Abstract
Currently, MSWI (municipal solid waste incineration) ashes are predominantly landfilled, although they can have copper contents comparable to those of low-grade ores. Based on a previously published characterization of MSWI-BA, this paper presents investigations on the identification of potential collectors for copper recovery from MSWI-BA by flotation. The studies were conducted with single minerals (mainly copper oxide and sulfide) and synthetic slag components. Collector screening included thiourea-, thiophosphate-, and thiocarbamate-based collectors. In addition to commercial collector mixtures, pure ureas were also examined. At least one representative from each collector group was selected for the more in-depth studies: the thiourea S-n-dodecyle-iso-thiourea hydrochloride, the thiophosphate Danaflot 245, AERO 3473, and AERO MX-5160 as a mixture of a thiocarbamate and thiophosphates. Studies of the influence of collector concentration and pH were carried out with these. In addition, the contact angles of various metal oxides and the matrix composition with and without collector treatment were determined. Subsequently, flotation tests were carried out with mixtures of copper oxide and the individual matrix components (quartz, glass, cement, gypsum). AERO MX-5160 proved to be the most suitable collector, although alginic acid was added as a depressant due to a lack of selectivity towards gypsum.
1. Introduction
Over the past decades, the demand for and production of copper have risen steadily. This trend is expected to continue, especially against the energy- and transport-transition background. In the meantime, it is assumed that a “peak copper” level will be reached in upcoming decades, which means that the availability of copper from primary raw material sources will decrease [1].
Against this backdrop, the importance of copper recycling continues to increase. While recycling routes have been established for metallic copper scrap, the recovery of copper from low-concentration, fine-grained process residues is still the subject of research. In these process residues, the copper is often not present in its metallic form and they thus show more similarities with copper ores than with copper scrap. Therefore, processing these “synthetic ores” using flotation seems to be a promising approach. Nevertheless, the process residues often differ significantly from natural ores in the bonding form of the copper, as well as in the composition of the matrix, so there is still a considerable need for research here.
An example of a processing residue that could be of interest for the recovery of copper is municipal solid waste incineration bottom ash (MSWI-BA). In the European Union (EU), about 470 MSW incineration plants are in operation. They process 63.7 Mt of waste per year (as of 2015) [2]. Since the compositions of the inputs of the incineration plants differ significantly due to different collection categories, the amount and composition of the bottom ash can also differ. Furthermore, the processing of the ash differs significantly in the individual countries of the EU. While there is no separation—or only separation of ferrous metals—in some countries, MSWI-BA is almost entirely processed and recycled in others, such as Germany, Austria, and Switzerland. In order to recover valuable metals and produce defined mineral products, the industrial processing of MSWI-BA typically includes screening, crushing, sifting, manual sorting, and magnetic and eddy current separation. While the applied unit operations are similar, the detailed flowcharts for each processing plant may vary [2].
While established treatment processes already exist for fractions above 2 mm, treatment processes for finer particles are under research or being implemented. The processes currently under development have the fact that they are designed to recover metallic copper in common [2,3,4,5,6,7]. However, due to the thermal treatment, significant amounts of copper are present in mineral forms, such as oxides or sulfides. A study published in 2020 by Keber et al. showed that the concentrations of non-metallic copper in the fine fractions of MSWI-BA are comparable to those in currently mined copper ores [8].
Due to the small particle size of the copper particles [8] and the high complexity of the MSWI-BA, flotation of the copper represents an interesting research approach. However, since little data are currently available from initial tactile tests, detailed experiments on the flotation behavior of the copper phases and the behavior of the matrix have to be carried out.
1.1. Mineral Composition of MSWI-BA
Previous investigations have shown that, even after mechanical processing using magnetic and eddy current separation, as well as selective comminution, significant amounts of copper were still present in the fraction of MSWI-BA smaller than 2 mm. Therefore, flotation tests were carried out, which showed feasibility in principle but were still unsatisfactory concerning recovery and concentration results [4].
A detailed chemical and mineralogical characterization was carried out in a subsequent project to understand the flotation properties better. For this purpose, a sampling campaign was conducted. Samples of MSWI-BA from the fraction smaller than 2 mm were obtained and processed using dry (magnetic and eddy current separation) and wet mechanical (selective comminution) methods. The remaining fine fraction was characterized in terms of its chemical and mineralogical composition. The details of the sampling and preparation can be found in the publication by Keber et al. [8]. A summary of the most important findings is given here.
Chemical analysis showed a copper concentration between 2604 and 3125 ppm. Since the samples were obtained over several months, slight variations occurred over time. Other authors also determined comparable grades in previous studies [8]. In Table 1, metal concentrations for two MSWI-BA samples are given.

Table 1.
Metal concentrations in processed and unprocessed MSWI-BA samples [8].
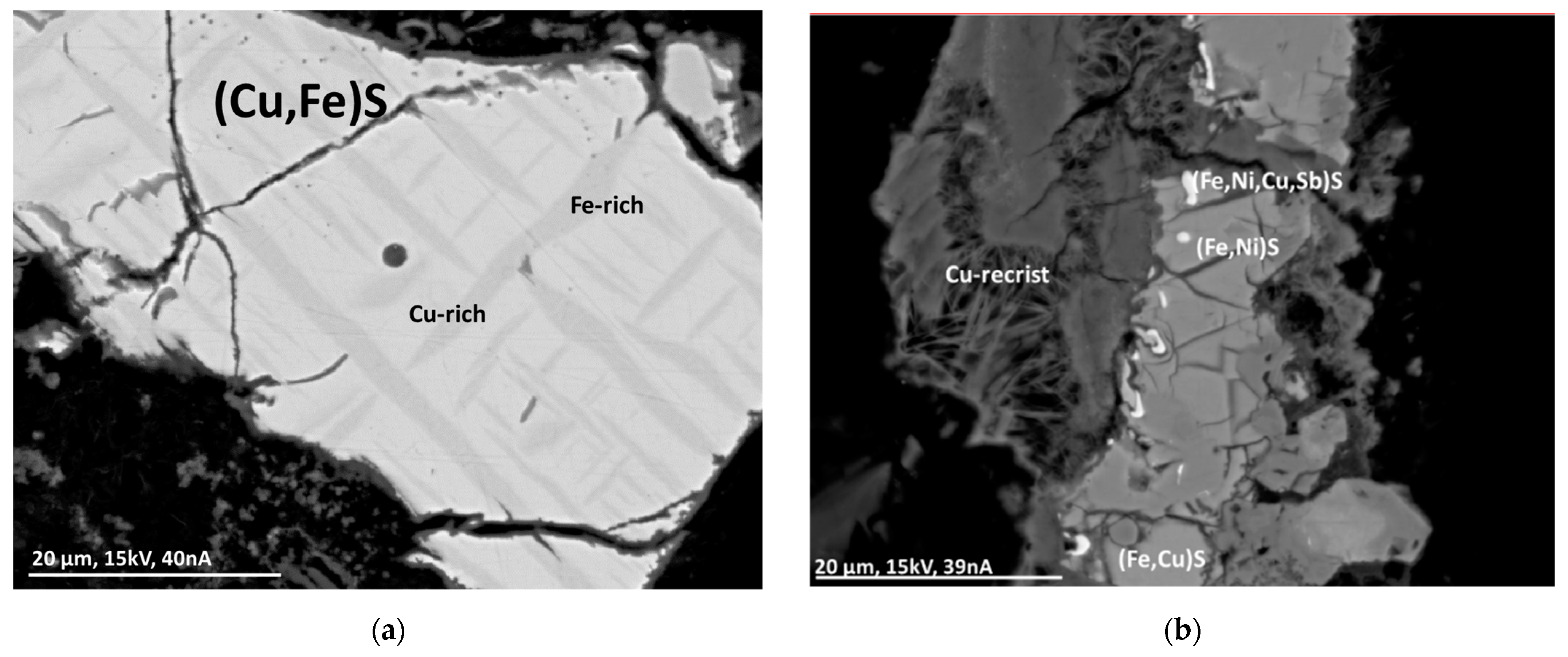
Mineralogical analyses were performed using an electron beam microprobe. No pure copper was found in metallic form; however, its presence in very small quantities cannot be completely excluded. Copper can be found in mineral-like compounds, minor compounds of alloys, and finely dispersed and fused in glass. Copper is predominantly bound in the form of oxides. To a lesser extent, copper can be found as copper sulfides, where these particles can be coated with a copper oxide layer. The copper-containing particles show significant differences in morphology and size. The particles often show sharp fracture edges and cracks, probably due to rapid cooling after incineration. Recrystallization has been observed with some particles, indicating formation during combustion. In addition to copper, other elements, such as zinc and iron, may also occur in the particles. Due to the comparatively fast combustion process, thermodynamic equilibrium is often not reached, leading to a spatial distribution of the elements within the particles. Figure 1 shows examples of copper-containing particles found in MSWI-BA [8].
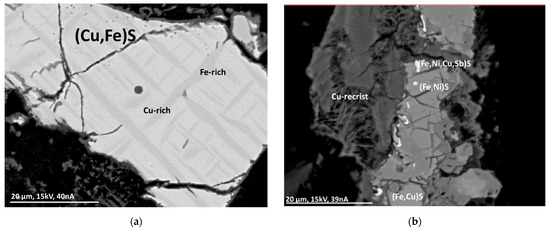
Figure 1.
BSE(Z) micrographs of sulfide particles in an MSWI-BA sample. (a) Segregation lamellae of Fe-rich (Cu,Fe)S (dark gray) in Cu-rich (Cu,Fe)S (light gray) with an oxide layer on the outside. (b) Sulfide particles with recrystallization (“Cu-recrist”) [8].
The investigations show the considerable value-potential of MSWI-BA in terms of the extraction of copper but also very high complexity in the mineralogy. To develop a recovery process, the focus should be on the compounds copper(II) oxide, copper(I) oxide, and copper(II) sulfide (Table 2), which correspond to the naturally occurring minerals cuprite, tenorite, and covellite [8]. However, these compounds are expected to also occur in other stoichiometries due to the non-uniform combustion conditions. In the case of copper sulfide, phases similar to chacocite (Cu2S) or djurleite (Cu31S16)—or, in the case of chalcopyrite, phases similar to valerite (Cu2Fe4S7)—can also be expected. Other elements, such as iron, might be incorporated in the compounds. Furthermore, mineral-like compounds can occur that have no naturally occurring counterparts.

Table 2.
Typical Cu-containing oxide and sulfide compounds from MSWI-BA samples analyzed with an electron beam microprobe [8].
In addition to copper, other metals can be found in the ashes. In particular, aluminum, iron, zinc, and lead are notable. These may be present in the form of oxides or metals, but especially in the form of alloys. In many cases, the composition of the alloys indicates that they have been formed during the incineration process. Depending on the origin of the ashes in mixed waste processing, many more elements may be found in low concentrations in the ashes. Due to their low concentrations, metals other than copper are not of economic interest but may prevent the utilization of the ashes [8].
One study used PXRD (Powder X-ray diffraction) to determine the main phases of the ashes. These were quartz, calcite, ettringite, anhydrite, and gypsum. It can be assumed that these originated from building materials, such as concrete. Quartz glass represented another significant part of the ashes. This originated from disposed glass containers and goods [8].
Table 3 shows an overview of the copper-bearing phases most likely to be recovered by flotation and the main matrix phases.

Table 3.
Overview of the matrix phases identified by PXRD [8].
1.2. Investigations
The investigations presented in this paper were designed to identify collectors and flotation parameters suitable for MSWI-BA. For this purpose, investigations were carried out with individual minerals—in particular, copper oxide and sulfide—as well as matrix components.
To characterize copper oxide and sulfide, the zeta potential was determined as a function of pH, and contact angles were measured. A screening was conducted to select suitable collectors. Due to the large proportion of alkaline-soluble Ca-containing matrix phases, the flotation of waste incineration bottom ash, in contrast to the flotation of natural sulfidic ores, must be carried out at substantially higher pH levels. Depressing the pH leads to massive leaching and acid consumption. In this respect, classic anion-active sulfhydryl collectors are hardly applicable. As a result, the focus was on thiourea-, thiophosphate-, and thiocarbamate-based collectors. These collectors are known to have a wider pH range [9]. Furthermore, for this reason, all experiments were carried out in a pH range above 7. One main goal of the investigation was to find flotation reagents that allowed the flotation of the copper without the need to adjust the pH.
Four collectors were selected for in-depth studies after screening. The influences of concentration, pH and various ions on these collectors were investigated in a Hallimond tube. In addition, conclusions were developed regarding the interaction of the collectors with various metal oxides and the matrix components by measuring the contact angle.
In a final step, the two most promising collectors were tested in a mechanical flotation cell against the matrix components with admixed copper oxide. Copper oxide was chosen for these experiments because the majority of copper is bound in this form. Furthermore, it was assumed that the ashes would be floated in a future process only after storage and transport, so the copper sulfide particles would be covered with an oxide layer. To simplify the experiments, complete liberation of the copper oxide and matrix phases was assumed so that they could simply be mixed for the experiments. Citric and alginic acid were used as depressants.
2. Materials and Methods
2.1. Materials
2.1.1. Collectors and Other Flotation Reagents
The thioureas were purchased from TCI Chemicals. The commercially available collector mixtures were provided by Danafloat and Cytec, respectively. A list of the collectors used is shown in Table 4 and Table 5.

Table 4.
Investigated thioureas.

Table 5.
Investigated collector mixtures.
Since the commercial collector mixtures were predominantly pre-dissolved in non- or poorly water-soluble organic solvents, they were dispersed in approximately 10 mL of water immediately before addition using an Ultra-Turrax. The thioureas were present as solids and were dissolved in butanol before use.
Depressants were also tested in the experiments to investigate the matrix phases’ influences. The tested depressants were citric acid (Carl Roth AG, Karlsruhe, Germany) and alginic acid (Carl Roth AG). Flotanol C7 (Clariant AG, Muttenz, Switzerland) and 4-methyl-2-pentanol (MIBC) were used as frothers.
2.1.2. Synthesis of Copper Oxide and Copper Sulfide
It was not possible to obtain copper oxide and copper sulfide with the required particle sizes and characteristics, so both were synthesized from copper powder. Copper powder with particle sizes between 50 and 100 µm (0–50 µm for zeta potential measurement) and a purity of at least 99% was used as the starting material. The powder was purchased from Carl Schlenk AG (Roth, Germany).
The copper was thermally oxidized to simulate the formation during the combustion process. For this purpose, the copper was roasted twice in a furnace at 400 °C for one hour followed by a further pass at 900 °C for another 1 h. Between the individual passes, the material was deagglomerated in a mortar. While the first two passes were intended to prevent the agglomeration and sintering of the material, the temperature and residence time in the last pass were selected in accordance with the conditions during waste incineration. This was intended to create surfaces as similar as possible to the original material.
In the case of copper sulfide, the exact mechanism of its formation during waste incineration has not yet been clarified. Therefore, a wet chemical process was used for the synthesis. Potassium polysulfide was chosen as a sulfurizing agent, since it is known to form copper sulfide [10]. The parameters used were determined experimentally by the authors. For the synthesis, 25 g of potassium polysulfide per liter of water was added to the solution and heated to 80 °C. Once the solution reached this temperature, copper powder with a solid concentration of 10 g/L was added. After one hour, the solid was dewatered and dried in a drying oven under a vacuum at 40 °C.
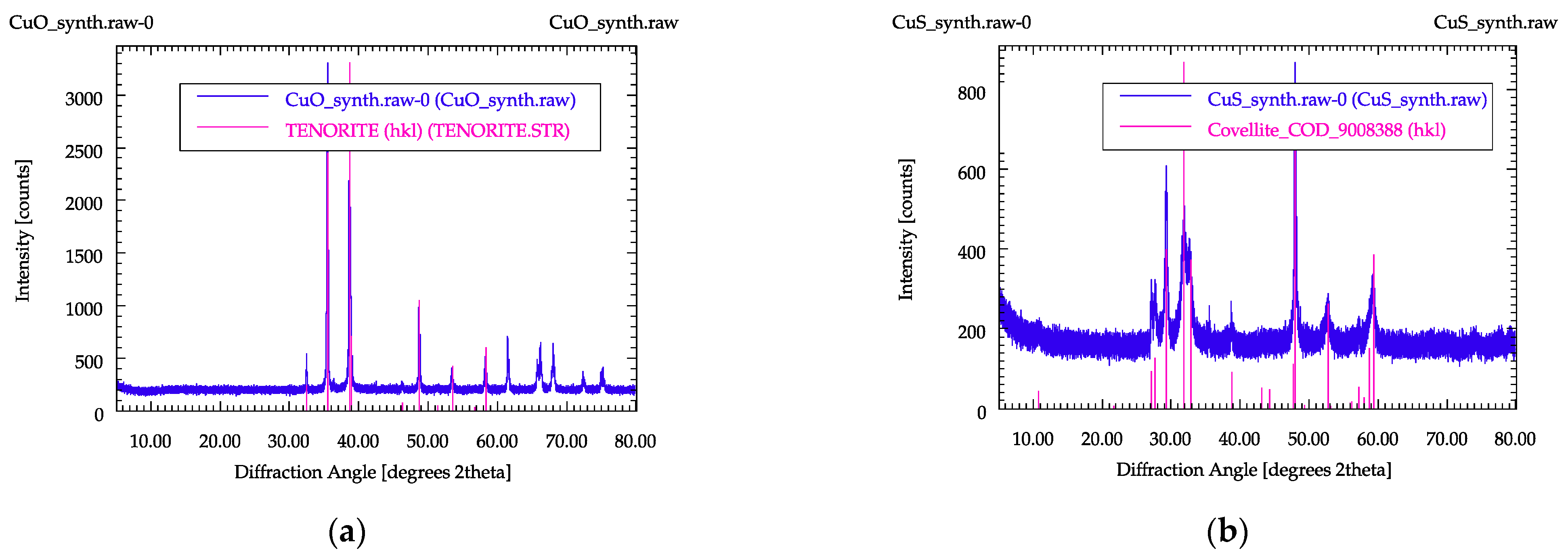
In order to control the phases formed during the syntheses, they were determined using PXRD. In Figure 2, PXRD patterns for synthesized copper oxide and copper sulfide are shown. Due to the large variation in the copper phases occurring in the ashes, not all possible copper carriers could be reproduced with these two materials, but they represented a good starting point for the investigations.
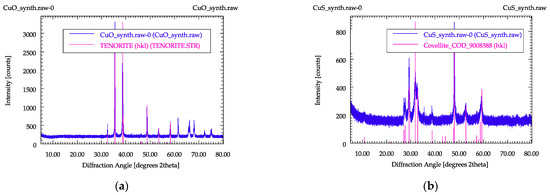
Figure 2.
PXRD patterns of the synthesized samples: (a) copper oxide, (b) copper sulfide.
2.1.3. Other Sample Materials
The previous investigations identified cement, quartz, gypsum, and glass as matrix materials. The first three presumably originated from building materials, while the glass might have originated from bottles and other glassware.
To achieve a chemical composition as in the MSWI-BA, instead of chemically pure single-mineral samples, the same sources were used as would be expected for the ashes; for example, commercial model-gypsum instead of pure calcium sulfate dihydrate and waste glass instead of pure quartz glass.
In the case of the cement, commercially available Portland cement was selected. This was set with a suitable amount of water (0.5 L water per kg cement) and then crushed. The gypsum was model gypsum. This was also (0.7 L water per kg gypsum) set and crushed. Waste glass, predominantly in the form of bottle and container glass, was used as glass. The materials were first crushed using a jaw crusher to a particle size <2 mm and then ground to the target particle size of <160 µm using a rod mill. Particles larger than 160 µm were sieved out. The glass was additionally roasted at 550 °C to exclude contamination with organic matter.
Quartz sand was purchased from Carl Roth AG in particle sizes between 0.4 and 0.8 mm and crushed directly in the rod mill.
The metal oxides for the investigation of the selectivity were purchased from Carl Roth AG and SCS GmbH. In addition to the synthesized copper oxide, chemically pure copper(I) oxide and copper (II) oxide were purchased for the zeta potential and contact angle measurements (see Table 6).

Table 6.
Investigated oxides.
2.2. Methods
2.2.1. Hallimond Tube Tests
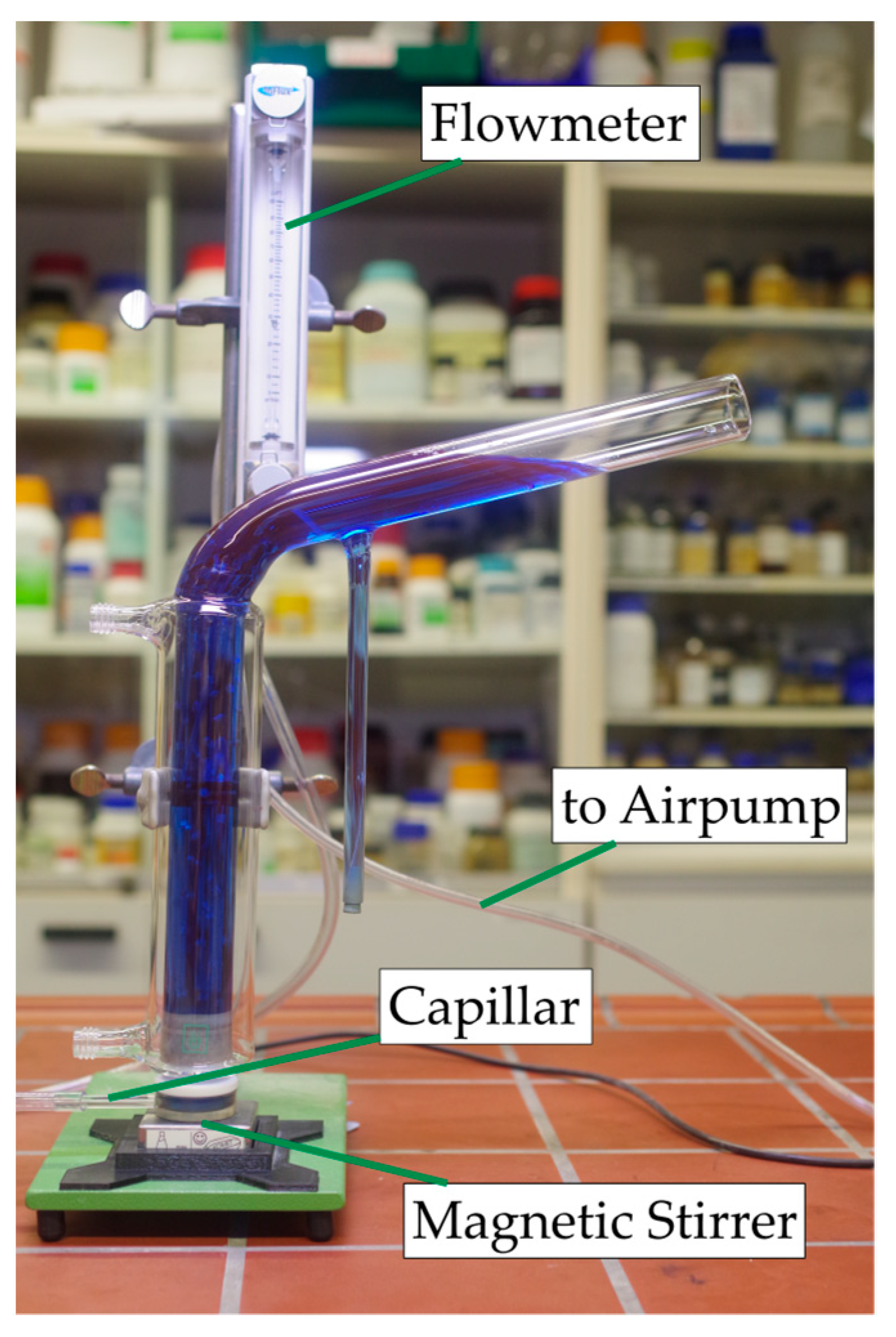
A modified Hallimond tube was used for the flotation examinations. The air was supplied via a capillary with a diameter of 300 µm to create small bubbles. A magnetic stirrer was used to keep the particles in suspension and swirl the bubbles. One gram of solid was used. This was first conditioned outside the Hallimond tube in a beaker with 30 mL distilled water for 5 min while stirring with the respective collector and then transferred into the Hallimond tube. After a flotation time of 7 min, the solids were dewatered and weighed after drying. The conditioning and flotation times were chosen based on preliminary tests. The flotation time had to be long enough to show a clear difference between the experiments but short enough so that entrainment and natural hydrophobicity did not predominate in the yield. The airflow rate was kept constant at 30 cm3·min−1 during all experiments. The Hallimond tube used is shown in Figure 3.
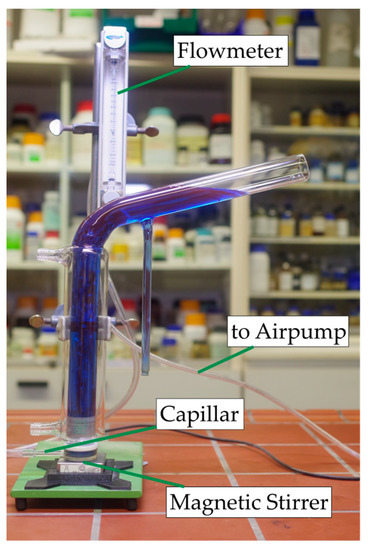
Figure 3.
Modified Hallimond tube used in the experiments filled with colorized water.
A NaOH solution with a pH of 10 was used, except in the experiments where the pH value was examined. The solution used had a similar pH as that observed in MSWI-BA pulps. In the experiments with pH adjustment, NaOH controlled by a pH meter was used. The test parameters were determined based on previous tests.
Over the course of the experiments, differences in the results could be observed; e.g., between screening and the more detailed tests. These differences probably had their origin in small material differences, as the samples originated from different synthesized batches, in combination with the high sensitivity of the Hallimond tube tests. Therefore, we ensured that only results from tests where the materials originated from the same batch and which were performed in a timely manner (ideally on the same day) were compared.
2.2.2. Analytical Methods
A DCAT25 tensiometer from DataPhysics Instruments GmbH, Filderstadt, Germany was used to determine the contact angle using the Washburn method. For this purpose, about 5 g of the material was loosely poured into the sample holder and then compacted with a stainless steel rod. To determine the c-value, measurements were carried out with n-hexane; these measurements were conducted three times, and the mean value was determined. The actual determination of the contact angle was then carried out in ultrapure water.
The conditioning was carried out in the same way as the flotation tests for the contact angle after treatment with collectors. After conditioning, the solid was dewatered and dried at 40 °C under a vacuum to prevent oxidation of the collector.
A Malvern Nanosizer equipped with a dip cell was used for the measurement. Before the measurement, the sample material was suspended utilizing ultrasound in distilled water.
The bulk phase determination was carried out with powder X-ray diffraction (PXRD) using a Bruker D4 Endeavor Powder diffractometer equipped with a Cu X-ray tube (Bruker Corporation, Billerica, MA, USA).
2.2.3. Flotation in a Mechanical Flotation Cell
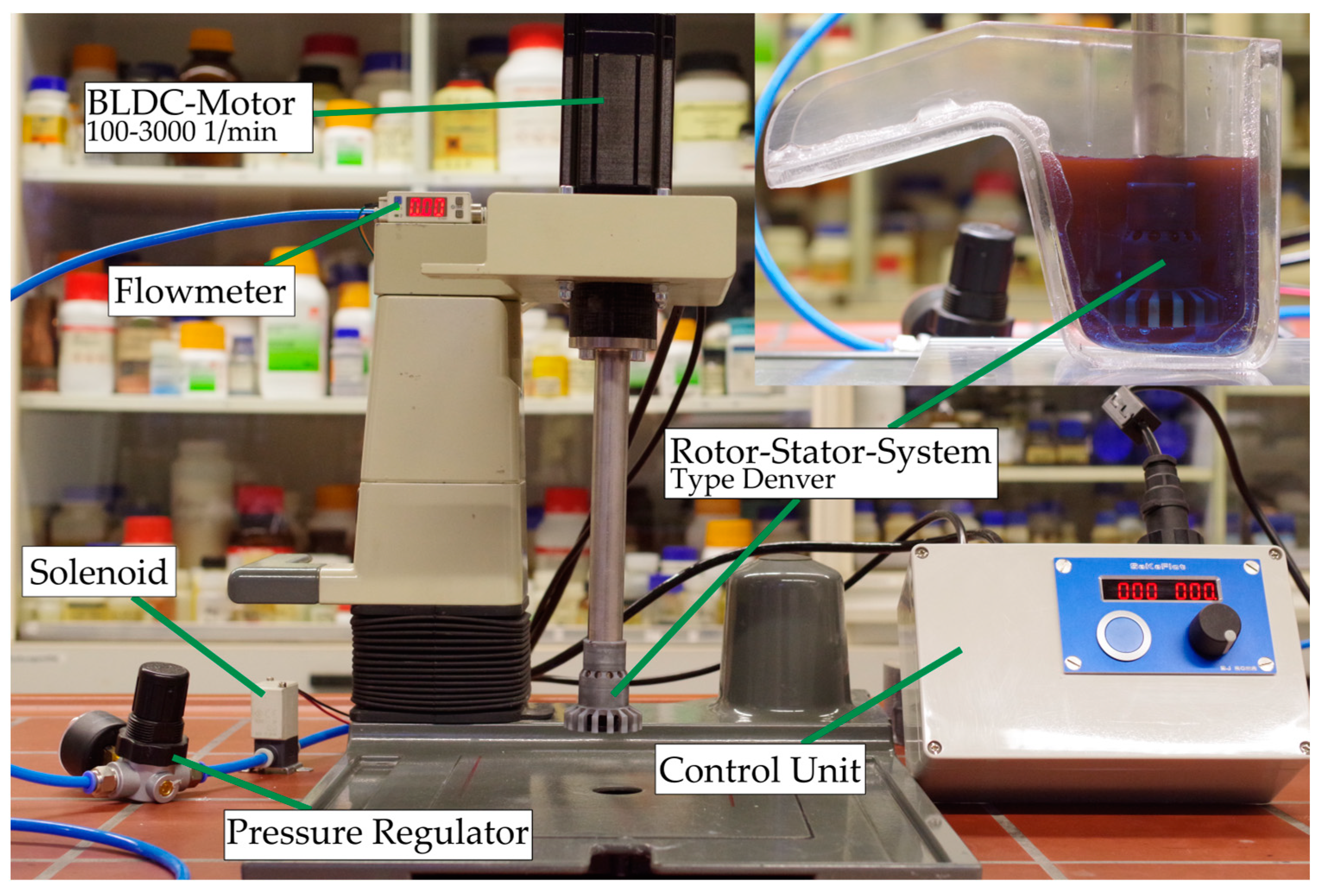
The individual matrix phases’ influences were investigated in a mechanical Denver-type flotation machine. The floatation machine was a modernized version of the IA92 constructed and built by TU Clausthal. The flotation volume was 125 mL and the rotational speed was 3000 1/min during conditioning and 2700 1/min during flotation. The rotor had a diameter of 24 mm. Flotation time was fixed at 5 min using a timer-controlled solenoid. Preliminary tests of the flotation kinetics showed that the floatation time could be shortened compared to the Hallimond tube test due to greater agitation and air flow. The airflow during flotation was 200 cm3/min at a pressure of 100 kPa. The conditioning time for the collector was 5 min, the same as in the Hallimond tube tests. The used flotation machine is shown in Figure 4
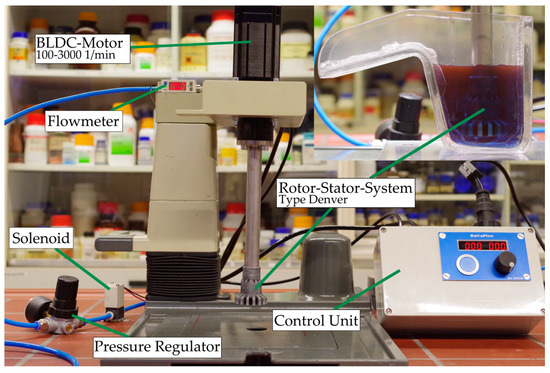
Figure 4.
Modernized IA92 flotation machine constructed and built by Technical University of Clausthal, Clausthal-Zellerfeld, Germany.
2.3. Evaluation of the Flotation Tests
In order to characterize the success of the flotation process, three parameters were determined in this work: the recovery of mass, the recovery of copper, and the enrichment factor for copper. The recovery of mass was used for the evaluation of the Hallimond tube tests. For the evaluation of the mechanical flotation cell with a material mixture, the copper recovery and enrichment factor were used.
The mass recovery (Rm) indicates how much of the total feed mass is discharged into the concentrate. Equation (1) shows the calculation of the mass output into the concentrate.
The copper recovery (RCu) indicates the percentage of copper recovered into the concentrate. Equation (2) shows the calculation of the copper recovery into the concentrate or tailings. In addition to the masses, the concentration of copper in the concentrate and tailings is required for the calculation (wi).
The enrichment factor (E) is a measure of how much a substance is concentrated or depleted in a fraction. If the enrichment factor is greater than 1, the substance is concentrated; if it is less than 1, the substance is depleted. The enrichment factor for copper in the concentrate was calculated according to Equation (3) using the copper contents of the concentrate and the concentration in the feed.
3. Results
3.1. Zeta Potential
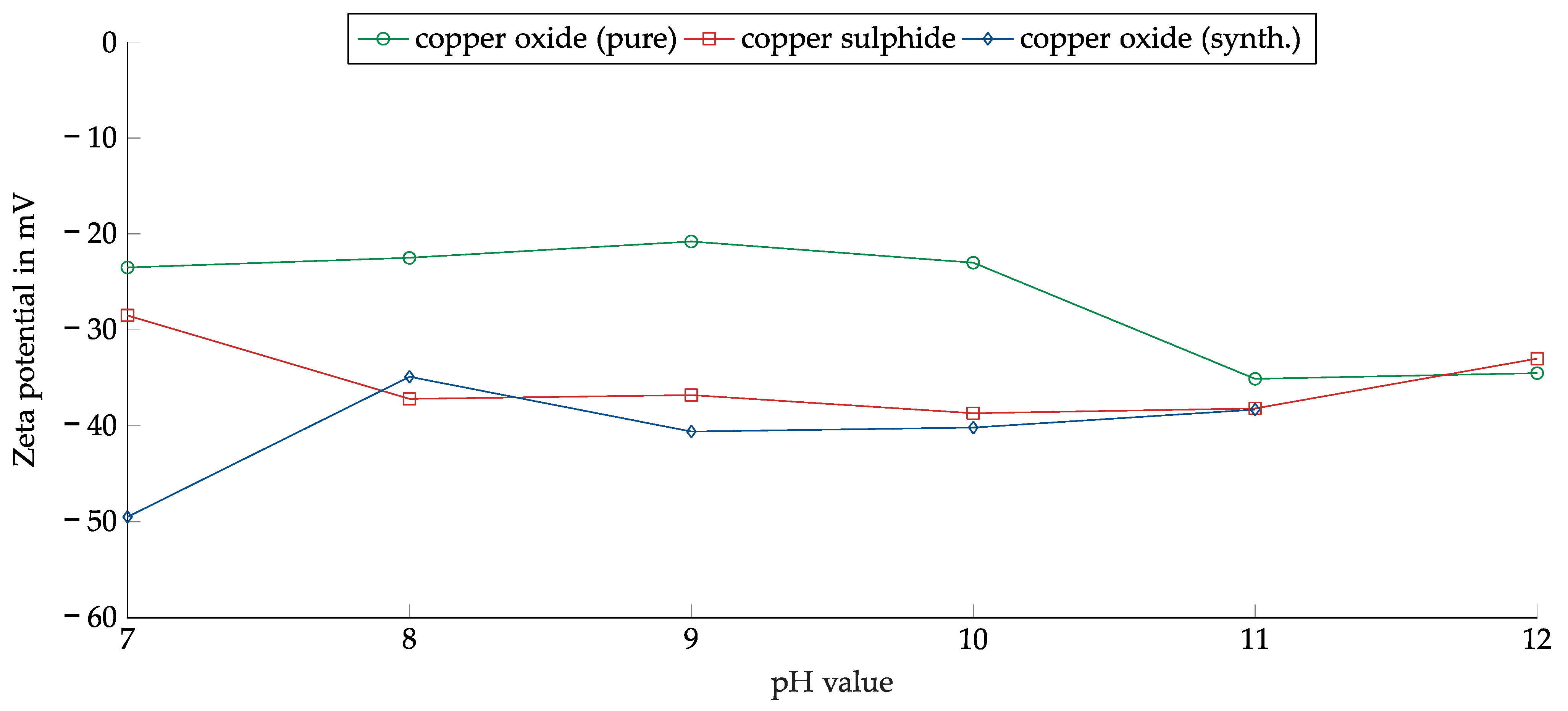
The zeta potential was measured as a function of pH for chemically pure copper(II) oxide, thermally synthesized copper oxide, and copper sulfide prepared as described in Section 2.1.2 but with a smaller particle size of less than 50 µm. The zeta potential of copper(I) oxide was not measured because it could not be suspended in water due to its hydrophobicity (see Section 3.3.4). Figure 5 shows the results of the measurements.
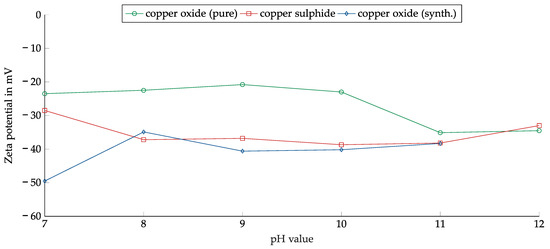
Figure 5.
Zeta potential as a function of pH for copper(II) oxide, thermally synthesized copper oxide, and copper sulfide.
All measured zeta potentials were in the negative range. A slight dependence on pH could be observed in the case of copper(II) oxide. The zeta potential rose slightly from −25 mV at pH 7 to −20 mV at pH 9 and then started to fall. At pH 11, the minimum of −35 mV was reached.
Both copper sulfide and the thermally synthesized copper oxide had lower zeta potential. Between 8 and 11, both showed a weak dependence on the pH value and had a similar course. Only the difference of approximately 20 mV at a pH of 7 was remarkable. The similar zeta potential should have facilitated the joint flotation of copper oxide and sulfide.
The weak dependence of the zeta potential on the pH value made it more feasible to float copper in the alkaline range without adjusting the pH value. Since the zeta potential was consistently negative, the cationic collectors may have had an advantage.
3.2. Collector Screening
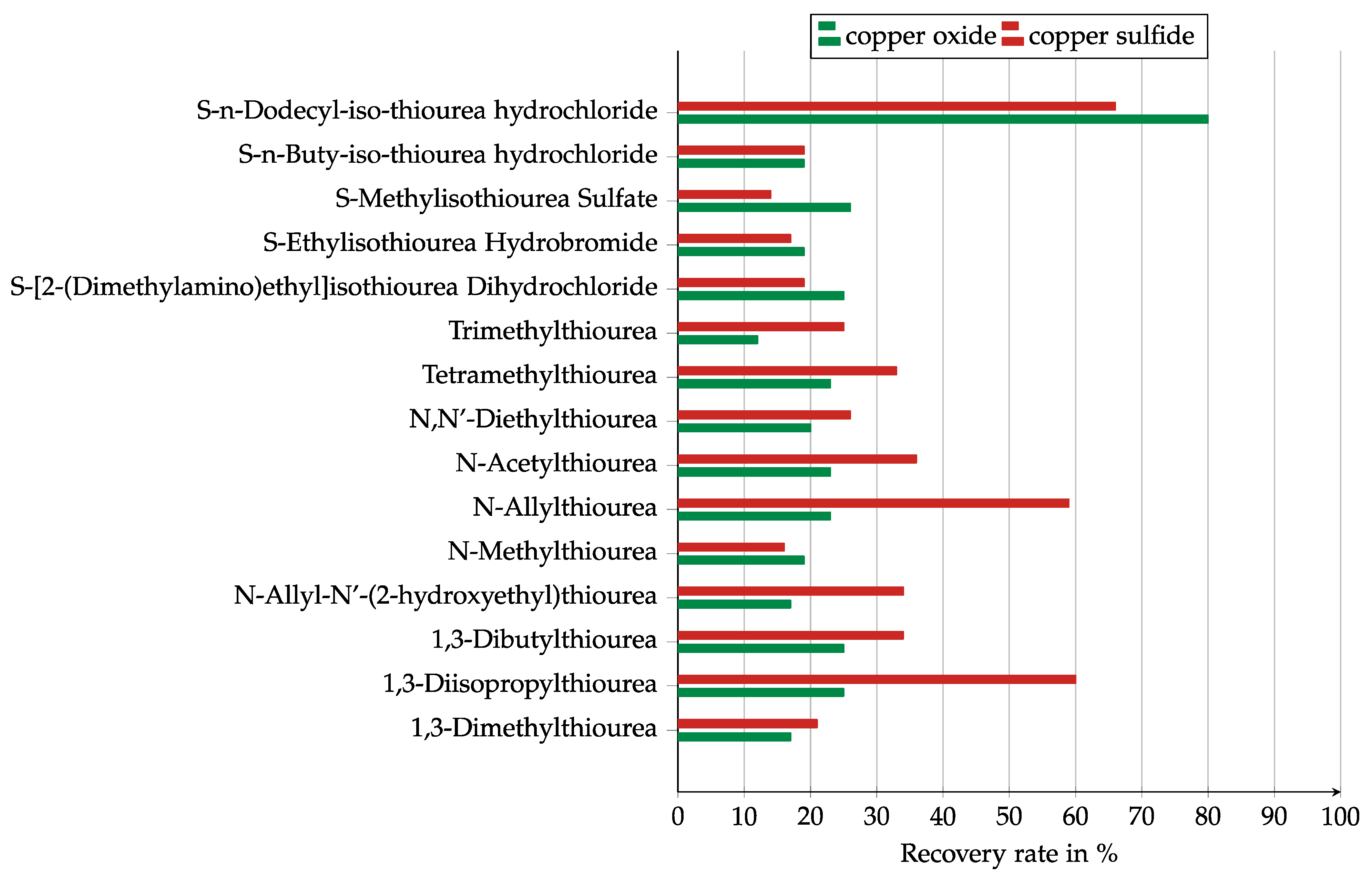
The results for the collector screening are presented in Figure 6 and Figure 7, split into the results for the pure thioureas and the collector mixtures. Most of the thioureas were stronger collectors for copper sulfide than for copper oxide. Only five of them were stronger for copper oxide. Both 1,3-diisopropyl and N-allylthiourea showed high recovery of nearly 60% for copper sulfide, but the recovery for copper oxide was below 25%. The only collector with a high recovery of 80% for copper oxide was S-n-dodecyle-iso-thiourea hydrochloride. It also showed the best results for copper sulfide.
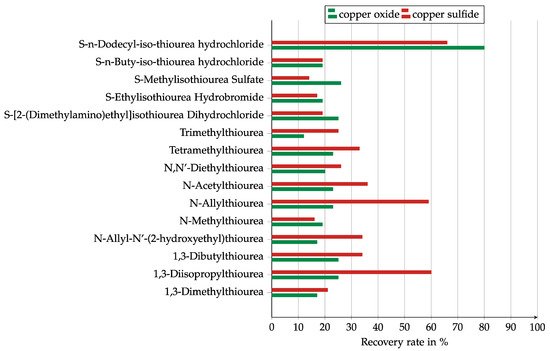
Figure 6.
Results of the collector screening in a Hallimond tube for the investigated thioureas, Collector dosage: 6 kg/t, conditioning time: 5 min, airflow: 30 cm3 min−1, flotation time: 7 min, pH: 10.
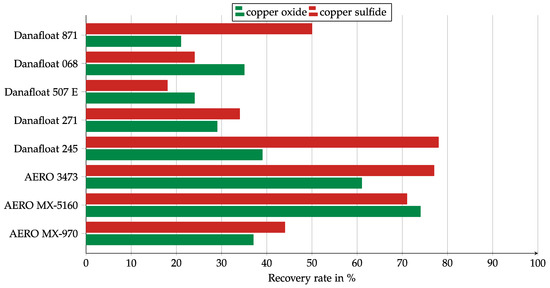
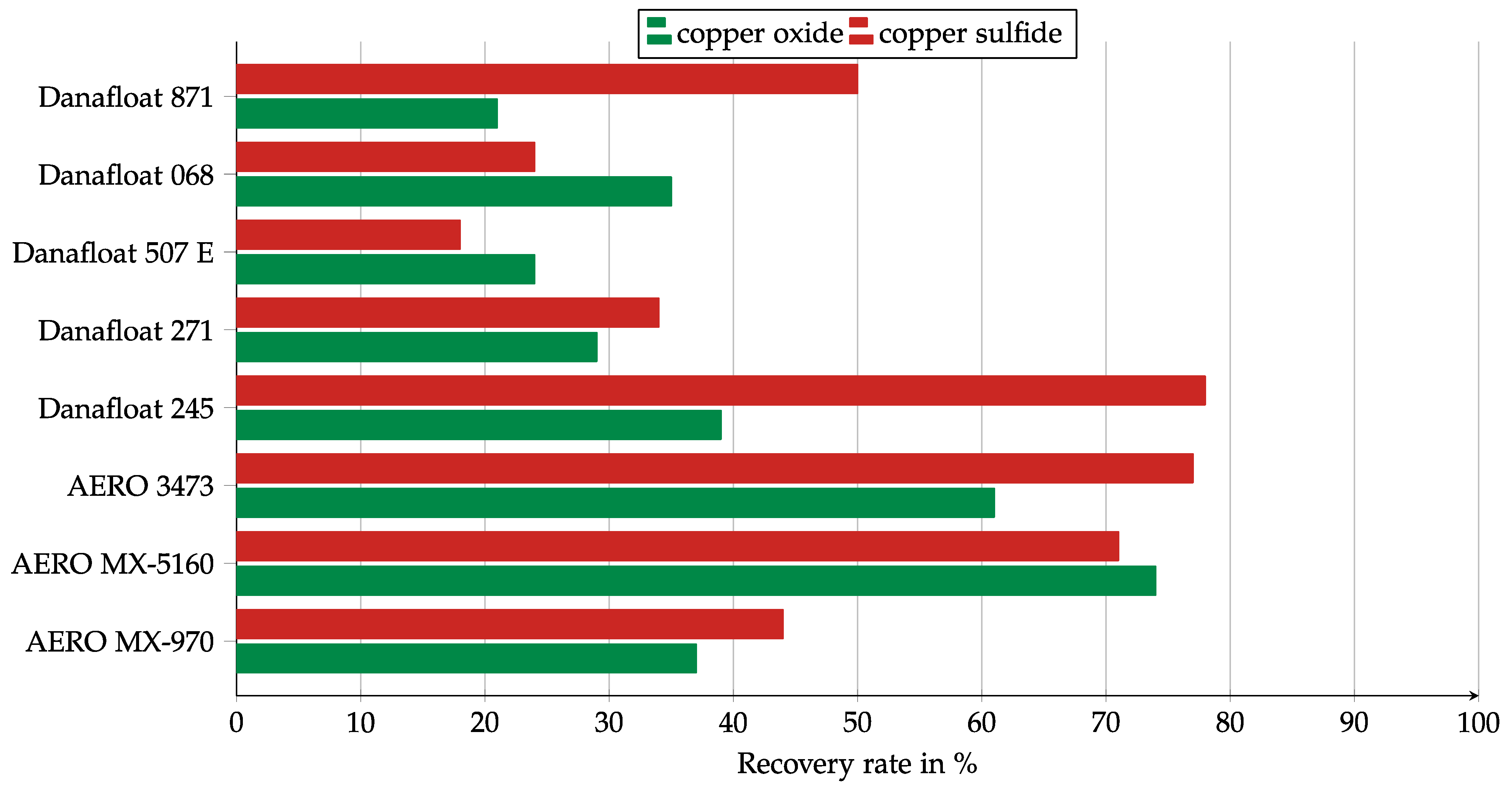
Figure 7.
The results of the collector screening in a Hallimond tube for the commercial collector mixtures. Collector dosage: 6 kg/t, conditioning time: 5 min, airflow: 30 cm3 min−1, flotation time: 7 min, pH: 10.
Due to the negative zeta potential, it was expected that cationic collectors would show a better effect. This could not be confirmed in this test as only S-n-dodecyle-iso-thiourea showed a significantly higher recovery than the non-cationic collectors. The reason for this could have been its particularly long carbon chain.
It should be noted that the collectors that showed only very low recovery rates might have had no effect and the recovery might have been caused by entrainment or the natural hydrobicity of the solids.
The collector mixtures showed higher recovery rates than the thioureas in most cases. The thiophosphate-based collectors of the Danafloat series showed weaker results than the Cytec collector mixtures. However, the concentrations of the stock solutions were not known, so there was a possibility that the effective dosages were different between Danafloat and Cytec. Danafloat 245 is a much stronger collector for copper sulfide than for copper oxide; indeed, it is the strongest copper sulfide collector among commercial collectors. Danafloat 871 (FMC Corporation, Philadelphia, PA, USA) was conspicuous because its copper sulfide recovery was more than twice as high as for copper oxide. Cytec AERO MX-5160 and AERO 3473 (Solvay S.A., Brussels, Belgium) both seemed to be promising collectors. While MX5160 showed a balanced recovery for copper oxide and copper sulfide, AERO 3473 worked more strongly for copper sulfide.
3.3. In-Depth Investigation of Selected Collectors
For the in-depth investigation, four collectors representing each investigated group were chosen. For the group of thiourea-based collectors, S-n-dodecyl-iso-thiourea hydrochloride (hereafter Thiourea) was investigated. As a representative of the thiophosphate-based collectors, Danafloat 245 (FMC Corporation, Philadelphia, PA, USA) was chosen. Cytec AERO 3473 (Solvay S.A., Brussels, Belgium) was chosen as a representative of the thiocarbamate-based mixtures and AERO MX-5160 (Solvay S.A., Brussles, Belgium) as a mixture of a thiocarbamate and thiophosphates.
3.3.1. Concentration
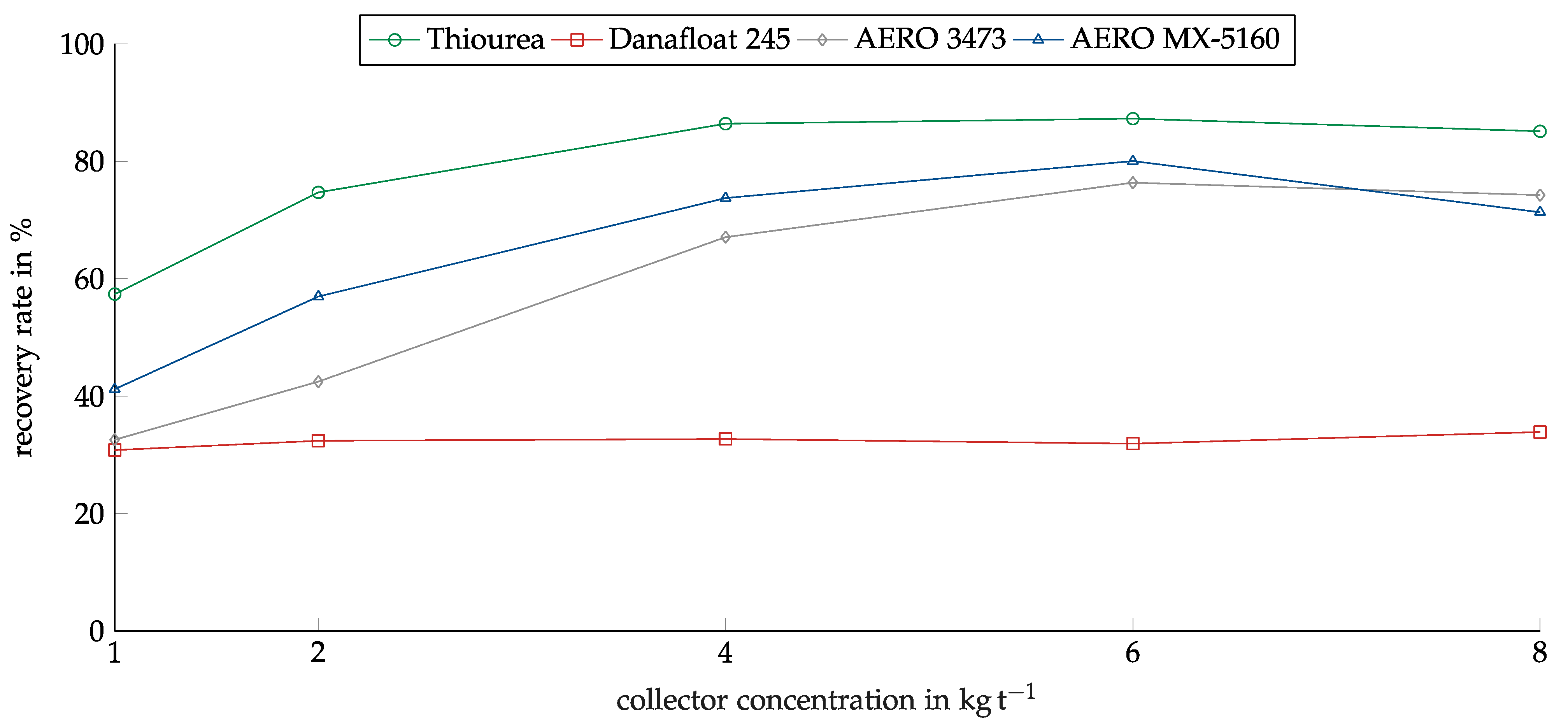
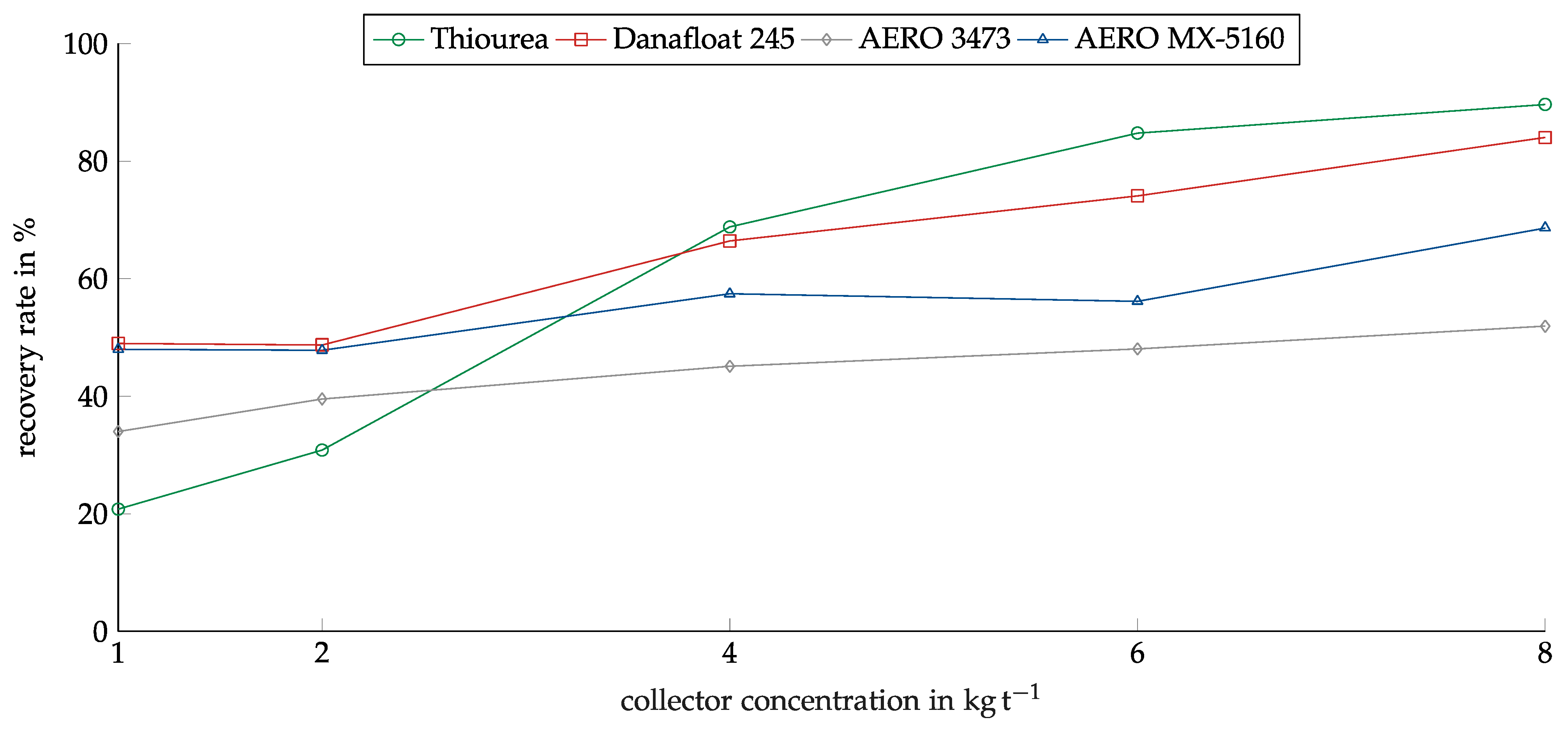
The concentration of the collectors was investigated in the range between 1 kg/t and 8 kg/t. The range was determined based on preliminary investigations. Figure 8 shows the recovery of copper oxide in relation to the collector concentration for the investigated collectors. As in the screening experiments, Thiourea showed the highest recovery. The recovery rate rose from 57% at 1 kg/t to 86% at 4 kg/t. After this, the recovery remained constant. AERO 3473 and MX-5160 showed similar results with slightly lower recovery. They only reached their maximums at a concentration of 6 kg/t.
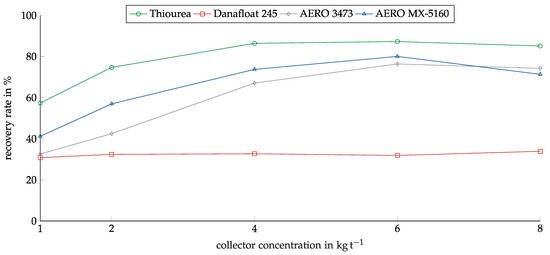
Figure 8.
Recovery of copper oxide for the investigated collectors in relation to the collector dosage. Conditioning time: 5 min, airflow: 30 cm3 min−1, flotation time: 7 min, pH: 10.
Danafloat 245 showed no dependency on concentration. The recovery for all concentrations lay slightly above 30%. For this reason, experiments with higher concentrations were conducted to ensure the correct concentration range was chosen, but no higher recovery was reached.
The results for copper sulfide are shown in Figure 9. The results differed from the results for copper oxide. At the lowest concentration of 1 kg/t, Thiourea showed the weakest recovery at only about 20%. However, Thiourea’s recovery strongly depended on the concentration; starting from 4 kg/t, it showed the highest recovery among the investigated collectors. At the maximum of 8 kg/t, it showed a recovery of 90%. For copper sulfide, Danafloat 245 showed the second highest efficiency. Starting at 50% at 1 kg/t, it reached about 85% at 8 kg/t. The Cytec collectors showed a much weaker dependency on the concentration and a lower recovery than for copper oxide.
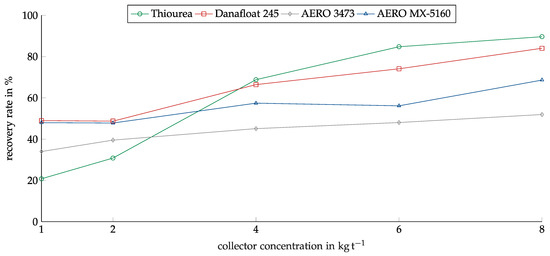
Figure 9.
Recovery of copper sulfide for the investigated collectors in relation to the collector dosage. Conditioning time: 5 min, airflow: 30 cm3 min−1, flotation time: 7 min, pH: 10.
3.3.2. pH Value
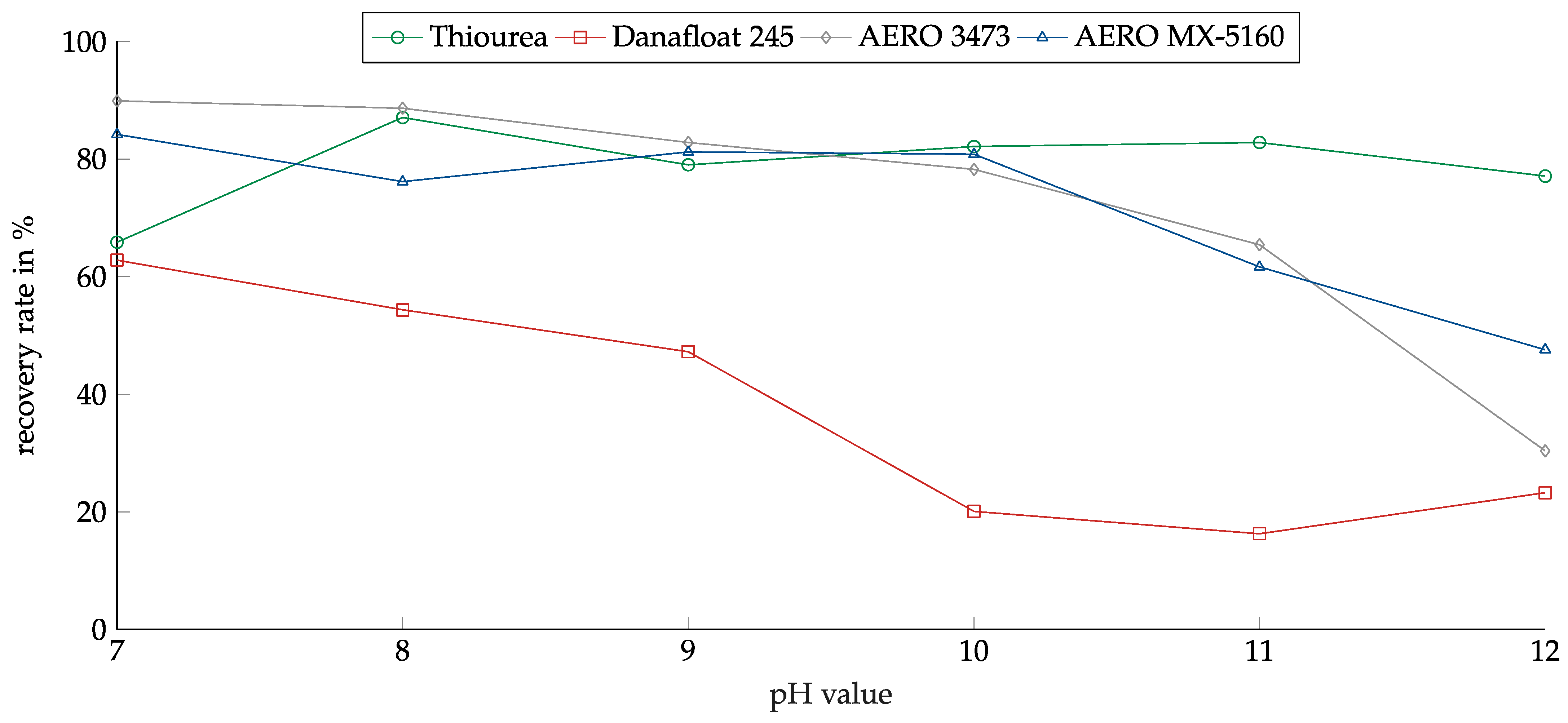
The influence of the pH was investigated in the range between 7 and 12. The range was chosen because MSWI-BA pulps have a basic pH, and adjusting the pH value into the acidic range did not appear reasonable. Figure 10 shows the recovery of copper oxide in relation to the pH value for the investigated collectors.
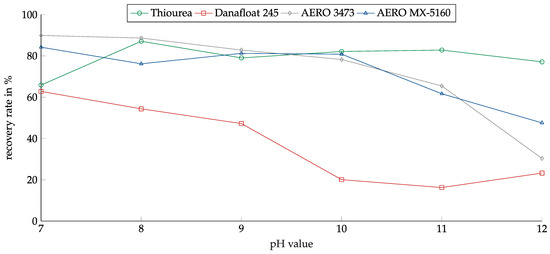
Figure 10.
Recovery of copper oxide for the investigated collectors in relation to the pH value. Collector dosage: 6 kg/t, conditioning time: 5 min, airflow: 30 cm3·min−1, flotation time: 7 min.
For copper oxide, Thiourea showed the lowest pH dependency. Except for at the pH of 7, the recovery reached around 80%. The other collectors showed decreasing recovery levels with rising pH values. Danafloat in particular showed a high dependency. At a pH of 7, the recovery reached 63%, dropping slowly to 47% at a pH of 9. After that, it dropped to around 20% and remained there. This explained the weak results for the thiophosphate-based collectors in the previous experiments, as they were conducted at a pH of 10. AERO 3473 and AREO MX-5160 showed the highest recovery rates at a pH of 7 at nearly 90%. Both collectors showed very good results up to a pH of 10. For higher pH values, the recovery dropped significantly.
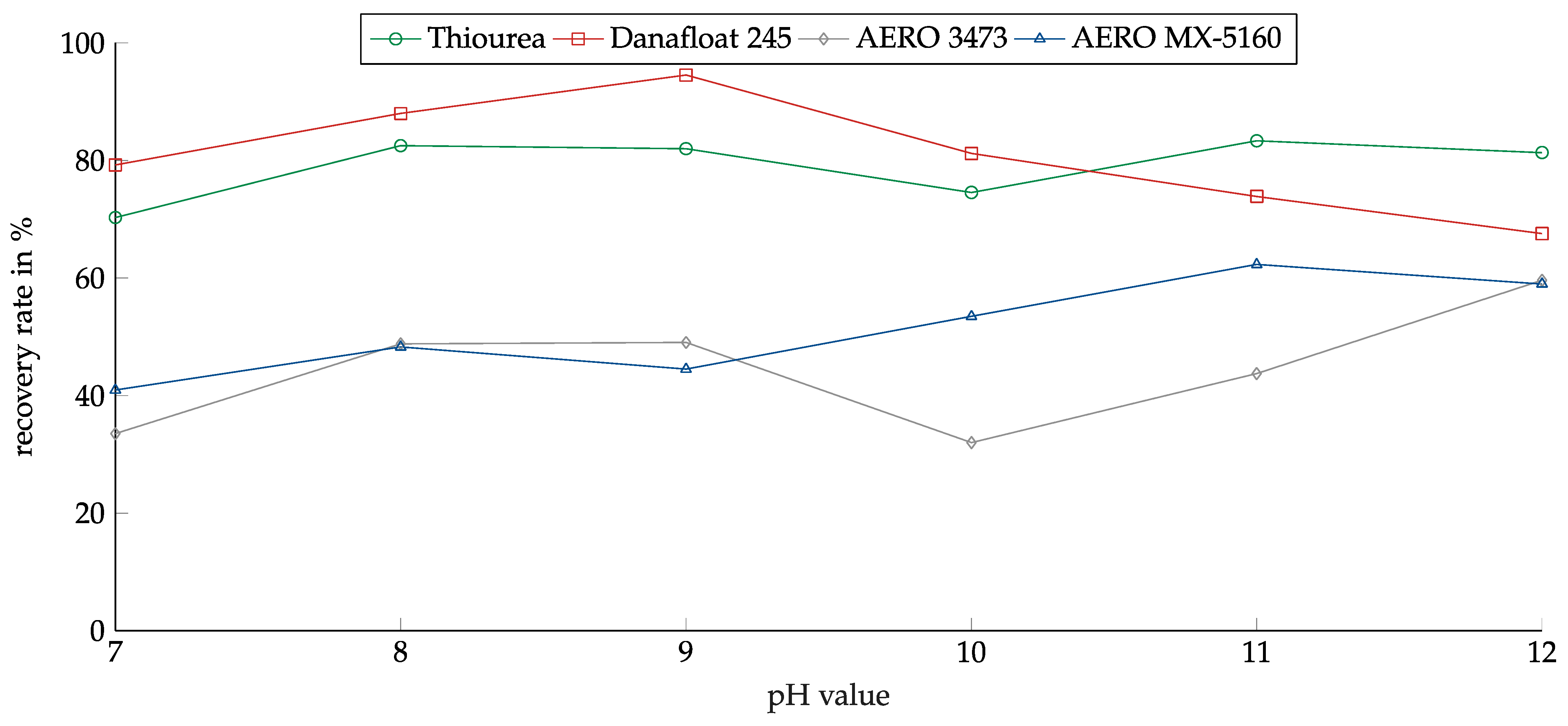
Figure 11 shows the recovery of copper sulfide in relation to the pH value for the investigated collectors. Again, Thiourea showed a low pH dependency. Recovery was between 70% and 83% over the whole pH range. Danafloat 245 showed much better results for copper sulfide than for copper oxide. In contrast to the copper oxide, for sulfide, the recovery reached a peak at a pH of 9 at over 90%. For lower and higher pH, the recovery decreased. The AERO 3473 showed only a weak pH dependency, and no peak could be determined. AERO MX-5160 showed slightly better results for higher pH values. The weak influence of the pH corresponded to the weak influence of the pH on the zeta potential.
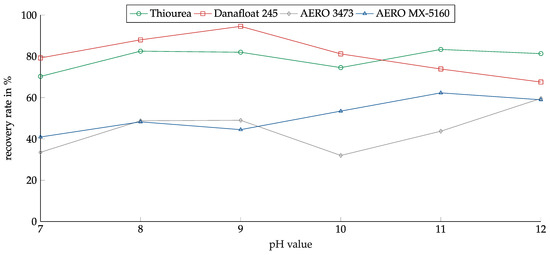
Figure 11.
Recovery of copper sulfide for the investigated collectors in relation to the pH value. Collector dosage: 6 kg/t, conditioning time: 5 min, airflow: 30 cm3·min−1, flotation time: 7 min.
3.3.3. Influence of Selected Ions
To investigate the influence of the ions, we first determined which ions dissolved from the MSWI-BA at which concentrations. To achieve this, a sample obtained for the characterization published by Keber et al. [8] was suspended in water with a solids concentration of 200 g/L and stirred for 30 min. After this, a water sample was filtered and analyzed using ICP-OES (Agilent 5100, Agilent, Santa Clara, SA, USA). Table 1 shows the concentrations for the elements found in relevant quantities. The element with the highest concentration was calcium at 500 mg/L. Sodium and potassium were also present in relevant quantities.
For the Hallimond test, solutions with comparable concentrations of calcium, sodium and potassium were prepared. Hydroxides and sulfates were used to investigate the influence of the anions. In addition to the solutions with only one cation, solutions with all three were also prepared. Ultrapure water was used to avoid contamination with other ions for all solutions. Table 7 shows an overview of all the solutions used.

Table 7.
Overview of the solutions used for the flotation experiments on ions.
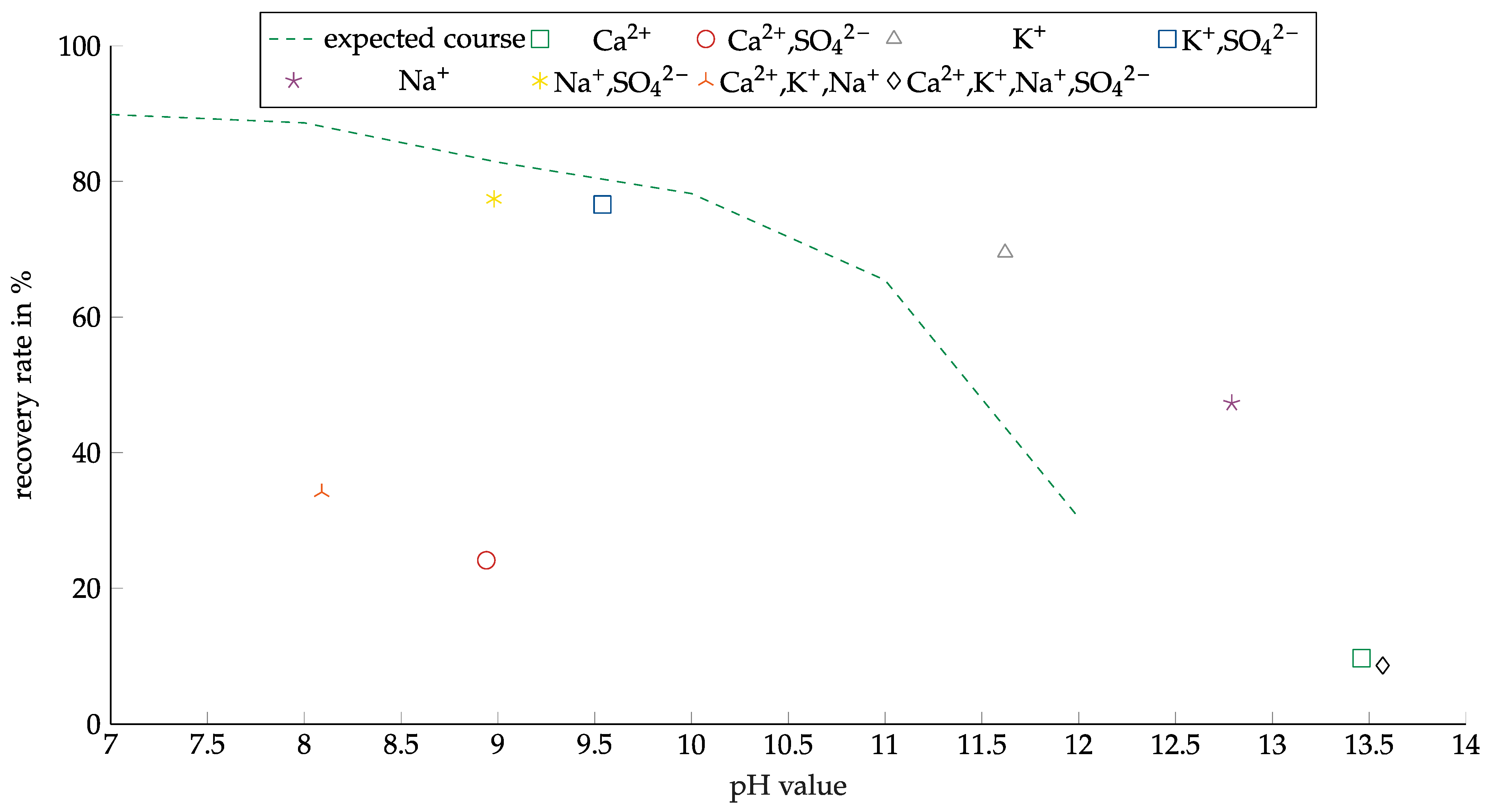
These tests were only carried out with copper oxide, which was the dominant copper carrier; most of the copper sulfide particles even had an oxide layer. Copper sulfide only occurred in much smaller quantities. AERO 3473 was investigated with all solutions for copper oxide. The results are shown in Figure 12. The recovery results are plotted against the pH value to account for the significantly different pH values of the solution. As the results presented in Figure 9 show, decreasing recovery with increasing pH was observed. The solutions that did not contain calcium showed worse results than expected from the previous experiments. The tests with the Ca-containing solution showed a significantly lower recovery. A good comparison can be drawn between the experiments with the CaSO4 and Na2SO4 solutions due to the similar pH value of about 9. For the Na2SO4 solution, the recovery was nearly 80%, while the recovery for the CaSO4 solution was only about 25%. The difference in output reached 53 percentage points (pp).
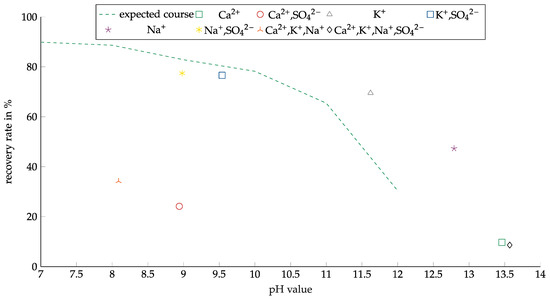
Figure 12.
Copper recovery with AERO 3473 as a collector in combination with different ions. Collector dosage: 6 kg/t, conditioning time: 5 min, airflow: 30 cm3·min−1; flotation time: 7 min.
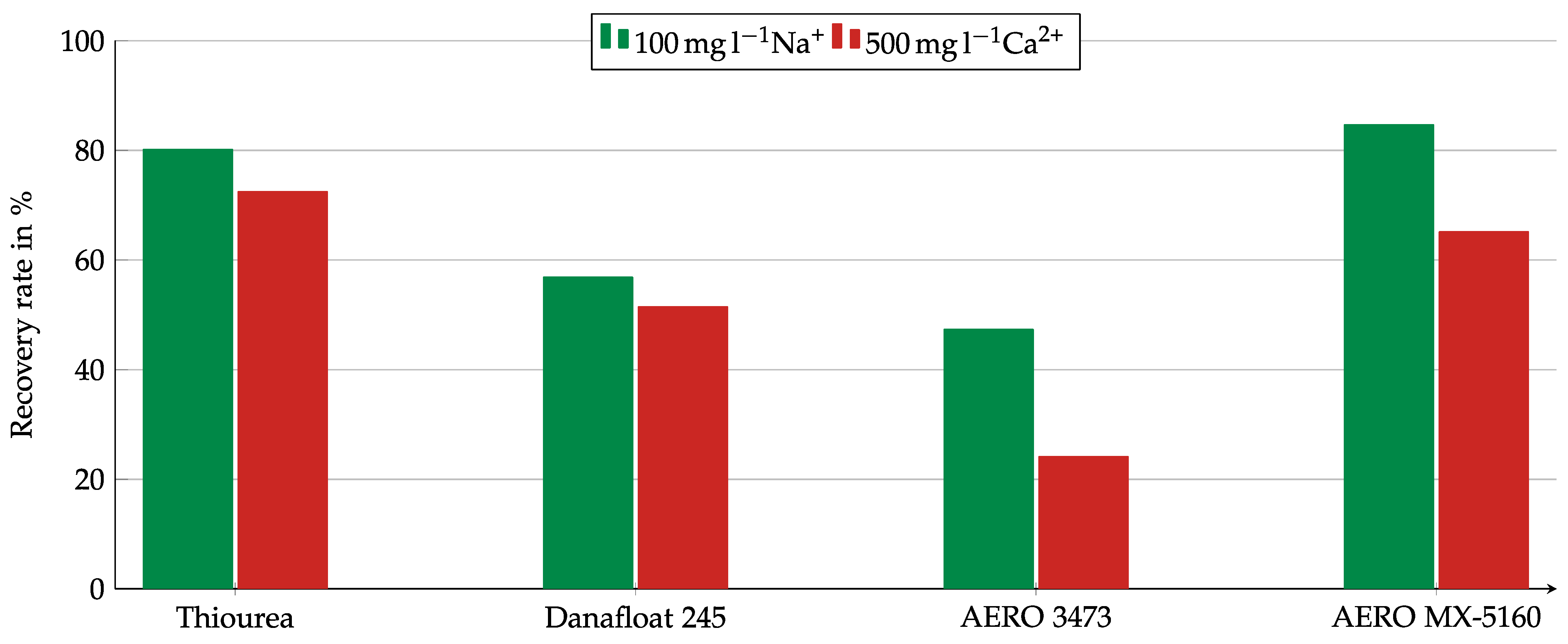
After Ca2+ was determined as the primary influence factor for the other collector, the tests were only conducted with the Na2SO4 and the CaSO4 solutions. Figure 13 shows the copper oxide recovery using a sulfate solution with 500 mg/L Ca2+ compared to a solution with 100 g/l Na+. The sodium solution was chosen for comparison because of the almost identical pH value. All collectors showed weaker results in the presence of calcium ions, but the intensity of the influence was different. While for AERO 3473 a strong influence with a difference in recovery of 20 pp could be observed, the differences for Thiourea and Danafloat 245 were much lower. Thiourea revealed a difference of 7.7 pp, while for Danafloat 245, the difference in recovery was only 5.4 pp.
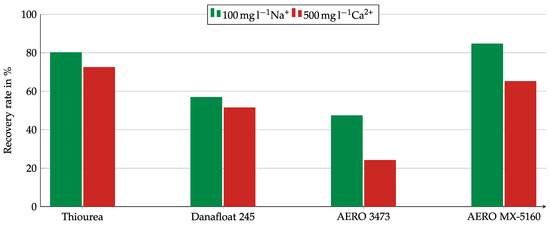
Figure 13.
Copper recovery for different collectors in the Hallimond tube in the presence of Na+ or Ca2+ ions. Collector dosage: 6 kg/t, conditioning time: 5 min, airflow: 30 cm3·min−1, flotation time: 7 min.
As one possible explanation for the worse results, a change in the zeta potential caused by the ca+− ions was suggested, but no difference could be measured.
3.3.4. Determination of Contact Angles
The results of the contact angle measurement are shown in Table 8. Since the Washburn method can only be used to measure contact angles up to 90°, no exact values are given for higher angles. The Washburn method does not precisely reproduce the conditions in flotation since it involves wetting a dry surface, whereas the surfaces are already wetted in flotation. The contact angles shown here may, therefore, deviate from those during flotation. However, it can be assumed that the high contact angles measured here would lead to better flotation results, especially for angles greater than 90°.

Table 8.
Contact angles with and without collector treatment for the investigated phases.
Among the copper-containing substances, copper(I) oxide had the highest contact angle of over 90°. The contact angles of copper(I) oxide and copper hydroxide were significantly lower at 42° and 48°. However, all the copper compounds had higher contact angles than the matrix minerals except for cement. Since copper oxide, which is formed during thermal processes, always represents a mixture of copper(I) and copper(II) oxide, it can be assumed that, in the case of MSWI-BA, the actual contact angle depends strongly on the degree of oxidation and, thus, the conditions during combustion.
After the collector treatment, copper(II) oxide and copper sulfide also had contact angles of >90° in all cases, confirming the collector screening results. The different results for the recovery with the individual collectors can be explained by the different contact angles in the range above 90°. In the case of the matrix components, high contact angles for quartz and glass after treatment with Thiourea were also noticeable; these were also over 90°. Interaction of the collector with SiO2 can therefore be expected, so Thiourea seems less suitable than the other collectors examined.
All collectors investigated showed hydrophobic effects on gypsum. This can lead to co-flotation of gypsum and a reduction in copper enrichment during flotation. The use of depressants would, therefore, be indispensable for successful flotation.
In the case of cement, a hydrophilic effect for the collector could be observed. Thiourea was an exception. The underlying mechanism has not yet been conclusively clarified. A possible explanation could be reactions with additives in the cement, which are added, for example, to adjust the setting time. Since Thiourea was the only collector supplied as a pure substance, while the other collectors were obtained as pre-dissolved mixtures, it cannot be ruled out that components other than the actual collector caused this effect.
Aluminum and iron oxide both had relatively high contact angles. However, it should be mentioned that this does not necessarily apply to the aluminum and iron compounds in ashes due to their different routes of genesis. Treatment with different collectors had no significant influence on either compound.
The contact angle of nickel oxide was 57° without the addition of a collector, which was higher than those of the copper compounds. Only after Thiourea treatment could a distinct angle increase be observed. The resulting angle of over 90° was in the hydrophobic range. The same behavior could be observed for zinc oxide. All collectors raised the contact angle of silver oxide above 90°. Both nickel and silver are by-products of copper metallurgy, so it is desirable to enrich them together with copper for recovery. This seemed possible, at least in the case of silver, with all collectors.
3.4. Investigation of the Mixtures in a Mechanical Flotation Cell
Since Thiourea showed poor selectivity against quartz and glass (which together made up more than half of the MSWI-BA) and Danafloat 245 did not show good results at high pH values, the following tests were carried out with the two Cytec collectors AERO 3473 and AERO MX-5160. The compositions of the matrices used and the pH levels of their suspensions are given in Table 9. The percentages of the components determined with PXRD were calculated from the Si/Ca ratios of various MSWI-BA samples and checked for plausibility using the results of the characterization previously performed by Keber et. al. For the experiments, 24 g of the respective matrix was mixed with 1 g of copper oxide. The collector dosage was kept the same as in the Hallimond tube tests in relation to the mass of copper. Thus, 6 kg/tCu resulted in a dosage of 240 g/t. The high dosage of frother and the combination of two different frothers were employed due to the observation of bad froth formation for the calcium-bearing matrix components cement and gypsum. Unless otherwise specified, the following floatation parameters were used: collector dosage—240 g/t, frother dosage—125 g/t Flotanol C7 and 125 g/t MIBC, solid concentration—200 g/t, no pH adjustment.

Table 9.
Matrices used for the experiments with the mechanical flotation cell.
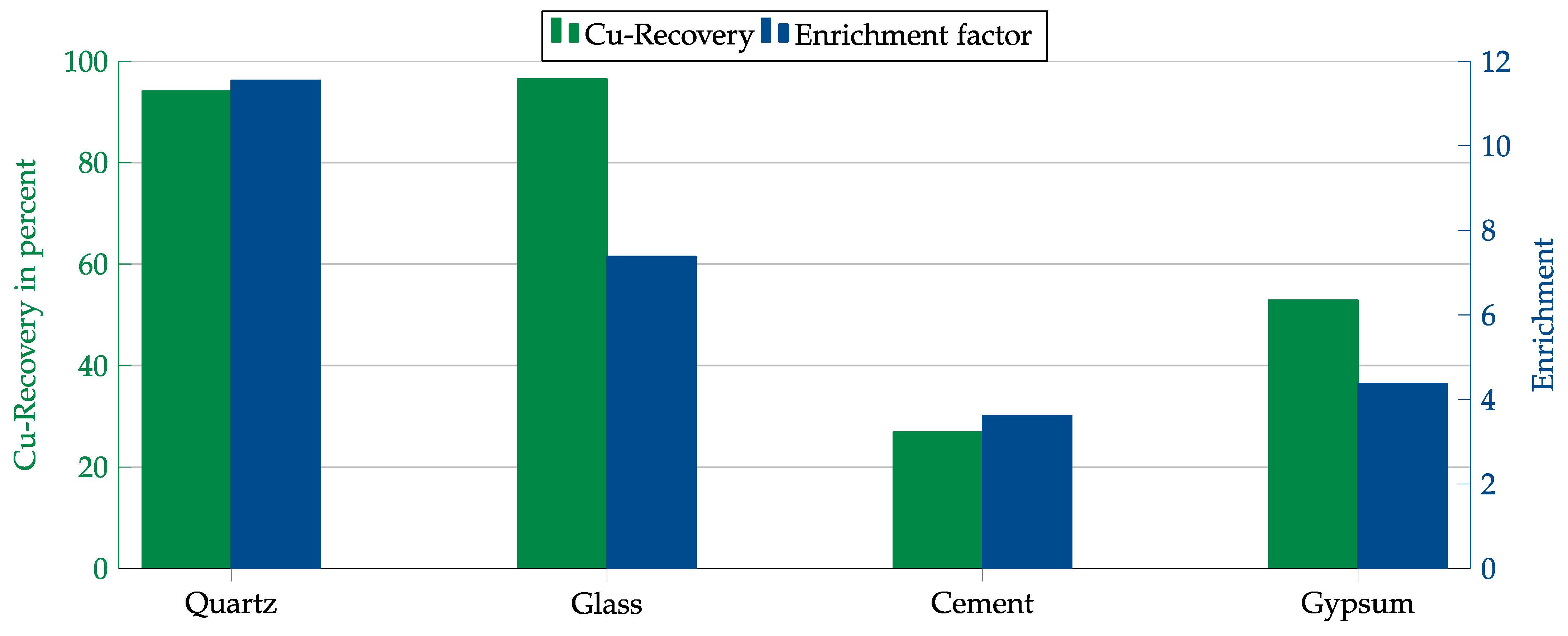
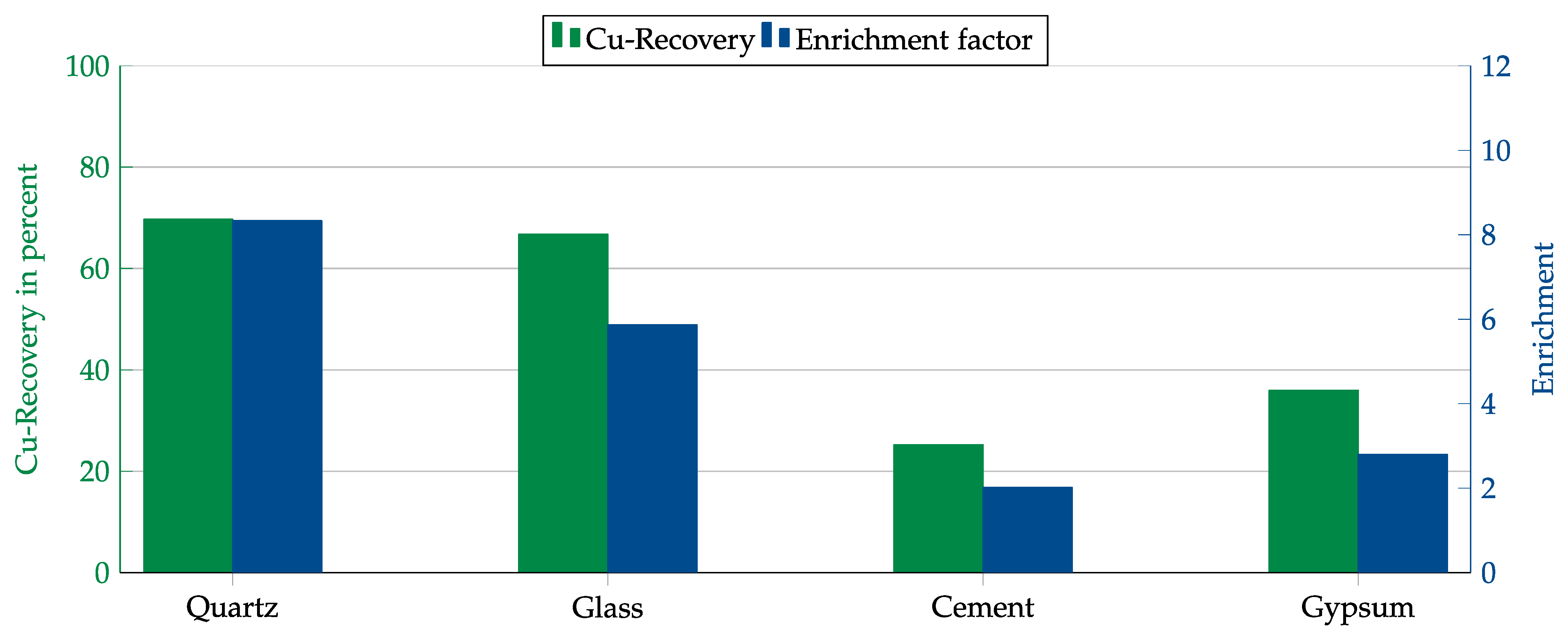
First, both collectors were tested with each matrix component. The results are shown in Figure 14 and Figure 15. In contrast to the Hallimond tube tests, AERO 3473 showed better results than AERO MX-5160 this time. Both collectors achieved the best results with quartz followed by glass. While, in both cases, the copper recovery rates for quartz and glass were comparable, the copper enrichment in glass was lower. This might have been due to impurities in the glass that were floated together with the copper or which allowed collector adsorption on the glass surface.
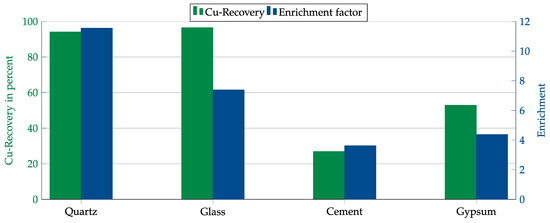
Figure 14.
Results for AERO 3473 in combination with different matrices. Flotation parameters: collector dosage—240 g/t, frother dosage—125 g/t Flotanol C7 and 125 g/t MIBC, solid concentration—200 g/t, natural pH.
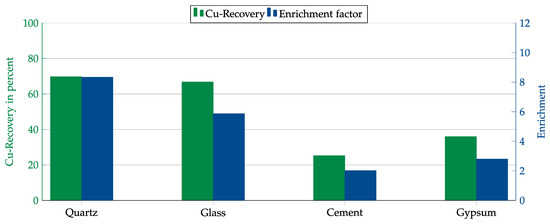
Figure 15.
Results for AERO MX-5160 in combination with different matrices. Flotation parameters: collector dosage—240 g/t, frother dosage—125 g/t Flotanol C7 and 125 g/t MIBC, solid concentration—200 g/t, natural pH.
For both collectors, the copper recovery and enrichment were much lower for the cement and gypsum. Cement showed the lowest results among all the tests. It could also be observed that the flotation speed was much lower in the case of cement. While, for the other matrices, the highest copper recovery was observed right after the beginning of the flotation, floating copper oxide particles could be observed over the whole 5 min flotation time for cement. Furthermore, only a small amount of froth formed for cement. This effect was even stronger in the preliminary tests, where only Flotanol C7 or MIBC were used as frothers. Therefore, a mixture of both frothers was used in the actual experiments.
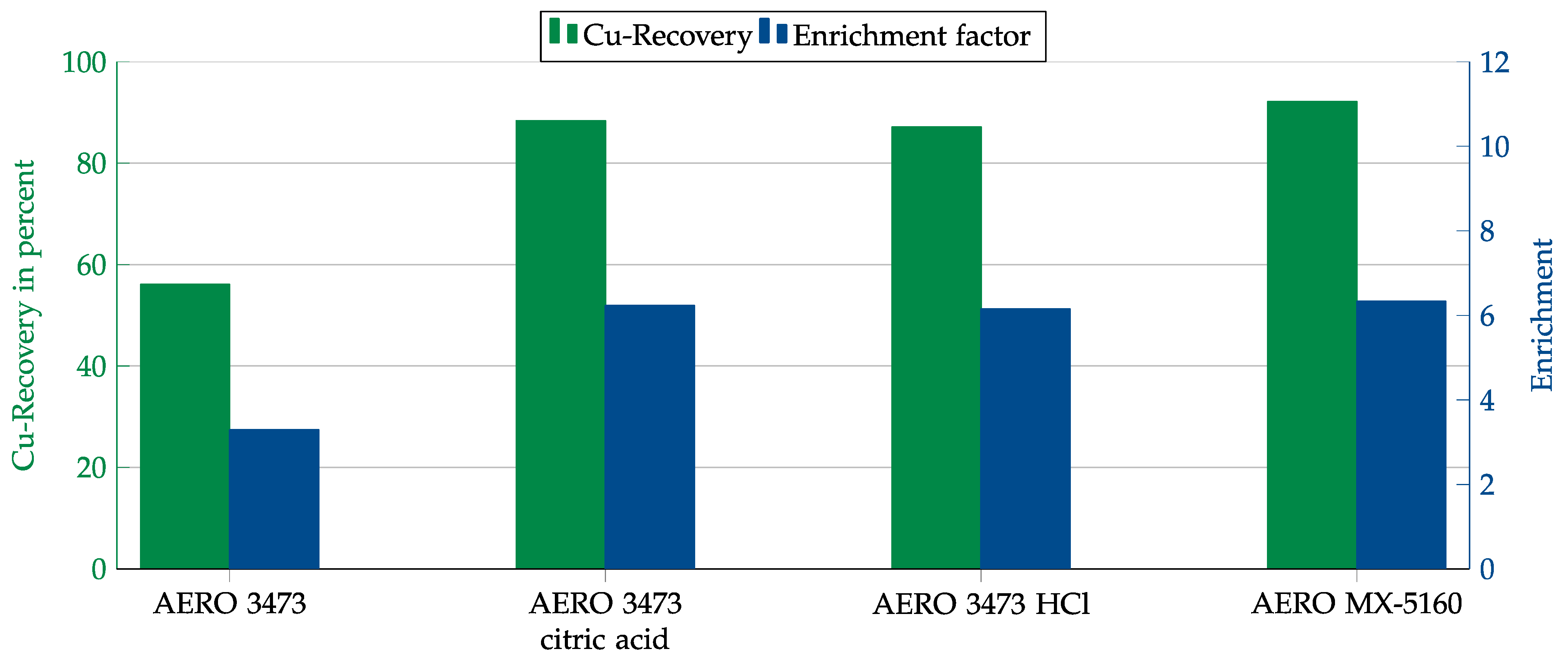
Due to the high pH value of the cement matrix of 12.4, we initially attempted to improve the results by adjusting the pH value. Since an exact adjustment was not possible, acid was added over the entire testing period so that the pH value ranged between 9.5 and 10.5. Hydrochloric acid and citric acid were used to adjust the pH. Citric acid was expected to have an additional depressant effect.
As seen from the results in Figure 16, adjusting the pH did not improve the results but worsened them.
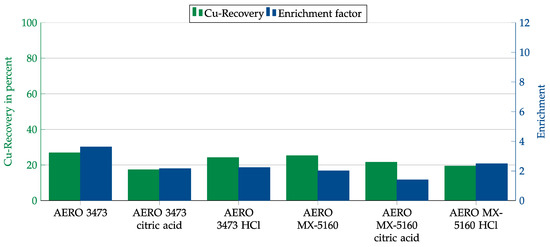
Figure 16.
Results for AERO 3473 and AERO MX-5160 without pH adjustment and with adjustment to 9.5 to 10.5 in combination with the cement matrix. Flotation parameters: collector dosage—240 g/t, frother dosage—125 g/t Flotanol C7 and 125 g/t MIBC, solid concentration—200 g/t.
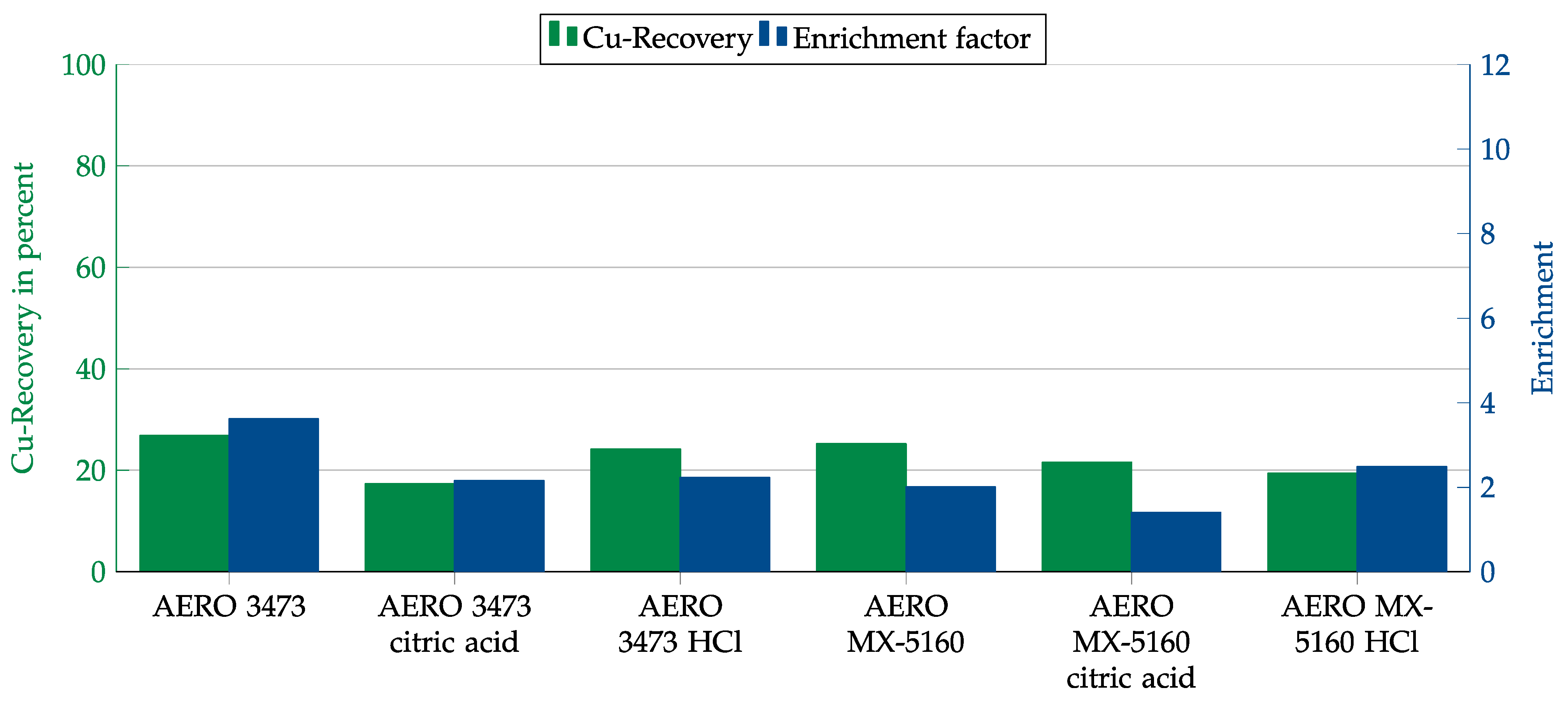
In another attempt to increase copper recovery and enrichment, the collector dosage was increased by a factor of five. In the case of AERO 3473, pH adjustment was again performed using hydrochloric and citric acid. The results are shown in Figure 17. With both collectors, recovery rates of about 90% could be achieved with an enrichment of just above 6. In the case of AERO MX-5160, this was achieved without adjusting the pH. For AERO 3473, recovery and enrichment were increased by adding acid, but a depressant effect from the citric acid could not be observed.
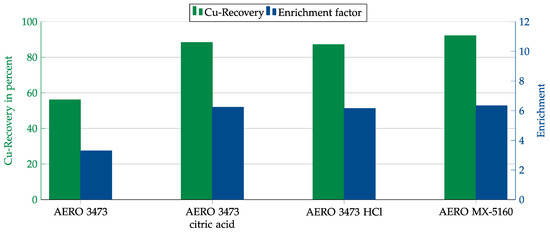
Figure 17.
Results for AERO 3473 at natural pH and with adjustment to 9.5 to 10.5 and AERO MX-5160 at natural pH in combination with the cement matrix. Flotation parameters: collector dosage—1200 g/t, frother dosage—125 g/t Flotanol C7 and 125 g/t MIBC, solid concentration—200 g/t.
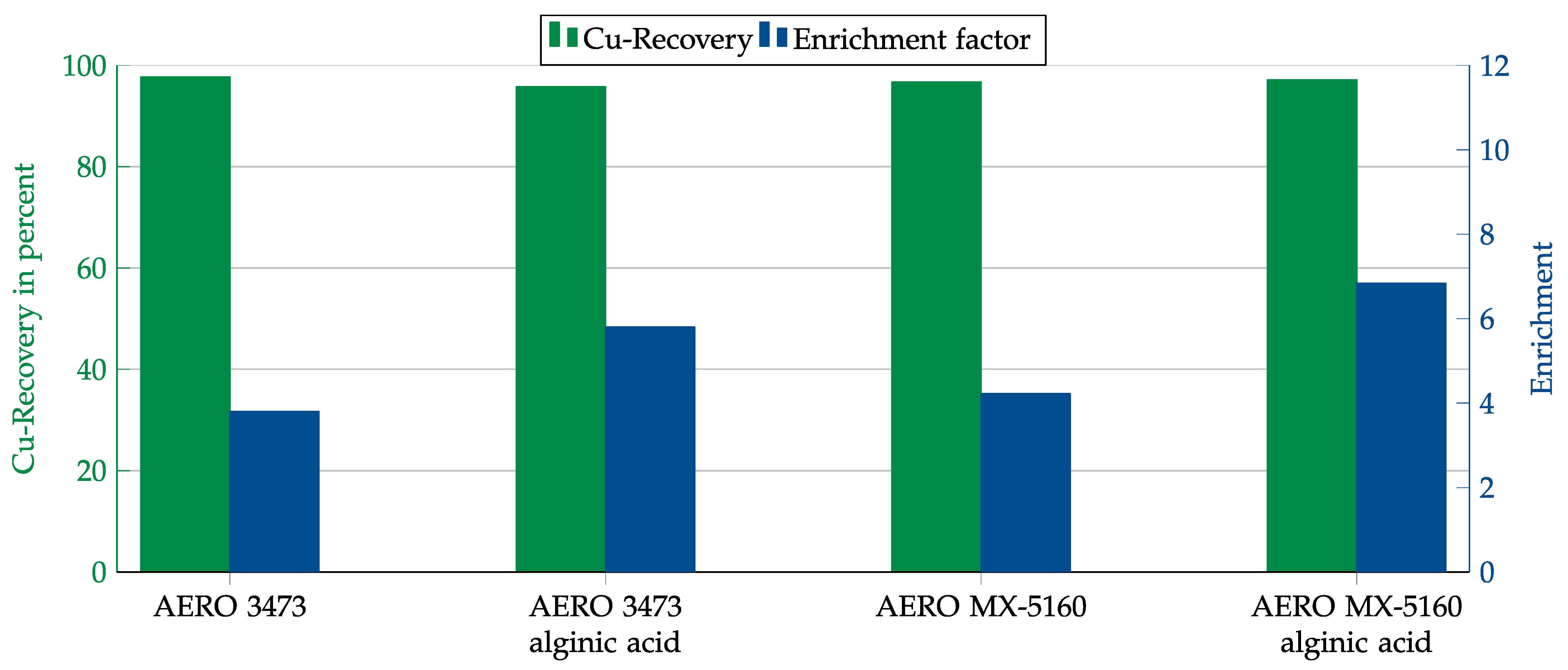
In order to improve the results in the presence of gypsum, the collector dosage was also increased. In addition, alginic acid was tested as a depressant. Alginic acid is known to have a depressant effect on calcium-bearing minerals such as gypsum [11]. As the test results in Figure 18 show, the copper recovery could be increased to over 95% by increasing the collector dosage. However, as with the previous dosage, the enrichment was only about 4. Using alginic acid could increase the enrichment to just under 6 for AERO 3473 and even over 6 for AERO MX-5160. It could also be observed that the copper oxide floated primarily at the beginning of the process, while, at the end of the experiment, only gypsum floated. It can therefore be assumed that the enrichment could be further increased by shortening the flotation time.
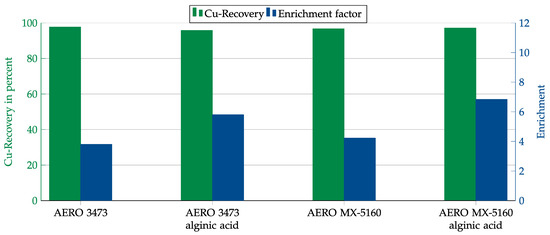
Figure 18.
Results for AERO 3473 and AERO MX-5160 with and without alginic acid as depressant in combination with the gypsum matrix. Flotation parameters: collector dosage—1200 g/t, depressant dosage—1000 g/t, frother dosage—125 g/t Flotanol C7 and 125 g/t MIBC, solid concentration—200 g/t, natural pH.
4. Discussion
The greatest challenges in recovering copper from MSWI-BA using flotation are the high complexity of the ashes, the large number of elements involved, and the high pH of the pulps. Adjusting the pH value is impractical, leading to high acid consumption and undesirable reactions. For this reason, the investigations presented in this paper aimed to identify collectors that, under the given circumstances, would have a good collection capacity for the contained copper phases, especially copper oxide. For this purpose, investigations were carried out with individual minerals and matrix components.
The main foci of the collector screening were thiourea-, thiophosphate-, and thiocarbamate-based collectors, as these were expected to show higher selectivity and higher tolerance to strongly alkaline conditions. The quite common method of sulfidation followed by flotation using xanthates was excluded due to the low prospects of success. The screening showed that the commercial collector mixtures, especially the carbamate-based ones, produced better results than the tested thioureas. The exception was S-n-dodecyle-iso-thiourea hydrochloride, which showed the highest recovery of all reagents tested in the screening. The reason for this could have been its long carbon chain in combination with its cationic nature, which facilitates adsorption of particles with a negative zeta potential.
At least one representative from each collector group was selected for the more in-depth studies: the thiourea S-n-dodecyle-iso-thiourea hydrochloride, the thiophosphate Danaflot 245, the thiocarbamate AERO 3473, and AERO MX-5160 as a mixture of a thiocarbamate and thiophosphates.
All collectors except S-n-dodecyle-iso-thiourea hydrochloride showed an optimum dosage of about 6 kg/t. S-n-dodecyle-iso-thiourea hydrochloride showed no dependence on dosage for copper oxide; for copper sulfide, the optimum was also 6 kg/ton. A copper content of up to 0.5% results in a theoretical dosage of 30 g/t for ashes. In reality, the dosage can be expected to be much higher
All collectors examined exhibited good collection capacities in the alkaline range. However, Danaflot 245 showed its optimum collection in the neutral pH range, while the effectiveness of AERO 3473 and MX-5160 decreased at pH levels over 10. For this reason, Danafloat 245 seemed less suitable for the ashes than the other collectors studied.
In experiments with different dissolved ions, it was found that calcium ions negatively affected the collectors’ effectiveness. This effect was observed with all collectors tested but was particularly noticeable with AERO 3473. An apparent effect was also observed with AERO MX-5160. Based on the observation from the studies with the individual matrix components that the flotation proceeded significantly slower with the presence of cement in the matrix, it can be assumed that the reason for this effect lay in slowed-down kinetics.
In the contact angle measurements, the commercial collectors showed good selectivity towards other metal oxides and over the matrix phases, except for gypsum. All collectors hydrophobized gypsum, so the use of depressants seemed unavoidable. S-n-dodecyle-iso-thiourea hydrochloride exhibited lower selectivity than the other collectors tested. In addition to the copper phases, quartz, glass, nickel, and zinc oxide were hydrophobized. Due to the lack of selectivity towards quartz and glass, S-n-dodecyle-iso-thiourea hydrochloride was excluded as a possible collector for the time being. However, the hydrophobization of nickel and zinc oxide showed some potential. If these could be concentrated together with the copper, they could represent additional value carriers. Silver oxide was hydrophobized by all collectors, so it is very likely that silver and possibly other precious metals can be recovered. In addition, high metal contents prevent the utilization of the ashes as, for example, mineral building materials. Hence, there would be a greater possibility of utilization after removing as many metals as possible. S-n-dodecyle-iso-thiourea hydrochloride showed high potential for the development of special collectors for use with MSWI-BA instead of already available collectors optimized for natural ores.
As final tests, experiments were conducted in a mechanical flotation machine with the individual matrix components admixed with copper oxide. These tests were only carried out with the two most promising collectors: AERO 3473 and AERO MX-5160. The best results were obtained with quartz as the dominant matrix material. With glass, a similar high recovery of over 90% was achieved, but the enrichment was lower, presumably due to mishydrophobization caused by impurities. The results obtained for MX-5160 were lower than those for AERO 3473. Therefore, a higher dosage would probably be required for MX-5160.
As the contact angle measurements suggested, for both collectors, copper recovery and enrichment were significantly lower when gypsum instead of quartz was the main matrix material. The recovery could be significantly increased by increasing the collector dosage while adding a depressant to increase the enrichment. In these investigations, alginic acid was used as a depressant, which is currently rarely used in the field of flotation, but, in this case, it provided good results.
Cement as a matrix phase turned out to be particularly problematic. In the experiments with cement, the flotation speed was significantly slower than in the other experiments. This led to meager copper recovery and copper enrichment due to the higher yield of hydrophilic particles. A significant increase in the collector dosage partially mitigated the effect. In the case of AERO 3473, a pH adjustment was necessary, while MX-5160 gave acceptable results even at natural pH. Citric acid was also tested as a depressant, but no effect was observed.
5. Conclusions
The flotation studies presented in this paper showed that some of the thiourea-, thiophosphate-, and thiocarbamate-based collectors could be suitable for the flotation of MSWI-BA. Due to the effects caused by the constituents of the matrix, especially gypsum and cement, the use of depressants seems to be indispensable. Various organic compounds, such as alginic acid, seem to be suitable as depressants.
Four collectors were investigated in more detail. These collectors were S-n-dodecyl-iso-thiourea hydrochloride, Danafloat 245, Cytec AERO 3473, and AERO MX-516. The investigations showed that calcium ions and the cement phases negatively influenced the flotation results. This could be partially compensated for by increasing the collector dosage. All collectors investigated showed poor selectivity towards gypsum. This effect could be compensated for by adding alginic acid.
Based on the investigations, AERO MX-5160—combined with alginic acid as a depressant for gypsum—appeared to be the most suitable collector for copper recovery from MSWI-BA. Therefore, our future investigations will focus on this collector. The interactions between the individual matrix components are still unknown and will be examined in more detail. After these have been investigated and further optimizations can be made, a transfer to real ash will be undertaken.
Author Contributions
Conceptualization, S.K. and T.E.; methodology, S.K.; formal analysis, S.K.; investigation, S.K. and M.M.; resources, S.K.; data curation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, T.E., M.M. and D.G.; visualization, S.K.; supervision, D.G. and T.E.; project administration, D.G.; funding acquisition, T.E. and D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft (DFG), grant number 403179546. The APC was funded by the Open Access Publishing Fund of Clausthal University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to the DFG for the financial support. We would also like to thank the analytical laboratory of the institute for the great teamwork. We acknowledge support from the Open Access Publishing Fund of Clausthal University of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Northey, S.; Mohr, S.; Mudd, G.M.; Weng, Z.; Giurco, D. Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resour. Conserv. Recycl. 2014, 83, 190–201. [Google Scholar] [CrossRef]
- Neuwahl, F.; Cusano, G.; Benavides Holbrook, S.; Roudler, S. Best Available Techniques (BAT) Reference Document for Waste Incineration. Available online: http://eippcb.jrc.ec.europa.eu/sites/default/files/2020-01/JRC118637_WI_Bref_2019_published_0.pdf (accessed on 13 December 2022).
- Breitenstein, B. Verfahren zur Rückgewinnung von NE-Metallen aus Feinkörnigen Rostaschen der Thermischen Abfallbehandlung. Ph.D. Thesis, Technische Unversität Clausthal, Clausthal, Germany, 2017. [Google Scholar]
- Breitenstein, B.; Elwert, T.; Goldmann, D.; Haas, A.; Schirmer, T.; Vogt, V. Froth Flotation of Copper and Copper Compounds from Fine Fractions of Waste Incineration Bottom Ashes. Chem. Ing. Tech. 2017, 89, 97–107. [Google Scholar] [CrossRef]
- Stockinger, G. Direct Wet Treatment of Fresh, Wet-Discharged Grate Ash from a Waste Incineration Plant. In Removal, Treatment and Utilisation of Waste Incineration Bottom Ash; Holm, O., Ed.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 47–52. ISBN 978-3-944310-44-2. [Google Scholar]
- Böni, D.; Morf, L. Thermo-Recycling: Efficient Recovery of Valuable Materials from Dry Bottom Ash. In Removal, Treatment and Utilisation of Waste Incineration Bottom Ash; Holm, O., Ed.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 25–37. ISBN 978-3-944310-44-2. [Google Scholar]
- Syc, M.; Simon, F.-G.; Biganzolli, L.; Grosso, M.; Hyks, J. Recource Recorvery from Incineration Bottom Ash: Basics, Concepts, Principles. In Removal, Treatment and Utilisation of Waste Incineration Bottom Ash; Holm, O., Ed.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 1–9. ISBN 978-3-944310-44-2. [Google Scholar]
- Keber, S.; Schirmer, T.; Elwert, T.; Goldmann, D. Characterization of Fine Fractions from the Processing of Municipal Solid Waste Incinerator Bottom Ashes for the Potential Recovery of Valuable Metals. Minerals 2020, 10, 838. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780080970530. [Google Scholar]
- Lautenschläger, K.-H.; Schröter, W.; Wanninger, A. Taschenbuch der Chemie, 20th ed.; Deutsch: Frankfurt am Main, Germany, 2005; ISBN 3-8171-1760-4. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-444-53082-0. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).