Abstract
Lead is the primary toxic element found in jarosite residue; it is necessary to synthesize simulated lead-containing jarosite residue (SLJS) to investigate its lead release behavior and predict the slag’s stability and potential for secondary environmental pollution. This study explores the ion release behavior, leaching toxicity, and stability of SLJS during freeze–thaw cycles with EDTA (E-FTC). Experimental results demonstrate that the release of lead, iron, and sulfate from SLJS under E-FTC is contingent upon multiple factors, including solution pH, EDTA concentration, freeze–thaw cycles, freezing temperature, and freeze–thaw mode. Specifically, employing an EDTA concentration of 200 mM, a pH of 6, a freezing temperature of −20 °C, and 12 freeze–thaw cycles, the lead release reaches 15.1 mM, accounting for 94.9% of the total lead content, while iron is negligibly released, thus enabling effective separation of lead from iron. Subsequent to E-FTC, the exchangeable lead content exhibits a substantial reduction, accompanied by a marked increase in residual lead, resulting in a remarkable 98% reduction in leaching toxicity. Moreover, the equilibrium concentration of lead in the continuous stable leaching solution is 0.13 mg/L, significantly below the lead toxicity threshold (5 mg/L). Therefore, environmental stability can be greatly enhanced. This study presents a novel approach for the safe disposal of jarosite residue under mild conditions and at low temperatures, contributing to the broader field of environmentally sustainable waste management.
1. Introduction
The jarosite process is widely utilized in hydrometallurgy for efficient iron removal, owing to its simplicity and the superior filtration performance of its slag [1,2,3]. However, it faces several challenges, including the generation of a large volume of slag, the need for extensive slag storage areas, the low grade of valuable metals such as lead and silver, and the significant difficulties in their economic recovery, particularly the high cost associated with the recovery of valuable metals such as lead [4,5]. Jarosite residue is classified as hazardous waste, necessitating the use of dedicated hazardous waste slag silos and incurring substantial treatment costs, with the potential for environmental pollution [6,7]. The primary component of jarosite residue is sodium jarosite, with a total sulfur content exceeding 12% and a water content generally surpassing 30%. The crystal phase characteristics of jarosite residue make it susceptible to adsorbing or replacing components such as Pb, Zn, Cd, Ag, In, and As, resulting in coprecipitation and subsequent loss of valuable metals, particularly lead jarosite [8,9]. Notably, Pb and Zn in the coprecipitation process will continuously dissolve under natural conditions such as wind, sunlight, and rainfall, leading to water and soil pollution and secondary contamination [10,11]. Therefore, studying the dissolution and release behavior of heavy metals in jarosite residue holds significant importance.
Currently, researches on jarosite residue primarily focus on the coprecipitation behavior of heavy metals, while the release of heavy metals and the continues stability of stacked jarosite residue remain poorly understood. Environmental factors such as solution pH, ion composition, dissolved organic matter, and microorganisms can lead to the dissolution or recrystallization of the mineral phase structure of jarosite in the residue [12,13]. During the mineral phase transformation of sodium jarosite, changes occur in surface properties, specific surface area, and crystal structure, potentially leading to the precipitation of mixed heavy metal ions. In the process of recrystallization, dissolved heavy metal ions may become fixed within the mineral structure, resulting in decreased concentrations in the environment [14]. Ethylenediaminetetraacetic acid (EDTA), a synthetic soluble organic compound containing amino and carboxyl groups, exhibits excellent chelating properties with most heavy metals. It finds wide applications in soil treatment, water treatment, detergents, textiles, papermaking, medicine, rubber processing, photography, and other fields [15,16,17,18]. The coordination ratio of EDTA and metal ions is 1:1, forming a charged complex that can dissolve in water. Sun et al. [19] investigated heavy metal removal from contaminated soil using EDTA leaching, and the results showed removal rates of 23.5% for Pb, 57.0% for Zn, and 54.7% for Cd. Guo et al. [20] employed an EDTA-citric acid mixed system to leach Cu-contaminated soil, reducing the Cu mass fraction from 344 mg/kg to 210 mg/kg. Yin et al. [21] used a 1:1 molar ratio of EDTA to citric acid as a mixed chelating agent to leach heavy-metal-contaminated soil, achieving removal rates of 27.2% for Pb, 24.4% for Cu, 43.4% for Cd, and 11.7% for As. Yi et al. [22] employed a 0.2 mol/L EDTA solution to leach heavy-metal-contaminated soil, achieving the best removal effect for Cu, Pb, and Zn with removal rates of 34.4%, 49.2%, and 29.9%, respectively. Yang et al. [23] washed heavy-metal-polluted soil using a 5 mM EDTA solution with a solid–liquid ratio of 1:2.5, resulting in removal rates of 19.3% for Pb, 21.9% for Cu, 20.9% for Zn, and 55.2% for Cd. Chen et al. [24] employed a 0.09 mol/L EDTA-tea saponin mixed solvent to shake and wash heavy-metal-contaminated soil from the Guangdong Dabaoshan tailings pond, achieving removal rates of 54.2% for Pb and 48.7% for Zn.
In previous studies, the lead removal rate using EDTA was found to be less than 60%. However, the reaction speed of lead complexation with EDTA at room temperature was very slow, resulting in poor lead removal efficiency. Freeze–thaw cycles have been shown to significantly promote various chemical reactions in the organic phase [25,26,27,28]. Numerous scholars have conducted extensive research on the subject, including the oxidation of nitrite ions into nitrate, sulfite into sulfate, polar bromine (iodine) promoting ozone and mercury reactions, and the release of ice-ring pollution by humic acid [29]. Freezing can accelerate catalytic reactions such as hydrolysis, ammonolysis, dehydration, oxidation, and peroxide decomposition [30]. Jeong et al. [31] investigated the dissolution behavior of iron oxide particles in ice and found that organic ligands and proton concentration in the ice crystal boundary region can enhance the dissolution of iron oxides during freezing. However, the ice-enhanced dissolution effect gradually decreased as the freezing temperature decreased from −10 °C to −196 °C, indicating that the presence and formation of a liquid-like ice crystal boundary region played a key role in accelerating the complexation dissolution reaction. Kim et al. [32] examined the oxidation and reduction reactions of chromium (Cr(VI)) and arsenic (As(III)) using model organic acids (citric acid and oxalic acid) at room temperature and freezing conditions, respectively. The results showed that redox conversions were significantly accelerated in ice, while almost no reaction occurred at room temperature. Takemoto et al. [33] found that freeze–thaw cycles could significantly increase the reaction rate of polymers. Agten et al. [34] conducted a systematic study on freezing methods and their influence on the reaction rate of oxime bond coupling. The results indicated that the reaction rate after freezing was 91%, much higher than the unfrozen reaction rate of 2%, suggesting that freezing promoted the reaction, particularly in the organic phase. Guo et al. [35] found that environmental freeze–thaw cycles or freezing could accelerate the redox dynamic transformation between silver nanoparticles (AgNPs) and Ag+. Min et al. [36] discovered that phenolic compounds and nitrite could accelerate the reaction under freezing conditions (−20 °C), while almost no reaction occurred at 20 °C. Freeze–thaw cycles have demonstrated significant potential in accelerating various chemical reactions, providing valuable insights for treating heavy metal-contaminated environments, managing wastewater, and understanding freezing-induced reactions in different fields.
Researchers have utilized the E-FTC for remediation of heavy-metal-contaminated soils containing lead, resulting in an enhanced removal effect compared to treatments without freeze–thaw. Rui et al. [37] employed EDTA as an eluant and successfully removed Pb and Cd from cohesive soil after multiple freeze–thaw cycles. The removal rate of heavy metals further increased with an escalation in freeze–thaw cycles and washing frequency. This improvement can be attributed to the disruptive impact of freeze–thaw on the soil particle structure, enabling optimal contact between eluant and heavy metals, thereby promoting their reaction. These findings suggest that the combination of freeze–thaw and chemical leaching can effectively remediate soils contaminated with heavy metals [38]. The successful application of EDTA freeze–thaw leaching in soil instills confidence in employing the EDTA freeze–thaw treatment for jarosite residue in the hydrometallurgical industry. Furthermore, studying the Pb release behavior of EDTA on jarosite residue under freeze–thaw conditions may present a novel approach for the safe disposal of jarosite residue under mild temperatures.
However, the presence of numerous impurities and complex structures in jarosite residue poses challenges in analyzing the migration patterns and environmental behavior of heavy metals within it. Previous research has indicated that Pb is the primary factor influencing the environmental stability of jarosite residue [39,40]. Therefore, investigating the release behavior of Pb from SLJS holds significant importance. The issue of heavy metal pollution caused by jarosite residue in the zinc hydrometallurgy industry requires urgent attention and resolution. To tackle this problem, it is crucial to explore effective strategies for reducing the leaching toxicity of simulated jarosite residue. In this study, we investigate the influence of the E-FTC on the release behavior of SLJS under freeze–thaw conditions. Our objective is to examine the effects of different freeze–thaw conditions on the migration behavior, morphology, and environmental stability of lead. By doing so, we aim to establish a solid basis for preventing heavy metal pollution and conducting risk assessments in smelting slag yards located in freeze–thaw areas. Furthermore, our research offers a fresh perspective on the treatment of smelting slag with high water content. The outcomes of this study contribute to addressing the pressing issue of heavy metal pollution in the zinc hydrometallurgy industry.
2. Materials and Methods
2.1. Materials
Chemicals used in this study were sourced from Sinopharm Chemical Reagent Co., Ltd., a reputable supplier based in Beijing, China, and were of analytical-reagent grade. The chemicals employed include ferric sulfate (Fe2(SO4)3·xH2O), sodium sulfate (Na2SO4), lead acetate (Pb(CH3COO)2), sodium hydroxide (NaOH), sulfuric acid (H2SO4), and EDTA (C10H16N2Na2O8). Due to the low solubility of EDTA, EDTA-2Na, which exhibits relatively higher solubility, was used instead. All solutions were prepared using ultrapure water with a resistivity of 18 MΩ·cm.
2.2. Synthesis of Simulated Lead-Containing Jarosite Residue
Simulated lead-containing jarosite residue (SLJS) was synthesized by adding 0.25 mol/L Fe2(SO4)3·xH2O and 0.06 mol/L Na2SO4 to a 1 L solution. The mixture was stirred continuously at 600 r/min and maintained at 95 °C. The pH was adjusted to 1.0 using a 7.0 mol/L NaOH solution, followed by the gradual addition of 100 mL of 0.3 mol/L Pb(CH3COO)2 solution at a rate of 50 mL/h while stirring. To maintain a pH of 2.0, NaOH solution was added during the reaction. After the precipitates were stirred for 3 h, the residual supernatant solutions were settled and decanted. The synthesized jarosite was then filtered using a vacuum, washed multiple times with ultrapure water (18 MΩ·cm), and dried at 80 °C for 24 h.
2.3. Procedure of E-FTC
The EDTA stock solution used in the experiment was prepared immediately and diluted to concentrations of 0, 5 mM, 20 mM, 100 mM, 200 mM, and 300 mM (mM: mmol/L). The pH of the EDTA solution was adjusted using H2SO4 or NaOH.
To begin the experiment, 1.00 g of SLJS was added to a series of 50 mL polyethylene centrifuge tubes. Then, 30 mL of EDTA solution was added, and the mixture was vigorously shaken and subjected to 2 min of ultrasonic treatment using an ultrasonic washing machine to ensure thorough mixing of the SLJS suspension. The centrifuge tubes were placed in a centrifuge tube rack and stored in a laboratory refrigerator at −20 °C for 12 h to freeze the suspension. After freezing, the centrifuge tubes were transferred to a thermostatic water bath set at 20 °C and allowed to thaw for 12 h. This process constituted one freeze–thaw cycle (E-FTC). Additionally, a control experiment (E) was conducted under the same conditions as the E-FTC samples, except without the freeze–thaw treatment.
Following the reaction, the suspension was filtered through a 0.45 μm microporous membrane, and the filtrate was collected for analysis of Pb, Fe, and SO42−. The filter cake was thoroughly washed with ultrapure water (18 MΩ/cm) and subsequently dried in a freeze-drying oven for solid phase analysis.
2.4. Sequential Extraction Scheme
The Community Bureau of Reference (BCR) has introduced a three-step extraction procedure for analyzing the forms of heavy metal combinations. In this study, we employed the Davidson’s three-stage BCR sequential extraction procedure to investigate the effective forms of heavy metal combinations in the jarosite residue. Detailed procedures for the BCR tests can be found in previous studies [41,42]. Each step was conducted in triplicate. The residual form was determined by subtracting the amounts obtained from the acidic extractable form, reducible form, oxidizable form, and residual form.
Step 1: Acidic extractable form. In a 50 mL polypropylene wide-mouthed bottle, 0.5 g of dry “residue” was mixed with 20 mL of acetic acid (0.11 mol/L). The bottle was shaken at ambient temperature (50 r/min) for 16 h. The resulting extract was separated from the solid residue by centrifugation (5000 r/min) for 10 min and transferred to a polyethylene container for storage at 4 °C until analysis. The residue was washed with 10 mL of distilled water, shaken for 60 min, and then centrifuged. The washings were discarded.
Step 2: Reducible form. The residue from Step 1 was mixed with a portion of 20 mL of hydroxyammonium chloride (0.1 mol/L, pH 2 adjusted with nitric acid) and quantitatively transferred back to the wide-mouthed bottle. The remaining reagent was added, and the extraction procedure was carried out as described above.
Step 3: Oxidizable form. To the residue from Step 2, 5 mL of hydrogen peroxide (8.8 mol/L) was added incrementally. The centrifuge tube was covered with a watch glass, and the contents were digested at room temperature for 1 h with intermittent manual shaking. The digestion was continued by heating the tube in a water bath at 85 °C for 2 h. The watch glass was removed, and the tube contents were evaporated to a small volume (approximately 1 mL). Another 5 mL aliquot of hydrogen peroxide was added, and the digestion procedure was repeated. The cooled moist residue was transferred back to the 50 mL bottle, and 25 mL of ammonium acetate (1.0 mol/L, pH 2 adjusted with nitric acid) was added. The sample was shaken and centrifuged, and the resulting extract was separated as described in Step 1. The solid residue was retained for microwave digestion. All samples were analyzed using ICP-AES.
2.5. Toxicity Characteristic Leaching Procedure
The Toxicity Characteristic Leaching Procedure (TCLP) is a widely recognized method endorsed by the US Environmental Protection Agency (EPA) to assess the environmental risk and classification of solid waste samples as hazardous waste. This method employs an extraction procedure specifically developed to simulate the leaching of metals and organic compounds from solid waste, resembling the conditions of acidic rainfall. Comprehensive details concerning the test procedure for TCLP can be found in the existing literature [40,42].
Dry samples weighing 50 g were introduced into polyethylene extraction bottles with a liquid extractant-to-sample ratio of 10:1. The extraction fluid consisted of a mixture of sulfuric acid and nitric acid in a mass ratio of 2:1, adjusted to a pH of 3.20 ± 0.05. Subsequently, the extraction bottles were sealed and subjected to tumbling in a standard tumbler for a period of (18 ± 2) h. Following tumbling, the samples were filtered under pressure using acid-treated (HNO3) 0.6 μm glass fiber filter paper. The solid phase was discarded, while the eluate was transferred to acid (HNO3)-rinsed polyethylene bottles. All samples were stored at 4 °C until further analysis.
2.6. Continuous Leaching Experiment
To simulate the continuous leaching effects of jarosite residue in an acid rain environment, a 20-day continuous leaching experiment (CLE) was conducted following the ANS (1986) standard [40]. Each CLE utilized a 30 g sample of the residue, which was subjected to a leaching agent composed of deionized water adjusted to a pH of 3.00 ± 0.05 using a mass ratio of 3:1:3 for H2SO4, HNO3, and CH3COOH, mimicking acid rain conditions. The leaching process was carried out at a constant rate of 1.25 mL/h. At 24h intervals, the leaching solution was collected and filtered using a 0.45 μm filter membrane. The filtrate was then analyzed to determine the concentration of heavy metals, allowing for the evaluation of the continuous stability of the residue.
2.7. Analysis
2.7.1. Determination of Elemental Composition
The solution underwent analysis to determine the concentrations of Pb and low-level Fe using atomic absorption fluorescence spectrometry. Additionally, the concentration of high-level Fe was assessed through phenanthroline spectrophotometry [43]. For the quantification of high- and low-level SO42− concentrations, barium sulfate turbidimetry and ion chromatography methods were employed, respectively. The concentration of EDTA was determined by gas chromatography [44].
2.7.2. Other Analyses
The microstructure, surface morphology, and specific chemical composition of the samples were observed using a scanning electron microscope (JEOL, Tokyo, Japan, JSM-7900F). The mineralogical composition was determined through X-ray diffraction analysis (XRD, BRUKER, D8 ADVANCE). The XRD analysis utilized Cu Kα radiation with a scanning rate of 10°/min and a step size of 0.02° over a 2θ range of 5° to 80°. The XRD measurements were conducted under operating conditions of 40 kV and 40 mA.
3. Results and Discussion
3.1. Effect of E-FTC on Release of Lead, Iron, and Sulfate from SLJS
3.1.1. Effect of Solution pH
Figure 1 illustrates lead and iron release from SLJS into solution at different pH values and freeze–thaw cycles. The concentration of lead in the solution showed an initial increase followed by stabilization with increasing solution pH. Conversely, the concentration of iron gradually decreased and reached a stable state as the pH increased. After subjecting the simulated jarosite residue to E-FTC, the release of lead was significantly higher compared to the control group without freeze–thaw treatment. This finding indicates that freeze–thaw cycles can facilitate the release of lead. Furthermore, as the freeze–thaw cycles increased, there was a slight increase in the release of lead, while the release of iron remained relatively constant.
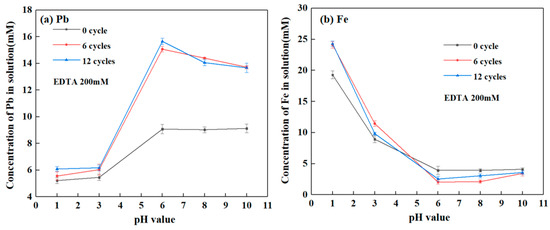
Figure 1.
Ion concentration in solution varied with pH value after E-FTC of SLJS. (a) Pb; (b) Fe.
At a solution pH of 6 and after undergoing 6 cycles of E-FTC, the released amount of lead in the solution was 15.07 mM (accounting for 94.93% of total Pb), whereas the control group without freeze–thaw treatment released only 9.08 mM of lead (accounting for 57.20% of total Pb). The freeze–thaw treatment resulted in an approximately 40% increase in lead release, suggesting that freeze–thaw cycles significantly enhance the reaction between lead in jarosite residue and EDTA, accelerating lead dissolution and release. This observation is consistent with previous literature reports [45].
When the solution pH was below 3, the release of lead was relatively low, ranging from 5.4 to 6.2 mM (accounting for 30% to 40% of total lead). This may be attributed to the increased concentration of H+ at low pH, which hinders the reaction between EDTA and PbSO4 due to the same-ion effect [46]. Conversely, the release of iron was relatively high at low pH values (pH < 3), ranging from 20 to 25 mM (accounting for 10% to 15% of total Fe), and increased as the pH decreased. This can be explained by the decomposition of sodium jarosite at low pH values. However, when the pH was above 6, the release of iron significantly decreased to less than 3.5 mM (accounting for 2.10% of total Fe) due to the secondary hydrolysis and precipitation of iron as iron hydroxide in the solution under high pH conditions [47].
Figure 2 and Figure 3 present the SEM and XRD patterns of SLJS, respectively, with different pH values after 12 E-FTC. The results indicate a significant reduction in the bright white lead sulfate phase of leaded jarosite following E-FTC treatment. At a solution pH of 3, the majority of the bright white lead sulfate particles on the surface of SLJS disappeared (Figure 2b), and XRD analysis revealed a substantial reduction in the characteristic diffraction peak at 20.9° (Figure 3). This indicates that the lead sulfate phase in SLJS reacts with EDTA during the freeze–thaw process, resulting in the dissolution and release of lead into the solution. At a pH of 6, the surface of SLJS exhibited the absence of bright white lead sulfate particles (Figure 2c). Upon magnification, numerous micro-pores were observed on the surface of the dark-colored large particles, corresponding to sodium jarosite crystals (Figure 2f). This phenomenon is likely caused by the detachment of the surface lead sulfate phase following its reaction with EDTA. XRD analysis confirms a significant weakening of the characteristic diffraction peak at 20.9°, indicating the reaction between lead sulfate and EDTA. At pH values of 8 and 10, the behavior of lead sulfate on the surface of SLJS remained consistent with that observed at pH 6, with the disappearance of bright white lead sulfate and a weakening of the characteristic peak. However, a new characteristic diffraction peak corresponding to EDTA appeared at 22.2°. This could be attributed to the excess EDTA remaining on the surface of SLJS or the re-adsorption and fixation of iron from the secondary precipitation of iron complexes formed by the reaction of EDTA with sodium jarosite under alkaline conditions. Hence, extreme pH values should be avoided as they have adverse effects. A low pH leads to the decomposition of sodium jarosite, reducing the selectivity of lead, while a high pH can cause the precipitation of dissolved iron as iron hydroxide, leading to the adsorption of lead and hindering its release [48]. Therefore, a pH of 6 is considered optimal for E-FTC.
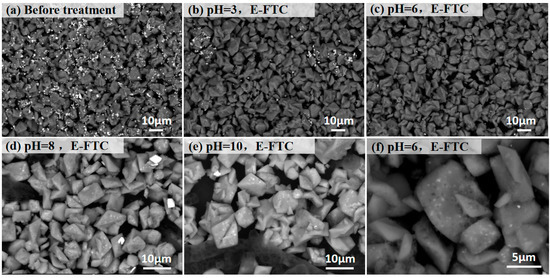
Figure 2.
SEM images of SLJS using 12 E-FTC cycles with different pH values. (a) Before treatment; (b) pH 3; (c) pH 6; (d) pH 8; (e) pH 10; (f) pH 6, Mag. 5000×.
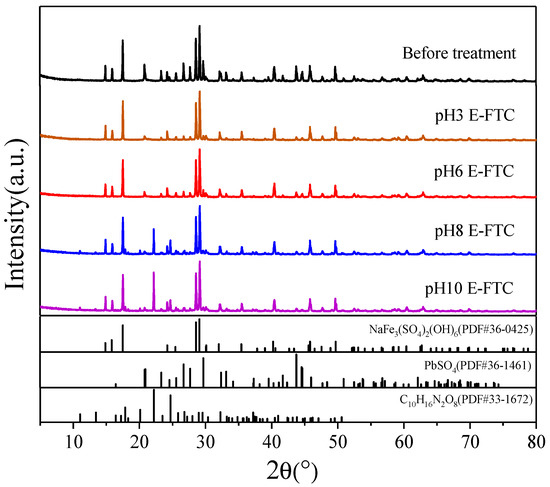
Figure 3.
XRD patterns of SLJS using 12 E-FTC with different pH value.
3.1.2. Effect of EDTA Concentration
Figure 4 presents lead and iron release from SLJS into solution with different EDTA concentration by freeze–thaw cycles. The results demonstrate that as the EDTA concentration increases, the concentrations of lead, iron, and sulfate in the solution exhibit a significant initial increase followed by a leveling off. This phenomenon occurs due to the increased concentration of EDTA per unit volume, resulting in enhanced contact between EDTA and the lead sulfate and sodium jarosite present in SLJS. As the reaction approaches equilibrium, the impact of concentration on the release of lead and iron diminishes. In fact, the reversible nature of the dissolution reaction may hinder the reaction progress with further concentration increments.
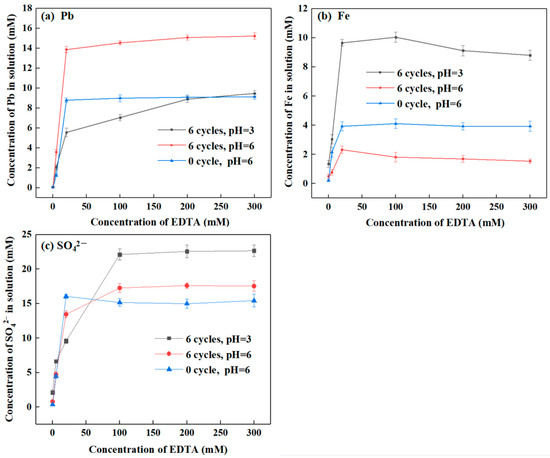
Figure 4.
Ion concentration in solution varied with EDTA concentration after E-FTC of SLJS. (a) Pb; (b) Fe, (c) SO42−.
For the direct EDTA treatment of SLJS without freeze–thaw (at pH 6), an EDTA concentration of 20 mM led to the release of 8.79 mM of lead (55.38% of the total), 3.93 mM of iron (2.38% of the total), and 16.04 mM of sulfate (12.25% of the total). Increasing the EDTA concentration further had a minimal effect on ion release, indicating that the direct EDTA leaching results in lead and iron leaching rates of approximately 55% and 2%, respectively. In contrast, under the same conditions with E-FTC, an EDTA concentration of 20 mM led to the release of 13.86 mM of lead (87.32% of the total), 2.32 mM of iron (1.41% of the total), and 13.44 mM of sulfate (10.27% of the total). The release of lead significantly increased, exceeding 35%, indicating that freeze–thaw cycles notably enhance the reaction between EDTA and lead. The release of iron was lower compared to the non-freeze–thaw condition, which can be attributed to the promotion of rehydration and reprecipitation of dissolved iron during freeze–thaw cycles, inhibiting the reaction between sodium jarosite and EDTA to some extent. Further increasing the EDTA concentration to 200 mM led to a further increase in the release of lead to 15.07 mM (94.93% of the total), while the release of iron remained nearly unchanged. However, caution should be exercised to avoid excessively high EDTA concentrations as they can lead to EDTA saturation, limiting the dissolution of lead.
Under acidic conditions (pH 3), the release of lead was significantly lower compared to pH 6. This can be attributed to the weakened complexing ability of EDTA under acidic conditions [49]. At an EDTA concentration of 20 mM, the release rate of lead was only 34.99% (5.56 mM Pb). Even with an increase in EDTA concentration to 300 mM, the release rate of lead only increased to 59.63% (9.47 mM). These findings align with the pH experimental results, indicating that acidic conditions are unfavorable for the reaction and release of lead. On the other hand, under acidic conditions, the release of iron intensified, and as the EDTA concentration increased, the release of iron also increased. This suggests that under acidic conditions, EDTA reacts with sodium jarosite, and freeze–thaw cycles facilitate the reaction. The release rate of iron was approximately 5%.
Taking into account reagent consumption, cost, and the efficiency of lead–iron separation, an EDTA concentration of 20 mM was deemed optimal.
3.1.3. Effect of Freeze–Thaw Cycles
Figure 5 shows lead and iron release from SLJS into solution with different cycles using E-FTC. The results demonstrate a consistent pattern in the release of lead and iron at different pH values. As the cycles increased, there was a slight elevation in the release of lead and sulfate, while the release of iron remained relatively stable. At pH 3, the leaching rate of lead was below 40% (6.23 mM Pb), and the leaching rate of iron was 5.8% (9.6 mM Fe). The acidic pH condition hampers the release of lead. However, at pH 6, the leaching rate of lead surpassed 80%, whereas the leaching rate of iron declined to approximately 1.5%. Subsequently, with an escalation in the freeze–thaw cycles, a marginal increase in the leaching rate of lead was observed. It is noteworthy that although a higher cycles moderately enhanced the leaching rate of lead, the increment was comparatively limited. This can be attributed to the rapid reaction between EDTA and lead, whereby a single freeze–thaw cycle already achieves an approximate 80% leaching rate, approaching a state of equilibrium. Consequently, further augmenting the cycles only marginally advances the reaction towards equilibrium, resulting in a modest increase in the leaching rate of lead. Nonetheless, in comparison to direct EDTA treatment without freeze–thaw, the leaching rate of lead demonstrated a substantial improvement, signifying the pronounced efficacy of freeze–thaw cycles in promoting the EDTA–lead reaction. To ensure optimal lead leaching efficiency, it is recommended to perform six freeze–thaw cycles.
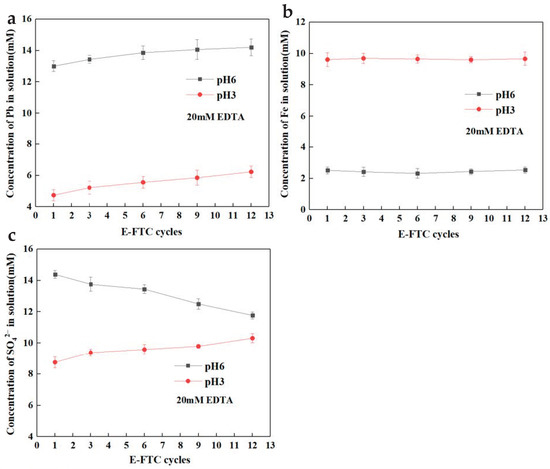
Figure 5.
Ion concentration in solution varied with cycles after E-FTC of SLJS (a) Pb; (b) Fe; (c) SO42−.
3.1.4. Effect of Freezing Temperature
Figure 6 illustrates the variations in ion concentration within the solution as freezing temperature varied following the E-FTC of SLJS. The results reveal that as the freezing temperature decreased, the release of Fe remained relatively stable, ranging from 2.1 to 2.5 mM (1.3% to 1.5% proportionally). In contrast, the release of lead initially increased and then reached a plateau. When the freezing temperature was reduced from −5 °C to −20 °C, the leaching rate of lead rose from 76.98% (12.22 mM Pb) to 87.32% (13.86 mM Pb). Subsequently, further reduction in the freezing temperature did not significantly affect the release of lead. The release pattern of SO42− in the solution mirrored that of lead, suggesting that the entry of SO42− into the solution is predominantly attributed to the dissolution and release of lead sulfate from the lead-bearing jarosite through the reaction with EDTA. The freezing and thawing process is intricately linked to the freezing temperature. Optimal freezing temperature is crucial as it affects the duration of freezing and allows for a longer suspension of the liquid before solidification. This extended period promotes the accumulation and concentration effects of freezing and thawing, leading to enhanced outcomes [50]. However, excessively high freezing temperatures can undermine the efficiency of the freezing and thawing process, necessitating careful temperature control. Considering all factors, a freezing temperature of −20 °C is deemed suitable.
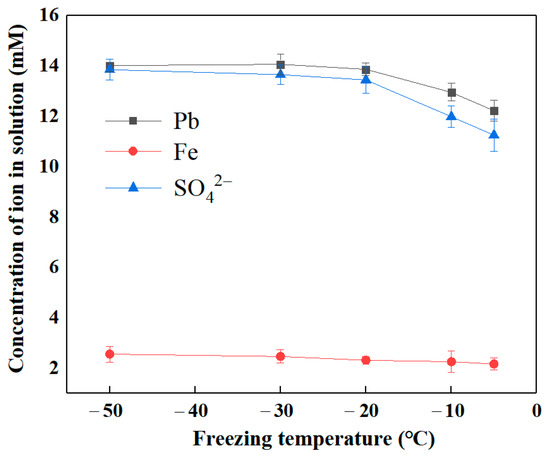
Figure 6.
Ion concentration in solution varied with freezing temperature after E-FTC of SLJS.
3.1.5. Effect of Freeze–Thaw Methods
The release of Pb, Fe, and SO42− in SLJS under different freeze–thaw methods are shown in Figure 7. The results demonstrate a significant decrease in lead release from SLJS after undergoing liquid nitrogen flash freezing. Compared to freezing at −20 °C for 12 h, the leaching rate of lead decreased from 87.32% (13.86 mM Pb) to 38.03% (6.04 mM Pb) after liquid nitrogen flash-freezing. This indicates that rapid freezing failed to effectively utilize the freeze concentration effect, leading to inadequate enrichment of EDTA in the unfrozen liquid and consequently weakening the reaction between EDTA and lead sulfate [51]. Furthermore, rapid thawing methods such as ultrasound or microwave thawing hinder the release of lead and iron. Although these methods result in a certain degree of reduction in lead and iron release compared to room temperature thawing, the impact is relatively minor. Therefore, it can be concluded that the crucial aspect of the freeze–thaw process lies in the freezing step, while the thawing step has a relatively negligible effect. Consequently, the use of rapid freezing methods is not recommended in the freeze–thaw cycle.
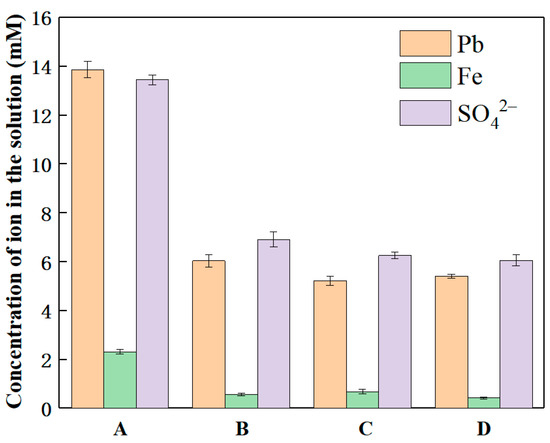
Figure 7.
Ion concentration in solution varied with freeze–thaw method after E-FTC of SLJS. (A: frozen at −20 °C for 12 h, thawed at 20 °C for 12 h; B: frozen in liquid nitrogen for 5 min and thawed at 20 °C for 12 h; C: frozen in liquid nitrogen for 5 min, thawed at 20 °C for 6 h; D: liquid nitrogen freezing for 5 min, microwave thawing for 5 min).
3.2. Effect of E-FTC on the Speciation of Lead from SLJS
Figure 8 illustrates the transformation of Pb in SLJS under various freeze–thaw treatments. FTC denotes a sample that undergoes a blank freeze–thaw cycle without EDTA treatment. On the other hand, E-FTC represents a sample treated with 200 mM EDTA and subjected to a pH value of 6, along with 12 freeze–thaw cycles during the freeze–thaw process. E-FTC with liquid nitrogen undergoes rapid freezing for 5 min using liquid nitrogen, while maintaining the same conditions as the E-FTC sample.
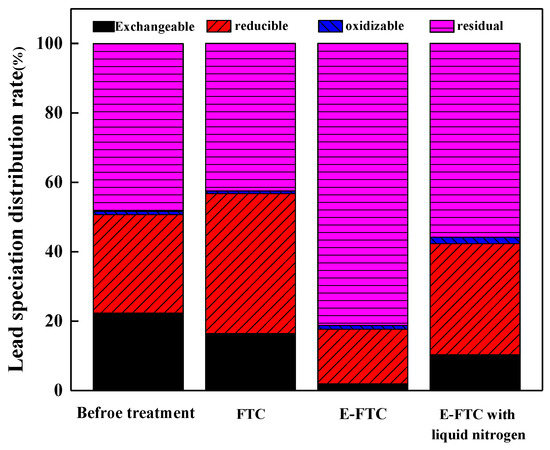
Figure 8.
Effects of different treatment conditions on the form of lead in SLJS.
The results demonstrate that the E-FTC significantly reduced the total lead content in SLJS, decreasing it from 9.86% to 0.51% when compared to untreated samples. This corresponded to a remarkable lead removal rate of 94.8%. Furthermore, the proportion of exchangeable lead decreased substantially from 22.3% to 1.92%, while the proportion of residual lead increased significantly from 48.3% to 81.2%. These findings indicate a significant enhancement in the environmental stability of lead following the EDTA freeze–thaw treatment. Under the influence of E-FTC, a considerable dissolution of lead occurred in SLJS, particularly in the form of exchangeable lead. Consequently, the remaining undissolved lead mainly existed as residual lead, which exhibited low environmental reactivity. Thus, the application of E-FTC leads to a substantial improvement in the environmental stability of lead. However, when the freezing method was altered to rapid freezing using liquid nitrogen, the environmental stability of lead diminished compared to the slow freezing process at −20 °C. The proportions of exchangeable lead and residual lead were 10.34% and 55.83%, respectively, indicating the less favorable environmental stability of lead. Nevertheless, even with rapid freezing, the stability of lead in treated SLJS was still superior to that in the untreated samples. Hence, it can be concluded that rapid freezing is less conducive to achieving optimal environmental stability of lead.
3.3. Environmental Stability
The SLJS sample was subjected to E-FTC treatment, involving an addition of 200 mM EDTA-2Na, a pH of 6, and 12 freeze–thaw cycles. As a result, the Pb content in the SLJS decreased to 0.51%. Subsequently, a leaching toxicity test was performed, and the obtained results are documented in Table 1.

Table 1.
The leaching toxicity of Pb from SLJS before and after E-FTC.
The results demonstrate the remarkable efficacy of the E-FTC in substantially reducing the leachability toxicity of lead in SLJS. Through the application of three different toxicity testing methods, the concentrations of leached lead were measured to be only 3.44 mg/L, 0.12 mg/L, and 3.89 mg/L, respectively. These values indicate a significant reduction of approximately 98% compared to the initial leachability toxicity levels. Importantly, all measured concentrations were well below the established identification standard for lead leachability toxicity in China and the stringent U.S. EPA lead standard of 5 mg/L.
The substantial reduction in lead leachability toxicity underscores the effectiveness of the EDTA treatment in mitigating the release of lead from SLJS. By forming stable complexes with lead ions, EDTA facilitates the dissolution and subsequent sequestration of lead, thereby minimizing its leachability and mitigating the associated environmental risks.
The observed reductions in lead leachability toxicity have significant implications for environmental remediation and the safe management of lead-contaminated sites. The results highlight the potential of E-FTC as a promising approach for mitigating lead release from lead-containing minerals or materials. This approach presents a viable strategy for minimizing the leachability and potential toxic effects of lead in various environmental contexts, addressing the challenges associated with lead contamination.
The results provide compelling evidence of the effectiveness of E-FTC in significantly reducing the leachability toxicity of lead in SLJS. These findings emphasize the importance of employing appropriate remediation techniques to effectively mitigate the environmental risks posed by lead contamination.
The present study investigated the environmental stability of SLJS when treated with E-FTC (200 mM EDTA, pH 6, 12 freeze–thaw cycles) during continuous stacking. To assess this, a continuous rinse experiment was conducted, and the experimental results are provided in Table 2.

Table 2.
Variation of leaching concentration of Pb with time in the continuous leaching experiment of SLJS.
The results demonstrate a remarkable reduction in the lead concentration in the leachate of SLJS after E-FTC. During the initial stages of continuous leaching (≤48 h), the leachate exhibited a lead concentration ranging from 2.2 to 2.5 mg/L. As the leaching process progressed, extending up to 264 h, the lead concentration in the leachate further decreased to 0.2 mg/L. Subsequently, a state of equilibrium was achieved, with the lead concentration stabilizing within the range of approximately 0.11–0.15 mg/L. Importantly, this equilibrium concentration falls well below the recognized toxicity identification standard of 5 mg/L for lead. These findings not only underscore the significant improvement in the environmental stability of SLJS following E-FTC but also highlight its potential for achieving safe and sustainable disposal of lead contaminants.
The observed decline in lead concentration in the leachate indicates the effective removal of lead from the treated jarosite. The E-FTC disrupts the stability of lead-bearing minerals, facilitating the release of lead into the leachate. The subsequent leaching process further contributes to the gradual reduction in lead concentration. The attainment of equilibrium suggests that the release and immobilization of lead reach a balanced state, where the leachate retains a consistently low lead concentration. This enhanced environmental stability significantly reduces the risk of lead leaching and underscores the efficacy of the E-FTC in mitigating lead contamination. These findings provide valuable insights into the development of sustainable strategies for the environmentally friendly management of lead-containing residues.
4. Conclusions
This study investigated the impact of EDTA on lead release, migration, and environmental stability in SLJS under freeze–thaw conditions, with factors of pH, EDTA concentration, freeze–thaw cycles, freezing temperature, and freeze–thaw mode taken into consideration.
Pb release shows a positive correlation with higher pH, EDTA concentration, and freeze–thaw cycles, but decreases with lower freezing temperatures. Rapid freezing significantly inhibits Pb release. The release of Fe is influenced by pH, with higher release observed at lower pH levels and lower release at higher pH levels. The release of sulfate radicals corresponds to lead, indicating its origin from lead sulfate dissolution.
Under optimized freeze–thaw conditions (EDTA concentration of 200 mM, pH 6, freezing at −20 °C, and 12 freeze–thaw cycles), 15.1 mM of lead is released, accounting for 94.9% of the total lead content. E-FTC treatment reduces exchangeable lead fraction, slightly decreases the reducible lead fraction, slightly increases the oxidizable lead fraction, and significantly increases the residual lead fraction, thereby improving the environmental stability. The leaching toxicity of the treated jarosite is reduced by 98%, with lead content in leaching solution below 5 mg/L, surpassing toxicity standards in China and the US. Continuous stability tests show an equilibrium lead concentration of approximately 0.13 mg/L, which is significantly below the 5 mg/L toxicity threshold, thus highlighting the enhanced environmental stability.
Author Contributions
J.P., H.L. and X.Y. conceived and designed the experiments; J.P., Y.W. and L.H. analyzed the data; J.P. wrote and edited the paper; X.Y. and H.L. revised the work critically for important intellectual content. Conceptualization, J.P., Y.S. and Y.W.; methodology, J.P., Y.S. and L.H.; software, J.P., Y.W. and L.H.; validation, J.P. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Major Program Natural Science Foundation of the Hunan Province of China (No. 2021JC0001), the National Natural Science Foundation of China (No. 22276218), and the National Key R and D Program of China (No. 2021YFC2902804).
Data Availability Statement
The data utilized in this study cannot be made publicly available at present, owing to concerns regarding privacy, ethics, and ongoing funding commitments. Nevertheless, we want to assure readers that our conclusions were derived from meticulous analysis and methodologies. Once all funding projects are finalized and the privacy/ethical concerns are adequately resolved, we will contemplate the possibility of sharing the data.
Acknowledgments
We gratefully acknowledge many important contributions from the researchers of all the reports cited in our paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asimi, A.; Gharibi, K.; Abkhoshk, E.; Moosakazemi, F.; Chelgani, S.C. Effects of Operational Parameters on the Low Contaminant Jarosite Precipitation Process-an Industrial Scale Study. Materials 2020, 13, 4662. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, N.; Kargar, M.; Rokhbakhsh-Zamin, F.; Rastakhiz, N.; Manafi, Z. A Review on Various Aspects of Jarosite and Its Utilization Potentials. Ann. Chim. Sci. Mat. 2020, 44, 43–52. [Google Scholar] [CrossRef]
- Kushwaha, P.; Agarwal, M.; Ghosh, A. Value-added products from jarosite hazardous waste: A review. Mater. Today Proc. 2022, 76, 201–205. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, Y.; Zhang, Y.; Xue, P.; Wang, Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J. Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Pappu, A.; Saxena, M.; Asolekar, S.R. Jarosite characteristics and its utilisation potentials. Sci. Total Environ. 2006, 359, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Srivastava, P.; Webster, G.; Weightman, A.J.; Sapsford, D.J. Biostimulation of jarosite and iron oxide-bearing mine waste enhances subsequent metal recovery. J. Hazard. Mater. 2023, 445, 130498. [Google Scholar] [CrossRef] [PubMed]
- Pappu, A.; Thakur, V.K.; Patidar, R.; Asolekar, S.R.; Saxena, M. Recycling marble wastes and Jarosite wastes into sustainable hybrid composite materials and validation through Response Surface Methodology. J. Clean. Prod. 2019, 240, 118249. [Google Scholar] [CrossRef]
- Ryu, J.-G.; Kim, Y. Mineral transformation and dissolution of jarosite coprecipitated with hazardous oxyanions and their mobility changes. J. Hazard. Mater. 2022, 427, 128283. [Google Scholar] [CrossRef]
- Shi, M.; Min, X.; Tian, C.; Hao, T.; Zhu, S.; Ge, Y.; Wang, Q.; Yan, X.; Lin, Z. Mechanisms of Pb(II) coprecipitation with natrojarosite and its behavior during acid dissolution. J. Environ. Sci. 2022, 122, 128–137. [Google Scholar] [CrossRef]
- Trueman, A.; McLaughlin, M.; Mosley, L.; Fitzpatrick, R. Composition and dissolution kinetics of jarosite-rich segregations extracted from an acid sulfate soil with sulfuric material. Chem. Geol. 2020, 543, 119606. [Google Scholar] [CrossRef]
- Gao, K.; Jiang, M.; Guo, C.; Zeng, Y.; Fan, C.; Zhang, J.; Reinfelder, J.; Huang, W.; Lu, G.; Dang, Z. Reductive dissolution of jarosite by a sulfate reducing bacterial community: Secondary mineralization and microflora development. Sci. Total Environ. 2019, 690, 1100–1109. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, X.; Chang, R.; Zhao, Y.S.; Li, X.; Liu, J.; Lin, H.; Qi, C. Effects of Formation Pathways and Bromide Incorporation on Jarosite Dissolution Rates: Implications for Mars. J. Geophys. Res. Planets 2022, 127, e2022JE007202. [Google Scholar] [CrossRef]
- González, E.; Hernández, L.; Muñoz, J.; Blázquez, M.L.; Ballester, A.; González, F. Electron shuttles stimulate the reductive dissolution of jarosite by Acidiphilium cryptum. Hydrometallurgy 2020, 194, 105351. [Google Scholar] [CrossRef]
- Nolasco, M.C.; Flores, L.F.; Gutiérrez, E.J.; Aguilar, J.; Palacios, E.G.; Flores, M.U.; Rodríguez, I.; Reyes, I.A. Acid dissolution of jarosite-type compounds: Effect of the incorporation of divalent cations into the structure on the reaction rate. Hydrometallurgy 2022, 212, 105907. [Google Scholar] [CrossRef]
- Lo, I.M.; Yang, X.Y. EDTA extraction of heavy metals from different soil fractions and synthetic soils. Water Air Soil Pollut. 1999, 109, 219–236. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Haensch, R.; Schnug, E. EDTA application on agricultural soils affects microelement uptake of plants. Sci. Total Environ. 2017, 577, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, C.; Huang, A. EDTA-Functionalized Covalent Organic Framework for the Removal of Heavy-Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 32186–32191. [Google Scholar] [CrossRef] [PubMed]
- Gluhar, S.; Kaurin, A.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Technological feasibility, remediation efficiency and environmental sustainability. Chemosphere 2020, 257, 127226. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, F.; Lombi, E.; McGrath, S. Leaching of heavy metals from contaminated soils using EDTA. Environ. Pollut. 2001, 113, 111–120. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Z.; Wu, Q. Degradation and residue of ethylenediaminetetraacetic acid in the remediation process of heavy metal-contaminated soils. Trans. Chin. Soc. Agric. Eng. 2015, 31, 272–278. [Google Scholar]
- Yin, X.; Chen, J.; Cai, W. Study on the remediation effect of EDTA and citric acid compound washing on multi-metal-contaminated soil. Environ. Sci. 2014, 8, 3096–3101. (In Chinese) [Google Scholar]
- Yi, L.; Wang, W.; Liu, Y.; Tao, Y.; Wen, J. Leaching effects of citric acid, EDTA, and saponin on heavy metal-contaminated soil. J. Saf. Environ. 2014, 14, 225–228. (In Chinese) [Google Scholar]
- Yang, B.; Hu, P.; Li, Z.; Chen, L.; Wu, L.; Luo, Y. Preliminary investigation on EDTA leaching conditions for highly contaminated farmland soils with heavy metals. Soil 2013, 45, 928–932. (In Chinese) [Google Scholar]
- Chen, Z.; Lei, G.; Zhao, S.; Jiang, X.; Peng, X. Desorption effects of EDTA, saponin, and their mixtures on Pb and Zn in soil. Environ. Chem. 2014, 34, 1314–1320. (In Chinese) [Google Scholar]
- Du, J.; Kim, K.; Min, D.W.; Choi, W. Freeze–Thaw Cycle-Enhanced Transformation of Iodide to Organoiodine Compounds in the Presence of Natural Organic Matter and Fe(III). Environ. Sci. Technol. 2021, 56, 1007–1016. [Google Scholar] [CrossRef]
- Wu, S.; Liu, C.; Li, X.; Xiao, B.; Hu, Q. Freeze-thaw controlled aggregation mechanism of humic acid-coated goethite: Implications for organic carbon preservation. Geoderma 2022, 406, 115514. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, W.; Wang, W.; Wang, B.; Zhang, P.; Zhao, T. Influence of organic corrosion inhibitors on steel corrosion in concrete under the coupled action of freeze–thaw cycles and chloride attack. Constr. Build. Mater. 2023, 368, 130385. [Google Scholar] [CrossRef]
- Wu, H.; Xingkai, X.; Cheng, W.; Lin, H. Dissolved organic matter and inorganic N jointly regulate greenhouse gases fluxes from forest soils with different moistures during a freeze-thaw period. Soil Sci. Plant Nutr. 2020, 66, 163–176. [Google Scholar] [CrossRef]
- O’Concubhair, R.; Sodeau, J.R. The Effect of Freezing on Reactions with Environmental Impact. Acc. Chem. Res. 2013, 46, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Grant, N.H.; Alburn, H.E. Acceleration of Enzyme Reactions in Ice. Nature 1966, 212, 194. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, K.; Min, D.W.; Lee, W. Freezing-enhanced dissolution of iron oxides: Effects of inorganic acid anions. Environ. Sci. Technol. 2015, 49, 12816–12822. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, W. Enhanced Redox Conversion of Chromate and Arsenite in Ice. Environ. Sci. Technol. 2011, 45, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, H.; Miyata, K.; Ishii, T.; Ishii, T.; Oishi, M.; Nagatsugi, F. Accelerated polymer–polymer click conjugation by freeze–thaw treatment. Bioconjugate Chem. 2012, 23, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Agten, S.M.; Suylen, D.P.L.; Hackeng, T.M. Oxime Catalysis by Freezing. Bioconjugate Chem. 2015, 27, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, Y.; Tan, Z.; Liu, J.-F. Environmentally Relevant Freeze–Thaw Cycles Enhance the Redox-Mediated Morphological Changes of Silver Nanoparticles. Environ. Sci. Technol. 2018, 52, 6928–6935. [Google Scholar] [CrossRef]
- Min, D.W.; Kim, K.; Lui, K.H.; Kim, D.H.; Choi, W. Abiotic formation of humic-like substances through freezing-accelerated reaction of phenolic compounds and nitrite. Environ. Sci. Technol. 2019, 53, 7410–7418. [Google Scholar] [CrossRef]
- Rui, D.; Wu, Z.; Wu, Y.; Yang, J.; Yi, T. Remediation of heavy metal-contaminated cohesive soil using combined freezing-thawing and leaching methods. Trans. Chin. Soc. Agric. Eng. 2018, 34, 199–205. [Google Scholar]
- Rui, D.; Wu, W.; Zhang, H.; Li, Y.; Yin, X.; Li, J.; Wang, G. Optimization analysis of heavy metal pollutants removal from fine-grained soil by freeze-thaw and washing technology. Cold Reg. Sci. Technol. 2020, 173, 103025. [Google Scholar] [CrossRef]
- Jun, P.; Wei, Y.J.; Shi, M.Q.; Wu, J.H.; Wang, Q.W.; Zhang, L.; Hui, L.; Xu, Y. Spontaneous separation of Pb from PbSO4-coprecipitated jarosite using freeze-thaw cycling with thiourea. Trans. Nonferrous Met. Soc. China 2022, 32, 1019–1030. [Google Scholar]
- Jun, P.; He, L.H.; Hui, L.; Sun, Z.M.; Xu, Y. The Synthesis of Lead-Bearing Jarosite and Its Occurrence Characteristic and Leaching Toxicity Evaluation. Metals 2023, 13, 941. [Google Scholar]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef]
- Min, X.-B.; Xie, X.-D.; Chai, L.-Y.; Liang, Y.-J.; Li, M.; Ke, Y. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans. Nonferrous Met. Soc. China 2013, 23, 208–218. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron (II) with 1, 10-phenanthroline in the presence of large amounts of iron (III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Sorvari, J.; Sihvonen, M.L. Low-level determination of EDTA and DTPA in natural waters by gas chromatography. Chromatographia 1996, 42, 578–582. [Google Scholar] [CrossRef]
- Rui, D.; Wu, Z.; Ji, M.; Liu, J.; Wang, S.; Ito, Y. Remediation of Cd- and Pb- contaminated clay soils through combined freeze-thaw and soil washing. J. Hazard. Mater. 2019, 369, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-F.; Pan, L.; Chen, L.-F.; Yu, Z.; Wang, Q.; Yan, C.-C. Comparison of EDTA and SDS as potential surface impregnation agents for lead adsorption by activated carbon. Appl. Surf. Sci. 2014, 309, 38–45. [Google Scholar] [CrossRef]
- Dai, C.; Hu, Y. Fe (III) hydroxide nucleation and growth on quartz in the presence of Cu (II), Pb (II), and Cr (III): Metal hydrolysis and adsorption. Environ. Sci. Technol. 2015, 49, 292–300. [Google Scholar] [CrossRef]
- Zhang, S.; Peiffer, S.; Liao, X.; Yang, Z.; Ma, X.; He, D. Sulfidation of ferric (hydr)oxides and its implication on contaminants transformation: A review. Sci. Total Environ. 2021, 816, 151574. [Google Scholar] [CrossRef]
- Hasegawa, H.; Al Mamun, M.A.; Tsukagoshi, Y.; Ishii, K.; Sawai, H.; Begum, Z.A.; Asami, M.S.; Maki, T.; Rahman, I.M. Chelator-assisted washing for the extraction of lead, copper, and zinc from contaminated soils: A remediation approach. Appl. Geochem. 2019, 109, 104397. [Google Scholar] [CrossRef]
- He, Z.-W.; Zou, Z.-S.; Sun, Q.; Jin, H.-Y.; Yao, X.-Y.; Yang, W.-J.; Tang, C.-C.; Zhou, A.-J.; Liu, W.; Ren, Y.-X.; et al. Freezing-low temperature treatment facilitates short-chain fatty acids production from waste activated sludge with short-term fermentation. Bioresour. Technol. 2022, 347, 126337. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Feng, C.; Liu, X.; Qin, F.; Liang, C.; Yao, S. Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).