Impact of Solid Hydrocarbon on the Composition of Fluid Phase at the Subduction (Experimental Simulation)
Abstract
:1. Introduction
2. Materials and Methods
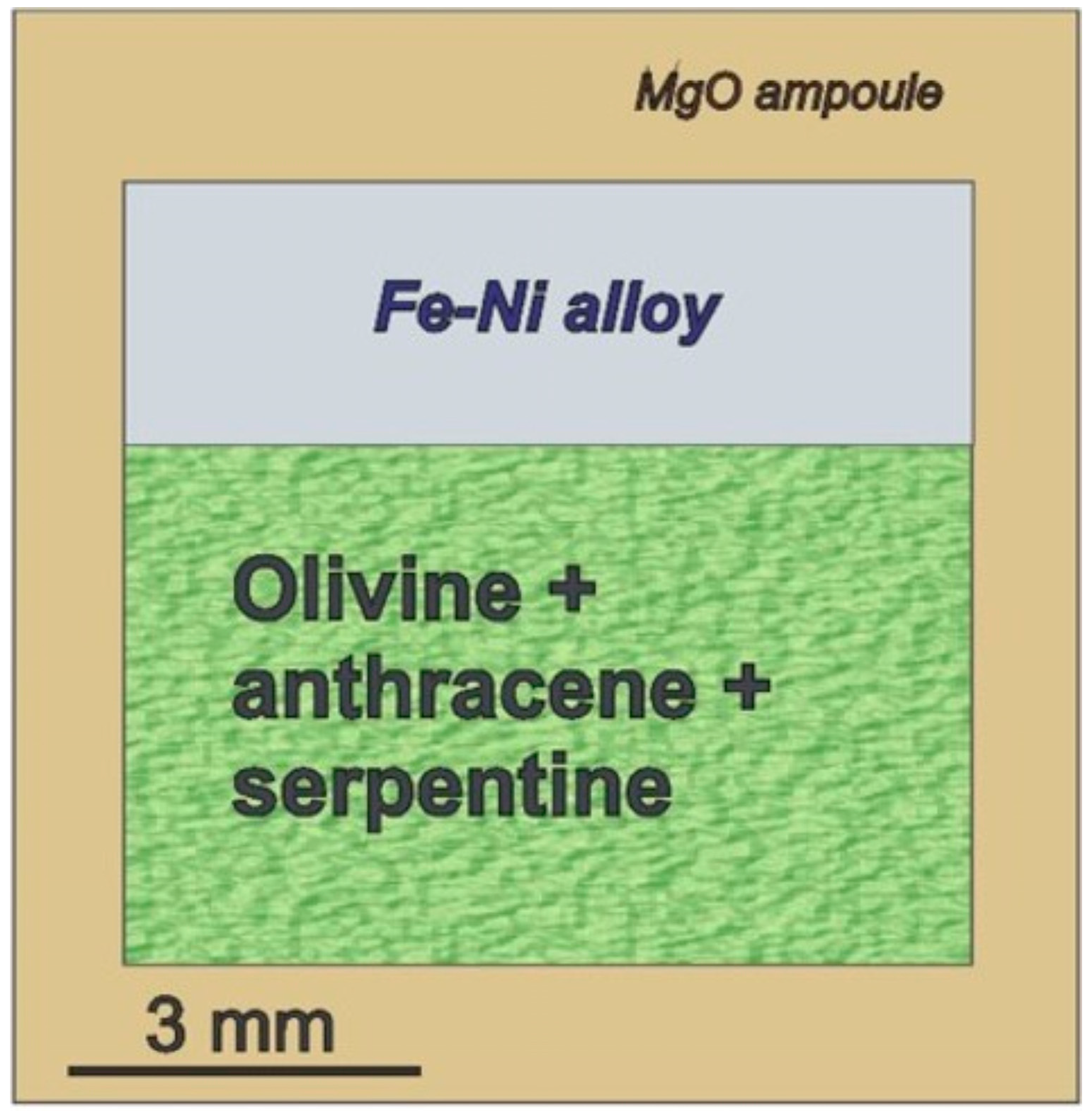
2.1. Experiments
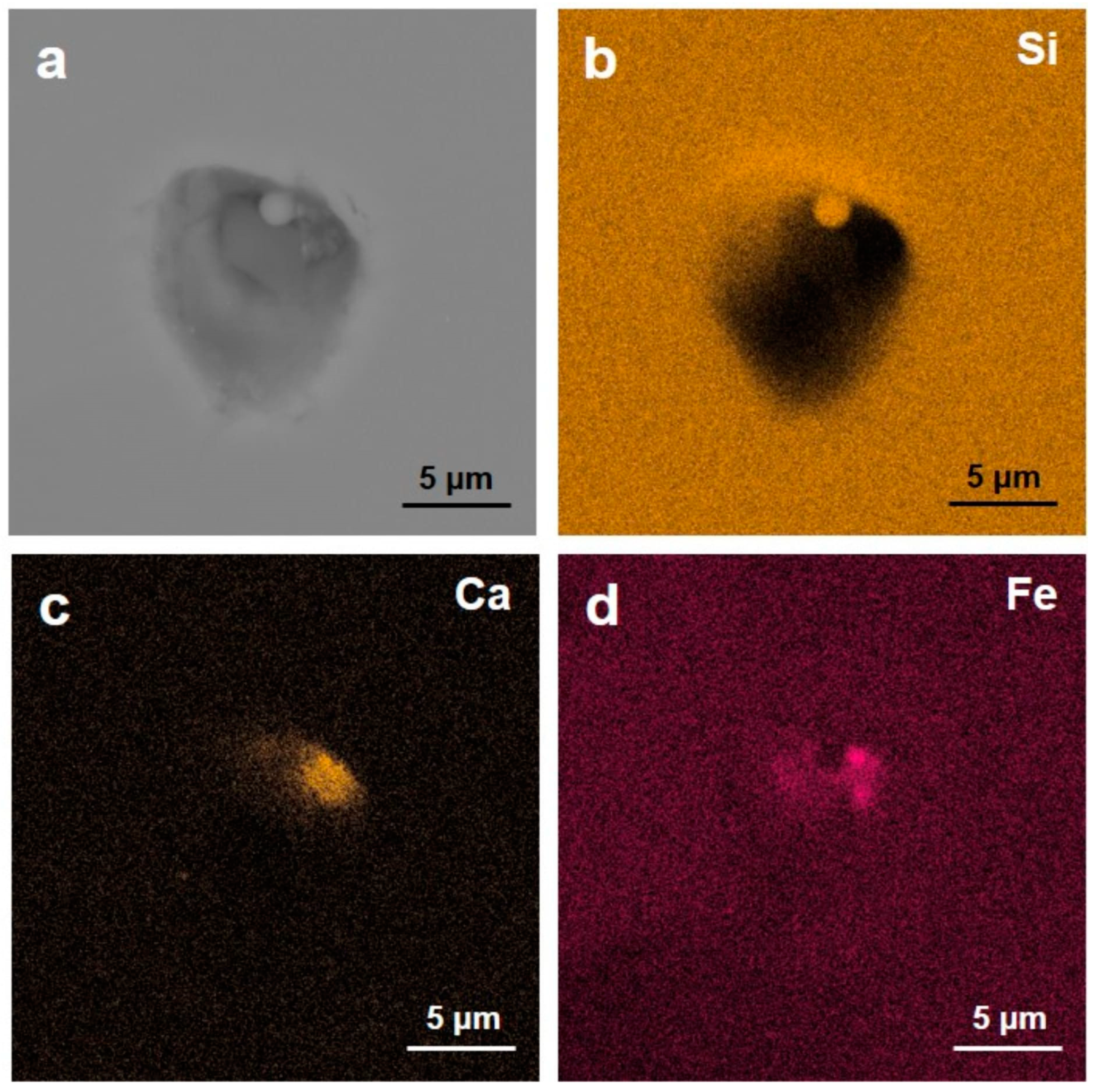
2.2. Scanning Electron Microscopy
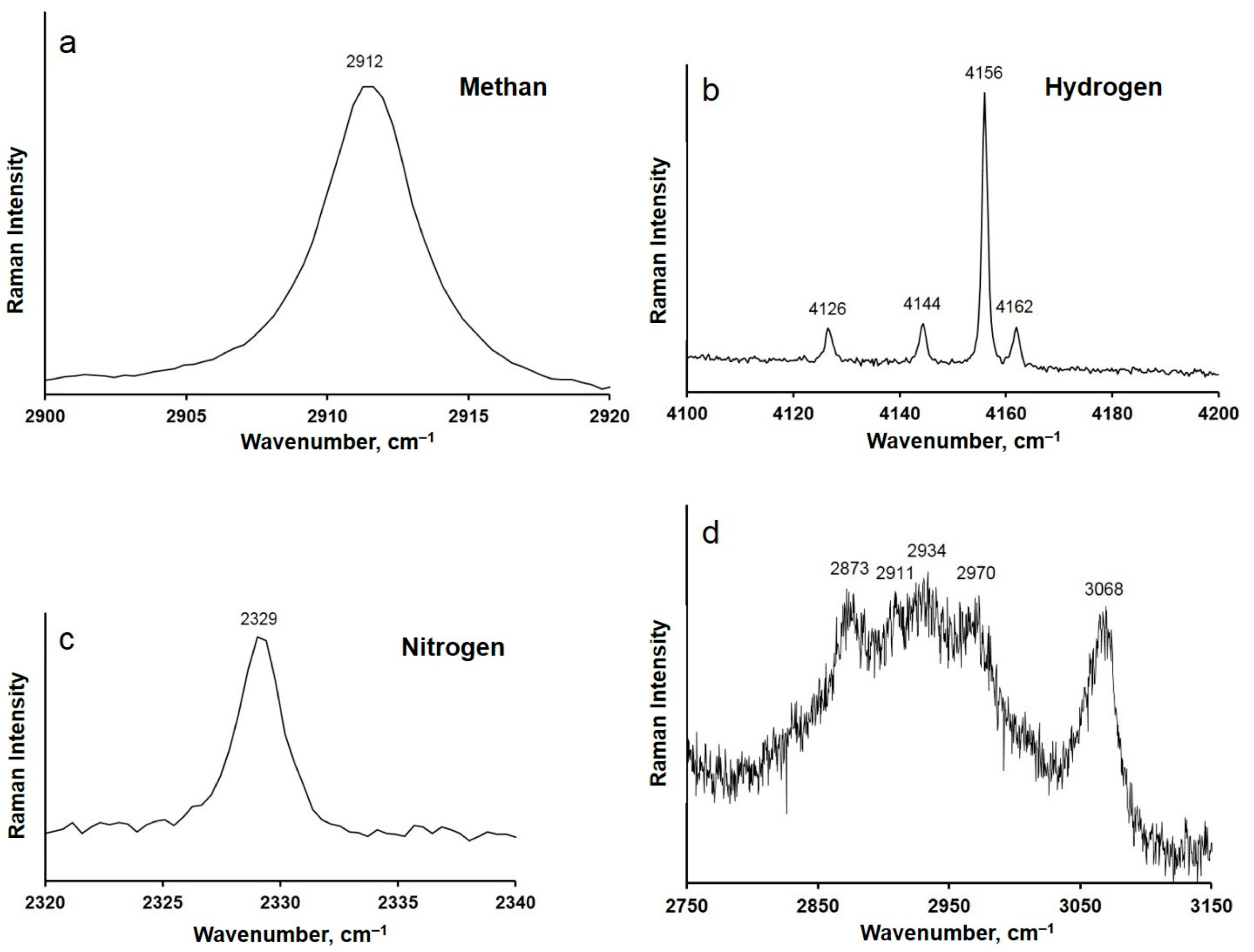
2.3. Raman Spectroscopy Analysis
2.4. GC–MS Analysis
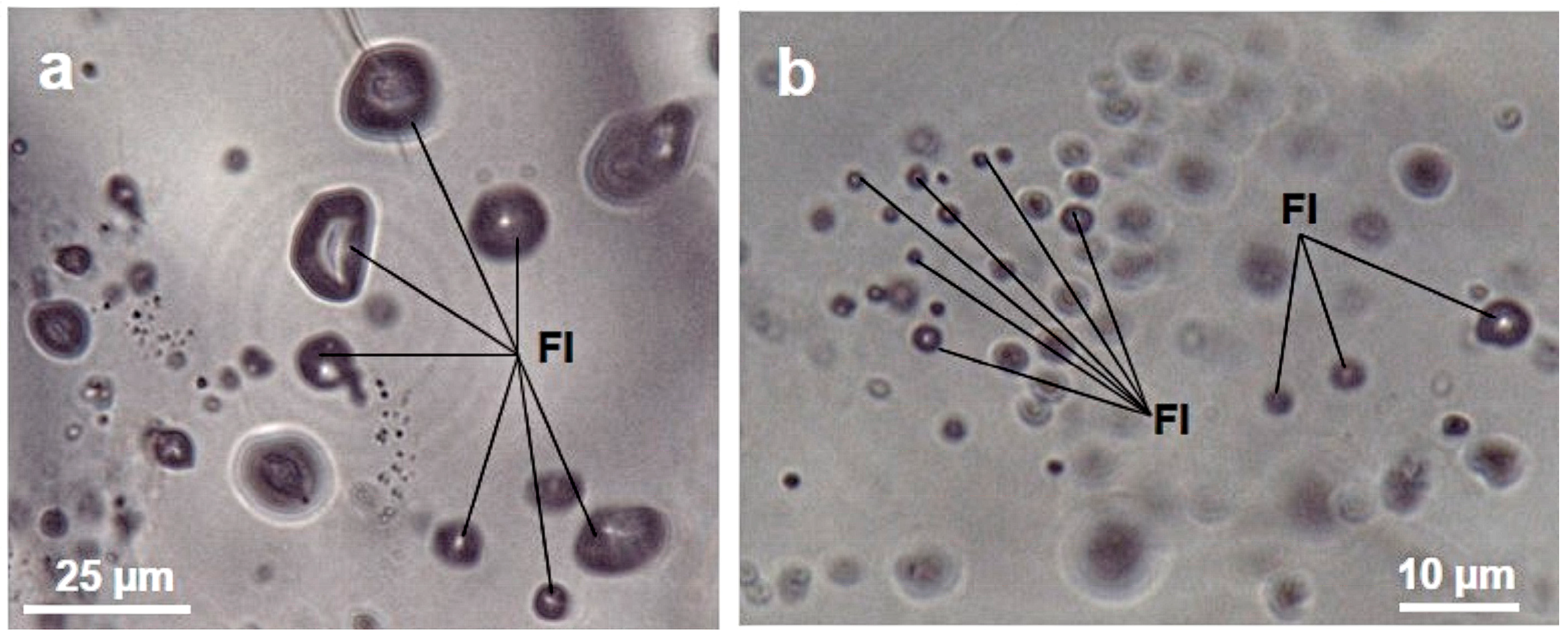
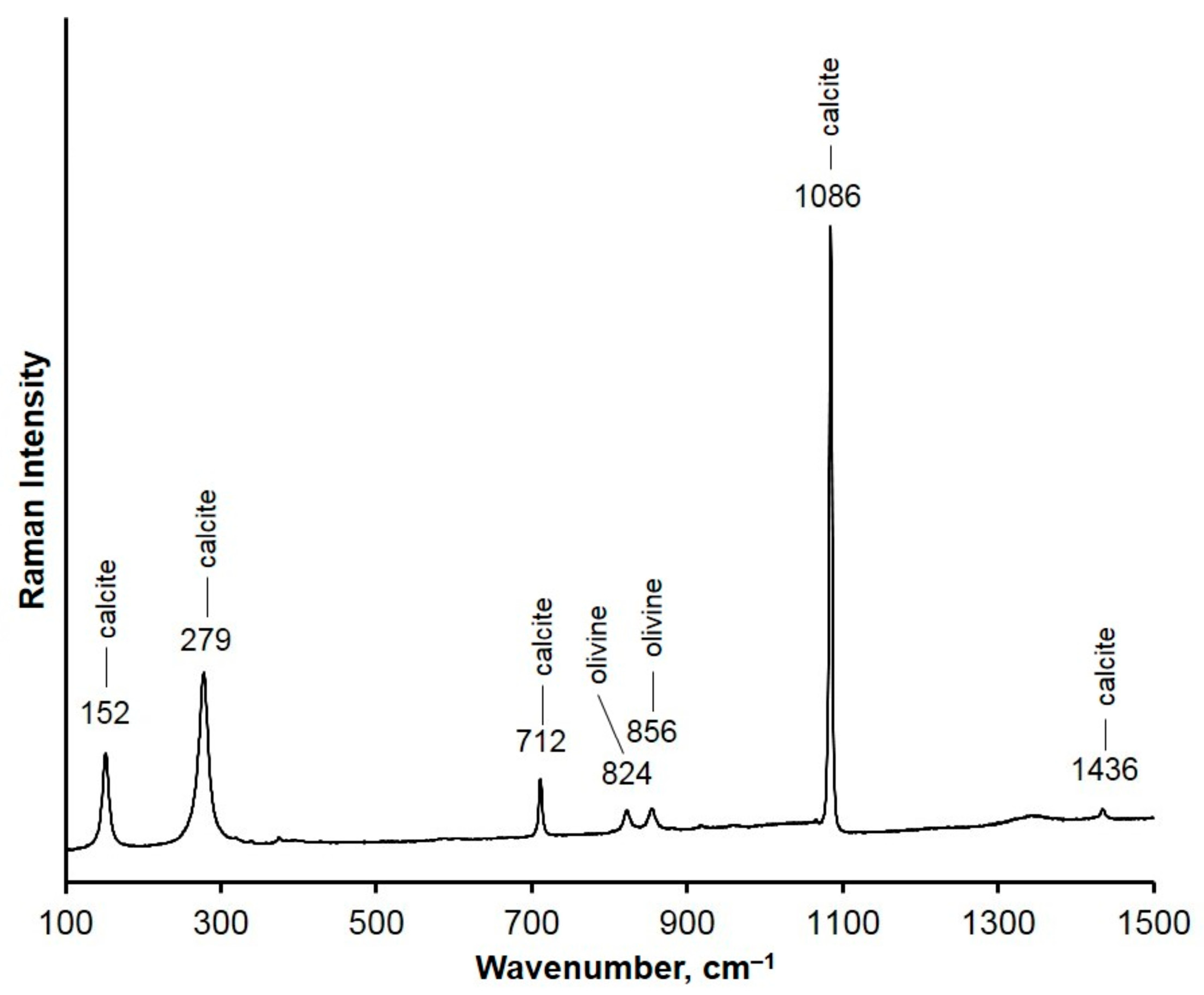
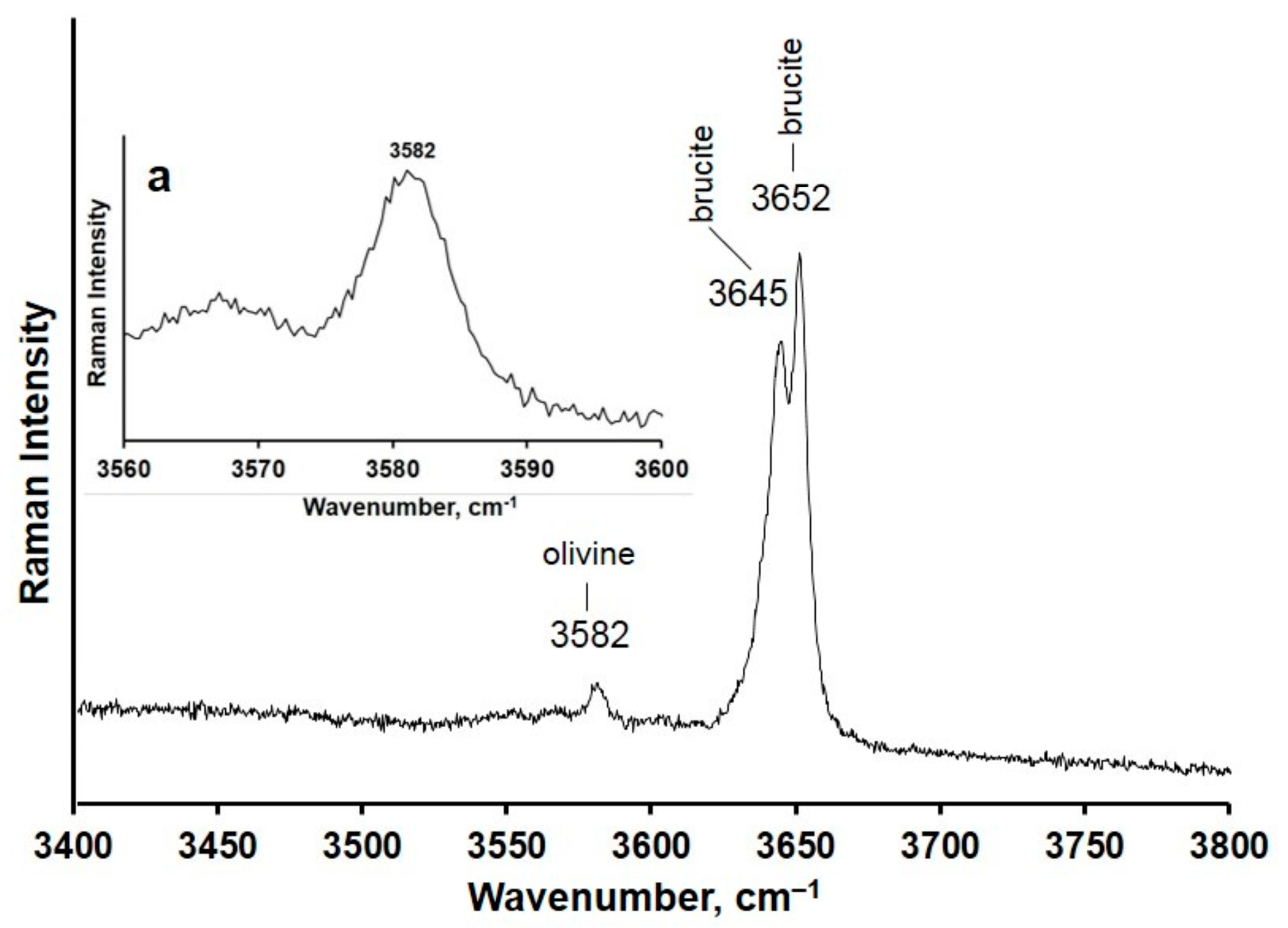
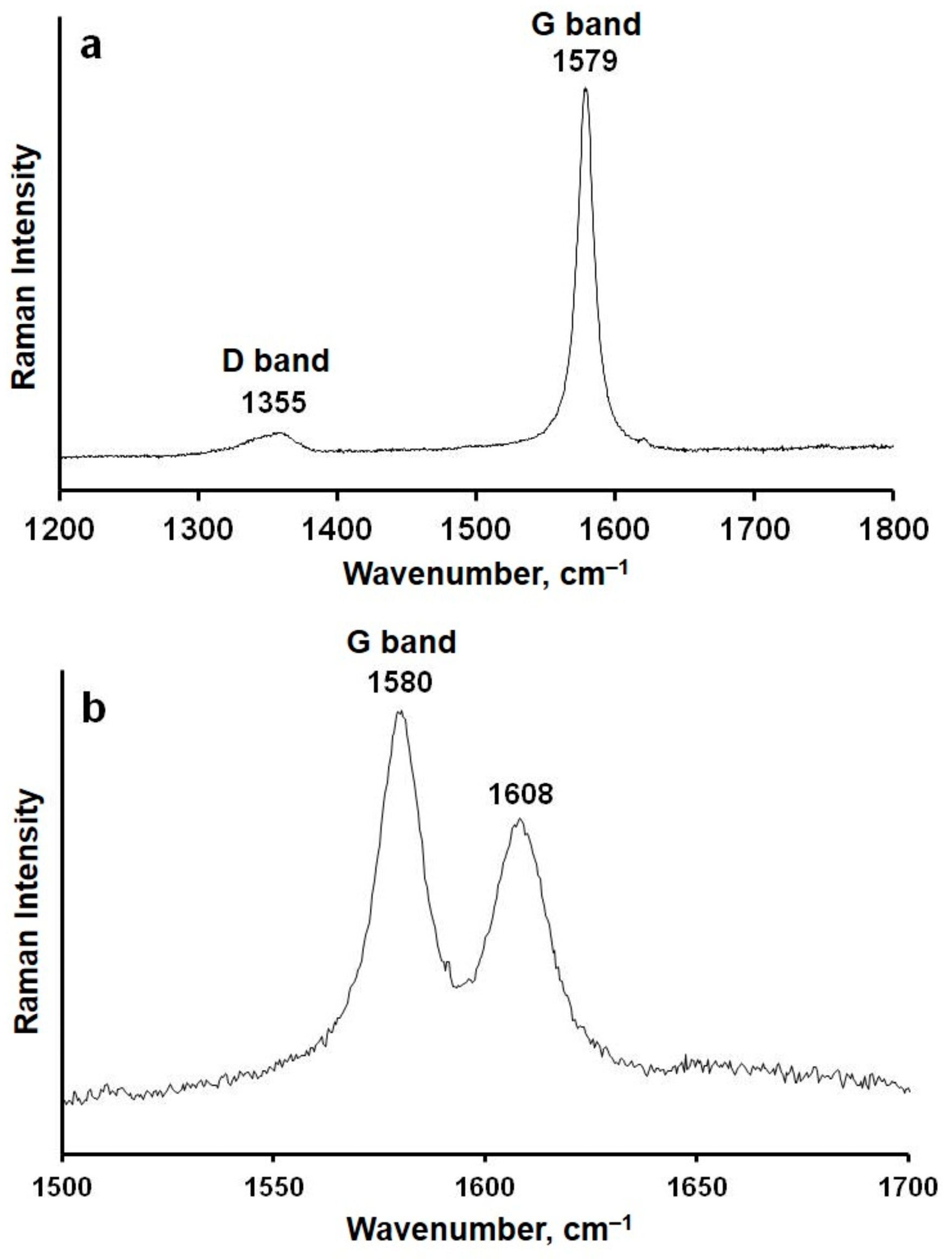
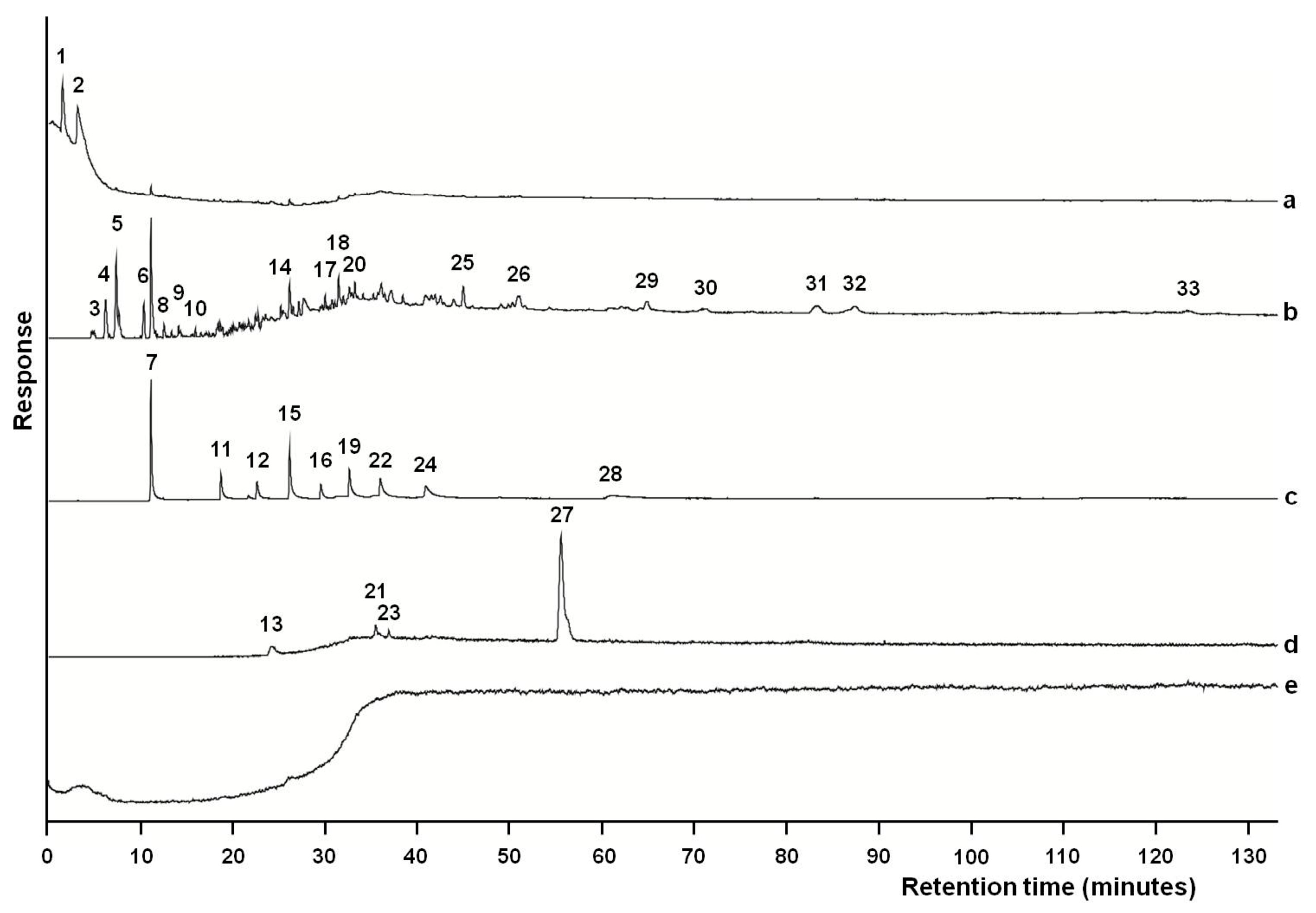
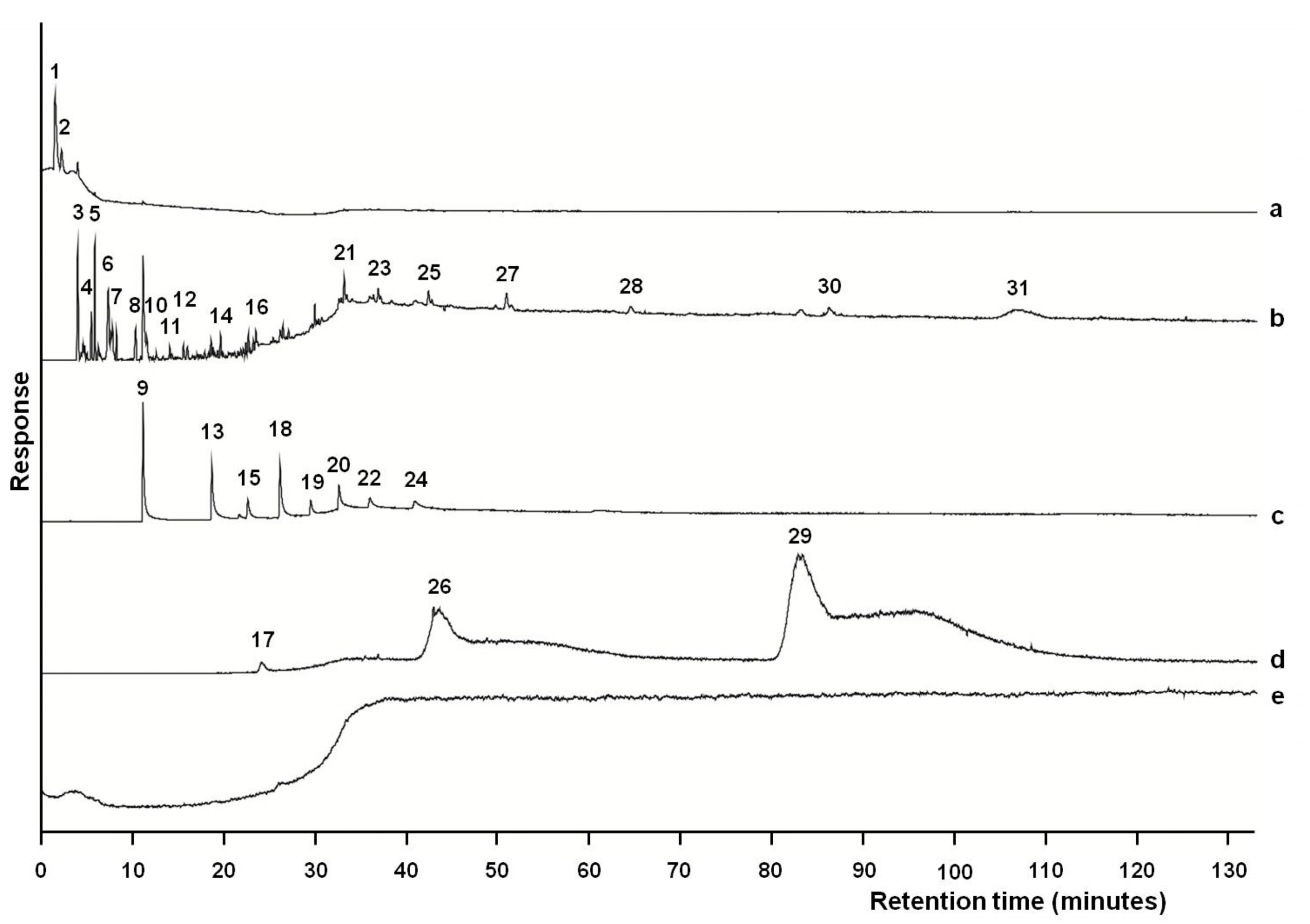
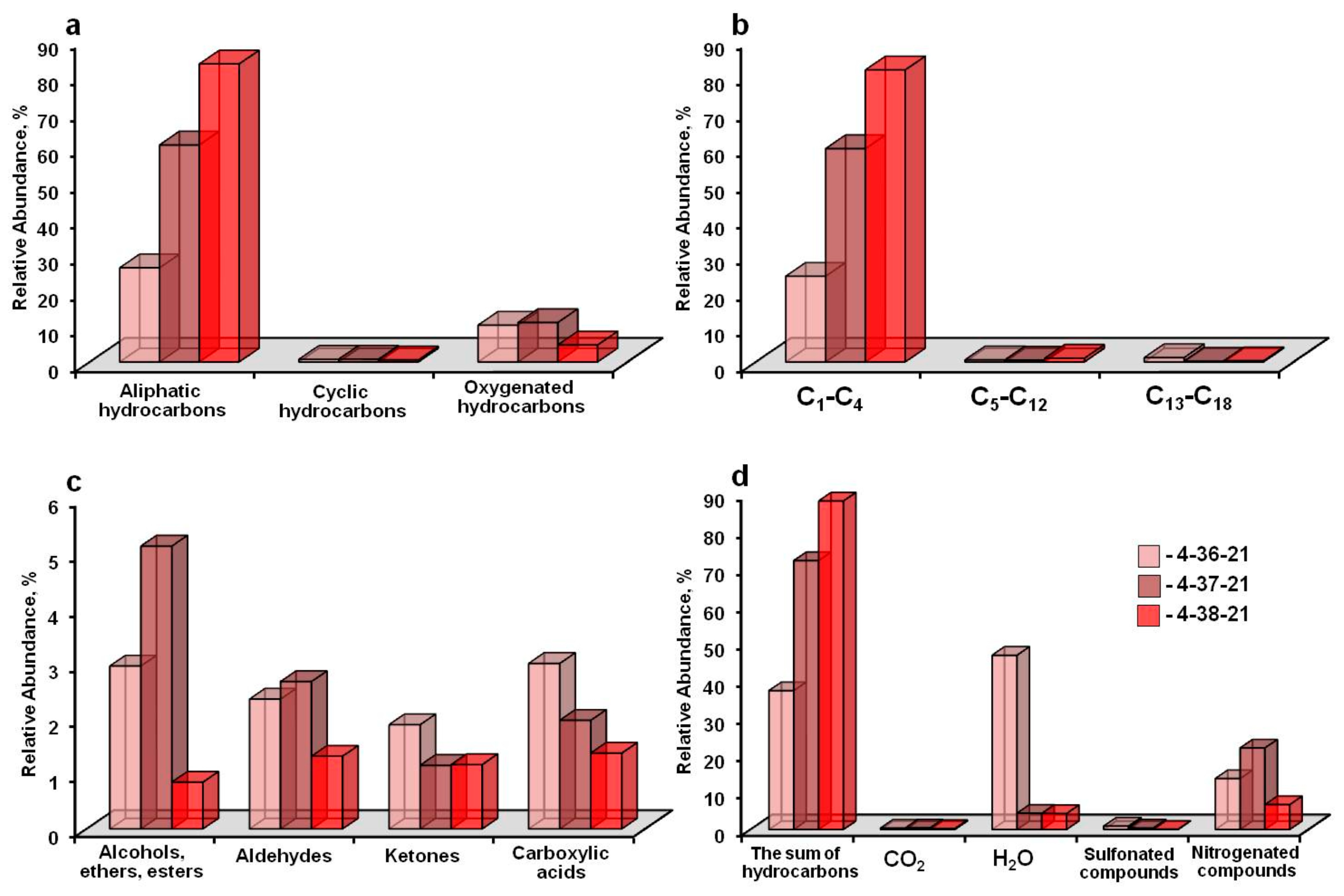
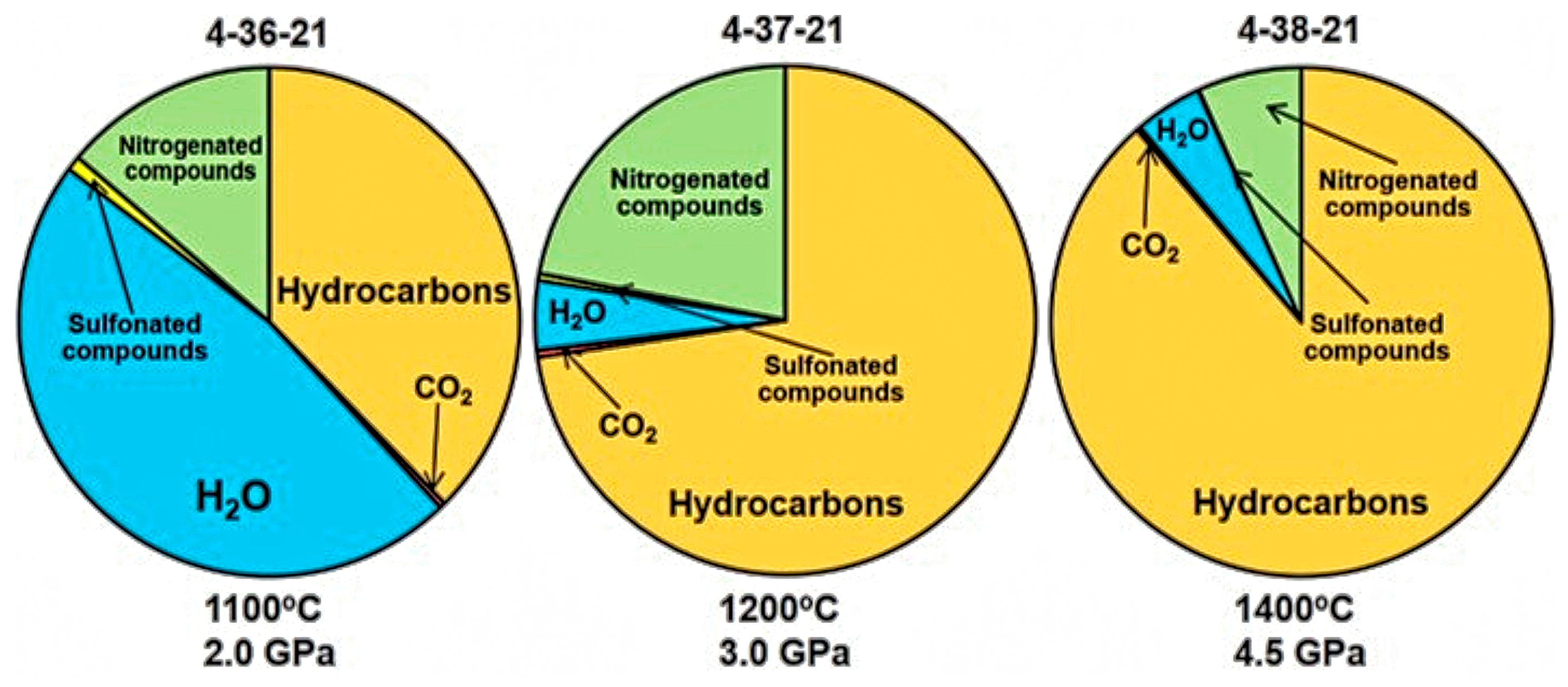
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molina, J.F.; Poli, S. Carbonate stability and fluid composition in subducted oceanic crust: Experimental study on H2O-CO2-bearing basalts. Earth Planet. Sci. Lett. 2000, 176, 295–310. [Google Scholar] [CrossRef]
- Kerrick, D.M.; Connoly, J.A.D. Metamorphic devolatilization of subducted oceanic metabasalts: Implication for seismicity, arc magmatism and votilate recycling. Erath Planet. Sci. Lett. 2001, 189, 19–29. [Google Scholar] [CrossRef]
- Presnall, D.C.; Gudfinnsson, G.H. Carbonate-rich in oceanic low-velocity zone and deep mantle. Geological Society of America. Spec. Pap. 2005, 388, 207–216. [Google Scholar]
- Thomsen, T.B.; Schmidt, M.W. Melting of carbonated pelites at 2.5-5.0 GPa, silicate-carbonatite liquid immiscibility, and potassium-carbon metasomatism of the mantle. Erath Planet. Sci. Lett. 2008, 267, 17–31. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Ague, J.J.; Nicolescu, S. Carbon dioxide released from subduction zones by fluid-mediated reactions. Nat. Geosci. 2014, 7, 355–360. [Google Scholar] [CrossRef]
- Kelement, P.B.; Manning, C.E. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl. Acad. Sci. USA 2015, 112, E3997–E4006. [Google Scholar] [CrossRef]
- Debret, B.; Sverjensky, D. Highly oxidizing fluids generated during serpentinite breakdown in subduction zones. Sci. Rep. 2017, 7, 10351. [Google Scholar] [CrossRef]
- Sverjensky, D.; Stagno, V.; Huang, F. Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nat. Geosci. 2014, 7, 909–913. [Google Scholar] [CrossRef]
- Malvoisin, B.; Chopin, C.; Brunet, F.; Galvez, M.E. Low-temperature wollastonite formed by carbonate reduction: A marker of serpentinite redox conditions. J. Petrol. 2012, 53, 159–176. [Google Scholar] [CrossRef]
- Galvez, M.E.; Beyssac, O.; Martinez, I.; Benzerara, K.; Chaduteau, C.; Malvoisin, B.; Malavieille, J. Graphite formation by carbonate reduction during subduction. Nat. Geosci. 2013, 6, 473–477. [Google Scholar] [CrossRef]
- Buseck, P.R.; Beyssak, O. From organic matter to graphite: Graphitization. Elements 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Vitale Brovarone, A.V.; Tumiati, S.; Piccoli, F.; Ague, J.J.; Connolli, J.A.D.; Beyssac, O. Fluid-mediated selective dissolution of subducting carbonaceous material: Implication for carbon recycling and fluid fluxes at forearc depths. Chem. Geol. 2020, 549, 119682. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshino, T.; Satish-Kumar, M. Pressure dependence of graphitization: Implications for rapid recrystallization of carbonaceous material in subduction zone. Contrib. Mineral. Petrol. 2020, 175, 32. [Google Scholar] [CrossRef]
- Duncan, M.S.; Dasgupta, R. Rise of Earth’s atmospheric oxygen controlled by efficient subduction of organic carbon. Nat. Geosci. 2017, 10, 387–392. [Google Scholar] [CrossRef]
- Plank, T.; Manning, G.E. Subducting carbon. Nature 2019, 574, 343–352. [Google Scholar] [CrossRef]
- Chanyshev, A.D.; Litasov, K.D.; Shatskiy, A.F.; Sharygin, I.S.; Higo, Y.; Ohtani, E. Transition from melting to carbonization of naphthaline, antracene, pyrene and coronene at high pressure. Phys. Earth Planet. Inter. 2017, 270, 29–39. [Google Scholar] [CrossRef]
- Evans, K.A.; Reddy, S.M.; Tomkins, A.G.; Crossley, R.J.; Frost, B.R. Effects of geodynamic setting on the redox state of fluids released by subducted mantle lithosphere. Lithos 2017, 278-281, 26–42. [Google Scholar] [CrossRef]
- Vitale Brovarone, A.V.; Sverjensky, D.A.; Piccoli, F.; Ressico, F.; Giovannelli, D.; Daniel, I. Subduction hides high-pressure sources of energy that may feed the deep subsurface biosphere. Nat. Commun. 2020, 11, 3880–3911. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, L.; Tian, M.; Zhu, J.; Liu, X.; Liu, J.; Höfer, H.E.; Stagno, V.; Fei, Y. Formation of abiotic hydrocarbon from reduction of carbonate in subduction zones: Constraints from petrological observation and experimental simulation. Geochim. Cosmochim. Acta 2018, 239, 390–408. [Google Scholar] [CrossRef]
- Vacquand, C.; Deville, E.; Beaumont, V.; Guyot, F.; Sissmann, O.; Pillot, D.; Arcilla, C.; Prinzhofer, A. Reduced gas seepages in ophiolitic complexes: Evidences for multiple origins of the H2-CH4-N2 gas mixture. Geochim. Cosmochim. Acta 2018, 223, 437–461. [Google Scholar] [CrossRef]
- Maffeis, A.; Ferrando, S.; Connolly, J.A.D.; Groppo, C.; Frezzotti, M.L.; Castelli, D. Thermodynamic analysis of HP-UHP fluid inclusions: The solute load and chemistry of metamorphic fluids. Geochim. Cosmochim. Acta 2021, 315, 207–229. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Ding, X.; Li, W.-C. Divers serpentinization and associated abiotic methanogenesis within multiple types of olivine-hosted fluid inclusions in orogenic peridotite from northern Tibet. Geochim. Cosmochim. Acta 2021, 296, 1–17. [Google Scholar] [CrossRef]
- McCollom, T.M.; Seewald, J.S.; German, C.R. Investigation of extractable organic compounds in deep-sea hydrothermal vent fluids along the Mid-Atlantic Ridge. Geochem. Cosmochim. Acta 2015, 156, 122–144. [Google Scholar] [CrossRef]
- Ménez, B.; Pisapia, C.; Andreani, M.; Jamme, F.; Vanbellingen, Q.; Brunelle, A.; Richard, L.; Dumas, P.; Réfrégiers, M. Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere. Nature 2018, 564, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Schwandner, F.M.; Seward, T.M.; Gize, A.P.; Hall, K.; Dietrich, V.J. Halocarbons and other trace heteroatomic organic compounds in volcanic gases from Vulcano (Aeolian Islands, Italy). Geochim. Cosmochim. Acta 2013, 101, 191–221. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Bul’bak, T.A.; Logvinova, A.M.; Sonin, V.M.; Sobolev, N.V. The composition features of volatile components in diamonds from the placers in the northeastern part of the Siberian platform by gas chromatography–mass spectrometry. Dokl. Earth Sci. 2018, 481, 953–957. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Sobolev, A.V.; Tomilenko, A.A.; Kuz’min, D.V.; Grakhanov, S.A.; Batanova, V.G.; Logvinova, A.M.; Bul’bak, T.A.; Kostrovitskii, S.I.; Yakovlev, D.A.; et al. Prospects of searching for diamondiferous kimberlites in the northeastern Siberian Platform. Russ. Geol. Geophys. 2018, 59, 1385–1399. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Tomilenko, A.A.; Wirth, R.; Bul’bak, T.A.; Luk’yanova, L.I.; Fedorova, E.N.; Reutsky, V.N.; Efimova, E.S. Mineral and fluid inclusions in diamonds from the Urals placers, Russia: Evidence for solid molecular N2 and hydrocarbons in fluid inclusions. Geochim. Cosmochim. Acta 2019, 266, 197–219. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Tomilenko, A.A.; Bul’bak, T.A.; Logvinova, A.M. Composition of hydrocarbons in diamonds, garnet, and olivine from diamondiferous peridotites from the Udachnaya pipe in Yakutia, Russia. Engineering 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Nizametdinov, I.R.; Kuzmin, D.V.; Smirnov, S.Z.; Bul’bak, T.A.; Tomilenko, A.A.; Maksimovich, I.A.; Kotov, A.A. Hydrocarbons in Magmatic Fluid in Phenocrysts of Eruption Products of Men’shii Brat Volcano (Iturup Island): Data from Pyrolysis-Free Gas Chromatography–Mass Spectrometry of Melt and Fluid Inclusions. Russ. Geol. Geophys. 2022, 63, 890–900. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Bul’bak, T.A.; Timina, T.Y.; Shaparenko, E.O.; Simonov, V.A.; Laptev, Y.V. Composition of volatiles of sulfide deposits and carbonate structures in submarine hydrothermal fields of the Mid-Atlantic Ridge. Marine Geol. 2022, 444, 106713. [Google Scholar] [CrossRef]
- Zhimulev, E.I.; Sonin, V.M.; Bil’bak, T.A.; Chepurov, A.I.; Tomilenko, A.A.; Pokhilenko, N.P. Volatile Compounds of Sulfur in the Fe-C-S System at 5.3 GPa and 1300 °C. Dokl. Earth Sci. 2015, 462, 528–533. [Google Scholar] [CrossRef]
- Poli, S.; Schmidt, M.W. H2O transport and release in subduction zones—Experimental constraints on basaltic and andesitic systems. J. Geophys. Res. 1995, 100, 22299–22314. [Google Scholar] [CrossRef]
- Huang, R.; Sun, W.; Ding, X.; Zhao, Y.; Song, M. Effect of pressure on the kinetics of peridotite serpentinization. Phys. Chem. Minerals 2020, 47, 33. [Google Scholar] [CrossRef]
- Ulmer, P.; Trommsdorff, V. Serpentine stability to mantle depths and subduction-related magmatism. Science 1995, 268, 858–861. [Google Scholar] [CrossRef]
- Wunder, B.; Schreyer, W. Antigorite: High-pressure stability in the system MgO-SiO2-H2O (MSH). Lithos 1997, 41, 213–227. [Google Scholar] [CrossRef]
- Chepurov, A.I.; Tomilenko, A.A.; Zhimulev, E.I.; Sonin, V.M.; Chepurov, A.A.; Surkov, N.V.; Kovyazin, S.V. Problem of water in the upper mantle: Antigorite breakdown. Dokl. Earth Sci. 2010, 434, 1275–1278. [Google Scholar] [CrossRef]
- Chepurov, A.I.; Tomilenko, A.A.; Zhimulev, E.I.; Sonin, V.M.; Chepurov, A.A.; Kovyazin, S.V.; Timina, T.Y.; Surkov, N.V. The conservation of an aqueous fluid in inclusions in minerals and their interstices at high pressures and temperatures during the decomposition of antigorite. Russ. Geol. Geophys. 2012, 53, 234–246. [Google Scholar] [CrossRef]
- Stagno, V.; Frost, D.J. Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at carbonate-bearing melts coexist with graphite or diamonds in peridotite assemblages. Earth Planet. Sci. Lett. 2010, 300, 72–84. [Google Scholar] [CrossRef]
- Stagno, V.; Frost, D.J.; McCammon, C.A.; Mohseni, H.; Fei, Y. The oxygen fugacity at which graphite or diamond forms from carbonate-bearing melts in eclogitic rocks. Contrib. Mineral. Petrol. 2015, 169, 16. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 35, 1403–1405. [Google Scholar] [CrossRef]
- Chepurov, A.I.; Tomilenko, A.A.; Sonin, V.M.; Zhimulev, E.I.; Bul’bak, T.A.; Chepurov, A.A.; Sobolev, N.V. Interaction of an Fe-Ni melt with anthracene (C14H10) in presence of olivine at 3 GPa: Fluid phase composition. Dokl. Earth Sci. 2020, 492, 333–337. [Google Scholar] [CrossRef]
- Turkin, A.I. Lead selenide as a continuous internal indicator of pressure in solid-media cells of high-pressure apparatus in the range of 4–6.8 GPa. High Temp. High Press. 2004, 36, 371–376. [Google Scholar] [CrossRef]
- Chepurov, A.; Zhimulev, E.; Chepurov, A.; Sonin, V. Where did the largest diamonds grow? The experiments on percolation of Fe-Ni melt through olivine matrix in the presence of hydrocarbons. Lithos 2021, 404–405, 106437. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Chepurov, A.I.; Sonin, V.M.; Bul’bak, T.A.; Zhimulev, E.I.; Chepurov, A.A.; Timina, T.Y.; Pokhilenko, N.P. The synthesis of methane and neavier hydrocarbons in the system graphite-iron-serpentine at 2 and 4 GPa and 1200 °C. High Temp. High Press. 2015, 44, 451–465. [Google Scholar]
- Sokol, A.G.; Palyanov, Y.N.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanova, G.A. Carbon and nitrogen speciation in nitrogen-rich C-O–H–N fluids at 5.5–7.8 GPa. Earth Planet. Sci. Lett. 2017, 60, 234–243. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Tomilenko, A.A.; Gibsher, N.A.; Sazonov, A.M.; Shaparenko, E.O.; Ryabukha, M.A.; Khomenko, M.O.; Sil’yanov, S.A.; Nekrasova, N.A. Hydrocarbons in Fluid Inclusions from Native Gold, Pyrite, and Quartz of the Sovetskoe Deposit (Yenisei Ridge, Russia) According to Pyrolysis-Free Gas Chromatography-Mass Spectrometry Data. Russ. Geol. Geophys. 2020, 61, 1260–1282. [Google Scholar] [CrossRef]
- Sonin, V.; Tomilenko, A.; Zhimulev, E.; Bul’bak, T.; Chepurov, A.; Babich, Y.; Logvinova, A.; Timina, Y.; Chepurov, A. The composition on the fluid phase in inclusions in synthetic HPHT diamonds grown in system Fe-Ni-Ti-C. Sci. Rep. 2022, 12, 1246. [Google Scholar] [CrossRef]
- Swartzendruber, L.J.; Itkin, V.P.; Alcock, C.B. The Fe-Ni (Iron-Nickel) System. J. Phase Equilibria 1991, 12, 288–312. [Google Scholar] [CrossRef]
- Kocherzhinskii, Y.A.; Kulik, O.G.; Turkevich, V.Z. Phase equilibria in the Fe-Ni-C and Fe-Co-C systems under high temperatures and high pressures. High Temp. High Pres. 1993, 25, 113–116. [Google Scholar]
- Lakovino, K.; Guild, M.R.; Till, C.B. Aqueous fluids are effective oxidizing agents of the mantle in subduction zones. Contrib. Mineral. Petrol. 2020, 175, 36. [Google Scholar] [CrossRef]
- Sawada, Y.; Sampei, Y.; Hada, O.; Taguchi, S. Thermal degradation and polymerization of carbonaceous materials in a metapelite-granitiod magma system in the Ryoke metamorphic belt, SW Japan. J. Asian Earth Sci. 2008, 33, 91–105. [Google Scholar] [CrossRef]
- Manning, C.E.; Shock, E.L.; Sverjensky, D.A. The chemistry of carbon in aqueous fluids at crustal and upper-mantle conditions: Experimental and theoretical constraints. Rew. Mineral. Geochem. 2013, 75, 109–148. [Google Scholar] [CrossRef]
- Stephton, M.A.; Hazen, R.M. On the origins of deep hydrocarbons. Rew. Mineral. Geochem. 2013, 75, 449–465. [Google Scholar] [CrossRef]
- McCollom, T.M. Laboratory simulations of abiotic hydrocarbon formation in Earth’s deep subsurface. Rev. Mineral. Geochem. 2013, 75, 467–494. [Google Scholar] [CrossRef]
- McCollom, T.M. Abiotic methane formation during experimental serpentinization of olivine. Proc. Natl. Acad. Sci. USA 2016, 113, 13965–13970. [Google Scholar] [CrossRef]
- Etiope, G.; Sherwood Lollar, B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Zubkov, V.S. Tendencies in distribution and hypotheses of the genesis of condensed naphthides in magmatic rocks from various geodynamic environments. Geochem. Int. 2009, 47, 741–757. [Google Scholar] [CrossRef]
- Savel’ev, V.S.; Ogloblina, A.I.; Florovskaya, V.N.; Rudenko, A.P.; Kulakova, I.I. Polycondensation of carbon monoxide with hydrogen is a possible source of PAHs in nature. Dokl. Akad. Nauk SSSR 1984, 275, 733–736. [Google Scholar]
- Ellis, D.E.; Wyllie, P.J. Hydration and melting reactions in the system MgO-SiO2-H2O at pressure up to 100 kbar. Am. Mineral. 1979, 64, 41–48. [Google Scholar]
- Johnson, M.C.; Walker, D. Brucite [Mg(OH)2] dehydration and molar volume of H2O to 15 GPa. Am. Mineral. 1993, 78, 271–284. [Google Scholar]
- Newton, R.C.; Manning, C.E. Experimental determination of calcite solubility in H2O-NaCl solutions at deep-crust/upper mantle pressures and temperatures: Implications for metasomatic processes in shear zones. Am. Mineral. 2002, 87, 1401–1409. [Google Scholar] [CrossRef]
- Caciagli, N.C.; Manning, C.E. The solubility of calcite in water at 6–16 kbar and 500–800 °C. Contrib. Mineral. Petrol. 2003, 146, 355–360. [Google Scholar] [CrossRef]
- Facq, S.; Daniel, I.; Montagnac, G.; Cardon, H.; Sverjensky, D.A. In situ Raman study and thermodynamic model of aqueous carbonate speciation in equilibrium with aragonite under subduction zone conditions. Geochim. Cosmochim. Acta 2014, 132, 375–390. [Google Scholar] [CrossRef]
- Farsang, S.; Louvel, M.; Zhao, C.; Mezouar, M.; Rosa, A.D.; Widmer, R.N.; Feng, X.; Liu, J.; Redfern, S.A.T. Deep carbon cycle constrained by carbonate solubility. Nat. Commun. 2021, 12, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, K.; Xiao, Y.; Hoefs, J.; Liou, J.G. Mineral and fluid inclusions in zircon of UHP metamorphic rocks from CCSD-main drill hole: A record of metamorphism and fluid activity. Lithos 2006, 92, 378–398. [Google Scholar] [CrossRef]
- Frezzotti, M.; Selverstone, J.; Sharp, Z.; Compagnoni, R. Carbonate dissolution during subduction revealed by diamond-bearing rocks from the Alps. Nat. Geosci. 2011, 4, 703–706. [Google Scholar] [CrossRef]


| Name | MW * | 4-36-21 | 4-37-21 | 4-38-21 |
|---|---|---|---|---|
| Aliphatic hydrocarbons: | 26.4 | 60.4 | 82.9 | |
| Paraffins (CH4-C19H40) | 16–268 | 25.8 | 60.1 | 82.6 |
| Olefins (C2H4-C15H30) | 28–210 | 0.6 | 0.3 | 0.3 |
| Cyclic hydrocarbons: | 0.72 | 0.75 | 0.6 | |
| Cycloalkanes (naphthenes)) (C5H8-C12H20) | 68–164 | - | - | - |
| Arenes (C6H6-C16H26) | 78–218 | 0.67 | 0.74 | 0.07 |
| Polycyclic aromatic hydrocarbons (C10H8-C14H10) | 128–178 | 0.05 | 0.01 | 0.53 |
| Oxygenated hydrocarbons: | 10.3 | 11.1 | 4.8 | |
| Alcohols, esters and ethers (CH4O-C14H18O4) | 32–250 | 3.0 | 5.2 | 0.9 |
| Aldehydes (C2H4O-C15H30O) | 44–226 | 2.4 | 2.7 | 1.3 |
| Ketones (C3H6O-C15H30O) | 58–226 | 1.9 | 1.2 | 1.2 |
| Carboxylic acids (C2H4O2-C14H28O2) | 60–228 | 3.0 | 2.0 | 1.4 |
| Heterocyclic compounds: | 0.18 | 0.25 | 0.1 | |
| Dioxanes (C4H8O2) | 88 | 0.01 | 0.01 | 0.01 |
| Furans (ethers) (C5H6O-C12H20O) | 82–180 | 0.17 | 0.24 | 0.09 |
| Nitrogenated compounds (N2-C10H21NO) | 28–171 | 14.0 | 22.0 | 6.7 |
| Sulfonated compounds (H2S-C12H26O3S) | 34–250 | 1.0 | 0.5 | 0.2 |
| CO2 | 44 | 0.4 | 0.5 | 0.3 |
| H2O | 18 | 47.0 | 4.5 | 4.4 |
| The number of identified components | 248 | 213 | 218 | |
| H/(O+H) | 0.82 | 0.97 | 0.98 | |
| Alkanes/Alkenes | 43.0 | 200.0 | 275.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomilenko, A.; Sonin, V.; Bul’bak, T.; Zhimulev, E.; Timina, T.; Chepurov, A.; Shaparenko, E.; Chepurov, A. Impact of Solid Hydrocarbon on the Composition of Fluid Phase at the Subduction (Experimental Simulation). Minerals 2023, 13, 618. https://doi.org/10.3390/min13050618
Tomilenko A, Sonin V, Bul’bak T, Zhimulev E, Timina T, Chepurov A, Shaparenko E, Chepurov A. Impact of Solid Hydrocarbon on the Composition of Fluid Phase at the Subduction (Experimental Simulation). Minerals. 2023; 13(5):618. https://doi.org/10.3390/min13050618
Chicago/Turabian StyleTomilenko, Anatoly, Valeriy Sonin, Taras Bul’bak, Egor Zhimulev, Tatiana Timina, Aleksey Chepurov, Elena Shaparenko, and Anatoly Chepurov. 2023. "Impact of Solid Hydrocarbon on the Composition of Fluid Phase at the Subduction (Experimental Simulation)" Minerals 13, no. 5: 618. https://doi.org/10.3390/min13050618






