Magmatic-Hydrothermal Fluid Processes of the Sn-W Granites in the Maniema Province of the Kibara Belt (KIB), Democratic Republic of Congo
Abstract
:1. Introduction


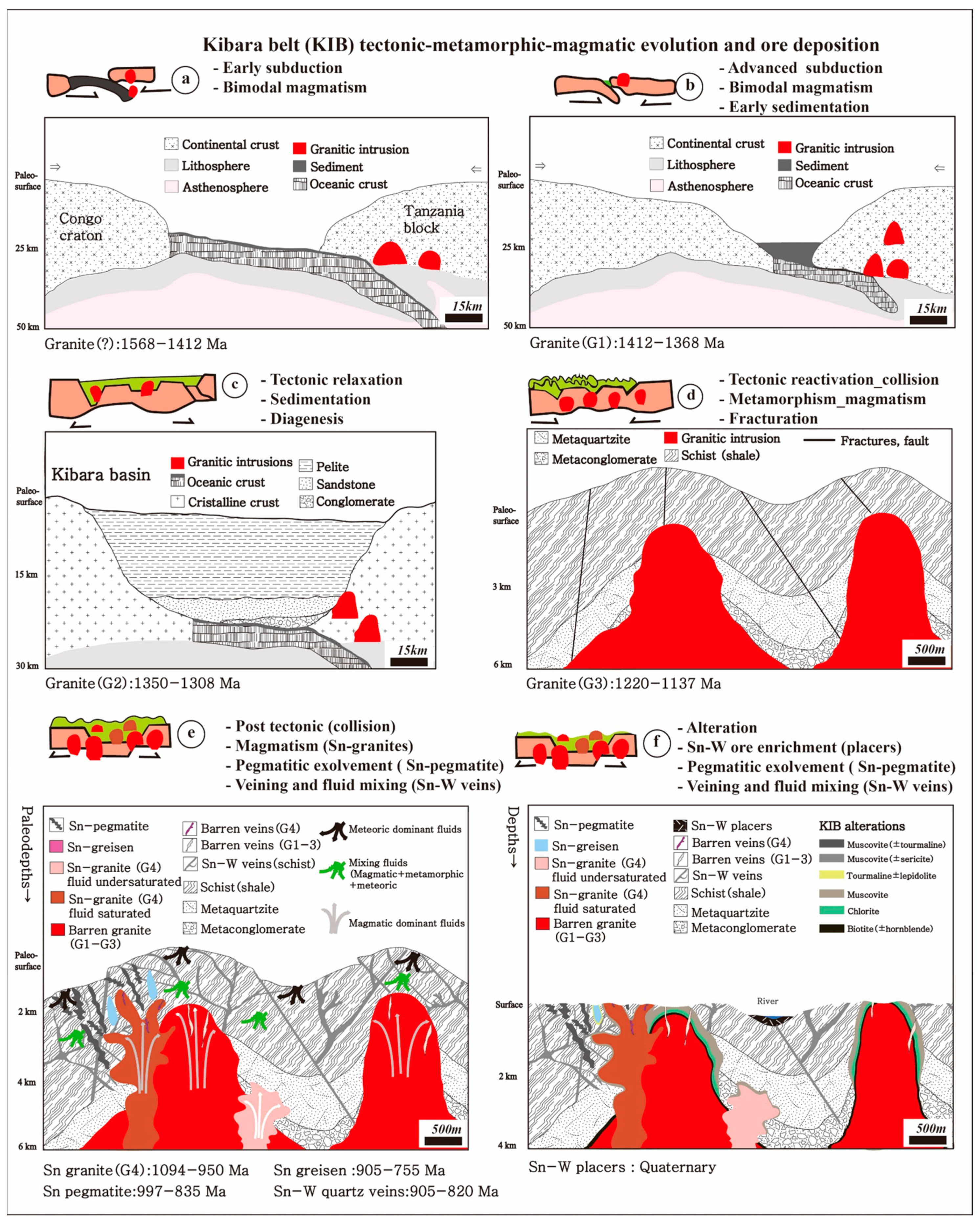
2. Background Geology
2.1. Geological Settings of the KIB
2.2. The Tectono-Magmatism, Rock and Ore Classifications and Chronology
2.3. The Petrography of the Sn(-W) Mineralized Granites
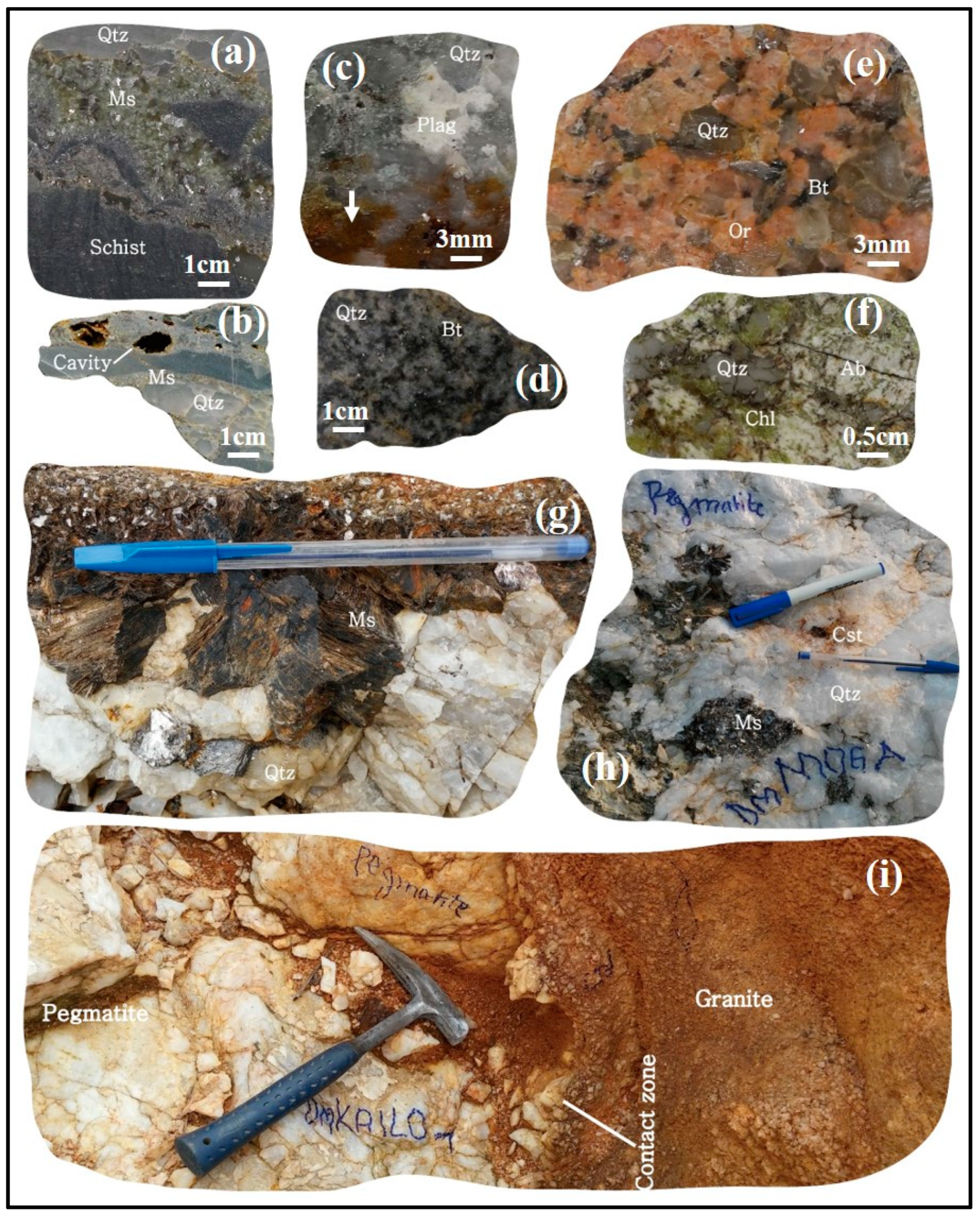
2.4. The Petrography of the Sn-W Quartz Veins
2.5. Alteration Features
3. Materials and Methods
3.1. Sample Preparation for Mineral Geochemistry and Fluid Inclusions
3.2. Microanalyses of Ore Minerals (SEM-CL, EPMA, EDS, and LA-ICP-MS)
| Major and Trace Element Distributions in Textured Cassiterite for Cross-Section 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orogen/Age: | Kibara belt (KIB)/Mesoproterozoic | ||||||||||
| Locality: | Yubuli (DMYUB) | ||||||||||
| Rock type: | Quartz vein | ||||||||||
| Mineral: | Cassiterite | ||||||||||
| Textures: | Oscillatory and Replacement | ||||||||||
| Ore type: | Type I | Type I | Type II | Type I | Type I | Type I | Type I | Type II | Type I | Type II | Type I |
| Samples | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB |
| 1_9_1 | 1_9_2 | 1_9_3 | 1_9_4 | 1_9_5 | 1_9_6 | 1_9_7 | 1_9_8 | 1_9_9 | 1_9_10 | 1_9_11 | |
| Major elements | |||||||||||
| SnO₂ (wt.%) | 99.1 | 98.9 | 98.3 | 97.9 | 98.5 | 99.0 | 98.7 | 97.6 | 98.3 | 99.6 | 98.8 |
| Total (wt.%) | 99.1 | 98.9 | 98.3 | 97.9 | 98.5 | 99.0 | 98.7 | 97.6 | 98.3 | 99.6 | 98.8 |
| Minor elements | |||||||||||
| Li (ppm) | 11.79 | 8.70 | bdl | 1.97 | 2.50 | 1.03 | 1.18 | 2.61 | bdl | 1.83 | 0.62 |
| B (ppm) | 15.74 | 13.53 | 13.49 | 13.63 | 14.48 | 14.12 | 12.44 | 12.17 | 12.06 | 11.44 | 13.19 |
| Na (ppm) | 49.53 | 28.89 | bdl | 15.33 | 32.27 | bdl | bdl | 38.83 | bdl | 47.41 | bdl |
| Mg (ppm) | 1.00 | 0.03 | bdl | bdl | 0.05 | bdl | bdl | 1.00 | bdl | bdl | 1.00 |
| Al (ppm) | 12.55 | 9.56 | 101.34 | 19.12 | 15.48 | 7.71 | 8.54 | 71.50 | 11.84 | 80.78 | 8.37 |
| Si (ppm) | 1353.59 | 1167.90 | 1224.77 | 1424.93 | 1132.53 | 1243.20 | 1153.29 | 1130.62 | 1284.81 | 1045.29 | 1063.83 |
| P (ppm) | 5.00 | bdl | 19.79 | 23.97 | bdl | bdl | 3.00 | 11.00 | 3.00 | 9.00 | bdl |
| K (ppm) | 13.39 | 15.53 | bdl | bdl | 6.98 | bdl | bdl | 18.81 | bdl | 20.00 | bdl |
| Ca (ppm) | 2.00 | bdl | 1.00 | bdl | bdl | 3.00 | bdl | bdl | bdl | 5.00 | 4.00 |
| Sc (ppm) | 1.67 | 2.58 | 4.53 | 3.69 | 4.67 | 2.85 | 2.52 | 4.15 | 2.84 | 3.48 | 2.45 |
| Ti (ppm) | 310.61 | 161.93 | 65.72 | 462.91 | 503.36 | 638.53 | 856.18 | 396.39 | 595.92 | 740.65 | 832.54 |
| V (ppm) | 9.49 | 8.09 | 2.00 | 17.35 | 22.66 | 20.77 | 29.95 | 18.16 | 16.41 | 35.30 | 12.89 |
| Mn (ppm) | 3.00 | 3.13 | 1.39 | 8.54 | 0.83 | 0.71 | 0.62 | 3.68 | 0.86 | 5.69 | 1.27 |
| Fe (ppm) | 91.51 | 145.37 | 1456.51 | 182.58 | 222.50 | 109.42 | 142.63 | 601.87 | 161.67 | 490.51 | 203.06 |
| Co (ppm) | 13.52 | 13.77 | 13.67 | 14.17 | 13.39 | 14.18 | 14.14 | 14.06 | 12.45 | 13.80 | 13.58 |
| Ni (ppm) | 242.73 | 248.12 | 222.86 | 246.34 | 230.96 | 229.10 | 238.69 | 220.71 | 210.97 | 236.77 | 224.47 |
| Cu (ppm) | 0.50 | bdl | 1.00 | bdl | 0.30 | bdl | bdl | 2.06 | bdl | 6.15 | bdl |
| Zn (ppm) | 3.95 | 3.68 | 11.81 | 3.77 | 3.79 | 4.22 | 3.99 | 11.95 | 3.90 | 8.75 | 3.56 |
| Ga (ppm) | 0.31 | 0.43 | 3.99 | 0.43 | 0.67 | 0.32 | 0.31 | 1.57 | 0.45 | 0.43 | 0.31 |
| Ge (ppm) | bdl | bdl | 1.81 | bdl | 0.03 | bdl | bdl | 2.30 | bdl | 2.40 | bdl |
| As (ppm) | 4.70 | 1.53 | 11.52 | 1.47 | 1.51 | 1.66 | 1.52 | 9.29 | 1.64 | 29.47 | 1.52 |
| Rb (ppm) | 0.67 | 1.82 | bdl | bdl | 1.00 | bdl | 0.50 | bdl | 0.40 | bdl | 1.50 |
| Sr (ppm) | 2.06 | 0.76 | 0.21 | 0.80 | 0.70 | 2.18 | bdl | 0.73 | bdl | 0.69 | 0.34 |
| Y (ppm) | bdl | bdl | 0.15 | bdl | bdl | 0.01 | bdl | 0.04 | bdl | 0.14 | bdl |
| Zr (ppm) | 8.23 | 3.51 | 2.07 | 9.01 | 9.86 | 17.98 | 20.61 | 7.01 | 19.40 | 12.61 | 25.80 |
| Nb (ppm) | 2.08 | 0.35 | 0.25 | 0.33 | 0.82 | 1.76 | 0.73 | 1.19 | 0.17 | 2.38 | 107.36 |
| Ag (ppm) | 0.13 | bdl | 1.67 | bdl | 42.00 | bdl | 0.33 | 1.30 | bdl | 1.20 | bdl |
| In (ppm) | 2.12 | 2.76 | 28.19 | 4.60 | 5.89 | 2.95 | 2.85 | 13.98 | 4.06 | 4.31 | 2.33 |
| Sb (ppm) | 0.76 | 0.41 | 1.43 | 0.44 | 0.61 | 0.42 | 0.50 | 0.62 | bdl | 0.39 | 0.45 |
| Cs (ppm) | 2.91 | 2.38 | 0.46 | 0.88 | 0.80 | 0.37 | 0.76 | 1.09 | 0.18 | 0.71 | 0.27 |
| Ba (ppm) | 4.11 | 3.78 | 2.89 | 2.99 | 3.30 | 3.04 | 2.93 | 3.13 | 3.50 | 3.44 | 3.54 |
| La (ppm) | 0.00 | bdl | bdl | bdl | bdl | bdl | bdl | 0.18 | bdl | 0.22 | bdl |
| Ce (ppm) | 0.50 | bdl | bdl | bdl | bdl | bdl | bdl | 0.77 | bdl | 1.72 | bdl |
| Pr (ppm) | 0.06 | 0.06 | 0.06 | 0.06 | bdl | bdl | bdl | 0.09 | 0.07 | 0.11 | 0.05 |
| Nd (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Sm (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Eu (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Gd (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Tb (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Dy (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Ho (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Er (ppm) | bdl | bdl | bdl | bdl | bdl | 0.58 | bdl | bdl | bdl | bdl | bdl |
| Tm (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Yb (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Lu (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Hf (ppm) | 0.16 | bdl | bdl | 0.17 | 0.15 | 0.53 | 0.56 | bdl | 0.49 | 0.20 | 0.70 |
| Ta (ppm) | 0.34 | 0.14 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.02 | 0.05 | 0.05 | 0.65 |
| W (ppm) | 25.79 | 5.94 | 67.44 | 20.16 | 22.56 | 5.88 | 4.77 | 88.34 | 7.56 | 152.52 | 4503.61 |
| Au (ppm) | bdl | bdl | 1.90 | bdl | 0.02 | bdl | bdl | 2.00 | bdl | 3.00 | bdl |
| Tl (ppm) | bdl | bdl | bdl | bdl | bdl | 0.40 | bdl | bdl | bdl | bdl | bdl |
| Pb (ppm) | 1.06 | 1.64 | 0.32 | 0.13 | 0.11 | 0.13 | 0.15 | 0.94 | 0.12 | 2.15 | 0.14 |
| Bi (ppm) | 0.89 | 0.07 | 0.07 | 0.07 | 0.06 | 0.07 | 0.07 | 1.05 | 0.07 | 3.86 | 0.06 |
| Th (ppm) | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 | bdl | 0.05 | 0.06 | 0.05 | 0.26 | bdl |
| U (ppm) | 0.12 | 0.11 | 2.82 | 0.25 | 0.24 | 0.10 | 0.13 | 0.64 | 0.13 | 0.21 | 2.46 |
| ∑REE (ppm) | 0.6 | 0.1 | 0.1 | 0.1 | bdl | 0.6 | bdl | 1.0 | 0.1 | 2.1 | 0.1 |
| Ti/Fe | 3.39 | 1.11 | 0.05 | 2.54 | 2.26 | 5.84 | 6.00 | 0.66 | 3.69 | 1.51 | 4.10 |
| In/Ta | 6.24 | 19.75 | 563.78 | 91.98 | 117.84 | 58.96 | 57.10 | 698.79 | 81.27 | 86.29 | 3.58 |
| Al/Nb | 6.04 | 27.18 | 403.96 | 57.34 | 18.81 | 4.38 | 11.78 | 60.29 | 68.46 | 33.94 | 0.08 |
| V/Fe | 0.10 | 0.06 | n.a. | 0.10 | 0.10 | 0.19 | 0.21 | 0.03 | 0.10 | 0.07 | 0.06 |
3.3. Fluid Inclusion Study (Microthermometry and Laser Raman Spectroscopy)
| LA ICPMS Major and Trace Element Data for Cassiterite Hosted in Granite, and Wolframite in Quartz Vein | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orogen/Age: | Kibara belt (KIB)/Mesoproterozoic | ||||||||||||||
| Locality: | Nakenge (DMNAKE) | Yubuli (DMYUB) | |||||||||||||
| Rock type: | Granite (greisen) | Quartz vein | |||||||||||||
| Mineral: | Cst | Cst | Cst | Cst | Wft | Wft | Wft | Wft | Wft | Wft | Wft | Wft | Wft | Wft | Wft |
| Textures: | Oscillatory and Replacement | Homogenous (No internal texture observed). | |||||||||||||
| Ore type: | Type II | Type II | Type I | Type II | |||||||||||
| Samples | DMNAKE | DMNAKE | DMNAKE | DMNAKE | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB | DMYUB |
| 117_12 | 117_10 | 117_5 | 118_2 | 1_14_1 | 1_14_2 | 1_14_3 | 1_14_4 | 1_14_5 | 1_11_1 | 1_11_2 | 1_11_9 | 1_11_10 | 1_11_11 | 1_11_12 | |
| Major elements | |||||||||||||||
| SnO₂ (wt.%) | 98.5 | 97.6 | 98.3 | 97.9 | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. |
| WO₃ (wt.%) | t.e. | t.e. | t.e. | t.e. | 74.8 | 75.2 | 73.9 | 74.2 | 74.1 | 75.8 | 74.5 | 75.5 | 74.1 | 73.6 | 74.2 |
| FeO (wt.%) | t.e. | t.e. | t.e. | t.e. | 17.6 | 18.4 | 17.9 | 17.6 | 17.6 | 16.7 | 17.8 | 17.2 | 18.5 | 18.4 | 17.0 |
| MnO (wt.%) | t.e. | t.e. | t.e. | t.e. | 6.6 | 5.9 | 6.9 | 6.8 | 6.4 | 6.7 | 6.2 | 6.1 | 6.5 | 6.3 | 6.9 |
| Total (wt.%) | 98.5 | 97.6 | 98.3 | 97.9 | 99.0 | 99.5 | 98.7 | 98.6 | 98.1 | 99.2 | 98.5 | 98.8 | 99.1 | 98.3 | 98.1 |
| Minor elements | |||||||||||||||
| Li (ppm) | 4686.1 | bdl | 19.3 | bdl | 14.1 | 1.2 | 7.9 | 5.7 | 0.0 | bdl | bdl | bdl | bdl | bdl | bdl |
| B (ppm) | 15.3 | 10.7 | 8.0 | 15.91 | 4.7 | 7.3 | 0.0 | 6.6 | 8.8 | bdl | bdl | bdl | bdl | bdl | bdl |
| Na (ppm) | 58.4 | 0.0 | 20.1 | 0.00 | 224.7 | 0.0 | 51.8 | 81.5 | 30.6 | bdl | bdl | bdl | bdl | bdl | bdl |
| Mg (ppm) | 2123.6 | 0.0 | 12.9 | 10.64 | 180.2 | 190.8 | 191.7 | 206.0 | 280.6 | bdl | bdl | 204.0 | bdl | bdl | bdl |
| Al (ppm) | 2140.3 | 10.6 | 23.2 | 14.38 | 10.0 | 11.0 | 9.3 | 10.9 | 13.8 | bdl | bdl | bdl | bdl | bdl | bdl |
| Si (ppm) | 5950.7 | 2331.3 | 3671.1 | 2017.06 | 0.0 | 783.5 | 1241.0 | 975.7 | 796.7 | bdl | bdl | bdl | bdl | bdl | bdl |
| P (ppm) | 0.0 | 0.0 | 242.4 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | bdl | bdl | bdl | bdl | bdl | bdl |
| K (ppm) | 3636.0 | 0.0 | 33.7 | 0.0 | 38.4 | 0.0 | 0.0 | 21.4 | 0.0 | bdl | bdl | bdl | bdl | bdl | bdl |
| Ca (ppm) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | bdl | bdl | bdl | bdl | bdl | bdl |
| Sc (ppm) | 4.8 | 2.5 | 1.9 | 2.9 | 12.8 | 12.3 | 14.8 | 21.0 | 15.6 | bdl | bdl | bdl | bdl | bdl | bdl |
| Ti (ppm) | 1540.0 | 1634.3 | 2031.4 | 1709.6 | 0.0 | 0.0 | 0.0 | 16.9 | 20.6 | bdl | bdl | bdl | bdl | bdl | bdl |
| V (ppm) | 9.0 | 6.0 | 6.4 | 6.1 | 0.0 | 0.0 | 0.0 | 0.5 | 1.2 | bdl | bdl | bdl | bdl | bdl | bdl |
| Mn (ppm) | 126.0 | 6.8 | 11.8 | 10.3 | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. |
| Fe (ppm) | 842.7 | 87.4 | 69.3 | 153.2 | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. |
| Co (ppm) | 13.0 | 11.9 | 12.8 | 13.4 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Ni (ppm) | 176.6 | 173.7 | 180.5 | 210.4 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Cu (ppm) | 2.2 | 0.0 | 0.0 | 0.0 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Zn (ppm) | 24.5 | 9.2 | 15.1 | 12.1 | 37.1 | 38.1 | 44.3 | 40.0 | 45.9 | bdl | bdl | bdl | bdl | bdl | bdl |
| Ga (ppm) | 6.6 | bdl | 0.7 | 0.6 | 0.5 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Ge (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| As (ppm) | bdl | bdl | bdl | 4.6 | bdl | bdl | bdl | 2.5 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Rb (ppm) | 452.4 | bdl | bdl | bdl | 0.6 | bdl | bdl | 0.5 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Sr (ppm) | 0.3 | bdl | 0.1 | 0.1 | 2.3 | bdl | 1.0 | 1.7 | 0.6 | 1.2 | bdl | bdl | bdl | 1.2 | bdl |
| Y (ppm) | 0.0 | 2.8 | 1.1 | 0.0 | 2.1 | 3.4 | 1.1 | 2.3 | 3.9 | bdl | 7.9 | bdl | 2.1 | 3.6 | 9.3 |
| Zr (ppm) | 157.7 | 169.5 | 188.7 | 167.9 | bdl | bdl | bdl | 0.5 | 4.8 | 1.0 | bdl | 1.3 | bdl | bdl | bdl |
| Nb (ppm) | 1085.3 | 1197.7 | 278.6 | 587.1 | 31.1 | 36.5 | 12.0 | 180.1 | 160.9 | 188.8 | 121.5 | 133.5 | 95.8 | 365.1 | 246.9 |
| Ag (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | 1.9 | 1.0 | bdl | 3.2 | bdl | bdl | bdl | 3.6 | 6.9 |
| In (ppm) | bdl | bdl | bdl | bdl | 4.1 | 3.7 | 3.9 | 5.9 | 5.6 | bdl | bdl | bdl | bdl | bdl | bdl |
| Sn (ppm) | m.e. | m.e. | m.e. | m.e. | bdl | bdl | 7.0 | bdl | 26.5 | bdl | bdl | bdl | bdl | bdl | bdl |
| Sb (ppm) | 1.1 | bdl | 1.7 | 0.9 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Cs (ppm) | 73.4 | 0.5 | 0.5 | 0.4 | 2.2 | bdl | 0.5 | 1.3 | 0.3 | bdl | bdl | bdl | bdl | bdl | bdl |
| Ba (ppm) | 26.8 | 10.3 | 9.0 | 5.7 | 1.7 | bdl | 2.0 | 1.8 | 1.1 | bdl | bdl | bdl | bdl | bdl | 23.3 |
| La (ppm) | 0.22 | 0.16 | 0.17 | 0.15 | bdl | bdl | 0.20 | 0.22 | bdl | 0.85 | bdl | bdl | bdl | bdl | 3.79 |
| Ce (ppm) | 0.12 | 0.24 | 0.09 | 0.12 | bdl | bdl | 0.29 | 1.07 | 0.27 | 4.43 | bdl | 1.25 | bdl | bdl | 9.19 |
| Pr (ppm) | 0.18 | 0.21 | 0.15 | 0.12 | bdl | bdl | bdl | 0.09 | 0.49 | 0.37 | bdl | 0.14 | bdl | bdl | 1.43 |
| Nd (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.51 | bdl | 2.01 | bdl | bdl | bdl | bdl | 7.21 |
| Sm (ppm) | bdl | bdl | bdl | bdl | 0.89 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 3.00 |
| Eu (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 1.13 |
| Gd (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Tb (ppm) | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.08 | 0.12 | bdl | bdl | bdl | bdl | bdl | bdl |
| Dy (ppm) | bdl | bdl | bdl | bdl | 0.53 | 0.46 | 0.52 | 0.74 | 1.29 | 1.28 | bdl | bdl | bdl | bdl | 4.17 |
| Ho (ppm) | bdl | 0.09 | bdl | bdl | 0.18 | 0.21 | 0.16 | 0.25 | 0.48 | bdl | 1.07 | 0.23 | bdl | bdl | bdl |
| Er (ppm) | bdl | 0.50 | bdl | bdl | 1.34 | 1.48 | 0.74 | 1.68 | 2.68 | bdl | bdl | 1.28 | 1.33 | bdl | 4.17 |
| Tm (ppm) | bdl | 0.13 | bdl | bdl | 0.45 | 0.47 | 0.31 | 0.49 | 0.76 | bdl | 1.87 | bdl | 0.36 | bdl | 1.04 |
| Yb (ppm) | bdl | 0.61 | 0.45 | bdl | 5.29 | 5.60 | 3.52 | 6.32 | 9.14 | 8.28 | 21.64 | bdl | 4.76 | 8.63 | 12.08 |
| Lu (ppm) | 0.11 | 0.19 | 0.11 | 0.07 | 1.06 | 1.14 | 1.01 | 1.32 | 1.95 | 1.68 | bdl | 1.28 | 1.02 | bdl | 2.39 |
| Hf (ppm) | 16.9 | 15.4 | 20.1 | 19.4 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Ta (ppm) | 3578.0 | 2822.3 | 1528.2 | 4177.9 | 0.9 | 0.9 | 0.9 | 1.2 | 3.9 | 4.7 | bdl | 1.2 | 1.0 | 7.4 | 8.6 |
| W (ppm) | 2.8 | 6.4 | 121.5 | 27.5 | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. |
| Au (ppm) | 5.4 | 4.2 | 1.8 | 3.1 | 1.8 | 0.6 | 0.6 | 0.6 | 0.5 | bdl | bdl | 0.5 | bdl | bdl | bdl |
| Tl (ppm) | 2.5 | bdl | bdl | bdl | 0.1 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Pb (ppm) | 0.3 | bdl | bdl | bdl | bdl | bdl | 9.6 | 33.6 | 3.6 | 93.3 | 49.9 | 63.9 | bdl | bdl | 75.5 |
| Bi (ppm) | bdl | 1.1 | 0.3 | bdl | bdl | bdl | 2.0 | 25.3 | 3.9 | 48.9 | bdl | 13.2 | bdl | bdl | 9.7 |
| Th (ppm) | bdl | 0.7 | 0.1 | bdl | bdl | bdl | bdl | 0.1 | 0.1 | bdl | bdl | bdl | bdl | bdl | bdl |
| U (ppm) | 0.4 | 2.7 | 1.0 | 0.5 | bdl | bdl | 0.2 | 0.1 | 0.3 | bdl | bdl | 0.3 | bdl | bdl | bdl |
| ∑REE (ppm) | 0.6 | 2.1 | 1.0 | 0.5 | 9.7 | 9.4 | 6.7 | 12.7 | 17.2 | 18.9 | 24.6 | 4.2 | 7.5 | 8.6 | 49.6 |
| Ti/Fe | 1.83 | 18.70 | 29.30 | 11.16 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| In/Ta | n.a | n.a | n.a | n.a | 4.47 | 3.97 | 4.30 | 4.70 | 1.42 | n.a | n.a | n.a | n.a | n.a | n.a |
| Al/Nb | 1.97 | 0.01 | 0.08 | 0.02 | 0.32 | 0.30 | 0.78 | 0.06 | 0.09 | n.a | n.a | n.a | n.a | n.a | n.a |
| V/Fe | 0.01 | 0.07 | 0.09 | 0.04 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
4. Results
4.1. Sn-W Minerals and Their Micro-Textures
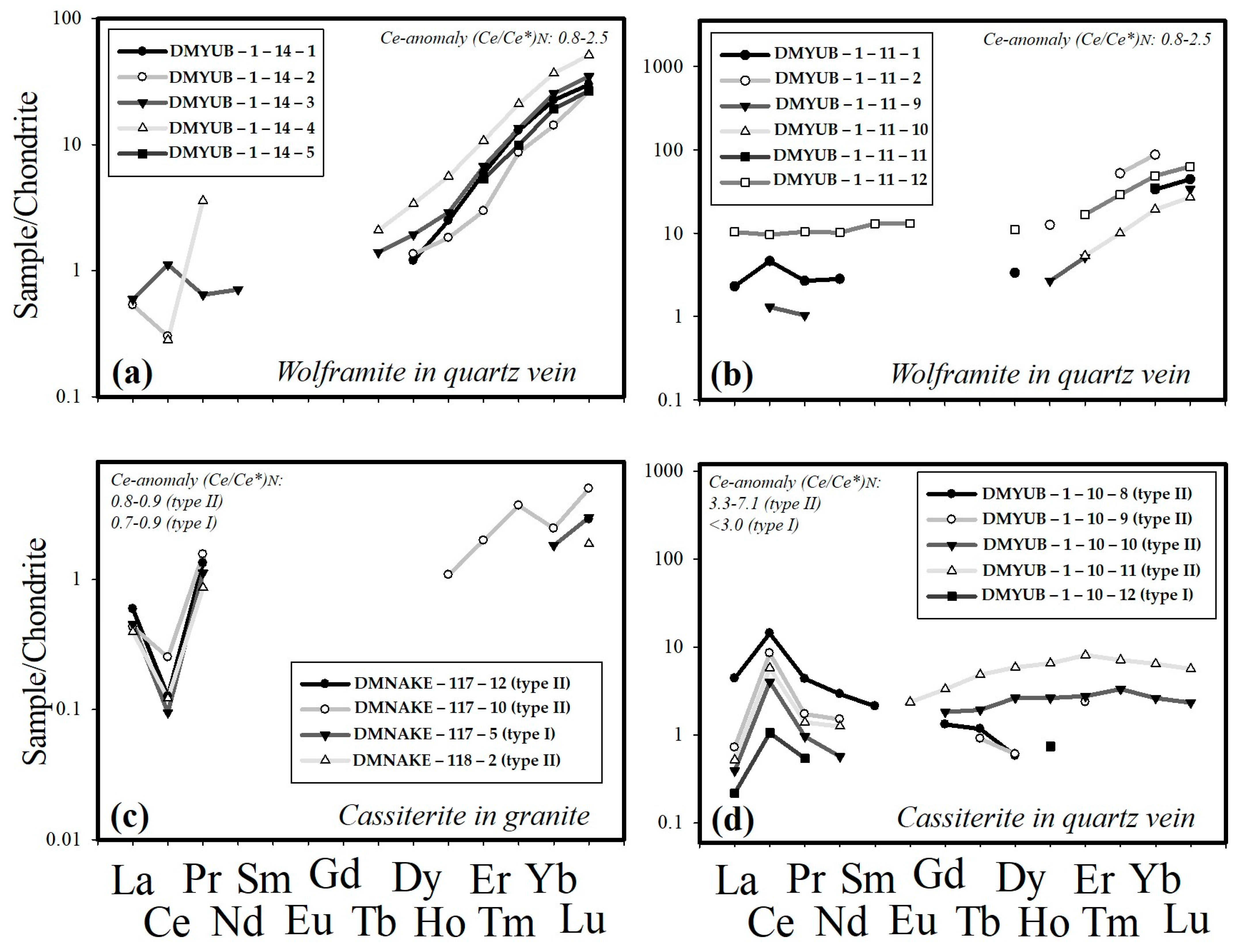
4.2. Compositions of Cassiterite and Wolframite
4.3. The Petrographic Descriptions of the KIB Fluid Inclusions
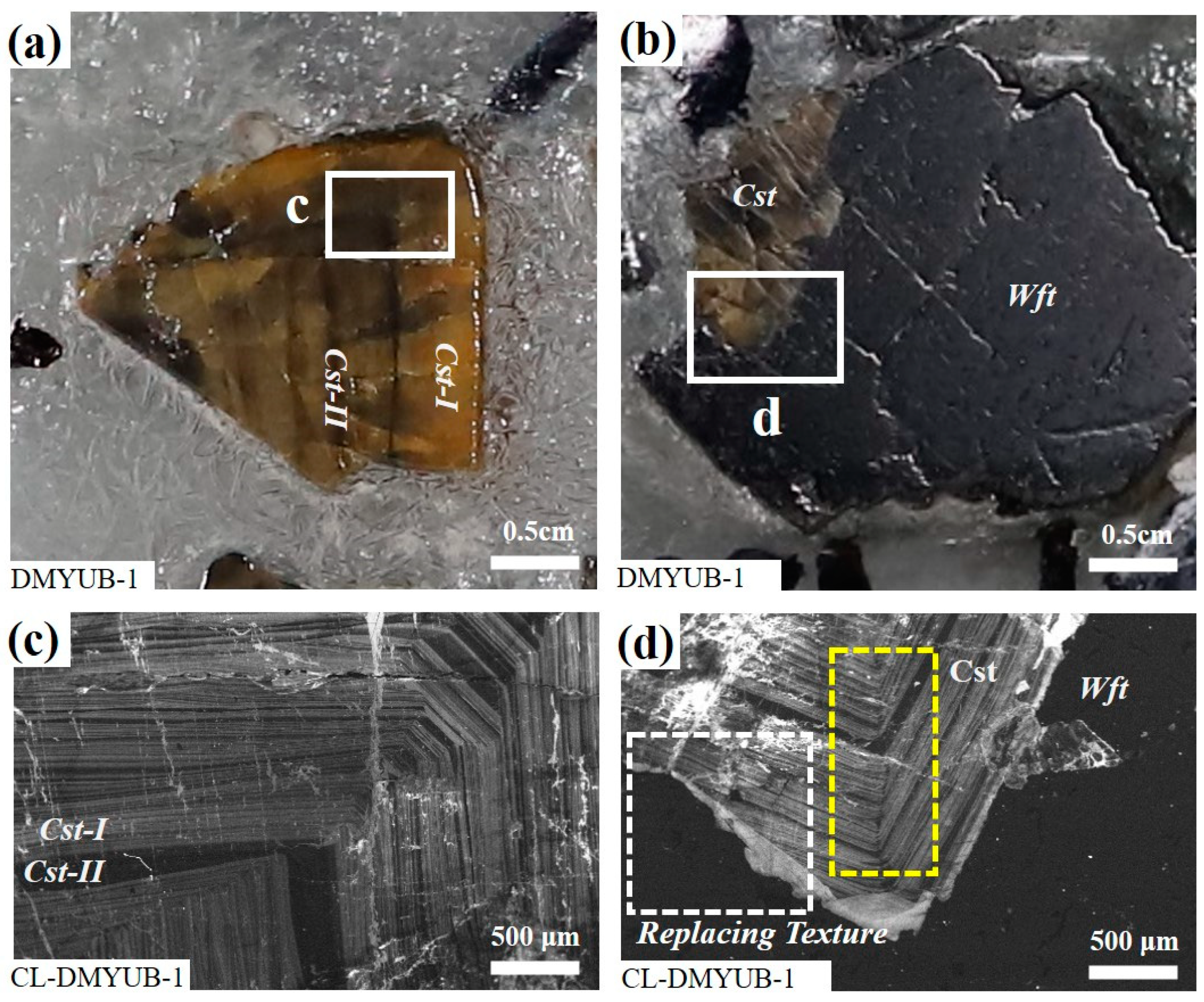
| Summarized Data of LA ICP MS Analyses of Cassiterite and Wolframite | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country: | Democratic Republic of Congo (DRC) | |||||||||||
| Orogenic belt/Age: | Kibara belt (KIB)/Mesoproterozoic | |||||||||||
| Rock type: | Granites and quartz veins | Quartz veins | ||||||||||
| Mineral: | Cassiterite | Wolframite | ||||||||||
| Ore texture: | Oscillatory and replacement | Homogenous | ||||||||||
| Ore type: | Type I (n = 68 spots) | Type II (n = 40 spots) | (n = 13 spots) | |||||||||
| Statistics: | Min. | Max. | Avg. | Std. | Min. | Max. | Avg. | Std. | Min. | Max. | Avg. | Std. |
| Major Oxides | ||||||||||||
| SO₂ (wt.%) | 97.4 | 99.6 | 98.5 | 1.1 | 97.4 | 99.6 | 98.5 | 1.1 | t.e. | t.e. | t.e. | t.e. |
| WO₃ (wt.%) | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | 72.4 | 76.0 | 74.2 | 1.8 |
| FeOt (wt.%) | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | 16.7 | 18.5 | 17.6 | 0.9 |
| MnO (wt.%) | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | t.e. | 5.9 | 6.9 | 6.4 | 0.5 |
| Total | 97.4 | 99.6 | 98.5 | 1.1 | 97.4 | 99.6 | 98.5 | 1.1 | 95.0 | 101.4 | 98.2 | 3.2 |
| Trace elements | ||||||||||||
| Li (ppm) | 8.5 | 67.9 | 2.7 | 8.5 | 15.5 | 686.1 | 22.4 | 109.0 | 1.1 | 14.1 | 2.2 | 4.2 |
| Be (ppm) | bdl | bdl | bdl | bdl | 0.0 | 3.6 | 0.1 | 0.7 | 0.0 | 5.6 | 0.4 | 1.5 |
| B (ppm) | 6.5 | 19.1 | 11.7 | 3.7 | 6.5 | 16.3 | 12.2 | 2.0 | 0.9 | 8.8 | 2.1 | 3.3 |
| Na (ppm) | 9.9 | 94.2 | 9.9 | 16.7 | bdl | 88.1 | 17.3 | 21.9 | 0.5 | 224.7 | 29.9 | 61.5 |
| Mg (ppm) | 0.1 | 20.8 | 0.9 | 3.7 | bdl | 2123.6 | 64.2 | 331.4 | 1.1 | 280.6 | 96.4 | 106.6 |
| Al (ppm) | 1.5 | 487.8 | 51.3 | 109.9 | 5.5 | 2140.3 | 207.0 | 468.0 | 0.9 | 13.8 | 2.6 | 4.9 |
| Si (ppm) | 500.0 | 10,563.4 | 1432.1 | 1266.8 | 850.4 | 6105.8 | 1711.3 | 1223.9 | 108.0 | 1241.0 | 292.1 | 449.9 |
| P (ppm) | 3.8 | 242.4 | 6.7 | 30.1 | 5.1 | 65.5 | 9.8 | 17.2 | bdl | bdl | bdl | bdl |
| K (ppm) | 4.4 | 618.7 | 12.5 | 74.4 | 5.6 | 3636.0 | 160.3 | 655.0 | 1.1 | 38.4 | 4.6 | 11.3 |
| Ca (ppm) | 3.2 | 814.5 | 31.0 | 147.0 | 4.3 | 2233.7 | 73.0 | 356.9 | bdl | bdl | bdl | bdl |
| Sc (ppm) | 0.3 | 14.3 | 3.0 | 3.0 | 1.8 | 15.0 | 3.9 | 3.4 | 3.2 | 21.0 | 5.9 | 7.7 |
| Ti (ppm) | 238.4 | 3077.3 | 1037.5 | 833.4 | 65.7 | 2951.8 | 883.6 | 767.8 | 1.7 | 20.6 | 2.9 | 6.8 |
| V (ppm) | 10.2 | 91.6 | 14.4 | 15.6 | 5.4 | 88.1 | 12.0 | 17.0 | 0.0 | 1.2 | 0.1 | 0.3 |
| Cr (ppm) | 0.2 | 12.0 | 0.4 | 1.7 | 0.5 | 5.5 | 0.2 | 1.0 | bdl | bdl | bdl | bdl |
| Mn (ppm) | 103.8 | 4876.9 | 206.9 | 840.9 | 542.3 | 4692.9 | 281.5 | 979.6 | m.e. | m.e. | m.e. | m.e. |
| Fe (ppm) | 28.2 | 294.0 | 118.5 | 67.5 | 87.4 | 2565.9 | 444.6 | 439.7 | m.e. | m.e. | m.e. | m.e. |
| Co (ppm) | 8.5 | 15.8 | 13.3 | 2.5 | 11.9 | 15.5 | 13.7 | 0.9 | bdl | bdl | bdl | bdl |
| Ni (ppm) | 138.5 | 381.7 | 222.8 | 70.8 | 143.9 | 355.4 | 241.7 | 49.4 | bdl | bdl | bdl | bdl |
| Cu (ppm) | 0.4 | 3.4 | 0.2 | 0.7 | 1.1 | 8.8 | 1.8 | 2.3 | bdl | bdl | bdl | bdl |
| Zn (ppm) | 2.1 | 35.9 | 3.3 | 5.9 | 7.4 | 57.3 | 8.9 | 11.9 | 8.9 | 45.9 | 15.8 | 20.1 |
| Ga (ppm) | 1.8 | 33.2 | 2.3 | 7.0 | 2.0 | 37.4 | 2.9 | 7.1 | bdl | 0.5 | 0.0 | 0.1 |
| Ge (ppm) | bdl | bdl | bdl | bdl | 0.0 | 1.8 | 0.1 | 0.4 | bdl | bdl | bdl | bdl |
| As (ppm) | 1.9 | 30.2 | 2.3 | 4.9 | 8.7 | 63.6 | 11.9 | 15.1 | bdl | 2.5 | 0.2 | 0.7 |
| Rb (ppm) | 0.5 | 8.6 | 0.2 | 1.1 | 9.9 | 452.4 | 12.0 | 70.6 | bdl | 0.6 | 0.1 | 0.2 |
| Sr (ppm) | 0.1 | 6.9 | 0.5 | 0.9 | 0.0 | 2.0 | 0.5 | 0.6 | bdl | 2.3 | 0.6 | 0.7 |
| Y (ppm) | 0.0 | 1.6 | 0.1 | 0.3 | 0.5 | 20.0 | 1.0 | 3.3 | bdl | 9.3 | 2.9 | 2.8 |
| Zr (ppm) | 1.0 | 1324.5 | 137.1 | 283.4 | 2.1 | 1319.2 | 152.5 | 321.0 | bdl | 4.8 | 0.6 | 1.3 |
| Nb (ppm) | 720.2 | 9060.7 | 993.2 | 1551.9 | 425.3 | 7320.2 | 809.9 | 1621.1 | 12.0 | 365.1 | 133.7 | 94.5 |
| Mo (ppm) | 0.1 | 4.7 | 0.2 | 0.7 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Ag (ppm) | 0.0 | 1.7 | 0.1 | 0.3 | 0.0 | 2.7 | 0.1 | 0.5 | 1.1 | 6.9 | 1.3 | 2.0 |
| In (ppm) | 1.3 | 7.1 | 1.6 | 1.9 | 3.8 | 48.5 | 4.4 | 8.9 | 1.5 | 5.9 | 1.8 | 2.3 |
| Sn (ppm) | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | m.e. | 2.1 | 26.5 | 2.6 | 7.1 |
| Sb (ppm) | 0.2 | 1.7 | 0.7 | 0.4 | 0.1 | 1.8 | 0.7 | 0.4 | bdl | bdl | bdl | bdl |
| Cs (ppm) | 0.1 | 2.9 | 0.6 | 0.5 | 1.8 | 73.4 | 2.5 | 11.4 | 0.1 | 2.2 | 0.3 | 0.6 |
| Ba (ppm) | 1.4 | 10.5 | 4.2 | 2.4 | 2.3 | 26.8 | 4.9 | 4.0 | 2.0 | 23.3 | 2.3 | 6.1 |
| La (ppm) | 0.1 | 0.6 | 0.1 | 0.1 | 0.3 | 1.6 | 0.1 | 0.3 | 0.5 | 3.8 | 0.4 | 1.0 |
| Ce (ppm) | 0.5 | 2.4 | 0.2 | 0.4 | 0.8 | 13.8 | 1.5 | 2.7 | 0.1 | 9.2 | 1.3 | 2.6 |
| Pr (ppm) | 0.0 | 0.3 | 0.1 | 0.1 | 0.0 | 0.6 | 0.1 | 0.1 | 0.1 | 1.4 | 0.2 | 0.4 |
| Nd (ppm) | 0.0 | 0.4 | 0.0 | 0.0 | 0.1 | 2.1 | 0.2 | 0.4 | 0.4 | 7.2 | 0.7 | 1.9 |
| Sm (ppm) | bdl | bdl | bdl | bdl | 0.1 | 0.5 | 0.2 | 0.1 | 0.1 | 3.0 | 0.3 | 0.8 |
| Eu (ppm) | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.2 | 0.0 | 0.5 | 2.2 | 0.3 | 0.6 |
| Gd (ppm) | bdl | bdl | bdl | bdl | 0.3 | 1.0 | 0.5 | 0.2 | bdl | bdl | bdl | bdl |
| Tb (ppm) | bdl | bdl | bdl | bdl | 0.3 | 0.3 | 0.3 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 |
| Dy (ppm) | bdl | bdl | bdl | bdl | 2.0 | 2.3 | 0.1 | 0.4 | 2.2 | 4.2 | 2.7 | 1.1 |
| Ho (ppm) | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.6 | 0.2 | 0.1 | 0.1 | 1.1 | 0.2 | 0.3 |
| Er (ppm) | 0.1 | 0.7 | 0.3 | 0.1 | 1.7 | 2.0 | 1.8 | 0.4 | 0.8 | 4.2 | 1.2 | 1.2 |
| Tm (ppm) | 0.0 | 0.3 | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 | 1.9 | 0.4 | 0.5 |
| Yb (ppm) | 1.6 | 7.3 | 2.3 | 1.4 | 2.0 | 7.0 | 2.5 | 1.6 | 3.5 | 21.6 | 6.6 | 5.7 |
| Lu (ppm) | 0.5 | 1.5 | 0.8 | 0.3 | 1.2 | 1.6 | 1.3 | 0.3 | bdl | 2.4 | 1.1 | 0.7 |
| Hf (ppm) | 12.4 | 261.0 | 21.7 | 57.2 | 15.4 | 286.5 | 23.5 | 63.6 | bdl | bdl | bdl | bdl |
| Ta (ppm) | 0.1 | 35,161.3 | 1781.7 | 5941.2 | 1.0 | 34,131.8 | 3201.4 | 7741.0 | 1.0 | 8.6 | 2.8 | 2.6 |
| W (ppm) | 4.8 | 4503.6 | 299.1 | 776.3 | 0.9 | 3210.4 | 342.1 | 726.6 | m.e. | m.e. | m.e. | m.e. |
| Re (ppm) | 0.1 | 0.5 | 0.3 | 0.2 | bdl | bdl | bdl | bdl | 1.0 | 4.3 | 1.6 | 2.0 |
| Au (ppm) | 0.5 | 29.5 | 1.5 | 5.0 | 1.1 | 33.3 | 2.6 | 5.8 | 0.1 | 1.8 | 0.4 | 0.5 |
| Tl (ppm) | 0.0 | 0.9 | 0.5 | 0.1 | 0.0 | 2.5 | 0.1 | 0.4 | bdl | bdl | bdl | bdl |
| Pb (ppm) | 0.1 | 3.8 | 0.3 | 0.7 | 1.1 | 15.3 | 1.6 | 2.7 | 3.6 | 93.3 | 25.3 | 32.7 |
| Bi (ppm) | 0.1 | 102.8 | 1.7 | 12.4 | 0.2 | 13.3 | 1.8 | 2.9 | 2.0 | 48.9 | 8.9 | 13.7 |
| Th (ppm) | 0.1 | 0.5 | 0.3 | 0.1 | bdl | 0.9 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 |
| U (ppm) | 0.1 | 12.2 | 1.4 | 2.7 | 0.1 | 18.5 | 3.6 | 2.4 | 0.1 | 0.3 | 0.2 | 0.1 |

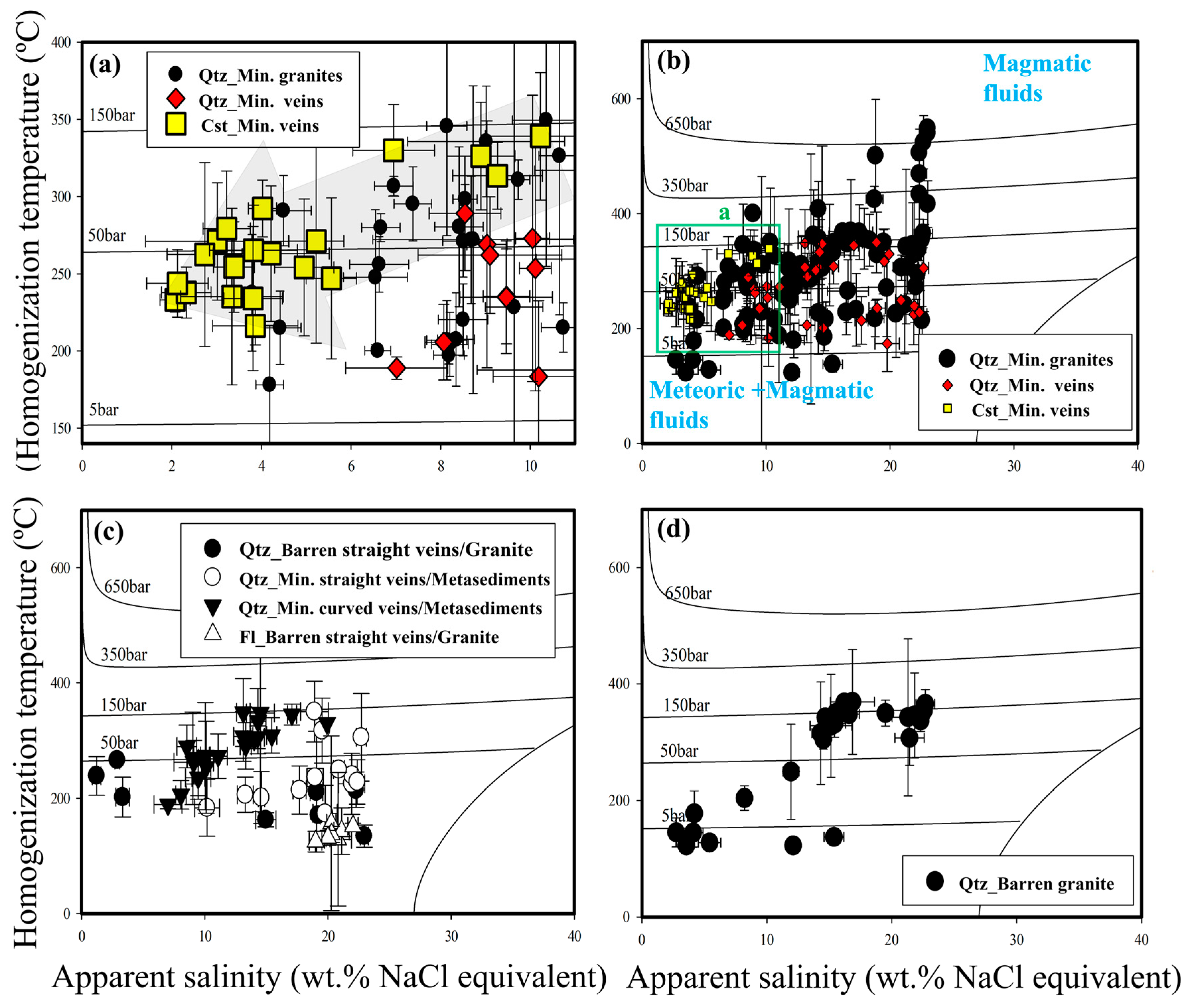
4.4. FI Microthermometry and Calculation of Fluid Density-Pressure-Paleodepths
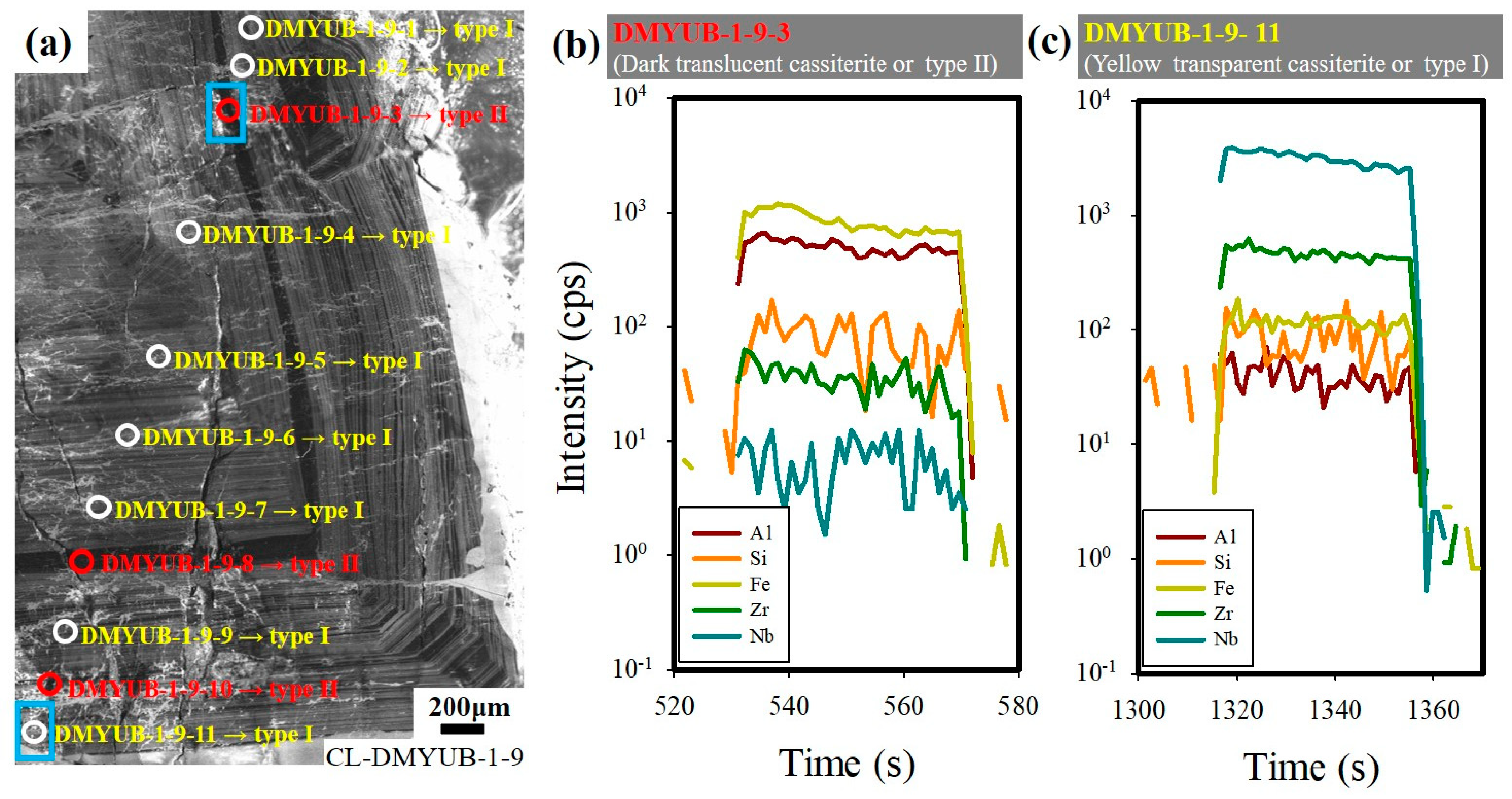
4.5. Raman Spectroscopy on FIs
5. Discussions
5.1. Ore Texture and Geochemistry
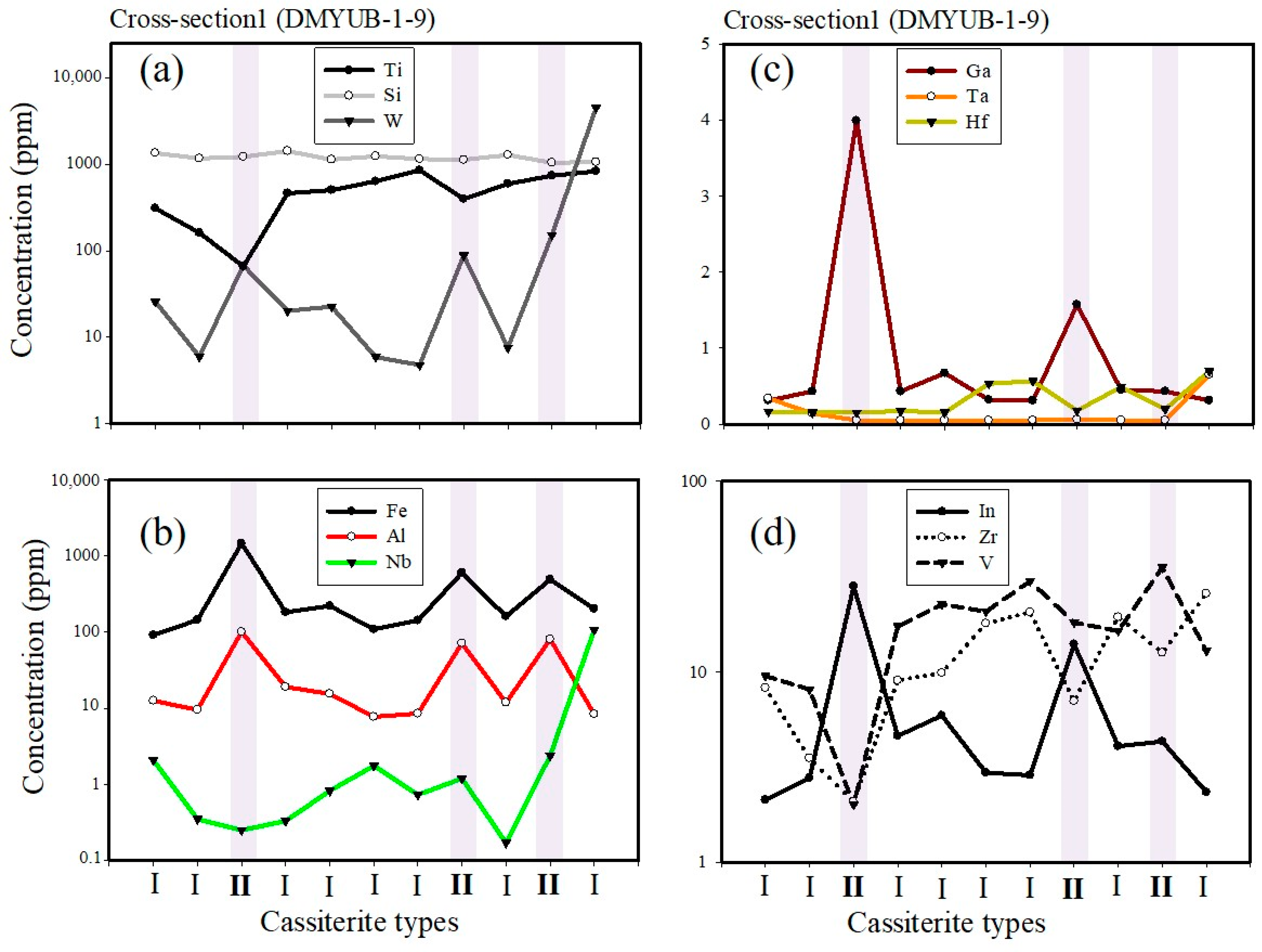
| Kibara Belt Cassiterite and Wolframite Summarized Textures, Trace Element Distributions, and Other Properties | |||
|---|---|---|---|
| Properties | Cassiterite | Wolframite | |
| Cassiterite type I | Cassiterite type II | ||
| Colors under naked eyes and microscope | Yellow transparent | Dark (to dark-reddish) translucent | Homogenous dark |
| Textures under microscope | Growth zone (oscillatory zoning), and replacement | Oscillatory zoning, replacement, and massive | Massive |
| Cathodoluminescence (CL) | Relatively higher luminescent (lighter) | Relatively low luminescent (darker) | Relatively low luminescent (darker) |
| Textures under CL | Growth zone (oscillatory zoning) | Oscillatory zoning and massive | Massive |
| Relative chronology | Earlier (old) | Later (young) | Very later (youngest) |
| Geochemistry (traces) | High Ti, V, Zr, Nb, Ta, Hf, and low Ce anomaly | High Fe, Al, Ga, In, As, U, Pb, Au, total REE, and high Ce anomaly | Al, Zn, Pb, Bi, Nb, high HREE |
| Mineral alteration evolution | Replaced by darker translucent cassiterite (type II) | Replaced by wolframite | No scheelite observed. |
5.2. Hydrothermal Alterations
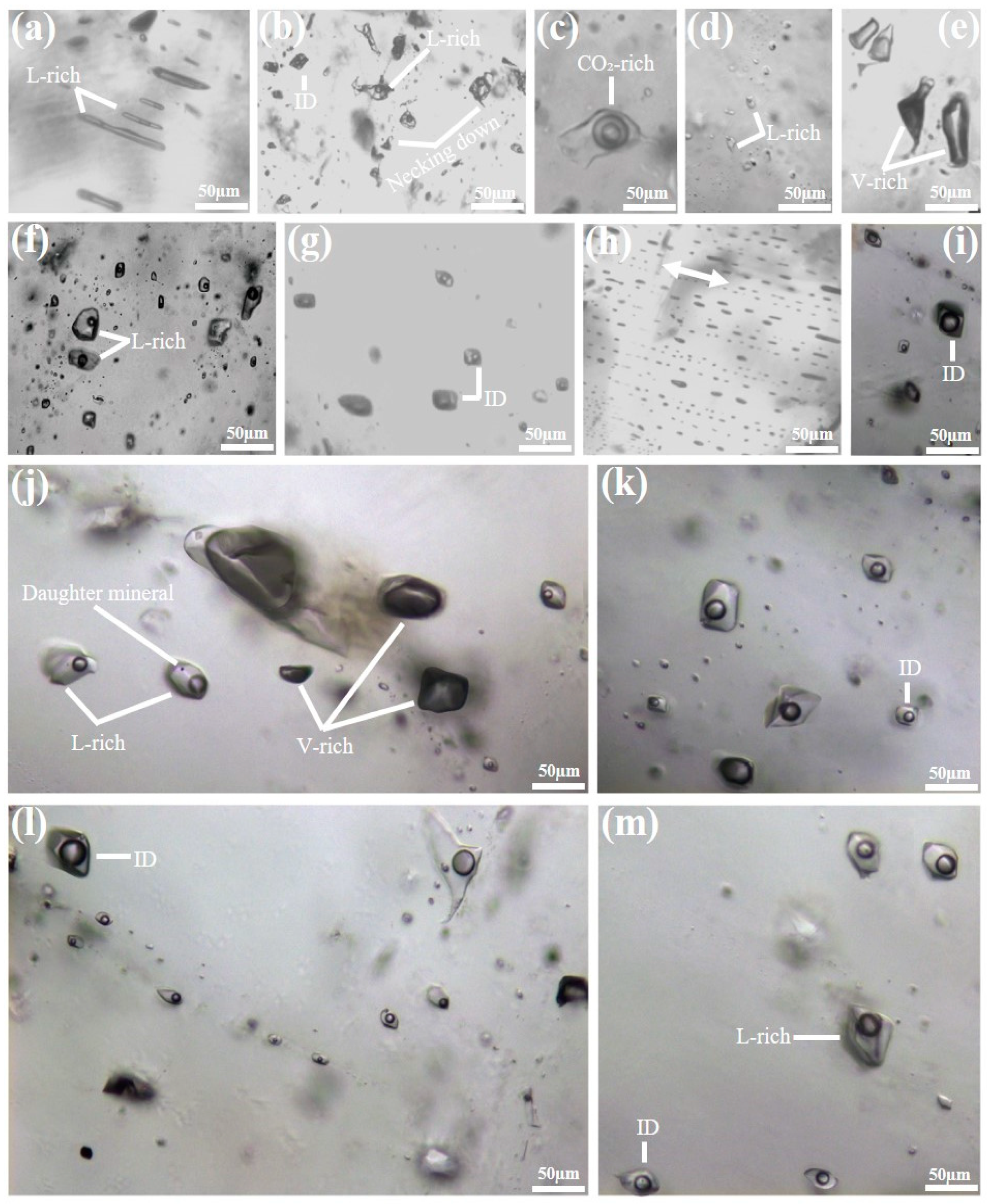
5.3. Fluid Inclusion Constraints
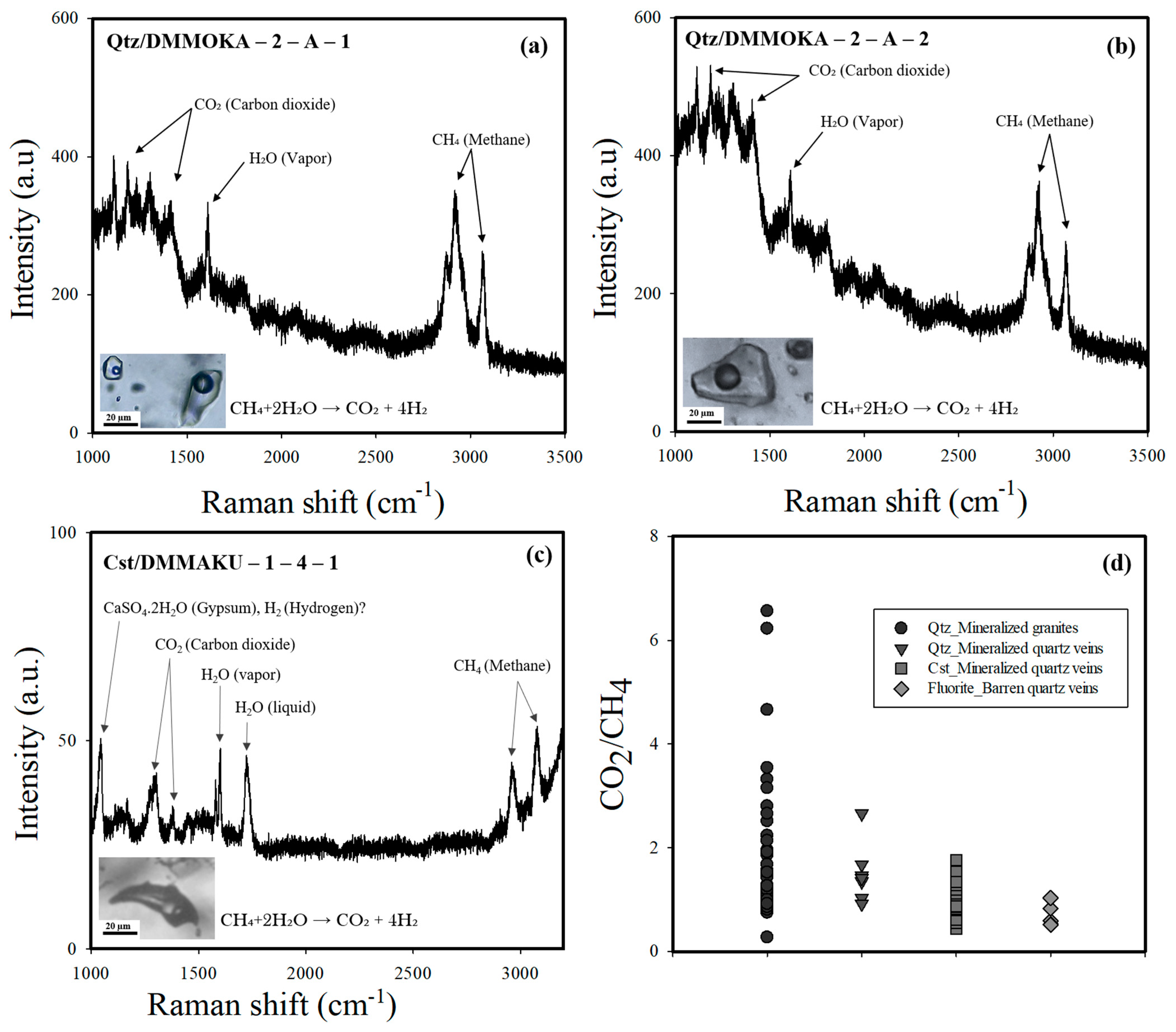
5.4. Sn-W Precipitation Processes
6. Conclusions
| FIAs | Host/Rock | Type | Bubble | Tm_Avg. | Tm_Std. | S_Avg. | S_Std. | Th_Avg. | Th_Std. | P_Avg. | P_Std. | Density |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vol.% | Degree (°C) | Wt.% NaCl equiv. | Degree (°C) | Bar | g/cm3 | |||||||
| DMMOKA-2-A-1 | Qz/M. granite | L-rich | 30 | −8.8 | 0.0 | 12.6 | 0.0 | 293.2 | 0.0 | 72.4 | 0.0 | 0.8577 |
| DMMOKA-2-A-2 | Qz/M. granite | ID | 40 | −9.9 | 0.6 | 13.8 | 0.6 | 362.1 | 15.9 | 173.2 | 13.0 | 0.7666 |
| DMMOKA-2-A-5 | Qz/M. granite | L-rich | 30 | −10.3 | 0.2 | 14.2 | 0.3 | 408.2 | 9.5 | 280.1 | 35.6 | 0.6929 |
| DMMOKA-2-B-1 | Qz/M. granite | L-rich | 20 | −5.2 | 0.1 | 8.2 | 0.2 | 197.4 | 1.2 | 13.9 | 0.9 | 0.9320 |
| DMMOKA-2-B-4 | Qz/M. granite | L-rich | 30 | −7.5 | 0.9 | 11.0 | 1.1 | 187.8 | 16.4 | 11.0 | 2.9 | 0.9646 |
| DMMOKA-2-C-1 | Qz/M. granite | L-rich | 30 | −5.5 | 0.2 | 8.5 | 0.3 | 220.2 | 4.7 | 22.0 | 0.6 | 0.9111 |
| DMMOKA-2-C-4 | Qz/M. granite | ID | 40 | −5.5 | 0.1 | 8.5 | 0.1 | 298.3 | 1.9 | 80.0 | 0.5 | 0.8106 |
| DMMOKA-2-C-6 | Qz/M. granite | L-rich | 30 | −9.7 | 0.2 | 13.6 | 0.2 | 286.3 | 43.5 | 65.0 | 5.0 | 0.8762 |
| DMMOKA-2-7-1-1 | Qz/B. vein | L-rich | 30 | −2.0 | 0.2 | 3.3 | 0.3 | 201.8 | 6.8 | 15.8 | 0.7 | 0.8899 |
| DMMOKA-2-7-3-1 | Qz/B. vein | L-rich | 20 | −1.7 | 0.0 | 2.9 | 0.0 | 265.8 | 0.0 | 50.7 | 2.9 | 0.8036 |
| DMMOGA-1-1-1 | Qz/M. peg. | L-rich | 20 | −6.3 | 0.2 | 9.6 | 0.3 | 228.5 | 47.2 | 25.6 | 14.5 | 0.9106 |
| DMMOGA-1-1-4 | Qz/M. peg. | L-rich | 20 | −7.1 | 0.2 | 10.7 | 0.2 | 326.5 | 20.6 | 115.6 | 27.9 | 0.7883 |
| DMMOGA-1-1-6 | Qz/M. peg. | L-rich | 20 | −8.5 | 1.7 | 12.2 | 2.0 | 179.3 | 6.0 | 9.0 | 0.3 | 0.9817 |
| DMMOKA-2-8-2-1 | Fl/B. vein | L-rich | 30 | −17.8 | 1.1 | 20.8 | 0.8 | 128.6 | 23.1 | 2.2 | 0.5 | 1.0900 |
| DMMOKA-2-8-2-6 | Fl/B. vein | L-rich | 30 | −17.1 | 0.2 | 20.3 | 0.2 | 156.4 | 30.3 | 4.7 | 0.8 | 1.0650 |
| DMMOKA-2-8-2-9 | Fl/B. vein | L-rich | 20 | −18.2 | 0.7 | 21.1 | 0.5 | 142.9 | 8.0 | 3.2 | 0.3 | 1.0820 |
| DMMOKA-2-8-6-1 | Fl/B. vein | L-rich | 20 | −17.1 | 0.1 | 20.3 | 0.1 | 137.4 | 2.8 | 2.8 | 0.1 | 1.0790 |
| DMMOKA-2-8-6-2 | Fl/B. vein | L-rich | 20 | −16.7 | 0.1 | 20.0 | 0.1 | 131.5 | 2.6 | 2.4 | 0.2 | 1.0810 |
| DMMOKA-2-8-6-4 | Fl/B. vein | L-rich | 20 | −15.4 | 0.3 | 19.0 | 0.3 | 124.9 | 3.6 | 2.0 | 0.5 | 1.0780 |
| DMMOKA-2-8-6-8 | Fl/B. vein | L-rich | 20 | −19.5 | 0.3 | 22.0 | 0.2 | 151.4 | 1.1 | 4.0 | 0.8 | 1.0830 |
| DMMOKA-2-8-4-1 | Qz/B. vein | L-rich | 30 | −15.7 | 0.1 | 19.2 | 0.1 | 170.7 | 1.3 | 6.8 | 0.1 | 1.0440 |
| DMMOKA-2-8-4-3 | Qz/B. vein | L-rich | 30 | −11.0 | 0.7 | 15.0 | 0.7 | 162.7 | 2.5 | 5.9 | 0.1 | 1.0170 |
| DMMOKA-2-8-4-5 | Qz/B. vein | L-rich | 10 | −20.9 | 0.3 | 22.9 | 0.2 | 134.4 | 3.8 | 2.5 | 0.2 | 1.1040 |
| DMMOKA-2-8-4-7 | Qz/B. vein | L-rich | 30 | −19.9 | 0.0 | 22.3 | 0.0 | 214.5 | 6.1 | 17.4 | 0.3 | 1.0320 |
| DMMOKA-2-8-4-9 | Qz/B. vein | L-rich | 30 | −15.5 | 0.7 | 19.1 | 0.6 | 211.1 | 20.9 | 16.8 | 1.1 | 1.0070 |
| DMMOKA-2-6-1-1 | Qz/M. granite | L-rich | 20 | −16.4 | 0.3 | 19.7 | 0.2 | 270.4 | 16.8 | 48.4 | 0.8 | 0.9519 |
| DMMOKA-2-6-1-2 | Qz/M. granite | L-rich | 30 | −19.6 | 0.3 | 22.1 | 0.2 | 273.1 | 4.4 | 49.4 | 1.7 | 0.9704 |
| DMMOKA-2-6-2-1 | Qz/M. granite | ID | 40 | −8.1 | 0.7 | 11.8 | 0.9 | 317.1 | 18.2 | 101.4 | 12.4 | 0.8156 |
| DMMOKA-2-6-2-4 | Qz/M. granite | ID | 40 | −10.3 | 0.6 | 14.2 | 0.6 | 302.4 | 23.3 | 81.6 | 8.4 | 0.8610 |
| DMMOKA-2-6-2-6 | Qz/M. granite | ID | 40 | −2.3 | 0.0 | 3.8 | 0.1 | 237.7 | 7.6 | 31.4 | 5.6 | 0.8512 |
| DMYUB-1-6-1 | Qz/M. vein | L-rich | 30 | −9.4 | 0.3 | 13.3 | 0.3 | 205.9 | 6.1 | 16.0 | 3.7 | 0.9645 |
| DMYUB-1-6-4 | Qz/M. vein | L-rich | 20 | −6.8 | 0.6 | 10.2 | 0.7 | 183.4 | 9.8 | 10.1 | 4.9 | 0.9620 |
| DMYUB-1-4-1 | Qz/M. vein | L-rich | 30 | −19.4 | 0.8 | 21.9 | 0.5 | 224.7 | 10.5 | 21.3 | 2.5 | 1.0190 |
| DMNAKE-1-3-1 | Qz/M. greisen | ID | 40 | −9.3 | 0.3 | 13.1 | 0.3 | 348.4 | 11.8 | 148.8 | 15.8 | 0.7815 |
| DMNAKE-1-3-2 | Qz/M. greisen | L-rich | 20 | −5.9 | 0.2 | 9.0 | 0.2 | 269.0 | 16.0 | 51.4 | 3.7 | 0.8569 |
| DMNAKE-1-5-1 | Qz/M. greisen | L-rich | 30 | −5.9 | 0.6 | 9.1 | 0.8 | 262.1 | 7.6 | 46.1 | 4.0 | 0.8659 |
| DMBALE-1-1-1 | Qz/B. granite | ID | 40 | −11.6 | 0.4 | 15.5 | 0.3 | 349.9 | 0.3 | 148.6 | 16.4 | 0.8059 |
| DMBALE-1-1-2 | Qz/B. granite | ID | 40 | −13.0 | 1.0 | 16.8 | 0.9 | 368.9 | 18.0 | 181.7 | 18.2 | 0.7915 |
| DMBALE-1-4-1 | Qz/B. granite | L-rich | 20 | −11.2 | 0.8 | 15.1 | 0.8 | 327.9 | 17.7 | 113.9 | 13.1 | 0.8345 |
| DMBALE-1-4-5 | Qz/B. granite | L-rich | 20 | −10.4 | 0.3 | 14.3 | 0.3 | 315.3 | 17.6 | 97.2 | 7.6 | 0.8442 |
| DMMOKA-2-4-3-1 | Qz/M. granite | L-rich | 30 | −10.8 | 0.1 | 14.7 | 0.0 | 185.3 | 4.8 | 10.1 | 0.8 | 0.9959 |
| DMMOKA-2-4-3-2 | Qz/M. granite | L-rich | 20 | −10.3 | 0.3 | 14.2 | 0.4 | 227.2 | 14.5 | 24.1 | 1.8 | 0.9508 |
| DMMOKA-2-4-3-3 | Qz/M. granite | L-rich | 10 | −19.8 | 0.4 | 22.2 | 0.3 | 295.2 | 5.0 | 68.8 | 6.9 | 0.9465 |
| DMMOKA-2-4-3-5 | Qz/M. granite | L-rich | 30 | −12.5 | 0.2 | 16.5 | 0.2 | 228.0 | 7.7 | 24.0 | 3.4 | 0.9683 |
| DMMOKA-2-4-3-10 | Qz/M. granite | L-rich | 20 | −4.4 | 0.2 | 7.0 | 0.2 | 306.9 | 1.3 | 91.1 | 10.2 | 0.7814 |
| DMMOKA-2-4-3-14 | Qz/M. granite | L-rich | 30 | −20.0 | 0.1 | 22.4 | 0.1 | 433.3 | 12.5 | 329.3 | 34.5 | 0.7654 |
| DMMOKA-2-4-3-16 | Qz/M. granite | L-rich | 10 | −4.1 | 0.2 | 6.6 | 0.3 | 256.1 | 3.6 | 42.4 | 8.9 | 0.8514 |
| DMMOKA-2-4-3-21 | Qz/M. granite | L-rich | 30 | −4.2 | 0.2 | 6.7 | 0.2 | 279.9 | 1.8 | 61.8 | 11.1 | 0.8200 |
| DMMOKA-2-7-7-1 | Qz/B. vein | L-rich | 20 | −0.7 | 0.1 | 1.2 | 0.2 | 238.5 | 6.6 | 32.4 | 3.2 | 0.8268 |
| DMMOKA-2-7-7-3 | Qz/M. granite | L-rich | 20 | −12.7 | 0.9 | 16.6 | 0.8 | 265.2 | 10.9 | 45.8 | 6.1 | 0.9294 |
| DMMOKA-2-2-5-1 | Qz/M. granite | ID | 50 | −20.5 | 0.3 | 22.7 | 0.2 | 524.2 | 9.3 | 651.6 | 48.6 | 0.6872 |
| DMMOKA-2-2-5-3 | Qz/M. granite | ID | 40 | −20.0 | 0.2 | 22.4 | 0.1 | 506.0 | 2.8 | 587.2 | 39.1 | 0.6964 |
| DMMOKA-2-2-1-1 | Qz/M. granite | L-rich | 30 | −21.0 | 0.0 | 23.1 | 0.0 | 416.6 | 8.1 | 280.2 | 22.1 | 0.7953 |
| DMMOKA-2-2-1-4 | Qz/M. granite | ID | 30 | −21.0 | 0.0 | 23.1 | 0.0 | 548.4 | 0.0 | 736.9 | 50.8 | 0.6751 |
| DMMOKA-2-2-1-5 | Qz/M. granite | L-rich | 20 | −15.4 | 0.0 | 19.0 | 0.0 | 328.8 | 0.0 | 111.7 | 11.8 | 0.8721 |
| DMMOKA-2-2-2-1 | Qz/M. granite | ID | 60 | −21.0 | 0.0 | 23.1 | 0.0 | 539.8 | 0.0 | 706.3 | 42.4 | 0.6807 |
| DMMOKA-2-2-2-2 | Qz/M. granite | ID | 40 | −20.0 | 0.4 | 22.4 | 0.3 | 468.9 | 15.7 | 452.3 | 32.6 | 0.7279 |
| DMBAR-1-4-1 | Qz/B. granite | L-rich | 30 | −18.6 | 0.8 | 21.4 | 0.6 | 307.1 | 9.4 | 82.1 | 8.8 | 0.9242 |
| DMBAR-1-4-3 | Qz/B. granite | L-rich | 30 | −20.5 | 0.6 | 22.7 | 0.4 | 365.5 | 4.9 | 165.8 | 12.3 | 0.8615 |
| DMBAR-1-4-6 | Qz/B. granite | L-rich | 40 | −5.3 | 0.2 | 8.2 | 0.2 | 204.3 | 4.2 | 16.1 | 2.6 | 0.9260 |
| DMKAILO-1-2-1 | Qz/B. granite | L-rich | 30 | −12.7 | 0.5 | 16.6 | 0.4 | 347.9 | 2.3 | 143.9 | 9.9 | 0.8204 |
| DMKAILO-1-2-3 | Qz/B. granite | L-rich | 20 | −18.5 | 0.9 | 21.3 | 0.6 | 342.7 | 26.9 | 129.8 | 10.2 | 0.8778 |
| DMKAILO-1-3-1 | Qz/B. granite | L-rich | 30 | −19.2 | 0.2 | 21.8 | 0.2 | 345.7 | 14.6 | 133.8 | 10.9 | 0.8789 |
| DMKAILO-1-3-4 | Qz/B. granite | L-rich | 30 | −20.3 | 0.4 | 22.6 | 0.2 | 354.4 | 4.4 | 146.9 | 13.3 | 0.8752 |
| DMKAILO-1-3-5 | Qz/B. granite | L-rich | 30 | −19.9 | 0.3 | 22.3 | 0.2 | 337.0 | 3.8 | 120.0 | 12.0 | 0.8955 |
| DMNAKE-1-3-1 | Qz/M. greisen | L-rich | 10 | −14.2 | 0.4 | 17.9 | 0.3 | 356.3 | 10.6 | 156.7 | 13.9 | 0.8226 |
| DMNAKE-1-3-2 | Qz/M. greisen | ID | 40 | −15.1 | 0.4 | 18.7 | 0.3 | 425.1 | 4.5 | 315.3 | 10.8 | 0.7292 |
| DMNAKE-1-6-1 | Qz/M. greisen | ID | 60 | −15.3 | 0.2 | 18.8 | 0.1 | 500.8 | 19.6 | 578.1 | 22.8 | 0.6536 |
| DMNAKE-1-4-1 | Qz/M. greisen | L-rich | 20 | −7.7 | 0.1 | 11.5 | 0.2 | 269.8 | 12.6 | 51.3 | 3.9 | 0.8765 |
| DMNAKE-1-4-3 | Qz/M. greisen | L-rich | 20 | −8.1 | 0.0 | 11.8 | 0.1 | 276.3 | 13.9 | 56.6 | 4.1 | 0.8725 |
| DMNAKE-1-4-5 | Qz/M. greisen | L-rich | 20 | −7.7 | 0.5 | 11.3 | 0.6 | 259.6 | 1.3 | 43.5 | 2.0 | 0.8892 |
| DMMOKA-2-8-17-1 | Qz/M. granite | L-rich | 30 | −13.7 | 0.2 | 17.5 | 0.2 | 367.7 | 9.5 | 178.4 | 7.4 | 0.8006 |
| DMMOKA-2-8-17-2 | Qz/M. granite | ID | 40 | −14.7 | 0.2 | 18.3 | 0.1 | 353.8 | 9.1 | 151.7 | 9.0 | 0.8309 |
| DMMOKA-2-8-17-4 | Qz/M. granite | L-rich | 30 | −19.4 | 0.4 | 22.0 | 0.3 | 329.0 | 23.6 | 109.0 | 8.1 | 0.9023 |
| DMMOKA-2-8-13-1 | Qz/M. granite | L-rich | 20 | −8.5 | 0.2 | 12.3 | 0.3 | 271.4 | 9.5 | 52.3 | 2.2 | 0.8829 |
| DMMOKA-2-8-13-4 | Qz/M. granite | L-rich | 30 | −10.9 | 0.2 | 14.8 | 0.2 | 216.5 | 6.1 | 19.5 | 0.8 | 0.9669 |
| DMMOKA-2-8-13-6 | Qz/M. granite | L-rich | 20 | −18.0 | 0.4 | 20.9 | 0.3 | 305.9 | 4.3 | 81.0 | 13.2 | 0.9215 |
| DMMOKA-2-8-13-8 | Qz/M. granite | L-rich | 30 | −20.3 | 0.3 | 22.6 | 0.2 | 214.3 | 4.9 | 20.0 | 2.3 | 1.0270 |
| DMYUB-1-9-1 | Qz/M. vein | L-rich | 30 | −13.9 | 0.3 | 17.7 | 0.3 | 214.1 | 8.3 | 18.1 | 0.9 | 0.9929 |
| DMYUB-1-9-3 | Qz/M. vein | L-rich | 30 | −19.4 | 0.2 | 22.0 | 0.1 | 239.4 | 0.8 | 28.0 | 3.1 | 1.0050 |
| DMYUB-1-9-5 | Qz/M. vein | L-rich | 20 | −15.3 | 0.7 | 18.9 | 0.6 | 349.8 | 10.6 | 144.2 | 11.8 | 0.8421 |
| DMYUB-1-10-3 | Qz/M. vein | L-rich | 30 | −20.5 | 0.3 | 22.7 | 0.2 | 305.2 | 15.2 | 78.9 | 5.5 | 0.9393 |
| DMYUB-1-10-5 | Qz/M. vein | L-rich | 30 | −10.6 | 0.1 | 14.6 | 0.1 | 201.0 | 8.9 | 14.3 | 2.7 | 0.9796 |
| DMYUB-1-10-8 | Qz/M. vein | L-rich | 20 | −20.0 | 0.1 | 22.4 | 0.1 | 228.1 | 7.6 | 22.6 | 2.7 | 1.0190 |
| DMYUB-1-8-1 | Qz/M. vein | L-rich | 30 | −16.4 | 1.0 | 19.8 | 0.8 | 173.7 | 9.7 | 7.3 | 0.5 | 1.0460 |
| DMYUB-1-8-3 | Qz/M. vein | L-rich | 20 | −17.9 | 0.3 | 20.9 | 0.2 | 249.2 | 0.9 | 33.7 | 11.0 | 0.9851 |
| DMBAT-1-2-1 | Qz/M. vein | L-rich | 30 | −9.4 | 0.2 | 13.3 | 0.2 | 290.4 | 8.1 | 69.2 | 15.1 | 0.8678 |
| DMBAT-1-2-2 | Qz/M. vein | L-rich | 30 | −10.3 | 0.3 | 14.3 | 0.3 | 305.2 | 4.0 | 84.8 | 12.6 | 0.8572 |
| DMBAT-1-1-1 | Qz/M. vein | L-rich | 20 | −9.3 | 0.4 | 13.1 | 0.4 | 306.8 | 8.6 | 87.4 | 14.5 | 0.8444 |
| DMBAT-1-1-2 | Qz/M. vein | L-rich | 30 | −4.4 | 0.4 | 7.0 | 0.6 | 189.0 | 1.5 | 11.7 | 1.9 | 0.9318 |
| DMBAT-1-3-1 | Qz/M. vein | L-rich | 35 | −10.6 | 0.5 | 14.5 | 0.6 | 347.4 | 34.1 | 145.5 | 10.5 | 0.7987 |
| DMBAT-1-5-1 | Qz/M. vein | L-rich | 35 | −5.2 | 0.2 | 8.1 | 0.2 | 205.7 | 4.9 | 16.6 | 4.3 | 0.9234 |
| DMBAT-1-5-3 | Qz/M. vein | L-rich | 35 | −6.7 | 0.2 | 10.1 | 0.2 | 273.0 | 18.6 | 54.4 | 4.6 | 0.8611 |
| DMBAT-1-5-5 | Qz/M. vein | L-rich | 35 | −6.2 | 0.2 | 9.5 | 0.3 | 235.4 | 6.2 | 29.1 | 3.3 | 0.9017 |
| DMBAT-1-6-1 | Qz/M. vein | L-rich | 20 | −6.7 | 0.1 | 10.1 | 0.2 | 253.6 | 15.9 | 39.8 | 5.9 | 0.8857 |
| DMBAT-1-6-3 | Qz/M. vein | L-rich | 20 | −10.1 | 0.2 | 14.0 | 0.3 | 301.3 | 0.4 | 80.5 | 13.0 | 0.8604 |
| DMMOKA-2-E-1 | Qz/M. granite | L-rich | 30 | −5.4 | 0.2 | 8.4 | 0.3 | 280.5 | 10.4 | 61.7 | 11.1 | 0.8351 |
| DMMOKA-2-E-3 | Qz/M. granite | L-rich | 30 | −9.0 | 0.2 | 12.8 | 0.2 | 311.1 | 9.7 | 92.9 | 11.9 | 0.8350 |
| DMMOKA-2-E-5 | Qz/M. granite | L-rich | 30 | −5.5 | 0.2 | 8.5 | 0.2 | 271.1 | 4.7 | 53.4 | 10.1 | 0.8493 |
| DMMOKA-2-E-8 | Qz/M. granite | L-rich | 30 | −10.3 | 0.1 | 14.3 | 0.1 | 356.4 | 4.1 | 161.9 | 23.9 | 0.7809 |
| DMMOKA-2-F-1 | Qz/M. granite | L-rich | 30 | −18.3 | 0.1 | 21.2 | 0.1 | 240.9 | 17.0 | 29.0 | 6.3 | 0.9963 |
| DMMOKA-2-F-2 | Qz/M. granite | L-rich | 30 | −15.2 | 0.4 | 18.8 | 0.4 | 217.5 | 6.3 | 19.1 | 6.9 | 0.9989 |
| DMMOKA-2-F-6 | Qz/M. granite | L-rich | 30 | −13.4 | 0.3 | 17.3 | 0.2 | 232.6 | 12.6 | 25.9 | 5.2 | 0.9706 |
| DMMOKA-2-G-1 | Qz/M. granite | ID | 40 | −6.9 | 0.3 | 10.4 | 0.4 | 349.5 | 19.0 | 154.0 | 15.6 | 0.7466 |
| DMMOKA-2-G-3 | Qz/M. granite | L-rich | 30 | −4.1 | 0.2 | 6.5 | 0.4 | 247.8 | 8.7 | 36.9 | 7.7 | 0.8621 |
| DMMOKA-2-G-6 | Qz/M. granite | L-rich | 30 | −9.4 | 0.3 | 13.2 | 0.3 | 285.3 | 4.8 | 64.2 | 9.9 | 0.8745 |
| DMMOKA-2-H-1 | Qz/M. granite | ID | 40 | −5.9 | 0.7 | 9.0 | 0.9 | 335.7 | 7.2 | 131.3 | 19.0 | 0.7556 |
| DMMOKA-2-I-1 | Qz/M. granite | ID | 40 | −5.7 | 1.1 | 8.7 | 1.4 | 272.1 | 19.9 | 54.1 | 8.7 | 0.8504 |
| DMMOKA-2-I-2 | Qz/M. granite | ID | 40 | −5.2 | 0.2 | 8.1 | 0.2 | 345.7 | 14.2 | 149.4 | 11.3 | 0.7259 |
| DMMOKA-2-I-3 | Qz/M. granite | ID | 60 | −5.8 | 0.0 | 9.0 | 0.0 | 400.1 | 0.0 | 269.9 | 30.4 | 0.6267 |
| DMMOKA-2-M-1 | Qz/M. granite | ID | 50 | −2.7 | 0.2 | 4.5 | 0.3 | 291.0 | 4.6 | 73.8 | 14.2 | 0.7805 |
| DMMOKA-2-M-2 | Qz/M. granite | L-rich | 30 | −5.6 | 0.1 | 8.7 | 0.1 | 272.0 | 8.3 | 54.1 | 10.2 | 0.8493 |
| DMMOKA-2-M-3 | Qz/M. granite | L-rich | 30 | −2.7 | 0.2 | 4.4 | 0.2 | 215.1 | 4.8 | 20.5 | 2.1 | 0.8838 |
| DMMOKA-2-M-4 | Qz/M. granite | L-rich | 30 | −17.4 | 0.3 | 20.5 | 0.2 | 225.7 | 15.2 | 22.0 | 3.6 | 1.0060 |
| DMMOKA-2-M-8 | Qz/M. granite | ID | 40 | −8.1 | 0.1 | 11.8 | 0.1 | 305.2 | 5.0 | 86.3 | 13.2 | 0.8331 |
| DMKAILO-1-12-1 | Qz/B. granite | ID | 40 | −11.5 | 0.5 | 15.4 | 0.4 | 331.6 | 4.7 | 119.1 | 15.6 | 0.8322 |
| DMKAILO-1-8-1 | Qz/B. granite | L-rich | 30 | −10.7 | 0.1 | 14.7 | 0.1 | 342.4 | 2.3 | 136.9 | 11.3 | 0.8075 |
| DMKAILO-1-8-3 | Qz/B. granite | ID | 30 | −12.3 | 0.2 | 16.2 | 0.1 | 367.8 | 2.6 | 180.6 | 14.0 | 0.7857 |
| DMKAILO-1-11-1 | Qz/B. granite | L-rich | 20 | −8.2 | 0.3 | 11.9 | 0.3 | 249.2 | 16.3 | 36.4 | 2.8 | 0.9067 |
| DMBAR-1-5-1 | Qz/B. granite | L-rich | 40 | −11.4 | 0.4 | 15.4 | 0.4 | 137.7 | 2.7 | 3.0 | 0.1 | 1.0400 |
| DMBAR-1-5-2 | Qz/B. granite | L-rich | 20 | −2.1 | 0.1 | 3.6 | 0.2 | 123.0 | 2.4 | 2.1 | 0.3 | 0.9653 |
| DMBAR-1-5-4 | Qz/B. granite | L-rich | 30 | −1.6 | 0.1 | 2.7 | 0.2 | 145.5 | 5.0 | 4.1 | 1.5 | 0.9408 |
| DMBAR-1-5-6 | Qz/B. granite | L-rich | 30 | −3.3 | 0.3 | 5.4 | 0.5 | 127.9 | 2.3 | 2.4 | 0.4 | 0.9745 |
| DMBAR-1-5-7 | Qz/B. granite | L-rich | 30 | −8.4 | 0.3 | 12.1 | 0.3 | 123.0 | 2.4 | 2.0 | 0.7 | 1.0270 |
| DMBAR-1-5-11 | Qz/B. granite | L-rich | 30 | −2.5 | 0.1 | 4.2 | 0.2 | 178.1 | 7.7 | 9.3 | 1.1 | 0.9209 |
| DMBAR-1-5-12 | Qz/B. granite | L-rich | 30 | −2.5 | 0.3 | 4.1 | 0.4 | 145.2 | 5.0 | 4.1 | 1.3 | 0.9514 |
| DMBALE-1-8-1 | Qz/B. granite | L-rich | 20 | −16.1 | 0.1 | 19.5 | 0.0 | 349.8 | 4.4 | 143.4 | 14.3 | 0.8490 |
| DMBALE-1-8-3 | Qz/B. granite | L-rich | 20 | −10.6 | 0.1 | 14.5 | 0.2 | 304.8 | 3.4 | 84.2 | 10.2 | 0.8608 |
| DMMOGA-1-2-1 | Qz/M. peg. | L-rich | 30 | −4.7 | 0.2 | 7.4 | 0.2 | 295.3 | 4.8 | 77.2 | 9.2 | 0.8041 |
| DMMOGA-1-2-3 | Qz/M. peg. | L-rich | 20 | −4.1 | 0.1 | 6.6 | 0.1 | 200.2 | 0.2 | 15.0 | 3.4 | 0.9170 |
| DMMOGA-1-2-4 | Qz/M. peg. | L-rich | 20 | −7.2 | 0.2 | 10.7 | 0.2 | 215.2 | 3.2 | 19.7 | 5.0 | 0.9343 |
| DMMOGA-1-3-1 | Qz/M. peg. | L-rich | 30 | −5.2 | 0.2 | 8.1 | 0.2 | 206.9 | 5.1 | 17.0 | 5.1 | 0.9222 |
| DMMOGA-1-4-1 | Qz/M. peg. | L-rich | 20 | −5.4 | 0.2 | 8.3 | 0.2 | 207.5 | 6.1 | 17.2 | 3.1 | 0.9237 |
| DMMOGA-1-4-2 | Qz/M. peg. | ID | 40 | −6.4 | 0.1 | 9.7 | 0.1 | 311.0 | 2.6 | 94.8 | 14.2 | 0.8033 |
| DMBAT-1-16-1 | Qz/M. vein | L-rich | 30 | −10.4 | 0.3 | 14.3 | 0.3 | 333.1 | 11.3 | 122.4 | 10.8 | 0.8183 |
| DMBAT-1-18-1 | Qz/M. vein | L-rich | 30 | −13.2 | 0.2 | 17.1 | 0.2 | 344.7 | 3.7 | 138.0 | 13.8 | 0.8301 |
| DMBAT-1-18-2 | Qz/M. vein | L-rich | 30 | −11.5 | 0.2 | 15.4 | 0.2 | 308.4 | 6.1 | 87.8 | 6.8 | 0.8648 |
| DMBAT-1-18-4 | Qz/M. vein | L-rich | 35 | −5.5 | 0.3 | 8.5 | 0.4 | 289.2 | 7.4 | 70.2 | 8.8 | 0.8240 |
| DMBAT-1-15-1 | Qz/M. vein | L-rich | 30 | −16.6 | 0.0 | 19.9 | 0.0 | 329.7 | 0.0 | 112.1 | 8.9 | 0.8806 |
| DMBAT-1-15-2 | Qz/M. vein | L-rich | 20 | −7.5 | 0.3 | 11.1 | 0.4 | 272.5 | 7.7 | 53.6 | 11.4 | 0.8708 |
| DMBAT-1-15-4 | Qz/M. vein | L-rich | 30 | −6.2 | 0.4 | 9.5 | 0.5 | 234.8 | 7.3 | 28.8 | 4.3 | 0.9024 |
| DMYUB-1-2-1 | Qz/M. vein | L-rich | 30 | −16.1 | 0.1 | 19.5 | 0.1 | 316.8 | 11.3 | 95.1 | 7.6 | 0.8936 |
| DMYUB-1-2-2 | Qz/M. vein | L-rich | 30 | −15.4 | 0.4 | 19.0 | 0.3 | 235.6 | 3.1 | 27.0 | 6.3 | 0.9822 |
| DMNAKE-1-1 | Cst/M. greisen | L-rich | 30 | −1.8 | 0.5 | 3.0 | 0.8 | 271.0 | 19.0 | 56.8 | 17.2 | 0.7960 |
| DMNAKE-1-2 | Cst/M. greisen | L-rich | 30 | −1.6 | 0.3 | 2.7 | 0.4 | 262.5 | 29.8 | 52.1 | 20.2 | 0.8035 |
| DMNAKE-1-3 | Cst/M. greisen | ID | 40 | −5.8 | 0.3 | 8.9 | 0.4 | 326.2 | 17.4 | 118.7 | 27.7 | 0.7687 |
| DMNAKE-1-4 | Cst/M. greisen | L-rich | 20 | −1.2 | 0.1 | 2.1 | 0.2 | 233.2 | 4.0 | 29.3 | 2.1 | 0.8414 |
| DMNAKE-1-5 | Cst/M. greisen | L-rich | 20 | −1.4 | 0.2 | 2.3 | 0.3 | 237.9 | 8.3 | 32.1 | 4.8 | 0.8373 |
| DMNAKE-1-6 | Cst/M. greisen | L-rich | 30 | −2.3 | 0.3 | 3.9 | 0.5 | 216.3 | 6.2 | 21.2 | 2.5 | 0.8772 |
| DMNAKE-1-7 | Cst/M. greisen | L-rich | 30 | −1.2 | 0.2 | 2.1 | 0.3 | 243.8 | 10.9 | 35.8 | 6.8 | 0.8275 |
| DMNAKE-1-8 | Cst/M. greisen | L-rich | 20 | −2.0 | 0.4 | 3.3 | 0.6 | 235.2 | 28.6 | 33.5 | 19.2 | 0.8469 |
| DMMAKU-1-4-9 | Cst/M. vein | L-rich | 30 | −3.2 | 0.2 | 5.2 | 0.3 | 271.2 | 33.1 | 60.6 | 35.6 | 0.8143 |
| DMMAKU-1-4-10 | Cst/M. vein | L-rich | 20 | −6.8 | 0.5 | 10.2 | 0.6 | 339.1 | 20.6 | 138.8 | 32.4 | 0.7622 |
| DMMAKU-1-4-11 | Cst/M. vein | L-rich | 30 | −3.4 | 0.2 | 5.6 | 0.3 | 247.1 | 26.1 | 39.6 | 19.3 | 0.8522 |
| DMBAT-1-2-12 | Cst/M. vein | L-rich | 30 | −3.0 | 0.2 | 5.0 | 0.3 | 254.1 | 22.2 | 43.6 | 15.0 | 0.8380 |
| DMYUB-1B-13 | Cst/M. vein | ID | 40 | −4.4 | 0.3 | 6.9 | 0.5 | 330.0 | 14.8 | 125.3 | 23.4 | 0.7399 |
| DMYUB-1B-14 | Cst/M. vein | L-rich | 30 | −2.5 | 0.4 | 4.2 | 0.6 | 263.1 | 21.9 | 50.4 | 16.5 | 0.8190 |
| DMYUB-1B-15 | Cst/M. vein | L-rich | 20 | −2.0 | 0.1 | 3.4 | 0.2 | 254.4 | 14.7 | 43.0 | 10.6 | 0.8240 |
| DMYUB-1B-16 | Cst/M. vein | ID | 50 | −1.9 | 0.2 | 3.2 | 0.3 | 279.3 | 18.7 | 64.3 | 17.5 | 0.7849 |
| DMYUB-1C-17 | Cst/M. vein | L-rich | 40 | −6.1 | 0.4 | 9.3 | 0.5 | 313.5 | 10.8 | 99.1 | 14.4 | 0.7921 |
| DMNAKE-1-2-18 | Cst/M. greisen | ID | 40 | −2.4 | 0.1 | 4.0 | 0.2 | 292.3 | 9.2 | 75.9 | 10.0 | 0.7731 |
| DMNAKE-1-2-19 | Cst/M. greisen | L-rich | 30 | −2.3 | 0.3 | 3.8 | 0.5 | 265.2 | 14.7 | 51.0 | 10.7 | 0.8128 |
| DMNAKE-1-2-20 | Cst/M. greisen | L-rich | 30 | −2.3 | 0.2 | 3.8 | 0.3 | 234.0 | 23.4 | 31.4 | 12.1 | 0.8541 |
| DMNAKE-1-2-21 | Cst/M. greisen | ID | 50 | −4.2 | 0.6 | 6.7 | 0.6 | 325 | 26.3 | 122.8 | 28.5 | 0.7398 |
| DMNAKE-1-2-22 | Cst/M. greisen | ID | 40 | −5.7 | 0.3 | 8.8 | 0.3 | 320.7 | 15.9 | 116.3 | 38.5 | 0.7688 |
| DMNAKE-1-2-23 | Cst/M. greisen | L-rich | 30 | −2.3 | 0.4 | 3.9 | 0.5 | 241 | 12.4 | 33.5 | 18.5 | 0.8543 |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cacho, A.; Melgarejo, J.C.; Camprubi, A.; Torro, L.; Castillo-Oliver, M.; Torres, B.; Artiaga, D.; Tauler, E.; Martinez, A.; Campeny, M.; et al. Mineralogy and Distribution of Critical Elements in the Sn-W-Pb-Ag-Zn Huanuni Deposit, Bolivia. Minerals 2019, 9, 753. [Google Scholar] [CrossRef] [Green Version]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef] [Green Version]
- Černý, P.; Ercit, T.S. Some recent advances in the mineralogy and geochemistry of Nb and Ta in rare-element granitic pegmatites. Bull. Minéralogie 1985, 108, 499–532. [Google Scholar] [CrossRef]
- Che, X.D.; Linnen, R.L.; Wang, R.C.; Aseri, A.; Thibault, Y. Tungsten solubility in evolved granitic melts: An evaluation of magmatic wolframite. Geochim. Cosmochim. Acta 2013, 106, 84–98. [Google Scholar] [CrossRef]
- Dill, H.G. Pegmatites and aplites: Their genetic and applied ore geology. Ore Geol. Rev. 2015, 69, 417–561. [Google Scholar] [CrossRef]
- Hulsbosch, N. Nb-Ta-Sn-W Distribution in Granite-related Ore Systems: Fractionation mechanisms and examples from the Karagwe-Ankole Belt of Central Africa. In Ore Deposits; Geophysical Monograph Series; John Wiley and Sons: Hoboken, NJ, USA, 2019; Volume 242, pp. 75–107. [Google Scholar]
- Tchunte, P.M.F.; Tchameni, R.; André-Mayer, A.S.; Dakoure, H.S.; Turlin, F.; Poujol, M.; Nomo, E.N.; Fouotsa, A.N.S.; Rouer, O. Evidence for Nb-Ta Occurrences in the Syn-Tectonic Pan-African Mayo Salah Leucogranite (Northern Cameroon): Constraints from Nb-Ta Oxide Mineralogy, Geochemistry and U-Pb LA-ICP-MS Geochronology on Columbite and Monazite. Minerals 2018, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Varlamoff, N. Central and West African Rare-Metal Granitic Pegmatites, related aplites, quartz veins and mineral deposits. Miner. Depos. 1972, 7, 202–216. [Google Scholar] [CrossRef]
- Chen, Y.; Song, S.; Niu, Y.; Wei, C. Melting of continental crust during subduction initiation: A case study from the Chaidanuo peraluminous granite in the North Qilian suture zone. Geochim. Cosmochim. Acta 2014, 132, 311–336. [Google Scholar] [CrossRef]
- Debruyne, D.; Hulsbosch, N.; Wilderode, J.V.; Balcaen, L.; Vanhaecke, F.A.; Muchez, P. Regional geodynamic context for the Mesoproterozoic Kibara Belt (KIB) and the Karagwe-Ankole Belt: Evidence from geochemistry and isotopes in the KIB. Precambrian Res. 2015, 264, 82–97. [Google Scholar] [CrossRef]
- Kemp, A.I.S.; Hawkesworth, C.J.; Foster, G.L.; Paterson, B.A.; Woodhead, J.D.; Hergt, J.M.; Gray, C.M.; Whitehouse, M.J. Magmatic and Crustal Differentiation History of Granitic Rocks from Hf-O Isotopes in Zircon. Am. Assoc. Adv. Sci. 2007, 315, 980–983. [Google Scholar] [CrossRef]
- Sylvester, P.J. Post-collisional strongly peraluminous granites. Lithos 1998, 45, 29–44. [Google Scholar] [CrossRef]
- Clemens, J.; Watkins, J. The fluid regime of high-temperature metamorphism during granitoid magma genesis. Contrib. Mineral. Petrol. 2001, 140, 600–606. [Google Scholar] [CrossRef]
- Van Daele, J.; Hulsbosch, N.; Dewaele, S.; Boiron, M.C.; Piessens, K.; Boyce, A.; Muchez, P. Mixing of magmatic-hydrothermal and metamorphic fluids and the origin of peribatholitic Sn vein-type deposits in Rwanda. Ore Geol. Rev. 2018, 101, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, C.A. The chemistry of hydrothermal tin (-tungsten) ore deposition. Econ. Geol. 1990, 90, 705–729. [Google Scholar] [CrossRef]
- Audetat, A.; Gunther, D.; Heinrich, C.A. Formation of a magmatic-hydrothermal ore deposit: Insights with LA-ICP-MS analysis of fluid inclusions. Science 1998, 279, 2091–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.J.; Pirajno, F.; Qi, J.P. Origin of Gold Metallogeny and Sources of Ore-Forming Fluids, Jiaodong Province, Eastern China. Int. Geol. Rev. 2005, 47, 530–549. [Google Scholar] [CrossRef]
- Baker, T.; Pollard, P.J.; Mustard, R.; Mark, G.; Graham, J. A comparison of granite-related tin, tungsten, and gold-bismuth deposits: Implications for exploration. SEG Discov. 2005, 61, 5–17. [Google Scholar] [CrossRef]
- Audetat, A.A.; Edmonds, M. Magmatic-Hydrothermal Fluids. Elements 2020, 16, 401–406. [Google Scholar] [CrossRef]
- Lerouge, C.; Gloaguen, E.; Wille, G.; Bailly, L. Distribution of In and other rare metals in cassiterite and associated minerals in Sn ± W ore deposits of the western Variscan Belt. Eur. J. Mineral. 2017, 29, 739–753. [Google Scholar] [CrossRef] [Green Version]
- Serranti, S.; Ferrini, V.; Masi, U.; Cabri, L.J. Trace element distribution in cassiterite and sulfides from rubane and massive ores of the corvo deposit, Portugal. Can. Mineral. 2002, 40, 815–835. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.H.; Audetat, A. Magmatic-Hydrothermal Evolution of the Barren Huangshan Pluton, Anhui Province, China: A Melt and Fluid Inclusion Study. Econ. Geol. 2018, 113, 803–824. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, R.-Q.; Gao, J.-F.; Lu, J.-J.; Wu, J.-W. In-situ LA-ICP-MS trace element analyses of scheelite and wolframite: Constraints on the genesis of veinlet-disseminated and vein-type tungsten deposits, South China. Ore Geol. Rev. 2018, 99, 166–179. [Google Scholar] [CrossRef]
- Wille, G.; Lerouge, C.; Schmidt, U. A multimodal microcharacterisation of trace-element zonation and crystallographic orientation in natural cassiterite by combining cathodoluminescence, EBSD, EPMA and contribution of confocal Raman-in-SEM imaging. J. Microsc. 2018, 270, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Hagemann, S.; Fiorentini, M.; Roberts, M.P. Sn-W-Critical Metals & Associated Magmatic Systems, the chemical variability of cassiterite microstructures. In Proceedings of the International Geological Conference, Tinaroo, QLD, Australia, 24–28 June 2019; pp. 1–121. [Google Scholar]
- Cheng, Y.; Spandler, C.; Kemp, A.; Mao, J.; Rusk, B.; Hu, Y.; Blake, K. Controls on cassiterite (SnO2) crystallization: Evidence from cathodoluminescence, trace-element chemistry, and geochronology at the Gejiu Tin District. Am. Mineral. 2019, 104, 118–129. [Google Scholar] [CrossRef]
- Farmer, C.B.; Searl, A.; Halls, C. Cathodoluminescence and growth of cassiterite the composite lodes at South Crofty Mine, Cornwall, England. Mineral. Soc. 1991, 55, 447–458. [Google Scholar] [CrossRef]
- Nambaje, C.; Eggins, S.M.; Yaxley, G.M.; Sajeev, K. Micro-characterisation of cassiterite by geology, texture and zonation: A case study of the Karagwe Ankole Belt, Rwanda. Ore Geol. Rev. 2020, 124, 103609. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Steele-MacInnis, M.; Bodnar, R.J. Synthetic fluid inclusions XIX: Experimental determination of the vapor-saturated liquidus of the system H2O-NaCl-FeCl2. Geochim. Cosmochim. Acta 2015, 148, 34–49. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Steele-MacInnis, M.; Weis, P.; Driesner, T.; Bodnar, R.J. Salt precipitation in magmatic-hydrothermal systems associated with upper crustal plutons. Geology 2015, 43, 1063–1066. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, Y.; Lee, T.; Guillong, M. Periodically Released Magmatic Fluids Create a Texture of Unidirectional Solidification (UST) in Ore-Forming Granite: A Fluid and Melt Inclusion Study of W-Mo Forming Sannae-Eonyang Granite, Korea. Minerals 2021, 11, 888. [Google Scholar] [CrossRef]
- Seo, J.H.; Yoo, B.C.; Villa, I.M.; Lee, J.H.; Lee, T.; Kim, C.; Moon, K.J. Magmatic–hydrothermal processes in Sangdong W–Mo deposit, Korea: Study of fluid inclusions and 39Ar–40Ar geochronology. Ore Geol. Rev. 2017, 91, 316–334. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Bakker, R.J.a.; Diamond, L.W. Determination of the composition and molar volume of H2O-CO2 fluid inclusions by microthermometry. Geochim. Cosmochim. Acta 2000, 64, 1753–1764. [Google Scholar] [CrossRef]
- Diamond, L.W. Stability of CO2 clathrate hydrate + CO2, liquid + CO2 vapour + aqueous KCI-NaCl solutions: Experimental determination and application to salinity estimates of fluid inclusions. Geochim. Cosmochim. Acta 1992, 56, 273–280. [Google Scholar] [CrossRef]
- Diamond, L.W. Review of the systematics of CO2–H2O fluid inclusions. Lithos 2001, 55, 69–99. [Google Scholar] [CrossRef]
- Audetat, A. Quantitative analysis of melt and fluid inclusions by LA-ICP-MS: Practical aspects and selected results. Acta Petrol. Sin. 2000, 16, 715. [Google Scholar]
- Audétat, A. The Metal Content of Magmatic-Hydrothermal Fluids and Its Relationship to Mineralization Potential. Econ. Geol. 2019, 114, 1033–1056. [Google Scholar] [CrossRef]
- Audetat, A.; Gunther, D.; Heinrich, C.A. Causes for large-scale metal zonation around mineralized plutons: Fluid inclusion LA-ICP-MS evidence from the Mole Granite, Australia. Econ. Geol. Bull. Soc. 2000, 95, 1563–1581. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, C.A.; Gunther, D.; Audetat, A.; Ulrich, T.; Frischknecht, R. Metal fractionation between magmatic brine and vapor, determined by microanalysis of fluid inclusions. Geology 1999, 27, 755–758. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Pettke, T.; Halter, W.E.; Aigner-Torres, M.; Audetat, A.; Gunther, D.; Hattendorf, B.; Bleiner, D.; Guillong, M.; Horn, I. Quantitative multi-element analysis of minerals, fluid and melt inclusions by laser-ablation inductively-coupled-plasma mass-spectrometry. Geochim. Cosmochim. Acta 2003, 67, 3473–3497. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Romer, R.L.; Luders, V.; Bodnar, R.J. Genetic relationship between silver-lead-zinc mineralization in the Wutong deposit, Guangxi Province and Mesozoic granitic magmatism in the Nanling belt, southeast China. Miner. Depos. 2014, 49, 353–369. [Google Scholar] [CrossRef]
- Lerchbaumer, L.; Audetat, A. Partitioning of Cu between vapor and brine—An experimental study based on LA-ICP-MS analysis of synthetic fluid inclusions. Geochim. Cosmochim. Acta 2009, 73, 743–744. [Google Scholar]
- Melcher, F.; Graupner, T.; Gabler, H.E.; Sitnikova, M.; Henjes-Kunst, F.; Oberthur, T.; Gerdes, A.; Dewaele, S. Tantalum-(niobium-tin) mineralisation in African pegmatites and rare metal granites: Constraints from Ta-Nb oxide mineralogy, geochemistry and U-Pb geochronology. Ore Geol. Rev. 2015, 64, 667–719. [Google Scholar] [CrossRef]
- Dewaele, S.; De Clercq, F.; Hulsbosch, N.; Piessens, K.; Boyce, A.; Burgess, R.; Muchez, P. Genesis of the vein-type tungsten mineralization at Nyakabingo (Rwanda) in the Karagwe-Ankole belt, Central Africa. Miner. Depos. 2016, 51, 283–307. [Google Scholar] [CrossRef]
- Dewaele, S.; DeClerq, F.; Muchez, P.; Schneider, J.; Burgess, R.; Boyce, A.; Fernandez-Alonso, M. Geology of the cassiterite mineralisation in the Rutongo area, Rwanda (Central Africa): Current state of knowledge. Geol. Belg. 2010, 13, 91–112. [Google Scholar]
- Dewaele, S.; Goethal, H.; Thys, T. Mineralogical characterization of cassiterite concentrates from quartz vein and pegmatite mineralization of the Karagwe-Ankole and Kibara Belts, Central Africa. Geol. Belg. 2013, 16, 66–75. [Google Scholar]
- Dewaele, S.; Henjes-Kunst, F.; Melcher, F.; Sitnikova, M.; Burgess, R.; Gerdes, A.; Fernandez, M.A.; Clercq, F.D.; Muchez, P.A.; Lehmann, B. Late Neoproterozoic overprinting of the cassiterite and columbite-tantalite bearing pegmatites of the Gatumba area, Rwanda (Central Africa). J. Afr. Earth Sci. 2011, 61, 10–26. [Google Scholar] [CrossRef]
- Dewaele, S.; Hulsbosch, N.; Cryns, Y.; Boyce, A.; Burgess, R.A.; Muchez, P. Geological setting and timing of the world-class Sn, Nb-Ta and Li mineralization of Manono-Kitotolo (Katanga, Democratic Republic of Congo). Ore Geol. Rev. 2016, 72, 373–390. [Google Scholar] [CrossRef]
- Dewaele, S.; Muchez, P.; Burgess, R.A.; Boyce, A. Geological setting and timing of the cassiterite vein type mineralization of the Kalima area (Maniema, Democratic Republic of Congo). J. Afr. Earth Sci. 2015, 112, 199–212. [Google Scholar] [CrossRef]
- Pohl, W.; Biryabarema, M.a.; Lehmann, B. Early Neoproterozoic rare metal (Sn, Ta, W) and gold metallogeny of the Central Africa Region: A review. Appl. Earth Sci. 2013, 122, 66–82. [Google Scholar] [CrossRef]
- Pohl, W. The origin of Kibaran (late Mid-Proterozoic) tin, tungsten and gold quartz vein deposits in Central Africa: A fluid inclusions study. Miner. Depos. 1991, 26, 51–59. [Google Scholar] [CrossRef]
- Wouters, S.; Hulsbosch, N.; Kaskes, P.; Claeys, P.; Dewaele, S.; Melcher, F.; Onuk, P.; Muchez, P. Late orogenic gold mineralization in the western domain of the Karagwe-Ankole Belt (Central Africa): Auriferous quartz veins from the Byumba deposit (Rwanda). Ore Geol. Rev. 2020, 125, 103666. [Google Scholar] [CrossRef]
- Fernandez-Alonso, M.; Mupande, J.-F.; Badosa, T.; Baudet, D.; Dewaele, S.; Kalenga, H.; Kampata, D.; Kanda, N.V.; Lahmouch, M.; Lahogue, P.; et al. New 1/2,5 Million Scale Geologic and Mineral Occurrences Maps of the Democratic Republic of Congo; Royal Museum for Central Africa: Tervuren, Belgium, 2016. [Google Scholar]
- RMCA. Geological Map of the Democratic Republic of Congo, Scale of 1/25 000000; Royal Museum for Central Africa: Tervuren, Belgium, 2006. [Google Scholar]
- Fernandez-Alonso, M.; Cutten, H.; De Waele, B.; Tack, L.; Tahon, A.; Baudet, D.; Barritt, S.D. The Mesoproterozoic Karagwe-Ankole Belt (formerly the NE Kibara Belt): The result of prolonged extensional intracratonic basin development punctuated by two short-lived far-field compressional events. Precambrian Res. 2012, 216, 63–86. [Google Scholar] [CrossRef]
- Kampunzu, A.B. The Mesoproterozoic Kibaran belt system in Africa: A key for the reconstruction of Rodinia Supercontinent. Gondwana Res. 1998, 1, 412–414. [Google Scholar] [CrossRef]
- Kampunzu, A.B.; Cailteux, J. Tectonic evolution of the Lufilian Arc (Central Africa Copper belt) during the neoproterozoic Pan-African orogenesis. Gondwana Res. 1997, 1, 149. [Google Scholar] [CrossRef]
- De Waele, B.; Johnson, S.P.; Pisarevsky, S.A. Palaeoproterozoic to Neoproterozoic growth and evolution of the eastern Congo Craton: Its role in the Rodinia puzzle. Precambrian Res. 2008, 160, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.P.; Rivers, T.; De Waele, B. A review of the Mesoproterozoic to early Palaeozoic magmatic and tectonothermal history of south–central Africa: Implications for Rodinia and Gondwana. J. Geol. Soc. 2005, 162, 433–450. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.; Halder, S.; Ruzindana Munana, J.; de la Paix Ngizimana, J.; Biryabarema, M. The geochemical signature of rare-metal pegmatites in Central Africa: Magmatic rocks in the Gatumba tin–tantalum mining district, Rwanda. J. Geochem. Explor. 2014, 144, 528–538. [Google Scholar] [CrossRef]
- Tack, L.; Bowden, P. Post-collisional granite magmatism in the central Damaran (Pan-African) Orogenic Belt, western Namibia. J. Afr. Earth Sci. 1999, 28, 653–674. [Google Scholar] [CrossRef]
- Tack, L.; Wingate, M.T.D.; De Waele, B.; Meert, J.; Belousova, E.; Griffin, B.; Tahon, A.; Fernandez-Alonso, M. The 1375Ma “Kibaran event” in Central Africa: Prominent emplacement of bimodal magmatism under extensional regime. Precambrian Res. 2010, 180, 63–84. [Google Scholar] [CrossRef]
- Buurman, I. An Assessment of Sn-W-Nb-Ta Oxide Minerals from the Bugesera District of Rwanda and Geological Context. Ph.D. Thesis, University of Witwatersrand, Johannesburg, South Africa, 2018. [Google Scholar]
- Buyse, F.; Dewaele, S.; Decrée, S.; Mees, F. Mineralogical and geochemical study of the rare earth element mineralization at Gakara (Burundi). Ore Geol. Rev. 2020, 124, 103659. [Google Scholar] [CrossRef]
- De Clercq, F. Metallogenis of Tin and Tungsten Vein-Type Deposit in the Karagwe-Ankole Belt (Rwanda). Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2012. [Google Scholar]
- Villeneuve, M.; Gärtner, A.; Kalikone, C.; Wazi, N.; Hofmann, M.; Linnemann, U. U-Pb ages and provenance of detrital zircon from metasedimentary rocks of the Nya-Ngezie and Bugarama groups (D.R. Congo): A key for the evolution of the Mesoproterozoic Kibaran-Burundian Orogen in Central Africa. Precambrian Res. 2019, 328, 81–98. [Google Scholar] [CrossRef]
- Key, R.; Kampunzu, A.B. IGCP 418 “Evolution of the Kibaran belt system in southwestern Africa: Comparison with equatorial and southern Africa”. J. Afr. Earth Sci. 1997, 24, R9–R10. [Google Scholar] [CrossRef]
- Kokonyangi, J. Geological fieldwork in the Kibaran-type region, Mitwaba district, Congo (former Zaire), central Africa. Gondwana Res. 2001, 4, 255–259. [Google Scholar] [CrossRef]
- Kokonyangi, J.; Armstrong, R.; Kampunzu, A.B.; Yoshida, M.; Okudaira, T. U-Pb zircon geochronology and petrology of granitoids from Mitwaba (Katanga, Congo): Implications for the evolution of the Mesoproterozoic Kibaran belt. Precambrian Res. 2004, 132, 79–106. [Google Scholar] [CrossRef]
- Kokonyangi, J.; Kampunzu, A.B.; Poujol, M.; Okudaira, T.; Yoshida, M.; Shabeer, K.P. Petrology and geochronology of Mesoproterozoic mafic-intermediate plutonic rocks from Mitwaba (D. R. Congo): Implications for the evolution of the Kibaran belt in central Africa. Geol. Mag. 2005, 142, 109–130. [Google Scholar] [CrossRef]
- Kokonyangi, J.; Kampunzu, A.B.; Yoshida, M. Lithostratigraphy and structural evolution of the Kalima-Moga tin district, Kibaran belt (Maniema, Congo). Gondwana Res. 2000, 3, 257–259. [Google Scholar] [CrossRef]
- Kokonyangi, J.W.; Kampunzu, A.B.; Armstrong, R.; Yoshida, M.; Okudaira, T.; Arima, M.; Ngulube, D.A. The Mesoproterozoic Kibaride belt (Katanga, SE DR Congo). J. Afr. Earth Sci. 2006, 46, 1–35. [Google Scholar] [CrossRef]
- Tack, L.; Liegeois, J.P.; Deblond, A.; Duchesne, J.C. Kibaran-a-Type Granitoids and Mafic Rocks Generated by 2 Mantle Sources in a Late Orogenic Setting (Burundi). Precambrian Res. 1994, 68, 323–356. [Google Scholar] [CrossRef]
- Kadima, E.; Delvaux, D.; Sebagenzi, S.N.; Tack, L.a.; Kabeya, S.M. Structure and geological history of the Congo Basin: An integrated interpretation of gravity, magnetic and reflection seismic data. Basin. Res. 2011, 23, 499–527. [Google Scholar] [CrossRef]
- Melcher, F.; Graupner, T.; Gäbler, H.-E.; Sitnikova, M.; Oberthür, T.; Gerdes, A.; Badanina, E.; Chudy, T. Mineralogical and chemical evolution of tantalum–(niobium–tin) mineralisation in pegmatites and granites. Part 2: Worldwide examples (excluding Africa) and an overview of global metallogenetic patterns. Ore Geol. Rev. 2017, 89, 946–987. [Google Scholar] [CrossRef]
- Van Daele, J.; Hulsbosch, N.; Dewaele, S.A.; Muchez, P. Metamorphic and metasomatic evolution of Western Domain of the Karagwe-Ankole Belt (Central Africa). J. Afr. Earth Sci. 2020, 165, 103783. [Google Scholar] [CrossRef] [Green Version]
- Maier, W.D.; Barnes, S.J.; Bandyayera, D.; Livesey, T.; Li, C.; Ripley, E. Early Kibaran rift-related mafic–ultramafic magmatism in western Tanzania and Burundi: Petrogenesis and ore potential of the Kapalagulu and Musongati layered intrusions. Lithos 2008, 101, 24–53. [Google Scholar] [CrossRef]
- De Clercq, F.; Muchez, P.; Dewaele, S.; Fernandez-Alonso, M.; Boyce, A.A.; Piessens, K. The evolution of W vein-type deposits in Rwanda: A fluid inclusion and stable isotope study. Geol. Belg. 2008, 11, 251–258. [Google Scholar]
- De Clercq, S.; Chew, D.; O’Sullivan, G.; De Putter, T.; De Grave, J.; Dewaele, S. Characterisation and geodynamic setting of the 1 Ga granitoids of the Karagwe-Ankole belt (KAB), Rwanda. Precambrian Res. 2021, 356, 106124. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Van Daele, J.; Reinders, N.; Dewaele, S.; Jacques, D.; Muchez, P. Structural control on the emplacement of contemporaneous Sn-Ta-Nb mineralized LCT pegmatites and Sn bearing quartz veins: Insights from the Musha and Ntunga deposits of the Karagwe-Ankole Belt, Rwanda. J. Afr. Earth Sci. 2017, 134, 24–32. [Google Scholar] [CrossRef]
- Muchez, P.; Hulsbosch, N.; Dewaele, S. Geological Mapping and Implications for Nb-Ta, Sn and W Prospection in Rwanda. Bull. Séanc. Acad. R. Sci. Outre-Mer 2014, 60, 515–530. [Google Scholar]
- Cahen, L.; Ledent, D. Précision sur l’âge, la pétrogenèse et la position stratigraphique des granites a étain de l’Est de l’Afrique centrale. Bull. Soc. Belg. Géologie 1979, 88, 33–49. [Google Scholar]
- Cailteux, J.; Binda, P.L.; Katekesha, W.M.; Kampunzu, A.B.; Intiomale, M.M.; Kapenda, D.; Kaunda, C.; Ngongo, K.; Tshiauka, T.a.; Wendorff, M. Lithostratigraphical Correlation of the Neoproterozoic Roan Supergroup from Shaba (Zaire) and Zambia, in the Central African Copper-Cobalt Metallogenic Province. J. Afr. Earth Sci. 1994, 19, 265–278. [Google Scholar] [CrossRef]
- Cailteux, J.L.H.; Kampunzu, A.B.; Lerouge, C.; Kaputo, A.K.; Milesi, J.P. Genesis of sediment-hosted stratiform copper-cobalt deposits, central African Copperbelt. J. Afr. Earth Sci. 2005, 42, 134–158. [Google Scholar] [CrossRef]
- Cailteux, J.L.H.; Muchez, P.; De Cuyper, J.; Dewaele, S.; De Putter, T. Origin of the megabreccias in the Katanga Copperbelt (D.R.Congo). J. Afr. Earth Sci. 2018, 140, 76–93. [Google Scholar] [CrossRef]
- Dewaele, S.; Muchez, P.; Vets, J.; Fernandez-Alonzo, M.; Tack, L. Multiphase origin of the Cu-Co ore deposits in the western part of the Lufilian fold-and-thrust belt, Katanga (Democratic Republic of Congo). J. Afr. Earth Sci. 2006, 46, 455–469. [Google Scholar] [CrossRef]
- Johnson, J.E.; Webb, S.M.; Ma, C.; Fischer, W.W. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim. Cosmochim. Acta 2016, 173, 210–231. [Google Scholar] [CrossRef] [Green Version]
- Kampunzu, A.B.; Cailteux, J.L.H.; Kamona, A.F.; Intiomale, M.M.; Melcher, F. Sediment-hosted Zn-Pb-Cu deposits in the Central African Copperbelt. Ore Geol. Rev. 2009, 35, 263–297. [Google Scholar] [CrossRef]
- Kampunzu, A.B.; Kanika, M.; Kapenda, D.; Tshimanga, K. Geochemistry and Geotectonic Setting of Late Proterozoic Katangan Basic Rocks from Kibambale in Central Shaba (Zaire). Geol. Rundsch. 1993, 82, 619–630. [Google Scholar] [CrossRef]
- Lerouge, C.; Cailteux, J.; Kampunzu, A.B.; Milesi, J.P.; Flehoc, C. Sulphur isotope constraints on formation conditions of the Luiswishi ore deposit, Democratic Republic of Congo (DRC). J. Afr. Earth Sci. 2005, 42, 173–182. [Google Scholar] [CrossRef]
- Mambwe, P.; Delpomdor, F.; Lavoie, S.; Mukonki, P.; Batumike, J.; Muchez, P. Sedimentary evolution and stratigraphy of the similar to 765-740 Ma Kansuki-Mwashya platform succession in the Tenke-Fungurume Mining District, Democratic Republic of the Congo. Geol. Belg. 2020, 23, 69–85. [Google Scholar] [CrossRef]
- Mambwe, P.; Kipata, L.; Chabu, M.; Muchez, P.; Lubala, T.; Jebrak, M.; Delvaux, D. Sedimentology of the Shangoluwe breccias and timing of the Cu mineralisation (Katanga Supergroup, D.R. of Congo). J. Afr. Earth Sci. 2017, 132, 1–15. [Google Scholar] [CrossRef]
- Mambwe, P.; Milan, L.; Batumike, J.; Lavoie, S.; Jebrak, M.; Kipata, L.; Chabu, M.; Mulongo, S.; Lubala, T.; Delvaux, D.; et al. Lithology, petrography and Cu occurrence of the Neoproterozoic glacial Mwale Formation at the Shanika syncline (Tenke Fungurume, Congo Copperbelt; Democratic Republic of Congo). J. Afr. Earth Sci. 2017, 129, 898–909. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Hertogen, J.; Dewaele, S.; Andre, L.; Muchez, P. Petrographic and mineralogical characterisation of fractionated pegmatites culminating in the Nb-Ta-Sn pegmatites of the Gatumba area (western Rwanda). Geol. Belg. 2013, 16, 105–117. [Google Scholar]
- Büttner, S.H.; Reid, W.; Glodny, J.; Wiedenbeck, M.; Chuwa, G.; Moloto, T.; Gucsik, A. Fluid sources in the Twangiza–Namoya Gold Belt (Democratic Republic of Congo): Evidence from tourmaline and fluid compositions, and from boron and Rb–Sr isotope systematics. Precambrian Res. 2016, 280, 161–178. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Boiron, M.C.; Dewaele, S.; Muchez, P. Fluid fractionation of tungsten during granite-pegmatite differentiation and the metal source of peribatholitic W quartz veins: Evidence from the Karagwe-Ankole Belt (Rwanda). Geochim. Cosmochim. Acta 2016, 175, 299–318. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Miner. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Melt composition control of Zr/Hf fractionation in magmatic processes. Geochim. Cosmochim. Acta 2002, 66, 3293–3301. [Google Scholar] [CrossRef]
- Gagnon, J.E.; Samson, I.M.; Fryer, B.J.; Williams-Jones, A.E. The Composition and Origin of Hydrothermal Fluids in a Nyf-Type Granitic Pegmatite, South Platte District, Colorado: Evidence from La Icp Ms Analysis of Fluorite-and Quartz-Hosted Fluid Inclusions. Can. Mineral. 2004, 42, 1331–1355. [Google Scholar] [CrossRef]
- Hatert, F.; Lefèvre, P.; Fransolet, A.-M.; Spirlet, M.-R.; Rebbouh, L.; Fontan, F.; Keller, P. Ferrorosemaryite, NaFe2+Fe3+Al(PO4)3, a new phosphate mineral from the Rubindi pegmatite, Rwanda. Eur. J. Mineral. 2005, 17, 749–759. [Google Scholar] [CrossRef]
- Goldmann, S. Mineralogical-Geochemical Characterisation of Cassiterite and Wolframite Ores for an Analytical Fingerprint: Focus on Trace Element Analysis by LA-ICP-MS. Ph.D. Thesis, Faculty of Natural Sciences, Leibniz University Hannover, Hannover, Germany, 2016; pp. 1–288. [Google Scholar]
- Goldmann, S.; Melcher, F.; Gabler, H.E.; Dewaele, S.; De Clercq, F.; Muchez, P. Mineralogy and Trace Element Chemistry of Ferberite/Reinite from Tungsten Deposits in Central Rwanda. Minerals 2013, 3, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 646. [Google Scholar]
- Chou, I.M.; Wang, A. Application of laser Raman micro-analyses to Earth and planetary materials. J. Asian Earth Sci. 2017, 145, 309–333. [Google Scholar] [CrossRef]
- Chi, G.; Diamond, L.W.; Lu, H.; Lai, J.; Chu, H. Common Problems and Pitfalls in Fluid Inclusion Study: A Review and Discussion. Minerals 2020, 11, 7. [Google Scholar] [CrossRef]
- Randive, K.R.; Hari, K.R.; Dora, M.L.; Malpe, D.B.; Bhondwe, A.A. Study of Fluid Inclusions: Methods, Techniques and Applications. Gondwana Geol. Mag. 2014, 29, 19–28. [Google Scholar]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O-NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Application; De Vivo, B., Frezzotti, M.L., Eds.; Short Course IMA: Pontignano-Siena, Italy, 1994; pp. 117–130. [Google Scholar]
- Driesner, T.; Heinrich, C.A. The system H2O–NaCl. Part I: Correlation formulae for phase relations in temperature–pressure–composition space from 0 to 1000 °C, 0 to 5000 bar, and 0 to 1 XNaCl. Geochim. Cosmochim. Acta 2007, 71, 4880–4901. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Luo, M.C.; Steele-MacInnis, M.; Runyon, S.E.; Sublett, D.M.; Klyukin, Y.I.; Bodnar, R.J. Synthetic fluid inclusions XXII: Properties of H2O-NaCl ± KCl fluid inclusions trapped under vapor- and salt-saturated conditions with emphasis on the effect of KCl on phase equilibria. Geochim. Cosmochim. Acta 2020, 272, 78–92. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Steele-MacInnis, M.; Bodnar, R.J. A numerical model to estimate trapping conditions of fluid inclusions that homogenize by halite disappearance. Geochim. Cosmochim. Acta 2012, 92, 14–22. [Google Scholar] [CrossRef]
- Murciego, A.; Sanchez, A.G.; Dusausoy, Y.; Pozas, J.M.M.; Ruck, R. Geochemistry and EPR of cassiterites from the Iberian Hercynian Massif. Miner. Mag. 1997, 61, 357–365. [Google Scholar] [CrossRef]
- Giuliani, G. La Cassitérite Zonée du Gisement De Sokhret Allal (Granite des Zaer; Maroc Central): Composition chimique et phases fluides associées. Miner. Depos. 1987, 22, 253–261. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Zeng, Z.; Lou, F. Petrogenesis of the Xihuashan granites in southeastern China: Constraints from geochemistry and in-situ analyses of zircon UPbHfO isotopes. Lithos 2012, 148, 209–227. [Google Scholar] [CrossRef]
- Haskin, L.A.; Wang, A.; Rockow, K.M.; Jolliff, B.L.; Korotev, R.L.a.; Viskupic, K.M. Raman spectroscopy for mineral identification and quantification for in situ planetary surface analysis: A point count method. J. Geophys. Res. 1997, 102, 19293–19306. [Google Scholar] [CrossRef] [Green Version]
- Izoret, L.; Marnier, G.a.; Dusausoy, Y. Caratérisation cristallochimique de la cassitérite des gisements d’étain et de tungsten de Galice, Espagne. Can. Miner. 1985, 23, 221–231. [Google Scholar]
- Möller, P.; Černý, P.; Saupé, F. Lanthanides, Tantalum, and Niobium: Mineralogy, Geochemistry, Characteristics of Primary ore Deposits, Prospecting, Processing, and Applications: Proceedings of a Workshop in Berlin, November 1986; Springer: Berlin, Germany; New York, NY, USA, 1989. [Google Scholar]
- Remond, G. Exemples d’identification et de localisation des éléments en traces dans des minéraux luminescents (cassitérites) à l’aide de l’analyseur ionique. Bull. Société Française Minéralogie Cristallogr. 1973, 96, 183–198. [Google Scholar] [CrossRef]
- Remond, G.; Cesbron, F.; Chapoulie, R.; Ohnenstetter, D.; Roques-Carmes, C.; Schvoerer, M. Cathodoluminescence Applied to the Microcharacterization of Mineral Materials: A Present Status in Experimentation and Interpretation. Scanning Microsc. 1992, 6, 23–68. [Google Scholar]
- Li, Y.; Liu, Z.; Shao, Y.; Chen, K.; Zhang, J.; Zhang, Y.; Zhang, T. Genesis of the Huanggangliang Fe-Sn polymetallic deposit in the southern Da Hinggan Range, NE China: Constraints from geochronology and cassiterite trace element geochemistry. Ore Geol. Rev. 2022, 151, 105226. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Ni, P.; Pan, J.-Y.; Cui, J.-M.; Li, W.-S.; Fang, G.-J.; Zhao, Z.-H.; Xu, Y.-M.; Ding, J.-Y.; Han, L. Fluid evolution and metallogenic mechanism of the Xianghualing skarn-type Sn deposit, South China: Evidence from petrography, fluid inclusions and trace-element composition of cassiterite. Ore Geol. Rev. 2023, 154, 105351. [Google Scholar] [CrossRef]
- Audetat, A.; Zhang, D.H. Abundances of S, Ga, Ge, Cd, In, Tl and 32 other major to trace elements in high-temperature (350–700 degrees C) magmatic-hydrothermal fluids. Ore Geol. Rev. 2019, 109, 630–642. [Google Scholar] [CrossRef]
- Raith, J.G.; Melcher, F.; Lichtervelde, M.V. Rare-element pegmatites; from natural systems to the experimental lab. Mitt. Österr. Miner. Ges. 2014, 160, 11–57. [Google Scholar]
- Bellot, N.; Boyet, M.; Doucelance, R.; Bonnand, P.; Savov, I.P.; Plank, T.A.; Elliott, T. Origin of negative cerium anomalies in subduction-related volcanic samples: Constraints from Ce and Nd isotopes. Chem. Geol. 2018, 500, 46–63. [Google Scholar] [CrossRef]
- Braun, J.J.; Pagel, M.; Muller, J.P.; Bilong, P.; Michard, A.A.; Guillet, B. Cerium anomalies in lateritic profiles. Geochim. Cosmochim. Acta 1990, 54, 771–795. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- De Baar, H.; Faure, G. On cerium anomalies in the Sargasso Sea. Geochim. Cosmochim. Acta 1991, 55, 2981–2983. [Google Scholar] [CrossRef] [Green Version]
- Kölling, M. Comparison of different methods for redox potential determination in natural waters. In Redox; Springer: Berlin/Heidelberg, Germany, 2000; pp. 42–54. [Google Scholar]
- Kraemer, D.; Tepe, N.; Pourret, O.; Bau, M. Negative cerium anomalies in manganese (hydr)oxide precipitates due to cerium oxidation in the presence of dissolved siderophores. Geochim. Cosmochim. Acta 2017, 196, 197–208. [Google Scholar] [CrossRef]
- Möller, P.; Muecke, G.K. Significance of Europium anomalies in silicate melts and crystal-melt equilibria: A re-evaluation. Contrib. Miner. Petr. 1984, 87, 242–250. [Google Scholar] [CrossRef]
- Tostevin, R.; Shields, G.A.; Tarbuck, G.M.; He, T.; Clarkson, M.O.; Wood, R.A. Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings. Chem. Geol. 2016, 438, 146–162. [Google Scholar] [CrossRef] [Green Version]
- Carocci, E.; Truche, L.; Cathelineau, M.; Caumon, M.-C.; Bazarkina, E.F. Tungsten (VI) speciation in hydrothermal solutions up to 400°C as revealed by in-situ Raman spectroscopy. Geochim. Cosmochim. Acta 2022, 317, 306–324. [Google Scholar] [CrossRef]
- Vonopartis, L.; Nex, P.; Kinnaird, J.; Robb, L. Evaluating the Changes from Endogranitic Magmatic to Magmatic-Hydrothermal Mineralization: The Zaaiplaats Tin Granites, Bushveld Igneous Complex, South Africa. Minerals 2020, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- McLennan, S.M.; Hemming, S.R.; Taylor, S.R.; Ericksson, K.A. Early Proterozoic crustal evolution: Geochemical and Nd-Pb isotopic evidence from metasedimentary rocks, southwestern North America. Geochim. Cosmochim. Acta 1995, 59, 1153–1177. [Google Scholar] [CrossRef]
- Turimumahoro, D.; Hulsbosch, N.; Nahimana, L.; Dewaele, S.; Muchez, P. Geochemical signature of muscovites as pathfinder for fractionation of pegmatites in the Kabarore-Mparamirundi area (northwestern Burundi, Central Africa). Geol. Belg. 2020, 23, 53–67. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Boiron, M.C.; Thomas, R.; Van Daele, J.; Dewaele, S.; Muchez, P. Evaluation of the petrogenetic significance of melt inclusions in pegmatitic schorl-dravite from graphic tourmaline-quartz assemblages: Application of LA-ICP-QMS analyses and volume ratio calculations. Geochim. Cosmochim. Acta 2019, 244, 308–335. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Muchez, P. Tracing fluid saturation during pegmatite differentiation by studying the fluid inclusion evolution and multiphase cassiterite mineralisation of the Gatumba pegmatite dyke system (NW Rwanda). Lithos 2020, 354–355, 105285. [Google Scholar] [CrossRef]
- Audetat, A.; Gunther, D.; Heinrich, C.A. Magmatic-hydrothermal evolution in a fractionating granite: A microchemical study of the Sn-W-F-mineralized Mole Granite (Australia). Geochim. Cosmochim. Acta 2000, 64, 3373–3393. [Google Scholar] [CrossRef] [Green Version]
- Linnen, R.L.; Pichavant, M.; Holtz, F. The combined effects of fO2 and melt composition on SnO2 solubility and tin diffusivity in haplogranitic melts. Geochim. Cosmochim. Acta 1996, 60, 4965–4976. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Lu, J.; Ma, D.; Ding, T.; Gao, S.; Zhang, Q. Neoproterozoic tin mineralization in South China: Geology and cassiterite U–Pb age of the Baotan tin deposit in northern Guangxi. Miner. Depos. 2019, 54, 1125–1142. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Lecumberri-Sanchez, P.; Moncada, D.; Steele-MacInnis, M. Fluid Inclusions in Hydrothermal Ore Deposits. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 119–142. [Google Scholar]
- Elongo, V.; Lecumberri-Sanchez, P.; Legros, H.; Falck, H.; Adlakha, E.; Roy-Garand, A. Paragenetic constraints on the Cantung, Mactung and Lened tungsten skarn deposits, Canada: Implications for grade distribution. Ore Geol. Rev. 2020, 125, 103677. [Google Scholar] [CrossRef]
- Korges, M.; Weis, P.; Lüders, V.; Laurent, O. Depressurization and boiling of a single magmatic fluid as a mechanism for tin-tungsten deposit formation. Geology 2018, 46, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Legros, H.; Lecumberri-Sanchez, P.; Elongo, V.; Laurent, O.; Falck, H.; Adlakha, E.; Chelle-Michou, C. Fluid evolution of the Cantung tungsten skarn, Northwest Territories, Canada: Differentiation and fluid-rock interaction. Ore Geol. Rev. 2020, 127, 103866. [Google Scholar] [CrossRef]
- Legros, H.; Marignac, C.; Tabary, T.; Mercadier, J.; Richard, A.; Cuney, M.; Wang, R.-C.; Charles, N.; Lespinasse, M.-Y. The ore-forming magmatic-hydrothermal system of the Piaotang W-Sn deposit (Jiangxi, China) as seen from Li-mica geochemistry. Am. Mineral. 2018, 103, 39–54. [Google Scholar] [CrossRef]
- Legros, H.; Richard, A.; Tarantola, A.; Kouzmanov, K.; Mercadier, J.; Vennemann, T.; Marignac, C.; Cuney, M.; Wang, R.-C.; Charles, N. Multiple fluids involved in granite-related W-Sn deposits from the world-class Jiangxi province (China). Chem. Geol. 2019, 508, 92–115. [Google Scholar] [CrossRef]
- Scholten, L.; Schmidt, C.; Lecumberri-Sanchez, P.; Newville, M.; Lanzirotti, A.; Sirbescu, M.L.C.; Steele-MacInnis, M. Solubility and speciation of iron in hydrothermal fluids. Geochim. Cosmochim. Acta 2019, 252, 126–143. [Google Scholar] [CrossRef]
- Wade, J.; Wood, B.J.; Norris, C.A. The oxidation state of tungsten in silicate melt at high pressures and temperatures. Chem. Geol. 2013, 335, 189–193. [Google Scholar] [CrossRef]
- Duc-Tin, Q.; Audetat, A.; Keppler, H. Solubility of tin in (Cl, F)-bearing aqueous fluids at 700 degrees C, 140 MPa: A LA-ICP-MS study on synthetic fluid inclusions. Geochim. Cosmochim. Acta 2007, 71, 3323–3335. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, S.-Y.; Li, W.-Q.; Peng, N.-J.; Zhao, K.-D. Fluid inclusion and isotopic (C, H, O, S and Pb) constraints on the origin of late Mesozoic vein-type W mineralization in northern Guangdong, South China. Ore Geol. Rev. 2019, 112, 103007. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Vieira, R.; Heinrich, C.A.; Pinto, F.; Walle, M. Fluid-rock interaction is decisive for the formation of tungsten deposits. Geology 2017, 45, 579–582. [Google Scholar] [CrossRef]
- Bali, E.; Audetat, A.; Keppler, H. Mobility of U and Th in subduction zone fluids—A synthetic fluid inclusion study. Geochim. Cosmochim. Acta 2009, 73, 54–80. [Google Scholar]
- Bali, E.; Audetat, A.A.; Keppler, H. The mobility of U and Th in subduction zone fluids: An indicator of oxygen fugacity and fluid salinity. Contrib. Miner. Petr. 2011, 161, 597–613. [Google Scholar] [CrossRef]
- Heinrich, C.A. From fluid inclusion microanalysis to large-scale hydrothermal mass transfer in the Earth’s interior. J. Mineral. Petrol. Sci. 2006, 101, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, C.A.; Candela, P.A. Fluids and Ore Formation in the Earth’s Crust. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–28. [Google Scholar]
- Li, X.; Wang, G.; Mao, W.; Wang, C.; Xiao, R.; Wang, M. Fluid inclusions, muscovite Ar–Ar age, and fluorite trace elements at the Baiyanghe volcanic Be–U–Mo deposit, Xinjiang, northwest China: Implication for its genesis. Ore Geol. Rev. 2015, 64, 387–399. [Google Scholar] [CrossRef]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The Chemistry of Metal Transport and Deposition by Ore-Forming Hydrothermal Fluids. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 29–57. [Google Scholar]
- Steele-MacInnis, M.; Manning, C.E. Hydrothermal Properties of Geologic Fluids. Elements 2020, 16, 375–380. [Google Scholar] [CrossRef]
- Gherardi, F.; Droghieri, E.; Magro, G. Hydrothermal Processes at Aluto-Langano (Ethiopia): Insights from the Stable Carbon Isotope Composition of Fluid Inclusions. GRC Trans. 2014, 38, 439–444. [Google Scholar]
- Green, D. The role of oxidation-reduction and CHO fluids in determining melting conditions and magma compositions in the upper mantle. Proc. Indian Acad. Sci. Earth Planet. Sci. 1990, 99, 153–165. [Google Scholar] [CrossRef]
- Mark, M.F.; Maier, W.F. CO2-reforming of methane on supported Rh and Ir catalysts. J. Catal. 1996, 164, 122–130. [Google Scholar] [CrossRef]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. Hokieflincs_H2O-NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O-NaCl. Comput. Geosci. UK 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Zhong, S.; Seltmann, R.; Qu, H.; Song, Y. Characterization of the Zircon Ce anomaly for estimation of oxidation state of magmas: A revised Ce/Ce* method. Miner. Petrol. 2019, 113, 755–763. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makutu, D.K.; Seo, J.H.; Lee, I.; Oh, J.; Kang, P.; Ongendangenda, A.T.; Makoka, F.M. Magmatic-Hydrothermal Fluid Processes of the Sn-W Granites in the Maniema Province of the Kibara Belt (KIB), Democratic Republic of Congo. Minerals 2023, 13, 458. https://doi.org/10.3390/min13040458
Makutu DK, Seo JH, Lee I, Oh J, Kang P, Ongendangenda AT, Makoka FM. Magmatic-Hydrothermal Fluid Processes of the Sn-W Granites in the Maniema Province of the Kibara Belt (KIB), Democratic Republic of Congo. Minerals. 2023; 13(4):458. https://doi.org/10.3390/min13040458
Chicago/Turabian StyleMakutu, Douxdoux Kumakele, Jung Hun Seo, Insung Lee, Jihye Oh, Pilmo Kang, Albert Tienge Ongendangenda, and Frederic Mwanza Makoka. 2023. "Magmatic-Hydrothermal Fluid Processes of the Sn-W Granites in the Maniema Province of the Kibara Belt (KIB), Democratic Republic of Congo" Minerals 13, no. 4: 458. https://doi.org/10.3390/min13040458





