Actualistic Testing of the Influence of Groundwater Chemistry on Degradation of Collagen I in Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Trial Apparatus
2.2.2. Solutions and Trials
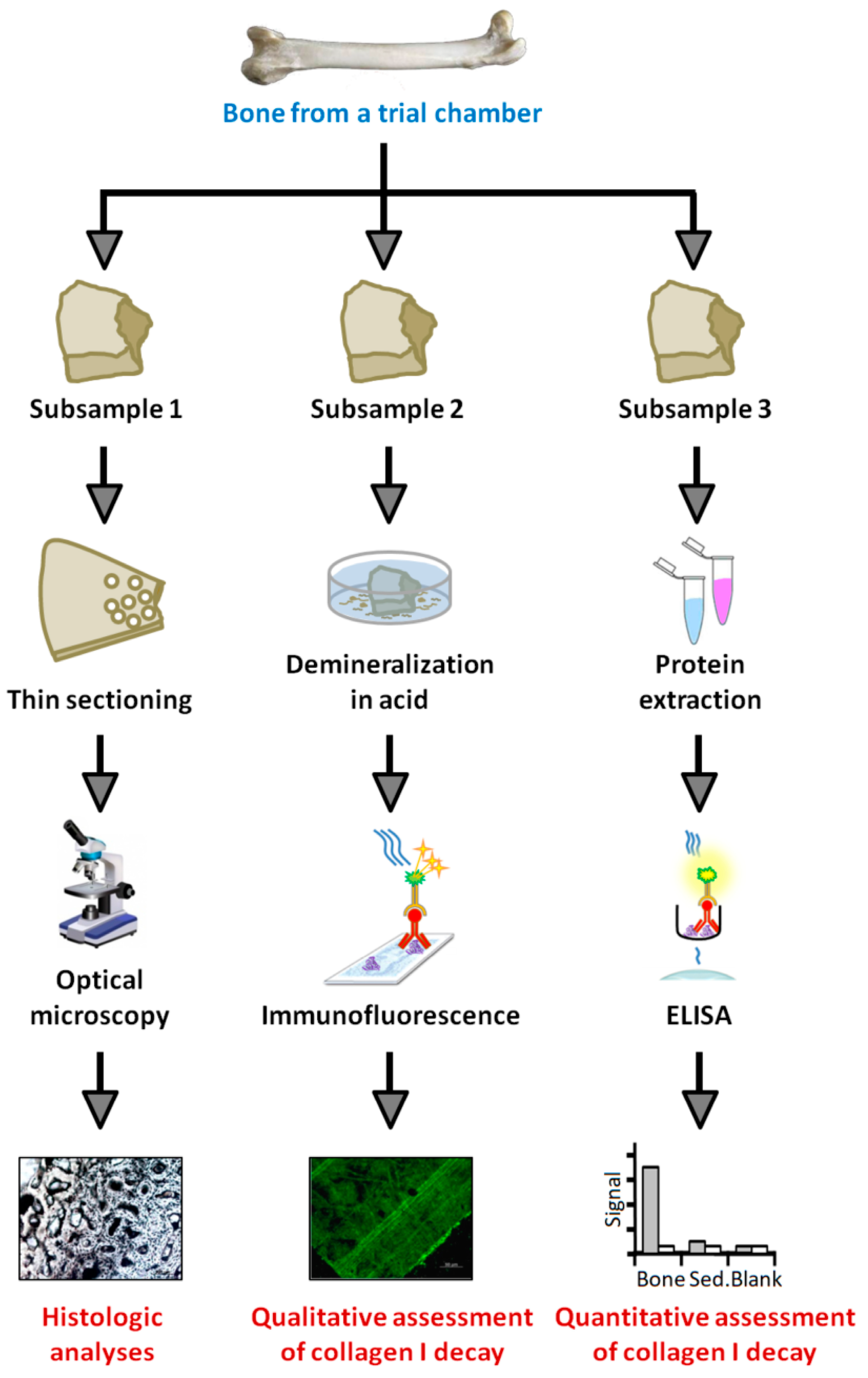
2.2.3. Histology
2.2.4. Protein Extraction
2.2.5. ELISA
2.2.6. Immunofluorescence
3. Results
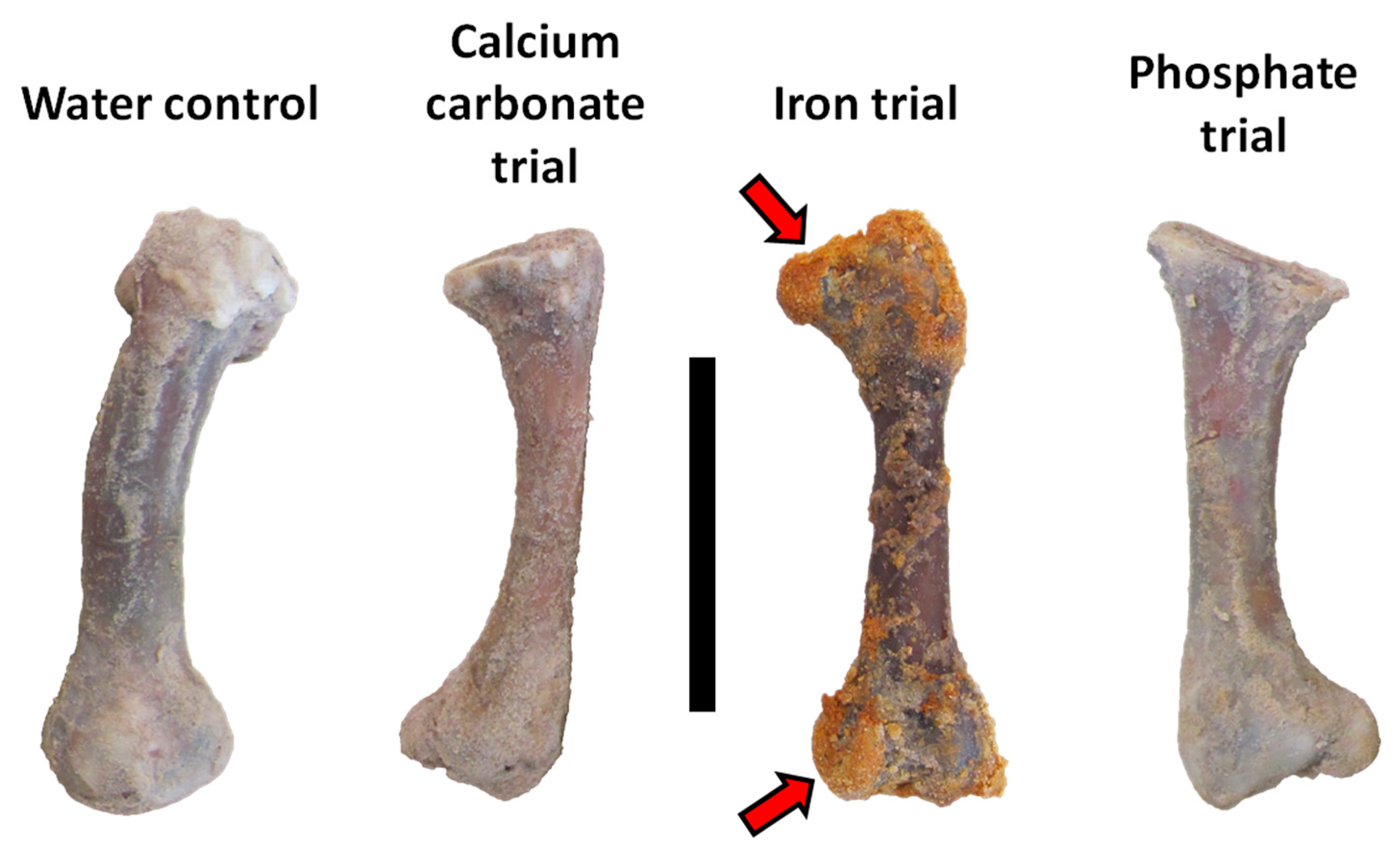
3.1. General Observations
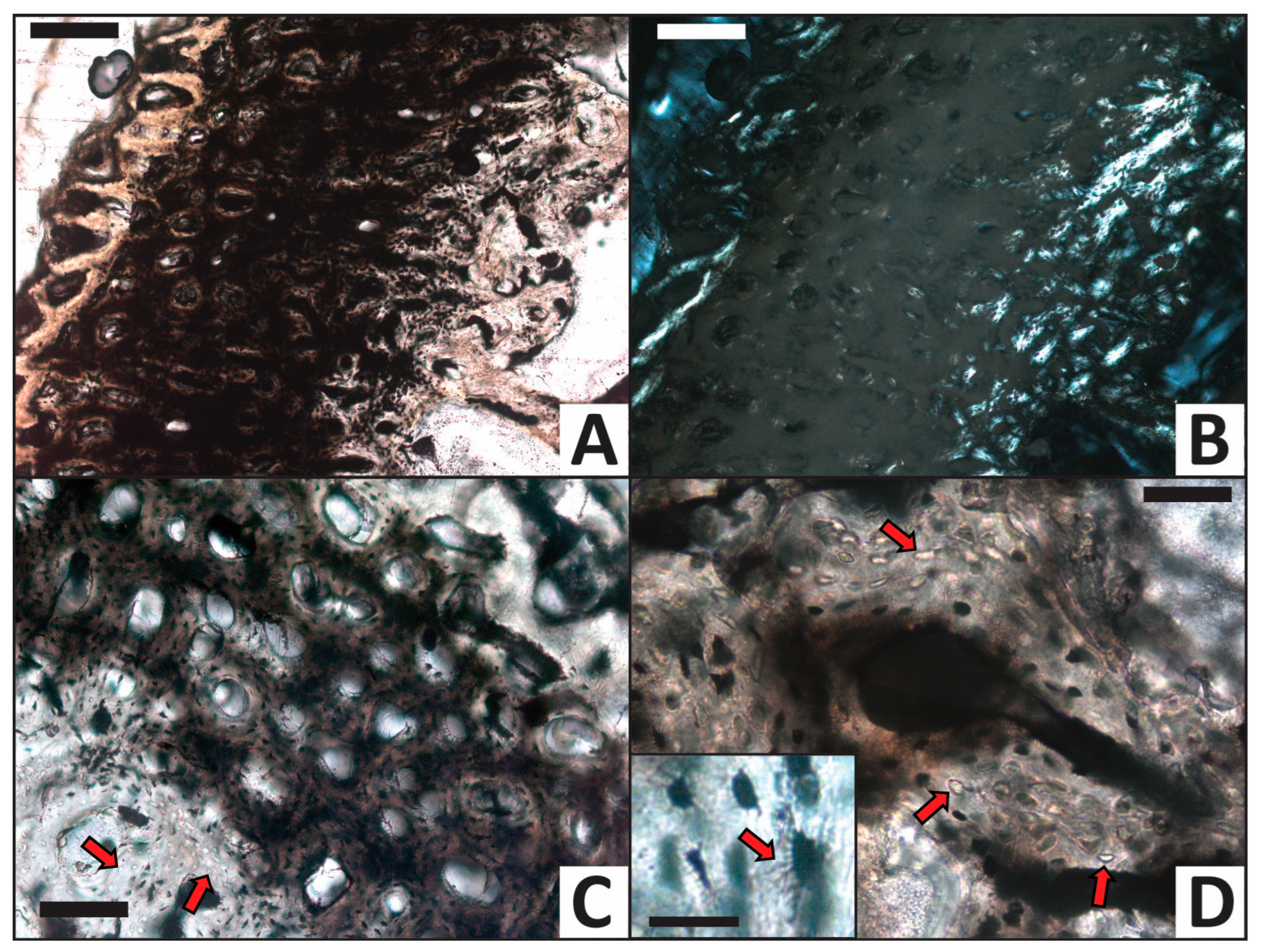
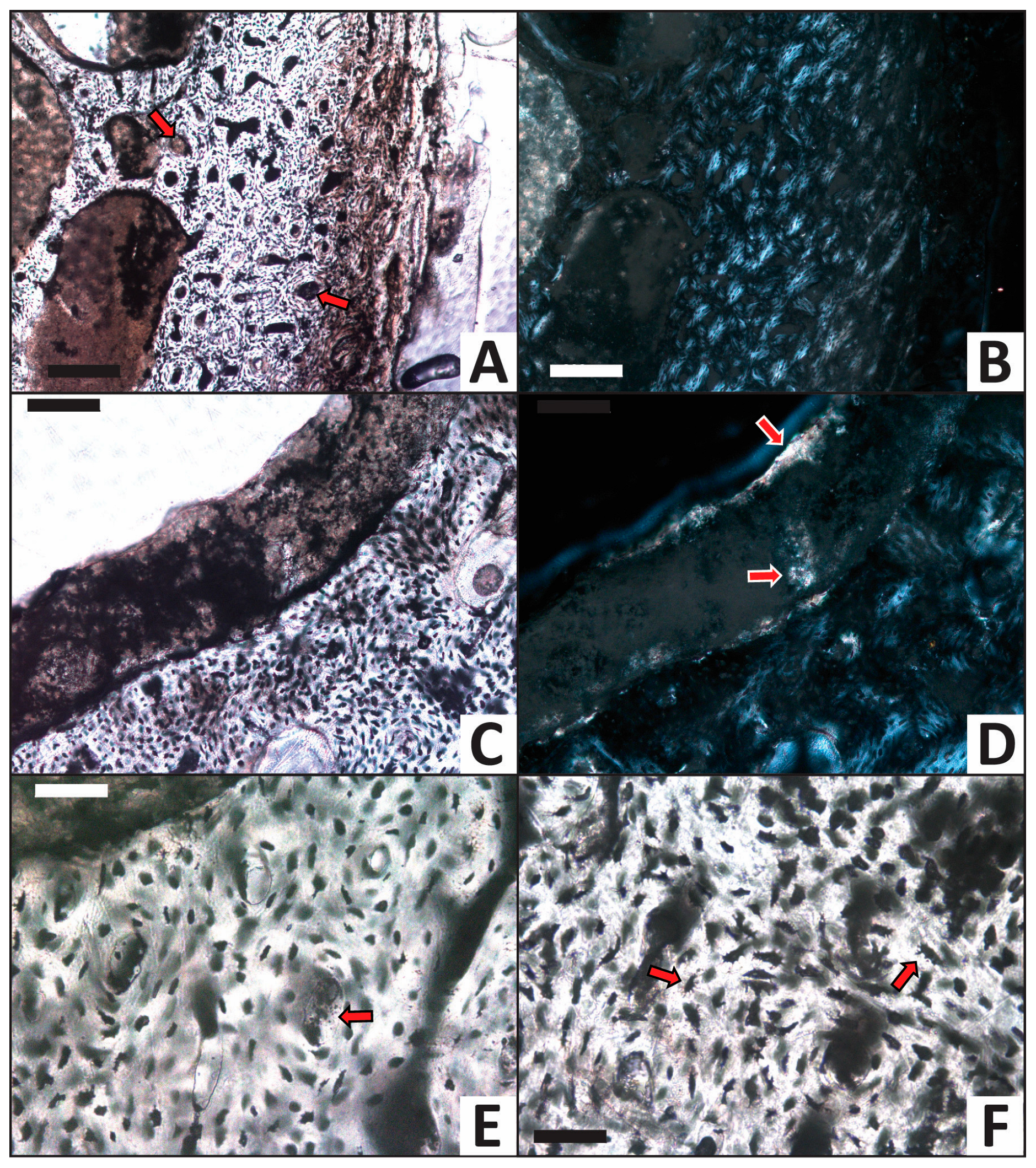
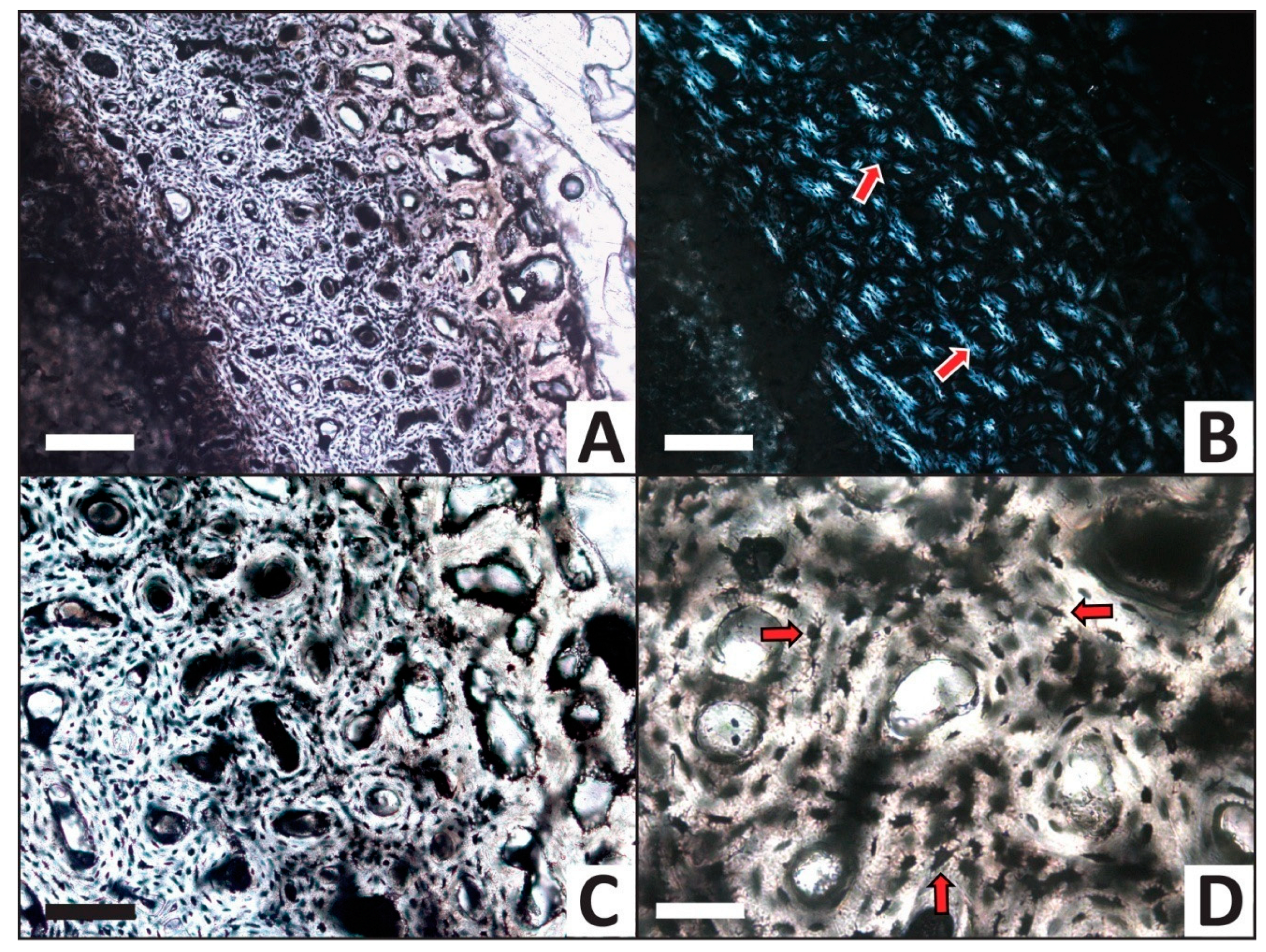
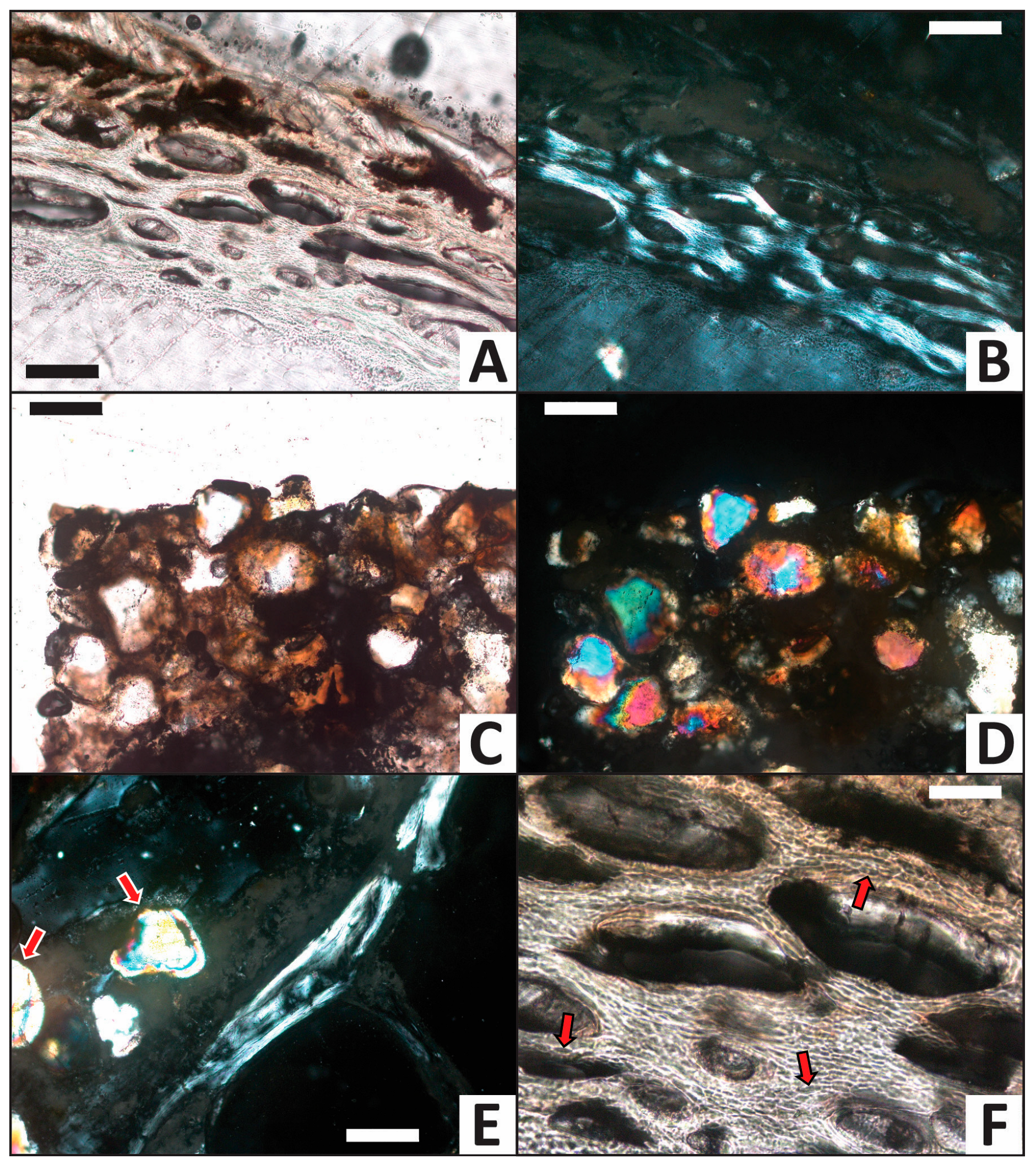
3.2. Histology
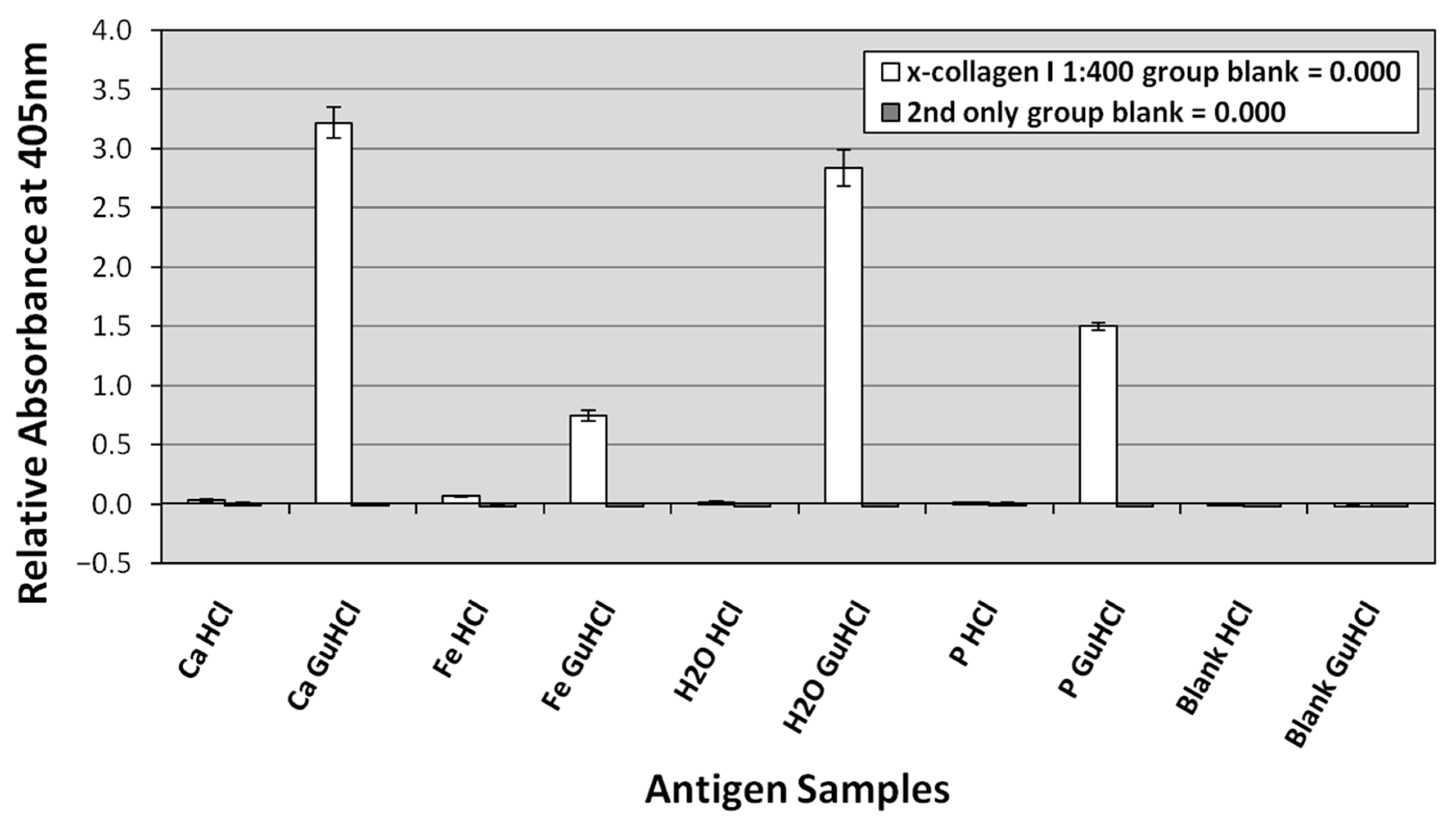
3.3. ELISA
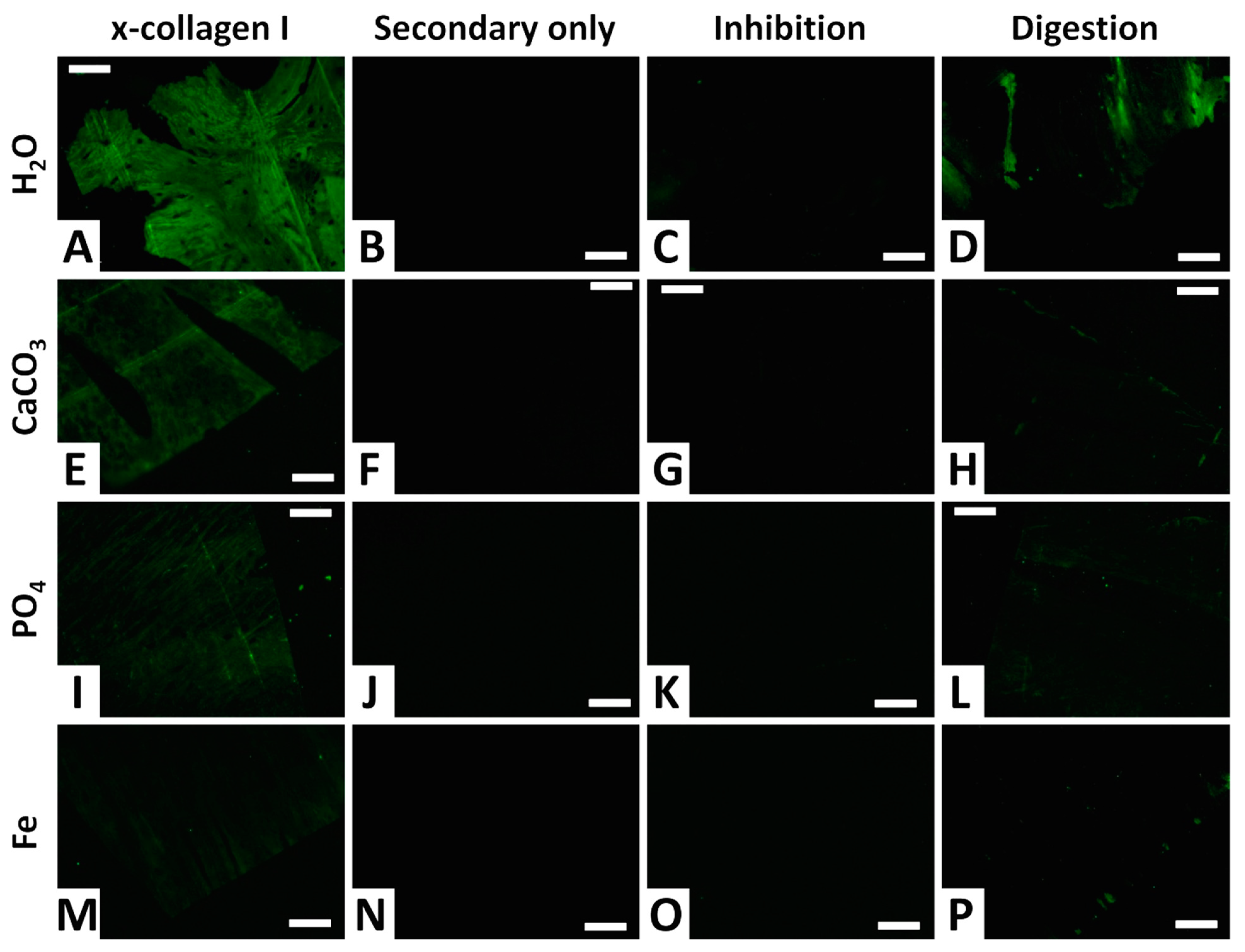
3.4. Immunofluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlando, L.; Bonjean, D.; Bocherens, H.; Thenot, A.; Argant, A.; Otte, M.; Hanni, C. Ancient DNA and the population genetics of cave bears (Ursus spelaeus) through space and time. Mol. Biol. Evol. 2002, 19, 1920–1933. [Google Scholar] [CrossRef] [PubMed]
- Asara, J.M.; Schweitzer, M.H.; Freimark, L.M.; Phillips, M.; Cantley, L.C. Protein sequences from mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science 2007, 316, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Vander Heiden, M.G.; Neveu, J.M.; et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Bern, M. Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone 2013, 52, 414–423. [Google Scholar] [CrossRef]
- Cappellini, E.; Jensen, L.J.; Szklarczyk, D.; Ginolhac, A.; da Fonseca, R.A.R.; Stafford, T.W., Jr.; Holen, S.R.; Collins, M.J.; Orlando, L.; Willerslev, E.; et al. Proteomic analysis of a Pleistocene mammoth femur reveals more than one hundred ancient bone proteins. J. Proteome Res. 2012, 11, 917–926. [Google Scholar] [CrossRef]
- Lindgren, J.; Uvdal, P.; Engdahl, A.; Lee, A.H.; Alwmark, C.; Bergquist, K.-E.; Nilsson, E.; Ekström, P.; Rasmussen, M.; Douglas, D.A.; et al. Microspectroscopic evidence of Cretaceous bone proteins. PLoS ONE 2011, 6, e19445. [Google Scholar] [CrossRef]
- Hofreiter, M.; Collins, M.; Stewart, J.R. Ancient biomolecules in Quaternary palaeoecology. Quat. Sci. Rev. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R.; Zamdborg, L.; Zheng, W.; Lee, J.E.; Tran, J.C.; Bern, M.; Duncan, M.B.; Lebleu, V.S.; Ahlf, D.R.; et al. Mass spectrometry and antibody-based characterization of blood vessels from Brachylophosaurus canadensis. J. Proteome Res. 2015, 14, 5252–5262. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R.; Feranec, R.S.; Vashishth, D. Peptide sequences from the first Castoroides ohioensis skull and the utility of old museum collections for palaeoproteomics. Proc. R. Soc. B 2016, 283, 20160593. [Google Scholar] [CrossRef]
- Schroeter, E.R.; DeHart, C.J.; Cleland, T.P.; Zheng, W.; Thomas, P.M.; Kelleher, N.L.; Bern, M.; Schweitzer, M.H. Expansion for the Brachylophosaurus canadensis collagen I sequence and additional evidence of the preservation of Cretaceous protein. J. Proteome Res. 2017, 16, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, E.R.; Ullmann, P.V.; Zheng, W.; Schweitzer, M.H.; Lacovara, K.J. Soft-tissue, rare earth element, and molecular analyses of Dreadnoughtus schrani, an exceptionally complete titanosaur from Argentina. Biology 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskiy, I.; Hope, J.M.; Ivantsov, A.; Nettersheim, B.J.; Hallmann, C.; Brocks, J.J. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 2018, 361, 1246–1249. [Google Scholar] [CrossRef]
- Barnett, R.; Westbury, M.V.; Sandoval-Velasco, M.; Vieira, F.G.; Jeon, S.; Zazula, G.; Martin, M.D.; Ho, S.Y.W.; Mather, N.; Gopalakrishnan, S.; et al. Genomic adaptations and evolutionary history of the extinct scimitar-toothed cat. Homotherium latidens. Curr. Biol. 2020, 30, 5018–5025. [Google Scholar] [CrossRef]
- Ullmann, P.V.; Voegele, K.K.; Grandstaff, D.E.; Ash, R.D.; Zheng, W.; Schroeter, E.R.; Schweitzer, M.H.; Lacovara, K.J. Molecular tests support the viability of rare earth elements as proxies for fossil biomolecule preservation. Sci. Rep. 2020, 10, 15566. [Google Scholar] [CrossRef]
- Voegele, K.K.; Ullmann, P.V.; Boles, Z.M.; Schroeter, E.R.; Zheng, W.; Schweitzer, M.H.; Lacovara, K.J. Soft tissue and biomolecular preservation in vertebrate fossils from glauconitic, shallow marine sediments of the Hornerstown Formation, Edelman Fossil Park, New Jersey. Biology 2022, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.; Collins, M.; Thomas-Oates, J.; Wilson, J.C. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 3843–3854. [Google Scholar] [CrossRef]
- Buckley, M.; Larkin, N.; Collins, M. Mammoth and Mastodon collagen sequences; survival and utility. Geochim. Cosmochim. Acta 2011, 75, 2007–2016. [Google Scholar] [CrossRef]
- Organ, C.L.; Schweitzer, M.H.; Zheng, W.; Freimark, L.M.; Cantley, L.C.; Asara, J.M. Molecular phylogenetics of Mastodon and Tyrannosaurus rex. Science 2008, 320, 499. [Google Scholar] [CrossRef]
- Welker, F.; Collins, M.J.; Thomas, J.A.; Wadsley, M.; Brace, S.; Cappellini, E.; Turvey, S.T.; Requero, M.; Gelfo, J.N.; Kramarz, A.; et al. Ancient proteins resolve the evolutionary history of Darwin’s South American ungulates. Nature 2015, 522, 81–84. [Google Scholar] [CrossRef]
- Cappellini, E.; Welker, F.; Pandolfi, L.; Ramos-Madrigal, J.; Samodova, D.; Rüther, P.L.; Fotakis, A.K.; Lyon, D.; Moreno-Mayar, J.V.; Bukhsianidze, M.; et al. Early Pleistocene enamel proteome from Dmanis resolves Stephanorhinus phylogeny. Nature 2019, 574, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.; Recabarren, O.P.; Lawless, C.; García, N.; Pino, M. A molecular phylogeny of the extinct South American gomphothere through collagen sequence analysis. Quat. Sci. Rev. 2019, 224, 105882. [Google Scholar] [CrossRef]
- Buckley, M.; Harvey, V.L.; Orihuela, J.; Mychajliw, A.M.; Keating, J.N.; Milan, J.N.A.; Lawless, C.; Chamberlain, A.T.; Egerton, V.M.; Manning, P.L. Collagen sequence analysis reveals evolutionary history of extinct West Indies Nesophontes (island-shrews). Mol. Biol. Evol. 2020, 37, 2931–2943. [Google Scholar] [CrossRef] [PubMed]
- Presslee, S.; Slater, G.J.; Pujos, F.; Forasiepi, A.M.; Fischer, R.; Molloy, K.; Mackie, M.; Olsen, J.V.; Kramarz, A.; Taglioretti, M.; et al. Palaeoproteomics resolves sloth relationships. Nat. Ecol. Evol. 2019, 3, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Pääbo, S.; Poinar, H.; Serre, D.; Jaenicke-Després, V.; Hebler, J.; Rohland, N.; Kuch, M.; Krause, J.; Vigilant, L.; Hofreiter, M. Genetic analyses from ancient DNA. Annu. Rev. Genet. 2004, 38, 645–679. [Google Scholar] [CrossRef] [PubMed]
- Rogaev, E.I.; Moliaka, Y.K.; Malyarchuk, B.A.; Kondrashov, F.A.; Derenko, M.V.; Chumakov, I.; Grigorenko, A.P. Complete mitochondrial genome and phylogeny of Pleistocene mammoth Mammuthus primigenius. PLoS Biol. 2006, 4, e73. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, T.; Pečnerová, P.; Díez-del-Molino, D.; Bergström, A.; Oppenheimer, J.; Hartmann, S.; Xenikoudakis, G.; Thomas, J.A.; Dehasque, M.; Sağlican, E.; et al. Million-year-old DNA sheds light on the genomic history of mammoths. Nature 2021, 591, 265–269. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, W.; Sawyer, R.H.; Pennington, M.W.; Zheng, X.; Wang, X.; Wang, M.; Hu, L.; O’Connor, J.; Zhao, T.; et al. The molecular evolution of feathers with direct evidence from fossils. Proc. Natl Acad. Sci. USA 2019, 116, 3018–3023. [Google Scholar] [CrossRef]
- Zimmerman, E.A.; Schaible, E.; Gludovatz, B.; Schmidt, F.N.; Riedel, C.; Krause, M.; Vettorazzi, E.; Acevedo, C.; Hahn, M.; Püshcel, K.; et al. Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions. Sci. Rep. 2016, 6, 21072. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K.; Hook, R.W.; Badgley, C.E.; Boy, J.A.; Chapman, R.E.; Dodson, P.; Gastaldo, R.A.; Graham, R.W.; Martin, L.D.; Olsen, P.E.; et al. Paleoenvironmental contexts and taphonomic modes. In Evolutionary Paleoecology of Terrestrial Plants and Animals; Behrensmeyer, A.K., Damuth, J.D., DiMichele, W.A., Potts, R., Sues, H.-D., Wing, S.L., Eds.; Chicago University Press: Chicago, IL, USA, 1992; pp. 15–136. [Google Scholar]
- Hubert, J.F.; Panish, P.T.; Chure, D.J.; Prostak, K.S. Chemistry, microstructure, petrology, and diagenetic model of Jurassic dinosaur bones, Dinosaur National Monument, Utah. J. Sediment. Res. 1996, 66, 531–547. [Google Scholar]
- Berna, F.; Matthews, A.; Weiner, S. Solubilities of bone mineral from archaeological sites: The recrystallization window. J. Anthropol. Sci. 2004, 31, 867–882. [Google Scholar] [CrossRef]
- Tütken, T.; Vennemann, T.W.; Pfretzschner, H.-U. Early diagenesis of bone and tooth apatite in fluvial and marine settings: Constraints from combined oxygen isotope, nitrogen and REE analysis. Palaeogeogr. Palaeocl. 2008, 266, 254–268. [Google Scholar] [CrossRef]
- Kowal-Linka, M.; Jochum, K.P.; Surmik, D. LA-ICP-MS analysis of rare earth elements in marine reptile bones from the Middle Triassic bonebed (Upper Silesia, S Poland): Impact of long-lasting diagenesis, and factors controlling the uptake. Chem. Geol. 2014, 363, 213–228. [Google Scholar] [CrossRef]
- Kowal-Linka, M.; Jochum, K.P. Variability of trace element uptake in marine reptile bones from three Triassic sites (S Poland): Influence of diagenetic processes on the host rock and significance of the applied methodology. Chem. Geol. 2015, 397, 1–13. [Google Scholar] [CrossRef]
- Kowalewski, M.; Labarbera, M. Actualistic taphonomy: Death, decay, and disintegration in contemporary settings. PALAIOS 2004, 19, 423–427. [Google Scholar] [CrossRef]
- Carpenter, K. Experimental investigation of the role of bacteria in bone fossilization. Neues Jahrb. Geol. Paläontologie Mon. 2005, 2, 83–94. [Google Scholar] [CrossRef]
- Daniel, J.C.; Chin, K. The role of bacterially mediated precipitation in the permineralization of bone. PALAIOS 2010, 25, 507–516. [Google Scholar] [CrossRef]
- Varricchio, D.J.; Jackson, F.J.; Scherzer, B.; Shelton, J. Don’t have a cow, man! It’s only actualistic taphonomy on the Yellowstone River of Montana. J. Vert. Paleo. 2005, 25 (Suppl. 3), 126A. [Google Scholar]
- Voorhies, M.R. Taphonomy and Population Dynamics of an Early Pliocene Vertebrate Fauna, Knox County, Nebraska; Contributions to Geology Special Paper No. 1; University of Wyoming: Laramie, WY, USA, 1969; Volume 1, pp. 1–69. [Google Scholar]
- Aslan, A.; Behrensmeyer, A.K. Taphonomy and time resolution of bone assemblages in a contemporary fluvial system: The East Fork River, Wyoming. PALAIOS 1996, 11, 411–421. [Google Scholar] [CrossRef]
- Kohn, M.J.; Moses, R.J. Trace element diffusivities in bone rule out simple diffusive uptake during fossilization but explain in vivo uptake and release. Proc. Natl. Acad. Sci. USA 2013, 110, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Krajcarz, M.T. Alteration of the metal content in animal bones after 2.5-year experimental exposure to sediments. Achaeol. Anthropol. Sci. 2019, 11, 361–372. [Google Scholar] [CrossRef]
- Peterson, J.E.; Lenczewski, M.E.; Clawson, S.R.; Warnock, J.P. Role of sediment size and biostratinomy on the development of biofilms in recent avian vertebrate remains. Front. Earth Sci. 2017, 5, 30. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K. Taphonomic and ecologic information from bone weathering. Paleobiology 1978, 4, 150–162. [Google Scholar] [CrossRef]
- Brett, C.E.; Baird, G.C. Comparative taphonomy: A key to paleoenvironmental interpretation based on fossil preservation. PALAIOS 1986, 1, 207–227. [Google Scholar] [CrossRef]
- Madgwick, R.; Mulville, J. Investigating variation in the prevalence of weathering in faunal assemblages in the UK: A multivariate statistical approach. Int. J. Osteoarchaeol. 2011, 22, 509–522. [Google Scholar] [CrossRef]
- Pokines, J.T.; Faillace, K.; Berger, J.; Pirtle, D.; Sharpe, M.; Curtis, A.; Lombardi, K.; Admans, J. The effects of repeated wet-dry cycles as a component of bone weathering. J. Archaeol. Sci. Rep. 2018, 17, 433–441. [Google Scholar] [CrossRef]
- Schroeter, E.R.; DeHart, C.J.; Schweitzer, M.H.; Thomas, P.M.; Keller, N.L. Bone protein “extractomics”: Comparing the efficiency of bone protein extractions of Gallus gallus in tandem mass spectrometry. PeerJ 2016, 4, e2603. [Google Scholar] [CrossRef] [PubMed]
- Ayars, J.; Gao, Y. Atmospheric nitrogen deposition to the Mullica River-Great Bay Estuary. Mar. Environ. Res. 2007, 64, 590–600. [Google Scholar] [CrossRef]
- Iannuzzi, T.J.; Armstrong, T.N.; Thelen, J.B.; Ludwig, D.F.; Firstenberg, C.E. Characterization of chemical contamination in shallow-water estuarine habitats of an industrialized river. Part I: Organic compounds. Soil Sediment Contam. 2005, 14, 13–33. [Google Scholar] [CrossRef]
- Armstrong, T.N.; Iannuzzi, T.J.; Thelen, J.B.; Ludwig, D.F.; Firstenberg, C.E. Characterization of chemical contamination in shallow-water estuarine habitats of an industrialized river. Part II: Metals. Soil Sediment Contam. 2005, 14, 35–52. [Google Scholar] [CrossRef]
- Leduc, M.; Kasra, R.; van Heijenoort, J. Induction and control of the autolytic system of Escherichia coli. J. Bacteriol. 1982, 152, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Coolbear, T.; Whittaker, J.M.; Daniel, R.M. The effect of metal ions on the activity and thermostability of the extracellular proteinase from a thermophilic Bacillus, strain EA.1. Biochem. J. 1992, 287, 367–374. [Google Scholar] [CrossRef] [PubMed]
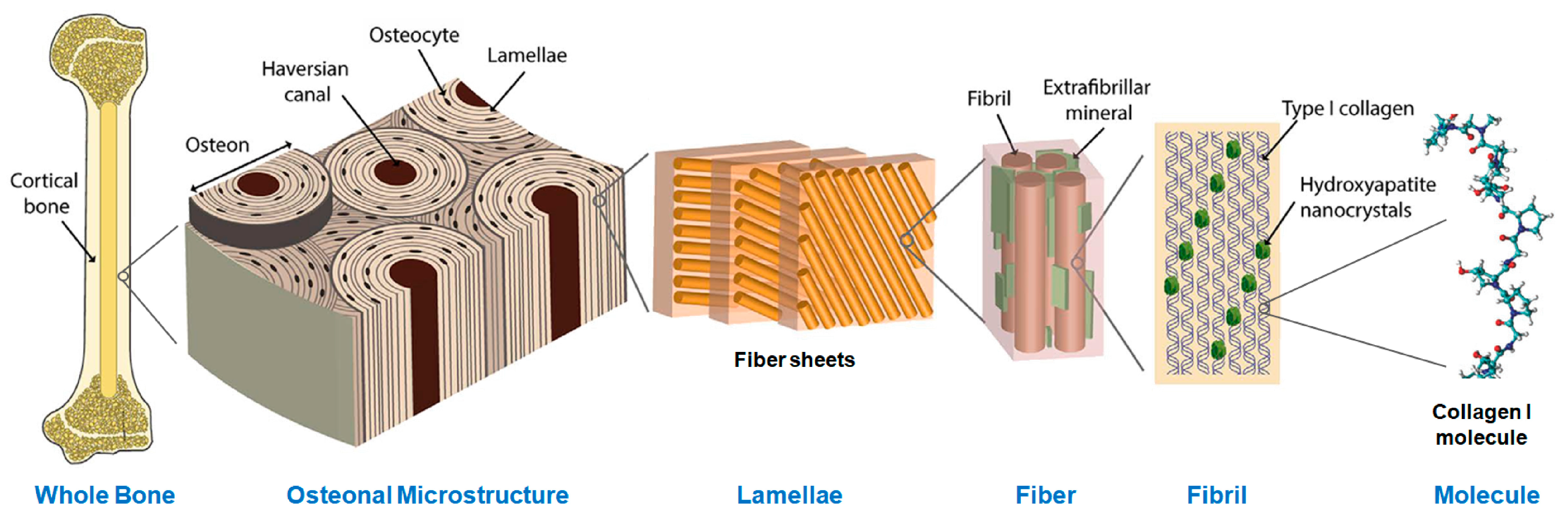
- Francillon-Vieillot, H.; de Buffrénil, V.; Castanet, J.; Géraudie, J.; Meunier, F.J.; Sire, J.Y.; Zylberberg, L.; de Ricqlès, A. Microstructure and mineralization of vertebrate skeletal tissues. In Skeletal Biomineralization Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; pp. 471–547. [Google Scholar]
- Ehrlich, H.L. How microbes influence mineral growth and dissolution. Chem. Geol. 1996, 132, 5–9. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Wings, O. Authigenic minerals in fossil bones from the Mesozoic of England: Poor correlation with depositional environments. Palaeogeog. Palaeocl. 2004, 204, 15–32. [Google Scholar] [CrossRef]
- Rogoz, A.; Sawlowicz, Z.; Wojtal, P. Diagenetic history of Woolly Mammoth (Mammuthus primigenius) skeletal remains from the archaeological site Cracow Spadzista Street (B), southern Poland. PALAIOS 2012, 27, 541–549. [Google Scholar] [CrossRef]
- Bodzioch, A. Idealized model of mineral infillings in bones of fossil freshwater animals, on the example of Late Triassic metoposaurs from Krasiejów (Poland). Austin J. Earth Sci. 2015, 2, 1008. [Google Scholar]
- Coto, B.; Martos, C.; Peña, J.L.; Rodríguez, R.; Pastor, G. Effects in the solubility of CaCO3: Experimental study and model description. Fluid Phase Equilibr. 2012, 324, 1–7. [Google Scholar] [CrossRef]
- Livingstone, D.A. Chemical composition of rivers and lakes. In Data of Geochemistry; Fleischer, M., Ed.; USGS: Washington, DC, USA, 1963; pp. 1–64. [Google Scholar]
- Chapelle, F.H.; Lovley, D.R. Competitive exclusion of sulfate reduction by Fe(III)-reducing bacteria: A mechanism for producing discrete zones of high-iron ground water. Groundwater 1992, 30, 29–36. [Google Scholar] [CrossRef]
- Previtera, E.; Mancuso, A.C.; de la Fuente, M.S.; Sánchez, E.S. Diagenetic analyses of tetrapod from the Upper Triassic, Puesto Viejo Group, Argentina. Andean Geol. 2016, 43, 197–214. [Google Scholar] [CrossRef]
- Previtera, E. Bone microstructure and diagenesis of saurischian dinosaurs from the Upper Cretaceous (Neuquén Group), Argentina. Andean Geol. 2017, 44, 39–58. [Google Scholar] [CrossRef]
- Ullmann, P.V.; Pandya, S.H.; Nellermoe, R. Patterns of soft tissue and cellular preservation in relation to fossil bone microstructure and overburden depth at the Standing Rock Hadrosaur Site, Maastrichtian Hell Creek Formation, South Dakota, USA. Cretaceous Res. 2019, 99, 1–13. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R. Soft tissue and cellular preservation in vertebrate skeletal elements from the Cretaceous to the present. Proc. R. Soc. B 2007, 274, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Goodwin, M.B.; Boatman, E.; Theil, E.; Marcus, M.A.; Fakra, S.C. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proc. R. Soc. B 2014, 281, 20132741. [Google Scholar] [CrossRef]
- Boatman, E.M.; Goodwin, M.B.; Holman, H.-Y.N.; Fakra, S.; Zheng, W.; Gronsky, R.; Schweitzer, M.H. Mechanisms of soft tissue and protein preservation in Tyrannosaurus rex. Sci. Rep. 2019, 9, 15678. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Metallic ion binding by Bacillus subtilis: Implications for the fossilization of microorganisms. Geology 1988, 16, 149–152. [Google Scholar] [CrossRef]
- Hirschler, A.; Lucas, J.; Hubert, J.-C. Apatite genesis: A biologically induced or biologically controlled mineral formation process? Geomicrobiol. J. 1990, 7, 47–57. [Google Scholar] [CrossRef]
- Prevot, L.; Lucas, J. Phosphate. In Paleobiology: A Synthesis; Briggs, D.E.G., Crowther, P.R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1990; pp. 256–257. [Google Scholar]
- Martill, D.M. Preservation of fish in the Cretaceous Santana Formation of Brazil. Paleontology 1988, 32, 1–18. [Google Scholar]
- Martill, D.M. Macromolecular resolution of fossilized muscle tissue from an elopomorph fish. Nature 1990, 346, 171–172. [Google Scholar] [CrossRef]
- Kellner, A.W.A. Fossilized theropod soft tissue. Nature 1996, 379, 32. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Wilby, P.R.; Perez-Moreno, B.P.; Sanz, J.L.; Fregenal-Martinez, M. The mineralization of dinosaur soft tissue in the Lower Cretaceous of Las Hoyas, Spain. J. Geol. Soc. 1997, 154, 587. [Google Scholar] [CrossRef]
- Briggs, D.E.G. The role of decay and mineralization in the preservation of soft-bodied fossils. Annu. Rev. Earth Pl. Sc. 2003, 31, 275–301. [Google Scholar] [CrossRef]
- Zhu, M.; Babcock, L.E.; Steiner, M. Fossilization modes in the Chengjiang Lagerstatte (Cambrian of China): Testing the roles of organic preservation and diagenetic alteration in exceptional preservation. Palaeogeogr. Palaeocl. 2005, 220, 31–46. [Google Scholar] [CrossRef]
- Sheldon, R.P. Ancient marine phosphates. Annu. Rev. Earth Pl. Sc. 1981, 9, 251–284. [Google Scholar] [CrossRef]
- McNamara, M.E.; Orr, P.J.; Kearns, S.L.; Alcalá, L.; Anadón, P.; Peñalver-Mollá, E. High-fidelity organic preservation of bone marrow in ca. 10 Ma amphibians. Geology 2006, 34, 641–644. [Google Scholar] [CrossRef]
- Lamm, E.-T. Preparation and sectioning of specimens. In Bone Histology of Fossil Tetrapods; Padian, K., Lamm, E.-T., Eds.; University of California Press: Berkeley, CA, USA, 2013; pp. 55–160. [Google Scholar]
- Schroeter, E.A.R. The Morphology, Histology, and Molecular Preservation of an Exceptionally Complete Titanosaur from Southernmost Patagonia. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2013. [Google Scholar]
- Zheng, W.; Schweitzer, M.H. Chemical analyses of fossil bone. In Forensic Microscopy for Skeletal Tissues: Methods and Protocols; Bell, L., Ed.; Humana Press: New York, NY, USA, 2012; pp. 153–172. [Google Scholar]
- Hedges, R.E.M.; Millard, A.R.; Pike, A.W.G. Measurements and relationships of diagenetic alteration of bone from three archaeological sites. J. Archaeol. Sci. 1995, 22, 201–209. [Google Scholar] [CrossRef]
- Jans, M.M.E. Microbial bioerosion of bone—A review. In Current Developments in Bioerosion; Wisshak, M., Tapanila, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 397–413. [Google Scholar]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of activity changes increases the fatigue life of the porous magnesium scaffold, as observed in dynamic immersion tests, over time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Jans, M.M.E.; Kars, H.; Nielsen-Marsh, C.M.; Smith, C.I.; Nord, A.G.; Arthur, P.; Earl, N. In situ preservation of archaeological bone: A histological study within a multidisciplinary approach. Archaeometry 2002, 44, 343–352. [Google Scholar] [CrossRef]
- Ostlund, E.N.; Crom, R.L.; Pedersen, D.D.; Johnson, D.J.; Williams, W.O.; Schmitt, B.J. Equine West Nile encephalitis, United States. Emerg. Infect. Dis. 2001, 7, 665–669. [Google Scholar] [CrossRef]
- Appiah, A.S.; Amoatey, H.M.; Klu, G.Y.P.; Afful, N.T.; Owusu, G.K. Spread of African cassava mosaic virus from cassava (Manihot esculenta Crantz) to physic nut (Jatropha curcas L.) in Ghana. J. Phytol. 2012, 4, 31–37. [Google Scholar]
- Schmidt-Schultz, T.H.; Schultz, M. Bone protects proteins over thousands of years: Extraction, analysis, and interpretation of extracellular matrix proteins in archaeological skeletal remains. Am. J. Phys. Anthropol. 2004, 128, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.; Kang, A.H. Studies on the specificity of bacterial collagenase. Biochem. Bioph. Res. Co. 1970, 41, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.A.; McLean, R.J.C.; Upchurch, G.R.; Folk, R.L. Enhancement of leaf fossilization potential by bacterial biofilms. Geology 1997, 25, 1119–1122. [Google Scholar] [CrossRef]
- Child, A.M. Microbial taphonomy of archaeological bone. Stud. Conserv. 1995, 40, 19–30. [Google Scholar]
- Retallack, G.J. Completeness of the rock and fossil record: Some estimates using fossil soils. Paleobiology 1984, 10, 59–78. [Google Scholar] [CrossRef]
- Retallack, G.J. Dinosaurs and dirt. In Dinofest International Proceedings; Wolberg, D.L., Stump, E., Rosenberg, G.D., Eds.; Academy of Natural Sciences: Philadelphia, PA, USA, 1997; pp. 345–359. [Google Scholar]
- Ullmann, P.V.; Shaw, A.; Nellermoe, R.; Lacovara, K.J. Taphonomy of the Standing Rock Hadrosaur Site, Corson County, South Dakota. PALAIOS 2017, 32, 779–796. [Google Scholar] [CrossRef]
- Peterson, J.E.; Lenczewski, M.E.; Scherer, R.P. Influence of microbial biofilms on the preservation of primary soft tissue in fossil and extant archosaurs. PLoS ONE 2010, 5, e13334. [Google Scholar] [CrossRef]
- Ullmann, P.V.; Macauley, K.; Ash, R.D.; Shoup, B.; Scannella, J.B. Taphonomic and diagenetic pathways to protein preservation, part I: The case of Tyrannosaurus rex specimen MOR 1125. Biology 2021, 10, 1193. [Google Scholar] [CrossRef]
- Berner, R.A. Calcium carbonate concretions formed by the decomposition of organic matter. Science 1968, 159, 195–197. [Google Scholar] [CrossRef]
- Allison, P.A. Konservat-Lagerstätten: Cause and classification. Paleobiology 1988, 14, 331–344. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Kear, A.J. Fossilization of soft tissue in the laboratory. Science 1993, 259, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Sagemann, J.; Bale, S.J.; Briggs, D.E.G.; Parkes, R.J. Controls on the formation of authigenic minerals in association with decaying organic matter: An experimental approach. Geochim. Cosmochim. Acta 1999, 63, 1083–1095. [Google Scholar] [CrossRef]
- Martin, D.; Briggs, D.E.G.; Parkes, R.J. Experimental attachment of sediment particles to invertebrate eggs and the preservation of soft-bodied fossils. J. Geol. Soc. 2004, 161, 735–738. [Google Scholar] [CrossRef]
- Kral, A.G.; Ziegler, A.; Tütken, T.; Geisler, T. Experimental aqueous alteration of cortical bone microarchitecture analyzed by quantitative micro-computed tomography. Front. Earth Sci. 2021, 9, 609496. [Google Scholar] [CrossRef]
- Kral, A.G.; Lagos, M.; Guagliardo, P.; Tütken, T.; Geisler, T. Rapid alteration of cortical bone in fresh- and seawater solutions visualized and quantified from the millimeter down to the atomic scale. Chem. Geol. 2022, 609, 121060. [Google Scholar] [CrossRef]
- Trueman, C.N.; Martill, D.M. The long-term survival of bone: The role of bioerosion. Archaeometry 2002, 44, 371–382. [Google Scholar] [CrossRef]
- Javan, G.T.; Finley, S.J.; Can, I.; Wilkinson, J.E.; Hanson, J.D.; Tarone, A.M. Human thanatomicrobiome succession and time since death. Sci. Rep. 2016, 6, 29598. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Qi, X.; Shi, L.; Zhang, J.; Zhang, X.; Yang, T.; Ren, J.; Liu, F.; Zhang, G.; et al. Predicting the postmortem interval of burial cadavers based on microbial community succession. Forensic Sci. Int.-Gen. 2021, 52, 102488. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth-Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Farmer, J. Taphonomic models in microbial fossilization. In Size Limits of Very Small Organisms: Proceedings of a Workshop; Farmer, J., Fogel, M.L., Lawrence, J., Lester, M.I., Olsen, G.J., Eds.; National Academy Press: Washington, DC, USA, 1999; pp. 94–102. [Google Scholar]
- Liebig, K. Bacteria. In Palaeobiology II; Crowther, P.R., Ed.; Blackwell Publishing: Oxford, UK, 2001; pp. 253–256. [Google Scholar]
- Hitchcock, A.P.; Dynes, J.J.; Lawrence, J.R.; Obst, M.; Swerhone, G.D.W.; Korber, D.R.; Leppard, G.G. Soft X-ray spectromicroscopy of nickel sorption in a natural river biofilm. Geobiology 2009, 7, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Elmore, K.L.; Hatfield, J.D.; Dunn, R.L.; Jones, A.D. Dissociation of phosphoric acid solution at 25°. J. Phys. Chem. 1965, 69, 3520–3525. [Google Scholar] [CrossRef]
- Toporski, J.K.W.; Steele, A.; Westall, F.; Avci, R.; Martill, D.M.; McKay, D.S. Morphological and spectral investigation of exceptionally well-preserved bacterial biofilms from the Oligocene Enspel Formation, Germany. Geochim. Cosmochim. Acta 2002, 66, 1773–1791. [Google Scholar] [CrossRef]
- Atkins, P.; Jones, L. Chemical Principles, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2009; pp. 1–1024. [Google Scholar]
- Schweitzer, M.H.; Schroeter, E.R.; Cleland, T.P.; Zheng, W. Paleoproteomics of Mesozoic dinosaurs and other Mesozoic fossils. Proteomics 2019, 19, 1800251. [Google Scholar] [CrossRef]
- Fernández-Jalvo, Y.; Andrews, P.; Pesquero, D.; Smith, C.; Marín-Monfort, D.; Sánchez, B.; Geigl, E.-M.; Alonso, A. Early bone diagenesis in temperate environments Part I: Surface features and histology. Palaeogeogr. Palaeocl. 2010, 288, 62–81. [Google Scholar] [CrossRef]
- Trueman, C.N.G.; Behrensmeyer, A.K.; Tuross, N.; Weiner, S. Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenyz: Diagenetic mechanisms and the role of sediment pore fluids. J. Archaeol. Sci. 2004, 31, 721–739. [Google Scholar] [CrossRef]


| Trial | HI after Experiment | Histologic Alterations | Relative ELISA Signal | Relative IF Signal |
|---|---|---|---|---|
| H2O (control) | 5 | Some osteocytes lost to decay in internal cortex | High | High |
| CaCO3 | 5 | Common calcite infilling of Haversian canals, linings in medullary cavity | High | High |
| PO4 | 5 | Minimal signs of alteration, no mineral precipitate/linings | Moderate | Moderate |
| Fe | 5 | Common iron hydroxide infillings and linings, nearly all osteocytes lost to decay | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullmann, P.V.; Voegele, K.K.; Lacovara, K.J. Actualistic Testing of the Influence of Groundwater Chemistry on Degradation of Collagen I in Bone. Minerals 2023, 13, 596. https://doi.org/10.3390/min13050596
Ullmann PV, Voegele KK, Lacovara KJ. Actualistic Testing of the Influence of Groundwater Chemistry on Degradation of Collagen I in Bone. Minerals. 2023; 13(5):596. https://doi.org/10.3390/min13050596
Chicago/Turabian StyleUllmann, Paul V., Kristyn K. Voegele, and Kenneth J. Lacovara. 2023. "Actualistic Testing of the Influence of Groundwater Chemistry on Degradation of Collagen I in Bone" Minerals 13, no. 5: 596. https://doi.org/10.3390/min13050596







