Luminescence Characteristics of Green Grossular Garnets
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
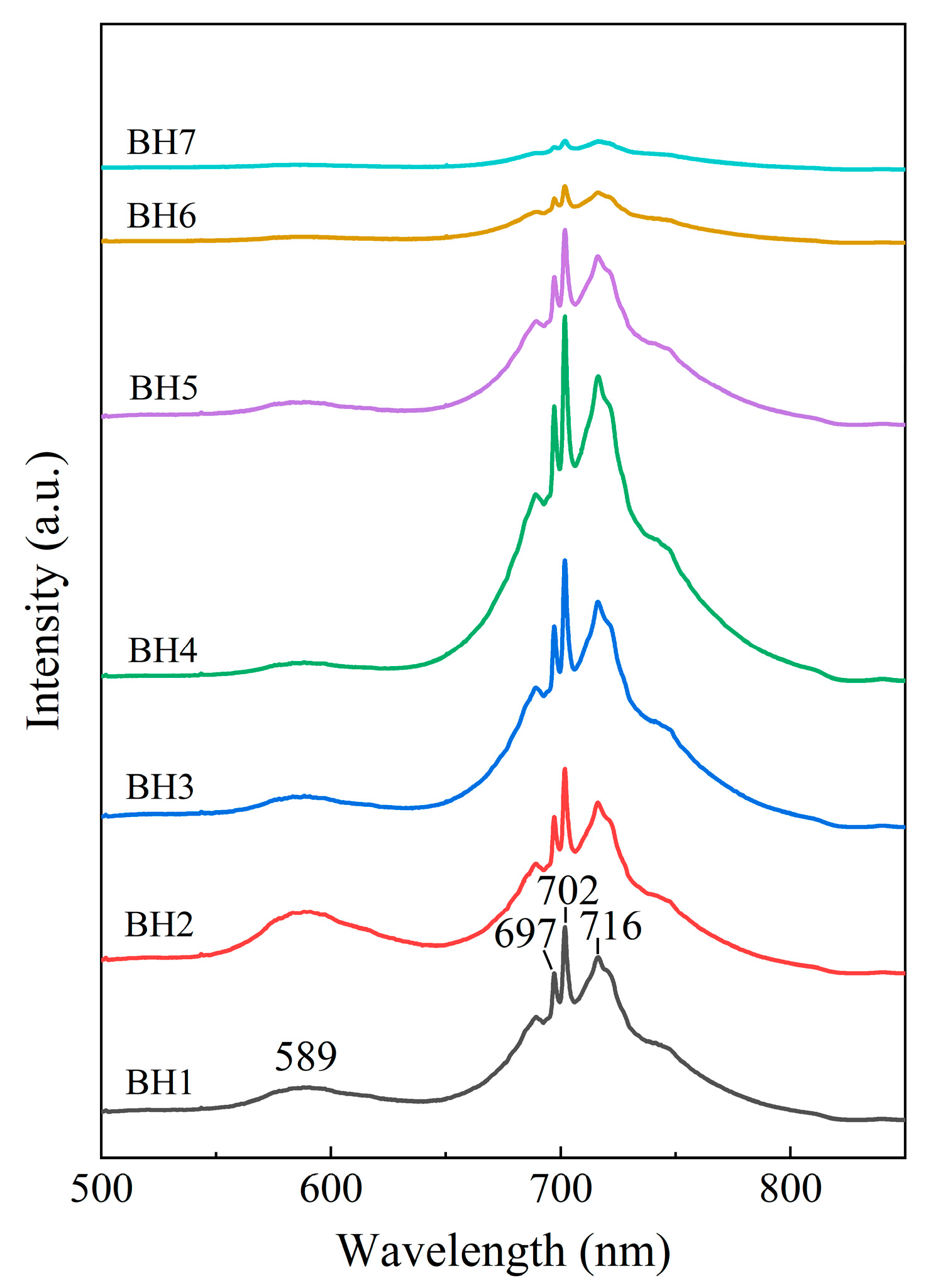
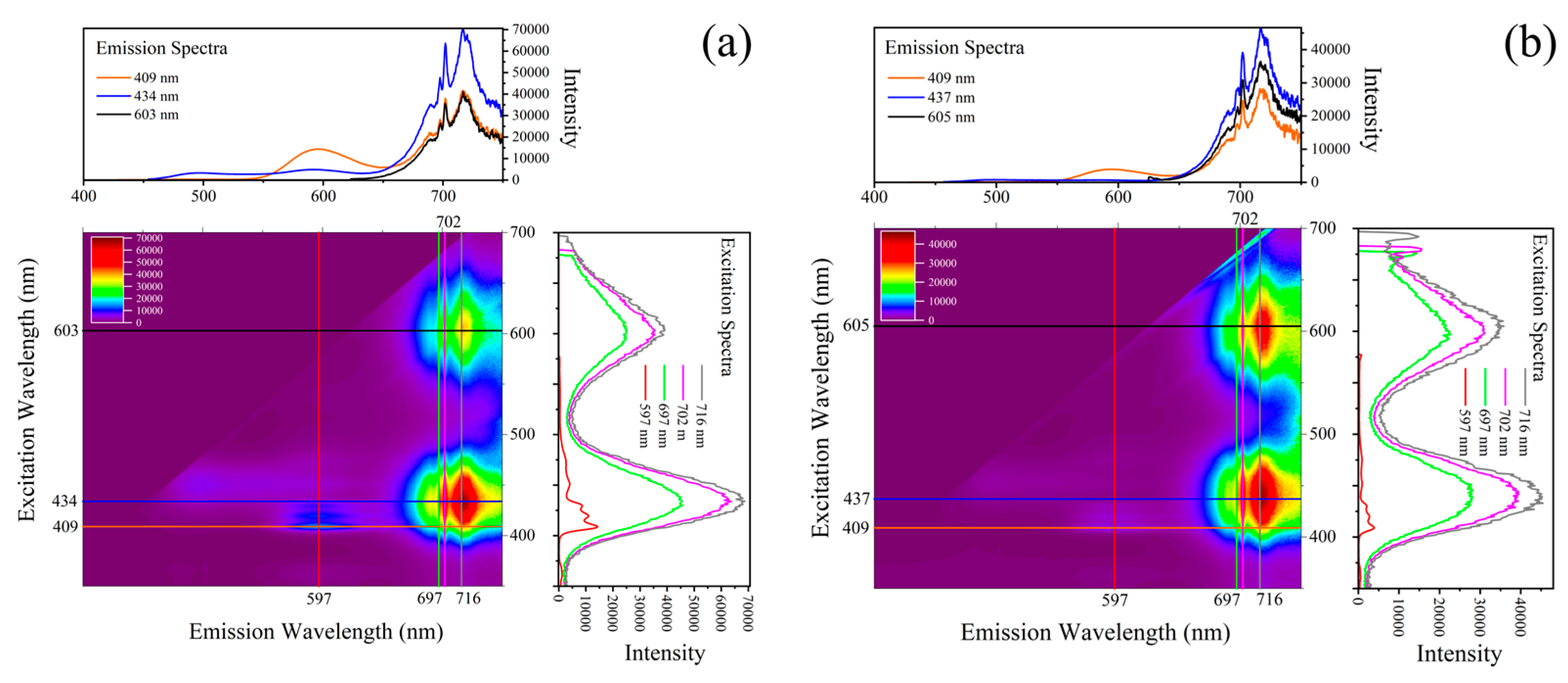
3.1. Photoluminescence Features
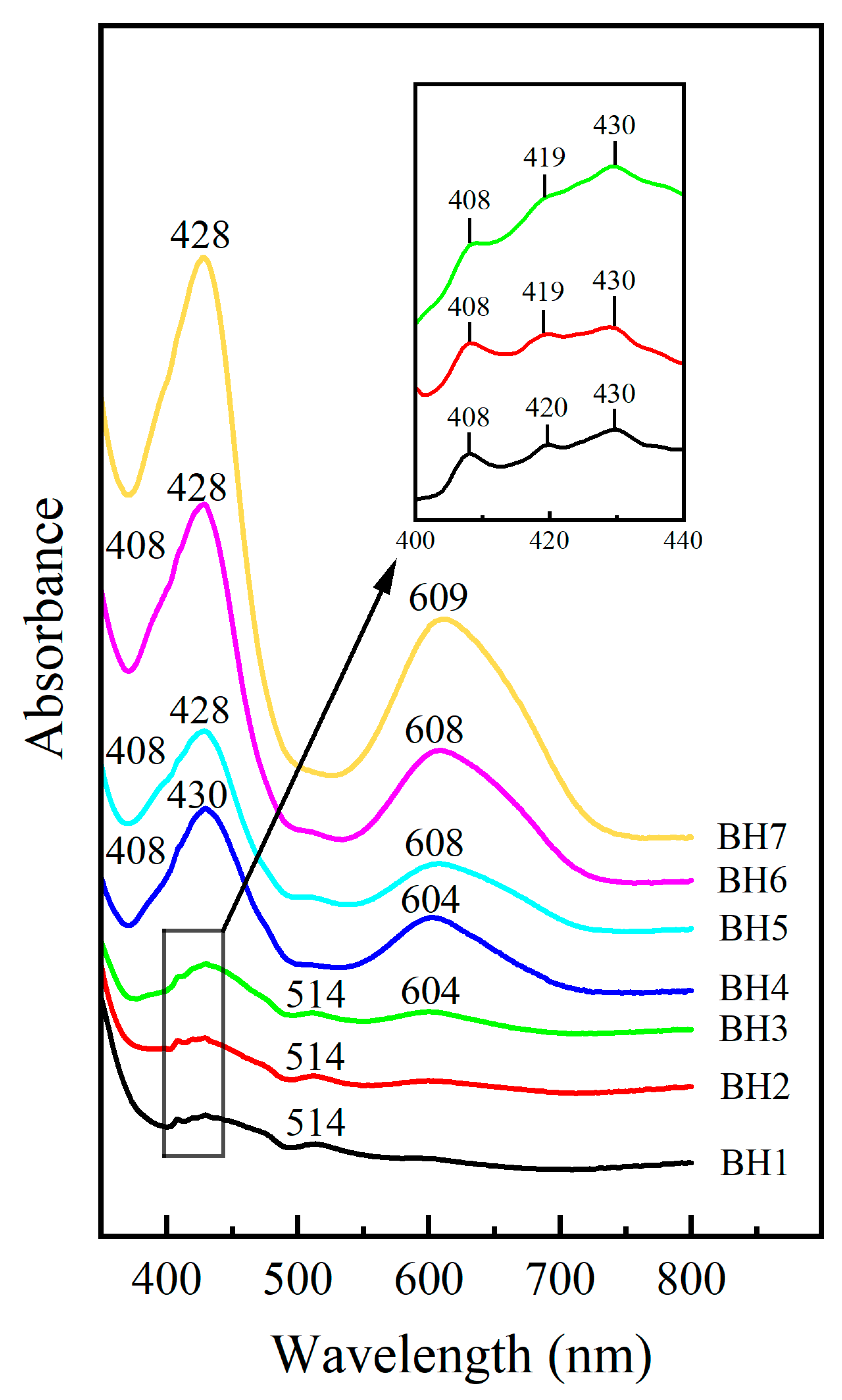
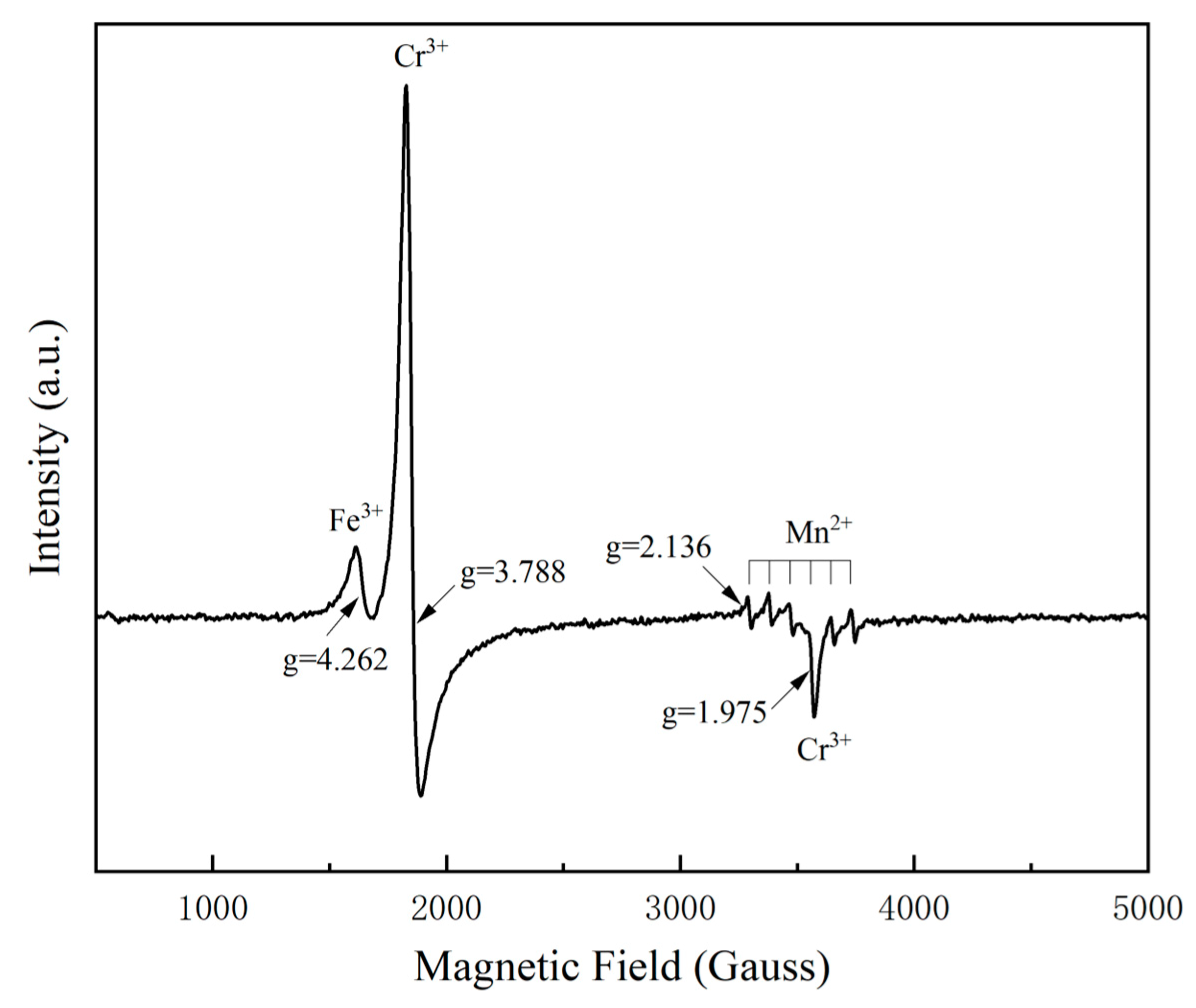
3.2. Chemical Analysis and Type Identification
3.3. Assignment of Luminescence and Coloration
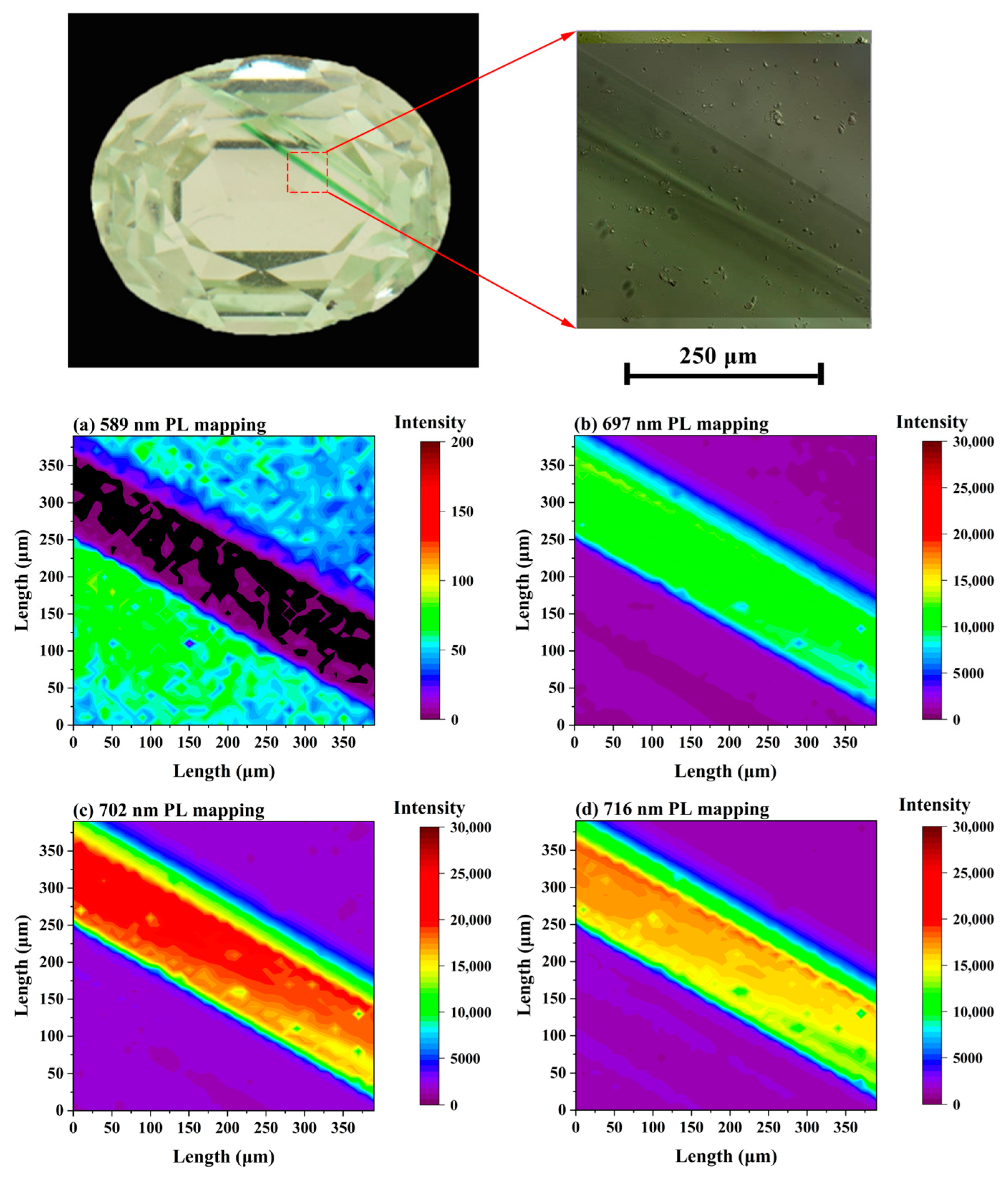
3.4. Suppression Influence on the Vanadium-Rich Sample
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bridges, C. Green Grossulanite Garnets (“Tsavorites”) in East Africa. Gems Gemol. 1974, 14, 290–295. [Google Scholar]
- Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Martelat, J.-E.; Monié, P.; Dubessy, J.; Rollion-Bard, C.; Goff, E.L.; Malisa, E.P.J.; et al. New Aspects and Perspectives on Tsavorite Deposits. Ore Geol. Rev. 2013, 53, 1–25. [Google Scholar] [CrossRef]
- Switzer, G. Composition of Green Garnet from Tanzania and Kenya. Gems Gemol. 1974, 14, 296–297. [Google Scholar]
- Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Rondeau, B.; Fritsch, E.; Fallick, A.E.; Ichang’i, D.; Omito, E.; Rakotondrazafy, M.; Ranatsenho, M.; et al. New Typology and Origin of Tsavorite Based on Trace-element Chemistry. Eur. J. Mineral. 2014, 26, 293–308. [Google Scholar] [CrossRef]
- Zeug, M.; Nasdala, L.; Chanmuang, N.C.; Hauzenberger, C. Gem Topaz from the Schneckenstein Crag, Saxony, Germany: Mineralogical Characterization and Luminescence. Gems Gemol. 2022, 58, 2–17. [Google Scholar] [CrossRef]
- Wang, C.; Shen, A.H.; Liu, Y. Characterization of Order-Disorder Transition in MgAl2O4: Cr3+ Spinel using Photoluminescence. J. Lumin. 2020, 227, 117552. [Google Scholar] [CrossRef]
- Nelson, D.F.; Sturge, M. Relation between Absorption and Emission in the Region of the R Lines of Ruby. Phys. Rev. 1965, 137, 1117–1130. [Google Scholar] [CrossRef]
- Gaft, M.; Reisfeld, R.; Panczer, G. Modern Luminescence Spectroscopy of Minerals and Materials; Springer: New York, NY, USA, 2015. [Google Scholar]
- Lueth, V.W.; Jones, R. Red grossular from the sierra de Cruces, coahuila, Mexico. Mineral. Rec. 2003, 34, 73–79. [Google Scholar]
- Mazurak, Z.; Czaja, M. Optical Properties of Tsavorite Ca3Al2(SiO4)3: Cr3+, V3+ from Kenya. J. Lumin. 1995, 65, 335–340. [Google Scholar] [CrossRef]
- Gaft, M.; Waychunas, G.; Rossman, G.R.; Nagli, L.; Goryachev, A.; Raichlin, Y. Zero-phonon Mn2+ luminescence in natural grossular Ca3Al2(SiO4)3. J. Lumin. 2022, 248, 119001. [Google Scholar] [CrossRef]
- Gaft, M.; Yeates, H.; Nagli, L.; Panczer, G. Laser-induced Time Resolved Luminescence of Natural Grossular Ca3Al2(SiO4)3. J. Lumin. 2013, 137, 43–53. [Google Scholar] [CrossRef]
- Malisa, E.P.J.; Muhongo, S. Tectonic Setting of Gemstone Mineralization in the Proterozoic Metamorphic Terrane of the Mozambique Belt in Tanzania. Precambrian Res. 1990, 46, 167–176. [Google Scholar] [CrossRef]
- Malisa, E. Trace Elements Characterization of the Hydrothermally Deposited Tanzanite and Green Grossular in the Merelani—Lelatema Shear Zone, Northeastern Tanzania. Tanz. J. Sci. 2003, 29, 45–60. [Google Scholar] [CrossRef]
- Giuliani, G.; Fallick, A.E.; Feneyrol, J.; Ohnenstetter, D.; Pardieu, V.; Saul, M. 18O/16O and V/Cr ratios in gem tsavorites from the Neoproterozoic Mozambique metamorphic belt: A clue towards their origins? Miner. Deposita 2011, 46, 671–676. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, A.H. Grossular with strong red fluorescence. Gems Gemol. 2022, 58, 508–509. [Google Scholar]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Rossman, G.R. Optical spectroscopy. Rev. Mineral. Geochem. 2014, 78, 371–398. [Google Scholar] [CrossRef]
- Manning, P.G. The optical absorption spectra of the garnets almandine-pyrope, pyrope, and spessartine and some structural interpretations of mineralogical significance. Can. Mineral. 1967, 9, 237–251. [Google Scholar]
- Geiger, C.A.; Stahl, A.; Rossman, G.R. Raspberry-red Grossular from Sierra de Cruces Range, Coahuila, Mexico. Eur. J. Mineral. 1999, 11, 1109–1113. [Google Scholar] [CrossRef]
- Pan, Y.; Nilges, M.J. Electron paramagnetic resonance spectroscopy: Basic principles, experimental techniques and applications to Earth and planetary sciences. Rev. Mineral. Geochem. 2014, 78, 655–690. [Google Scholar] [CrossRef]
- Kim, I.W.; Kaur, S.; Yadav, A.; Rao, A.S.; Saravanakumar, S.; Rao, J.L.; Singh, V. Structural, luminescence and EPR properties of deep red emitting MgY2Al4SiO12:Cr3+ garnet phosphor. J. Lumin. 2020, 220, 116975. [Google Scholar] [CrossRef]
- Yauri, J.; Watanabe, S. Defect Dependent Properties in Grossularite. Phys. Status Solidi C 2005, 2, 555–559. [Google Scholar] [CrossRef]
- Vazhenin, V.A.; Potapov, A.P.; Asatryan, G.R.; Artyomov, M.Y. Paramagnetic V2+ Centers in Yttrium–Aluminum Garnet. Phys. Solid State 2020, 62, 2116–2121. [Google Scholar] [CrossRef]
- Havlíček, V.; Novak, P. EPR Line-width Intensity of V4+ Ion in NaCa2Mg2V3O12 Garnet. Czech. J. Phys. B 1974, 24, 188–202. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y. Variation in Gemological Characteristics in Tsavorites with Different Tones from East Africa. Crystals 2022, 12, 1677. [Google Scholar] [CrossRef]

| Sample No. | Weight (ct) | Size (mm) | Specific Gravity | Refractive Index | Color |
|---|---|---|---|---|---|
| BH1 | 2.00 | 8.19 × 6.55 × 4.76 | 3.60 | 1.738 | light yellowish-green |
| BH2 | 1.27 | 7.05 × 4.96 × 3.79 | 3.61 | 1.739 | light yellowish-green |
| BH3 | 1.62 | 7.59 × 5.96 × 3.97 | 3.64 | 1.737 | light yellowish-green |
| BH4 | 1.12 | 5.63 × 5.56 × 3.92 | 3.61 | 1.738 | light mint-green |
| BH5 | 0.86 | 5.54 × 5.33 × 3.34 | 3.64 | 1.739 | light mint-green |
| BH6 | 0.73 | 6.79 × 4.99 × 2.80 | 3.65 | 1.739 | green |
| BH7 | 0.84 | 5.87 × 4.09 × 3.64 | 3.65 | 1.738 | green |
| Composition | BH1 | BH2 | BH3 | BH4 | BH5 | BH6 | BH7 | |
|---|---|---|---|---|---|---|---|---|
| Oxide (wt.%) | ||||||||
| CaO | Range | 36.62–36.96 | 36.85–37.04 | 36.12–36.59 | 36.86–36.99 | 36.36–36.97 | 36.35–36.70 | 36.55–37.13 |
| Average | 36.77 | 36.95 | 36.27 | 36.93 | 36.69 | 36.52 | 36.83 | |
| SiO2 | Range | 39.52–41.41 | 39.73–40.08 | 39.50–39.74 | 39.18–39.45 | 39.69–40.26 | 39.03–39.13 | 37.84–38.28 |
| Average | 40.10 | 39.96 | 39.60 | 39.29 | 39.94 | 39.07 | 38.05 | |
| Al2O3 | Range | 21.19–21.75 | 22.26–22.48 | 22.41–22.72 | 22.04–22.36 | 22.07–22.37 | 22.20–22.52 | 21.79–22.23 |
| Average | 21.45 | 22.37 | 22.60 | 22.20 | 22.22 | 22.38 | 22.01 | |
| MgO | Range | 0.13–0.53 | 0.56–0.60 | 0.47–0.51 | 0.53–0.56 | 0.60–0.67 | 0.41–0.48 | 0.49–0.54 |
| Average | 0.29 | 0.58 | 0.50 | 0.54 | 0.62 | 0.44 | 0.53 | |
| FeO | Range | 0.02–0.09 | 0.03–0.09 | 0.02–0.11 | 0.04–0.10 | 0.03–0.10 | 0.06–0.11 | 0.01–0.10 |
| Average | 0.05 | 0.06 | 0.06 | 0.06 | 0.07 | 0.08 | 0.04 | |
| MnO | Range | 0.20–0.30 | 0.34–0.44 | 0.24–0.32 | 0.38–0.44 | 0.28–0.34 | 0.61–0.70 | 0.54–0.65 |
| Average | 0.26 | 0.38 | 0.27 | 0.41 | 0.30 | 0.66 | 0.58 | |
| K2O | Range | 0.00–0.02 | 0.00–0.02 | 0.01–0.02 | 0.01–0.02 | 0.02–0.02 | 0.01–0.02 | 0.00–0.02 |
| Average | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| TiO2 | Range | 0.26–0.49 | 0.34–0.37 | 0.28–0.35 | 0.24–0.27 | 0.45–0.56 | 0.34–0.38 | 0.46–0.52 |
| Average | 0.38 | 0.35 | 0.31 | 0.26 | 0.51 | 0.37 | 0.49 | |
| Cr2O3 | Range | 0.00–0.04 | 0.00–0.05 | 0.02–0.04 | 0.06–0.11 | 0.01–0.09 | 0.02–0.10 | 0.05–0.09 |
| Average | 0.01 | 0.01 | 0.03 | 0.08 | 0.03 | 0.06 | 0.07 | |
| Na2O | Range | 0.00–0.02 | 0.01–0.02 | 0.03–0.04 | 0.01–0.04 | 0.01–0.03 | 0.01–0.04 | 0.00–0.02 |
| Average | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | |
| NiO | Range | 0.02–0.08 | 0.02–0.06 | 0.04–0.06 | 0.01–0.02 | 0.02–0.08 | 0.01–0.05 | 0.01–0.05 |
| Average | 0.04 | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.02 | |
| V2O3 | Range | 0.01–0.05 | 0.01–0.05 | 0.03–0.07 | 0.03–0.13 | 0.11–0.32 | 0.43–0.65 | 0.61–0.70 |
| Average | 0.01 | 0.02 | 0.04 | 0.10 | 0.23 | 0.51 | 0.67 | |
| Total | Range | 98.18–101.40 | 100.46–100.89 | 99.36–100.25 | 99.87–100.04 | 100.33–101.23 | 100.03–100.31 | 99.02–99.94 |
| Average | 99.39 | 100.72 | 99.71 | 99.91 | 100.68 | 100.13 | 99.31 |
| Sample No. | Chemical Formula |
|---|---|
| BH1 | (Ca2.98Mg0.03Mn0.02)3.03(Al1.91Ti0.02)1.93Si3.03O12 |
| BH2 | (Ca2.95Mg0.06Mn0.02)3.03(Al1.96Ti0.02)1.98Si2.97O12 |
| BH3 | (Ca2.92Mg0.06Mn0.02)3.00(Al2.00Ti0.02)2.02Si2.98O12 |
| BH4 | (Ca2.97Mg0.06Mn0.02)3.05(Al1.96Ti0.01)1.97Si2.95O12 |
| BH5 | (Ca2.93Mg0.07Mn0.02)3.02(Al1.95Ti0.03V0.01)1.99Si2.98O12 |
| BH6 | (Ca2.94Mg0.05Mn0.04)3.03(Al1.98V0.03Ti0.02)2.03Si2.93O12 |
| BH7 | (Ca2.99Mg0.06Mn0.04)3.09(Al1.96V0.04Ti0.03)2.03Si2.88O12 |
| Mg | P | Ti | V | Cr | Mn | Fe | |
|---|---|---|---|---|---|---|---|
| BH1 | 3531 | 157 | 2893 | 121 | 128 | 2232 | 613 |
| BH2 | 3463 | 136 | 2127 | 257 | 102 | 3266 | 597 |
| BH3 | 3534 | 71.6 | 1850 | 149 | 189 | 2287 | 620 |
| BH4 | 3259 | 54.0 | 1733 | 281 | 133 | 2328 | 579 |
| BH5 | 3653 | 197 | 2926 | 2470 | 318 | 2641 | 591 |
| BH6 | 3156 | 87.6 | 2281 | 3582 | 310 | 4947 | 710 |
| BH7 | 3400 | 137 | 3068 | 4721 | 425 | 4750 | 612 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Shi, Y.; Shao, T.; Li, X.; Xu, F.; Shen, A.H. Luminescence Characteristics of Green Grossular Garnets. Minerals 2023, 13, 639. https://doi.org/10.3390/min13050639
Zhang Q, Shi Y, Shao T, Li X, Xu F, Shen AH. Luminescence Characteristics of Green Grossular Garnets. Minerals. 2023; 13(5):639. https://doi.org/10.3390/min13050639
Chicago/Turabian StyleZhang, Qian, Yujing Shi, Tian Shao, Xingtong Li, Fengshun Xu, and Andy H. Shen. 2023. "Luminescence Characteristics of Green Grossular Garnets" Minerals 13, no. 5: 639. https://doi.org/10.3390/min13050639
APA StyleZhang, Q., Shi, Y., Shao, T., Li, X., Xu, F., & Shen, A. H. (2023). Luminescence Characteristics of Green Grossular Garnets. Minerals, 13(5), 639. https://doi.org/10.3390/min13050639








