Treatment Technology and Research Progress of Residual Xanthate in Mineral Processing Wastewater
Abstract
1. Introduction
2. The Characteristics and Hazards of Xanthate
3. Xanthate Wastewater Treatment Technology
3.1. Coagulation–Flocculation Method
3.2. Adsorption Methods
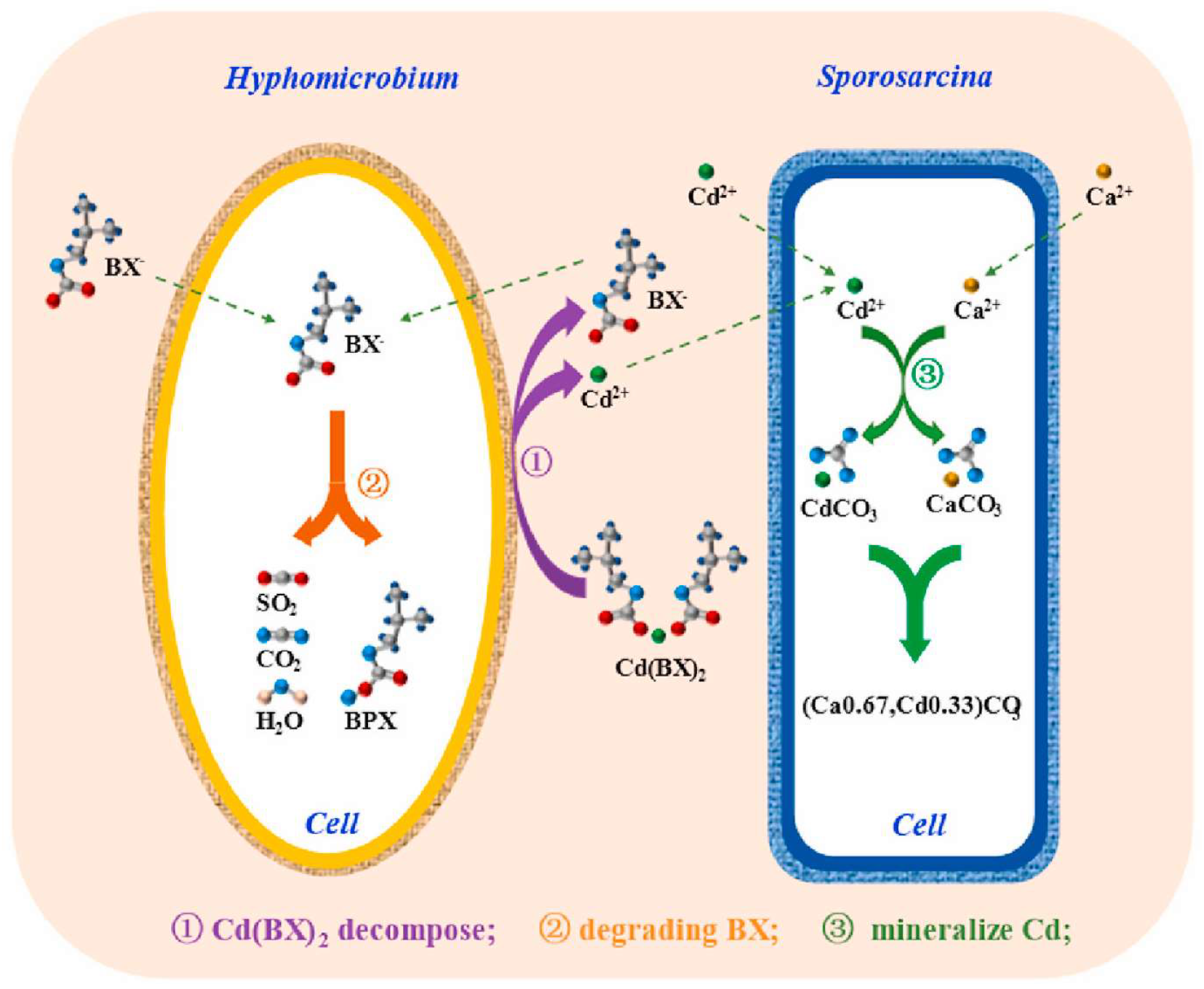
3.3. Biological Method
3.4. Oxidation Methods
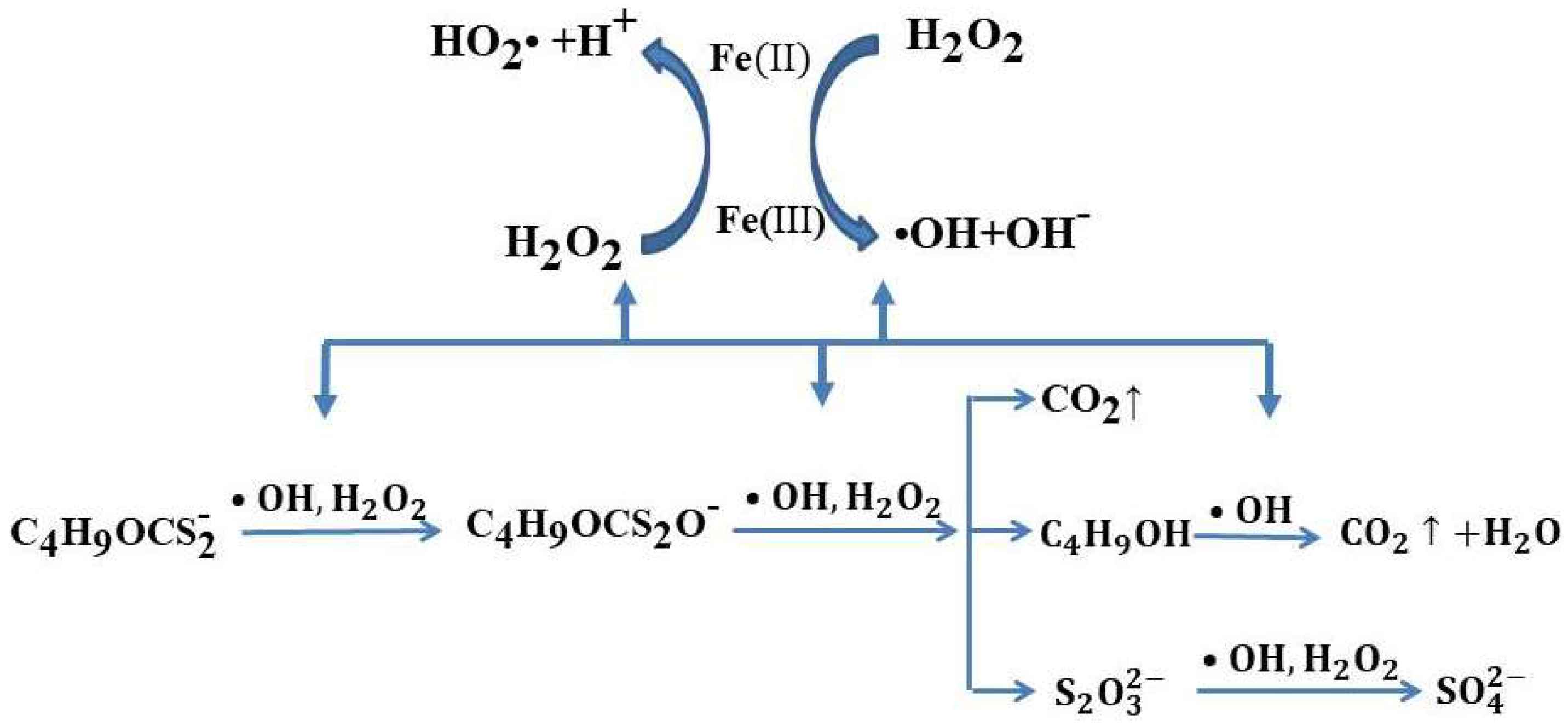
3.4.1. Fenton Methods
3.4.2. Ozone Oxidation
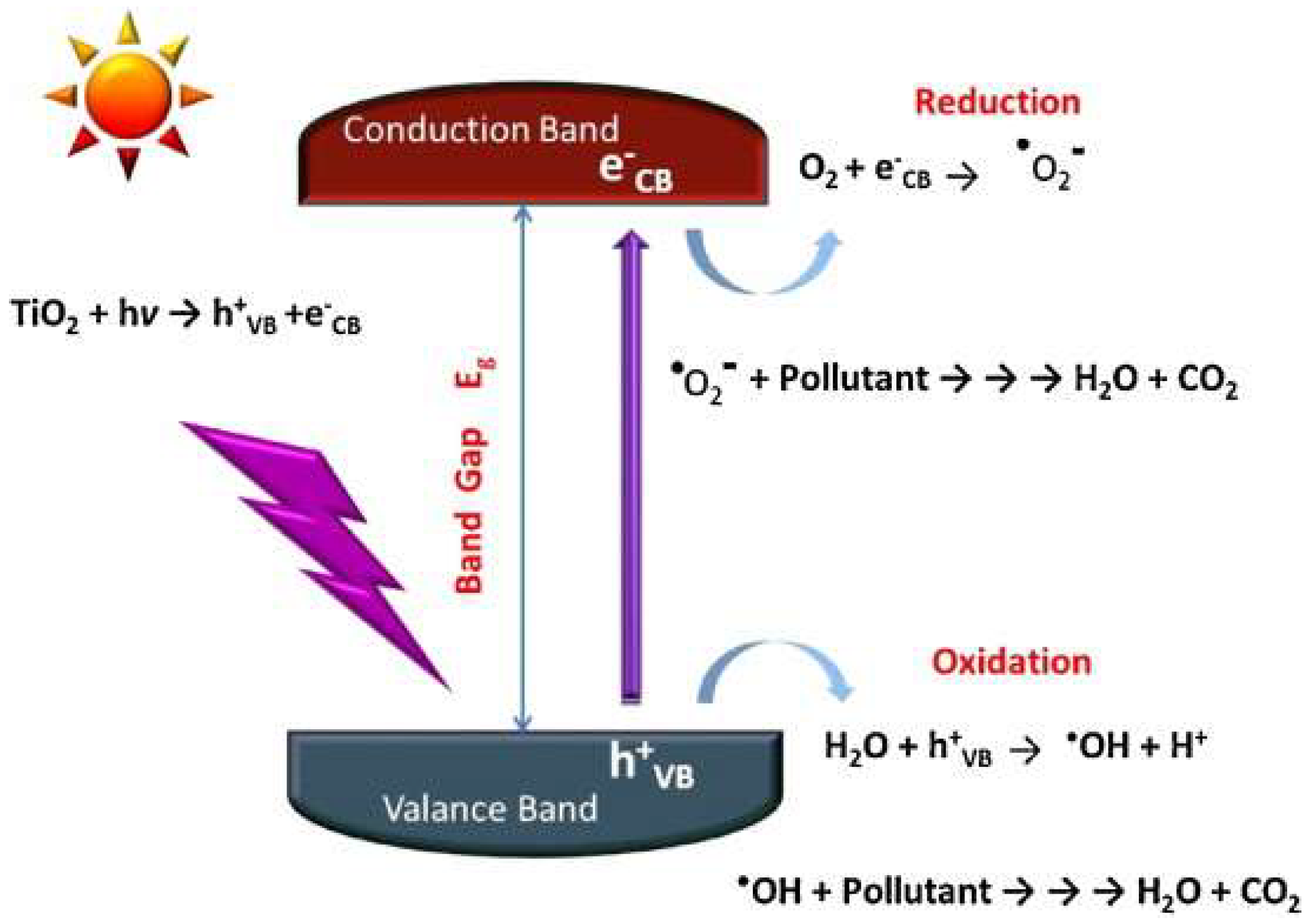
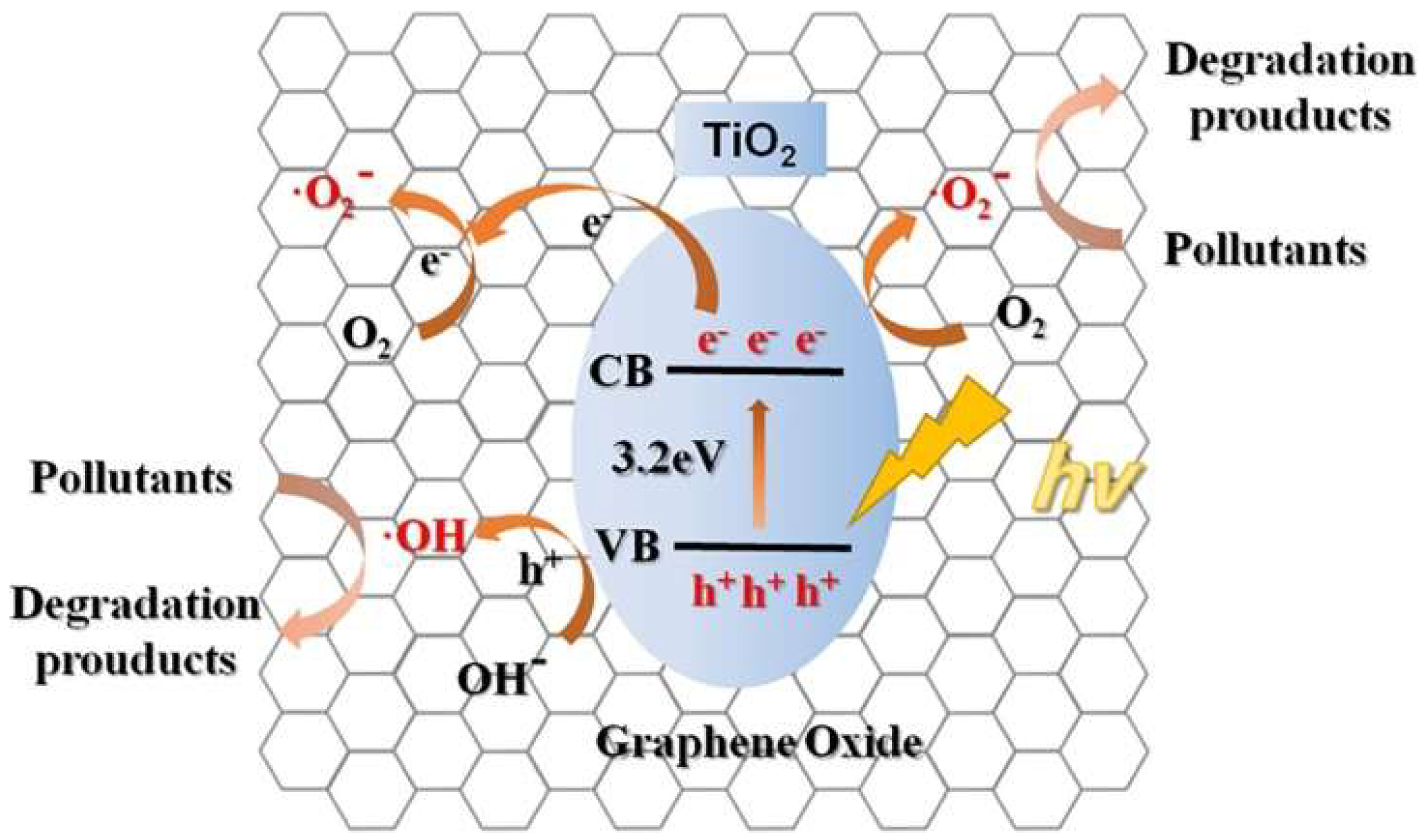
3.4.3. Photocatalytic Oxidations
TiO2 Photocatalysts
Bi-Based Oxide Photocatalysts
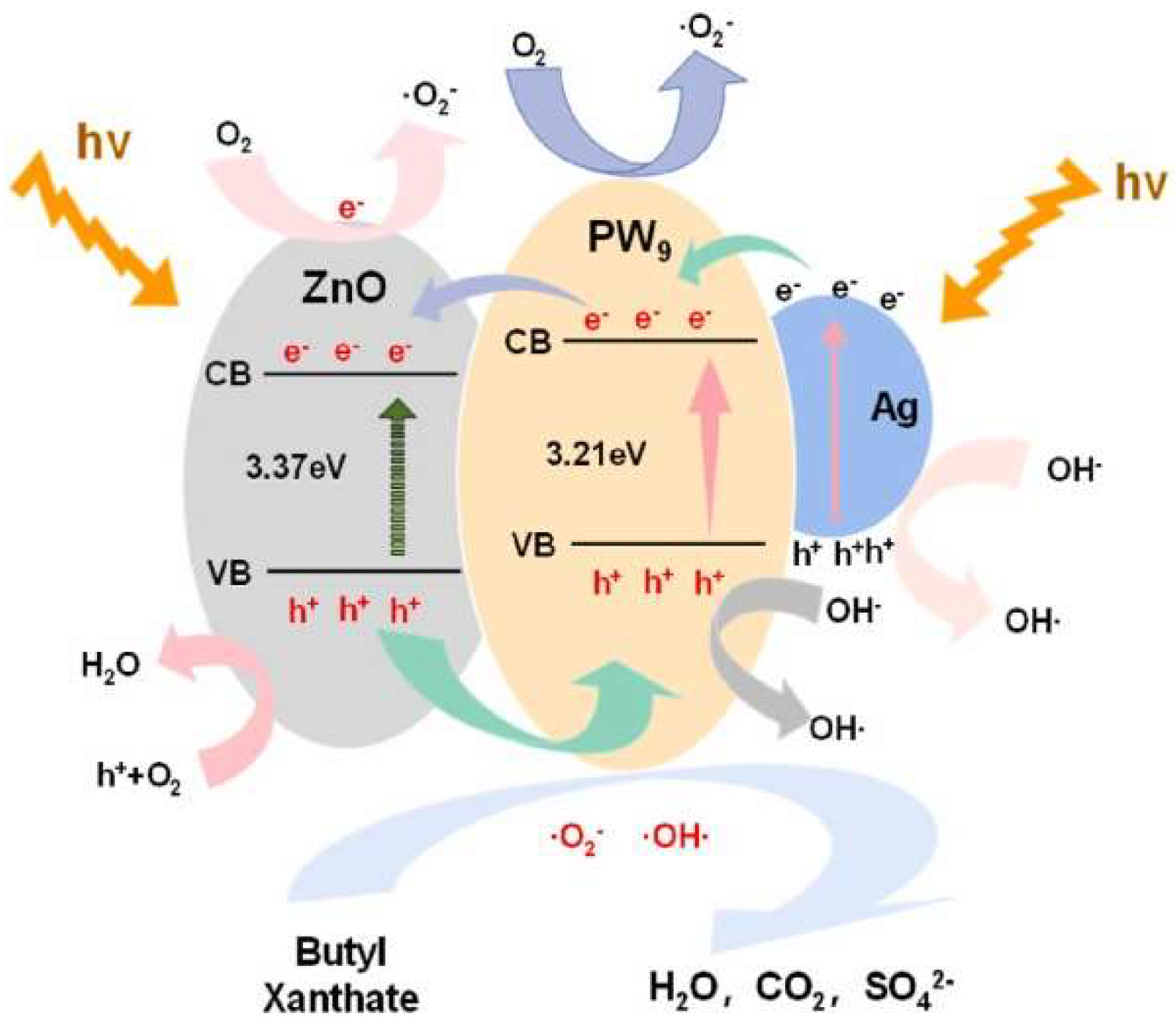
ZnO-Based Photocatalysts
4. Conclusions and Future Research Requirements for the Treatment of Xanthate Wastewater
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.; Lin, H.; Huo, H.; Dong, Y.; Xue, Q.; Cao, L. Continuous removal of ore floatation reagents by an anaerobic-aerobic biological filter. Bioresour. Technol. 2012, 114, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Amrollahi, A.; Massinaei, M.; Zeraatkar Moghaddam, A. Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nano-particles. Miner. Eng. 2019, 134, 142–155. [Google Scholar] [CrossRef]
- Natarajan, K.A.; Prakasan, M.R.S. Biodegradation of sodium isopropyl xanthate by paenibacillus polymyxa and pseudomonas putida. Miner. Metall. Process. 2013, 30, 226–232. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, Z.; Wang, S. Investigation of the interaction between xanthate and kaolinite based on experiments, molecular dynamics simulation, and density functional theory. J. Mol. Liq. 2021, 336, 116298. [Google Scholar] [CrossRef]
- García-Leiva, B.; Teixeira, L.A.C.; Torem, M.L. Degradation of xanthate in waters by hydrogen peroxide, fenton and simulated solar photo-fenton processes. J. Mater. Res. Technol. 2019, 8, 5698–5706. [Google Scholar] [CrossRef]
- Wang, L.; Fu, P.; Ma, Y.; Zhang, X.; Zhang, Y.; Yang, X. Steel slag as a cost-effective adsorbent for synergic removal of collectors, Cu(II) and Pb(II) ions from flotation wastewaters. Miner. Eng. 2022, 183, 107593. [Google Scholar] [CrossRef]
- Sun, Z.X.; Forsling, W. The degradation kinetics of ethyl-xanthate as a function of pH in aqueous solution. Miner. Eng. 1997, 10, 389–400. [Google Scholar] [CrossRef]
- Behnamfard, A.; Veglio, F. Estimation of xanthate decomposition percentage as a function of pH, temperature and time by least squares regression and adaptive neuro-fuzzy inference system. Int. J. Min. Geo-Eng. 2019, 53, 157–163. [Google Scholar]
- Yang, C.; Gao, P.; Dang, M.; Liu, Y.; Yao, F.; Ge, L.; Guo, L.; Tao, Y. Research progress on treatment process of xanthate in beneficiation wastewater. J. Xuzhou Inst. Technol. 2022, 37, 62–70+92. (In Chinese) [Google Scholar] [CrossRef]
- Rezaei, R.; Massinaei, M.; Zeraatkar Moghaddam, A. Removal of the residual xanthate from flotation plant tailings using modified bentonite. Miner. Eng. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Mielczarski, J. The role of impurities of sphalerite in the adsorption of ethyl xanthate and its flotation. Int. J. Miner. Process. 1986, 16, 179–194. [Google Scholar] [CrossRef]
- Dong, Y.B.; Lin, H.; Liu, Q.L.; Huo, H.X. Treatment of flotation wastewater using biological activated carbon. J. Cent. South Univ. 2014, 21, 3580–3587. [Google Scholar] [CrossRef]
- Li, G.; Teng, Q.; Sun, B.; Yang, Z.; Liu, S.; Zhu, X. Synthesis scaly Ag-TiO2 loaded fly ash magnetic bead particles for treatment of xanthate wastewater. Colloids Surf. Physicochem. Eng. Asp. 2021, 624, 126795. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núñez-Delgado, A.; Bokhari, A.; Race, M.; Khataee, A.; Jaromír Klemeš, J.; et al. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem. Eng. J. 2022, 431, 134104. [Google Scholar] [CrossRef]
- Fu, P.; Feng, J.; Yang, H.; Yang, T. Degradation of sodium n-butyl xanthate by vacuum UV-ozone (VUV/O3) in comparison with ozone and VUV photolysis. Process Saf. Environ. Prot. 2016, 102, 64–70. [Google Scholar] [CrossRef]
- Chen, S.H.; Du, D.Y. Degradation of n-butyl xanthate using fly ash as heterogeneous Fenton-like catalyst. J. Cent. South Univ. 2014, 21, 1448–1452. [Google Scholar] [CrossRef]
- Martins, T.A.G.; Falconi, I.B.A.; Pavoski, G.; de Moraes, V.T.; Galluzzi Baltazar, M.d.P.; Espinosa, D.C.R. Green synthesis, characterization, and application of copper nanoparticles obtained from printed circuit boards to degrade mining surfactant by Fenton process. J. Environ. 2021, 9, 106576. [Google Scholar] [CrossRef]
- Ai, G.H.; Tao, X.X.; Wang, Y.; Chen, Y.L. Removal of Xanthate in Flotation Wastewater by Ultrasound and Fenton Reagent. Int. Conf. Electr. Technol. Civ. Eng. 2011, 6106–6110. [Google Scholar]
- Chen, X.H.; Hu, Y.H.; Peng, H.; Cao, X.F. Degradation of ethyl xanthate in flotation residues by hydrogen peroxide. J. Cent. South Univ. 2015, 22, 495–501. [Google Scholar] [CrossRef]
- Elizondo-Álvarez, M.A.; Uribe-Salas, A.; Bello-Teodoro, S. Chemical stability of xanthates, dithiophosphinates and hydroxamic acids in aqueous solutions and their environmental implications. Ecotoxicol. Environ. Saf. 2021, 207, 111509. [Google Scholar] [CrossRef] [PubMed]
- Bararunyeretse, P.; Yao, J.; Dai, Y.; Bigawa, S.; Guo, Z.; Zhu, M. Toxic effect of two kinds of mineral collectors on soil microbial richness and activity: Analysis by microcalorimetry, microbial count, and enzyme activity assay. Environ. Sci. Pollut. Res. Int. 2017, 24, 1565–1577. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.; Zhang, C.; Zhou, W.; Fu, M.Y.; Chen, W.L.; Wang, S. Highly Sensitive Determination of Butyl Xanthate in Surface and Drinking Water by Headspace Gas Chromatography with Electron Capture Detector. Chromatographia 2015, 78, 1305–1310. [Google Scholar] [CrossRef]
- Mosevich, M.V.; Arshanitsa, N.M.; Glukhova, L.V. Substantiation of the maximum Permissible concentration of bytul sodium xanthate for fishery water bodies. Chem. Abstr. 1977, 87, 266. [Google Scholar]
- Li, H.; Yao, J.; Duran, R.; Liu, J.; Min, N.; Chen, Z.; Zhu, X.; Zhao, C.; Ma, B.; Pang, W.; et al. Toxic response of the freshwater green algae Chlorella pyrenoidosa to combined effect of flotation reagent butyl xanthate and nickel. Environ. Pollut. 2021, 286, 117285. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, W.; Ouyang, K.; Zhang, L.; Hu, Y. Decomposition of sodium butyl xanthate (SBX) in aqueous solution by means of OCF: Ozonator combined with flotator. Miner. Eng. 2015, 70, 222–227. [Google Scholar] [CrossRef]
- Lou, J.; Lu, G.; Wei, Y.; Zhang, Y.; An, J.; Jia, M.; Li, M. Enhanced degradation of residual potassium ethyl xanthate in mineral separation wastewater by dielectric barrier discharge plasma and peroxymonosulfate. Sep. Purif. Technol. 2022, 282, 119955. [Google Scholar] [CrossRef]
- Xin, Z.; Wang, S.; He, Q.; Han, X.; Fu, Z.; Xu, X.; Zhao, X. Preparation of a novel photocatalytic catalyst PW9@ZnO/Ag and the photocatalytic degradation of butyl xanthate under visible light. Environ. Res. 2022, 214, 113776. [Google Scholar] [CrossRef]
- Yanev, S.G.; Kent, U.M.; Roberts, E.S.; Ballou, D.P.; Hollenberg, P.F. Mechanistic studies of cytochrome P450 2B1 inactivation by xanthates. Arch. Biochem. Biophys. 2000, 378, 157–166. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhang, Y.M.; Liang, D.W. Applying hybrid coagulants and polyacrylamide flocculants in the treatment of high-phosphorus hematite flotation wastewater (HHFW): Optimization through response surface methodology. Sep. Purif. Technol. 2010, 76, 72–78. [Google Scholar] [CrossRef]
- Meng, X.; Wu, J.; Kang, J.; Gao, J.; Liu, R.; Gao, Y.; Wang, R.; Fan, R.; Khoso, S.A.; Sun, W.; et al. Comparison of the reduction of chemical oxygen demand in wastewater from mineral processing using the coagulation–flocculation, adsorption and Fenton processes. Miner. Eng. 2018, 128, 275–283. [Google Scholar] [CrossRef]
- Xia, C.; Luo, Y.; Yao, J.; Liu, W.; Wang, F.; Wu, X. Joint effects of Cd and thioglycollic acid on soil microbial activity. Int. Biodeterior. Biodegrad. 2018, 128, 164–170. [Google Scholar] [CrossRef]
- Sun, W.; Huang, H.; Cao, X.F.; Meng, W.; Dong, D. Study on the Treating Methods of High Concentration Organic Wastewater in Production of Xanthate. Nonferr. Met. 2012, 2, 47–50. (In Chinese) [Google Scholar] [CrossRef]
- Salarirad, M.M.; Behnamfard, A.; Veglio, F. Removal of xanthate from aqueous solutions by adsorption onto untreated and acid/base treated activated carbons. Desalin. Water Treat. 2021, 212, 220–233. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Ren, S.; Luo, W. Removal of ethyl, isobutyl, and isoamyl xanthates using cationic gemini surfactant-modified montmorillonites. Colloids Surf. Physicochem. Eng. Asp. 2019, 580, 123723. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Rubio, J. Isopropylxanthate ions uptake by modified natural zeolite and removal by dissolved air flotation. Int. J. Miner. Process. 2009, 90, 21–26. [Google Scholar] [CrossRef]
- Li, L.; He, M.; Feng, Y.; Wei, H.; You, X.; Yu, H.; Wang, Q.; Wang, J. Adsorption of xanthate from aqueous solution by multilayer graphene oxide: An experimental and molecular dynamics simulation study. Adv. Compos. Hybrid Mater. 2021, 4, 725–732. [Google Scholar] [CrossRef]
- Chen, S.; Gong, W.; Mei, G. Experimental Study on the Biodegradability of Alkyl Xanthate Collectors. Acta Sci. Nat. Univ. Pekinensis. 2010, 46, 351–357. (In Chinese) [Google Scholar] [CrossRef]
- Zeng, Y.; Tang, L.; Zhang, M. Progress research on the treatment technology of residual xanthate in mineral concentration wastewater and its mechanism. Ind. Water Treat. 2010, 30, 8–10+49. (In Chinese) [Google Scholar]
- Chen, S.; Gong, W.; Mei, G.; Zhou, Q.; Bai, C.; Xu, N. Primary biodegradation of sulfide mineral flotation collectors. Miner. Eng. 2011, 24, 953–955. [Google Scholar] [CrossRef]
- Lin, H.; Qin, K.; Dong, Y.; Li, B. A newly-constructed bifunctional bacterial consortium for removing butyl xanthate and cadmium simultaneously from mineral processing wastewater: Experimental evaluation, degradation and biomineralization. J. Environ. Manag. 2022, 316, 115304. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, E.; Subramanian, S.; Natarajan, K.A. Studies on biodegradation of organic flotation collectors using Bacillus polymyxa. Hydrometallurgy 2003, 71, 249–256. [Google Scholar] [CrossRef]
- Molina, G.C.; Cayo, C.H.; Rodrigues, M.A.S.; Bernardes, A.M. Sodium isopropyl xanthate degradation by advanced oxidation processes. Miner. Eng. 2013, 45, 88–93. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2022, 311, 136977. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Yong, T.; Cao, W.; Wu, J.; Shen, Y. Synergistic effects of oxidation, coagulation and adsorption in the integrated fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water. 2022, 14, 2385. [Google Scholar] [CrossRef]
- Bello, M.M.; Abdul Raman, A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Ai, G.H.; Tu, Y.Q. Degradation of Residual Xanthate of in Sulfide Mineral Processing Wastewater by Fenton Reagent. Int. Conf. Electr. Technol. Civ. Eng. 2011, 3046–3049. [Google Scholar]
- Zheng, Y.; Yuan, F.; Gao, D.; Liu, L.; Wang, L.; Hu, X. Improved visible-light-driven activity of micro graphite/BiOI nanocomposite for effective removal of xanthate from wastewater. Mater. Res. Bull. 2023, 158, 112080. [Google Scholar] [CrossRef]
- Wei, G.; Li, Y.; Cai, S.; Li, Z.; Mo, J.; Zhang, L. Photo-Fenton degradation of ethyl xanthate catalyzed by bentonite-supported Fe(II)/phosphotungstic acid under visible light irradiation. Water Sci. Technol. 2018, 2017, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Fard, M.S. A critical review in Fenton-like approach for the removal of pollutants in the aqueous environment. Environ. Chall. 2022, 7, 100534. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Shao, L.; Wei, G.; Wang, Y.; Li, Z.; Zhang, L.; Zhao, S.; Zhou, M. Preparation and application of acidified/calcined red mud catalyst for catalytic degradation of butyl xanthate in Fenton-like process. Environ. Sci. Pollut. Res. Int. 2016, 23, 15202–15207. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Adv. Chem. Eng. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Umar, M.; Roddick, F.; Fan, L.; Aziz, H.A. Application of ozone for the removal of bisphenol A from water and wastewater—A review. Chemosphere 2013, 90, 2197–2207. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.S. Study on degradation of flotation reagents butyl xanthate by zone. Chin. J. Environ. Eng. 2011, 5, 2712–2716. (In Chinese) [Google Scholar]
- Kiyanmehr, K.; Moussavi, G.; Mohammadi, S.; Naddafi, K.; Giannakis, S. The efficacy of the VUV/O3 process run in a continuous-flow fluidized bed reactor for simultaneous elimination of favipiravir and bacteria in aqueous matrices. Chemosphere 2022, 304, 135307. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Feng, J.; Yang, T.; Yang, H. Comparison of alkyl xanthates degradation in aqueous solution by the O3 and UV/O3 processes: Efficiency, mineralization and ozone utilization. Miner. Eng. 2015, 81, 128–134. [Google Scholar] [CrossRef]
- Fu, P.; Wang, L.; Ma, Y.; Hou, Z. A comparative study on the degradation of ethyl xanthate collector by O3, UV254nm, UV185+254nm, O3/UV254nm and O3/UV185+254nm processes. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Yan, P.; Chen, G.; Ye, M.; Sun, S.; Ma, H.; Lin, W. Oxidation of potassium n -butyl xanthate with ozone: Products and pathways. J. Clean. Prod. 2016, 139, 287–294. [Google Scholar] [CrossRef]
- Koelsch, M.; Cassaignon, S.; Ta Thanh Minh, C.; Guillemoles, J.F.; Jolivet, J.P. Electrochemical comparative study of titania (anatase, brookite and rutile) nanoparticles synthesized in aqueous medium. Thin Solid Films 2004, 451–452, 86–92. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef]
- Lim, T.-T.; Yap, P.-S.; Srinivasan, M.; Fane, A.G. TiO2/AC Composites for Synergistic Adsorption-Photocatalysis Processes: Present Challenges and Further Developments for Water Treatment and Reclamation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1173–1230. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis Transition metal ions in photocatalytic systems. Appl. Catal. B 1999, 23, 89–144. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef]
- Su, J.; Yu, S.; Xu, M.; Guo, Y.; Sun, X.; Fan, Y.; Zhang, Z.; Yan, J.; Zhao, W. Enhanced visible light photocatalytic performances of few-layer MoS2@TiO2 hollow spheres heterostructures. Mater. Res. Bull. 2020, 130, 110936. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; Zheng, L.; Wan, C. Fabrication of TiO2/MoS2@zeolite photocatalyst and its photocatalytic activity for degradation of methyl orange under visible light. Appl. Surf. Sci. 2015, 358, 468–478. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Hu, J.; Pan, J.; Fan, J.; Shao, G. One-dimensional Z-scheme TiO2 /WO3 /Pt heterostructures for enhanced hydrogen generation. Appl. Surf. Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef]
- Bian, Z.; Feng, Y.; Li, H.; Yu, H.; Wu, H. Adsorption-photocatalytic degradation and kinetic of sodium isobutyl xanthate using the nitrogen and cerium co-doping TiO2-coated activated carbon. Chemosphere 2021, 263, 128254. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, P.; Zhao, S.; Li, A.; Chen, Y.; Bai, J.; Han, C.; Wei, D.; Ao, Y. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Miner. Eng. 2020, 159, 106640. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, T.; Zheng, S.; Sun, Z.; Li, C. Adsorptive and photocatalytic behaviour of PANI/TiO2/metakaolin composites for the removal of xanthate from aqueous solution. Miner. Eng. 2021, 171, 107129. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Hydrothermal synthesis of novel ternary hierarchical MoS2/TiO2/clinoptilolite nanocomposites with remarkably enhanced visible light response towards xanthates. Appl. Surf. Sci. 2021, 542, 148578. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, L.; Shu, K.; Yang, J.; Tang, H. Fabrication of TiO2 @MoS2 heterostructures with improved visible light photocatalytic activity. Colloids Surf. Physicochem. Eng. Asp. 2022, 642, 128686. [Google Scholar] [CrossRef]
- Zhang, M.; Han, N.; Fei, Y.; Liu, J.; Xing, L.; Núñez-Delgado, A.; Jiang, M.; Liu, S. TiO2/g-C3N4 photocatalyst for the purification of potassium butyl xanthate in mineral processing wastewater. J. Environ. Manag. 2021, 297, 113311. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ouyang, L. Photocatalytic photodegradation of xanthate over C, N, S-tridoped TiO2 nanotubes under visible light irradiation. J. Phys. Chem. Solids 2011, 72, 39–44. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, G.; Duan, G.; Yang, Y.; Zhang, L.; Li, Z.; Pei, R.; Luo, D. Heterogeneous activation of persulfate by mechanically activated bentonite-based bismuth ferrite composites under visible light for ethyl xanthate degradation: Application performance and catalytic mechanism. Opt. Mater. 2022, 125, 112089. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Zhu, Y.; Wei, G.; Li, Z.; Mo, R. Three-dimensional photoelectrocatalytic degradation of ethyl xanthate catalyzed by activated bentonite-based bismuth ferrites particle electrodes: Influencing factors, kinetics, and mechanism. J. Environ. Chem. Eng. 2021, 9, 105559. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, G.; Zhu, Y.; Zhang, L.; Li, Z.; Li, Z.; Ding, X. Mechanism study of ethyl xanthate degradation catalyzed by activated bentonite-based bismuth ferrites composite in visible-light-driven photo-Fenton process. Opt. Mater. 2022, 132, 112855. [Google Scholar] [CrossRef]
- Yuan, F.; Zheng, Y.; Gao, D.; Wang, L.; Hu, X. Facile assembly and enhanced visible-light-driven photocatalytic activity of S-scheme BiOBr/g-C3N4 heterojunction for degrading xanthate in wastewater. J. Mol. Liq. 2022, 366, 120279. [Google Scholar] [CrossRef]
- Cui, K.; He, Y.; Jin, S. Enhanced UV-visible response of bismuth subcarbonate nanowires for degradation of xanthate and photocatalytic reaction mechanism. Chemosphere 2016, 149, 245–253. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, K.; Hu, M.; Jin, S. Fe(III) ions enhanced catalytic properties of (BiO)2CO3 nanowires and mechanism study for complete degradation of xanthate. Chemosphere 2017, 181, 190–196. [Google Scholar] [CrossRef]
- Zou, M.; Tan, C.; Yang, H.; Kuang, D.; Nie, Z.; Zhou, H. Facile preparation of recyclable and flexible BiOBr@TiO2/PU-SF composite porous membrane for efficient photocatalytic degradation of mineral flotation wastewater. J. Water Process. Eng. 2022, 50, 103127. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Li, G.; Cui, B.; Wei, D.; Shen, Y. Synthesis of clinoptilolite-supported BiOCl/TiO2 heterojunction nanocomposites with highly-enhanced photocatalytic activity for the complete degradation of xanthates under visible light. Chem. Eng. J. 2021, 407, 126697. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Serpone, N. Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef]
- Guo, M.; Du, J. First-principles study of electronic structures and optical properties of Cu, Ag, and Au-doped anatase TiO2. Phys. B Condens. Matter 2012, 407, 1003–1007. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Latif, S.; Tahir, K.; Ullah Khan, A.; Abdulaziz, F.; Arooj, A.; Alanazi, T.Y.A.; Rakic, V.; Khan, A.; Jevtovic, V. Green synthesis of Mn-doped TiO2 nanoparticles and investigating the influence of dopant concentration on the photocatalytic activity. Inorg. Chem. Commun. 2022, 146, 110091. [Google Scholar] [CrossRef]
- Alkorbi, A.S.; Muhammad Asif Javed, H.; Hussain, S.; Latif, S.; Mahr, M.S.; Mustafa, M.S.; Alsaiari, R.; Alhemiary, N.A. Solar light-driven photocatalytic degradation of methyl blue by carbon-doped TiO2 nanoparticles. Opt. Mater. 2022, 127, 112259. [Google Scholar] [CrossRef]
- Khairy, M.; Kamar, E.M.; Mousa, M.A. Photocatalytic activity of nano-sized Ag and Au metal-doped TiO2 embedded in rGO under visible light irradiation. Mater. Sci. Eng. 2022, 286, 116023. [Google Scholar] [CrossRef]
- Liu, R.; Ji, Z.; Wang, J.; Zhang, J. Solvothermal synthesized Ag-decorated TiO2/sepiolite composite with enhanced UV–vis and visible light photocatalytic activity. Microporous Mesoporous Mater. 2018, 266, 268–275. [Google Scholar] [CrossRef]
- Ullah, R.; Liu, C.; Panezai, H.; Gul, A.; Sun, J.; Wu, X. Controlled crystal phase and particle size of loaded-TiO2 using clinoptilolite as support via hydrothermal method for degradation of crystal violet dye in aqueous solution. Arab. J. Chem. 2020, 13, 4092–4101. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Bai, J.; Han, C.; Liu, W.; Wei, D. Facile synthesis of clinoptilolite-supported Ag/TiO2 nanocomposites for visible-light degradation of xanthates. J. Taiwan Inst. Chem. Eng. 2021, 122, 231–240. [Google Scholar] [CrossRef]
- Ma, C.; Wei, J.; Jiang, K.; Chen, J.; Yang, Z.; Yang, X.; Yu, G.; Zhang, C.; Li, X. Typical layered structure bismuth-based photocatalysts for photocatalytic nitrogen oxides oxidation. Sci. Total Environ. 2023, 855, 158644. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Lv, Q.; Wu, J. Bismuth-based photocatalyst for photocatalytic oxidation of flue gas mercury removal: A review. J. Hazard. Mater. 2021, 418, 126280. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wan, S.; Zhao, Y.; Gu, Y.; Wang, Y.; Qin, Y.; Zhang, Z.; Ge, X.; Zhong, Q.; Bu, Y. Recent advances in bismuth-based multimetal oxide photocatalysts for hydrogen production from water splitting: Competitiveness, challenges, and future perspectives. Mater. Rep. Energy 2021, 1, 100019. [Google Scholar] [CrossRef]
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M = W, Mo, Cr) for environmental remediation and clean energy production. J. Ind. Eng. Chem. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. Engl. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Li, Z.; Dai, J.; Cheng, C.; Feng, W.; Wang, Q. Tailoring photocatalytic activity and magnetic properties of BiFeO3/CeO2/Bi2Fe4O9 composites. J. Phys. Chem. Solids 2021, 156, 110171. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, S.; Li, B.; Cheng, X.; Cheng, Q. Fabrication of magnetic Co/BiFeO3 composite and its advanced treatment of pharmaceutical waste water by activation of peroxysulphate. Sep. Purif. Technol. 2018, 202, 242–247. [Google Scholar] [CrossRef]
- Lu, S.; Shen, L.; Li, X.; Yu, B.; Ding, J.; Gao, P.; Zhang, H. Advances in the photocatalytic reduction functions of graphitic carbon nitride-based photocatalysts in environmental applications: A review. J. Clean. Prod. 2022, 378, 134589. [Google Scholar] [CrossRef]
- Cui, K.; He, Y.; Jin, S.; Qian, H. Preparation, characterization, and photoluminescence property of core-shell (BiO)2CO3@Bi2S3 nanowires. Mater. Chem. Phys. 2018, 211, 65–71. [Google Scholar] [CrossRef]
- Raza, W.; Faisal, S.M.; Owais, M.; Bahnemann, D.; Muneer, M. Facile fabrication of highly efficient modified ZnO photocatalyst with enhanced photocatalytic, antibacterial and anticancer activity. RSC Adv. 2016, 6, 78335–78350. [Google Scholar] [CrossRef]
- Poongodi, G.; Anandan, P.; Kumar, R.M.; Jayavel, R. Studies on visible light photocatalytic and antibacterial activities of nanostructured cobalt doped ZnO thin films prepared by sol-gel spin coating method. Spectrochim. Acta A Mol. Biomol. 2015, 148, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Xiao, Q.; Ouyang, L. Photocatalytic photodegradation of xanthate over Zn1−xMnxO under visible light irradiation. J. Alloys Compd. 2009, 479, L4–L7. [Google Scholar] [CrossRef]
- Xiao, Q.; Yao, C. Preparation and visible light photocatalytic activity of Zn1−xFexO nanocrystalline. Mater. Chem. Phys. 2011, 130, 5–9. [Google Scholar] [CrossRef]



| AOPs | Advantages | Disadvantages | |
|---|---|---|---|
| Fenton | • Rapid degradation rate • Mild reaction conditions • Convenient operation | • High dosage of reagents • Limited pH range • Catalysts are not easily recovered •Excess Fe2+ increases the color and COD of the treated water • Secondary pollution | |
| Ozonation | • Short reaction time • High removal rate of xanthate | • Limited mass transfer efficiency and utilization productivity • High treatment costs •Low mineralization rate of the intermediates • Intermediate products increase the COD content of the effluent | |
| Photocatalytics | TiO2 photocatalysts | • Low cost • Innocuous and harmless • Strong redox capability • Excellent chemical stability | • Wide band gap • Electron-hole pairs are unstable • Narrow light absorption range • Low adsorption capacity •High concentration nano-TiO2 suspensions are easy to agglomerate |
| Bi-based oxide photocatalysts | • Innocuous and harmless • Excellent chemical stability • Unique layered structure | • Electron-hole pairs are unstable • Visible light absorption is limited to a specific wavelength range | |
| ZnO-based photocatalysts | • Excellent photophysical properties • Low cost • High light sensitivity and thermal stability | • Wide band gap • Photoinduced carriers are unstable • Low sunlight usage rate | |
| Photocatalyst | Light Source | Pollutant | Synthesis Methods | Degradation (%) | Refs. |
|---|---|---|---|---|---|
| Ce/NeTiO2@AC | Visible light irradiation | Sodium isobutyl xanthate | Sol-gel method | 95.80 | [77] |
| Ag-TiO2-FAMB | Visible light irradiation | Sodium butyl xanthate | Sol-gel method | 98.50 | [14] |
| TiO2/clinoptilolite | UV irradiation | Sodium isopropyl xanthate | Hydrothermal method | >90 | [78] |
| PANI/TiO2/metakaolin | Visible light irradiation | Butyl xanthate | Sol–gel and in-situ polymerization | 94.8 | [79] |
| MoS2/TiO2/clinoptilolite | Visible light irradiation | Sodium isopropyl xanthate | Moderate hydrothermal route | >90 | [80] |
| b-TiO2 @MoS2 | Visible light irradiation | Butyl xanthate | Sol-gel method | 94.80 | [81] |
| TiO2/ graphene nanocomposites | Visible light irradiation | Potassium butyl xanthate | Hydrothermal method | 97.03 | [15] |
| TiO2/g-C3N4 | Visible light irradiation | Potassium butyl xanthate | Hydrothermal method | 97.10 | [82] |
| C, N, S-tridoped TiO2 nanotubes | Visible light irradiation | Potassium ethyl xanthate | Hydrothermal method | - | [83] |
| A-BiFe/Bent | Visible light irradiation | Ethyl xanthate | Mechanically activated | 98.60 | [84] |
| A-BiFe/Bent | Visible light irradiation | Ethyl xanthate | Mechanically activated | 97.85 | [85] |
| A-BiFe/Bent | Visible light irradiation | Ethyl xanthate | Mechanically activated | 98.43 | [86] |
| micro graphite/BiOI | Visible light irradiation | Xanthate | Hydrothermal | 94.36 | [51] |
| S-scheme BiOBr/g-C3N4 | Visible light irradiation | Ethyl xanthate | Hydrothermal | 96.10 | [87] |
| (BiO)2CO3 nanowires | UV-visible light irradiation | Sodium isopropyl xanthate | Hydrothermal | 96.00 | [88] |
| Fe3+-doped (BiO)2CO3 | UV-visible light irradiation | Sodium isopropyl xanthate | Hydrothermal | 95.71 | [89] |
| BiOBr@TiO2/PU-SF | Visible light irradiation | Ethyl xanthate | A facile blending-phase separation-impregnation-precipitation | 97.85 | [90] |
| BiOCl/TiO2/clinoptilolite | Visible light irradiation | Sodium isopropyl xanthate | A facile hydrothermal route combining with a water bath precipitation procedure | >90 | [91] |
| PW9@ZnO/Ag | UV-visible light irradiation | Butyl xanthate | Hydrothermal | 99.83 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Li, S.; Ding, Z.; Li, J.; Yu, A.; Wen, S.; Bai, S. Treatment Technology and Research Progress of Residual Xanthate in Mineral Processing Wastewater. Minerals 2023, 13, 435. https://doi.org/10.3390/min13030435
Yuan J, Li S, Ding Z, Li J, Yu A, Wen S, Bai S. Treatment Technology and Research Progress of Residual Xanthate in Mineral Processing Wastewater. Minerals. 2023; 13(3):435. https://doi.org/10.3390/min13030435
Chicago/Turabian StyleYuan, Jiaqiao, Suqi Li, Zhan Ding, Jie Li, Anmei Yu, Shuming Wen, and Shaojun Bai. 2023. "Treatment Technology and Research Progress of Residual Xanthate in Mineral Processing Wastewater" Minerals 13, no. 3: 435. https://doi.org/10.3390/min13030435
APA StyleYuan, J., Li, S., Ding, Z., Li, J., Yu, A., Wen, S., & Bai, S. (2023). Treatment Technology and Research Progress of Residual Xanthate in Mineral Processing Wastewater. Minerals, 13(3), 435. https://doi.org/10.3390/min13030435







