Genesis of the Giant Huoshaoyun Non-Sulfide Zinc–Lead Deposit in Karakoram, Xinjiang: Constraints from Mineralogy and Trace Element Geochemistry
Abstract
:1. Introduction
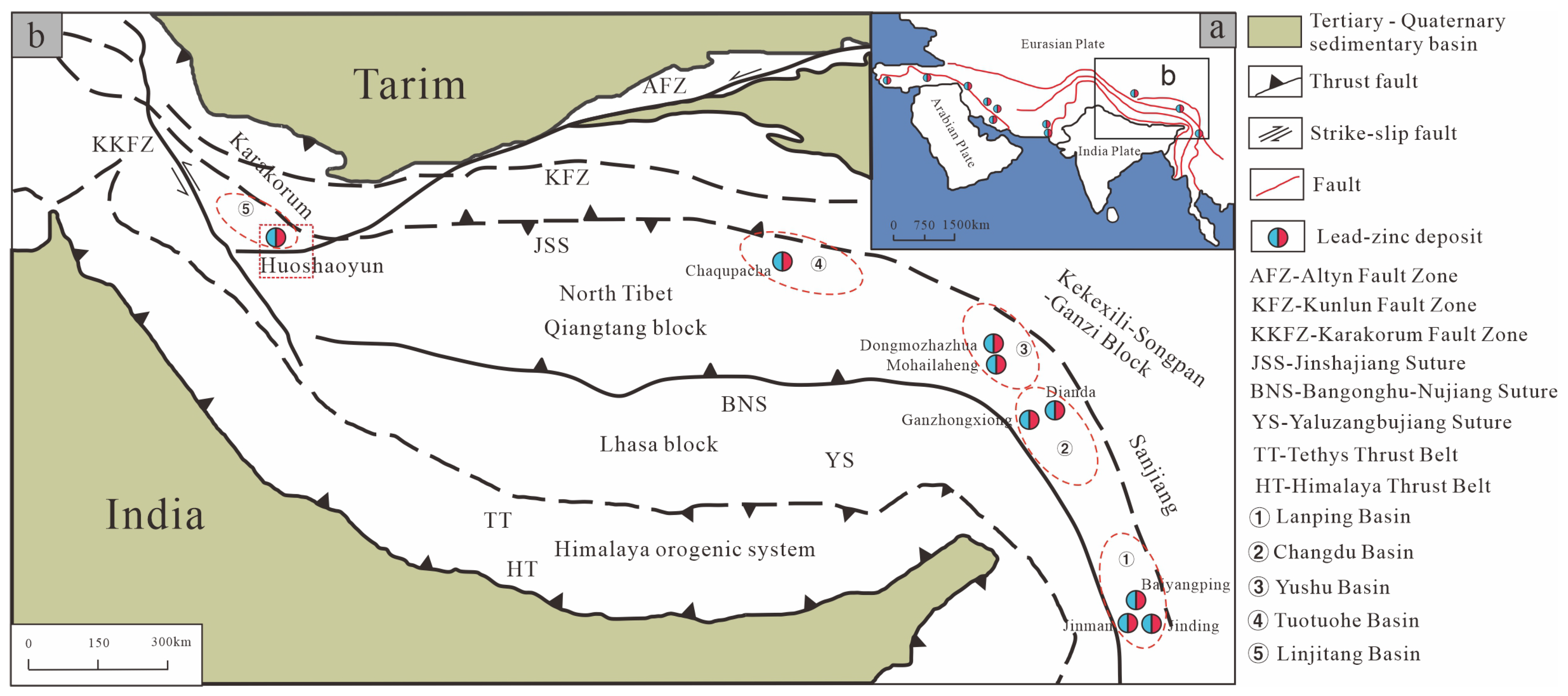
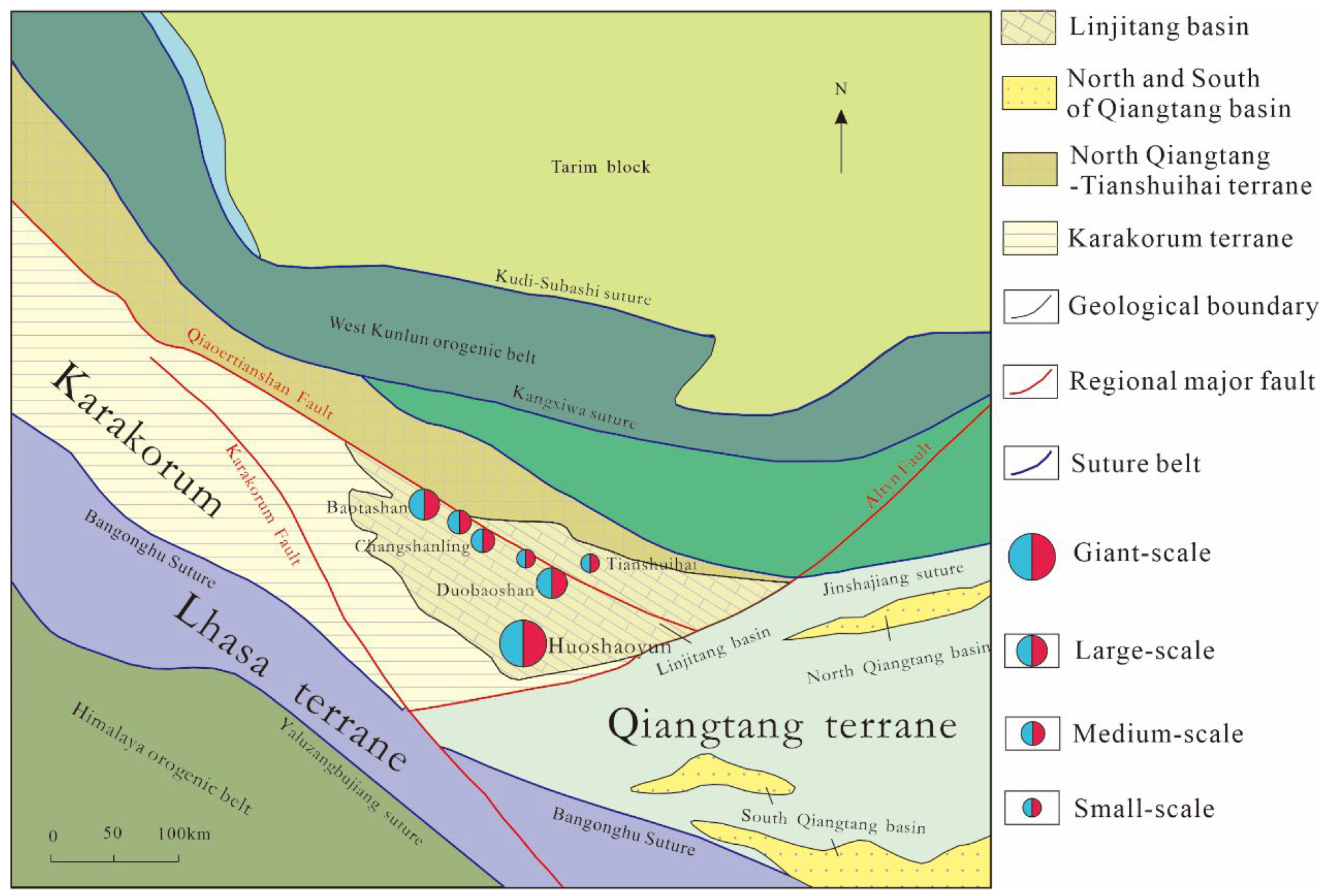
2. Regional Geology
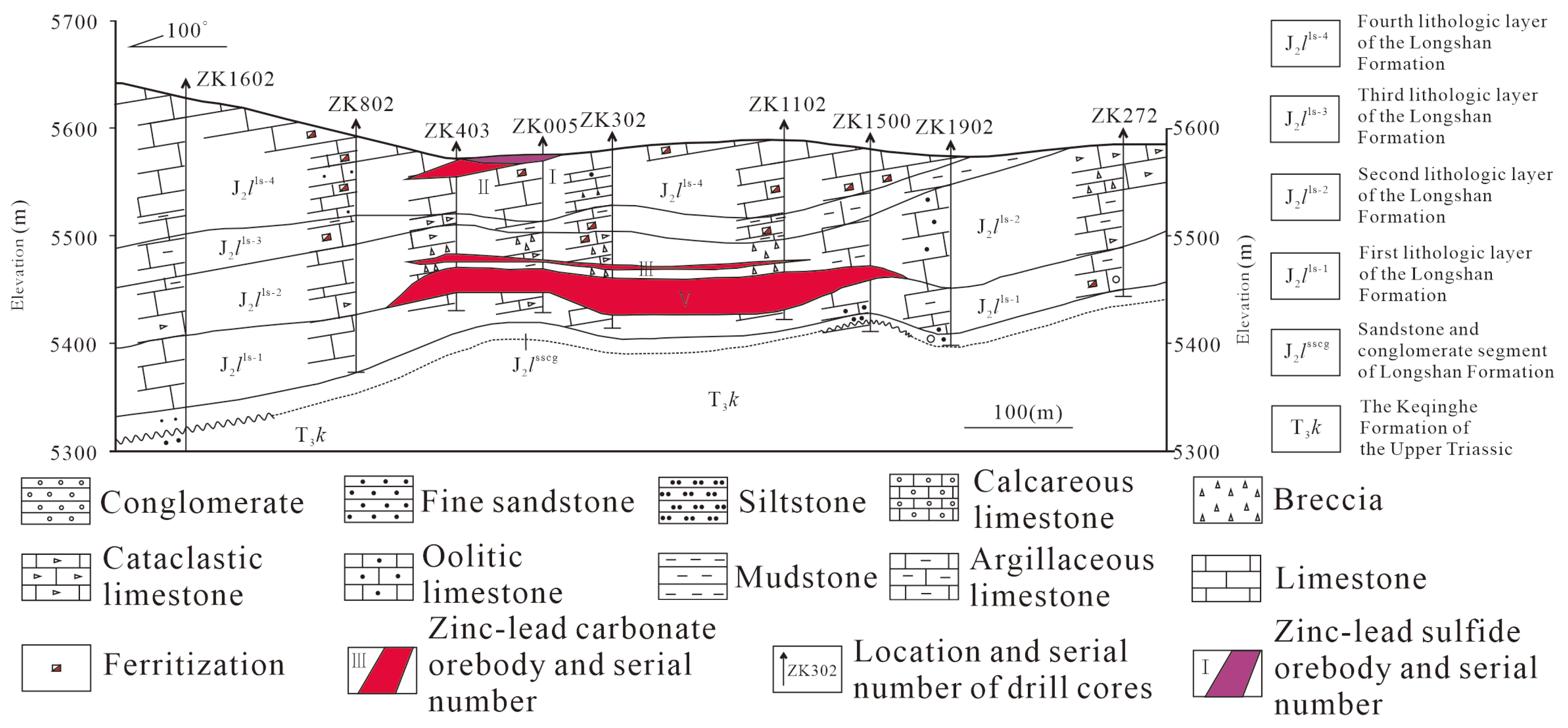
3. Geology of the Huoshaoyun Deposit
4. Sampling and Analytical Methods
5. Results
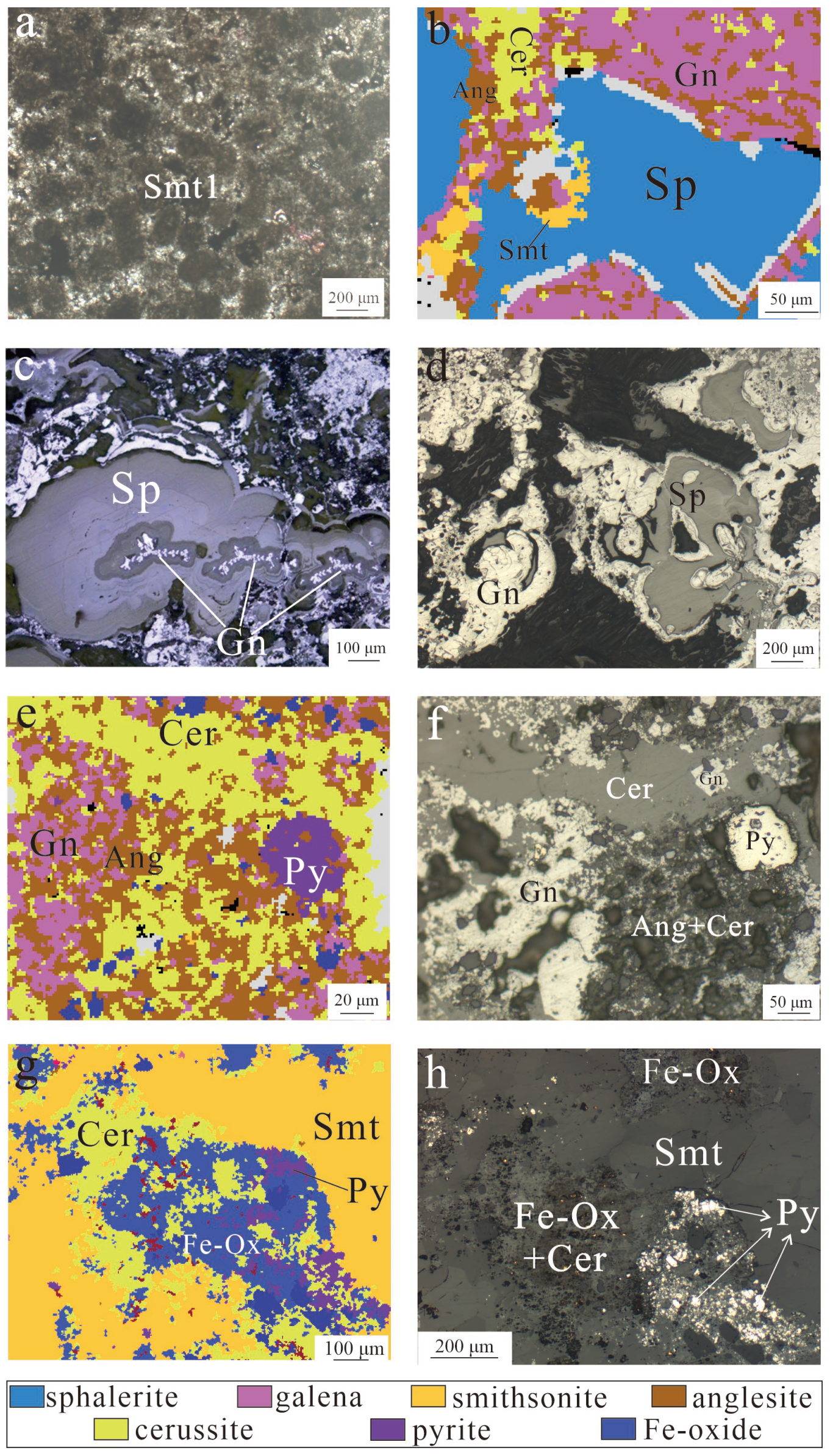
5.1. Type and Texture of the Zn-Pb Ores
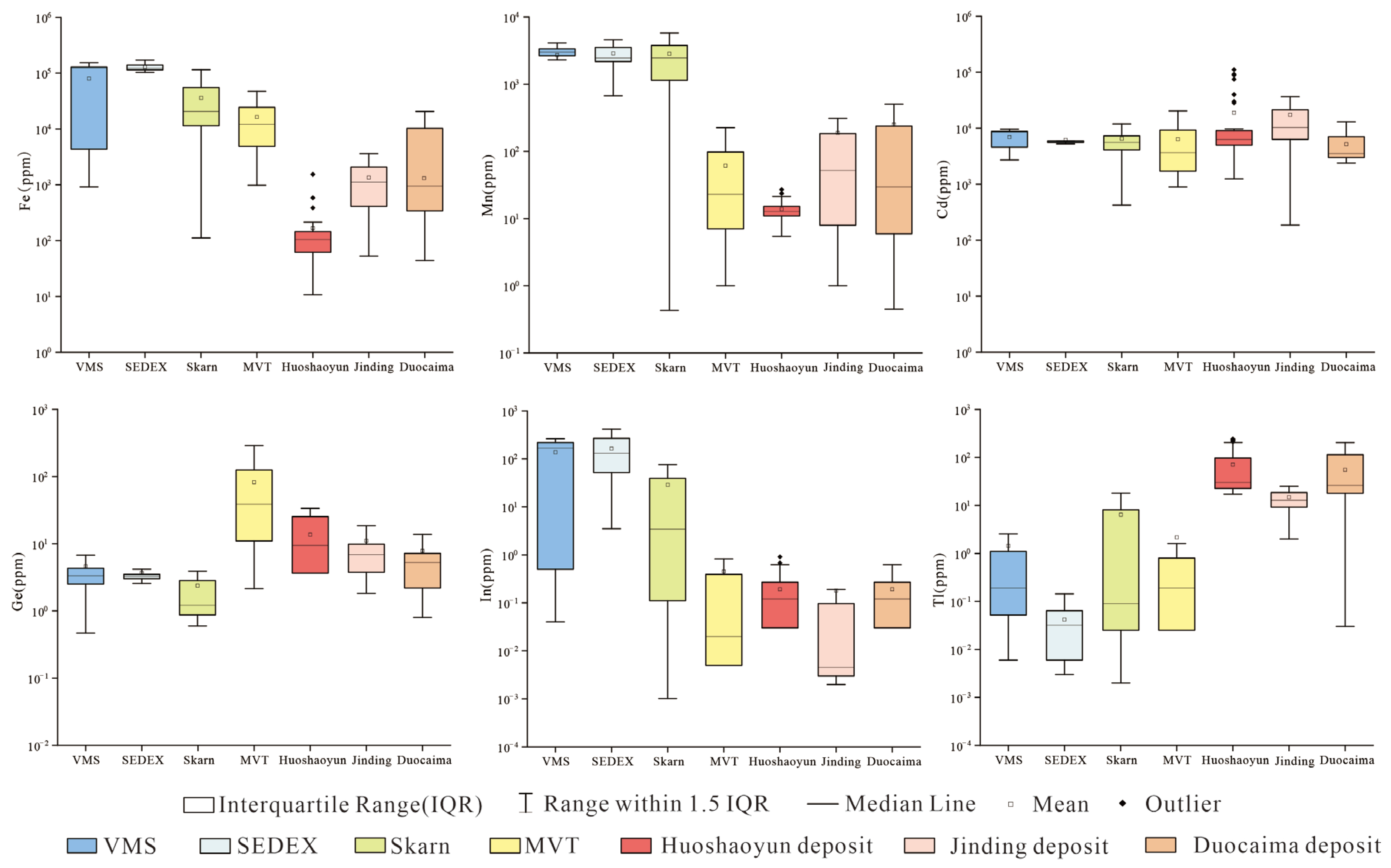
5.2. The Results of LA-ICP-MS
- (1)
- Sphalerite has low Fe and Mn element contents, with Mn content ranging from 10 ppm to 23.7 ppm and an average content of 13.2 ppm. The Fe content varies greatly, ranging from 10.7 ppm to 1540 ppm, with an average content of 248 ppm.
- (2)
- Test on sphalerite reveals that Cd is the most enriched element among the rare dispersed elements, followed by Tl and Ge, while the contents of Ga and In are relatively low. Some measurement points of Ge, Ga, and In are below the detection limit. The Cd content in sphalerite is high and varies greatly, ranging from 3130 ppm to 6282 ppm with an average content of 4962 ppm. The Tl content ranges from 17.2 ppm to 52.5 ppm with an average content of 29.7ppm. The Ge content is unevenly distributed, with some samples having low content ranging from 0.06 ppm to 1.12 ppm, while some samples have higher content ranging from 9.45 ppm to 28.4 ppm. The Ga content is generally low, ranging from 0.02 ppm to 1.65 ppm with an average content of 0.35 ppm. The In content is only detected in a few measurement points, ranging from 0.01 ppm to 0.55 ppm with an average content of 0.05 ppm.
- (3)
- The sphalerite has relatively high Pb content and low As content. The Pb content ranges from 3954 ppm to 9057 ppm, with an average content of 5613 ppm. The distribution of As content is uneven, ranging from 5.24 ppm to 269 ppm, with an average content of 66.5 ppm.
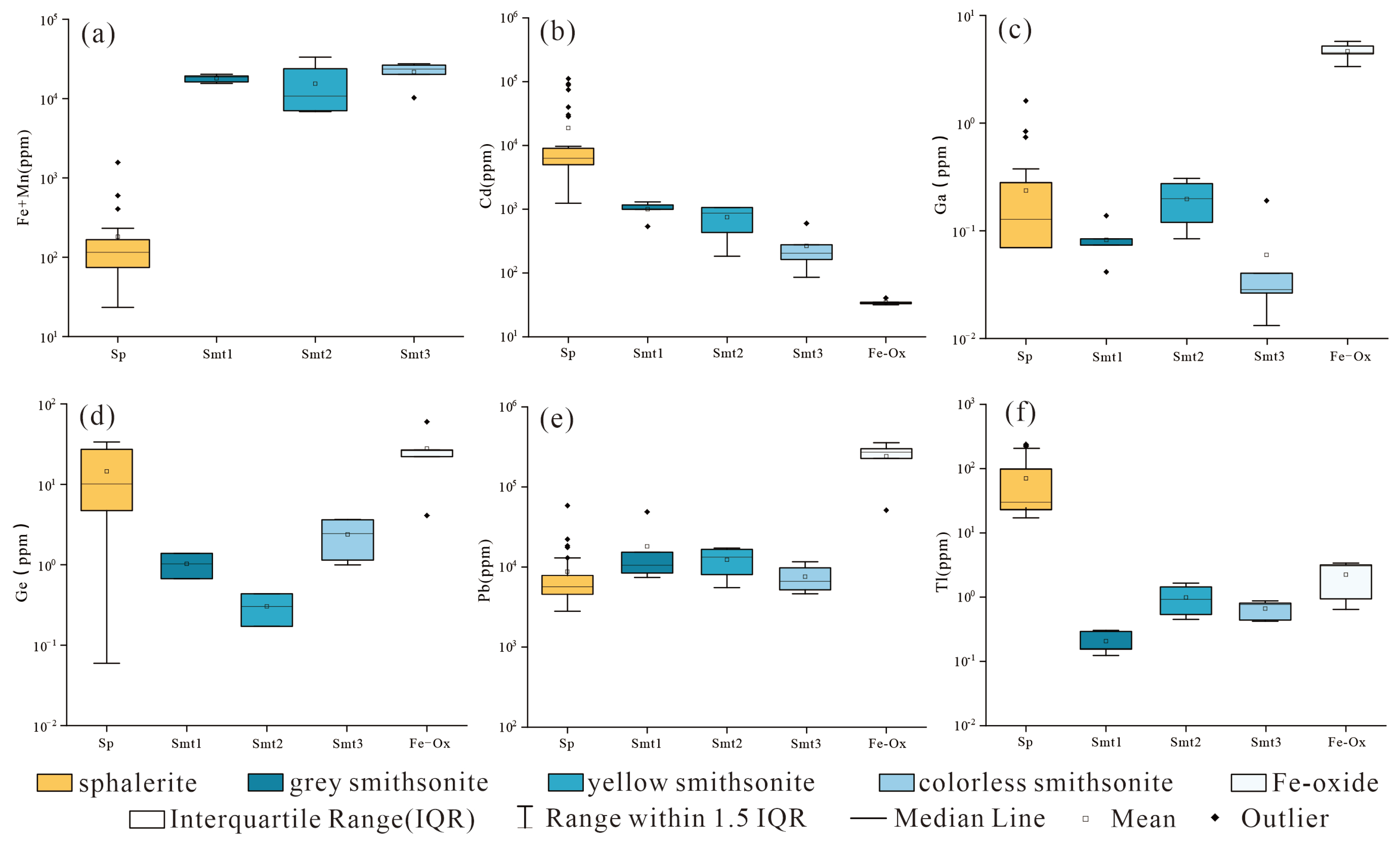
- (1)
- The smithsonite enriches Fe and Mn elements, with Fe content ranging from 4849 ppm to 27,649 ppm and an average content of 14,748 ppm, and Mn content ranging from 1598 ppm to 5646 ppm with an average content of 3542 ppm.
- (2)
- Cd is the most abundant rare dispersed element in smithsonite, while Tl, Ga, and Ge are of lower concentrations. The Cd content significantly varies among the different types of smithsonite. In Smt1, Cd content is relatively high, ranging from 994 ppm to 1302 ppm, with an average content of 1002 ppm. In Smt2, Cd content is lower, ranging from 183 ppm to 1067 ppm, with an average content of 748 ppm. In Smt3, Cd content is the lowest, ranging from 83.9 ppm to 580 ppm, with an average content of 258 ppm. Tl and Ga contents are low, ranging from 0.12 ppm to 1.66 ppm and 0.01 ppm to 0.31 ppm, respectively, while Ge content is the lowest, with only 8 points detected, ranging from 0.17 ppm to 3.75 ppm.
- (3)
- Pb content is relatively high, ranging from 4646 ppm to 48,890 ppm, with an average content of 12,713 ppm.
6. Discussion
6.1. Genesis of the Sulfide Ores
6.1.1. Characteristics of Trace Elements in Sphalerite and Ore-Forming Temperature
6.1.2. Genesis of the Sulfide Ores in the Huoshaoyun Deposit

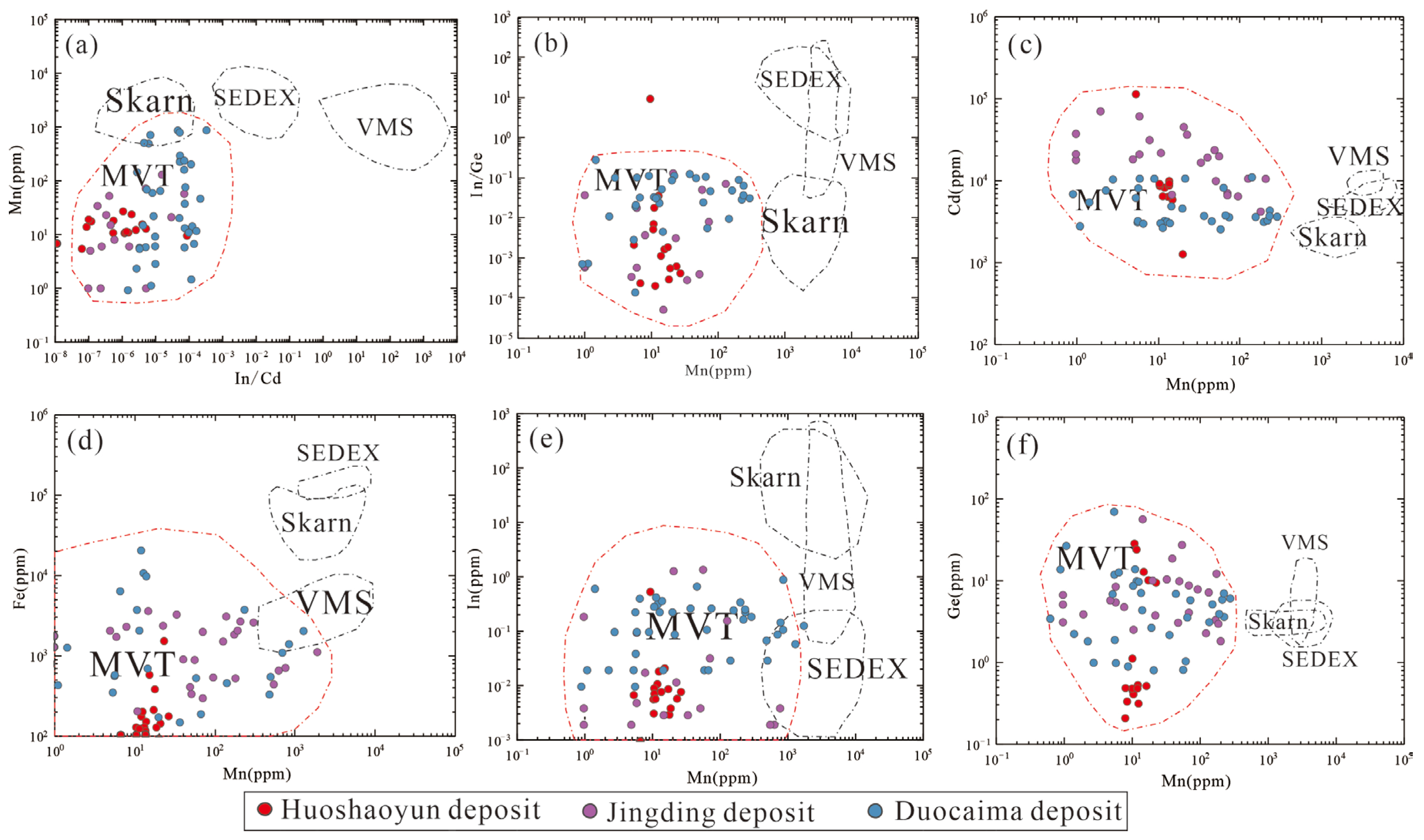
6.2. Trace Element Characteristics of the Non-Sulfide Minerals and Implications for Their Origins
6.3. From MVT Deposits to Non-Sulfide Ores
- (1)
- Smt1: Sphalerite oxidation generates Zn2+ which migrates through fractures and reacts with host rocks, replacing the calcite and forming grey smithsonite: Zn2+ + CaCO3 → ZnCO3 (Smithsonite) + Ca2+. The oolitic textures (Figure 7a) indicates its replacement of the host limestone, while the development of a small amount of fine-grained grey smithsonite (Figure 8a) suggests that a portion of Zn2+ in the fluid could have directly crystallized into smithsonite: Zn2+ + H2O + CO2 → ZnCO3 (Smithsonite) + 2H+.
- (2)
- (3)
- Smt3: The colorless smithsonite was formed by the third-stage fluid carrying Zn2+ filling along the fractures from both sides to the center, or replacing Smt1 and Smt2.
7. Conclusions
- (1)
- The Huoshaoyun zinc–lead deposit is composed of sulfide and non-sulfide ores. The former is mainly composed of sphalerite, galena, and pyrite, whereas the latter is primarily composed of smithsonite, with minor cerussite, anglesite, and Fe-oxide. The non-sulfide minerals clearly replaced the sulfides, suggesting the oxidation of the primary sulfide ores.
- (2)
- The trace element analysis of sphalerite indicates that it is rich in Cd, Tl, and Ge, but poor in Fe and Mn. The ore-forming temperature, calculated using the GGIMFis geothermometer, is most within the range of 100~150 °C. Moreover, the trace element characteristics, ore-forming temperature, and S and Pb isotope compositions of the sulfide ores of the Huoshaoyun deposit are similar to those of the Jinding and Duocaima MVT lead–zinc deposits, which are also located in the eastern Tethyan zinc–lead belt. This suggests that the sulfide orebody in the Huoshaoyun Zn-Pb deposit could be also the MVT deposit.
- (3)
- The trace element of the non-sulfide minerals shows that the Mn and Cd are relatively enriched in smithsonite, while Ga, Ge, and Pb are enriched in Fe-oxide. This characteristic is consistent with that of the other oxidized MVT deposits worldwide, thus indicating the supergene oxidation process of the precursor MVT ores in the Huoshaoyun deposit.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hitzman, M.W.; Reynolds, N.A.; Sangster, D.F.; Allen, C.R.; Carmen, C.E. Classification, genesis, and exploration guides for nonsulfide zinc deposits. Econ. Geol. 2003, 98, 685–714. [Google Scholar] [CrossRef]
- Fan, T.B.; Li, H.; Xu, X.W.; Dong, L.H. Research Status and Progress of Nonsulfide Zinc-Lead Deposit. Northwestern Geol. 2018, 51, 147–159. (In Chinese) [Google Scholar]
- Harkins, S.A.; Appold, M.S.; Nelson, B.K.; Brewer, A.M.; Groves, L.M. Lead isotope constraints on the origin of nonsulfide zinc and sulfide zinc-lead deposits in the Flinders Ranges, South Australia. Econ. Geol. 2008, 103, 353–364. [Google Scholar] [CrossRef]
- Rezaeian, A.; Rasa, I.; Amiri, A.; Jafari, M.R. Geochemistry of Oxygen and Carbon Stable Isotopes in Non-Sulfide Zn-Pb Deposits, Case Study: Chah-Talkh non-Sulfide Zn-PbDeposit (Sirjan-South of Iran). World Appl. Sci. J. 2013, 24, 1163–1171. [Google Scholar]
- Boni, M.; Mondillo, N. The “Calamines” and the “Others”: The great family of supergene nonsulfide zinc ores. Ore Geol. Rev. 2015, 67, 208–233. [Google Scholar] [CrossRef]
- Large, D. The geology of non-sulphide zinc deposits: An overview. Erzmetall 2001, 54, 264–276. [Google Scholar]
- Gao, Y.B.; Li, K.; Teng, J.X.; Zhao, X.M.; Zhao, X.J.; Yan, Z.Q.; Jin, M.S.; Zhao, H.B.; Li, X.T. Mineralogy, Geochemistry and Genesis of Giant Huoshaoyun Zn-Pb Deposit in Karakoram Area, Xinjiang, NW China. Northwestern Geol. 2019, 52, 152–169. (In Chinese) [Google Scholar]
- Dong, L.H.; Xu, X.W.; Fan, T.B.; Qu, X.; Li, H.; Wan, J.L.; An, H.T.; Zhou, G.; Li, J.H.; Chen, G.; et al. Discovery of the Huoshaoyun Super-Large Exhalative-Sedimentary Carbonate Pb-Zn Deposit in the Western Kunlun Area and its Great Significance for Regional Metallogeny. Xinjiang Geol. 2015, 33, 41–50. (In Chinese) [Google Scholar]
- Xu, Z.P.; Xu, Y. Genesis of the Huoshoaoyun deposit in the Karakorum district in Hotan, Xinjiang Province. Resour. Inf. Eng. 2017, 32, 26–27. (In Chinese) [Google Scholar]
- Fan, T.B.; Yu, Y.J.; Xia, M.Y.; Jiang, G.P.; Wang, M. Geological Features and Prospect for the Huoshaoyun Pb-Zn Deposit in Hotan, Xinjiang. Acta Geol. Sichuan 2017, 37, 578–582. (In Chinese) [Google Scholar]
- Jin, H.Z. Analysis of metallogenic condition and prospecting potential of lead-zinc deposit in Tianshuihai-Huoshaoyun area, Karakoram. J. Geol. 2018, 42, 17–22. (In Chinese) [Google Scholar]
- Song, Y.C.; Hou, Z.Q.; Liu, Y.C.; Zhang, H.R. Mississippi Valley-Type (MVT) Pb-Zn deposits in the Tethyan domain: A review. Geol. China 2017, 44, 664–689. (In Chinese) [Google Scholar]
- Gao, L.; Du, Y.; Song, L.S.; Ren, X.D.; Ding, J.H.; Wei, H.T.; Cui, N.; Zhang, Q. Geological characteristics and genesis of the Huoshaoyun hypogene smithsonite deposit, Karakoram Mountains, northwest China. Geol. Rev. 2020, 66, 365–379. (In Chinese) [Google Scholar]
- Yuan, X.; Wu, Y.; Duan, D.F.; Zhu, J.; Ouyang, H.G.; Cao, L.; Zhou, B. Trace (Dispersed) Elements in Sphalerite from the Giant Huoshaoyun Lead-Zinc Deposit, Xinjiang and Their Geological Implications. Geol. Explor. 2022, 58, 545–560. (In Chinese) [Google Scholar]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Yuping, L.; Qian, Z.; Tiegeng, L.; Wei, G.; Yulong, Y.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Qian, Z. Trace elements in galena and sphalerite and their geochemical significance in distinguishing the genetic types of Pb-Zn ore deposits. Chin. J. Geochem. 1987, 6, 177–190. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, Y.X.; Li, Z.K.; Zhao, X.F.; Zuo, R.G.; Xiao, F.; Zheng, Y. Discrimination of Pb-Zn deposit types using sphalerite geochemistry: New insights from machine learning algorithm. Geosci. Front. 2023, 14, 101580. [Google Scholar] [CrossRef]
- Mondillo, N.; Arfè, G.; Herrington, R.; Boni, M.; Wilkinson, C.; Mormone, A. Germanium enrichment in supergene settings: Evidence from the Cristal nonsulfide Zn prospect, Bongará district, northern Peru. Miner. Depos. 2018, 53, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.P. Tectonic, magmatic, and metallogenic evolution of the Tethyan orogen: From subduction to collision. Ore Geol. Rev. 2015, 70, 323–345. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, H. Geodynamics and metallogeny of the eastern Tethyan metallogenic domain. Ore Geol. Rev. 2015, 70, 346–384. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.W.; Borg, G.; Gilg, H.A.; Dong, L.H.; Fan, T.B.; Zhou, G.; Liu, R.L.; Hong, T.; Ke, Q.; et al. Geology and Geochemistry of the giant Huoshaoyun zinc-lead deposit, Karakorum Range, northwestern Tibet. Ore Geol. Rev. 2019, 106, 251–272. [Google Scholar] [CrossRef]
- Deng, W.M. Geological Features of Ophiolite and Tectonic Significance in the Karakorum-West Kunlun Mts. Acta Petrol. Sin. 1995, 11, 98–111. (In Chinese) [Google Scholar]
- Li, R.S.; Ji, W.H.; Yang, Y.C.; Pan, X.P. Geology of Kunlun Mountains and Adjacent Areas. Geology Publishing House: Beijing, China, 2008; pp. 1–400. (In Chinese) [Google Scholar]
- Dong, L.H.; Feng, J.; Liu, D.Q.; Tang, Y.L.; Qu, X.; Wang, K.Z.; Yang, Z.F. Research for classification of metallogenic unit of Xinjiang. Xinjiang Geol. 2010, 28, 1–15. (In Chinese) [Google Scholar]
- Zhang, Z.; Shen, N.; Peng, J.; Yang, X.; Feng, G.; Yu, F.; Zhou, L.; Li, Y.; Wu, C. Syndeposition and epigenetic modification of the strata-bound Pb–Zn–Cu deposits associated with carbonate rocks in western Kunlun, Xinjiang, China. Ore Geol. Rev. 2014, 62, 227–244. [Google Scholar] [CrossRef]
- Fan, T.B.; Jin, H.Z.; Yu, Y.J.; Jiang, G.P.; Xia, M.Y. Metallogenic characteristics and prospecting progress of lead-zinc deposits in the Tianshuihai area of west Kunlun. J. Geol. 2019, 43, 184–197. (In Chinese) [Google Scholar]
- Ren, G.L.; Fan, T.B.; Yu, Y.J.; Yang, M.; Liang, N.; Zhang, Z.; Li, J.Q.; Yang, J.L. Application of Multi-Source Remote Sensing Information to Metallogenic Prediction in the Huoshaoyun Region of Karakorum, Xinjiang. Geol. Explor. 2017, 53, 1164–1173. (In Chinese) [Google Scholar]
- Tang, J.L.; Li, H.; Tan, K.B.; Ke, Q.; Dong, L.H.; Xu, X.W.; Ha, Y.R.T. Ore-Bearing Strata of Lead-Zinc Deposits in the Linjitang Basin of Karakorum in a Marine Sedimentary Environment: Constraints from Trace Elements in Limestone and Sulfur Isotope of Gypsum in Jurassic. Geol. Explor. 2020, 56, 1134–1144. (In Chinese) [Google Scholar]
- Wan, J.L. Exploration Report on the Huoshaoyun Lead-Zinc Deposit in Hetian County, Xinjiang; The Eighth Geological Brigade of the Geological and Mineral Exploration and Development Bureau of Xinjiang Uygur Autonomous Region: Xinjiang, China, 2017. (In Chinese) [Google Scholar]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Cook, N.J.; Etschmann, B.; Cristiana, C.L.; Geraki, K.; Howard, D.L.; Williams, T.; Rae, N.; Pring, A.; Chen, G.; Johannessen, B.; et al. Distribution and Substitution Mechanism of Ge in a Ge−(Fe)−Bearing Sphalerite. Minerals 2015, 5, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.S.; Ni, P.; Pan, J.Y.; Wang, G.G.; Ding, J.Y.; Chu, S.W.; Li, W.S.; Huang, W.Q.; Zhu, R.Z.; Chi, Z. Rare disperse elements in epithermal deposit: Insights from LA–ICP–MS study of sphalerite at Dalingkou, South China. J. Geochem. Explor. 2023, 244, 107124. [Google Scholar] [CrossRef]
- Ivashchenko, V. Geology, geochemistry and mineralogy of indium resources at Pitkäranta Mining District, Ladoga Karelia, Russia. J. Geochem. Explor. 2022, 240, 107046. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, Y.; Liu, Z.; Chen, K. Sphalerite as a record of metallogenic information using multivariate statistical analysis: Constraints from trace element geochemistry. J. Geochem. Explor. 2022, 232, 106883. [Google Scholar] [CrossRef]
- Duan, H.Y.; Wang, C.M. Geochemistry and mineralization of a critical element: Thallium. Acta Petrol. Sin. 2022, 38, 1771–1794. (In Chinese) [Google Scholar]
- Nestmeyer, M.; Keith, M.; Haase, K.M.; Klemd, R.; Voudouris, P.; Schwarz-Schampera, U.; Strauss, H.; Kati, M.; Magganas, A. Trace element signatures in pyrite and marcasite from shallow marine island arc-related hydrothermal vents, Calypso Vents, New Zealand, and Paleochori Bay, Greece. Front. Earth Sci. 2021, 9, 641654. [Google Scholar] [CrossRef]
- Kelley, K.D.; Leach, D.L.; Johnson, C.A.; Clark, J.L.; Fayek, M.; Slack, J.F.; Anderson, V.M.; Ayuso, R.A.; Ridley, W.I. Textural, compositional, and sulfur isotope variations of sulfide minerals in the Red Dog Zn-Pb-Ag deposits, Brooks Range, Alaska: Implications for ore formation. Econ. Geol. 2004, 99, 1509–1532. [Google Scholar] [CrossRef]
- Pfaff, K.; Koenig, A.; Wenzel, T.; Ridley, I.; Hildebrandt, L.H.; Leach, D.L.; Markl, G. Trace and minor element variations and sulfur isotopes in crystalline and colloform ZnS: Incorporation mechanisms and implications for their genesis. Chem. Geol. 2011, 286, 118–134. [Google Scholar] [CrossRef]
- Heijlen, W.; Muchez, P.; Banks, D.A.; Schneider, J.; Kucha, H.; Keppens, E. Carbonate-hosted Zn-Pb deposits in Upper Silesia, Poland: Origin and evolution of mineralizing fluids and constraints on genetic models. Econ. Geol. 2003, 98, 911–932. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Bagas, L.; Evans, N.J.; Chen, J.; Du, B. Mineralization processes at the giant Jinding Zn–Pb deposit, Lanping Basin, Sanjiang Tethys Orogen: Evidence from in situ trace element analysis of pyrite and marcasite. Geol. J. 2018, 53, 1279–1294. [Google Scholar] [CrossRef]
- Zhang, H.S. Mineralization of Sediment-hosted Lead-Zinc Deposits in the Middle-East Segment of the Neo-Tethys Tectonic Domain: Examples from the Duocaima Deposit in Qinghai, China and the Duddar Deposit in Pakistan. Doctoral Dissertation, University of Science and Technology of China, Beijing, China, 2021. (In Chinese). [Google Scholar]
- Shen, H.; Zhang, Y.; Zuo, C.; Shao, Y.; Zhao, L.; Lei, J.; Shi, G.; Han, R.; Zheng, X. Ore-forming process revealed by sphalerite texture and geochemistry: A case study at the Kangjiawan Pb–Zn deposit in Qin-Hang Metallogenic Belt, South China. Ore Geol. Rev. 2022, 150, 105153. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Basuki, N.I.; Spooner, E.T.C. A review of fluid inclusion temperatures and salinities in Mississippi Valley-type Zn-Pb deposits: Identifying thresholds for metal transport. Explor. Min. Geol. 2002, 11, 1–17. [Google Scholar] [CrossRef]
- Xingyu, L.; Bo, L.; Xinyue, Z.; Chengnan, Z.; Huaikun, Q.; Gao, L. Trace element composition and genesis mechanism of the Fuli Pb-Zn deposit in Yunnan: LA-ICP-MS and in situ S-Pb isotopic constraints. Front. Earth Sci. 2023, 11, 352. [Google Scholar]
- Liu, S.; Zhang, Y.; Ai, G.; Xue, X.; Li, H.; Shah, S.A.; Wang, N.; Chen, X. LA-ICP-MS trace element geochemistry of sphalerite: Metallogenic constraints on the Qingshuitang Pb–Zn deposit in the Qinhang Ore Belt, South China. Ore Geol. Rev. 2022, 141, 104659. [Google Scholar] [CrossRef]
- Xing, B.; Mao, J.; Xiao, X.; Liu, H.; Zhang, C.; Guo, S.; Li, H.; Huang, W.; Lai, C. Metallogenic discrimination by sphalerite trace element geochemistry: An example from the Fengyan Zn-Pb deposit in central Fujian, SE China. Ore Geol. Rev. 2022, 141, 104651. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, L.; Wei, C.; Li, Z.; Huang, Z.; Wang, H. Trace Elements in Sphalerite from the Dadongla Zn-Pb Deposit, Western Hunan–Eastern Guizhou Zn-Pb Metallogenic Belt, South China. Acta Geol. Sin.-Engl. Ed. 2020, 94, 2152–2164. [Google Scholar] [CrossRef]
- Yuan, X. Study on Genesis of Primary Sulfide Ore Body from the Giant Huoshaoyun Lead-Zinc Deposit, Xinjiang. Master’s Dissertation, Yangtze University, Wuhan, China, 2022. (In Chinese). [Google Scholar]
- Wu, Z.Y.; Song, Y.C.; Hou, Z.Q.; Liu, Y.C.; Zhuang, L.L. The World-Class Huoshaoyun Nonsulfide Zinc-Lead Deposit, Xinjiang, NW China: Formation by Supergene Oxidization of a Mississippi Valley-Type Deposit. Earth Sci. 2019, 44, 1987–1997. (In Chinese) [Google Scholar]
- Tang, Y.Y.; Bi, X.W.; Fayek, M.; Hu, R.Z.; Wu, L.Y.; Zou, Z.C.; Feng, C.X.; Wang, X.S. Microscale sulfur isotopic compositions of sulfide minerals from the Jinding Zn–Pb deposit, Yunnan Province, Southwest China. Gondwana Res. 2014, 26, 594–607. [Google Scholar] [CrossRef]
- Dai, Z.J. Bacteriogenic Textures and Bacterial Fossils in Sulfide Ores and Their Metallogenic Implication in the Giant Jinding Pb-Zn Deposit, Yunnan, China. Master’s Dissertation, China University of Geosciences, Beijing, China, 2016. (In Chinese). [Google Scholar]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. In Stable Isotopes in High Temperature Geological Processes; De Gruyter: Berlin, Germany, 2018; pp. 491–560. [Google Scholar]
- Gagnevin, D.; Menuge, J.F.; Kronz, A.; Barrie, C.; Boyce, A.J. Minor elements in layered sphalerite as a record of fluid origin, mixing, and crystallization in the Navan Zn-Pb ore deposit, Ireland. Econ. Geol. 2014, 109, 1513–1528. [Google Scholar] [CrossRef]
- Chen, X.; Xue, C.J. Origin of H2S in Uragen large-scale Zn-Pb mineralization, western Tien Shan: Bacteriogenic structure and S-isotopic constraints. Acta Petrol. Sin. 2016, 32, 1301–1314. (In Chinese) [Google Scholar]
- Leach, D.L.; Sangster, D.F.; Kelley, K.D.; Large, R.R.; Garven, G.; Allen, C.R.; Gutzmer, J.; Walters, S. Sediment-Hosted Lead-Zinc Deposits: A Global Perspective. Econ. Geol. 2005, 100, 561–607. [Google Scholar]
- Wu, Z.Y. The World-Class Huoshaoyun Nonsulfide Zinc-Lead Deposit, Xinjiang, NW China: Formation by Supergene Oxidization of a Mississippi Valley-Type Deposit. Master’s Dissertation, China University of Geosciences (Beijing), Beijing, China, 2019. (In Chinese). [Google Scholar]
- Zhang, R.J. Metallogenic Metal Source of Jinding Giant Lead-Zinc Deposit, Yunnan Province: Constraints of Trace Elements of Sphalerite and Lead, Zinc and Strontium Isotopic Compositions. Master’s Dissertation, China University of Geosciences (Beijing), Beijing, China, 2020. (In Chinese). [Google Scholar]
- Xiong, S.F.; Jiang, S.Y.; Chen, Z.H.; Zhou, J.X.; Ma, Y.; Zhang, D.; Duan, Z.P.; Niu, P.P.; Xu, Y.M. A Mississippi Valley–type Zn-Pb mineralizing system in South China constrained by in situ U-Pb dating of carbonates and barite and in situ S-Sr-Pb isotopes. Bulletin 2022, 134, 2880–2890. [Google Scholar] [CrossRef]
- Mu, L.; Hu, R.; Bi, X.; Tang, Y.; Lan, T.; Lan, Q.; Zhu, J.; Peng, J.; Oyebamiji, A. New insights into the origin of the world-class Jinding sediment-hosted Zn-Pb deposit, Southwestern China: Evidence from LA-ICP-MS analysis of individual fluid inclusions. Econ. Geol. 2021, 116, 883–907. [Google Scholar] [CrossRef]
- Liu, C.Z.; Li, S.J.; Chen, Y.L.; Li, D.P.; Gao, Y.W.; Guo, H.M.; Li, L.S.; Wang, Y.K. Characteristics and Genetic Type of Fluid Inclusion of the Duocaima Pb-Zn Deposit in Qiangtang Area. Geotecton. Metallog. 2015, 39, 658–669. (In Chinese) [Google Scholar]
- Mondillo, N.; Herrington, R.; Boyce, A.J.; Wilkinson, C.; Santoro, L.; Rumsey, M. Critical elements in non-sulfide Zn deposits: A reanalysis of the Kabwe Zn-Pb ores (central Zambia). Mineral. Mag. 2018, 82, S89–S114. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Pan, Z.P.; Li, C.Y.; Liu, T.G.; Xia, B. The present situation and prospects of geochemical researches on cadmium. Acta Petrol. Mineral. 2005, 24, 339–348. (In Chinese) [Google Scholar]
- Santoro, L.; Putzolu, F.; Mondillo, N.; Boni, M.; Herrington, R. Influence of genetic processes on geochemistry of fe-oxy-hydroxides in supergene Zn non-sulfide deposits. Minerals 2020, 10, 602. [Google Scholar] [CrossRef]
- Chirico, R.; Mondillo, N.; Boni, M.; Joachimski, M.M.; Ambrosino, M.; Buret, Y.; Mormone, A.; Leigh, L.E.N.B.; Flores, W.H.; Balassone, G. Genesis of the Florida Canyon Nonsulfide Zn Ores (Northern Peru): New Insights Into the Supergene Mineralizing Events of the Bongará District. Econ. Geol. 2022, 117, 1339–1366. [Google Scholar] [CrossRef]
- Stavinga, D. Trace Element Geochemistry and Metal Mobility of Oxide Mineralization at the Prairie Creek Zinc-Lead-Silver Deposit. Doctoral Dissertation, Queen’s University, Kingston, ON, Canada, 2014. [Google Scholar]
- Martínez, C.E.; McBride, M.B. Cd, Cu, Pb, and Zn coprecipitates in Fe oxide formed at different pH: Aging effects on metal solubility and extractability by citrate. Environ. Toxicol. Chem. Int. J. 2001, 20, 122–126. [Google Scholar] [CrossRef]





| Period | Sulfide | Supergene | ||
|---|---|---|---|---|
| Stage | Sulfide | Stage1 | Stage2 | Stage3 |
| Galena | +++ | |||
| Sphalerite | +++ | |||
| Pyrite | + | |||
| Grey Smithsonite (Smt1) | ++ | |||
| Yellow Smithsonite (Smt2) | +++ | |||
| Colorless Smithsonite (Smt3) | + | |||
| Anglesite | ++ | ++ | + | |
| Cerussite | ++ | ++ | + | |
| Fe-oxide | ++ | |||
| Greenockite | + | |||
| Calcite | ++ | + | + | + |
| Quartz | + | + | + | + |
| Gypsum | + | |||
| Simple | Fe | Mn | Cu | Cd | Ga | Ge | Tl | Pb | In | As | PC1 | T (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSY-10-1-7J | 52.3 | 12.8 | 0 | 3673 | 0.05 | 0.53 | 32.3 | 4318 | 0.02 | 12.4 | 1.05 | 151 |
| 59.8 | 10.7 | 0.72 | 5940 | 0.15 | 0.45 | 28.4 | 5664 | 0 | 6.72 | 1.46 | 129 | |
| 62.1 | 12.1 | 0.53 | 4316 | 0.04 | 0 | 30.1 | 4772 | 0.01 | 8.4 | - | - | |
| 69.8 | 10.6 | 0.63 | 4995 | 0.86 | 1.12 | 28.0 | 5209 | 0.01 | 7.71 | 1.92 | 103 | |
| 61.5 | 10.9 | 0.66 | 6037 | 0.06 | 0 | 27.4 | 6056 | 0.01 | 6.45 | - | - | |
| 59.3 | 9.5 | 0.65 | 6282 | 0.02 | 0.06 | 27.7 | 6522 | 0.55 | 5.97 | 0.04 | 206 | |
| 59.4 | 11.0 | 0.02 | 6035 | 0 | 0.41 | 26.9 | 6265 | 0.01 | 5.24 | - | - | |
| HSY-17-1J | 32.5 | 11.3 | 0.13 | 4383 | 0 | 28.4 | 52.5 | 4206 | 0.01 | 269 | - | - |
| 10.7 | 12.4 | 0.17 | 3807 | 0.18 | 22.8 | 51.1 | 3954 | 0 | 262 | - | - | |
| HSY-17-9J | 584 | 15.6 | 1.36 | 5416 | 0.38 | 12.8 | 17.2 | 4560 | 0.02 | 81.48 | 1.27 | 139 |
| 1540 | 23.7 | 3.22 | 3130 | 1.65 | 9.45 | 17.3 | 9057 | 0.01 | 65.65 | 1.23 | 141 | |
| 387 | 18.2 | 0.48 | 5531 | 0.76 | 10.1 | 17.9 | 6772 | 0 | 67.37 | 1.71 | 115 |
| Simple | Mineral | Fe | Mn | Cd | Ga | Ge | Tl | Pb |
|---|---|---|---|---|---|---|---|---|
| HSY-1-1-2J | Smt1 | 12,189 | 3328 | 1010 | 0.14 | 0 | 0.16 | 8429 |
| Smt1 | 12,940 | 3267 | 1302 | 0.08 | 0.67 | 0.15 | 10,533 | |
| Smt1 | 15,186 | 3487 | 994 | 0.04 | 1.38 | 0.12 | 15,343 | |
| Smt1 | 17,221 | 3029 | 1165 | 0.07 | 0 | 0.29 | 48,890 | |
| Smt1 | 15,722 | 3539 | 538 | 0.07 | 0 | 0.3 | 7407 | |
| HSY-1-1-2J | Smt2 | 27,649 | 5646 | 183 | 0.24 | 0 | 0.45 | 5535 |
| HSY-11-4J | Smt2 | 5261 | 1598 | 1062 | 0.16 | 0.17 | 1.66 | 17,224 |
| Smt2 | 5512 | 1675 | 1067 | 0.31 | 0 | 1.23 | 16,044 | |
| Smt2 | 11,084 | 3198 | 681 | 0.08 | 0.44 | 0.63 | 10,559 | |
| HSY-1-1-2J | Smt3 | 16,227 | 3941 | 270 | 0.04 | 1.03 | 0.88 | 9812 |
| Smt3 | 20,917 | 5512 | 83.9 | 0.19 | 3.75 | 0.45 | 4646 | |
| Smt3 | 22,609 | 4827 | 199 | 0.03 | 0 | 0.78 | 6658 | |
| Smt3 | 19,109 | 4507 | 159 | 0.01 | 3.68 | 0.43 | 5211 | |
| HSY-11-4J | Smt3 | 4849 | 2040 | 580 | 0.03 | 1.33 | 0.82 | 11,694 |
| HSY-1-1-2J | Fe-Ox | 711,876 | 0.10 | 33.1 | 5.74 | 60 | 0.94 | 51,301 |
| Fe-Ox | 464,796 | 0.08 | 34.8 | 3.35 | 22.1 | 0.65 | 357,638 | |
| Fe-Ox | 473,549 | 0.02 | 33.7 | 5.2 | 26.2 | 3.06 | 272,532 | |
| Fe-Ox | 537,490 | 0.01 | 40.6 | 4.52 | 4.1 | 3.39 | 227,114 | |
| Fe-Ox | 460,070 | 0.02 | 31.8 | 4.4 | 26.7 | 3.17 | 300,398 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Duan, D.; Zhang, Y.; Zhou, F.; Yuan, X.; Wu, Y. Genesis of the Giant Huoshaoyun Non-Sulfide Zinc–Lead Deposit in Karakoram, Xinjiang: Constraints from Mineralogy and Trace Element Geochemistry. Minerals 2023, 13, 842. https://doi.org/10.3390/min13070842
Chen X, Duan D, Zhang Y, Zhou F, Yuan X, Wu Y. Genesis of the Giant Huoshaoyun Non-Sulfide Zinc–Lead Deposit in Karakoram, Xinjiang: Constraints from Mineralogy and Trace Element Geochemistry. Minerals. 2023; 13(7):842. https://doi.org/10.3390/min13070842
Chicago/Turabian StyleChen, Xiang, Dengfei Duan, Yuhang Zhang, Fanyan Zhou, Xin Yuan, and Yue Wu. 2023. "Genesis of the Giant Huoshaoyun Non-Sulfide Zinc–Lead Deposit in Karakoram, Xinjiang: Constraints from Mineralogy and Trace Element Geochemistry" Minerals 13, no. 7: 842. https://doi.org/10.3390/min13070842
APA StyleChen, X., Duan, D., Zhang, Y., Zhou, F., Yuan, X., & Wu, Y. (2023). Genesis of the Giant Huoshaoyun Non-Sulfide Zinc–Lead Deposit in Karakoram, Xinjiang: Constraints from Mineralogy and Trace Element Geochemistry. Minerals, 13(7), 842. https://doi.org/10.3390/min13070842





