Improvement of Carbon Dioxide Sequestration of Anorthite through Bacterial: Release of Calcium and Destruction of Crystal Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sample and Gas
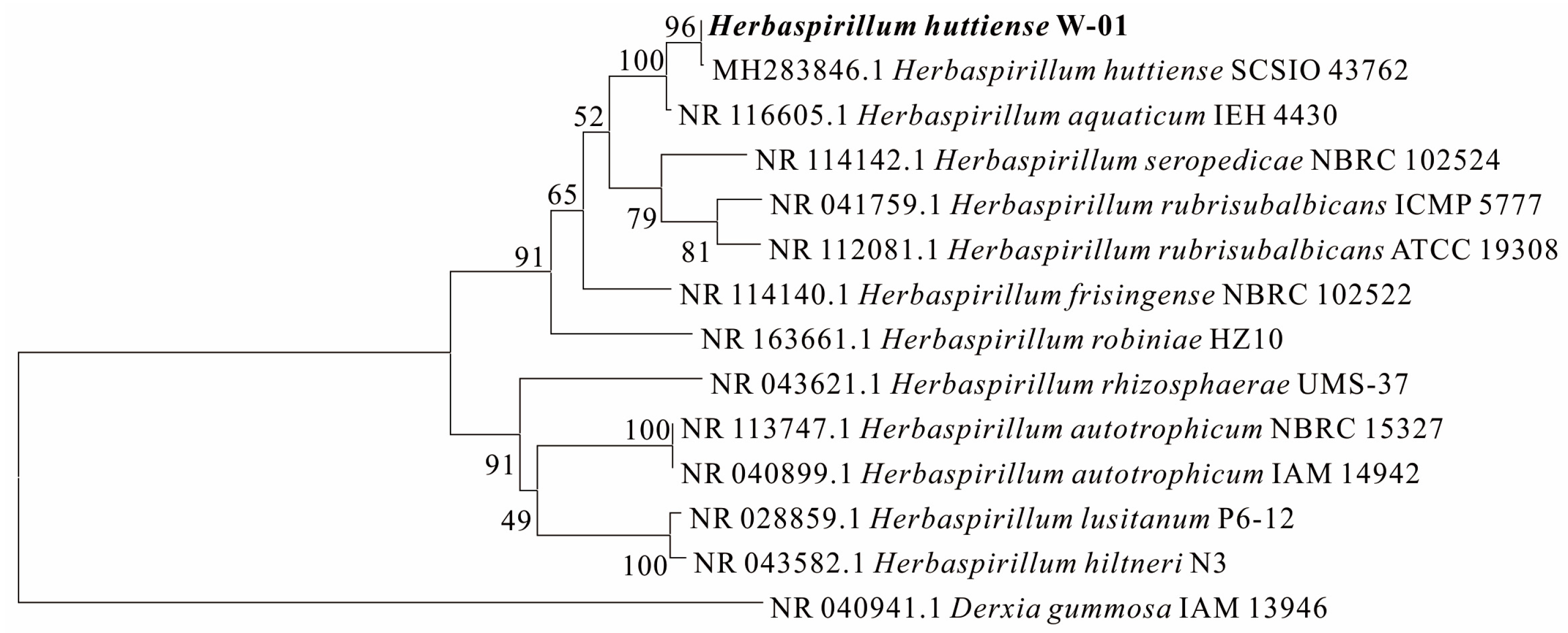
2.1.2. Strains
2.2. Methods
2.2.1. Culture and Preparation of Strains
2.2.2. Microbial Leaching Test
2.2.3. Determination of pH Value
2.2.4. Determination of Calcium Ion Concentration
2.2.5. Carbonation Test
2.2.6. Characterization Methods
2.2.7. Calculation of Carbonation Conversion Rate
3. Results and Discussion
3.1. Results of Microbial Leaching
3.1.1. Changes of pH Value
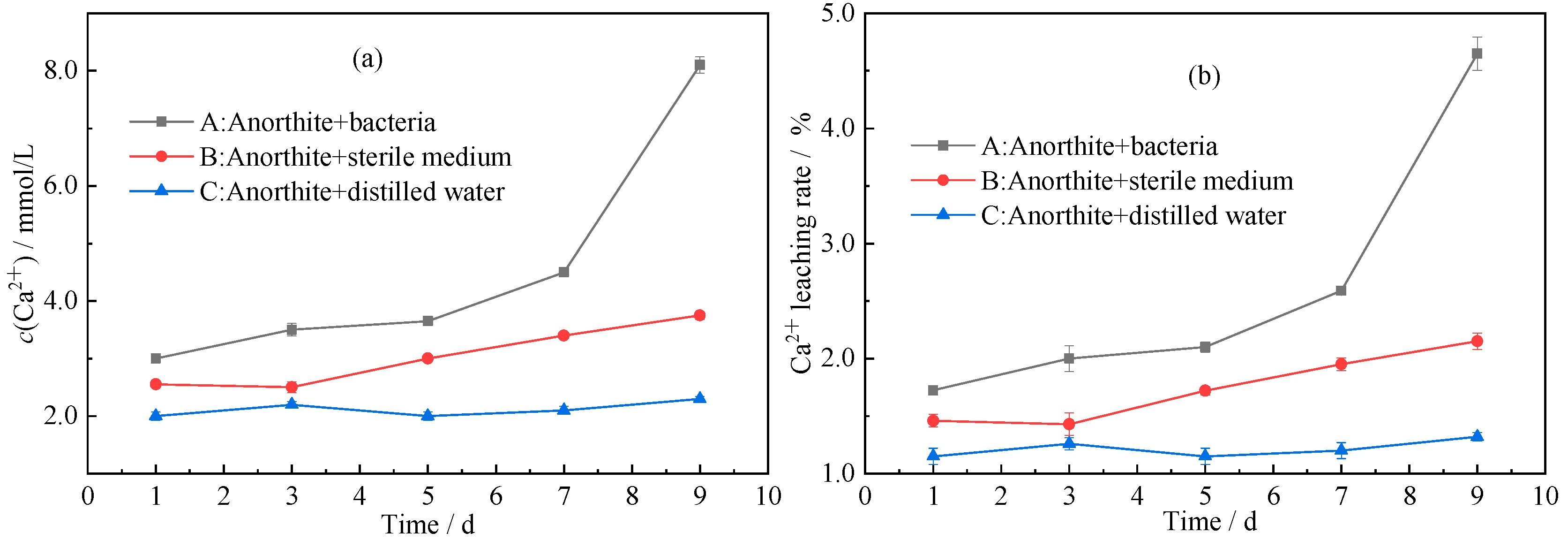
3.1.2. Ca2+ Leaching Rate
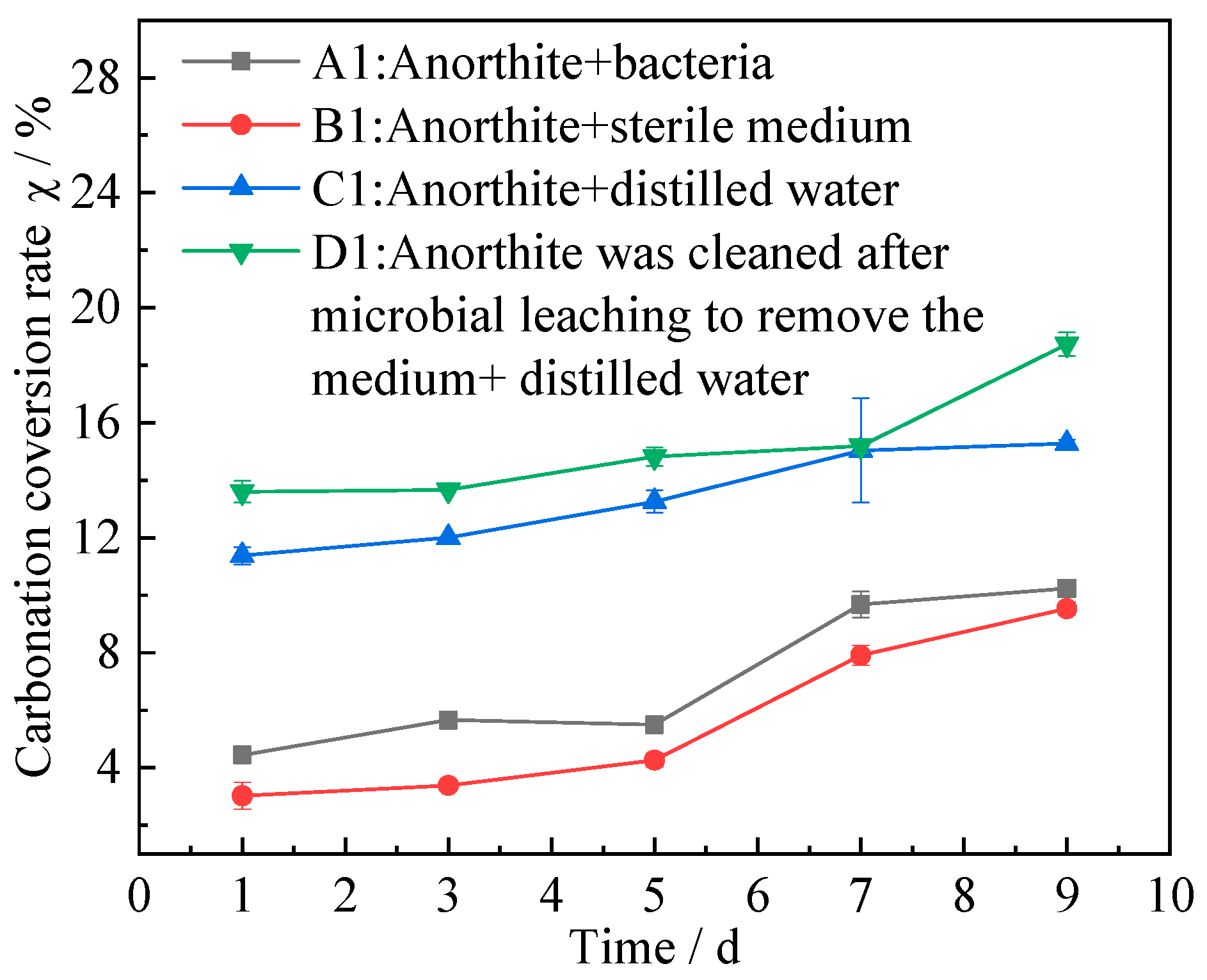
3.1.3. Carbonation Conversion of Anorthite
3.2. Analysis of Leaching Residue and Carbonation Products
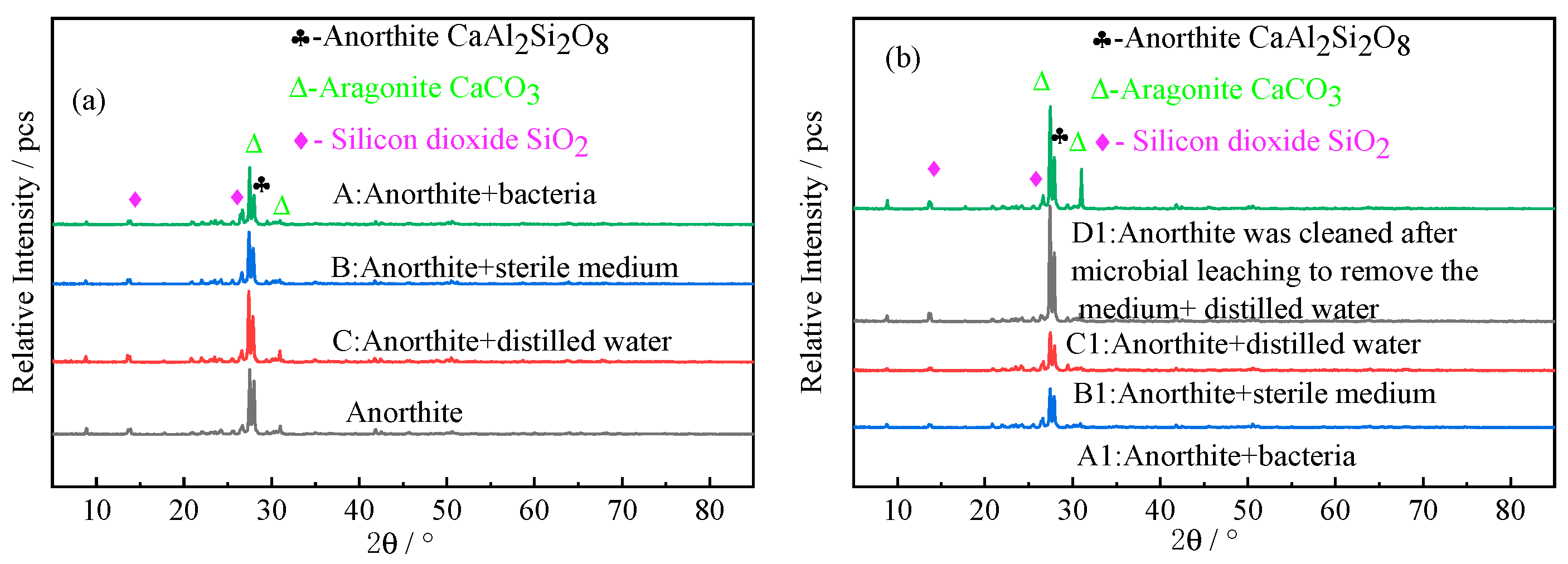
3.2.1. XRD Analysis
3.2.2. FT-IR Analysis
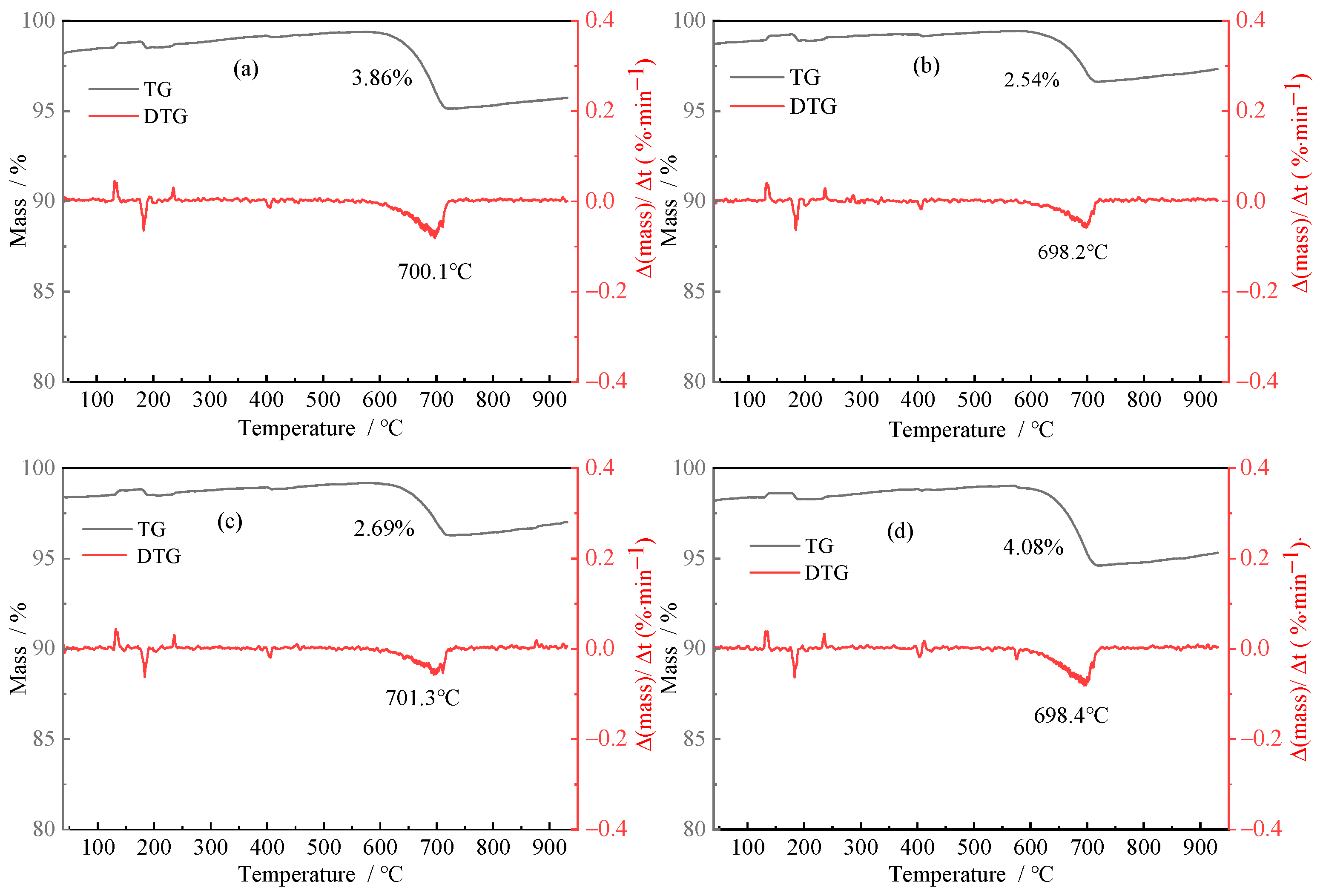
3.2.3. TG-DTG Analysis
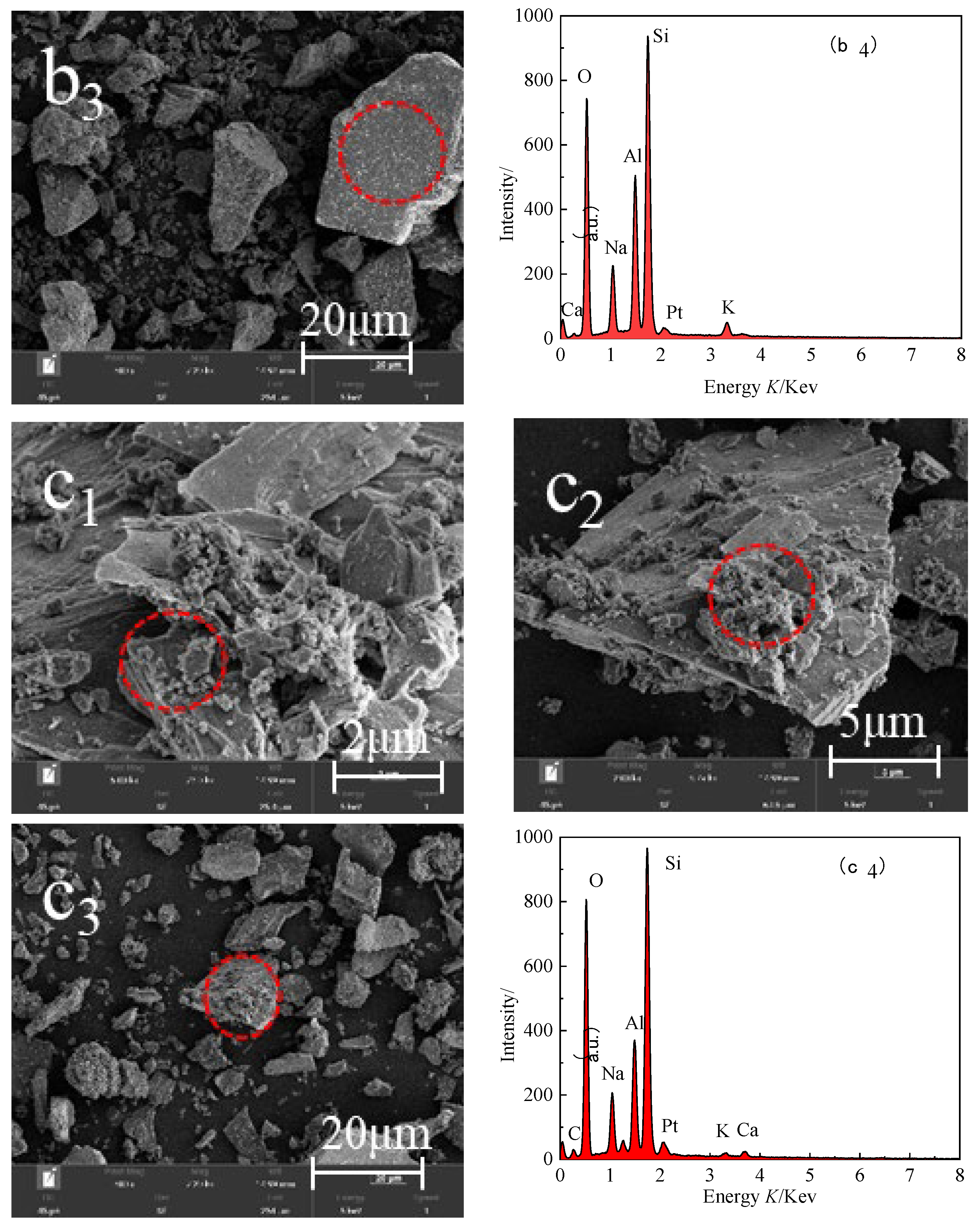
3.2.4. SEM-EDS Images
4. Conclusions
- Herbaspirillum huttiense W-01 was first found to accelerate the release of Ca2+ from anorthite. The concentration of Ca2+ in the fermentation solution reached 8.1 mmol/L after leaching 9 days with the corresponding leaching rate of 4.65%, which was about twice that of sterile medium.
- Herbaspirillum huttiense W-01 was adsorbed on the surface of anorthite and produced acidic metabolites to accelerate the weathering of anorthite, resulting in the roughness of the surface area, and then the breakage and decomposition of edges and corners and obvious corrosion phenomena.
- A carbonized product with higher crystallinity and greater thermal stability was obtained after the carbonation reaction. After 9 days of microbial pretreatment, the carbonation conversion rate of anorthite after bioleaching was increased to 18.74% with a 3.46% higher rate than that of distilled water pretreatment, which was mainly due to the destruction of the crystal structure of anorthite by microorganisms.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinehbaghizadeh, S.; Saptoro, A.; Mohammadi, A.H. CO2 hydrate properties and applications: A state of the art. Prog. Energy Combust. Sci. 2022, 93, 101026. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Jones, J.; Sun, G.; Wei, X.; Ellison, D.; Archer, E.; McNulty, S.; Asbjornsen, H.; Zhang, Z.; et al. Managing the forest-water nexus for climate change adaptation. For. Ecol. Manag. 2022, 525, 120545. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. IPCC, 2021: Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA. [CrossRef]
- Yuen, Y.T.; Sharratt, P.N.; Jie, B. Carbon dioxide mineralization process design and evaluation: Concepts, case studies, and considerations. Environ. Sci. Pollut. Res. 2016, 23, 22309–22330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jacobs, A.D.; Hitch, M. Direct aqueous carbonation on olivine at a CO2 partial pressure of 6.5 MPa. Energy 2019, 173, 902–910. [Google Scholar] [CrossRef]
- Park, A.H.A.; Fan, L.S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Gamba, N.; Farina, V.; Garroni, S.; Mulas, G.; Gennari, F. CO2 storage and conversion to CH4 by wet mechanochemical activation of olivine at room temperature. Powder Technol. 2021, 377, 857–867. [Google Scholar] [CrossRef]
- Julcour, C.; Bourgeois, F.; Bonfils, B.; Benhamed, I.; Guyot, F.; Bodenan, F.; Petiot, C.; Gaucher, E.C. Development of an attrition-leaching hybrid process for direct aqueous mineral carbonation. Chem. Eng. J. 2015, 262, 716–726. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Park, A.-H.A. Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3. Phys. Chem. Chem. Phys. 2014, 16, 4679–4693. [Google Scholar] [CrossRef]
- Eikeland, E.; Blichfeld, A.B.; Tyrsted, C.; Jensen, A.; Iversen, B.B. Optimized Carbonation of Magnesium Silicate Mineral for CO2 Storage. ACS Appl. Mater. Interfaces 2015, 7, 5258–5264. [Google Scholar] [CrossRef]
- Imani, M.; Tahmasebpoor, M.; Sánchez-Jiménez, P.E.; Valverde, J.M.; Moreno, V. Improvement in cyclic CO2 capture performance and fluidization behavior of eggshell-derived CaCO3 particles modified with acetic acid used in calcium looping process. J. CO2 Util. 2022, 65, 102207. [Google Scholar] [CrossRef]
- Lan, J.; Sun, Y.; Chen, X.; Zhan, W.; Du, Y.; Zhang, T.C.; Ye, H.; Du, D.; Hou, H. Bio-leaching of manganese from electrolytic manganese slag by Microbacterium trichothecenolyticum Y1: Mechanism and characteristics of microbial metabolites. Bioresour. Technol. 2021, 319, 124056. [Google Scholar] [CrossRef]
- Yao, M.; Lian, B.; Teng, H.H.; Tian, Y.; Yang, X. Serpentine dissolution in the presence of bacteria Bacillus mucilaginosus. Geomicrobiol. J. 2013, 30, 72–80. [Google Scholar] [CrossRef]
- Sun, J.-Z.; Wen, J.-K.; Chen, B.-W.; Wu, B. Mechanism of Mg2+ dissolution from olivine and serpentine: Implication for bioleaching of high-magnesium nickel sulfide ore at elevated pH. Int. J. Miner. Met. Mater. 2019, 26, 1069–1079. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Santos, R.M.; Monballiu, A.; Ghyselbrecht, K.; Martens, J.A.; Mattos, M.L.T.; Van Gerven, T.; Meesschaert, B. Effects of bioleaching on the chemical, mineralogical and morphological properties of natural and waste-derived alkaline materials. Miner. Eng. 2013, 48, 116–125. [Google Scholar] [CrossRef]
- Štyriaková, I.; Štyriak, I.; Oberhänsli, H. Rock weathering by indigenous heterotrophic bacteria of Bacillus spp. at different temperature: A laboratory experiment. Mineral. Petrol. 2012, 105, 135–144. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Chen, Z.; Liu, X.; Chen, B.; Zhang, M.; Ke, X.; Zhang, T.C. Effects of different silicate minerals on silicon activation by Ochrobactium sp. T-07-B. Environ. Sci. Pollut. Res. 2022, 29, 87393–87401. [Google Scholar] [CrossRef]
- Ogbughalu, O.T.; Vasileiadis, S.; Schumann, R.C.; Gerson, A.R.; Li, J.; Smart, R.S.C.; Short, M.D. Role of microbial diversity for sustainable pyrite oxidation control in acid and metalliferous drainage prevention. J. Hazard. Mater. 2020, 393, 122338. [Google Scholar] [CrossRef]
- Henne, A.; Craw, D.; Vasconcelos, P.; Southam, G. Bioleaching of waste material from the Salobo mine, Brazil: Recovery of refractory copper from Cu hosted in silicate minerals. Chem. Geol. 2018, 498, 72–82. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D. Advanced review on extraction of nickel from primary and secondary sources. Miner. Process. Extr. Metall. Rev. 2018, 40, 157–193. [Google Scholar] [CrossRef]
- Tucker, B.B.; Kurtz, L.T. Calcium and magnesium determinations by EDTA titrations. Soil Sci. Soc. Am. J. 1961, 25, 27–29. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhao, Y.; Liu, R.; Zheng, C. CO2 Sequestration by Direct Aqueous Mineral Carbonation under Low-Medium Pressure Conditions. J. Chem. Eng. Jpn. 2015, 48, 937–946. [Google Scholar] [CrossRef]
- Miura, A.; Nakazawa, K.; Takei, T.; Kumada, N.; Kinomura, N.; Ohki, R.; Koshiyama, H. Acid-, base-, and heat-induced degradation behavior of Chinese sepiolite. Ceram. Int. 2012, 38, 4677–4684. [Google Scholar] [CrossRef]
- Nirmala, G.; Viruthagiri, G. FT-IR characterization of articulated ceramic bricks with wastes from ceramic industries. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 126, 129–134. [Google Scholar] [CrossRef] [PubMed]
- González-García, D.M.; Téllez-Jurado, L.; Jiménez-Álvarez, F.J.; Balmori-Ramírez, H. Structural study of geopolymers obtained from alkali-activated natural pozzolan feldspars. Ceram. Int. 2017, 43, 2606–2613. [Google Scholar] [CrossRef]




| Composition | SiO2 | CaO | MgO | Fe2O3 | Al2O3 | SO3 | K2O | Na2O | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 30.93 | 24.41 | 19.17 | 16.50 | 4.06 | 2.83 | 1.25 | 0.21 | 0.16 | 0.15 |
| Test | A | B | C |
|---|---|---|---|
| Description | Anorthite + bacteria | Anorthite + sterile medium | Anorthite + distilled water |
| Test | A1 | B1 | C1 | D1 |
|---|---|---|---|---|
| Description | Anorthite + bacteria | Anorthite + sterile medium | Anorthite + distilled water | Anorthite was cleaned after microbial leaching to remove the medium + distilled water |
| Anorthite | BET Specific Area (m2/g) |
|---|---|
| Before microbial leaching | 0.5187 |
| After microbial leaching | 0.9883 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Zhang, L.; Guo, J.; Wen, Q.; Liu, S. Improvement of Carbon Dioxide Sequestration of Anorthite through Bacterial: Release of Calcium and Destruction of Crystal Structure. Minerals 2023, 13, 367. https://doi.org/10.3390/min13030367
Chang C, Zhang L, Guo J, Wen Q, Liu S. Improvement of Carbon Dioxide Sequestration of Anorthite through Bacterial: Release of Calcium and Destruction of Crystal Structure. Minerals. 2023; 13(3):367. https://doi.org/10.3390/min13030367
Chicago/Turabian StyleChang, Chengbing, Lei Zhang, Jianying Guo, Quanbao Wen, and Shengyu Liu. 2023. "Improvement of Carbon Dioxide Sequestration of Anorthite through Bacterial: Release of Calcium and Destruction of Crystal Structure" Minerals 13, no. 3: 367. https://doi.org/10.3390/min13030367
APA StyleChang, C., Zhang, L., Guo, J., Wen, Q., & Liu, S. (2023). Improvement of Carbon Dioxide Sequestration of Anorthite through Bacterial: Release of Calcium and Destruction of Crystal Structure. Minerals, 13(3), 367. https://doi.org/10.3390/min13030367








