Genetic Mechanism of Structurally Controlled Dolomites Derived from Seawater-Hydrothermal Mixed Fluids—A Case Study from Middle Permian, Central Sichuan Basin, South China
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
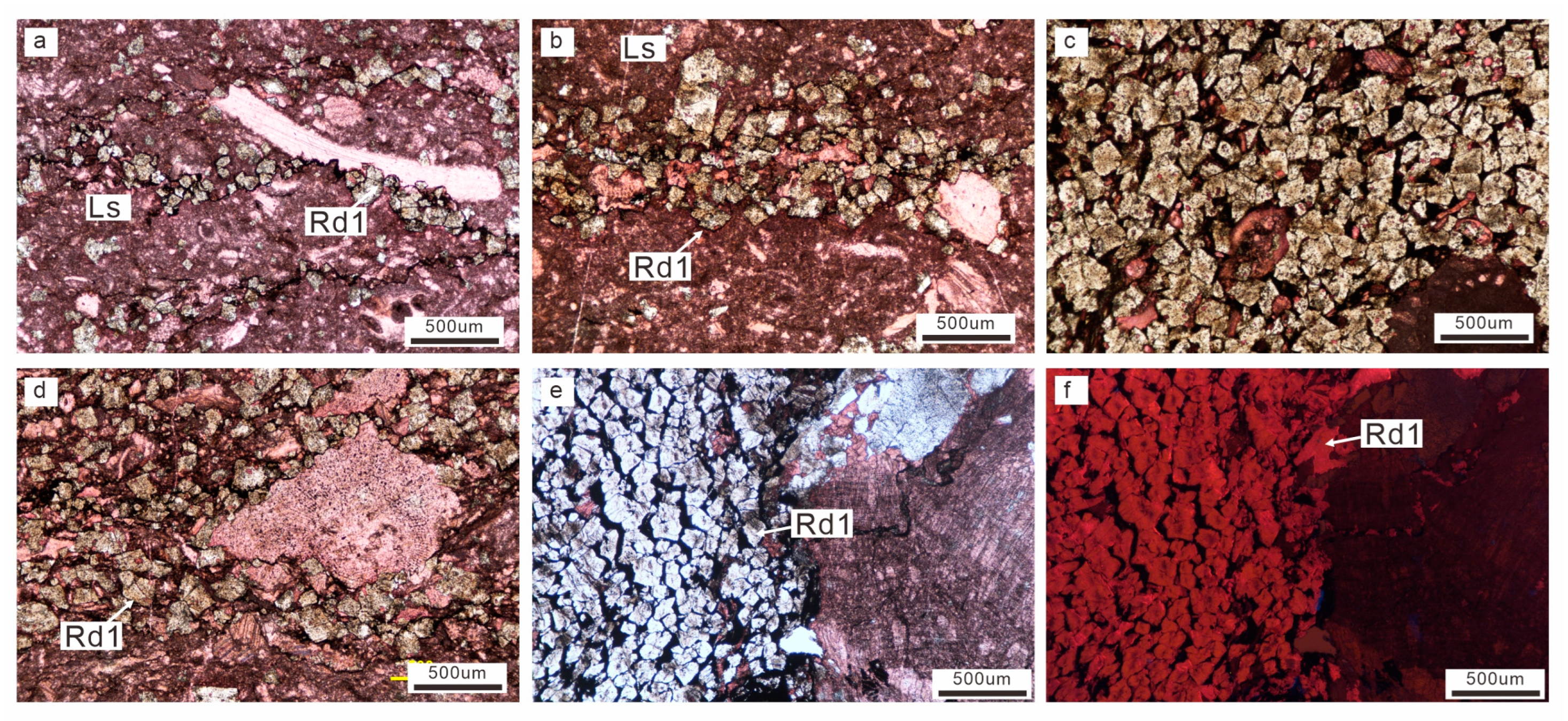
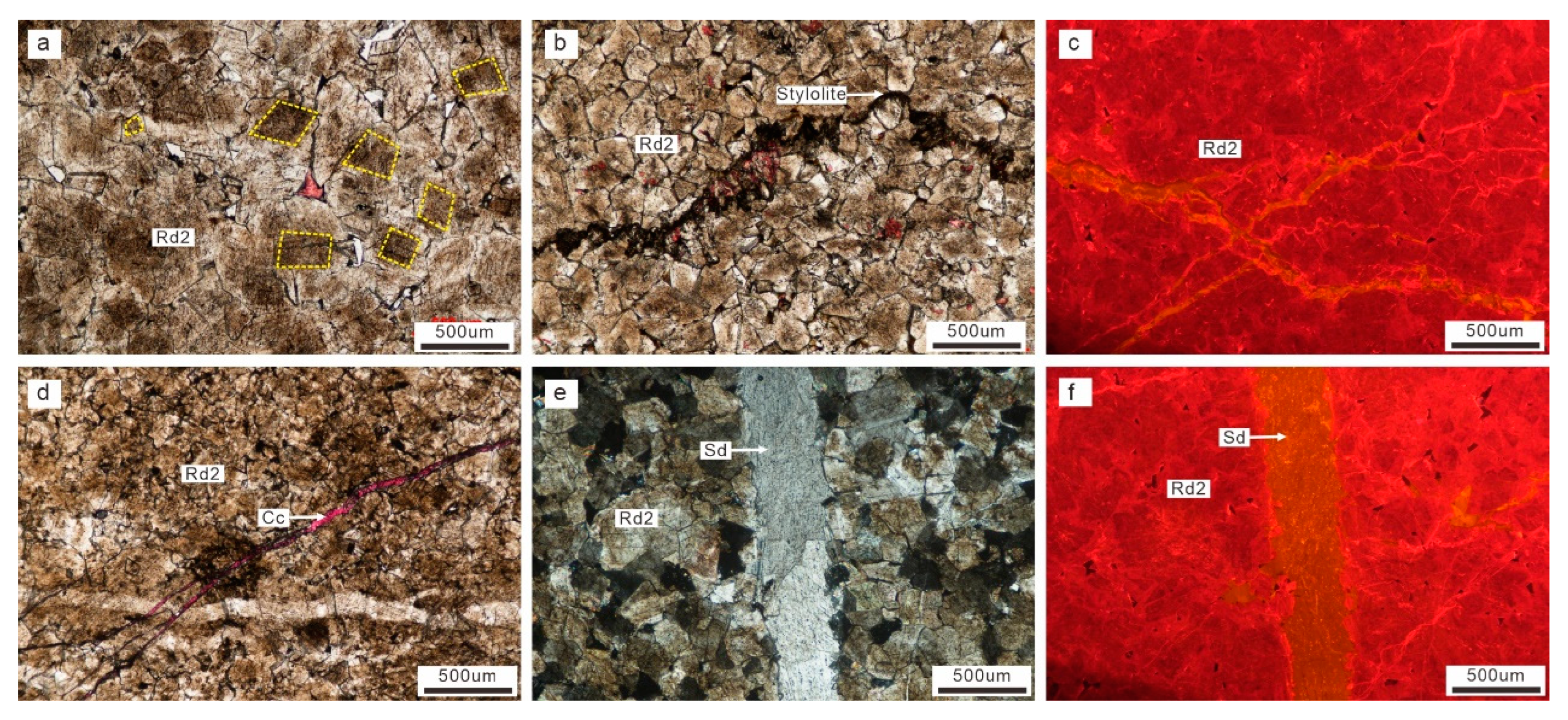
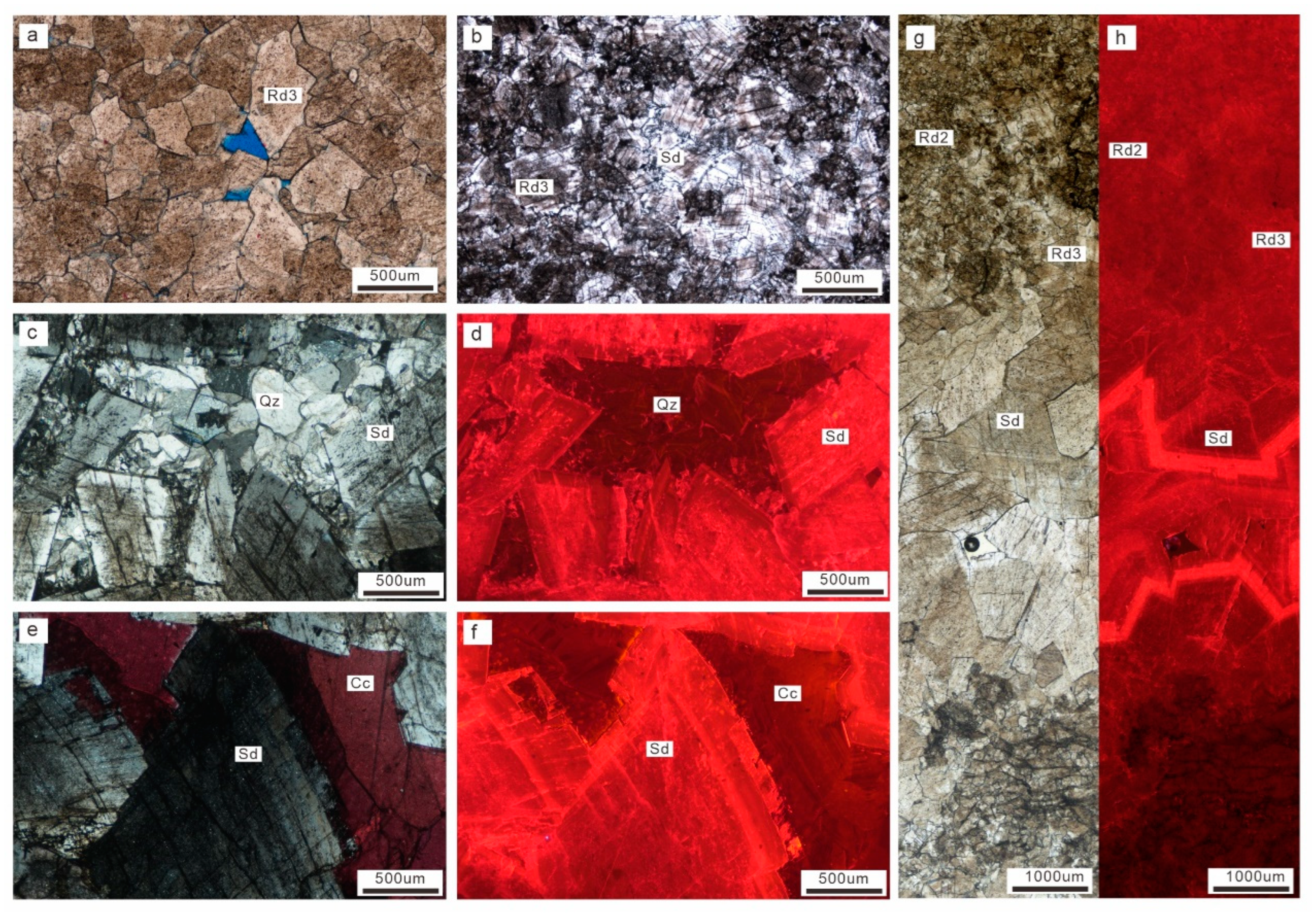
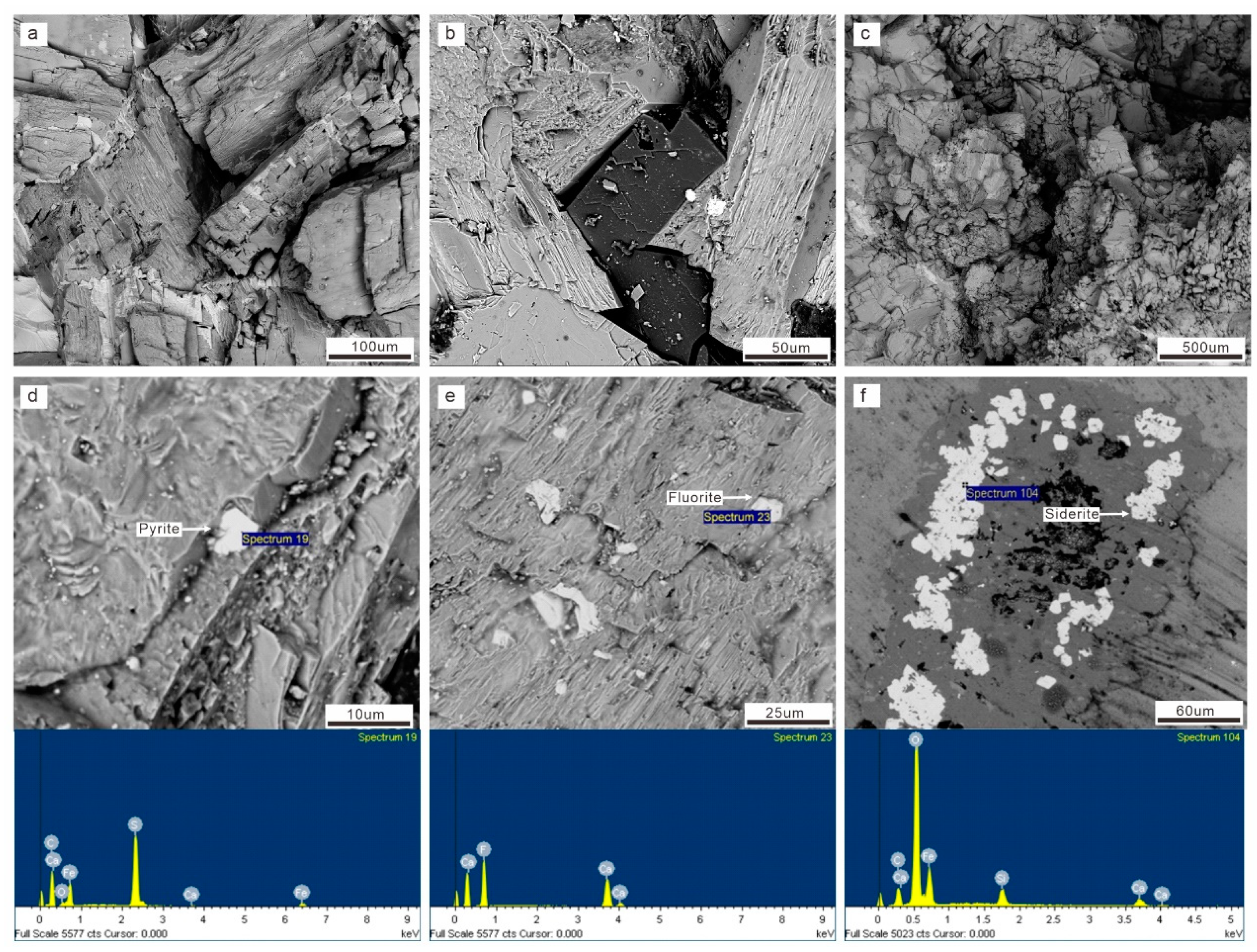
4.1. Petrography
4.1.1. Macroscopic Petrology
4.1.2. Microscopic Petrography
4.2. Microthermometry and Salinity
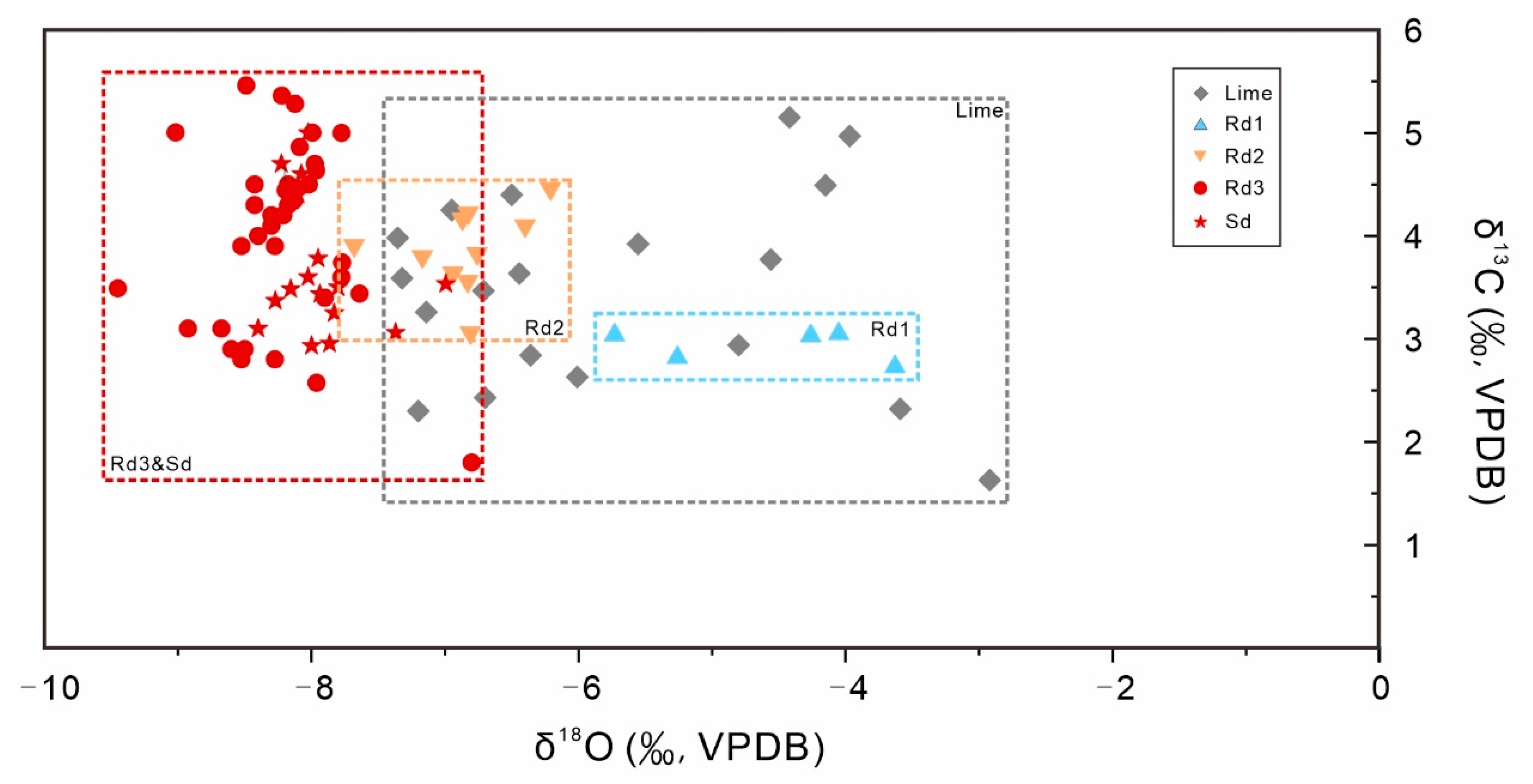
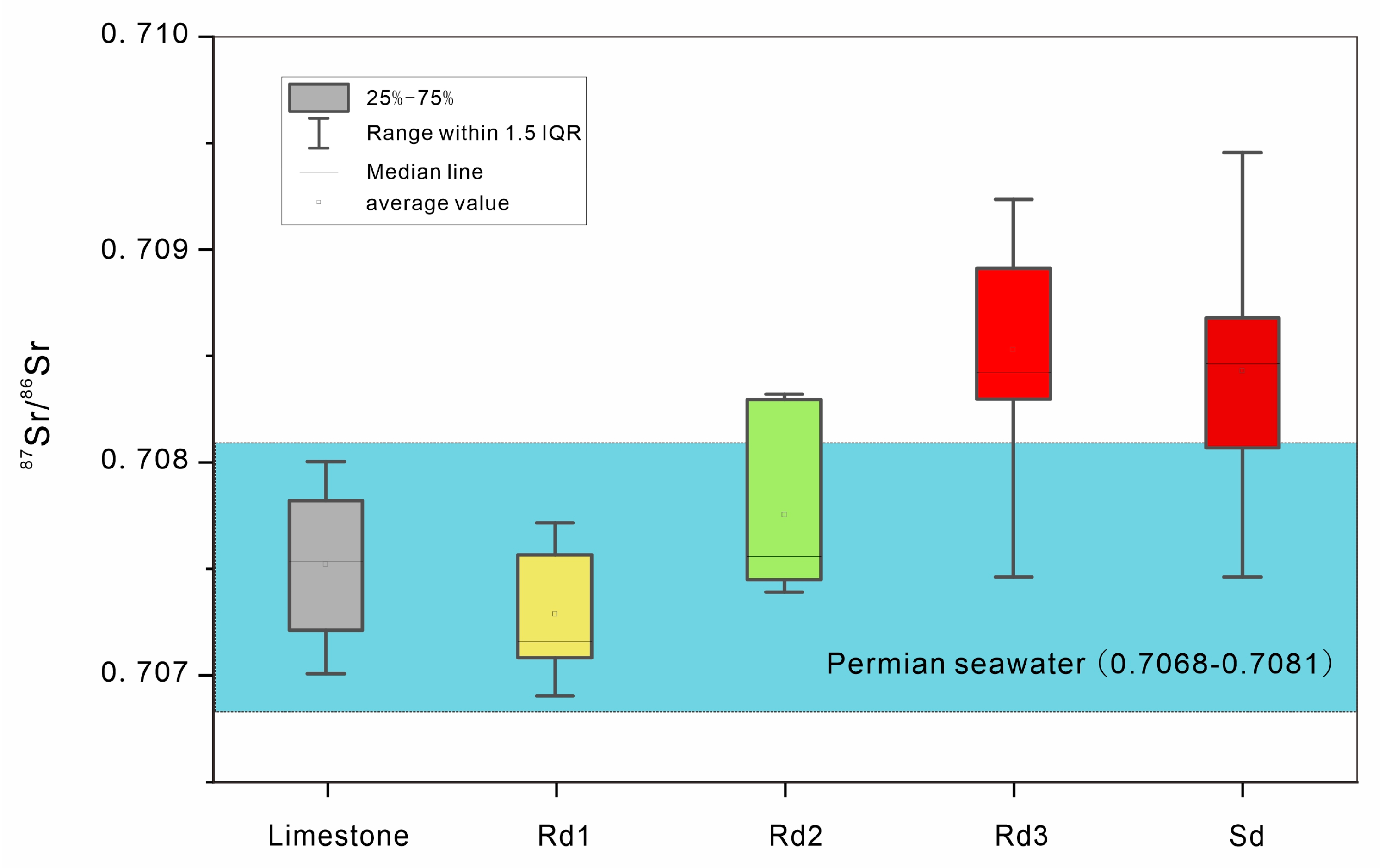
4.3. Carbon, Oxygen, and Strontium Isotopes
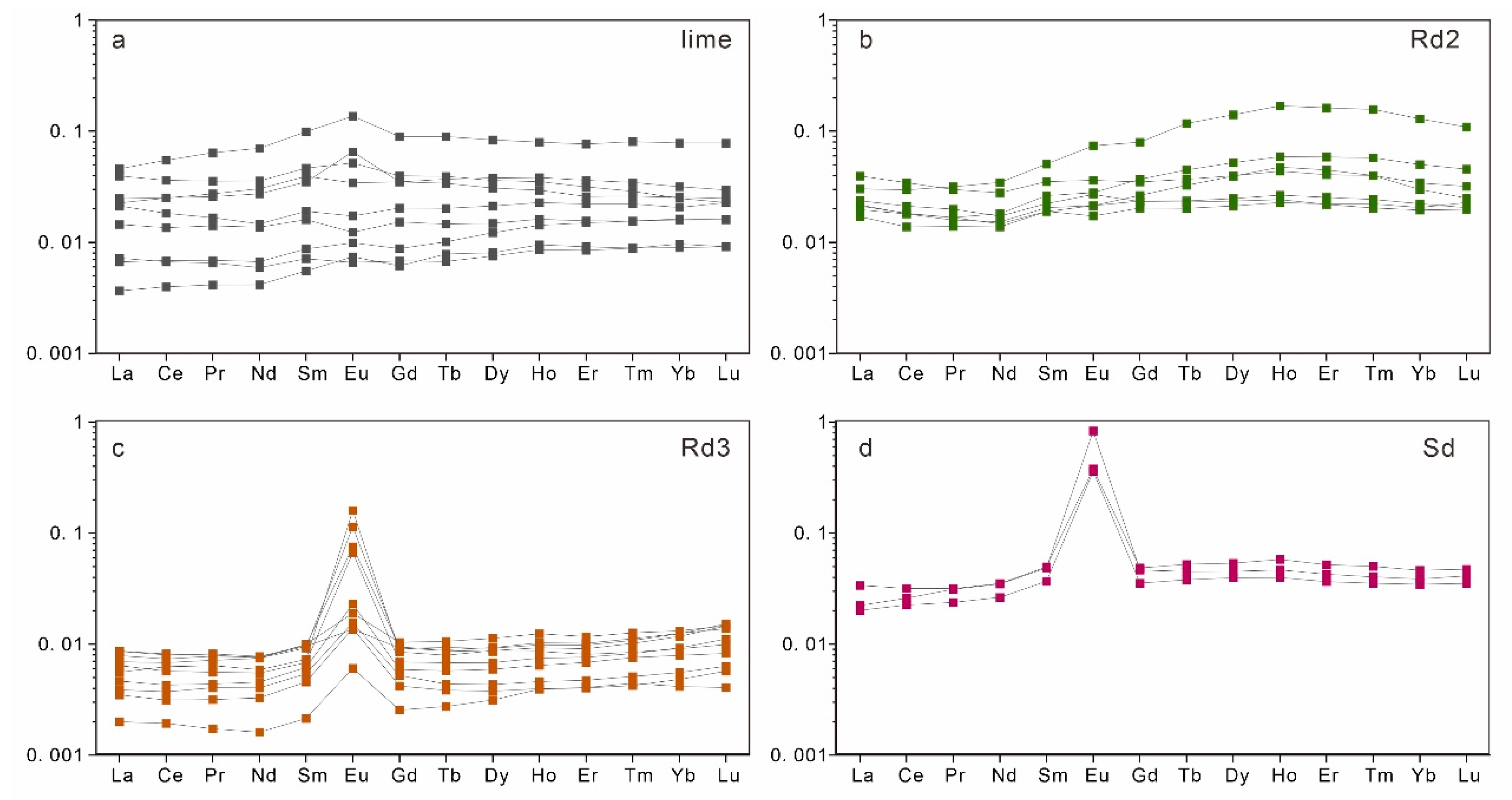
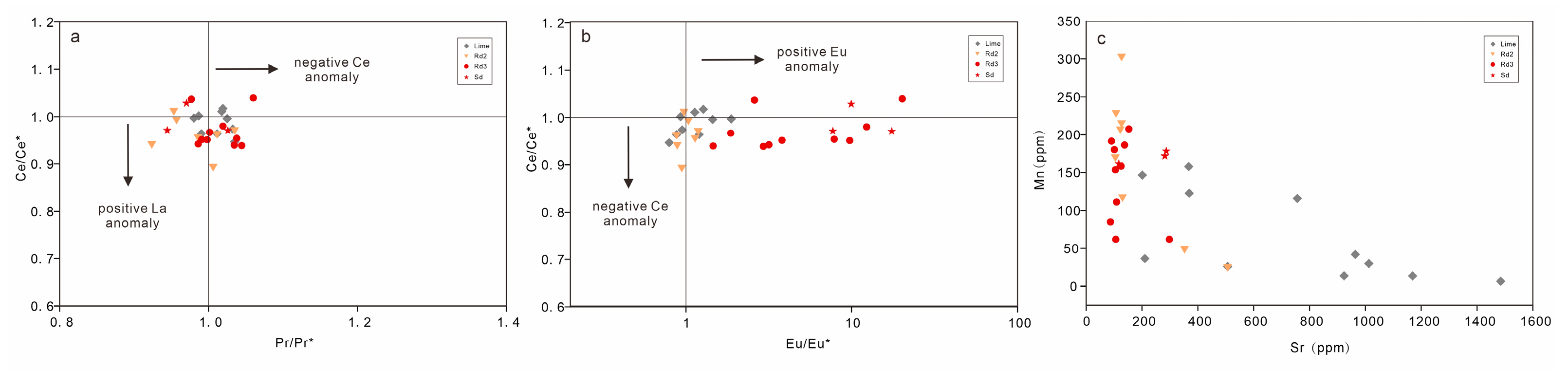
4.4. Trace and Rare Earth Elements
5. Discussion
5.1. Diagenetic Sequence
5.2. Sources of Dolomitizing Fluids
5.3. Genetic Mechanism of the Dolomites
6. Conclusions
- In the eastern part of the Middle Permian in the Sichuan Basin, dolomites mainly occur in the upper part of the Maokou Formation and the top of the Qixia Formation, consisting mainly of three types of replacive dolomites (Rd1, Rd2, and Rd3) and saddle dolomite cement (Sd).
- Petrological and geochemical evidence indicates that Rd1 formed during the initial stage of burial, mainly from residual seawater in the strata.
- Intense hydrothermal activity related to ELIP caused seawater–hydrothermal mixing dolomitization, which was the main cause of the formation of Rd2, Rd3, and Sd. Activated basement faults, freshwater dissolution cavities at the top of the strata, and high–permeability strata were the main pathways for fluid circulation. The diagenetic fluids of Rd3 and Sd contain more deep–seated hydrothermal fluids, reflected in their higher 87Sr/86Sr ratios, distinct Eu–positive anomalies, and very high temperature and salinity. Their distribution is also controlled by basement faults.
- This study provides a good example for investigating the formation mechanism of dolomite controlled by faults under the influence of anomalous thermal effects.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, G.R.; Smith, L.B., Jr. Structurally controlled hydrothermal dolomite reservoir facies: An overview: Reply. AAPG Bull. 2007, 91, 1342–1344. [Google Scholar] [CrossRef]
- Barale, L.; Bertok, C.; Salih Talabani, N.; d’Atri, A.; Martire, L.; Piana, F.; Préat, A.; Hollis, C. Very hot, very shallow hydrothermal dolomitization: An example from the Maritime Alps (north–west Italy–south–east France). Sedimentology 2016, 63, 2037–2065. [Google Scholar] [CrossRef]
- Koeshidayatullah, A.; Corlett, H.; Stacey, J.; Swart, P.K.; Boyce, A.; Hollis, C. Origin and evolution of fault–controlled hydrothermal dolomitization fronts: A new insight. Earth Planet. Sci. Lett. 2020, 541, 116291. [Google Scholar] [CrossRef]
- Lavoie, D.; Chi, G.; Urbatsch, M.; Davis, W.J. Massive dolomitization of a pinnacle reef in the Lower Devonian West Point Formation (Gaspé Peninsula, Quebec): An extreme case of hydrothermal dolomitization through fault–focused circulation of magmatic fluids. AAPG Bull. 2010, 94, 513–531. [Google Scholar] [CrossRef]
- Friedman, G.M. Structurally controlled hydrothermal dolomite reservoir facies: An overview: Discussion. AAPG Bull. 2007, 91, 1339–1341. [Google Scholar] [CrossRef]
- Mansurbeg, H.; Morad, D.; Othman, R.; Morad, S.; Ceriani, A.; Al-Aasm, I.; Kolo, K.; Spirov, P.; Proust, J.N.; Preat, A. Hydrothermal dolomitization of the Bekhme Formation (Upper Cretaceous), Zagros Basin, Kurdistan Region of Iraq: Record of oil migration and degradation. Sediment. Geol. 2016, 341, 147–162. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Wang, J.; Hou, M.; Xu, S.; Zhu, P.; Zhang, C.; Cui, Y. Thermal convection dolomitization induced by the Emeishan Large Igneous Province. Mar. Pet. Geol. 2020, 116, 104308. [Google Scholar] [CrossRef]
- Koeshidayatullah, A.; Corlett, H.; Stacey, J.; Swart, P.K.; Boyce, A.; Robertson, H.; Whitaker, F.; Hollis, C. Evaluating new fault–controlled hydrothermal dolomitization models: Insights from the Cambrian Dolomite, Western Canadian Sedimentary Basin. Sedimentology 2020, 67, 2945–2973. [Google Scholar] [CrossRef]
- Zheng, H.; Ma, Y.; Chi, G.; Qing, H.; Liu, B.; Zhang, X.; Shen, Y.; Liu, J.; Wang, Y. Stratigraphic and Structural Control on Hydrothermal Dolomitization in the Middle Permian Carbonates, Southwestern Sichuan Basin (China). Minerals 2019, 9, 32. [Google Scholar] [CrossRef]
- Guo, C.; Chen, D.; Qing, H.; Dong, S.; Li, G.; Wang, D.; Qian, Y.; Liu, C. Multiple dolomitization and later hydrothermal alteration on the Upper Cambrian–Lower Ordovician carbonates in the northern Tarim Basin, China. Mar. Pet. Geol. 2016, 72, 295–316. [Google Scholar] [CrossRef]
- Dong, S.; Chen, D.; Qing, H.; Zhou, X.; Wang, D.; Guo, Z.; Jiang, M.; Qian, Y. Hydrothermal alteration of dolostones in the Lower Ordovician, Tarim Basin, NW China: Multiple constraints from petrology, isotope geochemistry and fluid inclusion microthermometry. Mar. Pet. Geol. 2013, 46, 270–286. [Google Scholar] [CrossRef]
- Yang, T.; Azmy, K.; He, Z.; Li, S.; Liu, E.; Wu, S.; Wang, J.; Li, T.; Gao, J. Fault–controlled hydrothermal dolomitization of Middle Permian in southeastern Sichuan Basin, SW China, and its temporal relationship with the Emeishan Large Igneous Province: New insights from multi–geochemical proxies and carbonate U–Pb dating. Sediment. Geol. 2022, 439, 106215. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Jin, Z.-K.; Zhu, X.-E.; Li, Y.; Guo, Q.-H.; Shi, S.-T. Open thermal convection dolomitization: An example from East Yunnan (China). Geol. Mag. 2021, 158, 330–348. [Google Scholar] [CrossRef]
- Lin, P.; Peng, J.; Zhang, L.; Lan, X.; Wang, J.; Xia, Q.; Xia, J. Characteristics of multiple dolomitizing fluids and the genetic mechanism of dolomite formation in the Permian Qixia Formation, NW Sichuan Basin. J. Pet. Sci. Eng. 2022, 208, 109749. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L.; Huang, R.; Duan, J.; Xu, Z.; Pan, L. Characteristics and main controlling factors of the Middle Permian Maokou dolomite reservoirs in the eastern Sichuan Basin. Nat. Gas Ind. 2019, 39, 13–21. [Google Scholar]
- Tian, J.; Lin, X.; Zhang, X.; Peng, S.; Chenyu, Y.; Shoubing, L.; Liang, X. The genetic mechanism of shoal facies dolomite and its additive effect of Permian Qixia Formation in Sichuan Basin. Acta Petrol. Sin. 2014, 30, 679–686. [Google Scholar]
- Jiang, Q.; Hu, S.; Wang, Z.; Wang, T.; Li, Q.; Zhai, X. Genesis of medium–macro–crystalline dolomite in the Middle Permian of Sichuan Basin. Oil Gas Geol. 2014, 35, 503–510. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, W.; Liu, Y.; Zhou, H.; Jiang, Q. Characteristics and exploration strategy of the Middle Permian hydrothermal dolomite in southwestern Sichuan Basin. Acta Pet. Sin. 2013, 34, 460–466. [Google Scholar] [CrossRef]
- Chen, X.; ZHao, W.; Zhang, L. Discovery and exploration significance of structure–controlled hydrothermal dolomites in the Middle Permian of the central Sichuan Basin. Acta Pet. Sin. 2012, 33, 562–569. [Google Scholar] [CrossRef]
- Qi, L.; Gu, Y.; He, P.; Wang, Z.; Jiang, Y.; Li, S.; Zhou, Y. Hydrothermal dolomitization in the Middle Permian in the Central Sichuan Basin, SW China: Evidence from petrology, geochemistry, and fluid inclusions. Arab. J. Geosci. 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Huang, K.; Lan, Y.; Jie, L. Authigenic noncarbonate minerals in hydrothermal dolomite of Middle Permian Qixia Formation in the west of Sichuan Basin, China. J. Chengdu Univ. Technol. 2012, 7, 343–352. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Yuan, Y.; Zhao, Y.; Shan, J.; He, Z.; Tian, Y.; Hu, S. Palaeogeothermal response and record of the effusing of Emeishan basalts in the Sichuan basin. Chin. Sci. Bull. 2010, 55, 949–956. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Shan, J.; Yuan, Y.; Zhao, Y.; Hu, S. Quantifying the denudations of major tectonic events in Sichuan basin:Constrained by the paleothermal records. Geol. China 2009, 36, 1268–1277. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, S.; Qiu, N.; Jiang, Q.; Rao, S.; Liu, S. Geothermal constraints on Emeishan mantle plume magmatism: Paleotemperature reconstruction of the Sichuan Basin, SW China. Int. J. Earth Sci. 2018, 107, 71–88. [Google Scholar] [CrossRef]
- He, D.; Li, D.; Zhang, G.; Zhao, L.; Fan, C.; Lu, R.; Wen, Z. Formation and evolution of multi–cycle superposed Sichuan Basin, China. Dizhi Kexue/Chin. J. Geol. 2011, 46, 589–606. [Google Scholar]
- Wang, H.-J.; Yang, Z.-R.; Han, B. Superposed evolution of Sichuan Basin and its petroleum accumulation. Earth Sci. Front. 2015, 22, 161. [Google Scholar]
- Xiao, D.; Zhang, B.; Tan, X.; Liu, H.; Xie, J.; Wang, L.; Yang, X.; Ma, T. Discovery of a shoal–controlled karst dolomite reservoir in the Middle Permian Qixia Formation, northwestern Sichuan Basin, Southwest China. Energy Explor. Exploit. 2018, 36, 686–704. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, X.; Xi, A.; Liu, H.; Shan, S.; Xia, J.; Cheng, Y.; Lian, C. An inland facies–controlled eogenetic karst of the carbonate reservoir in the Middle Permian Maokou Formation, southern Sichuan Basin, SW China. Mar. Pet. Geol. 2016, 72, 218–233. [Google Scholar] [CrossRef]
- He, B.; Xu, Y.-G.; Guan, J.-P.; Zhong, Y.-T. Paleokarst on the top of the Maokou Formation: Further evidence for domal crustal uplift prior to the Emeishan flood volcanism. Lithos 2010, 119, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, W.; Li, Z.; Jiang, X.; Jun, L. Role of basement faults in gas accumulation of Xujiahe Formation, Sichuan Basin. Pet. Explor. Dev. 2008, 35, 541–547. [Google Scholar] [CrossRef]
- Xihua, Z.; Cong, C.; Jie, H.; Long, W.; Chen, X.; Shiwei, X.; Zhaolong, G. The discovery of Middle Permian Guangyuan–Bazhong extensional trough in the Sichuan Basin and its petroleum geological significance. China Pet. Explor. 2019, 24, 466. [Google Scholar]
- Xingzhi, W.; Bo, L.I.; Xiyan, Y.; Long, W.E.N.; Liang, X.U.; Shengyang, X.I.E.; Yao, D.U.; Mingyou, F.; Xuefei, Y.; Yaping, W. Characteristics of “Guangyuan–Wangcang” trough during Late Middle Permian and its petroleum geological significance in northern Sichuan Basin, SW China. Pet. Explor. Dev. 2021, 48, 655–669. [Google Scholar]
- Liu, X.; Qiu, N.; Søager, N.; Fu, X.; Liu, R. Geochemistry of Late Permian basalts from boreholes in the Sichuan Basin, SW China: Implications for an extension of the Emeishan large igneous province. Chem. Geol. 2022, 588, 120636. [Google Scholar] [CrossRef]
- Dickson, J. Carbonate identification and genesis as revealed by staining. J. Sediment. Res. 1966, 36, 491–505. [Google Scholar] [CrossRef]
- Pourmand, A.; Dauphas, N.; Ireland, T.J. A novel extraction chromatography and MC–ICP–MS technique for rapid analysis of REE, Sc and Y: Revising CI–chondrite and Post–Archean Australian Shale (PAAS) abundances. Chem. Geol. 2012, 291, 38–54. [Google Scholar] [CrossRef]
- Chi, G. Equations for calculation of NaCl/(NaCl + CaCl2) ratios and salinities from hydrohalite–melting and ice–melting temperatures in the H2O–NaCl–CaCl2 system. Acta Petrol. Sin. 2007, 23, 33–37. [Google Scholar]
- Steele–MacInnis, M.; Bodnar, R.; Naden, J. Numerical model to determine the composition of H2O–NaCl–CaCl2 fluid inclusions based on microthermometric and microanalytical data. Geochim. Et Cosmochim. Acta 2011, 75, 21–40. [Google Scholar] [CrossRef]
- Korte, C.; Jasper, T.; Kozur, H.W.; Veizer, J. 87Sr/86Sr record of Permian seawater. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 240, 89–107. [Google Scholar] [CrossRef]
- Korte, C.; Kozur, H.W.; Bruckschen, P.; Veizer, J. Strontium isotope evolution of Late Permian and Triassic seawater. Geochim. Et Cosmochim. Acta 2003, 67, 47–62. [Google Scholar] [CrossRef]
- Choquette, P.W.; Hiatt, E.E. Shallow–burial dolomite cement: A major component of many ancient sucrosic dolomites. Sedimentology 2008, 55, 423–460. [Google Scholar] [CrossRef]
- Gregg, J.M.; Sibley, D.F. Epigenetic dolomitization and the origin of xenotopic dolomite texture. J. Sediment. Res. 1984, 54, 908–931. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and models of dolomitization: A critical reappraisal. Geol. Soc. Lond. Spec. Publ. 2004, 235, 7–63. [Google Scholar] [CrossRef]
- Sibley, D.F.; Gregg, J.M. Classification of dolomite rock textures. J. Sediment. Res. 1987, 57, 967–975. [Google Scholar] [CrossRef]
- Smith, L.B., Jr. Origin and reservoir characteristics of Upper Ordovician Trenton–Black River hydrothermal dolomite reservoirs in New York. AAPG Bull. 2006, 90, 1691–1718. [Google Scholar] [CrossRef]
- Lavoie, D.; Chi, G. Hydrothermal dolomitization in the Lower Silurian La Vieille Formation in northern New Brunswick: Geological context and significance for hydrocarbon exploration. Bull. Can. Pet. Geol. 2006, 54, 380–395. [Google Scholar] [CrossRef]
- Koehn, D.; Renard, F.; Toussaint, R.; Passchier, C. Growth of stylolite teeth patterns depending on normal stress and finite compaction. Earth Planet. Sci. Lett. 2007, 257, 582–595. [Google Scholar] [CrossRef]
- Andrews, L.M.; Railsback, L.B. Controls on stylolite development: Morphologic, lithologic, and temporal evidence from bedding–parallel and transverse stylolites from the US Appalachians. J. Geol. 1997, 105, 59–73. [Google Scholar] [CrossRef]
- Banner, J.L.; Hanson, G.N. Calculation of simultaneous isotopic and trace element variations during water–rock interaction with applications to carbonate diagenesis. Geochim. Et Cosmochim. Acta 1990, 54, 3123–3137. [Google Scholar] [CrossRef]
- Qing, H.; Mountjoy, E.W. Formation of coarsely crystalline, hydrothermal dolomite reservoirs in the Presqu’ile Barrier, Western Canada Sedimentary Basin. AAPG Bull. 1994, 78, 55–77. [Google Scholar]
- Bau, M. Rare–earth element mobility during hydrothermal and metamorphic fluid–rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Azomani, E.; Azmy, K.; Blamey, N.; Brand, U.; Al-Aasm, I. Origin of Lower Ordovician dolomites in eastern Laurentia: Controls on porosity and implications from geochemistry. Mar. Pet. Geol. 2013, 40, 99–114. [Google Scholar] [CrossRef]
- Mahboubi, A.; Nowrouzi, Z.; Al-Aasm, I.S.; Moussavi-Harami, R.; Mahmudy-Gharaei, M.H. Dolomitization of the Silurian Niur Formation, Tabas block, east central Iran: Fluid flow and dolomite evolution. Mar. Pet. Geol. 2016, 77, 791–805. [Google Scholar] [CrossRef]
- Widodo, R.W.; Laya, J.C. Controls on diagenesis and dolomitization of peritidal facies, Early Cretaceous Lower Edwards Group, central Texas, USA. Facies 2017, 63, 1–25. [Google Scholar] [CrossRef]
- Vahrenkamp, V.C.; Swart, P.K. New distribution coefficient for the incorporation of strontium into dolomite and its implications for the formation of ancient dolomites. Geology 1990, 18, 387–391. [Google Scholar] [CrossRef]
- Horita, J.; Friedman, T.J.; Lazar, B.; Holland, H.D. The composition of Permian seawater. Geochim. Et Cosmochim. Acta 1991, 55, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, T.K.; Timofeeff, M.N.; Kovalevych, V.M.; Horita, J. The major–ion composition of Permian seawater. Geochim. Et Cosmochim. Acta 2005, 69, 1701–1719. [Google Scholar] [CrossRef]
- Mountjoy, E.W.; Machel, H.G.; Green, D.; Duggan, J.; Williams-Jones, A.E. Devonian matrix dolomites and deep burial carbonate cements: A comparison between the Rimbey–Meadowbrook reef trend and the deep basin of west–central Alberta. Bull. Can. Pet. Geol. 1999, 47, 487–509. [Google Scholar]
- Nasir, S.; Al-Saad, H.; Alsayigh, A.; Weidlich, O. Geology and petrology of the Hormuz dolomite, Infra–Cambrian: Implications for the formation of the salt–cored Halul and Shraouh islands, Offshore, State of Qatar. J. Asian Earth Sci. 2008, 33, 353–365. [Google Scholar] [CrossRef]
- Compton, J.; Harris, C.; Thompson, S. Pleistocene dolomite from the Namibian Shelf: High 87Sr/86Sr and δ18O values indicate an evaporative, mixed–water origin. J. Sediment. Res. 2001, 71, 800–808. [Google Scholar] [CrossRef]
- Meyers, W.J.; Lu, F.H.; Zachariah, J.K. Dolomitization by mixed evaporative brines and freshwater, upper Miocene carbonates, Nijar, Spain. J. Sediment. Res. 1997, 67, 898–912. [Google Scholar]
- Li, D.; Chen, H.; Chen, H.; Liang, H.; Peng, C.; Xia, M.; Duan, H. Relationship between reservoir development in the Middle Permian Maokou Formation and paleostructure evolution in the Sichuan Basin. Oil Gas Geol. 2016, 37, 756–763. [Google Scholar]
- Xu, G.; Xie, G.; Long, K.; Song, X. Sedimentary features and exploration targets of Middle Permian reservoirs in the SW Sichuan Basin. Nat. Gas Ind. B 2015, 2, 415–420. [Google Scholar] [CrossRef]
- Davies, G.R.; Smith, L.B., Jr. Structurally controlled hydrothermal dolomite reservoir facies: An overview. AAPG Bull. 2006, 90, 1641–1690. [Google Scholar] [CrossRef]
- Chen, D.; Qing, H.; Yan, X.; Li, H. Hydrothermal venting and basin evolution (Devonian, South China): Constraints from rare earth element geochemistry of chert. Sediment. Geol. 2006, 183, 203–216. [Google Scholar] [CrossRef]
- Hecht, L.; Freiberger, R.; Gilg, H.A.; Grundmann, G.; Kostitsyn, Y.A. Rare earth element and isotope (C, O, Sr) characteristics of hydrothermal carbonates: Genetic implications for dolomite–hosted talc mineralization at Göpfersgrün (Fichtelgebirge, Germany). Chem. Geol. 1999, 155, 115–130. [Google Scholar] [CrossRef]
- Pierson, B.J. The control of cathodoluminescence in dolomite by iron and manganese. Sedimentology 1981, 28, 601–610. [Google Scholar] [CrossRef]
- Machel, H.-G. Cathodoluminescence in calcite and dolomite and its chemical interpretation. Geosci. Can. 1985, 12. [Google Scholar]
- Katz, A.; Matthews, A. The dolomitization of CaCO3: An experimental study at 252–295 °C. Geochim. Et Cosmochim. Acta 1977, 41, 297–308. [Google Scholar] [CrossRef]
- Malone, M.J.; Baker, P.A.; Burns, S.J. Hydrothermal dolomitization and recrystallization of dolomite breccias from the Miocene Monterey Formation, Tepusquet area, California. J. Sediment. Res. 1996, 66, 976–990. [Google Scholar]
- Chi, G.; Xu, D.; Xue, C.; Li, Z.; Ledru, P.; Deng, T.; Wang, Y.; Song, H. Hydrodynamic Links between Shallow and Deep Mineralization Systems and Implications for Deep Mineral Exploration. Acta Geol. Sin.–Engl. Ed. 2022, 96, 1–25. [Google Scholar] [CrossRef]
- Dong, Y. Genetic Mechanism of the Middle Permian Dolomite in the Sichuan Basin; Chengdu University of Technology: Chengdu, China, 2020. [Google Scholar]
- Benjakul, R.; Hollis, C.; Robertson, H.A.; Sonnenthal, E.L.; Whitaker, F.F. Understanding controls on hydrothermal dolomitisation: Insights from 3D reactive transport modelling of geothermal convection. Solid Earth 2020, 11, 2439–2461. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, J.; Tang, H.; Liu, B.; Yang, X.; Shi, K.; Luo, K.; Qiu, Q.; Dong, Y. Origin of the Multiple–Sourced Cherts in Maokou Carbonates in Sichuan Basin, South China. Minerals 2021, 11, 1269. [Google Scholar] [CrossRef]
- Wendte, J.; Chi, G.; Al–Aasm, I.; Sargent, D. Fault/fracture controlled hydrothermal dolomitization and associated diagenesis of the Upper Devonian Jean Marie Member (Redknife Formation) in the July Lake area of northeastern British Columbia. Bull. Can. Pet. Geol. 2009, 57, 275–322. [Google Scholar] [CrossRef]
- Smith, L.B.; Davies, G.R. Structurally controlled hydrothermal alteration of carbonate reservoirs: Introduction. AAPG Bull. 2006, 90, 1635–1640. [Google Scholar] [CrossRef]
- Yang, H.; Li, K.K.; Pan, W.Q.; Xiao, Z.Y.; Cai, C.F. Burial hydrothermal dissolution fluid activity and its transforming effect on the reservoirs in Ordovician in Central Tarim. Acta Petrol. Sin. 2012, 28, 783–792. [Google Scholar]
- Liu, Q.; Zhu, D.; Jin, Z.; Liu, C.; Zhang, D.; He, Z. Coupled alteration of hydrothermal fluids and thermal sulfate reduction (TSR) in ancient dolomite reservoirs–An example from Sinian Dengying Formation in Sichuan Basin, southern China. Precambrian Res. 2016, 285, 39–57. [Google Scholar] [CrossRef]
- Corbella, M.; Gomez–Rivas, E.; Martín-Martín, J.D.; Stafford, S.; Teixell, A.; Griera, A.; Travé, A.; Cardellach, E.; Salas, R. Insights to controls on dolomitization by means of reactive transport models applied to the Benicàssim case study (Maestrat Basin, eastern Spain). Pet. Geosci. 2014, 20, 41–54. [Google Scholar] [CrossRef]
- Wilson, M.E.; Evans, M.J.; Oxtoby, N.H.; Nas, D.S.; Donnelly, T.; Thirlwall, M. Reservoir quality, textural evolution, and origin of fault–associated dolomites. AAPG Bull. 2007, 91, 1247–1272. [Google Scholar] [CrossRef]
- Sharp, I.; Gillespie, P.; Morsalnezhad, D.; Taberner, C.; Karpuz, R.; Vergés, J.; Horbury, A.; Pickard, N.; Garland, J.; Hunt, D. Stratigraphic architecture and fracture–controlled dolomitization of the Cretaceous Khami and Bangestan groups: An outcrop case study, Zagros Mountains, Iran. Geol. Soc. Lond. Spec. Publ. 2010, 329, 343–396. [Google Scholar] [CrossRef]
- Korneva, I.; Bastesen, E.; Corlett, H.; Eker, A.; Hirani, J.; Hollis, C.; Gawthorpe, R.L.; Rotevatn, A.; Taylor, R. The effects of dolomitization on petrophysical properties and fracture distribution within rift–related carbonates (Hammam Faraun Fault Block, Suez Rift, Egypt). J. Struct. Geol. 2018, 108, 108–120. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Sibley, D.F. Direct physical evidence of dolomite recrystallization. Sedimentology 2014, 61, 1862–1882. [Google Scholar] [CrossRef]


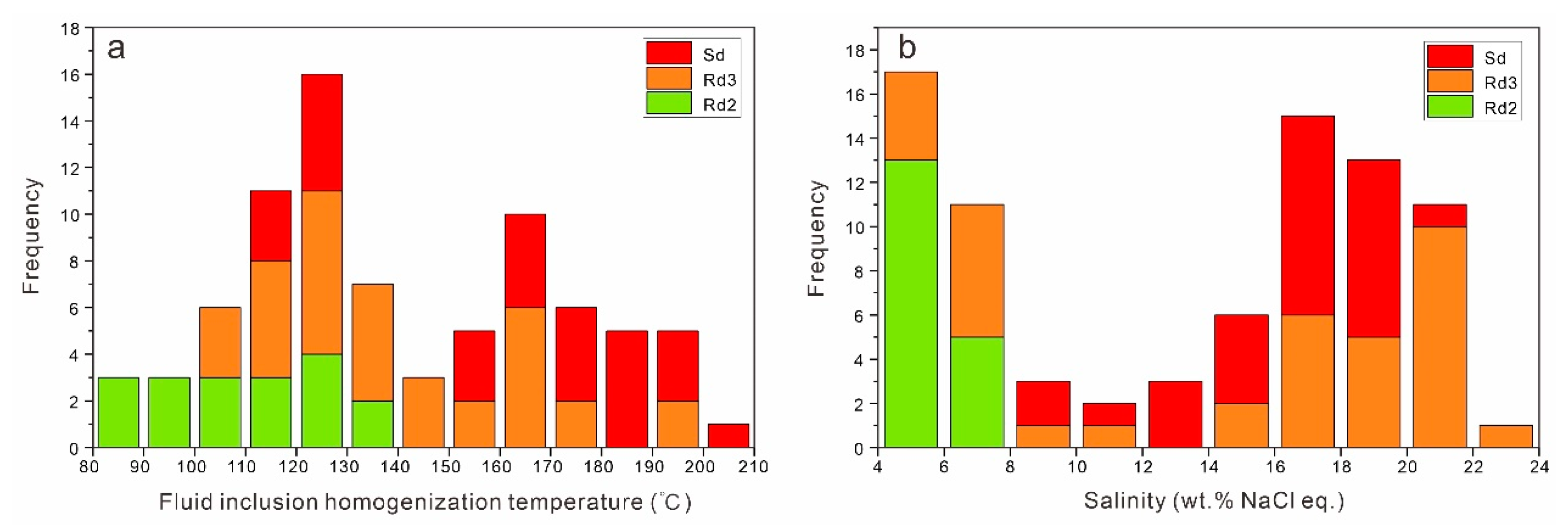
| Sample | Mineral Type | Occurrences | Size (μm) | V% | Th (°C) | Tm (°C) | Salinity (wt% NaCl eq.) |
|---|---|---|---|---|---|---|---|
| GT2-11 | Rd2 | Isolated | 4 | 5 | 98 | −2.6 | 4.3 |
| GT2-11 | Rd2 | Isolated | 3 | 5 | 83 | −4.3 | 6.9 |
| GT2-11 | Rd2 | Isolated | 3 | 5 | 102 | −3.2 | 5.3 |
| GT2-11 | Rd2 | Isolated | 5 | 5 | 86 | −3.3 | 5.4 |
| GT2-11 | Rd2 | Isolated | 2 | 5 | 94 | −3.5 | 5.7 |
| GT2-13 | Rd2 | Isolated | 4 | 5 | 113 | −4.1 | 6.6 |
| GT2-13 | Rd2 | Isolated | 4 | 5 | 132 | −2.9 | 4.8 |
| GT2-13 | Rd2 | Isolated | 4 | 5 | 121 | −3.4 | 5.6 |
| GT2-13 | Rd2 | Isolated | 5 | 10 | 108 | −3.7 | 6.0 |
| GT2-13 | Rd2 | Isolated | 5 | 5 | 115 | −3 | 5.0 |
| GT2-10 | Rd2 | Isolated | 4 | 5 | 125 | −3.3 | 5.4 |
| GT2-10 | Rd2 | Isolated | 5 | 5 | 107 | −3.5 | 5.7 |
| GT2-10 | Rd2 | Isolated | 3 | 5 | 116 | −4.5 | 7.2 |
| GT2-10 | Rd2 | Isolated | 4 | 5 | 128 | −4.6 | 7.3 |
| GC2-3 | Rd2 | Isolated | 3 | 5 | 95.00 | −3.1 | 5.1 |
| GC2-3 | Rd2 | Isolated | 4 | 5 | 86.00 | −3.5 | 5.7 |
| GC2-3 | Rd2 | Isolated | 4 | 5 | 127.00 | −2.9 | 4.8 |
| GC2-3 | Rd2 | Isolated | 3 | 5 | 134.00 | −3.2 | 5.3 |
| GC2-3 | Rd3 | Isolated | 5 | 10 | 197.00 | −17.6 | 20.7 |
| GC2-3 | Rd3 | Isolated | 5 | 10 | 199.00 | −16.9 | 20.1 |
| GC2-10 | Rd3 | Isolated | 6 | 10 | 160.00 | −18.3 | 21.2 |
| GC2-10 | Rd3 | Isolated | 4 | 20 | 162.00 | −16.2 | 19.6 |
| GC2-10 | Rd3 | Isolated | 4 | 10 | 170.00 | −18.6 | 21.4 |
| GC2-10 | Rd3 | Isolated | 4 | 5 | 147.00 | −17.3 | 20.4 |
| GC2-10 | Rd3 | Isolated | 4 | 5 | 170.00 | −15.2 | 18.8 |
| GC2-6 | Rd3 | Isolated | 4 | 5 | 168.00 | −14.1 | 17.9 |
| GC2-6 | Rd3 | Isolated | 5 | 10 | 169.00 | −15.8 | 19.3 |
| GC2-6 | Rd3 | Isolated | 5 | 10 | 166.00 | −19.6 | 22.1 |
| GC2-6 | Rd3 | Isolated | 4 | 5 | 159.00 | −18.7 | 21.5 |
| GC2-6 | Rd3 | Isolated | 4 | 5 | 166.00 | −14.2 | 18.0 |
| GC2-6 | Rd3 | Isolated | 4 | 5 | 153.00 | −13.3 | 17.2 |
| MX42-4 | Rd3 | Isolated | 6 | 5 | 148.00 | −12.9 | 16.8 |
| MX42-4 | Rd3 | Isolated | 4 | 5 | 128.00 | −10.7 | 14.7 |
| MX42-4 | Rd3 | Isolated | 4 | 5 | 135.00 | −11.4 | 15.4 |
| MX42-4 | Rd3 | Isolated | 5 | 5 | 121.00 | −12.3 | 16.2 |
| MX42-4 | Rd3 | Isolated | 4 | 5 | 104.00 | −3.1 | 5.1 |
| MX42-4 | Rd3 | Isolated | 4 | 5 | 106.00 | −4.2 | 6.7 |
| MX42-4 | Rd3 | Isolated | 3 | 5 | 126.00 | −5.9 | 9.1 |
| MX42-6 | Rd3 | Isolated | 3 | 5 | 118.00 | −4.3 | 6.9 |
| MX42-6 | Rd3 | Isolated | 4 | 5 | 119.00 | −3.7 | 6.0 |
| MX42-6 | Rd3 | Isolated | 4 | 5 | 121.00 | −4.2 | 6.7 |
| MX42-6 | Rd3 | Isolated | 5 | 5 | 123.00 | −4.4 | 7.0 |
| MX42-6 | Rd3 | Isolated | 4 | 5 | 123.00 | −3.2 | 5.3 |
| TL6-14 | Rd3 | Isolated | 4 | 5 | 127.60 | −7.7 | 11.3 |
| TL6-14 | Rd3 | Isolated | 5 | 5 | 132.30 | −18.2 | 21.1 |
| TL6-14 | Rd3 | Isolated | 5 | 10 | 134.50 | −18.8 | 21.5 |
| TL6-14 | Rd3 | Isolated | 5 | 10 | 141.60 | −19.3 | 21.9 |
| TL6-14 | Rd3 | Isolated | 5 | 5 | 132.70 | −17.4 | 20.5 |
| TL6-20 | Rd3 | Isolated | 5 | 5 | 105.30 | −4.6 | 7.3 |
| TL6-20 | Rd3 | Isolated | 3 | 10 | 113.20 | −3.2 | 5.3 |
| TL6-20 | Rd3 | Isolated | 3 | 5 | 111.30 | −3.5 | 5.7 |
| TL6-20 | Rd3 | Isolated | 4 | 5 | 112.90 | −13.2 | 17.1 |
| TL6-20 | Rd3 | Isolated | 4 | 5 | 139.6 | −16.4 | 19.8 |
| TL6-20 | Rd3 | Isolated | 4 | 5 | 132.1 | −15.7 | 19.2 |
| GC2-3 | Sd | Cluster | 6 | 5 | 185 | −16.8 | 20.1 |
| GC2-3 | Sd | Cluster | 6 | 5 | 182 | −15.3 | 18.9 |
| GC2-3 | Sd | Cluster | 5 | 10 | 178 | −14.7 | 18.4 |
| GC2-3 | Sd | Cluster | 4 | 5 | 187 | −15.9 | 19.4 |
| GC2-3 | Sd | Cluster | 4 | 5 | 191 | −15.5 | 19.0 |
| GC2-6 | Sd | Cluster | 4 | 10 | 162 | −13.2 | 17.1 |
| GC2-6 | Sd | Cluster | 3 | 5 | 157 | −12.4 | 16.3 |
| GC2-6 | Sd | Cluster | 5 | 10 | 168 | −14.7 | 18.4 |
| GC2–6 | Sd | Cluster | 4 | 5 | 174 | −14.1 | 17.9 |
| GC2-6 | Sd | Cluster | 6 | 5 | 208 | −14.8 | 18.5 |
| TL6-17 | Sd | Cluster | 5 | 10 | 157 | −8.8 | 12.6 |
| TL6-17 | Sd | Cluster | 6 | 10 | 191 | −11.6 | 15.6 |
| TL6-17 | Sd | Cluster | 4 | 10 | 187 | −13.5 | 17.3 |
| TL6-17 | Sd | Cluster | 4 | 5 | 196 | −14.2 | 18.0 |
| TL6-17 | Sd | Cluster | 4 | 5 | 183 | −12.3 | 16.2 |
| TL6-17 | Sd | Cluster | 5 | 10 | 164 | −14.5 | 18.2 |
| TL6-22 | Sd | Cluster | 4 | 10 | 121.4 | −5.4 | 8.4 |
| TL6-22 | Sd | Cluster | 3 | 10 | 117.6 | −14.1 | 17.9 |
| TL6-22 | Sd | Cluster | 7 | 5 | 119.3 | −6.2 | 9.5 |
| TL6-22 | Sd | Cluster | 4 | 5 | 117.6 | −8.3 | 12.0 |
| TL6-22 | Sd | Cluster | 4 | 10 | 120.4 | −13.4 | 17.3 |
| TL6-22 | Sd | Cluster | 3 | 5 | 177.4 | −12.5 | 16.4 |
| TL6-22 | Sd | Cluster | 3 | 10 | 162.2 | −10.9 | 14.9 |
| TL6-22 | Sd | Cluster | 4 | 10 | 174.3 | −14.4 | 18.1 |
| TL6-22 | Sd | Cluster | 4 | 10 | 157.3 | −11.7 | 15.7 |
| MX42-6 | Sd | Isolated | 4 | 5 | 124 | −9.3 | 13.2 |
| MX42-6 | Sd | Isolated | 4 | 5 | 121 | −7.8 | 11.5 |
| MX42-6 | Sd | Isolated | 4 | 5 | 120 | −11.2 | 15.2 |
| Sample | Mineral Type | δ13C | δ18O | 87Sr/86Sr | Error |
|---|---|---|---|---|---|
| MX108–1 | Limestone | 3.59 | −7.32 | ||
| MX42–1 | Limestone | 3.67 | −7.62 | ||
| EY–1 | Limestone | 2.94 | −4.8 | 0.707219 | 0.000018 |
| EY–2 | Limestone | 4.49 | −4.15 | ||
| EY–3 | Limestone | 4.97 | −3.97 | ||
| EY–4 | Limestone | 5.15 | −4.42 | 0.707523 | 0.000016 |
| EY–5 | Limestone | 3.77 | −4.56 | ||
| EY–6 | Limestone | 4.25 | −6.95 | ||
| LSX–1 | Limestone | 3.92 | −5.55 | ||
| LSX–2 | Limestone | 4.40 | −6.50 | ||
| HLC–1 | Limestone | 2.30 | −7.20 | 0.7072 | 0.000017 |
| HLC–2 | Limestone | 0.7074 | 0.000019 | ||
| HLC–3 | Limestone | 0.7077 | 0.000020 | ||
| HLC–4 | Limestone | 0.7080 | 0.000021 | ||
| HLC–5 | Limestone | 0.7076 | 0.000016 | ||
| HLC–6 | Limestone | 0.7076 | 0.000017 | ||
| HLC–7 | Limestone | 0.7070 | 0.000023 | ||
| GC2–1 | Limestone | 1.63 | −2.92 | 0.707512 | 0.000022 |
| GC2–2 | Limestone | 2.32 | −3.59 | 0.7070536 | 0.000019 |
| GT2–1 | Limestone | 2.43 | −6.7 | ||
| GT2–2 | Limestone | 2.84 | −6.36 | ||
| GT2–3 | Limestone | 2.63 | −6.01 | ||
| GT2–4 | Limestone | 3.26 | −7.14 | ||
| TL6–2 | Limestone | 3.23 | −7.76 | 0.707933 | 0.000017 |
| TL6–3 | Limestone | 3.47 | −6.71 | 0.707965 | 0.000019 |
| TL6–4 | Limestone | 3.98 | −7.35 | 0.707829 | 0.000020 |
| TL6–5 | Limestone | 0.707774 | 0.000021 | ||
| TL6–6 | Limestone | 0.707028 | 0.000023 | ||
| TL6–7 | Limestone | 0.708009 | 0.000019 | ||
| TL6–8 | Limestone | 3.64 | −6.44 | 0.707112 | 0.000016 |
| GT2–5 | Rd1 | 2.73 | −3.63 | 0.707165 | 0.000018 |
| GT2–6 | Rd1 | 3.05 | −4.05 | 0.706572 | 0.000017 |
| GT2–7 | Rd1 | 3.04 | −5.73 | 0.707091 | 0.000021 |
| GT2–8 | Rd1 | 3.03 | −4.26 | 0.705214 | 0.000022 |
| GT2–9 | Rd1 | 2.82 | −5.26 | 0.705723 | 0.000023 |
| EY–7 | Rd2 | 4.46 | −6.21 | 0.706548 | 0.000017 |
| EY–8 | Rd2 | 4.1 | −6.4 | 0.708002 | 0.000021 |
| EY–9 | Rd2 | 3.83 | −6.76 | 0.707566 | 0.000014 |
| GT2–10 | Rd2 | 3.06 | −6.81 | ||
| GT2–11 | Rd2 | 4.22 | −6.83 | 0.707457 | 0.000025 |
| EY–10 | Rd2 | 3.56 | −6.83 | ||
| GT2–12 | Rd2 | 4.17 | −6.87 | 0.708328 | 0.000023 |
| EY–11 | Rd2 | 3.8 | −7.17 | 0.707719 | 0.000016 |
| GT2–13 | Rd2 | 3.91 | −7.68 | ||
| EY–12 | Rd2 | 3.64 | −6.94 | 0.7074 | 0.000023 |
| HLC–8 | RD3 | 3.40 | −7.90 | 0.7086 | 0.000013 |
| HLC–9 | RD3 | 1.80 | −6.80 | ||
| HLC–7 | RD3 | −0.70 | −7.70 | ||
| GC2–3 | RD3 | 3.44 | −7.64 | ||
| GC2–4 | RD3 | 3.74 | −7.77 | 0.709194 | 0.000017 |
| MX108–2 | RD3 | 4.63 | −7.96 | ||
| MX42–2 | RD3 | 5.00 | −7.99 | 0.707457 | 0.000016 |
| MX42–3 | RD3 | 4.86 | −8.09 | ||
| MX42–4 | RD3 | 5.28 | −8.12 | ||
| MX108–3 | RD3 | 4.34 | −8.13 | 0.708571 | 0.000021 |
| MX42–5 | RD3 | 4.44 | −8.19 | ||
| MX108–5 | RD3 | 5.36 | −8.22 | ||
| M11–1 | RD3 | 6.14 | −8.45 | ||
| WJ1–1 | RD3 | 5.46 | −8.49 | ||
| MX42–6 | RD3 | 5.00 | −9.02 | ||
| GT2–12 | RD3 | 3.49 | −9.45 | ||
| TL6–10 | RD3 | 2.57 | −7.96 | ||
| TL6–11 | RD3 | 4.50 | −8.30 | ||
| TL6–12 | RD3 | 3.90 | −8.40 | ||
| TL6–13 | RD3 | 3.60 | −7.90 | ||
| TL6–14 | RD3 | 4.30 | −8.30 | 0.709234 | 0.000022 |
| TL6–15 | RD3 | 2.80 | −8.40 | 0.708309 | 0.000013 |
| TL6–16 | RD3 | 3.10 | −8.80 | 0.709088 | 0.000021 |
| TL6–17 | SD | 0.708508 | 0.000015 | ||
| TL6–18 | SD | 0.708399 | 0.000016 | ||
| TL6–19 | RD3 | 5.00 | −7.90 | 0.708418 | 0.000013 |
| TL6–20 | RD3 | 4.70 | −8.10 | 0.708201 | 0.000017 |
| EY–13 | RD3 | 4.10 | −8.30 | 0.708234 | 0.000018 |
| EY–14 | RD3 | 4.20 | −8.21 | 0.708332 | 0.000020 |
| GC2–5 | SD | 3.25 | −7.83 | 0.709286 | 0.000021 |
| GC2–6 | SD | 3.78 | −7.95 | 0.709453 | 0.000016 |
| GC2–8 | SD | 3.37 | −8.27 | ||
| GC2–9 | SD | 3.06 | −7.37 | ||
| TL6–21 | SD | 2.93 | −8.00 | 0.708655 | 0.000017 |
| TL6–22 | SD | 3.44 | −7.94 | 0.708009 | 0.000018 |
| TL6–23 | SD | 3.53 | −6.99 | 0.708508 | 0.000019 |
| TL6–24 | SD | 3.48 | −8.16 | 0.708399 | 0.000012 |
| TL6–25 | SD | 2.95 | −7.86 | ||
| HLC–10 | SD | 0.708571 | 0.000018 | ||
| HLC–11 | SD | 0.70782 | 0.000013 | ||
| HLC–12 | SD | 0.707728 | 0.000015 | ||
| HLC–13 | SD | 0.707457 | 0.000014 | ||
| HLC–14 | SD | 3.10 | −8.40 | 0.708462 | 0.000021 |
| GZS–1 | RD3 | 3.10 | −8.80 | 0.708653 | 0.000021 |
| GZS–2 | RD3 | 2.90 | −8.60 | 0.708752 | 0.000022 |
| GZS–3 | RD3 | 2.90 | −8.50 | 0.708321 | 0.000013 |
| GZS–4 | RD3 | 2.80 | −8.40 | 0.709026 | 0.000017 |
| GZS–5 | RD3 | 3.90 | −8.40 | 0.708908 | 0.000014 |
| GZS–7 | RD3 | 4.00 | −8.40 | 0.707982 | 0.000019 |
| GZS–9 | RD3 | 4.20 | −8.30 | 0.708132 | 0.000015 |
| GZS–10 | RD3 | 4.30 | −8.30 | 0.708293 | 0.000017 |
| GZS–11 | RD3 | 4.50 | −8.30 | 0.708398 | 0.000026 |
| GZS–12 | RD3 | 4.50 | −8.30 | 0.709031 | 0.000009 |
| GZS–13 | SD | 4.60 | −8.20 | 0.708432 | 0.000012 |
| GZS–14 | SD | 4.60 | −8.20 | 0.70889 | 0.000016 |
| GZS–15 | SD | 4.40 | −8.10 | 0.708768 | 0.000016 |
| GZS–16 | SD | 4.50 | −8.10 | 0.708807 | 0.000023 |
| GZS–17 | SD | 4.70 | −8.10 | 0.708219 | 0.000021 |
| GZS–18 | SD | 3.60 | −7.90 | 0.707893 | 0.000019 |
| GZS–19 | SD | 5.00 | −7.90 | 0.708675 | 0.000012 |
| GZS–20 | SD | 3.50 | −7.80 | 0.708065 | 0.000011 |
| Sample | Mineral Type | Mn | Sr | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Ce/Ce* | Pr/Pr* | Eu/Eu* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MX108–1 | Limestone | 146.466 | 201.047 | |||||||||||||||||
| MX42–1 | Limestone | 122.775 | 369.039 | |||||||||||||||||
| EY–1 | Limestone | 36.244 | 210.065 | 1.761 | 3.194 | 0.361 | 1.330 | 0.321 | 0.063 | 0.240 | 0.035 | 0.192 | 0.037 | 0.097 | 0.013 | 0.075 | 0.010 | 0.964 | 0.990 | 1.201 |
| EY–2 | Limestone | 6.353 | 1484.558 | 0.321 | 0.589 | 0.066 | 0.221 | 0.049 | 0.008 | 0.041 | 0.006 | 0.040 | 0.009 | 0.026 | 0.004 | 0.027 | 0.004 | 0.974 | 1.032 | 0.947 |
| EY–3 | Limestone | 29.669 | 1012.368 | 0.163 | 0.350 | 0.042 | 0.155 | 0.038 | 0.009 | 0.037 | 0.007 | 0.043 | 0.010 | 0.028 | 0.004 | 0.029 | 0.004 | 1.017 | 1.019 | 1.272 |
| EY–4 | Limestone | 13.350 | 1169.095 | 0.297 | 0.605 | 0.070 | 0.250 | 0.060 | 0.012 | 0.053 | 0.009 | 0.065 | 0.015 | 0.046 | 0.007 | 0.048 | 0.007 | 1.011 | 1.018 | 1.130 |
| EY–5 | Limestone | 25.780 | 506.701 | 0.947 | 1.612 | 0.169 | 0.547 | 0.131 | 0.021 | 0.123 | 0.018 | 0.113 | 0.024 | 0.068 | 0.010 | 0.062 | 0.010 | 0.964 | 1.011 | 0.878 |
| EY–6 | Limestone | 13.566 | 923.556 | 0.646 | 1.194 | 0.143 | 0.511 | 0.110 | 0.015 | 0.092 | 0.013 | 0.079 | 0.017 | 0.048 | 0.007 | 0.049 | 0.007 | 0.947 | 1.035 | 0.791 |
| TL6–1 | Limestone | 115.859 | 756.184 | 2.046 | 4.834 | 0.650 | 2.622 | 0.682 | 0.166 | 0.541 | 0.080 | 0.446 | 0.084 | 0.236 | 0.036 | 0.236 | 0.034 | 0.996 | 1.025 | 1.448 |
| TL6–5 | Limestone | 157.785 | 367.510 | 1.009 | 2.210 | 0.278 | 1.135 | 0.270 | 0.042 | 0.211 | 0.030 | 0.164 | 0.031 | 0.079 | 0.012 | 0.077 | 0.011 | 1.002 | 0.987 | 0.927 |
| TL6–7 | Limestone | 41.719 | 963.803 | 1.105 | 2.224 | 0.261 | 1.020 | 0.240 | 0.080 | 0.211 | 0.033 | 0.202 | 0.040 | 0.111 | 0.016 | 0.095 | 0.013 | 0.997 | 0.980 | 1.876 |
| EY–7 | Rd2 | 207.785 | 122.111 | 0.877 | 1.589 | 0.161 | 0.570 | 0.141 | 0.026 | 0.142 | 0.021 | 0.132 | 0.028 | 0.078 | 0.011 | 0.067 | 0.009 | 1.013 | 0.953 | 0.973 |
| EY–8 | Rd2 | 49.420 | 352.488 | 1.760 | 3.044 | 0.303 | 1.041 | 0.242 | 0.044 | 0.211 | 0.033 | 0.211 | 0.046 | 0.125 | 0.018 | 0.103 | 0.014 | 0.995 | 0.957 | 1.034 |
| EY–9 | Rd2 | 229.331 | 107.109 | 0.757 | 1.219 | 0.141 | 0.515 | 0.130 | 0.026 | 0.160 | 0.029 | 0.210 | 0.050 | 0.138 | 0.018 | 0.090 | 0.011 | 0.895 | 1.006 | 0.943 |
| EY–10 | Rd2 | 303.567 | 126.992 | 1.348 | 2.613 | 0.321 | 1.289 | 0.349 | 0.090 | 0.482 | 0.105 | 0.750 | 0.178 | 0.500 | 0.071 | 0.389 | 0.048 | 0.957 | 0.986 | 1.136 |
| GT2–12 | Rd2 | 117.987 | 130.118 | 1.054 | 1.867 | 0.202 | 0.642 | 0.154 | 0.033 | 0.141 | 0.021 | 0.125 | 0.025 | 0.067 | 0.009 | 0.059 | 0.009 | 0.972 | 1.035 | 1.186 |
| EY–11 | Rd2 | 170.466 | 104.428 | 0.967 | 1.599 | 0.170 | 0.677 | 0.181 | 0.034 | 0.223 | 0.040 | 0.278 | 0.062 | 0.180 | 0.026 | 0.151 | 0.020 | 0.942 | 0.924 | 0.886 |
| EY–10 | Rd2 | 215.764 | 126.785 | |||||||||||||||||
| EY–12 | Rd2 | 25.780 | 506.701 | 0.947 | 1.612 | 0.169 | 0.547 | 0.131 | 0.021 | 0.123 | 0.018 | 0.113 | 0.024 | 0.068 | 0.010 | 0.062 | 0.010 | 0.964 | 1.011 | 0.878 |
| MX108–2 | RD3 | 186.117 | 137.456 | 0.309 | 0.601 | 0.073 | 0.279 | 0.069 | 0.023 | 0.063 | 0.009 | 0.060 | 0.013 | 0.036 | 0.006 | 0.040 | 0.006 | 0.967 | 1.002 | 1.864 |
| MX42–2 | RD3 | 179.965 | 101.122 | 0.155 | 0.277 | 0.032 | 0.123 | 0.031 | 0.017 | 0.025 | 0.003 | 0.020 | 0.004 | 0.012 | 0.002 | 0.014 | 0.003 | 0.942 | 0.986 | 3.169 |
| MX42–3 | RD3 | 158.206 | 125.373 | 0.387 | 0.721 | 0.081 | 0.280 | 0.064 | 0.138 | 0.056 | 0.008 | 0.049 | 0.010 | 0.030 | 0.005 | 0.037 | 0.007 | 0.980 | 1.019 | 12.347 |
| MX42–4 | RD3 | 153.474 | 104.804 | 0.173 | 0.328 | 0.041 | 0.151 | 0.037 | 0.019 | 0.032 | 0.004 | 0.023 | 0.005 | 0.015 | 0.002 | 0.017 | 0.003 | 0.938 | 1.045 | 2.936 |
| MX108–3 | RD3 | 111.122 | 108.942 | 0.250 | 0.552 | 0.065 | 0.219 | 0.050 | 0.194 | 0.051 | 0.007 | 0.047 | 0.010 | 0.028 | 0.005 | 0.035 | 0.006 | 1.040 | 1.060 | 20.268 |
| MX42–5 | RD3 | 207.578 | 152.937 | 0.384 | 0.707 | 0.083 | 0.290 | 0.067 | 0.092 | 0.057 | 0.008 | 0.050 | 0.011 | 0.031 | 0.005 | 0.038 | 0.006 | 0.954 | 1.038 | 7.858 |
| MX108–5 | RD3 | 61.930 | 106.087 | 0.207 | 0.379 | 0.045 | 0.170 | 0.043 | 0.028 | 0.036 | 0.005 | 0.032 | 0.007 | 0.021 | 0.003 | 0.024 | 0.004 | 0.951 | 0.991 | 3.806 |
| M11–1 | RD3 | 61.995 | 298.129 | 0.089 | 0.171 | 0.018 | 0.060 | 0.015 | 0.007 | 0.015 | 0.002 | 0.017 | 0.004 | 0.013 | 0.002 | 0.013 | 0.002 | 1.037 | 0.977 | 2.591 |
| WJ1–1 | RD3 | 84.911 | 86.910 | 0.351 | 0.649 | 0.079 | 0.287 | 0.066 | 0.017 | 0.056 | 0.008 | 0.046 | 0.009 | 0.025 | 0.004 | 0.027 | 0.004 | 0.939 | 1.035 | 1.459 |
| MX42–6 | RD3 | 191.254 | 90.783 | 0.286 | 0.504 | 0.057 | 0.205 | 0.047 | 0.081 | 0.042 | 0.006 | 0.036 | 0.008 | 0.023 | 0.004 | 0.028 | 0.005 | 0.951 | 0.998 | 9.765 |
| TL6–17 | SD | 161.454 | 116.765 | 1.511 | 2.809 | 0.321 | 1.312 | 0.342 | 0.461 | 0.294 | 0.047 | 0.286 | 0.061 | 0.160 | 0.023 | 0.139 | 0.021 | 0.971 | 0.945 | 7.716 |
| TL6–18 | SD | 171.624 | 281.809 | 0.994 | 2.292 | 0.317 | 1.301 | 0.334 | 1.012 | 0.281 | 0.040 | 0.240 | 0.049 | 0.131 | 0.018 | 0.117 | 0.018 | 0.971 | 1.026 | 17.510 |
| TL6–22 | SD | 177.983 | 286.753 | 0.897 | 1.991 | 0.241 | 0.985 | 0.253 | 0.438 | 0.214 | 0.034 | 0.211 | 0.042 | 0.113 | 0.016 | 0.104 | 0.015 | 1.029 | 0.970 | 9.969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zheng, H.; Liu, B.; Pan, L.; Li, R.; Wu, J.; Yang, X.; Tang, H.; Dong, Y. Genetic Mechanism of Structurally Controlled Dolomites Derived from Seawater-Hydrothermal Mixed Fluids—A Case Study from Middle Permian, Central Sichuan Basin, South China. Minerals 2023, 13, 758. https://doi.org/10.3390/min13060758
Gao J, Zheng H, Liu B, Pan L, Li R, Wu J, Yang X, Tang H, Dong Y. Genetic Mechanism of Structurally Controlled Dolomites Derived from Seawater-Hydrothermal Mixed Fluids—A Case Study from Middle Permian, Central Sichuan Basin, South China. Minerals. 2023; 13(6):758. https://doi.org/10.3390/min13060758
Chicago/Turabian StyleGao, Jinliang, Haofu Zheng, Bo Liu, Lei Pan, Rangbin Li, Junfeng Wu, Xiangyang Yang, Hailei Tang, and Yixin Dong. 2023. "Genetic Mechanism of Structurally Controlled Dolomites Derived from Seawater-Hydrothermal Mixed Fluids—A Case Study from Middle Permian, Central Sichuan Basin, South China" Minerals 13, no. 6: 758. https://doi.org/10.3390/min13060758





