonate Minerals in the BLS U Deposit, SW Songliao Basin, Northeast China
Abstract
:1. Introduction
2. Geological Setting
2.1. Songliao Basin
2.2. The Baolongshan U Deposit
3. Sampling and Analytical Methods
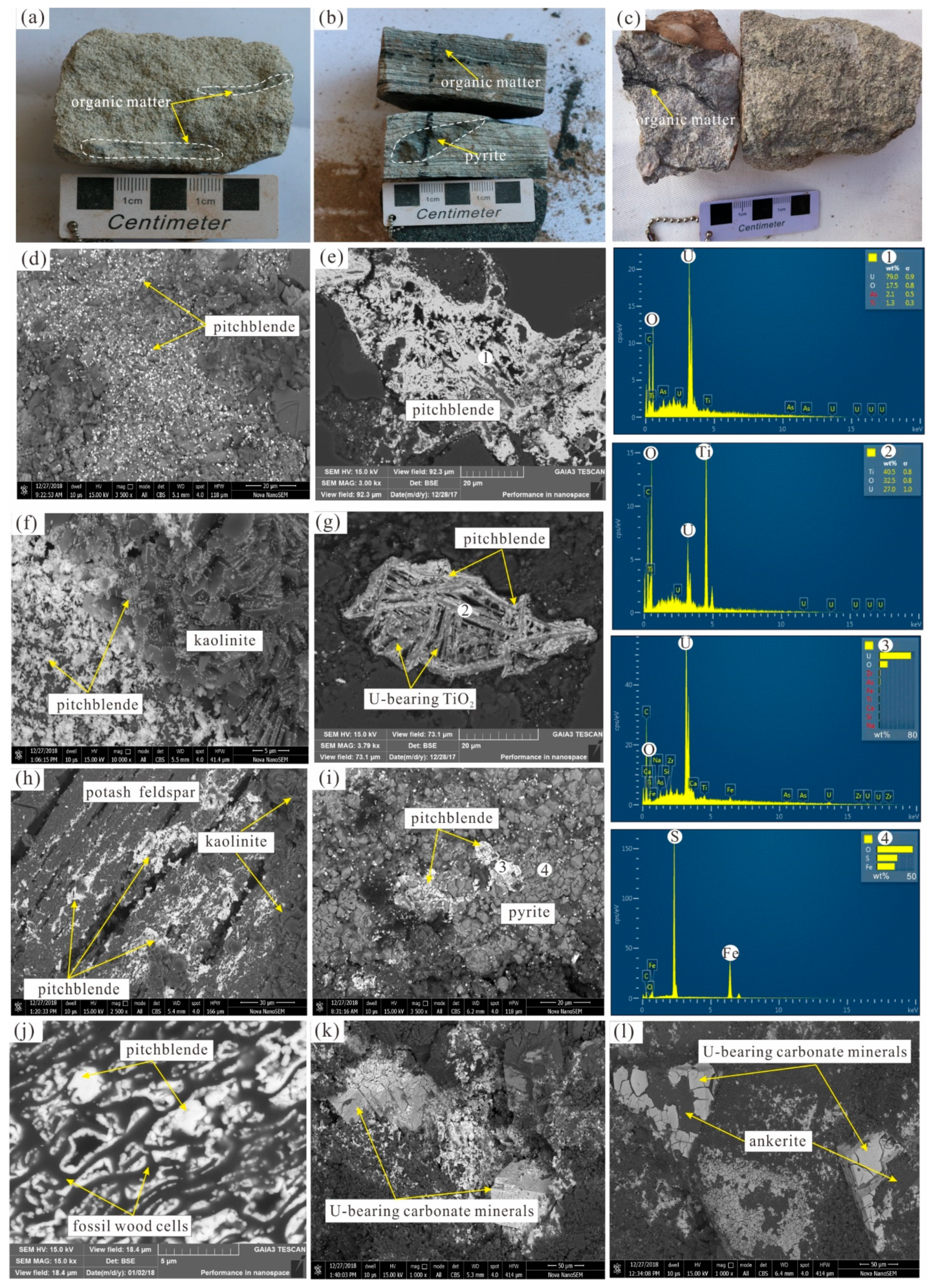
4. Results
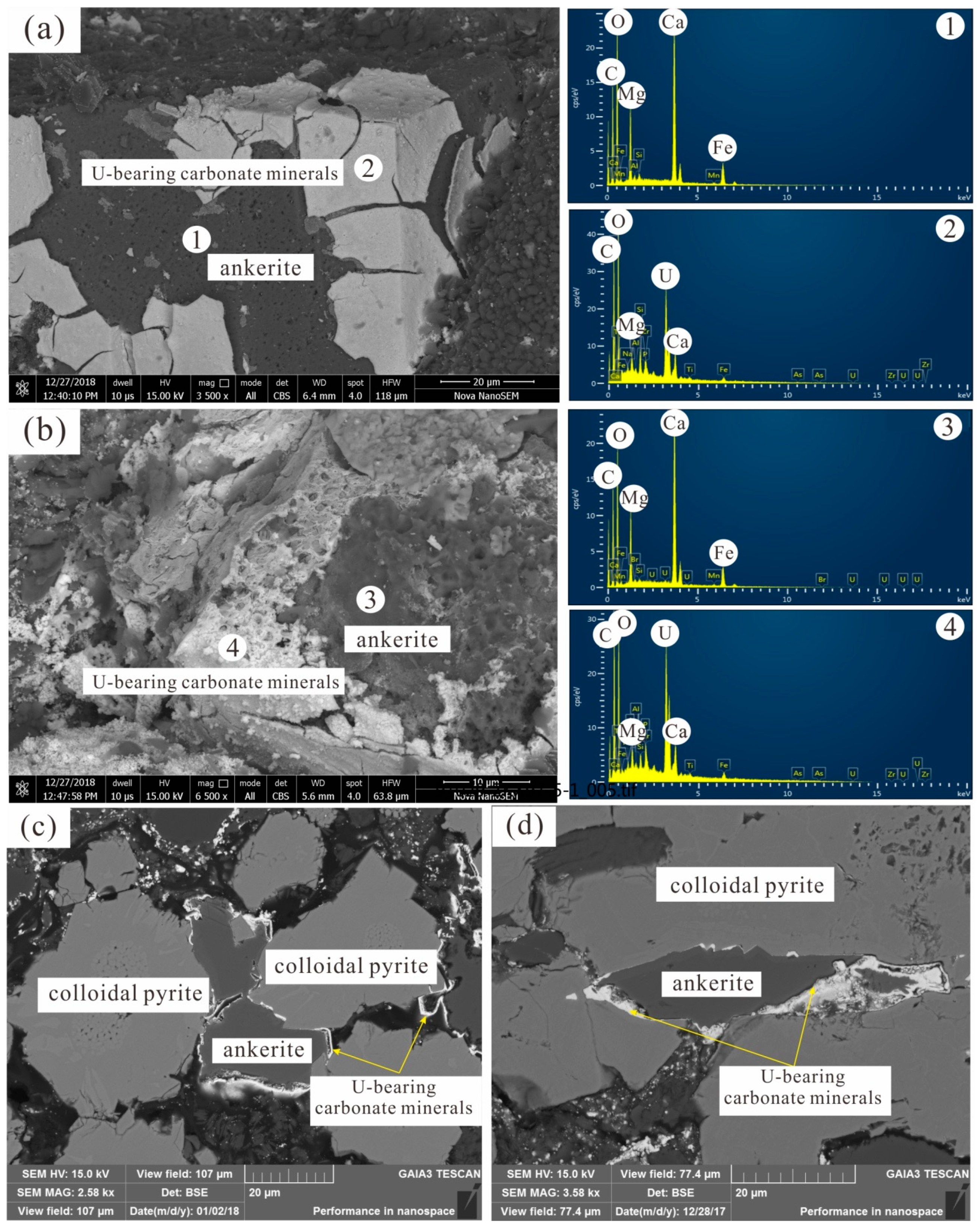
4.1. U Minerals and Their Occurrences
4.2. Chemical Composition of the U-Bearing Carbonate Minerals
5. Discussion
5.1. Mineral Types of Uranyl Carbonate Minerals
5.2. Genesis of Uranyl Carbonate Minerals
5.3. Hydrothermal U Mineralization in Sandstone-Type U Deposits
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, B.H.; Keeling, J.; Li, Z.Y. Paleovalley-related U deposits in Australia and China: A review of geological and exploration models and methods. Ore Geol. Rev. 2017, 88, 201–234. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.X. A growing sandstone type U district in South Yili Basin, NW China as a result of extension of Tienshan Orogen: Evidences from geochronology and hydrology. Gondwana Res. 2019, 76, 146–172. [Google Scholar] [CrossRef]
- Ding, B.; Liu, H.X.; Li, P.; Jiang, H.; Zhang, H.J.; Zhang, B. The genetic mechanism of kaolinite in ore-bearing sandstone in the Mengqiguer U Deposit, Yili, and its relation with U mineralization. Acta Geol. Sin. 2019, 93, 2021–2036, (In Chinese with English Abstract). [Google Scholar]
- Ding, B.; Liu, H.X.; Zhang, C.; Liu, H.; Li, P.; Zhang, B. Mineralogy, Fluid Inclusion and H-O-C-S Stable Isotopes of Mengqiguer U Deposit in the Southern Yili Basin, Xinjiang: Implication for Ore Formation. Acta Geol. Sin. (Engl. Ed.) 2020, 94, 1488–1503. [Google Scholar] [CrossRef]
- Ding, B.; Liu, H.X.; Zhang, B. The formation mechanism of tabular orebody of sandstone—Type U in northern Ordos basin: Constraints on the study of kaolinite content from different zones of ore—Bearing sandstone. Acta Geol. Sin. 2020, 94, 2874–2882, (In Chinese with English Abstract). [Google Scholar]
- Li, Z.Y.; Fang, X.H.; Chen, A.P.; Sun, Y.; Chen, F.; Liu, Z.; Zhang, K.; Zhou, W.; Li, M.; Jiao, Y. Superposition metallogenic model of sandstone-type U deposit in the Northeastern Ordos basin. Uranium Geol. 2009, 25, 65–70, (In Chinese with English Abstract). [Google Scholar]
- Yi, C.; Wang, G.; Li, X.D.; Zhang, K.; Wang, Y.J. A tentative discussion on U enrichment characteristics and metallogenic model in Zhiluo Formation, northeastern Ordos Basin. Miner. Depos. 2018, 37, 835–852, (In Chinese with English Abstract). [Google Scholar]
- Zhang, C.; Yi, C.; Dong, Q.; Cai, Y.Q.; Liu, H.X. Geological and geochronological evidence for the effect of Paleogene and Miocene uplift of the Northern Ordos Basin on the formation of the Dongsheng U district, China. J. Geodyn. 2018, 114, 1–18. [Google Scholar] [CrossRef]
- Bonnetti, C.; Cuney, M.; Malartre, F.; Michels, R.; Li, X.; Peng, Y. The Nuheting deposit, Erlian Basin, NE China: Synsedimentary to diagenetic U mineralization. Ore Geol. Rev. 2015, 69, 118–139. [Google Scholar] [CrossRef]
- Bonnetti, C.; Cuney, M.; Michels, R.; Truche, L.; Malartre, F.; Liu, X.; Yang, J. The multiple roles of sulfate-reducing bacteria and Fe-Ti oxides in the genesis of the Bayinwula roll front-type U deposit, Erlian Basin, NE China. Econ. Geol. 2015, 110, 1059–1081. [Google Scholar] [CrossRef]
- Nie, F.; Yan, Z.; Feng, Z.; Li, M.; Xia, F.; Zhang, C.; Wang, Y.; Yang, J.; Kang, S.; Shen, K. Genetic models and exploration implication of the paleo-channel sandstone-type U deposits in the Erlian Basin, North China—A review and comparative study. Ore Geol. Rev. 2020, 127, 103821. [Google Scholar] [CrossRef]
- Nie, F.; Yan, Z.; Xia, F.; Li, M.; Feng, Z.; Lu, Y.; Cai, J. Mineralisation from hot fluid flows in the sandstone-type U deposit in the Kailu Basin, Northeast China. Appl. Earth Sci. 2018, 127, 2–14, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Qin, M.K.; Huang, S.H.; Liu, J.L.; Liu, Z.Y.; Guo, Q.; Jia, L.C.; Jiang, W.J. Hydrothermal alteration and its superimposed enrichment for Qianjiadian tabular-type U deposit in southwestern Songliao Basin. Minerals 2022, 12, 52. [Google Scholar] [CrossRef]
- Bonnetti, C.; Liu, X.D.; Yan, Z.B.; Cuney, M.; Michels, R.; Malartre, F.; Mercadier, J.; Cai, J.F. Coupled U mineralisation and bacterial sulphate reduction for thegenesis of the Baxingtu sandstone-hosted U deposit, SW Songliao Basin, NE China. Ore Geol. Rev. 2017, 82, 108–129. [Google Scholar] [CrossRef]
- Jiao, Y.Q.; Wu, L.Q.; Rong, H. Model of inner and outer reductive media within U reservoir sandstone of sandstone-type U deposits and its orecontrolling mechanism: Case studies in Daying and Qianjiadian U deposits. Earth Sci. 2018, 43, 459–474, (In Chinese with English Abstract). [Google Scholar]
- Zhang, L.; Liu, C.Y.; Lei, K.Y. Green altered sandstone related to hydrocarbon migration from the U deposits in the northern Ordos Basin, China. Ore Geol. Rev. 2019, 109, 482–493. [Google Scholar] [CrossRef]
- Wu, B.L.; Zhang, W.Y.; Song, Z.S.; Cun, X.N.; Sun, L.; Luo, J.J.; Li, Y.Q.; Cheng, X.H.; Sun, B. Geological and geochemical characteristics of U minerals in the sandstone-type U deposits in the north of Ordos Basin and their genetic significance. Acta Geol. Sin. 2016, 90, 3393–3407, (In Chinese with English Abstract). [Google Scholar]
- Ding, B.; Liu, H.X.; Zhang, B.; Li, P.; Jiang, H.; Zhang, H.; Xie, X.L.; Guo, C.J. Mineralogical and isotopes evidence for origin of pyrite: Implication for formation mechanism of pyrite and its relationship with U mineralization in Mengqigu’er U deposit, Yili Basin. Miner. Depos. 2019, 38, 1379–1391, (In Chinese with English Abstract). [Google Scholar]
- Yue, L.; Jiao, Y.; Wu, L.; Rong, H.; Fayek, M.; Xie, H. Evolution and origins of pyrite in sandstone-type U deposits, northern Ordos basin, north-central China, based on micromorphological and compositional analysis. Ore Geol. Rev. 2020, 118, 103334. [Google Scholar] [CrossRef]
- Miao, A.S.; Lu, Q.; Liu, H.F.; Xiao, P. Occurrence and formation of coffinite in ancient interlayer oxidizing zone of sandstone type U-deposit in Ordos Basin. Geol. Sci. Technol. Inf. 2009, 28, 51–58, (In Chinese with English Abstract). [Google Scholar]
- Wang, G.; Wang, Q.; Miao, A.S.; Jiao, Y.Q.; Yi, C. Characteristic of U minerals in Nalinggou U deposit or Ordos Basin and their formation mechanism. Acta Miner. Sin. 2017, 37, 461–468, (In Chinese with English Abstract). [Google Scholar]
- Chen, L.; Chen, Y.; Feng, X.; Li, J.G.; Guo, H.; Miao, P.; Jin, R.; Tang, C.; Zhao, H.; Wang, G.; et al. U occurrence state in the Tarangaole area of the Ordos Basin, China: Implications for enrichment and mineralization. Ore Geol. Rev. 2019, 115, 103034. [Google Scholar] [CrossRef]
- Ding, B.; Liu, H.X.; Qiu, L.F.; Zhang, C.; Xu, D.R. Ilmenite alteration and its adsorption and catalytic reduction in U enrichment in sandstone-hosted U deposits from the northern Ordos Basin, North China. Minerals 2022, 12, 167. [Google Scholar] [CrossRef]
- Machel, H.G.; Lonnee, L. Hydrothermal dolomite-a product of poor definition and imagination. Sediment. Geol. 2002, 152, 163–171. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, M.K.; Fan, H.H.; Gu, D.Z.; Wang, S.Y. Preliminary discussion on ore-controlling factors of Azelik sandstone type U deposits in Niger. World Nucl. Geosci. 2012, 29, 149–155, (In Chinese with English Abstract). [Google Scholar]
- Liu, Z.Y.; Peng, S.P.; Qin, M.K.; He, Z.B.; Guo, Q.; Xu, Q. Multistage enrichment of the Sawafuqi U deposit: New Insights into Sandstone-hosted U deposits in the intramontane Basins of Tianshan, China. Acta Geol. Sin. (Engl. Ed.) 2017, 6, 2138–2152. [Google Scholar] [CrossRef]
- Xiao, X.J.; Li, Z.Y.; Fang, X.H.; Ou, G.X.; Sun, Y.; Chen, A.P. The evidences and significances of epithermal mineralization fluid in the Dongsheng sandstone type U deposit. Bull. Mineral. Petrol. Geochem. 2004, 23, 301–304, (In Chinese with English Abstract). [Google Scholar]
- Zhang, L.; Liu, C.-Y.; Zhao, Z.-Q.; Diao, Z.-B.; Wang, F.-F.; Song, Z.-S. Fluid evolution and mineralization of Hangjinqi sandstone-type U deposit, Ordos Basin. Earth Sci. Front. 2015, 22, 368–381, (In Chinese with English Abstract). [Google Scholar]
- Wang, F.G.; Hou, S.R.; Zhang, L.; Men, H.; Wang, J.L. Study on the Characteristics of Water-Rock Interaction and Its Relation to Uranium Mineralization in Tamusu Uranium Deposit, Southern Bayin Gobi Basin. Geol. Rev. 2018, 64, 633–646, (In Chinese with English Abstract). [Google Scholar]
- Zhang, C.Y.; Nie, F.J.; Jiao, Y.Q.; Deng, W.; Peng, Y.B.; Hou, S.R.; Dai, M.J.; Ye, T.F. Characterization of ore-forming fluids in the Tamusu sandstone-type uranium deposit, Bayingobi Basin, China: Constraints from trace elements, fluid inclusions and C–O–S isotopes. Ore Geol. Rev. 2019, 111, 102999. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Cai, Y.Q.; Cai, J.F.; Liu, Z.Y. Discussion on the relationship between diabase intrusion and sandstone-type uranium mineralization in the southwestern Songliao Basin: C-O isotope evidence from carbonate cement. Geol. Rev. 2019, 65, 113–114, (In Chinese with English Abstract). [Google Scholar]
- Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Zhao, X.F.; Gao, M.Q.; Liu, W.H. Effects of igneous intrusions on diagenesis and reservoir quality of sandstone in the Songliao Basin, China. Mar. Pet. Geol. 2021, 127, 104980. [Google Scholar] [CrossRef]
- Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Ji, D.M.; Li, H.L.; Zhu, Q.; Cao, M.Q.; Wang, X.M.; Li, Q.C.; Xie, H.L. Epigenetic Alteration and Its Constraints on Uranium Mineralization from the Qianjiadian Uranium Deposit, Southern Songliao Basin. Earth Sci. 2016, 41, 153–166, (In Chinese with English Abstract). [Google Scholar]
- Jia, J.M.; Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Guo, X.J.; Cao, M.Q.; Cui, Z.J.; Tao, Z.P. Occurrence of Carbonate Cements and Rela-tionship between Carbonate Cementation and Uranium Mineralization of Qianjiadian Uranium Deposit, Songliao Basin. Earth Sci. 2018, 43, 149–161, (In Chinese with English Abstract). [Google Scholar]
- Yang, D.G.; Wu, J.H.; Nie, F.J.; Bonnetti, C.; Xia, F.; Yan, Z.B.; Cai, J.F.; Wang, C.D.; Wang, H.T. Petrogenetic constraints of early Cenozoic mafic rocks in the southwest Songliao Basin, NE China: Implications for the genesis of sandstone-hosted Qianjiadian U deposits. Minerals 2020, 10, 1014. [Google Scholar] [CrossRef]
- Yan, X.L. Characteristics of upper Cretaceous diabase and uranium mineralization in Qianjiadian area, Songliao Basin. J. Northeast. Pet. Univ. 2018, 42, 40–48, (In Chinese with English Abstract). [Google Scholar]
- Liu, H.B.; Jin, G.S.; Han, J.; Zhang, J.F.; Li, J.J.; Zhang, J.; Shi, X. Element and isotope geochemical characteristics of diabase in Qianjiadian uranium deposit. World Nucl. Geosci. 2021, 38, 135–143, (In Chinese with English Abstract). [Google Scholar]
- Deng, C.L.; He, H.Y.; Pan, Y.X.; Zhu, R.X. Chronology of the terrestrial Upper Cretaceous in the Songliao Basin, Northeast Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 385, 44–54. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, C.; Wang, X.; Feng, Z. Late Cretaceous (Campanian) provenance change in the Songliao Basin, NE China: Evidence from detrital zircon U-Pb ages from the Yaojia and Nenjiang formations. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 385, 83–94. [Google Scholar] [CrossRef]
- Dong, T.; He, S.; Wang, D.; Hou, Y. Hydrocarbon migration and accumulation in the Upper Cretaceous Qingshankou Formation, Changling Sag, Southern Songliao Basin: Insights from integrated analyses of fluid inclusion, oil source correlation and basin modelling. J. Asian Earth Sci. 2014, 90, 77–87. [Google Scholar] [CrossRef]
- Ren, J.; Tamaki, K.; Li, S.; Zhang, J. Late Mesozoic and Cenozoic rifting and its dynamic setting in Eastern China and adjacent areas. Tectonophysics 2002, 344, 175–205. [Google Scholar] [CrossRef]
- Li, S.Q.; Chen, F.; Siebel, W.; Wu, J.D.; Zhu, X.Y. Late Mezosoic tectonic evolution of the Songliao basin, NE China: Evidence from detrital zircon ages and Sr-Nd isotopes. Gondwana Res. 2012, 22, 943–955. [Google Scholar] [CrossRef]
- Xia, Y.L.; Zheng, J.W.; Li, Z.Y.; Li, L.Q.; Tian, S.F. Metallogenic characteristics and metallogenic model of Qianjiadian U deposit in Songliao Basin. Miner. Depos. 2003, 29, 154–155, (In Chinese with English Abstract). [Google Scholar]
- Zhang, M.Y.; Zheng, J.W.; Tian, S.F.; Xia, Y.L.; Liu, H.B. Research on existing state of U and U ore-formation age at Qianjiadian U deposit in Kailu depression. Uranium Geol. 2005, 21, 213–218. [Google Scholar]
- Renaud, V.; Michel, D.; Olaf, M. Oswaldpeetersite, (UO2)2CO3(OH)2·4H2O, a new basic uranyl carbonate mineral from the Jomac Uranium Mine, San Juan Country, Utah, U.S.A. Can. Mineral. 2001, 39, 1685–1689. [Google Scholar]
- Samer, A.; Thuro, A.; Harald, F.; Gerhard, G.; Gert, B. Spectroscopic characterization of synthetic Becquerelite Ca[(UO2)6O4(OH)6]·8H2O, and Swartzite CaMg[UO2(CO3)3]·12H2O. Can. Mineral. 2004, 42, 953–962. [Google Scholar]
- Bernhard, G.; Geipel, G.; Reich, T.; Brendler, V.; Amayri, S.; Nitsche, H. Uranyl(VI) carbonate complex formation: Validation of the Ca2UO2(CO3)3(aq) species. Radiochim. Acta 2001, 89, 511–518. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yun, J.I. Formation of ternary CaUO2(CO3)32− and Ca2UO2(CO3)3(aq) complexes under neutral to weakly alkaline conditions. Dalton Trans. 2013, 42, 9862–9869. [Google Scholar] [CrossRef]
- Endrizzi, F.; Rao, L. Chemical speciation of U(VI) in marine environments: Complexation of calcium and magnesium ions with [(UO2)(CO3)3]4− and the effect on the extraction of U from seawater. Chem. Eur. J. 2014, 20, 14499–14506. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.C.; Fredrickson, J.K.; Carroll, S.L. Inhibition of bacterial U(Ⅵ) reduction by calcium. Environ. Sci. Technol. 2003, 37, 1850–1858. [Google Scholar] [CrossRef] [Green Version]
- Stewart, B.D.; Mayes, M.A.; Fendorf, S. Impact of uranyl-calcium- carbonate complexes on U(VI) adsorption to synthetic and natural sediments. Environ. Sci. Technol. 2010, 44, 928–934. [Google Scholar] [CrossRef]
- Slimane, D.D.; Vaughan, D.J.; Livens, F.R.; Burton, N.A. Atomistic Simulations of Calcium Uranyl(VI) Carbonate Adsorption on Calcite and Stepped-Calcite Surfacs. Environ. Sci. Technol. 2012, 46, 7587–7594. [Google Scholar]
- Carroll, S.A.; Bruno, J. Mineral-solution interactions in the U(VI)-CO2-H2O system. Radiochim. Acta 1991, 52–53, 187–193. [Google Scholar] [CrossRef]
- Geipel, G.; Reich, T.; Brendler, V.; Bernhard, G.; Nitsche, H. Laser and X-ray spectroscopic studies of U-calcite interface phenomena. J. Nucl. Mater. 1997, 248, 408–411. [Google Scholar] [CrossRef]
- Christian, D.; Dimosthenis, S.; Thomas, K.; John, R.B.; Scott, F. Calcium-uranyl- carbonate species kinetically limit U(VI) reduction by Fe(II) and lead to U(V)-bearing ferrihydrite. Environ. Sci. Technol. 2020, 54, 6021–6030. [Google Scholar] [CrossRef]
- Pablo, J.D.; Casas, I.; Cimenez, J. The oxidative dissolution mechanism of U dioxide.Ⅰ. The effect of temperature in hydrogen carbonate medium. Geochim. Cosmochim. Acta 1999, 63, 3097–3103. [Google Scholar] [CrossRef]
- Goldhaber, M.B.; Hemingway, B.S.; Mohagheghi, A.; Reynolds, R.L.; Northrop, H.R. Origin of coffinite in sedimentary rocks by a sequential adsorption- reduction mechanism. Bull. Mineral. 1987, 110, 131–144. [Google Scholar] [CrossRef]


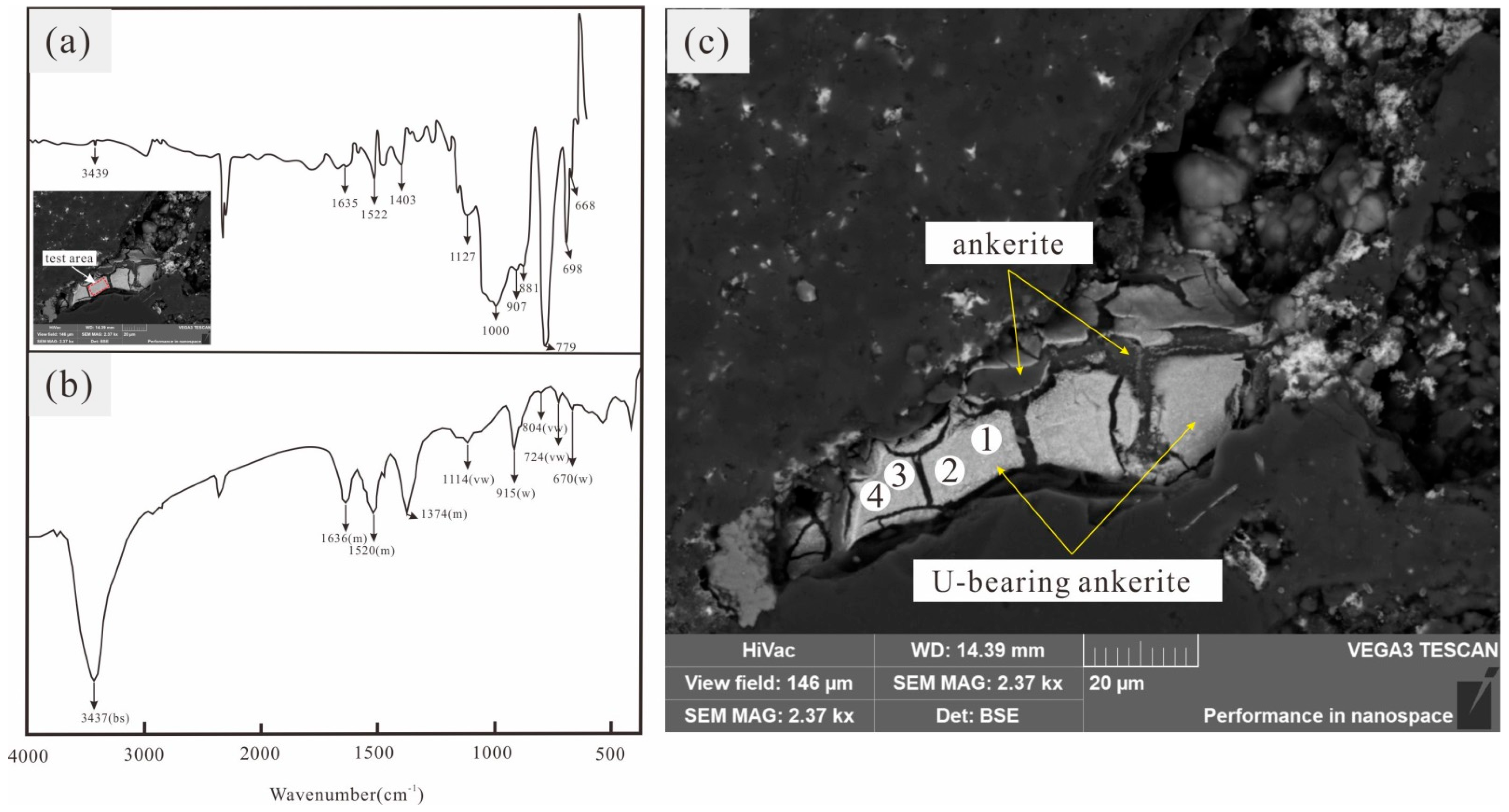
| Wavenumber (cm−1) of Oswaldpeetersite from Renaud [45] | Vibrational Mode | Wavenumber (cm−1) of U-Bearing Carbonate Minerals from This Study |
|---|---|---|
| 1636 | δ (H-O-H) bending | 1635 |
| 1520, 1374 | ν3 (CO32−) antisymmetric stretching | 1522, 1403 |
| 1114 | ν1 (CO32−) symmetric stretching | 1127, 1000 |
| 915 | ν3 (O-U-O) antisymmetric stretching | 907, 881 |
| 804 | ν2 (CO32−) out-of-plane bending | 779 |
| 724 | ν4 (CO32−) in-plane bending | 698 |
| Test Point | FeO (%) | UO2 (%) | MgO (%) | CaO (%) | Test Total (%) | CO32− (%) | H2O (%) |
|---|---|---|---|---|---|---|---|
| 1 | 6.46 | 28.8 | 1.48 | 15.08 | 51.82 | ||
| 2 | 7.22 | 25.6 | 1.46 | 15.18 | 49.46 | ||
| 3 | 6.44 | 28.02 | 1.65 | 15.35 | 51.46 | ||
| 4 | 6.66 | 27.3 | 1.34 | 15.71 | 51.01 | ||
| average | 6.70 | 27.43 | 1.48 | 15.33 | 50.90 | 30.48 | 17.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, B.; Liu, H.; Xu, D.; Qiu, L.; Liu, W. Mineralogical Evidence for Hydrothermal Uranium Mineralization: Discovery and Genesis of the Uranyl Carbonate Minerals in the BLS U Deposit, SW Songliao Basin, Northeast China. Minerals 2023, 13, 339. https://doi.org/10.3390/min13030339
Ding B, Liu H, Xu D, Qiu L, Liu W. Mineralogical Evidence for Hydrothermal Uranium Mineralization: Discovery and Genesis of the Uranyl Carbonate Minerals in the BLS U Deposit, SW Songliao Basin, Northeast China. Minerals. 2023; 13(3):339. https://doi.org/10.3390/min13030339
Chicago/Turabian StyleDing, Bo, Hongxu Liu, Deru Xu, Linfei Qiu, and Weihong Liu. 2023. "Mineralogical Evidence for Hydrothermal Uranium Mineralization: Discovery and Genesis of the Uranyl Carbonate Minerals in the BLS U Deposit, SW Songliao Basin, Northeast China" Minerals 13, no. 3: 339. https://doi.org/10.3390/min13030339




