Coal-Based Activated Carbon via Microwave-Assisted ZnCl2 Activation for Methyl Violet 2B Dye Removal: Optimization, Desirability Function, and Adsorption Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MCAC’s Preparation
2.3. Characterization
2.4. Statistical Optimization Methodology
2.5. Adsorption Study of MV 2B on MCAC
3. Results and Discussion
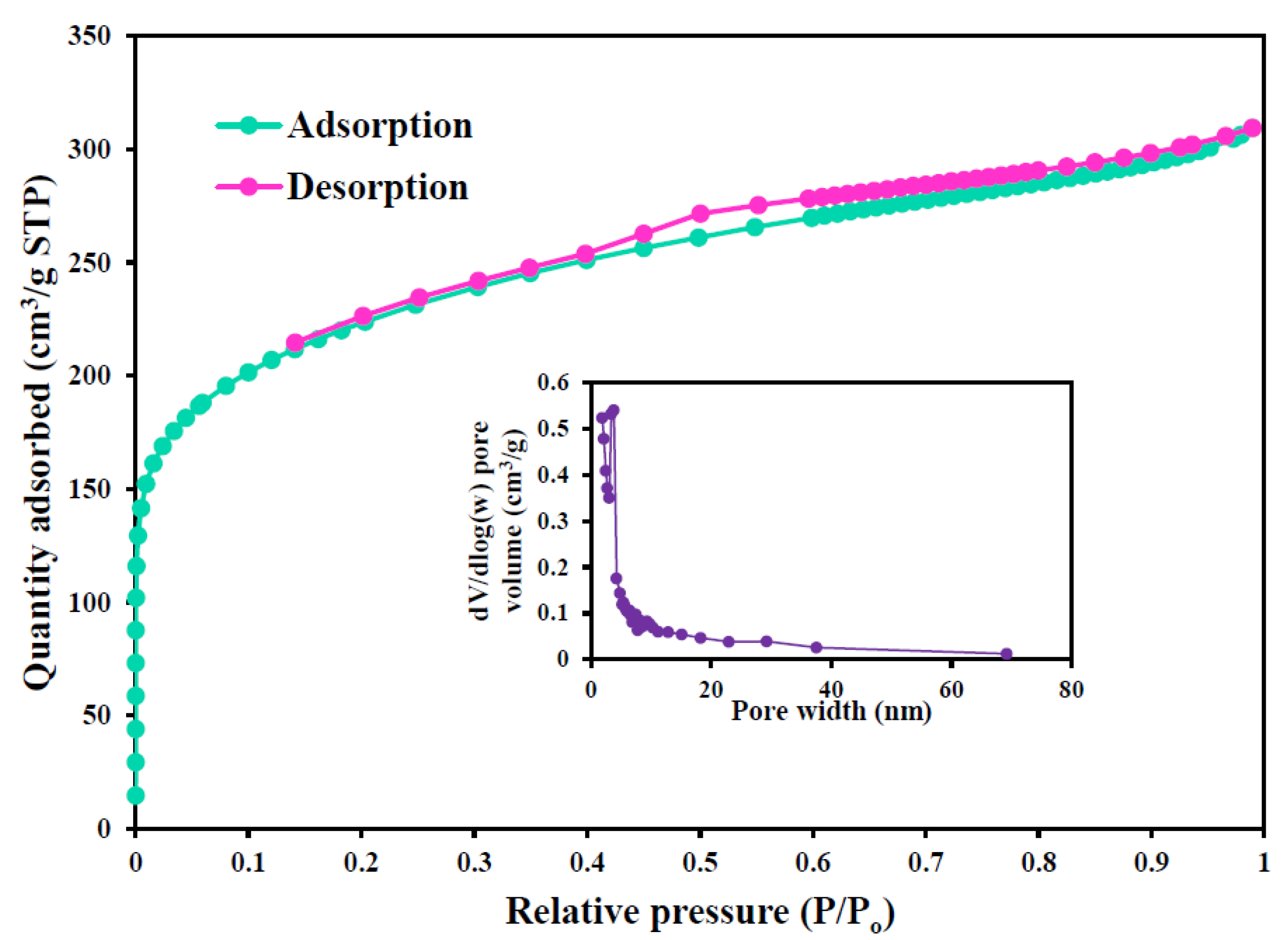
3.1. Characterization of MCAC
3.2. Model Validation
3.3. Dual Effects of the Variables on MV 2B Removal
3.4. Optimization by the Desirability Functions
3.5. Adsorption Study
3.6. Adsorption Kinetics
3.7. Adsorption Isotherms
3.8. Adsorption Mechanism of MV 2B
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamil, L.D.O.; Georgin, J.; Franco, D.S.; Netto, M.S.; Grassi, P.; Piccilli, D.G.; Dotto, G.L. Powdered biosorbent from pecan pericarp (Carya illinoensis) as an efficient material to uptake methyl violet 2B from effluents in batch and column operations. Adv. Powder Technol. 2020, 31, 2843–2852. [Google Scholar]
- Wang, X.; Xu, Q.; Zhang, L.; Pei, L.; Xue, H.; Li, Z. Adsorption of methylene blue and Congo red from aqueous solution on 3D MXene/carbon foam hybrid aerogels: A study by experimental and statistical physics modeling. J. Environ. Chem. Eng. 2023, 11, 109206. [Google Scholar] [CrossRef]
- Arslan, H.; Bouchareb, R.; Arikan, E.B.; Dizge, N. Iron-loaded leonardite powder for Fenton oxidation of Reactive Red 180 dye removal. Environ. Sci. Pollut. Res. 2022, 29, 77071–77080. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Ullah, R.; Gulfam, N. Biological degradation of the azo dye basic orange 2 by Escherichia coli: A sustainable and ecofriendly approach for the treatment of textile wastewater. Water 2022, 14, 2063. [Google Scholar] [CrossRef]
- Mohammadhosseini, S.; Al-Musawi, T.J.; Romero Parra, R.M.; Qutob, M.; Gatea, M.A.; Ganji, F.; Balarak, D. UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite. Minerals 2022, 12, 1417. [Google Scholar] [CrossRef]
- Pasichnyk, M.; Gaálová, J.; Minarik, P.; Václavíková, M.; Melnyk, I. Development of polyester filters with polymer nanocomposite active layer for effective dye filtration. Sci. Rep. 2022, 12, 973. [Google Scholar] [CrossRef]
- Saghir, S.; Pu, C.; Fu, E.; Wang, Y.; Xiao, Z. Synthesis of high surface area porous biochar obtained from pistachio shells for the efficient adsorption of organic dyes from polluted water. Surf. Interface 2022, 34, 102357. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, H.; Jiang, M.; Zhang, Q. Preparation and characterization of activated carbon derived from deashing coal slime with ZnCl2 activation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128124. [Google Scholar] [CrossRef]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Jasri, K.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yousef, T.A.; Al Duaij, O.K. Mesoporous activated carbon produced from mixed wastes of oil palm frond and palm kernel shell using microwave radiation-assisted K2CO3 activation for toxic dye removal: Optimization by response surface methodology. Diamond Relat. Mater. 2022, 131, 109581. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, G.; Zheng, Y.; Zhao, Y. Preparation of activated carbon from Guhanshan coal and its effect on methane adsorption thermodynamics at different temperatures. Powder Technol. 2022, 395, 424–442. [Google Scholar] [CrossRef]
- Xue, H.; Gao, X.; Seliem, M.K.; Mobarak, M.; Dong, R.; Wang, X.; Li, Z. Efficient adsorption of anionic azo dyes on porous heterostructured MXene/biomass activated carbon composites: Experiments, characterization, and theoretical analysis via advanced statistical physics models. Chem. Eng. J. 2023, 451, 138735. [Google Scholar] [CrossRef]
- Abdollahzadeh, H.; Fazlzadeh, M.; Afshin, S.; Arfaeinia, H.; Feizizadeh, A.; Poureshgh, Y.; Rashtbari, Y. Efficiency of activated carbon prepared from scrap tires magnetized by Fe3O4 nanoparticles: Characterisation and its application for removal of reactive blue19 from aquatic solutions. Int. J. Environ. Anal. Chem. 2022, 102, 1911–1925. [Google Scholar] [CrossRef]
- Jawad, A.H.; Surip, S.N. Upgrading low rank coal into mesoporous activated carbon via microwave process for methylene blue dye adsorption: Box Behnken Design and mechanism study. Diamond Relat. Mater. 2022, 127, 109199. [Google Scholar] [CrossRef]
- Thi, V.H.T.; Lee, B.K. Great improvement on tetracycline removal using ZnO rod-activated carbon fiber composite prepared with a facile microwave method. J. hazard. Mater. 2017, 324, 329–339. [Google Scholar]
- Yağmur, H.K.; Kaya, İ. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J. Mol. Struct. 2021, 1232, 130071. [Google Scholar] [CrossRef]
- Pezoti, O., Jr.; Cazetta, A.L.; Souza, I.P.; Bedin, K.C.; Martins, A.C.; Silva, T.L.; Almeida, V.C. Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J. Ind. Eng. Chem. 2014, 20, 4401–4407. [Google Scholar] [CrossRef]
- Madankar, C.S.; Bhagwat, S.S.; Meshram, P.D. Cd2+ removal from synthetic waters by ZnCl2-activated carbon. Mater. Today Proceed. 2021, 45, 4684–4688. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Zubair, M.; Jarrah, N.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Sewage sludge ZnCl2-activated carbon intercalated MgFe—LDH nanocomposites: Insight of the sorption mechanism of improved removal of phenol from water. Int. J. Mol. Sci. 2020, 21, 1563. [Google Scholar] [CrossRef] [Green Version]
- Cifci, D.İ.; Aydin, N.; Atav, R.; Gunes, Y.; Gunes, E. Synthesis of ZnCl2 Activated Raising Powder of Cotton Fabrics for Acid and Basic Dye Adsorption: A Way to Reuse Cellulosic Wastes for Sustainable Production. J. Nat. Fib. 2022, 19, 14299–14317. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, H.; Jiang, Y.; Li, H.; Tang, P.; Li, D.; Feng, Y. Porous ZnCl2-activated carbon from shaddock peel: Methylene blue adsorption behavior. Materials 2022, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Z.; Ahmad Zaini, M.A. One-step ZnCl2/FeCl3 composites preparation of magnetic activated carbon for effective adsorption of rhodamine B dye. Toxin Rev. 2022, 41, 64–81. [Google Scholar] [CrossRef]
- Azmi, A.S.; Yusup, S.; Muhamad, S. The influence of temperature on adsorption capacity of Malaysian coal. Chem. Eng. Process. Process Intensif. 2006, 45, 392–396. [Google Scholar] [CrossRef]
- Dalvand, A.; Nabizadeh, R.; Ganjali, M.R.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magnet. Magnet. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Hanafi, N.A.M.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yousef, T.A.; Al Duaij, O.K.; Alsaiari, N.S. Optimized removal process and tailored adsorption mechanism of crystal violet and methylene blue dyes by activated carbon derived from mixed orange peel and watermelon rind using microwave-induced ZnCl2 activation. Biomass Conver. Bioref. 2022. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kaur, B.R.K.; Gupta, H. Bhunia, Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Micropor. Mesopor. Mater. 2019, 282, 146–158. [Google Scholar] [CrossRef]
- Niu, J.; Miao, J.; Zhang, H.; Guo, Y.; Li, L.; Cheng, F. Focusing on the impact of inherent minerals in coal on activated carbon production and its performance: The role of trace sodium on SO2 and/or NO removal. Energy 2023, 263, 125638. [Google Scholar] [CrossRef]
- Oguz Erdogan, F.; Kopac, T. Comparison of activated carbons produced from Zonguldak Kozlu and Zonguldak Karadon hard coals for hydrogen sorption. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Ge, X.; Ma, X.; Wu, Z.; Xiao, X.; Yan, Y. Modification of coal-based activated carbon with nitric acid using microwave radiation for adsorption of phenanthrene and naphthalene. Res. Chem. Intermed. 2015, 41, 7327–7347. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Tang, M.; Hao, X.; Liu, J.; Zhang, G.; Zhang, Y. Effect of the pore structure of coal-based activated carbon and hydrogen addition on methane decomposition for the preparation of carbon nanotubes. Vacuum 2023, 207, 111584. [Google Scholar] [CrossRef]
- Wang, R.; Rish, S.K.; Lee, J.M.; Bahadur, R.; Vinu, A.; Tahmasebi, A.; Yu, J. N-doped porous carbon from direct KOH activation of Victorian brown coal for high-rate energy storage performance. J. Anal. Appl. Pyrol. 2022, 168, 105785. [Google Scholar] [CrossRef]
- Tamarkina, Y.V.; Kucherenko, V.A.; Shendrik, T.G. Nanoporous brown coal adsorbents prepared by alkaline activation with thermal shock. Solid Fuel Chem. 2012, 46, 289–294. [Google Scholar] [CrossRef]
- Hassani, A.; Alidokht, L.; Khataee, A.R.; Karaca, S. Optimization of comparative removal of two structurally different basic dyes using coal as a low-cost and available adsorbent. J. Taiwan Inst. Chem. Eng. 2014, 45, 1597–1607. [Google Scholar] [CrossRef]
- Zain, Z.M.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yaseen, Z.M. A pH-Sensitive Surface of Chitosan/Sepiolite Clay/Algae Biocomposite for the Removal of Malachite Green and Remazol Brilliant Blue R Dyes: Optimization and Adsorption Mechanism Study. J. Polym. Environ. 2022, 31, 501–518. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Puad, N.A.A.; Bello, O.S. Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour. Ind. 2014, 6, 18–35. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.A.; Eusoff, M.A.; Oladoye, P.O.; Adegoke, K.A.; Bello, O.S. Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel. Chem. Data Collect. 2020, 28, 100426. [Google Scholar] [CrossRef]
- Shengli, S.; Junping, L.; Qi, L.; Fangru, N.; Jia, F.; Shulian, X. Optimized preparation of Phragmites australis activated carbon using the Box-Behnken method and desirability function to remove hydroquinone. Ecotoxicol. Environ. Saf. 2018, 165, 411–422. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Jawad, A.H.; Kashi, E.; Radzun, K.A.; ALOthman, Z.A.; Wilson, L.D. Insight into adsorption mechanism, modeling, and desirability function of crystal violet and methylene blue dyes by microalgae: Box-Behnken design application. Algal Res. 2022, 67, 102864. [Google Scholar] [CrossRef]
- Baig, U.; Uddin, M.K.; Gondal, M.A. Removal of hazardous azo dye from water using synthetic nano adsorbent: Facile synthesis, characterization, adsorption, regeneration and design of experiments. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124031. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Vet. Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Theamwong, N.; Intarabumrung, W.; Sangon, S.; Aintharabunya, S.; Ngernyen, Y.; Hunt, A.J.; Supanchaiyamat, N. Activated carbons from waste Cassia bakeriana seed pods as high-performance adsorbents for toxic anionic dye and ciprofloxacin antibiotic remediation. Bioresour. Technol. 2021, 341, 125832. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Frenudlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Khan, T.A.; Nouman, M.; Dua, D.; Khan, S.A.; Alharthi, S.S. Adsorptive scavenging of cationic dyes from aquatic phase by H3PO4 activated Indian jujube (Ziziphus mauritiana) seeds based activated carbon: Isotherm, kinetics, and thermodynamic study. J. Saudi Chem. Soc. 2022, 26, 101417. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Lim, L.B.; Dahri, M.K.; Lim, L.H.; Sarath Bandara, J.M.R. Azolla pinnata: An efficient low cost material for removal of methyl violet 2B by using adsorption method. Waste Biomass Valori. 2015, 6, 547–559. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.; Lim, L.H.; Malik, O.A. Batch adsorption studies of the removal of methyl violet 2B by soya bean waste: Isotherm, kinetics and artificial neural network modelling. Environ. Earth Sci. 2016, 75, 1–14. [Google Scholar] [CrossRef]
- Lim, L.B.; Priyantha, N.; Hei Ing, C.; Khairud Dahri, M.; Tennakoon, D.T.B.; Zehra, T.; Suklueng, M. Artocarpus odoratissimus skin as a potential low-cost biosorbent for the removal of methylene blue and methyl violet 2B. Desalin. Water Treat. 2015, 53, 964–975. [Google Scholar]
- Mehr, H.V.; Saffari, J.; Mohammadi, S.Z.; Shojaei, S. The removal of methyl violet 2B dye using palm kernel activated carbon: Thermodynamic and kinetics model. Int. J. Environ. Sci. Technol. 2020, 17, 1773–1782. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Ahmadi, A.; Bikhabar, G.; Babaei, F.; Ramavandi, B. Impact of ZnO and Fe3O4 magnetic nanoscale on the methyl violet 2B removal efficiency of the activated carbon oak wood. Chemosphere 2022, 286, 131632. [Google Scholar] [CrossRef]
- Wathukarage, A.; Herath, I.; Iqbal, M.C.M.; Vithanage, M. Mechanistic understanding of crystal violet dye sorption by woody biochar: Implications for wastewater treatment. Environ. Geochem. Health 2019, 41, 1647–1661. [Google Scholar] [CrossRef] [PubMed]







| Codes | Variables | Level 1 (−1) | Level 2 (0) | Level 3 (+1) |
|---|---|---|---|---|
| A | MCAC dose (g) | 0.02 | 0.06 | 0.1 |
| B | pH | 4 | 7 | 10 |
| C | Time (min) | 5 | 15 | 25 |
| Run | A: Dose (g) | B: pH | C: Time (Min) | MV 2B Removal (%) |
|---|---|---|---|---|
| 1 | 0.02 | 4 | 15 | 28.2 |
| 2 | 0.1 | 4 | 15 | 56.9 |
| 3 | 0.02 | 10 | 15 | 25.1 |
| 4 | 0.1 | 10 | 15 | 72.6 |
| 5 | 0.02 | 7 | 5 | 11.4 |
| 6 | 0.1 | 7 | 5 | 31.1 |
| 7 | 0.02 | 7 | 25 | 17.5 |
| 8 | 0.1 | 7 | 25 | 59.6 |
| 9 | 0.06 | 4 | 5 | 32.3 |
| 10 | 0.06 | 10 | 5 | 34.2 |
| 11 | 0.06 | 4 | 25 | 48.5 |
| 12 | 0.06 | 10 | 25 | 57.2 |
| 13 | 0.06 | 7 | 15 | 39.2 |
| 14 | 0.06 | 7 | 15 | 34.3 |
| 15 | 0.06 | 7 | 15 | 35.2 |
| 16 | 0.06 | 7 | 15 | 33.5 |
| 17 | 0.06 | 7 | 15 | 37.4 |
| Characteristics | MCAC |
|---|---|
| Surface area (m2/g) | 798.18 |
| Langmuir surface area (m2/g) | 1011 |
| Pore volume (cm3/g) | 0.440 |
| Pore diameter (nm) | 3.67 |
| Coal | Activator | Surface Area (m2/g) | Ref. |
|---|---|---|---|
| MCAC | ZnCl2 | 798.18 | Present study |
| Hongshaquan coal | NaCl | 686 | [29] |
| Zonguldak Kozlu coal | H3PO4 | 636 | [30] |
| Anthracite coal | HNO3 | 677.73 | [31] |
| Shaanxi coal | KOH | 411–1733 | [32] |
| Victorian brown coal | KOH | 687 | [33] |
| Brown coal | NaOH | 585 | [34] |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 3973.23 | 9 | 441.47 | 60.58 | <0.0001 |
| A-Dose | 2380.50 | 1 | 2380.50 | 326.65 | <0.0001 |
| B-pH | 67.28 | 1 | 67.28 | 9.23 | 0.0189 |
| C-Time | 680.81 | 1 | 680.81 | 93.42 | <0.0001 |
| AB | 88.36 | 1 | 88.36 | 12.12 | 0.0102 |
| AC | 125.44 | 1 | 125.44 | 17.21 | 0.0043 |
| BC | 11.56 | 1 | 11.56 | 1.59 | 0.2482 |
| A² | 11.95 | 1 | 11.95 | 1.64 | 0.2411 |
| B² | 553.46 | 1 | 553.46 | 75.95 | <0.0001 |
| C² | 79.13 | 1 | 79.13 | 10.86 | 0.0132 |
| Residual | 51.01 | 7 | 7.29 | ||
| Lack of Fit | 29.06 | 3 | 9.69 | 1.77 | 0.2924 |
| Pure Error | 21.95 | 4 | 5.49 | ||
| Cor Total | 4024.24 | 16 |
| Models | Equations | Parameters |
|---|---|---|
| Pseudo-first order (PFO) | : pseudo-first-order rate constant (1 min) | |
| Pseudo-second order (PSO) | : pseudo-second-order rate constant (g/mg min) | |
| Langmuir | : monolayer capacity (mg/g) KL: Langmuir constant (L/mg) | |
| Freundlich | KF: Freundlich constant (mg/g) (L/mg)1/n n: adsorption intensity | |
| Temkin | : Temkin constant (L/mg) : heat of adsorption (J/mol) |
| Concentration (mg/L) | qe exp. (mg/g) | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|
| qe cal (mg/g) | k1 (1/min) | R2 | R2 | k2 × 10−2 (g/mg min) | qe cal (mg/g) | ||
| 20 | 17.77 | 17.29 | 0.2365 | 0.95 | 0.99 | 2.3550 | 17.94 |
| 40 | 28.08 | 30.51 | 0.1889 | 0.92 | 0.97 | 1.0135 | 31.73 |
| 80 | 43.97 | 42.03 | 0.2135 | 0.92 | 0.97 | 0.8213 | 43.84 |
| 150 | 63.04 | 55.44 | 0.1733 | 0.81 | 0.90 | 0.4494 | 58.54 |
| 250 | 99.47 | 91.26 | 0.0589 | 0.91 | 0.96 | 0.0906 | 98.68 |
| Adsorption Isotherm | Parameter | Value |
|---|---|---|
| Langmuir | qmax (mg/g) | 134.1 |
| Ka (L/mg) | 0.014 | |
| R2 | 0.90 | |
| Freundlich | Kf (mg/g) (L/mg)1/n | 9.21 |
| n | 2.18 | |
| R2 | 0.96 | |
| Temkin | KT (L/mg) | 1.77 |
| bT (J/mol) | 227.01 | |
| R2 | 0.78 |
| Adsorbents | qmax (mg/g) | References |
|---|---|---|
| MCAC | 134.1 | Present study |
| Azolla pinnata | 194.2 | [49] |
| Soya bean waste | 180.7 | [50] |
| Artocarpus odoratissimus (Tarap) skin | 137.3 | [51] |
| Palm kernel activated carbon | 107.3 | [52] |
| Activated carbon oak wood/ZnO/Fe3O4 | 48.59 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, S.A.; Abdulhameed, A.S.; Baharin, S.N.A.; ALOthman, Z.A.; Wilson, L.D.; Jawad, A.H. Coal-Based Activated Carbon via Microwave-Assisted ZnCl2 Activation for Methyl Violet 2B Dye Removal: Optimization, Desirability Function, and Adsorption Mechanism. Minerals 2023, 13, 438. https://doi.org/10.3390/min13030438
Musa SA, Abdulhameed AS, Baharin SNA, ALOthman ZA, Wilson LD, Jawad AH. Coal-Based Activated Carbon via Microwave-Assisted ZnCl2 Activation for Methyl Violet 2B Dye Removal: Optimization, Desirability Function, and Adsorption Mechanism. Minerals. 2023; 13(3):438. https://doi.org/10.3390/min13030438
Chicago/Turabian StyleMusa, Salis A., Ahmed Saud Abdulhameed, Siti Nor Atika Baharin, Zeid A. ALOthman, Lee D. Wilson, and Ali H. Jawad. 2023. "Coal-Based Activated Carbon via Microwave-Assisted ZnCl2 Activation for Methyl Violet 2B Dye Removal: Optimization, Desirability Function, and Adsorption Mechanism" Minerals 13, no. 3: 438. https://doi.org/10.3390/min13030438
APA StyleMusa, S. A., Abdulhameed, A. S., Baharin, S. N. A., ALOthman, Z. A., Wilson, L. D., & Jawad, A. H. (2023). Coal-Based Activated Carbon via Microwave-Assisted ZnCl2 Activation for Methyl Violet 2B Dye Removal: Optimization, Desirability Function, and Adsorption Mechanism. Minerals, 13(3), 438. https://doi.org/10.3390/min13030438










