Cu-Ag-Au Microspherules in Igneous Rocks: Morphology, Composition, Diagnostic Criteria and Possible Origin
Abstract
:1. Introduction
2. Materials and Methods
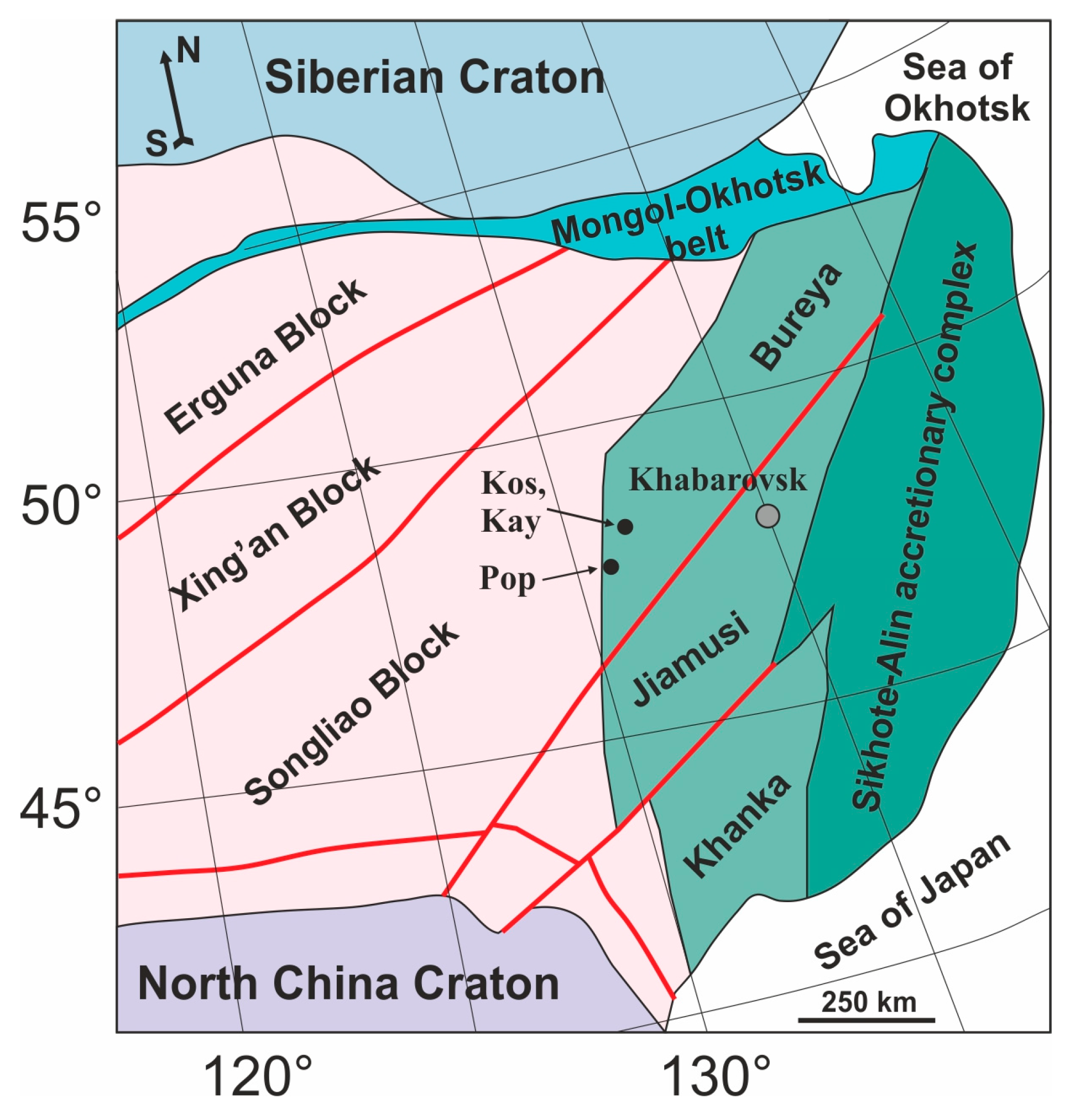
2.1. Geologic Background and Samples
2.2. Sample Preparation and Analytical Methods
3. Results
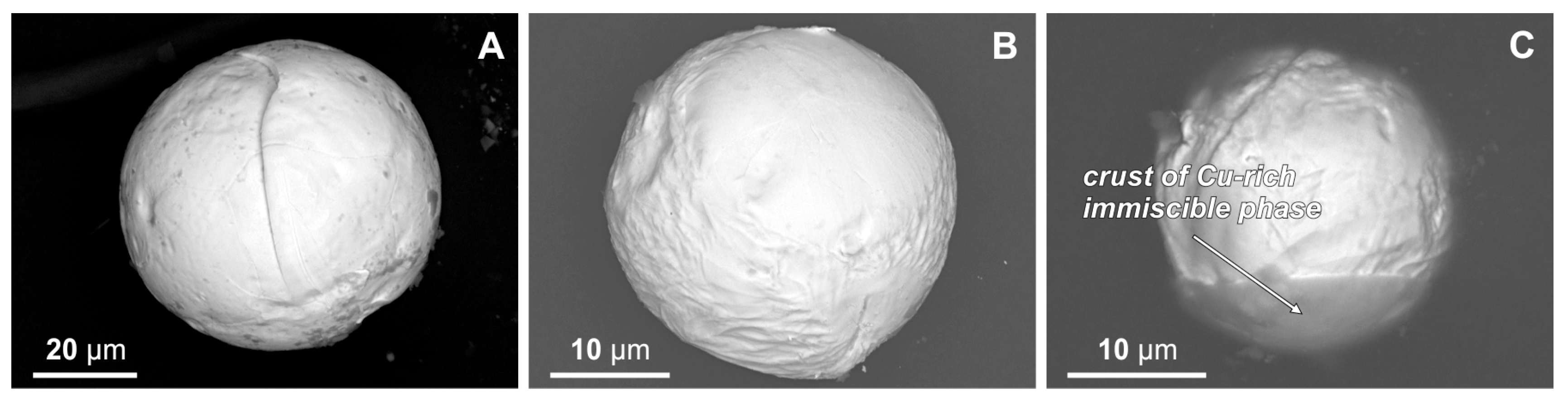
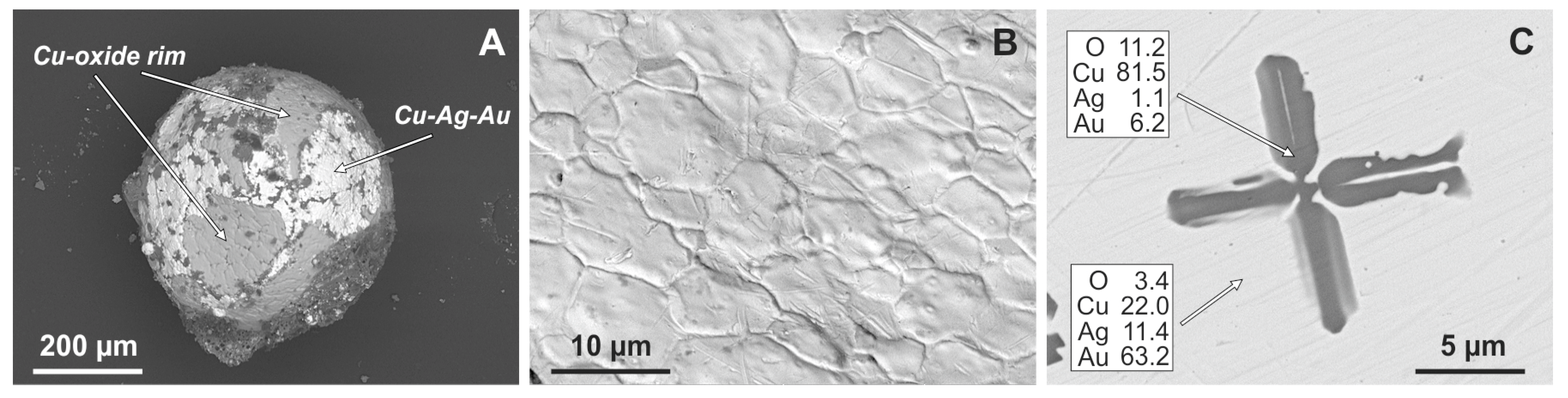
3.1. Exterior Features of Cu-Ag-Au Microspherules
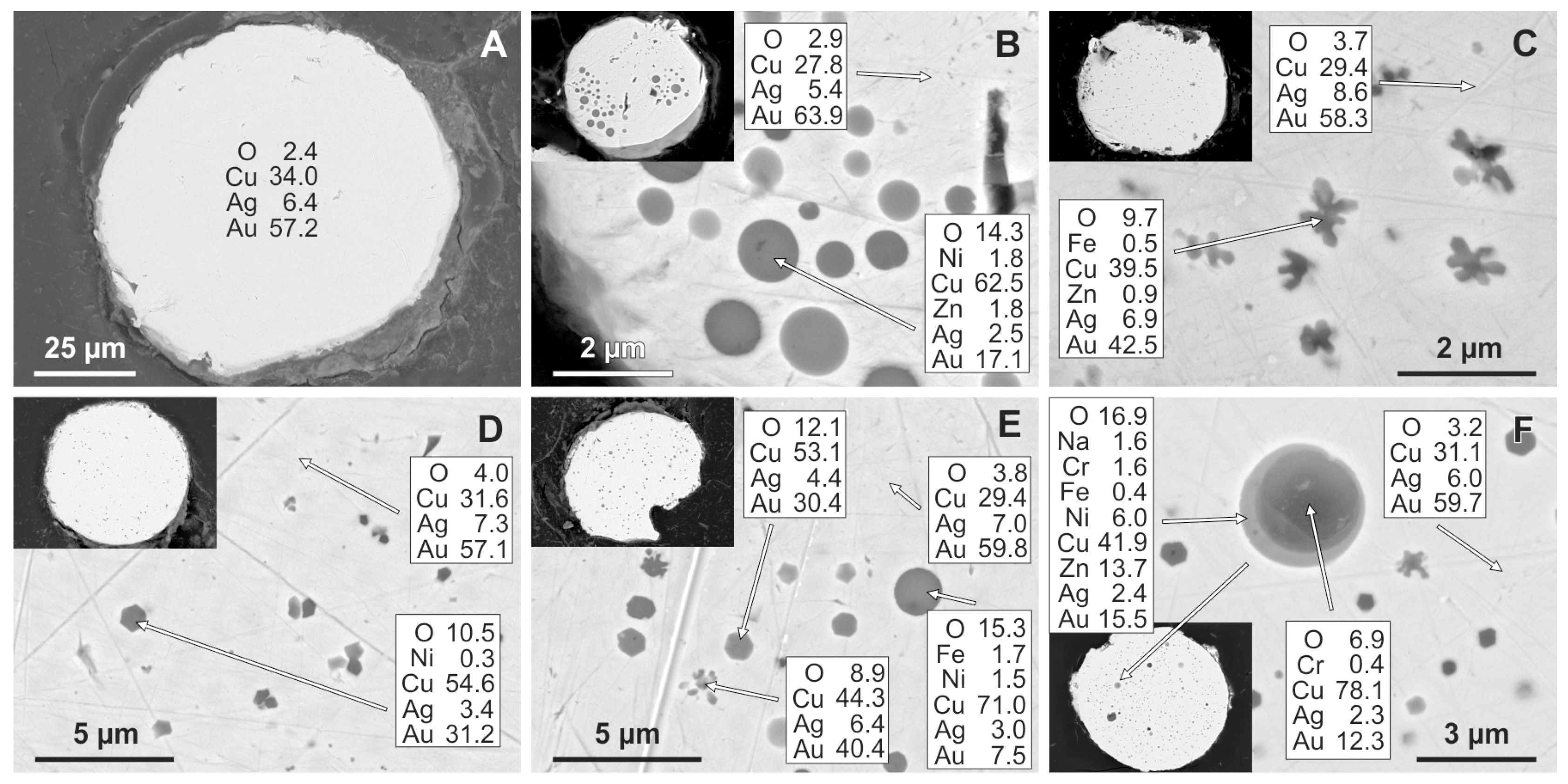
3.2. Internal Structure and Composition of Cu-Ag-Au Microspherules
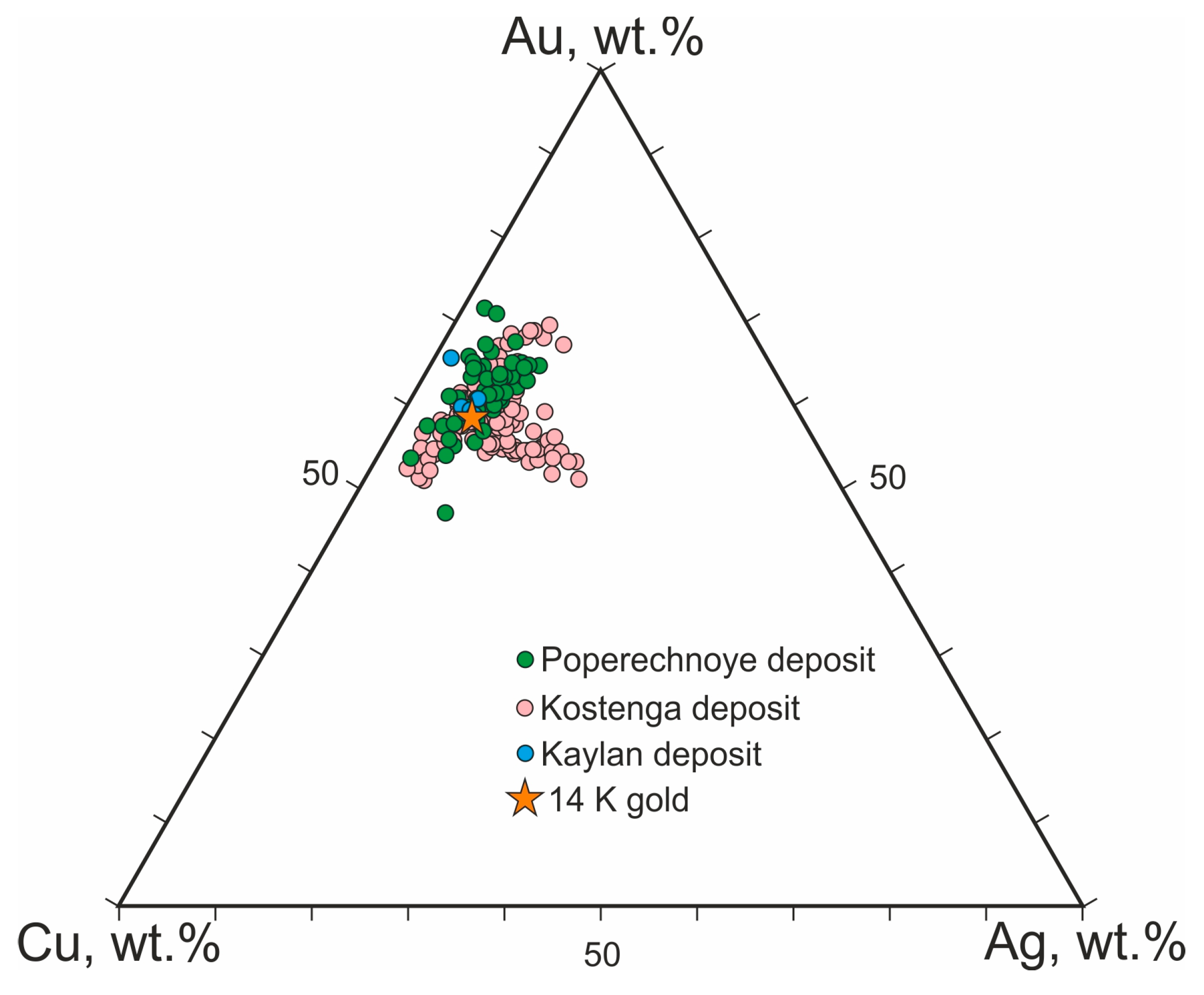
3.3. Diagnostic Criteria for Cu-Ag-Au Microspherule Identification
4. Discussion and Genesis of the Cu-Ag-Au Microspherules
5. Conclusions
- Surface relief and appearance. Natural microspherules are characterized by compaction-related surficial deformations, while technogenic products display cellular surfaces indicative of proto-crystallization processes.
- Natural microspherules do not show any solid solution evidence for exsolution or post-formation crystallization, while technogenic spherules display a well-defined internal domain microstructure due to decomposition of the initial homogenous alloy into several sub-crystalline phases.
- Technogenic spherules totally lack spherical copper-oxide micro-inclusions and micro-inclusions with meniscus-type textural boundaries, indicative of liquid immiscibility at the early stages of their formation and compaction.
- Copper-oxide micro-inclusions in natural Cu-Ag-Au spherules always contain some Fe, Ni, Zn, Cr and, to a lesser extent, Na, while inclusions in technogenic microspherules are composed exclusively of copper and oxygen, as suggested by SEM-EDX microanalysis.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berdnikov, N.; Nevstruev, V.; Kepezhinskas, P.; Astapov, I.; Konovalova, N. Gold in mineralized volcanic systems from the Lesser Khingan Range (Russian Far East): Textural types, composition and possible origins. Geosciences 2021, 11, 103. [Google Scholar] [CrossRef]
- Berdnikov, N.V.; Nevstruev, V.G.; Kepezhinskas, P.K.; Krutikova, V.P.; Konovalova, N.S.; Astapov, I.A. Silicate, Fe-oxide, and Au-Cu-Ag microspherules in ores and pyroclastic rocks of the Kostenga iron deposit, in the Far East of Russia. Russ. J. Pac. Geol. 2021, 15, 236–251. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Kepezhinskas, N.P.; Berdnikov, N.V.; Krutikova, V.O. Native metals and intermetallic compounds in subduction-related ultramafic rocks from the Stanovoy mobile belt (Russian Far East): Implications for redox heterogeneity in subduction zones. Ore Geol. Rev. 2020, 127, 127. [Google Scholar] [CrossRef]
- Berdnikov, N.; Kepezhinskas, P.; Konovalova, N.; Kepezhinskas, N. Formation of gold alloys during crustal differentiation of convergent zone magmas: Constraints from an Au-rich websterite in the Stanovoy Suture Zone (Russian Far East). Geosciences 2022, 12, 126. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Adakites, high-Nb basalts and copper-gold deposits in magmatic arcs and collisional orogens. Geosciences 2022, 12, 29. [Google Scholar] [CrossRef]
- Oen, I.S.; Kieft, C. Nickeline with pyrrhotite and cubanite exsolutions, Ni-Co rich loellingite and an AuCu alloy in Cr-Ni ores from Beni-Bousera, Morocco. Neues Jahrb. Miner. Monatsh. 1974, 443, 1–8. [Google Scholar]
- Grigorieva, A.V.; Damdinov, B.B.; Sluzhenikin, S.F. Ore mineralization in ultramafic and metasomatic rocks of the Ospin-Kitoi massif, Eastern Sayan. Geol. Ore Depos. 2018, 60, 121–141. [Google Scholar] [CrossRef]
- Yurichev, A.N. Accessory gold-silver mineralization in chromitites from the Kharcheruzky massif (Polar Urals). Proc. Tomsk. Polytech. Univ. Eng. Georesources 2021, 332, 229–236. (In Russian) [Google Scholar]
- Yurichev, A.N. Accessory minerals of gold and silver in ultramafic rocks from the Kyzyr-Burlyuksky massif (Western Sayan). Ores Met. 2021, 4, 109–120. (In Russian) [Google Scholar]
- Rudashevsky, N.S.; Rudashevsky, V.N.; Nielsen, T.F.D.; Shebanov, A.D. Au-Cu alloys and inter-metallides in Pd-Au ores of the Skaergaard intrusion (Greenland). Proc. Russ. Miner. Soc. 2014, 143, 1–23. [Google Scholar]
- Sluzhenikin, S.F.; Mokhov, A.V. Gold and silver in PGE-Cu-Ni and PGE ores of the Noril’sk deposits, Russia. Mineral. Depos. 2015, 50, 465–492. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Konovalova, N.; Kepezhinskas, N.; Krutikova, V.; Kirichenko, E. Native metals and alloys in trachytes and shoshonite from the continental United States and high-K dacite from the Bolivian Andes: Magmatic origins of ore metals in convergent and within-plate tectonic settings. Russ. J. Pac. Geol. 2022, 16, 405–426. [Google Scholar] [CrossRef]
- Li, P.; Boudreau, A.E. Vapor transport of silver and gold in basaltic lava flows. Geology 2019, 47, 877–880. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Metals in Avachinsky peridotite xenoliths with implications for redox heterogeneity and metal enrichment in the Kamchatka mantle wedge. Lithos 2022, 412–413, 106610. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Defant, M.J.; Widom, E. Abundance and distribution of PGE and Au in the island-arc mantle: Implications for sub-arc metasomatism. Lithos 2002, 60, 113–128. [Google Scholar] [CrossRef]
- Wang, Z.; Becker, H. Abundances of Ag and Cu in mantle peridotites and the implications for the behavior of chalcophile elements in the mantle. Geochim. Cosmochim. Acta 2015, 160, 209–226. [Google Scholar] [CrossRef]
- Richards, J.P. Postsubduction porphyry Cu-Au and epithermal Au deposits: Products of remelting of subduction-modified lithosphere. Geology 2009, 37, 247–250. [Google Scholar] [CrossRef]
- Tassara, S.; Reich, M.; Konecke, B.A.; González-Jiménez, J.M.; Simon, A.C.; Morata, D.; Barra, F.; Fiege, A.; Schilling, M.E.; Corgne, A. Unravelling the effects of melt-mantle interactions on the gold fertility of magmas. Front. Earth Sci. 2020, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Holwell, D.A.; Fiorentini, M.; McDonald, I.; Lu, Y.; Giuliani, A.; Smith, D.J.; Keith, M.; Locmelis, M. A metasomatized lithospheric mantle control on the metallogenic signature of post-subduction magmatism. Nat. Commun. 2019, 10, 3511. [Google Scholar] [CrossRef] [Green Version]
- Murzin, V.V.; Oydup, C.K.; Varlamov, D.A. A new finding of Cu-Au alloy in association with rodingite minerals in the Kaa-Khem ophiolitic belt, Tuva. Geol. Ore Depos. 2009, 51, 784–793. [Google Scholar] [CrossRef]
- Palyanova, G.A.; Murzin, V.V.; Zhuravkova, T.V.; Varlamov, D.A. Au-Cu-Ag mineralization in rodingites and nephritoids of the Agardag ultramafic massif (Southern Tuva, Russia). Russ. Geol. Geophys. 2018, 59, 238–256. [Google Scholar] [CrossRef]
- Chudnenko, K.V.; Palyanova, G.A. Thermodynamic properties of solid solutions in the Ag-Au-Cu system. Russ. Geol. Geophys. 2014, 55, 349–360. [Google Scholar] [CrossRef]
- Knight, J.; Leitch, C.H.B. Phase relations in the system Au-Cu-Ag at low temperatures, based on natural assemblages. Can. Miner. 2001, 39, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Symonds, R.B.; Rose, W.I.; Reed, M.H.; Lichte, F.E.; Finnegan, D.L. Volatilization, transport and sublimation of metallic and non-metallic elements in high-temperature gases at Merapi volcano, Indonesia. Geochim. Cosmochim. Acta 1987, 51, 2083–2101. [Google Scholar] [CrossRef]
- Cline, J.S.; Bodnar, R.J. Can economic porphyry mineralization be generated by a typical calc-alkaline melt? J. Geophys. Res. 1991, 96, 8113–8126. [Google Scholar] [CrossRef]
- Ulrich, T.; Günther, D.; Heinrich, C.A. Gold of magmatic brines and the metal budget of porphyry copper deposits. Nature 1999, 399, 676–679. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Heinrich, C.A. Vapor transport of metals and the formation of magmatic-hydrothermal ore deposits. Econ. Geol. 2005, 100, 1287–1312. [Google Scholar] [CrossRef]
- Halter, W.E.; Heinrich, C.A.; Pettke, T. Magma evolution and the formation of porphyry Cu-Au ore fluids: Evidence from silicate and sulfide melt inclusions. Miner. Depos. 2005, 39, 845–863. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Audetat, A. Partitioning of V, Mn, Co, Ni, Cu, Zn, Mo, Ag, Sn, Sb, W, Au, Pb and Bi between sulfide phases and hydrous basanite melt at upper mantle conditions. Earth Planet. Sci. Lett. 2012, 355, 327–340. [Google Scholar] [CrossRef]
- Zajacz, Z.; Candela, P.; Piccoli, P.M.; Sanchez-Valle, C.; Wälle, M. Solubility and partitioning behavior of Au, Cu, Ag and reduced S in magmas. Geochim. Cosmochim. Acta 2013, 112, 288–304. [Google Scholar] [CrossRef]
- Audetat, A. The metal content of magmatic-hydrothermal fluids and its relationship to mineralization potential. Geochim. Cosmochim. Acta 2019, 114, 1033–1056. [Google Scholar] [CrossRef]
- Kesler, S.E.; Simon, A.C. Mineral Resources, Economics and the Environment, 2nd ed.; Cambridge University Press: Cambridge, UK, 2015; p. 446. [Google Scholar]
- Morrison, G.W.; Rose, W.J.; Jaireth, S. Geological and geochemical controls on the silver content (fineness) of gold in gold-silver deposits. Ore Geol. Rev. 1991, 6, 333–364. [Google Scholar] [CrossRef]
- Banks, D.A.; Bozkaya, G.; Bozkaya, O. Direct observation and measurement of Au and Ag in epithermal mineralizing fluids. Ore Geol. Rev. 2019, 111, 102955. [Google Scholar] [CrossRef]
- Liu, H.; Beaudoin, G. Geochemical signatures in native gold derived from Au-bearing ore deposits. Ore Geol. Rev. 2021, 132, 104066. [Google Scholar] [CrossRef]
- Townley, B.K.; Hérail, G.; Maksaev, V.; Palacios, C.; de Parseval, P.; Sepulveda, F.; Orellana, R.; Rivas, P.; Ulloa, C. Gold grain morphology and composition as an exploration tool: Application to gold exploration in covered areas. Geochem. Explor. Environ. Anal. 2003, 3, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, A.A.; Kotov, A.B.; Kudryashov, N.M.; Kovach, V.P. Early Mesozoic granitoid and rhyolite magmatism of the Bureya Terrane of the Central Asian Orogenic Belt: Age and geodynamic setting. Lithos 2016, 261, 181–194. [Google Scholar] [CrossRef]
- Ge, M.-H.; Li, L.; Wang, T.; Zhang, J.-J.; Tong, Y.; Geo, L.; Liu, K.; Feng, L.; Song, P.; Yuan, J.-G. Hf isotopic mapping of the Paleozoic-Mesozoic granitoids fro thee Jiamusi and Songhen block, NE China: Implications for their tectonic division and juvenile continental crustal growth. Lithos 2021, 386–387, 106048. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Xu, Y.-G. Mesozoic intraplate tectonism of East Asia due to flat subduction of a composite terrane slab. Earth-Sci. Rev. 2021, 214, 103505. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.; Huang, J. Dynamics of the subducted Izanagi-Pacific plates since the Mesozoic and its implications for the formation of big mantle wedge beneath Eastern Asia. Front. Earth Sci. 2022, 10, 829163. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Y.-A.; Flament, N.; Wu, J.T.-J.; Liu, Y. Northwest Pacific-Izanagi plate tectonics since Cretaceous times from western Pacific mantle structure. Earth Planet. Sci. Lett. 2022, 583, 117445. [Google Scholar] [CrossRef]
- Berdnikov, N.V.; Nevstruev, V.G.; Kepezhinskas, P.K.; Mochalov, A.G.; Yakubovich, O.V. PGE mineralization in andesite explosive breccias associated with the Poperechny iron-manganese deposit (Lesser Khingan, Far East Russia): Whole-rock geochemical, 190Pt-4He isotopic, and mineralogical evidence. Ore Geol. Rev. 2020, 118, 103352. [Google Scholar] [CrossRef]
- Honour, V.; Holness, M.B.; Partridge, J.L.; Charlier, B. Microstructural evolution of silicate immiscible liquids in ferrobasalts. Contrib. Miner. Petrol. 2019, 174, 77. [Google Scholar] [CrossRef] [Green Version]
- Philpotts, A.R. Textural evidence for liquid immiscibility in tholeiites. Miner. Mag. 1978, 42, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Rapson, W.S. The metallurgy of the coloured carat gold alloys. Gold Bull. 1990, 23, 125–133. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, J.C.; Aithal, S.; Welch, P.R. Silicon microsegregation in 14K yellow gold jewelry alloys. Gold Bull. 2001, 34, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Ferro, R.; Saccone, A.; Macció, D.; Delfino, S. A survey of gold intermetallic chemistry. Gold Bull. 2003, 36, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Doh, S.J.; Yu, Y. Anthropogenic contribution of magnetic particulates in urban roadside dust. Atmos. Environ. 2009, 43, 3137–3144. [Google Scholar] [CrossRef]
- Niyogi, A.; Pati, J.K.; Patel, S.C.; Panda, D.; Patil, S.K. Anthropogenic and impact spherules: Morphological similarity and chemical distinction—A case study from India and its implications. J. Earth Syst. Sci. 2011, 120, 1043–1054. [Google Scholar] [CrossRef]
- Magiera, T.; Jablonska, M.; Strzyszcz, Z.; Rachwal, M. Morphological and mineralogical forms of technogenic magnetic particles in industrial dusts. Atmos. Environ. 2011, 45, 4281–4290. [Google Scholar] [CrossRef]
- Hegelson, H.C.; Garrels, R.M. Hydrothermal transport and deposition of gold. Econ. Geol. 1968, 63, 622–635. [Google Scholar]
- Henley, R.W. Geology and geochemistry of epithermal systems. Rev. Econ. Geol. 1985, 2, 1–24. [Google Scholar]
- Kepezhinskas, P.K.; Berdnikov, N.V.; Krutikova, V.O.; Kepezhinskas, N.P.; Astapov, I.A.; Kirichenko, E.A. Silver mineralizaion in deep magmatoogenic systems of ancient island arcs: The Ildeus ultrabasic massif, Stanovoy mobile belt (Russian Far East). Russ. J. Pacific Geol. 2023, 17, 322–349. [Google Scholar]
- Simmons, S.F.; Brown, K.L. Gold in magmatic hydrothermal solutions and the rapid formation of a giant ore deposit. Science 2006, 314, 288–291. [Google Scholar] [CrossRef]
- Frank, M.R.; Simon, A.C.; Pettke, T.; Candela, P.A.; Piccoli, P.M. Gold and copper partitioning in magmatic-hydrothermal systems at 800 °C and 100 MPa. Geochim. Cosmochim. Acta 2011, 75, 2470–2482. [Google Scholar] [CrossRef]
- Zajacz, Z.; Candela, P.A.; Piccoli, P.M.; Wälle, M.; Sanchez-Valle, C. Gold and copper in volatile saturated mafic to intermediate magmas: Solubilities, partitioning, and implications for ore deposit formation. Geochim. Cosmochim. Acta 2012, 91, 140–159. [Google Scholar] [CrossRef]
- Pokrovsky, G.S.; Borisova, A.Y.; Bychkov, A.Y. Speciation and transport of metals and metalloids in geological vapors. Rev. Miner. Geochem. 2013, 76, 165–218. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.P. Giant ore deposits formed by optimal alignments and combinations of geological processes. Nat. Geosci. 2013, 6, 911–916. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Q.; Zhang, H.; Xu, B.; Yu, N.; Wang, R.; Groves, D.I.; Zheng, Y.; Han, S.; Gao, L.; et al. Lithospheric architecture characterized by crust-mantle decoupling controls the formation of orogenic gold deposits. Nat. Sci. Rev. 2023, 10, nwac257. [Google Scholar] [CrossRef]
- Neumann, J.P.; Zhong, T.; Chang, Y.A. The Cu-O (Copper-Oxygen) system. Bull. Alloy Phase Diagr. 1984, 5, 136–140. [Google Scholar] [CrossRef]
- Gasparini, C. Gold and Other Precious Metals: From Ore to Market; Springer: Berlin/Heidelberg, Germany, 1993; p. 336. [Google Scholar]
- Pal’yanova, G.A. Physicochemical modeling of the coupled behavior of gold and silver in hydrothermal processes: Gold fineness, Au/Ag ratios and their possible implications. Chem. Geol. 2008, 255, 399–413. [Google Scholar] [CrossRef]
- Goryachev, N.A.; Pirajno, F. Gold deposits and gold metallogeny of Far East Russia. Ore Geol. Rev. 2014, 59, 123–151. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Driesner, T.; Stefánsson, A.; Seward, T.M. Magmatic vapor contraction and the transport of gold from the porphyry environment to epithermal ore deposits. Geology 2004, 32, 761–764. [Google Scholar] [CrossRef]
- Savva, N.E.; Kravtsova, R.G.; Anisimova, G.S.; Palyanova, G.A. Typomorphism of native gold (geological-industrial types of gold deposits in the north-east of Russia). Minerals 2022, 12, 561. [Google Scholar] [CrossRef]
- Tait, S.; Thomas, R.; Gardner, J.; Jaupart, C. Constraints on cooling rates and permeabilities of pumice in an explosive eruption jet from colour and magnetic mineralogy. J. Volcanol. Geotherm. Res. 1998, 86, 79–91. [Google Scholar] [CrossRef]
- Newcombe, M.E.; Plank, T.; Zhang, Y.; Holycross, M.; Barth, A.; Lloyd, A.S.; Ferguson, D.; Houghton, B.F.; Hauri, E. Magma pressure-temperature-time paths during mafic explosive eruptions. Front. Earth Sci. 2020, 8, 531911. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdnikov, N.; Kepezhinskas, P.; Krutikova, V.; Kozhemyako, N.; Konovalova, N. Cu-Ag-Au Microspherules in Igneous Rocks: Morphology, Composition, Diagnostic Criteria and Possible Origin. Minerals 2023, 13, 819. https://doi.org/10.3390/min13060819
Berdnikov N, Kepezhinskas P, Krutikova V, Kozhemyako N, Konovalova N. Cu-Ag-Au Microspherules in Igneous Rocks: Morphology, Composition, Diagnostic Criteria and Possible Origin. Minerals. 2023; 13(6):819. https://doi.org/10.3390/min13060819
Chicago/Turabian StyleBerdnikov, Nikolai, Pavel Kepezhinskas, Valeria Krutikova, Nadezhda Kozhemyako, and Natalia Konovalova. 2023. "Cu-Ag-Au Microspherules in Igneous Rocks: Morphology, Composition, Diagnostic Criteria and Possible Origin" Minerals 13, no. 6: 819. https://doi.org/10.3390/min13060819




