Abstract
First experimental modeling of decarbonation reactions resulting in the formation of CO2-fluid and Mg, Fe, Ca, and Mn garnets, with composition corresponding to the garnets of carbonated eclogites of types I and II (ECI and ECII), was carried out at a wide range of lithospheric mantle pressures and temperatures. Experimental studies were performed on a multi-anvil high-pressure apparatus of a “split sphere” type (BARS), in (Mg, Fe, Ca, Mn)CO3-Al2O3-SiO2 systems (with compositional variations according to those in ECI and ECII), in the pressure interval of 3.0–7.5 GPa and temperatures of 1050–1450 °C (t = 10–60 h). A specially designed high-pressure cell with a hematite buffering container—preventing the diffusion of hydrogen into the platinum capsule—was used, in order to control the fluid composition. Using the mass spectrometry method, it was proven that in all experiments, the fluid composition was pure CO2. The resulting ECI garnet compositions were Prp48Alm35Grs15Sps02–Prp44Alm40Grs14Sps02, and compositions of the ECII garnet were Prp57Alm34Grs08Sps01–Prp68Alm23Grs08Sps01. We established that the composition of the synthesized garnets corresponds strongly to natural garnets of carbonated eclogites of types I and II, as well as to garnets from xenoliths of diamondiferous eclogites from the Robert Victor kimberlite pipe; according to the Raman characteristics, the best match was found with garnets from inclusions in diamonds of eclogitic paragenesis. In this study, we demonstrated that the lower temperature boundary of the stability of natural garnets from carbonated eclogites in the presence of a CO2 fluid is 1000 (±20) °C at depths of ~90 km, 1150–1250 (±20) °C at 190 km, and 1400 (±20) °C at depths of about 225 km. The results make a significant contribution to the reconstruction of the fluid regime and processes of CO2/carbonate-related mantle metasomatism in the lithospheric mantle.
1. Introduction
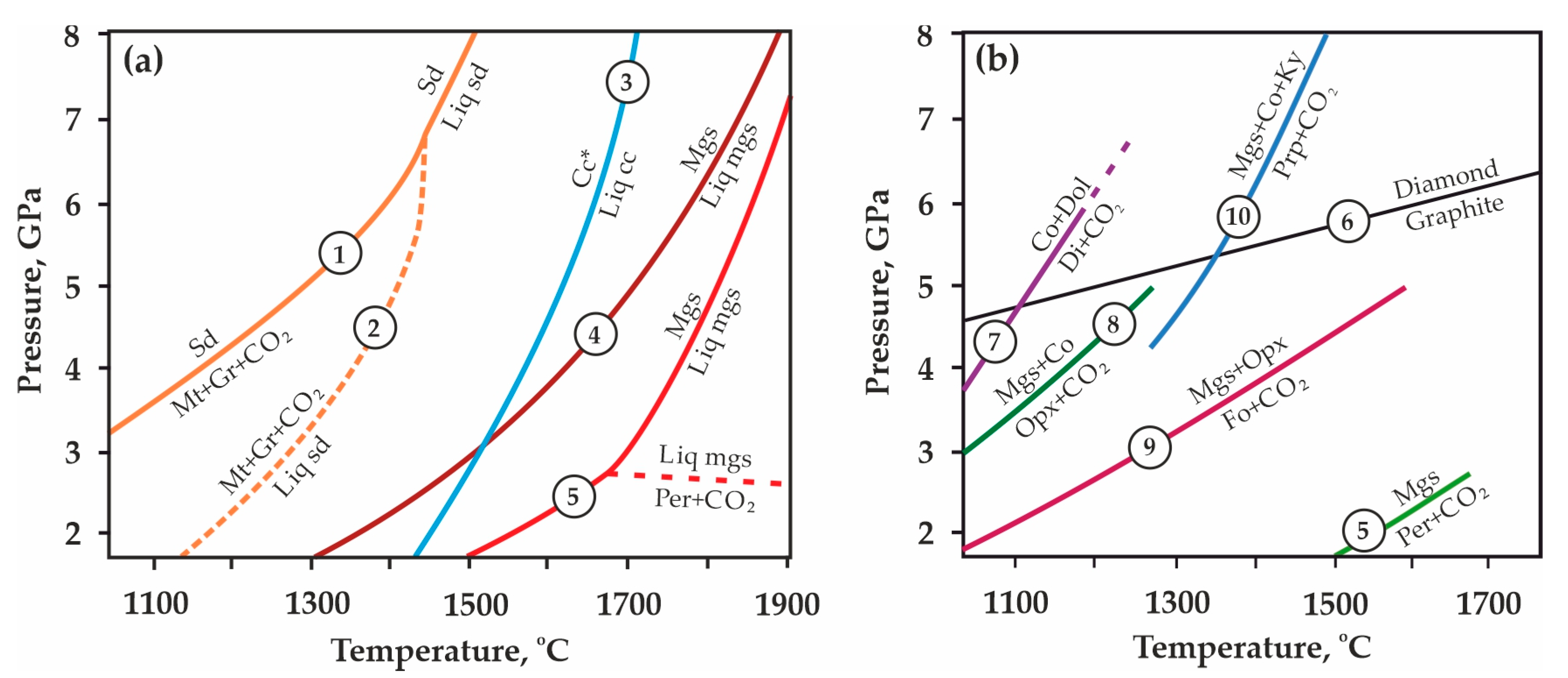
Decarbonation, as well as decomposition and the melting of carbonates (Figure 1a,b), is one of the most important fluid-generating processes under subduction conditions [1,2,3,4,5,6,7]. Experimental modeling of decarbonation reactions is a powerful approach for understanding mantle metasomatism, diamond genesis, the fluid regime in the mantle, and the global carbon cycle [1,2,3,4,5,6,7]. CO2-dominated fluid, formed by decarbonation reactions during the interaction of carbonate-bearing slab rocks with upper mantle silicate or silicate-oxide assemblages in a wide pressure range, is one of the most powerful agents of mantle metasomatism. The presence of CO2 fluid in mantle rocks is confirmed by multiple discoveries of inclusions in mantle silicates—olivine, pyroxenes, garnets [8,9,10,11,12,13,14,15], as well as in diamonds [16,17,18,19,20,21,22,23]. Moreover, CO2 fluid is found to be the predominant component of fluid inclusions in the minerals of mantle xenoliths from depths of <170 km [24]. Among the other indicators for the existence of a CO2-bearing fluid in the mantle, there are mineral assemblages, which are interpreted as the products of the carbonation of mantle rocks [25,26,27,28,29,30].
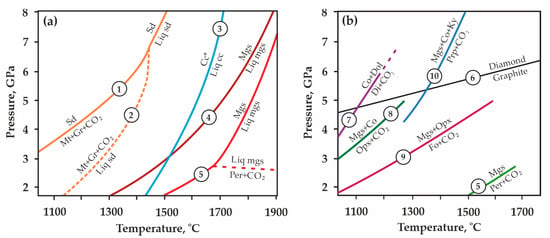
Figure 1.
Experimentally determined pressure-temperature boundaries of melting and decomposition regarding both Mg, Ca, and Fe carbonates (a) and decarbonation reaction curves (b): 1, 2–after [31,32]; 3–after [33]; 4, 5–after [34]; 6–after [35]; 7–after [36]; 8–after [37,38,39]; 9–after [40,41]; 10–after [42]; Sd—siderite, Liq sd—liquid FeCO3, Mt—magnetite, Gr—graphite, Mgs—magnesite, Per—periclase, Liq mgs—liquid MgCO3, Cc—calcite, Liq cc—liquid CaCO3, Co—coesite, Dol—dolomite, Di—diopside, Opx—orthopyroxene, Fo—forsterite, Ky—kyanite, Prp—pyrope.
Pioneer experimental studies performed to determine the position of carbonation/decarbonation curves in the P,T-field at mantle pressures and temperatures began in the 1970s. The relevance of these works was due to a number of unresolved issues that existed at that time and required explanation, in particular: (1) numerous new findings of CO2 inclusions in mantle minerals [43,44]; (2) the established fact of high CO2 solubility in silicate melts [45]; and (3) the potential role of CO2 in the genesis of kimberlites and carbonatites [46,47]. In these experimental studies, the parameters of decarbonation reactions in CaO-MgO-CO2, CaO-SiO2-CO2, MgO-SiO2-CO2, and CaO-MgO-SiO2-CO2 systems [25,26,27,28,29,30,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] were examined (Figure 1b, list of reactions (1)–(8) is shown in Supplementary Materials). Later (from the mid-1970s to the late 1990s), an experimental reconstruction of the carbonation reactions of olivine-bearing ultramafic mantle rocks was performed in a very wide pressure range (up to 50 GPa) [56,57,58,59,60]. It has been found that in these ultramafic associations, carbonation involves the interaction of olivine/pyroxene with CO2 to form carbonate, while the resulting carbonate remains stable and does not decompose [38,40,61,62,63] (reactions (9)–(10) in Supplementary Materials).
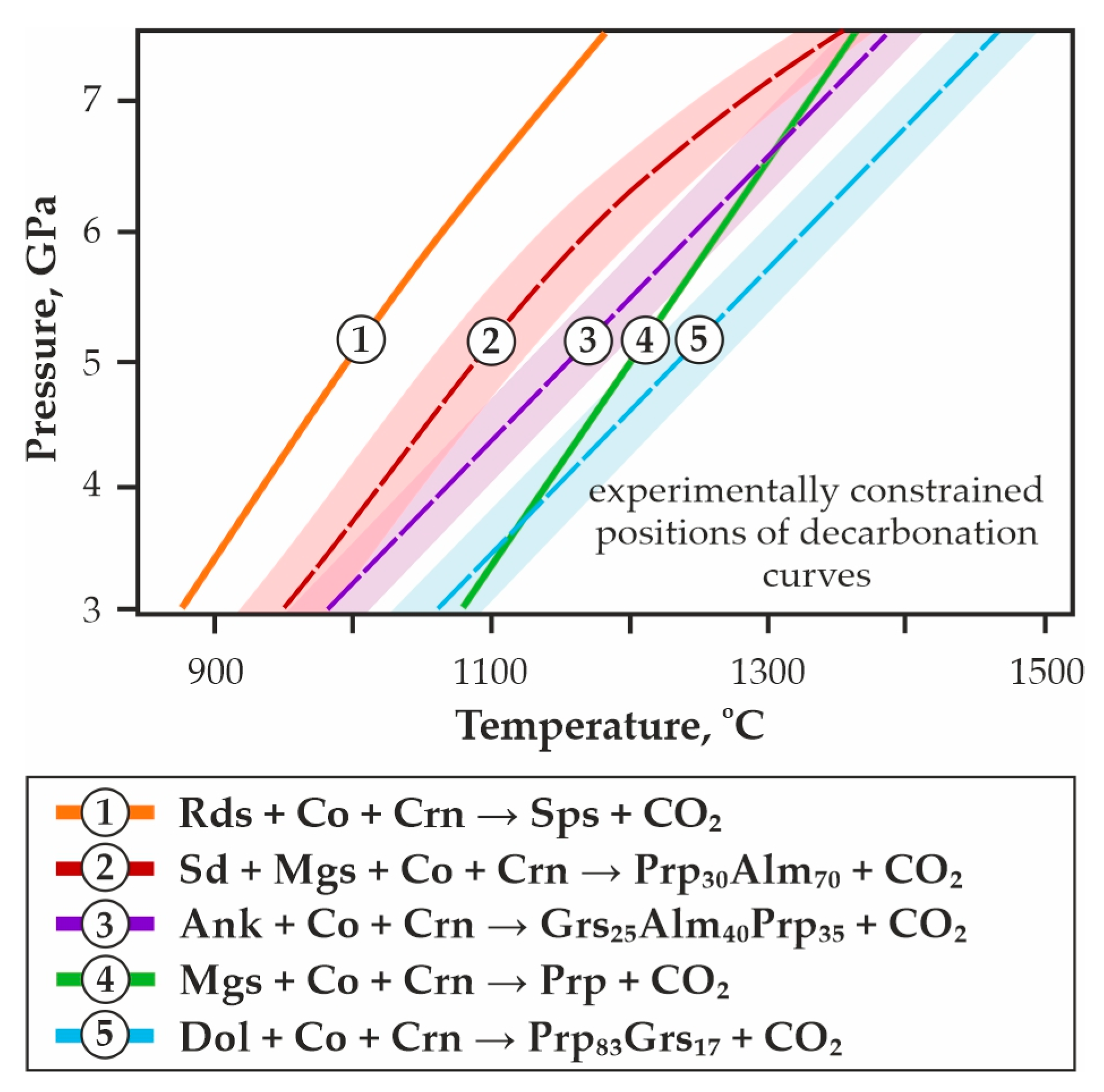
The first experimental modeling of decarbonation reactions associated with the formation of pyrope and CO2-fluid, and simulating the interaction in eclogite rocks-CO2 systems under conditions of the upper mantle, was performed in [42] (Figure 1b, reaction (11) in Supplementary Materials). Later, we carried out systematic experimental studies to determine the position of decarbonation lines in the P,T field, and established the stability regions of the CO2-fluid in association with pyrope, pyrope-almandine, pyrope-grossular, pyrope-almandine-grossular, and spessartine (Figure 1b and Figure 2, reactions (12)–(16) in Supplementary Materials) [64,65,66,67]. These experimental works were continued in studies [68,69].
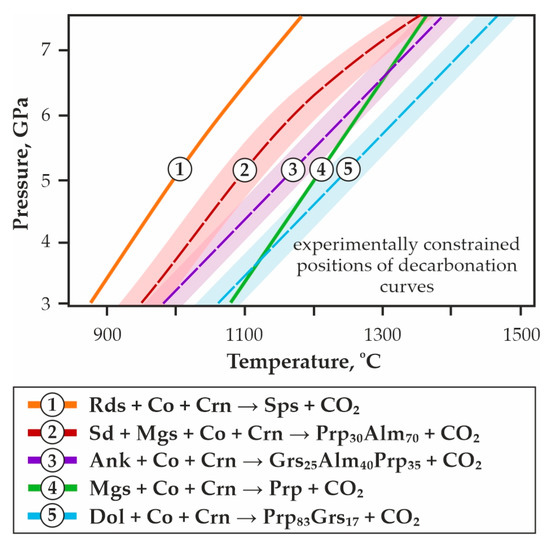
Figure 2.
P,T-diagram with the results of our previous experimental studies [65,66,67] and the positions of decarbonation curves, resulting in the formation of CO2 fluid and garnets of various compositions.
It is impossible not to notice that the systems in which the above reactions were modeled are greatly simplified relative to natural environments and poorly take into account variations in the composition of mantle minerals. Among the works mentioned above, only [67] considers a reaction with the participation of three-component garnet, while other compositions of silicates are represented by one end-member, and less often by two-component solid solutions. Considering that garnet group minerals are important components of mantle mafic and ultramafic rocks [70], experimental modeling of decarbonation reactions associated with the formation of garnets, corresponding in composition to natural ones, seems to be very relevant. Study of the multicomponent garnet would better approximate the expanded stability field of garnets via decarbonation reactions, as well as the eclogitization of oceanic crust, which is critical for the slab-pull driving force for plate tectonics.
A number of works have experimentally studied phase formation in carbonated eclogites at pressures of 2–10 GPa and temperatures of 1050–1400 °C [71,72,73,74], as well as the reactions of natural eclogite garnet with CO2 fluid in the temperature range of 950–1550 °C (6.3 GPa) [75]. Despite the indisputable significance of the obtained results, in experiments on the study of model eclogite systems, it is rather difficult to assess the contribution of individual minerals to the processes of carbonation and/or partial melting with the formation of carbonate-silicate melts, as well as the boundary conditions for the stability of eclogite garnets coexisting with CO2. In [71], phase relationships in carbonated eclogites in the pressure range of 2.5–5.5 GPa were experimentally studied, while the authors considered two contrasting eclogite systems with compositions based on the averaged compositions of type I and II model carbonated eclogites (ECI, ECII) (Table 1) [76].

Table 1.
Compositions of model carbonated eclogites of type I (ECI) and II (ECII), as well as garnets and carbonates synthesized in ECI and ECII model systems in [71] (5 GPa, 1100 °C).
In this paper, we present the experimental reconstruction of the decarbonation curve positions in the (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 systems (with the compositional variations according to those in ECI and ECII [74,76,77,78,79]) in the wide range of the lithospheric mantle P,T-parameters. The main goal of this study is the estimation of the stability field of garnets from type I and type II model carbonated eclogites coexisting with CO2-fluid.
2. Materials and Methods
2.1. Experimental Methods and Starting Materials
Experimental modeling of decarbonation reactions resulting in the formation of ECI and ECII model garnets and CO2-fluid, was performed in the (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 systems using a multi-anvil high-pressure split-sphere apparatus (BARS) [80]. Experiments were carried out at pressures of 3.0, 6.3, and 7.5 GPa, in the temperature range of 1050–1450 °C and durations from 10 to 60 h. The methodological features of the assembly, the design of the high-pressure cell, as well as data on the pressure and temperature calibration, have been published previously [81,82,83,84].
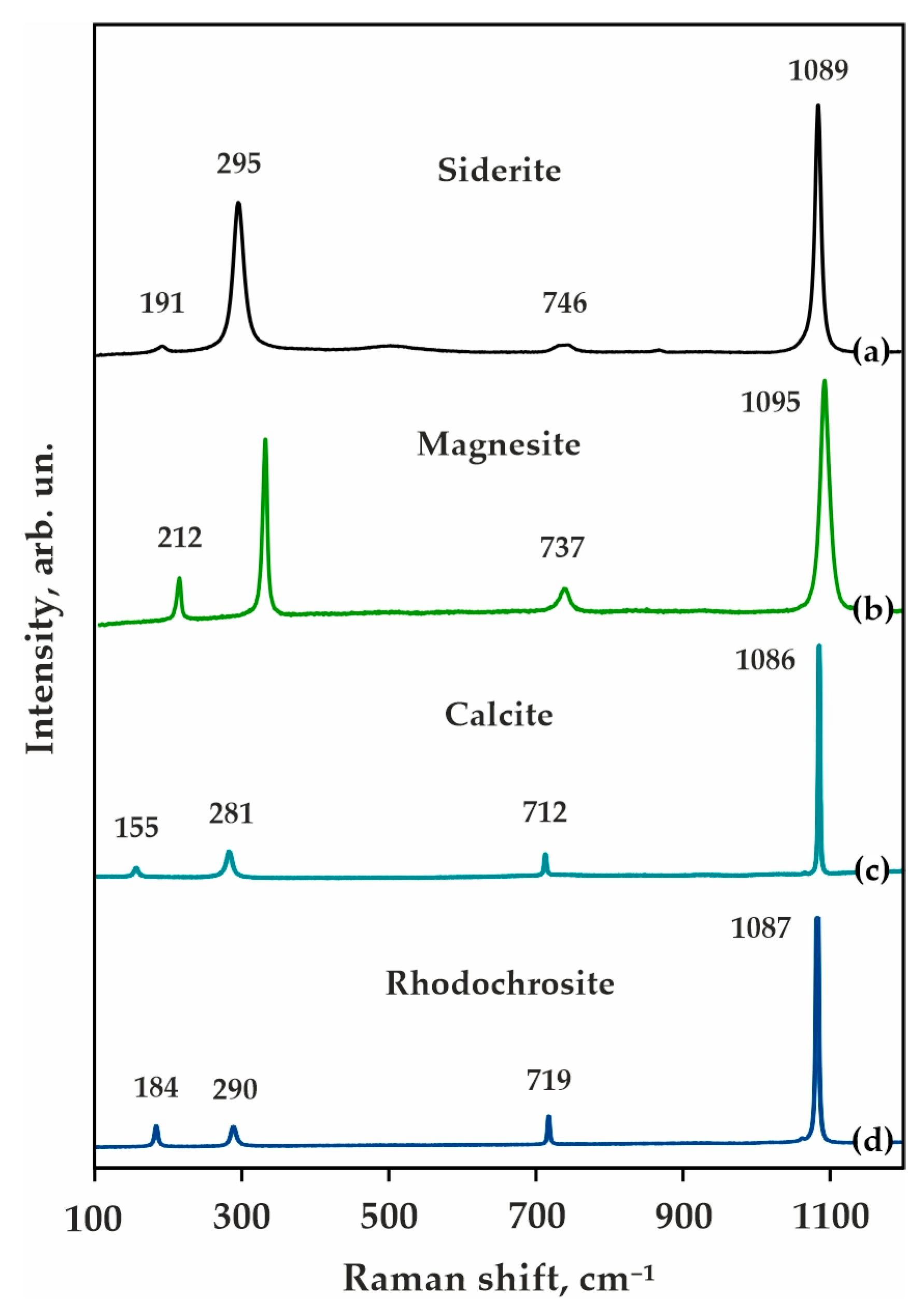
Starting materials were natural carbonates-magnesite (Satka deposit, Urals, Russia), magnesiosiderite (Mésage Mine, Saint-Pierre-de-Mésage, France), calcite (Tura deposit, Russia), and rhodochrosite (China, Guangxi Zhuang Autonomous Region, Wuzhou Prefecture, Cangwu Co., Wutong Mine (Wudong Mine)), as well as synthetic Al2O3 and SiO2 with a purity of 99.9%. Compositions of the initial reagents and the bulk compositions of the ECI and ECII systems are given in Table 2, and the Raman spectra of the initial carbonates are shown in Figure 3. The proportions of the initial carbonates were selected according to the cationic composition of garnets in model carbonated eclogites of types I and II (Table 1) [71]. The weight proportions were selected stoichiometrically according to the full completion of decarbonation reaction 3(Mg,Fe,Ca,Mn)CO3 + 3SiO2 + Al2O3 = (Mg,Fe,Ca,Mn)3Al2Si3O12 + 3CO2. It should be noted that both initial oxides and carbonates are known to be minor or accessory phases in natural carbonated eclogites. In previous experimental research devoted to the reconstruction of decarbonation reactions resulting in the garnet + CO2 formation, carbonate + SiO2 + Al2O3 or carbonate + kyanite were used as starting materials. As evident from our previous experiments [65,66,67] and from pioneer research [42], the SiO2 + Al2O3 assemblage is not stable under high pressures and high temperatures. SiO2 reacts with Al2O3 to form kyanite. Thus, the most probable real reaction under natural conditions will be carbonate + kyanite = garnet + CO2.

Table 2.
Compositions of initial carbonates and bulk compositions of (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 systems, modeling the formation of ECI (S-ECI) and ECII (S-ECII) garnet via decarbonation.
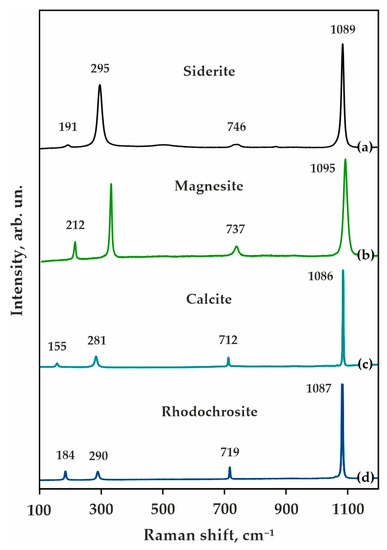
Figure 3.
Raman spectra of initial carbonates-natural Mg-siderite (Fe0.610Mg0.357Mn0.010Ca0.024)CO3 (a), magnesite Mg0.932Ca0.060Fe0.008CO3 (b), calcite (Ca0.996Mn0.003Fe0.001)CO3 (c), and rhodochrosite (Mn0.918Fe0.011Mg0.008Ca0.063)CO3 (d).
The initial reagents were ground into a fine powder using a tungsten carbide mortar under a layer of alcohol and thoroughly mixed, after which powders were dried at a temperature of 200 °C for at least 24 h. The exception was rhodochrosite, which was dried and stored in a vacuum oven. The reason for the different procedure for rhodochrosite is its thermal decomposition is at about 200 °C under atmospheric pressure. The weighed portions of the reaction powders were measured with an accuracy of ±0.1 mg.
Taking into account our previous experience of the studies on carbonate-oxide systems under high pressures and temperatures [65,66,67,85,86,87], platinum was chosen as the capsule material. The capsule volumes were selected according to the sizes of the high-pressure cell and to ensure the possibility of a number of modern analytical studies for each sample. For experiments, the dried reaction mixtures were carefully pressed into platinum capsules, which were then sealed by microarc welding. The internal diameter of the Pt capsules for experiments at 3.0 and 6.3 GPa was 1.5 mm at a length of 6 mm, and at 7.5 GPa, 1.5 mm at a length of 4 mm.
2.2. Control of Fluid Composition and Redox Conditions during Experiments
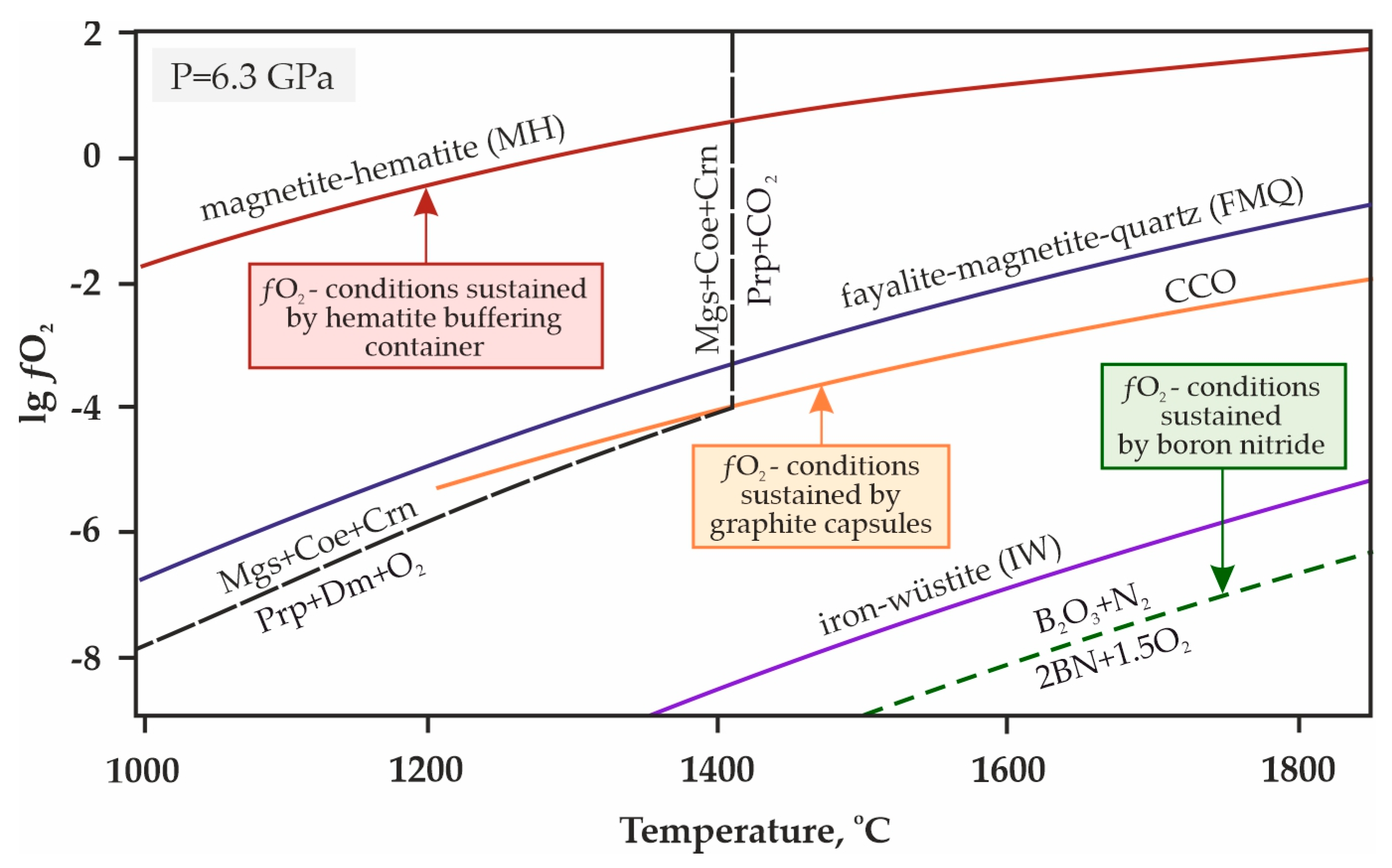
Taking into account the specifics of experiments in CO2-containing media, special attention should be paid to methodological aspects aimed at maintaining a stable fluid composition. In the experimental petrological studies, the phenomenon of hydrogen diffusion into platinum capsules at high pressures and temperatures is well known [88,89]. In this work, to prevent hydrogen diffusion into platinum capsules and a corresponding decrease of oxygen fugacity in the reaction volume, we used a high-pressure cell with an external hematite buffering container [84,90]. This technique makes it possible to maintain the ƒO2 values in a high pressure cell at the level of the magnetite/hematite (MH) buffer (Figure 4), and thus preserves the extremely low fugacity of hydrogen inside the buffering container, which ensures the minimum concentration of H2O in the fluid (no more than 0.1 mol. % [90]). The effective working time of this hematite buffering container at temperatures below 1200 °C is at least 150 h, and at 1500 °C, it is about 5 h [84].
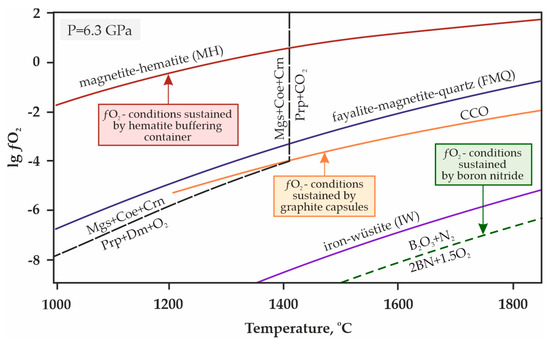
Figure 4.
T-ƒO2 diagram with buffer equilibria lines [91,92,93] and the decarbonation reaction [85]. Magnetite-hematite (MH), fayalite-magnetite-quartz (FMQ), iron-wüstite (IW), CCO—buffer equilibria; Ms—magnesite, Coe—coesite, Crn—corundum, Prp—pyrope, Mgt—magnetite, Dm—diamond.
When one performs unbuffered high pressure high temperature experiments, there is constant hydrogen diffusion through the high-pressure cell and the capsule materials throughout the run’s duration, and the hydrogen content in the fluid cannot be controlled. An influx of hydrogen (which is a highly reduced fluid component) results in a decrease of ƒO2 values. Considering the constant hydrogen diffusion through capsule materials throughout the run duration, the fluid composition will be shifted from pure CO2 to CO2-H2O. As it was shown in experimental studies (e.g., [42]), carbonation reactions in the presence of water is shifted far into the lower temperature region. Namely, for pyrope garnet and fluid composition of 20% CO2 × 80% H2O, this reaction shift is 300 °C at 7 GPa (relative to the parameters of pyrope carbonation reaction with 100% CO2). Thus, we believe it is crucial to control fluid composition and ƒO2 values to constrain the adequate position of decarbonation reaction curves.
The phase compositions of the buffer containers after the completion of the experiments were analyzed by powder X-ray diffraction. In the entire temperature range of 1050–1450 °C, these containers were composed only of hematite and magnetite. For the studied systems, a control analysis of the resulting fluid was carried out after experiments in the pressure range of 3.0–7.5 GPa and temperatures of 1050–1450 °C using mass spectrometry (see for details Section 2.3). The composition of the fluid corresponds to pure CO2, which confirms the efficiency of the buffer containers.
It should be noted that in previous works on the experimental modeling of decarbonation reactions with the formation of garnet by R. Knohe et al. [42] as well as Yu. Vinogradova et al. [68,69], either buffering was not used, or details made from boron nitride were present in the assembly of the experimental setup. Both the uncontrolled diffusion of hydrogen in the absence of buffering and the presence of BN parts reduce the oxygen fugacity in the high pressure cell (Figure 4), and thus, despite all the care taken in the preparation of these experiments, the reliability of the results obtained is rather controversial.
2.3. Analytical Methods
Analytical studies of the obtained samples were carried out at the Analytical Center for multi-elemental and isotope research SB RAS and at the Institute of Geology and Mineralogy Siberian Branch of the Russian Academy of Sciences. The obtained samples were studied using a number of modern methods, including optical and scanning electron microscopy, energy dispersive spectroscopy, elemental mapping, X-ray phase analysis, Raman spectroscopy, and mass spectrometry.
After the experiments, platinum capsules with samples were cut into two parts, one of which was impregnated with warm (42 °C) epoxy resin under reduced pressure. After polymerization of the resin, polished sections were prepared. The compositions of the starting materials and resulting phases, as well as the phase relationships in the samples, were studied on a MIRA 3LMU scanning electron microscope (TESCAN, Brno, Czech Republic) combined with an INCA Energy 450 energy-dispersive X-ray microanalyzer (Oxfords Instruments, High Wycombe, UK). The analysis was carried out at an accelerating voltage of 20 kV, a probe current of 1.6 nA, an exposure time of 20–30 s, and an electron beam diameter of 3 μm. Additionally, the chemical analysis of the obtained garnets and the recording of element distribution maps were carried out on a JXA-8100 microprobe X-ray spectral microanalyzer (manufactured by JEOL Ltd., Tokyo, Japan). The analyses were carried out at an accelerating voltage of 20 kV, a probe current of 20 nA, a counting time of 20 s, and an electron beam diameter of 2–3 μm. Pyrope-O-145 (SiO2, FeO), ferruginous spessartine (MnO), albite (Al2O3), and diopside (MgO, CaO) were used as standards.
The composition of the fluid phase was qualitatively determined by mass spectrometry. After each experiment, the platinum capsule was placed in a vacuum device connected to the sample injection system in the Delta V Advantage mass spectrometer (produced by Thermo Fisher Scientific, Bremen, Germany) and equipped with a special mechanism for piercing samples. After preliminary vacuuming of the device with the sample to a pressure of 2.7 × 10–2 mbar, which guarantees the absence of atmospheric gases in the device, the capsule was pierced, and the gas released at room temperature was injected into the analyzer of the mass spectrometer.
The structural features of the initial carbonates and the synthesized phases were studied using Raman spectroscopy on a Jobin Yvon LabRAM HR800 spectrometer (manufactured by Horiba, Tokyo, Japan) equipped with an Olympus BX41 stereomicroscope (manufactured by Olympus, Tokyo, Japan). The excitation source was a Torus diode-pumped solid-state laser with a wavelength of 532 nm (manufactured by Laser Quantum, Stockport, UK). The spectra were collected with a spectral resolution of 2 cm−1. Calibration was carried out using the emission lines of a neon discharge lamp at 540.06 nm and 585.25 nm. The obtained spectra were interpreted using the RRUFF database (www.rruff.info, accessed on 15 January 2023); the spectra were processed (noise elimination, baseline correction, peak identification) using the OPUS software version 5.5.
The contents of Fe, Mg, Mn, and Ca in the initial carbonates were analyzed with atomic absorption spectrometry using flame atomization (acetylene-air flame and nitrous oxide-acetylene) on a Solaar M6 spectrometer (produced by Thermo Scientific, Waltham, MA, USA) equipped with Zeeman and deuterium background correctors (See Supplementary Materials for details). The phase composition of the buffer containers after the experiments was analyzed using powder X-ray diffraction on a DRON-8 diffractometer (produced by Burevestnik, Saint Petersburg, Russia) with CuKα radiation.
3. Results
3.1. Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of ECI Garnets
The experimental conditions and results are given in Table 3 and Figure 5. At a pressure of 3.0 GPa and a temperature of 1050 °C, the formation of a polycrystalline aggregate of recrystallized magnesite, magnesiosiderite, coesite, corundum, as well as newly formed dolomite and kyanite was established. Corundum and kyanite form specific zonal rounded aggregates up to 100 µm in diameter (corundum is in the center). The resulting recrystallized phases show some variations in composition relative to the starting materials (Table 4). In particular, for magnesiosiderite, there was a decrease in the concentrations of CaO by 0.8 wt.% and FeO by 1 wt.%; for magnesite, there was an increase in MgO content by ~4.5 wt.% and a decrease in CaO concentration by ~3.3 wt.%. In the newly formed kyanite, impurities of FeO (up to 2.7 wt.%), CaO, and MgO (up to 1 wt.%) were found.

Table 3.
The experimental conditions and results in (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 systems.
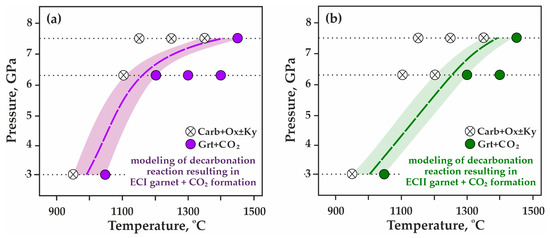
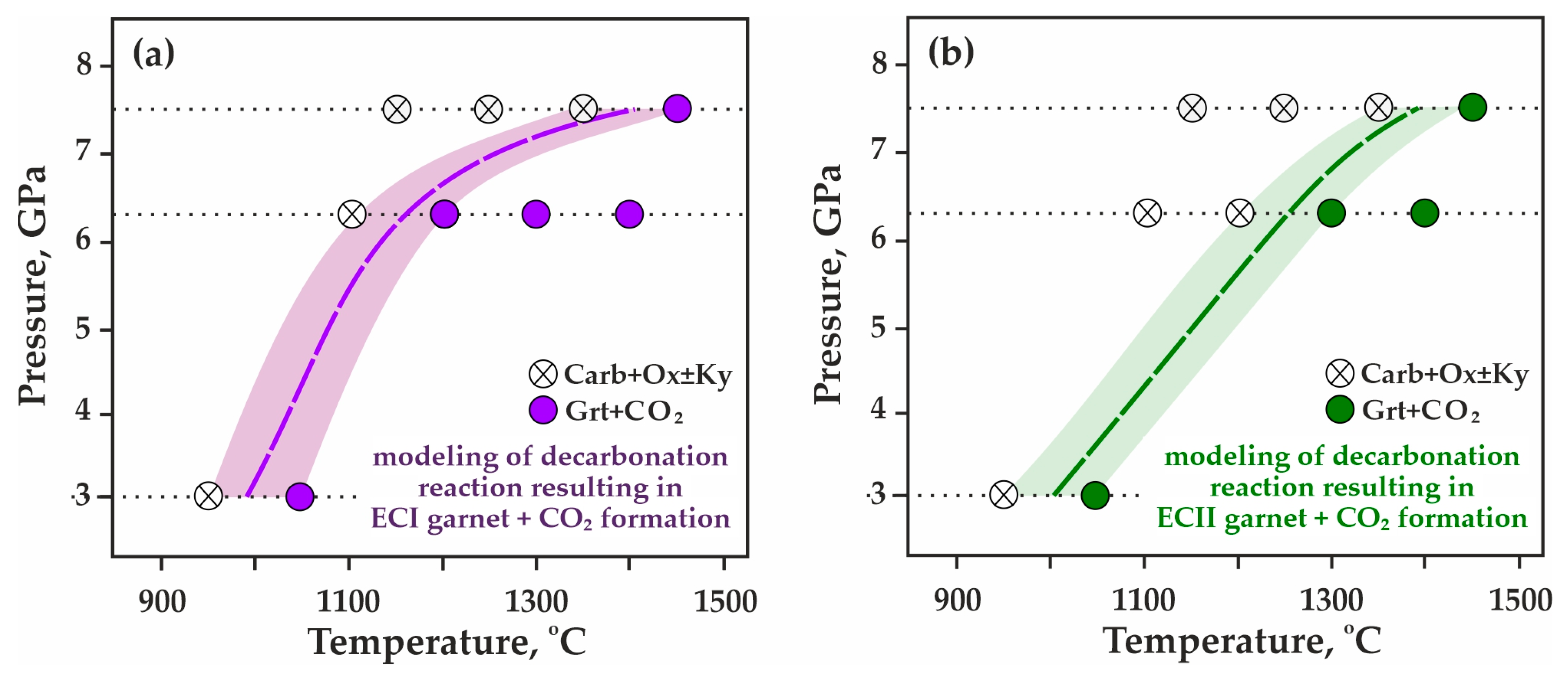
Figure 5.
P,T-diagrams showing the formation of Grt + CO2 from the breakdown of Carb + Ox ± Ky based on experimental results: (a) model ECI system, garnet compositions Prp48Alm35Grs15Sps02–Prp44Alm40Grs14Sps02, (b) model ECII system, garnet compositions Prp57Alm34Grs08Sps01–Prp68Alm23Grs08Sps01.

Table 4.
Compositions of mineral phases after experiments of S-ECI in the (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 system at the pressure range from 3.0–6.3 GPa.
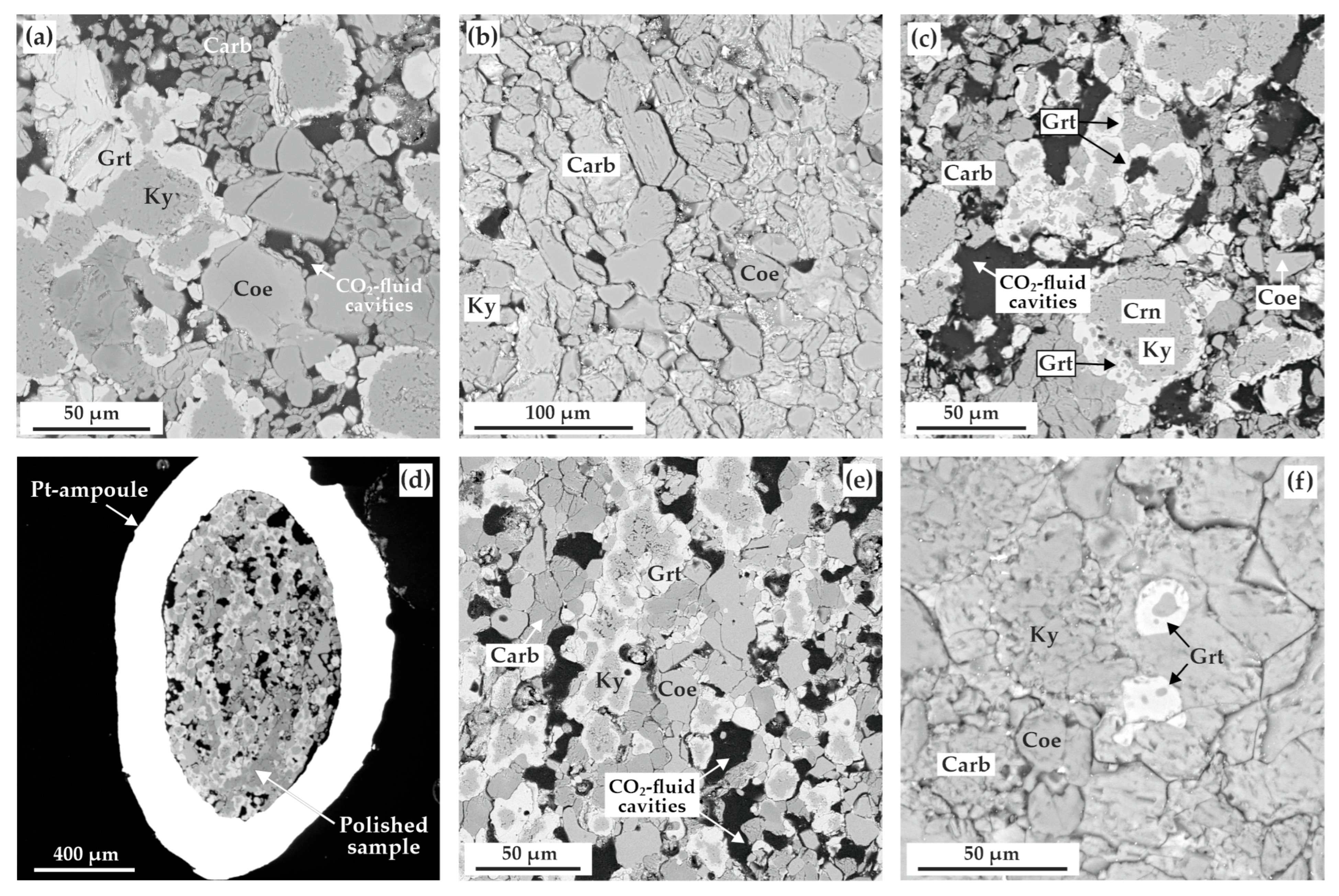
At a higher temperature of 1150 °C, there was garnet formation in association with kyanite, ferromagnesite and ferrodolomite; recrystallized coesite, magnesite, magnesiosiderite, and corundum also occurred. The obtained sample contains a large number of fluid cavities formed by segregated CO2 fluid as a result of decarbonation reactions. This interpretation of rounded cavities as “fluid cavities” or “fluid bubbles” is based on the data that CO2 fluid has high wetting angles, which results in formation of round, almost spherical segregations in host mineral aggregates under mantle pressures and temperatures. It has been established that garnet is predominantly present in zonal aggregates, with the central part consisting of corundum, surrounded by kyanite and covered, in turn, with garnet rims (the latter up to 30 µm thick) (Figure 6a).
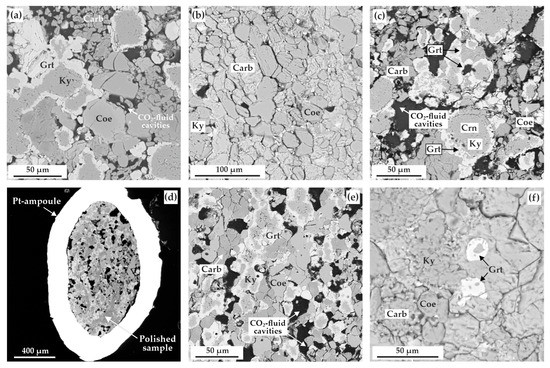
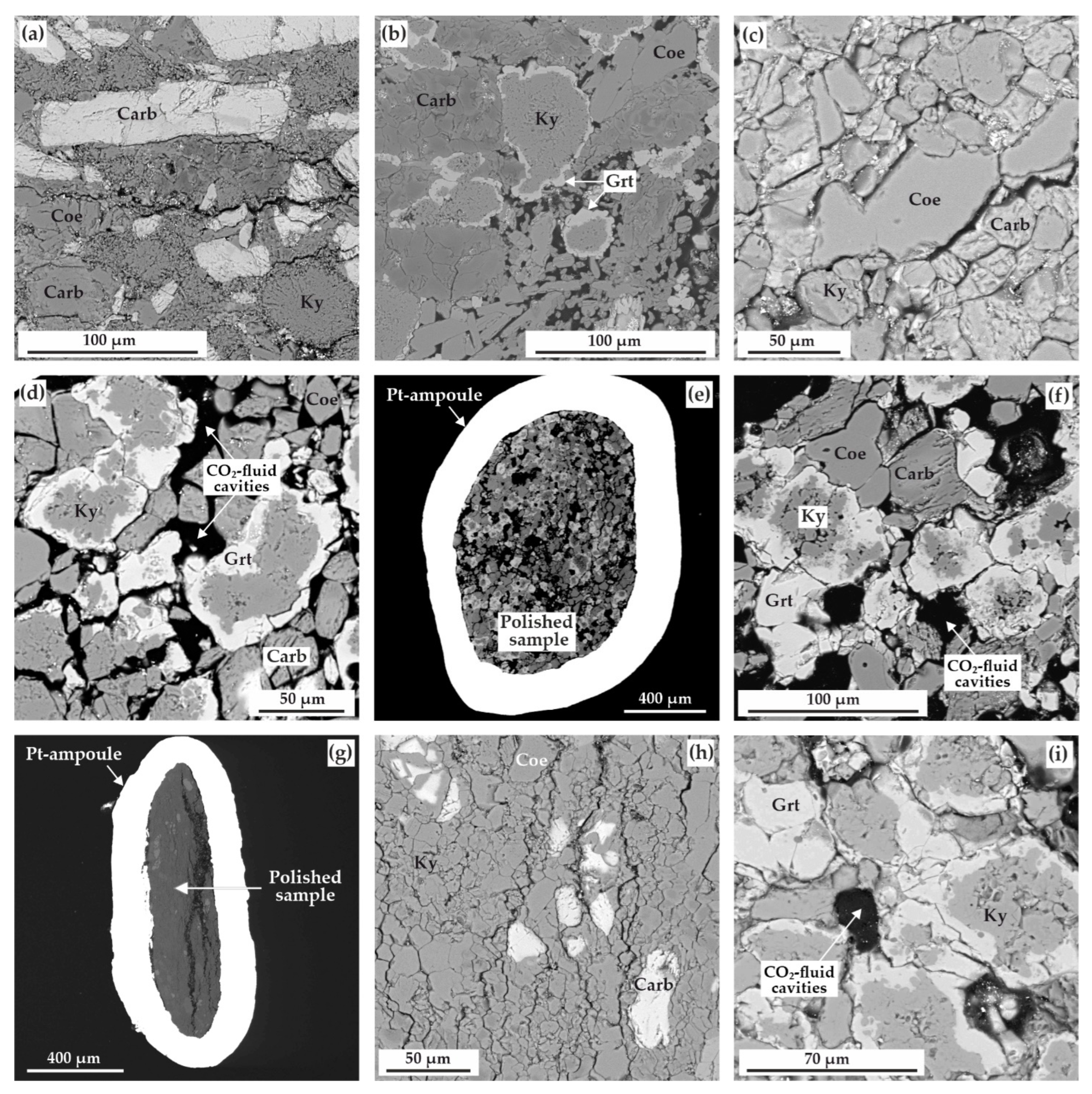
Figure 6.
SEM-micrographs (BSE regime) of polished sample fragments, after experiments in the ECI system: (a) zoned aggregates of kyanite and garnet as well as CO2-fluid cavities in the carbonate + coesite matrix (N 2122-I, 3.0 GPa, 1150 °C); (b) polycrystalline aggregate of magnesiosiderite, ferromagnesite, coesite, and kyanite (N 2117-I, 6.3 GPa, 1100 °C); (c) zoned aggregates of kyanite and garnet, CO2-fluid cavities, as well as minor ferromagnesite and coesite crystals (N 2115-I, 6.3 GPa, 1300 °C); (d) section of Pt-capsule with a sample and fluid cavities therein (N 2113-II, 6.3 GPa, 1400 °C); (e) polycrystalline aggregate of garnet, kyanite, carbonate, and coesite as well as CO2-fluid cavities therein (N 2113-I, 6.3 GPa, 1400 °C); (f) garnet crystals in the polycrystalline aggregate of kyanite, coesite, and carbonate (N 2144-I, 7.5 GPa, 1450 °C); Carb—carbonate (magnesiosiderite or ferromagnesite), Coe—coesite, Ky—kyanite, Grt—garnet.
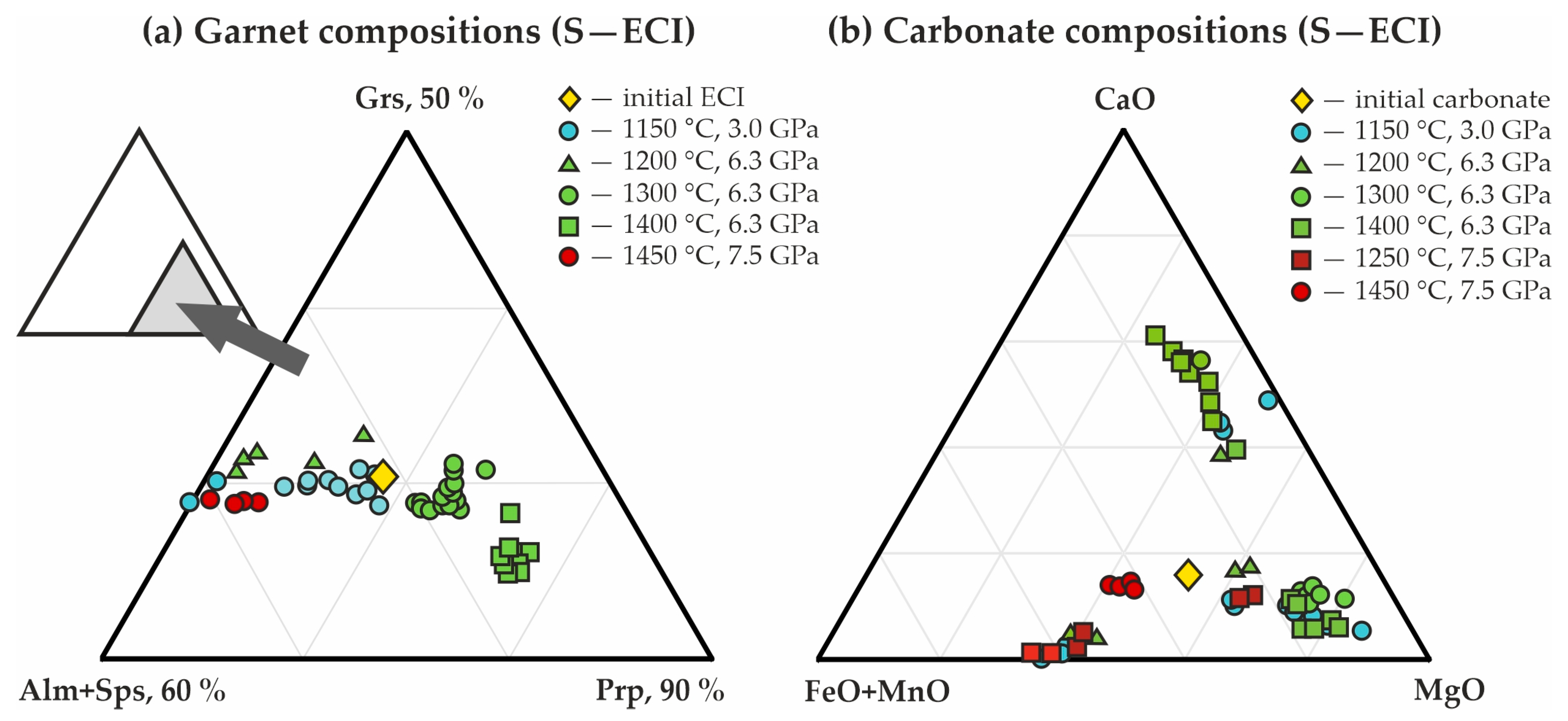
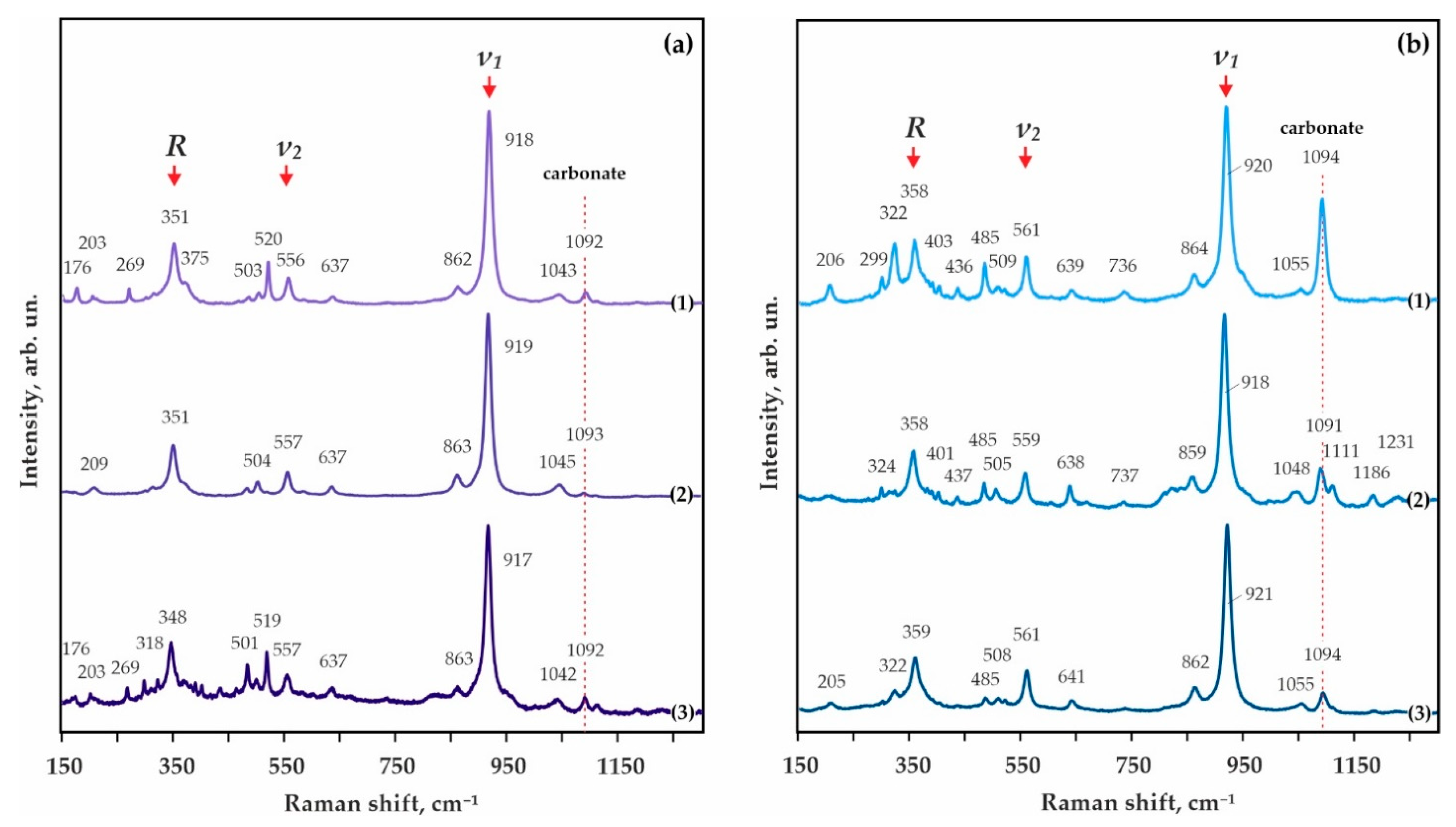
The resulting garnet corresponds to a pyrope-almandine-grossular with a composition varying from (Fe0.93Mg1.60Ca0.49Mn0.04)Al1.96Si3O12 to (Fe1.35Mg1.23Ca0.42Mn0.07)Al1.93Si3O12 (Table 4 and Figure 7a). Raman characteristics of the synthesized garnet are shown in Figure 8a. The main Raman modes of garnet are 351 cm−1 (librational R(SiO4)4−), 556 cm−1 (internal bending (Si-O)bend, υ2), and 918 cm−1 (stretching (Si-O)str, υ1). Secondary modes are at 176, 203, 269, 375, 503, 556, 637, 862, and 1043 cm–1 as characteristic bands for pyrope-almandine-grossular garnet formed as a result of decarbonation reactions.
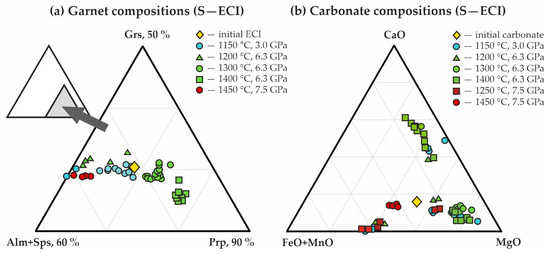
Figure 7.
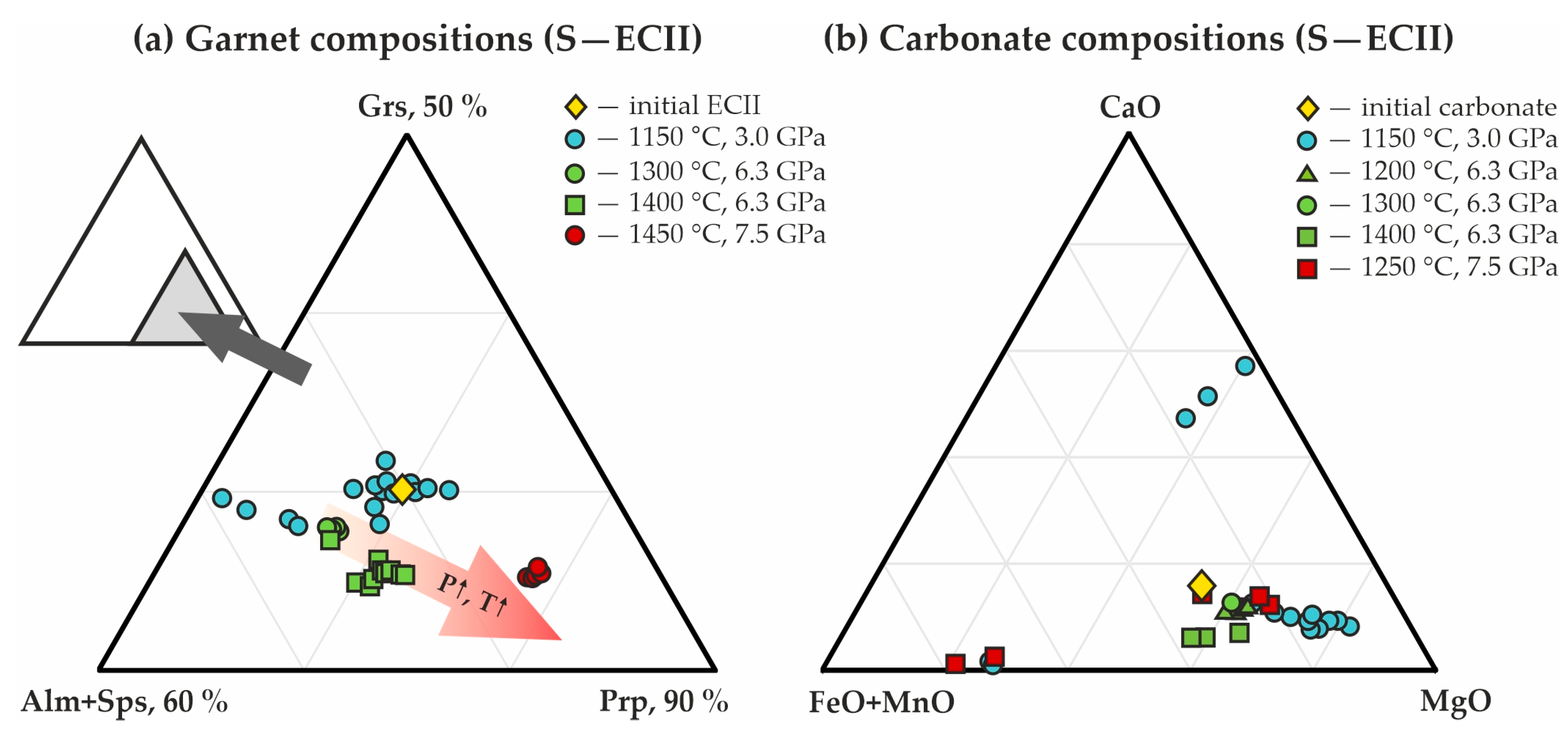
Triangle diagrams of the chemical compositions of (a) garnets, and (b) carbonates, synthesized in the S-ECI system.
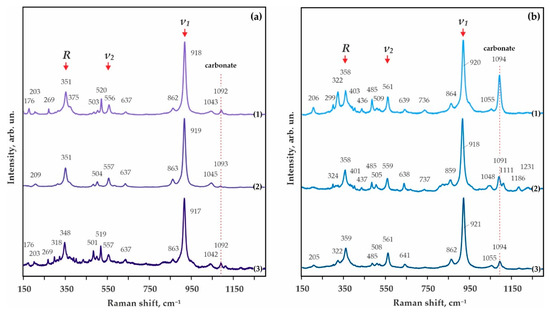
Figure 8.
Raman spectra of the synthesized pyrope-almandine-grossular garnets: (a) S-ECI: 1-N 2122-I, 3.0 GPa, 1150 °C, 2-N 2115-I, 6.3 GPa, 1300 °C, 3-N 2144-I, 7.5 GPa, 1450 °C; (b) S-ECII: 1-N 2122-II, 3.0 GPa, 1150 °C, 2–N 2113-II, 6.3 GPa, 1400 °C, 3-N 2140-II, 7.5 GPa, 1450 °C.
Kyanite composition is characterized by the presence of FeO, CaO, and MgO impurities (0.06, 0.03, and 0.05 formula units, respectively); compositions of newly formed carbonates correspond to the formulas Fe0.08-0.26Mg0.63-0.84Ca0.05-0.11Mn0.01CO3 (ferromagnesite) and Ca0.86Mg0.90Fe0.22Mn0.02(CO3)2 (ferrodolomite) (Table 4 and Figure 7b).
At the pressure of 6.3 GPa and a temperature of 1100 °C, the formation of a polycrystalline aggregate of newly formed kyanite (Al2.02(Si0.97,Fe0.01)O5) and ferromagnesite (Fe0.26Mg1.01Ca0.74Mn0.02CO3), as well as recrystallized magnesiosiderite (Fe0.52Mg0.38Ca0.04Mn0.01CO3), coesite, and corundum (Figure 6b and Table 4) takes place.
At higher temperatures (1200–1400 °C), garnet formation (Figure 6c), in association with kyanite, ferromagnesite, and recrystallized magnesiosiderite (±corundum and coesite), was found to occur.
Corundum, kyanite, and garnet form rounded zonal aggregates (Figure 6c), the central part of which is comprised of kyanite and corundum (crystal size < 5 µm), while garnet occurs as rims up to 30 µm thick. In the samples, there are a large quantity of CO2 fluid cavities (Figure 6c–e), the number of which increases with increasing temperature of the experiments. The resulting garnet composition varies from Fe0.89-1.17Mg1.29-1.52Ca0.53-0.59Mn0.05-0.07Al1.96Si3O12 (1200 °C) to Fe0.68Mg1.95Ca0.39Mn0.04Al1.92Si3O12 (1400 °C) (Figure 7a). The features of the garnet Raman spectra are shown in Figure 8a. They are characterized with 351, 557, and 919 cm−1 as the main Raman modes. The second-order lines are generally similar to those found in the spectra for the pyrope-almandine-grossular synthesized at 3.0 GPa; however, small shifts in the positions of the Raman bands by 1–2 cm–1 and the absence of several low and middle frequency bands—176, 269, 375, 520 cm−1—are noted. In the range of 1200–1400 °C, the composition of kyanite includes significant FeO up to 0.9 wt.%, and the compositions of carbonates correspond to the formulas Fe0.15-0.26Mg0.63-0.74Ca0.06-0.12Mn0.01CO3 (newly formed ferromagnesite) and Fe0.62Mg0.35Mn0.01Ca0.02CO3 (recrystallized magnesiosiderite) (Figure 7b and Table 4).
At a pressure of 7.5 GPa in the temperature range from 1150–1350 °C, the formation of a polycrystalline aggregate of kyanite (Al1.97-2.02(Si0.97-1.01,Fe0.02-0.04)O5) and Fe,Mg,Ca-carbonates (± coesite and corundum) (Table 5 and Figure 7b) occurs. At a higher temperature (1450 °C), garnet crystallizes in association with kyanite (Al2.02(Si0.97,Fe0.04)O5), ferromagnesite (Fe0.25Mg0.64Ca0.10Mn0.01CO3), and coesite (Figure 6f). CO2-fluid cavities are present in the sample. The resulting garnet composition corresponds to pyrope-almandine-grossular Fe1.22Mg1.34Ca0.42Mn0.07Al1.96Si3O12. According to the Raman characteristics, the garnet synthesized at 7.5 GPa is quite close to the garnet obtained at a pressure of 3.0 GPa (Figure 8a). The first-order modes are 348, 557, and 917 cm−1, and the second order bands are 176, 203, 269, 501, 519, 637, 863, and 1042 cm−1. It is also interesting to note the presence of a second-order band at 318 cm−1, which is not found in the spectra of garnets obtained at lower pressures.

Table 5.
Compositions of mineral phases after experiments of S-ECI in the (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 system at the pressure of 7.5 GPa.
3.2. Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of ECII Garnets
At a pressure of 3.0 GPa and 1050 °C, crystallization of a dense polycrystalline aggregate of newly formed kyanite (Al2.02(Si0.97,Fe0.01)O5) and ferromagnesite (Fe0.10Mg0.89Ca0.06CO3), as well as recrystallized coesite and magnesiosiderite (Fe0.60Mg0.41Ca0.01Mn0.01CO3) (Figure 9a and Table 6), occurs. At a higher temperature (1150 °C), the formation of garnet coexisting with kyanite (Al2.09(Si0.92,Fe0.02)O5), coesite, and carbonates of various compositions (magnesiosiderite, ferromagnesite and dolomite) was established in the system (Figure 9b and Table 6). Kyanite and garnet present as rounded zonal aggregates (20–100 µm), with kyanite in the center (crystals < 5 µm), and a dense garnet aggregate in the periphery (rims up to 15 µm thick). Throughout the sample, there are a large quantity of cavities formed by the CO2 fluid, which was segregated during the experiment. The composition of the resulting garnet corresponds to the formula Fe0.78-1.14Mg1.48-1.77Ca0.37-0.46Mn0.04-0.06Al1.95-1.98Si3O12 (Figure 10a and Table 6). The Raman characteristics of the synthesized garnet are shown in Figure 8b. Its main Raman bands are 358 cm−1 (librational R(SiO4)4-), 561 cm−1 (internal bending (Si-O)bend, υ2), and 920 cm−1 (stretching (Si-O)str, υ1). Secondary modes were recorded at 206, 299, 322, 403, 436, 485, 509, 639, 864, and 1055 cm–1 as characteristic bands for the pyrope-almandine-grossular garnet.
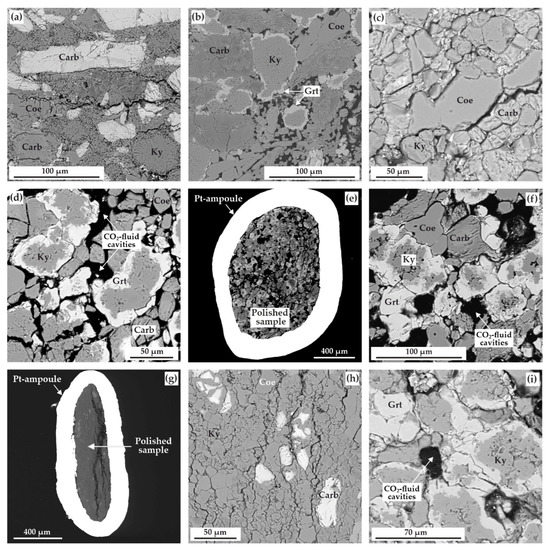
Figure 9.
SEM-micrographs (BSE regime) of polished sample fragments, after experiments in the ECII system: (a) polycrystalline aggregate of carbonates (magnesiosiderite and ferromagnesite), coesite, and kyanite (N 1738-II, 3.0 GPa, 1050 °C); (b) zoned aggregates of kyanite and garnet in a carbonate+coesite polycrystalline matrix (N 2122-II, 3.0 GPa, 1150 °C); (c) polycrystalline aggregate of magnesiosiderite, coesite, and kyanite (N 2119-II, 6.3 GPa, 1200 °C); (d) zoned aggregates of kyanite and garnet as well as CO2-fluid cavities in a ferromagnesite+coesite polycrystalline matrix (N 2115-II, 6.3 GPa, 1300 °C); (e) section of Pt-capsule with a sample and fluid cavities therein (N 2113-II, 6.3 GPa, 1400 °C); (f) zoned aggregates of kyanite and garnet as well as CO2-fluid cavities in a carbonate+coesite matrix (N 2113-II, 6.3 GPa, 1400 °C); (g) section of Pt-capsule with a sample (N 2135-II, 7.5 GPa, 1250 °C); (h) polycrystalline aggregate of kyanite with minor quantities of coesite and magnesiosiderite (N 2135-II, 7.5 GPa, 1250 °C); (i) zoned aggregates of kyanite and garnet as well as CO2-fluid cavities (N 2140-II, 7.5 GPa, 1450 °C); Carb—carbonate (magnesiosiderite or ferromagnesite), Coe—coesite, Ky—kyanite, Grt—garnet.

Table 6.
Phase compositions after experiments of S-ECII in the (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 system.
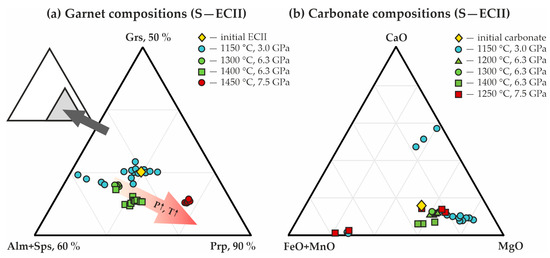
Figure 10.
Triangle diagrams of the chemical compositions of garnets (a) and carbonates (b), synthesized in the S-ECII system.
At a pressure of 6.3 GPa, in the temperature range of 1100–1200 °C, the formation of kyanite (Al1.99(Si1.00,Fe0.01)O5), coesite, magnesiosiderite (Fe0.58Mg0.38–0.40Ca0.01Mn0.01CO3), and ferromagnesite (Fe0.14–0.17Mg0.72–0.75Ca0.09–0.10Mn0.01CO3) was established (Figure 9c and Figure 10b, Table 6). At higher temperatures (1300 and 1400 °C), garnet crystallized in association with kyanite, coesite, and ferromagnesite (Fe0.17–0.23Mg0.70–0.73Ca0.05–0.10Mn0.01CO3); additionally, a large amount of fluid cavities were formed (Figure 9d–f). Silicates formed rounded zonal aggregates (50–150 µm), with kyanite in the center and garnet in the periphery (rims up to 30 µm thick) (Figure 9d,f). Garnets obtained in this temperature range practically do not show variations in iron content, and their composition corresponds to the formula Fe1.09–1.10Mg1.57–1.75Ca0.22–0.37Mn0.05-0.06Al1.90–1.94Si3O12 (Figure 10a, Table 6). According to the Raman characteristics, the synthesized garnet is quite close to the garnet obtained at a lower pressure of 3.0 GPa (Figure 8b). The main Raman bands are at 358, 559, and 918 cm−1, and the second-order lines are at 324, 401, 437, 485, 505, 638, 859, and 1048 cm−1.
At a pressure of 7.5 GPa and in the temperature range of 1250–1350 °C, the formation of a polycrystalline aggregate of kyanite (Al2.12(Si0.89,Fe0.03)O5), coesite, magnesiosiderite (Fe0.58Mg0.41Ca0.02–0.04Mn0.01CO3), and ferromagnesite (Fe0.13–0.23Mg0.59–0.77Ca0.05–0.12Mn0.01CO3) (Figure 9g,h and Figure 10b, Table 6) takes place.
As the temperature rises to 1450 °C, garnet crystallizes in the system, coexisting with kyanite (Al2.03(Si0.96,Fe0.03)O5), carbonate, and coesite; in this case, a large number of cavities formed by the CO2 fluid are observed in the sample. The composition of the resulting garnet corresponds to the Fe0.71Mg2.10Ca0.25Mn0.04Al1.91Si3O12 formula (Figure 10a). Characteristics of the Raman spectra of garnet are shown in Figure 8b. It was found that the main bands are at 359, 561, and 921 cm−1. The set of second-order lines generally coincides with the spectra for garnet synthesized at 3.0 GPa; however, there are shifts in the position of the Raman peaks by 1–3 cm−1.
4. Discussion and Conclusions
The carbonated eclogite is suggested to be derived from altered oceanic crustal material, recycled back into the mantle by subduction, and stored for billions of years in the mantle before incorporation into the carbonatites source regions. According to the thermal models of Earth’s interior, the experimentally studied reactions can happen in the mantle wedge, close to a downgoing slab. Redox conditions of the experiments corresponded to the oxygen fugacity values of the magnetite/hematite buffer, which are of about FMQ + 2.5 log units. At mantle depths, the oxygen fugacity in fluids and melts formed in the slab and migrating in the mantle wedge can reach very high values, up to FMQ + 5 log units [7,14]. Thus, the redox conditions of ƒO2 values of about FMQ + 2.5 log units do not contradict the tectonic environment in the mantle wedge, close to the downgoing slab.
As a result of a detailed study of the phase and chemical compositions of the obtained samples, as well as the structure of zonal aggregates, the phase formation processes in carbonate-oxide (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 systems was illuminated. It has been experimentally established that at temperatures below the onset of decarbonation reactions, coesite and corundum interaction results in the formation of kyanite (over the entire pressure range of 3.0–7.5 GPa and temperatures of 1050–1450 °C). A similar process occurred in all our earlier experimental studies on the modeling of decarbonation reactions with the formation of CO2 fluid in association with pyrope, pyrope-almandine, pyrope-grossular, pyrope-almandine-grossular, and spessartine [65,66,67], as well as in the carbonate–oxide–sulfide system (MgCO3–SiO2–Al2O3–FeS system, 6.3 GPa, 1250–1450 °C) [94]. Synthesized kyanite contains FeO impurities (0.4–2.6 wt.%), which indicate exchange reactions of kyanite with magnesiosiderite—the only iron-bearing phase in the reaction volume of the capsules. It should be emphasized that at 3.0–6.3 GPa, and temperatures insufficient for the onset of decarbonation, magnesiosiderite and magnesite remain stable in the samples. These carbonates demonstrate variations in the Mg/Fe contents, and do not undergo phase transitions, which is confirmed by the results of Raman spectroscopy, and agrees with modern experimental data (Figure 1a). In the entire range of pressures and temperatures, the initial calcite and rhodochrosite are unstable; they are completely consumed in decarbonation reactions and exchange reactions with other carbonates. At 3.0 GPa, calcium from the initial calcite is redistributed into newly formed dolomite, and at higher pressures, into ferromagnesite and magnesiosiderite as CaO impurities with concentrations of 0.5–7.3 wt.%.
It has been established that at temperatures above the onset of decarbonation, a number of processes occur in the reaction volume: (1) crystallization of kyanite; (2) exchange reactions of carbonates and their partial recrystallization with compositional changes; (3) interactions of kyanite + coesite + Mg,Fe,Ca,Mn-carbonate and corundum + coesite + Mg,Fe,Ca,Mn-carbonate, leading to the crystallization of pyrope-almandine-grossular garnet and the formation of CO2 fluid. It should be noted that the methodical approach with an external hematite buffer limits the duration of the experiments; therefore, only partial rather than complete decarbonation occurs in the samples. In the case of complete decarbonation, the molar ratio of divalent cations in the initial carbonate and newly formed garnet will be completely the same. This study demonstrated that the ratio of Fe, Ca, Mg, and Mn in the synthesized garnets differs from the bulk composition of the initial carbonates (Figure 7 and Figure 10). However, it is the established features of partially realized decarbonation reactions that make it possible to reconstruct natural processes. The main patterns were a decrease in Ca# (Ca# = Ca/(Ca + Mg + Fe + Mn), mol.) and an increase in Fe# (Fe# = Fe/(Ca + Mg + Fe + Mn), mol.) in garnets relative to the concentrations of Ca and Fe in the initial carbonates, and a corresponding increase in Ca# and a decrease in Fe# in the resulting carbonates relative to the initial ones (Figure 7 and Figure 10, Table 4, Table 5 and Table 6). Similar patterns were established by us earlier in the experimental studies on decarbonation reactions involving ankerite in the Ca(Mg,Fe)(CO3)2-Al2O3-SiO2 system [67], and were described for model carbonated eclogites of I and II types [71]. In particular, it was shown in [71] that at a pressure of 5.0 GPa and a temperature of 1100 °C, ECI garnet contains 15.4 wt.% FeO and 9.2 wt.% CaO, while ECI carbonate contains 5.7 wt.% FeO and 32.7 wt.% CaO.
We have to discuss the significantly different compositions of garnets crystallized at 7.5 GPa and 1450 °C in the ECI system. We believe that the main reason for these different garnet compositions is the very first stage of the decarbonation reaction. On this stage of the decarbonation, garnet species are more Fe-rich, since the temperatures needed for the crystallization of almandine garnet are much lower than the ones needed for the pyrope formation. We suppose that for the ECI garnets, either a longer run duration or a slightly higher temperature is required to stand in line with P,T-arrays of Figure 7a.
Taking into account the existing theoretical and experimental data on the parameters of the decarbonation of Fe,Ca,Mg-carbonate-oxide associations [42,64,65,66,67], the lowest temperatures are required for the formation of almandine garnet, and the highest ones for grossular garnet. In particular, it was experimentally demonstrated that the dilution of pyrope with an almandine component causes a decrease in the temperature of decarbonation reactions, while the addition of a grossular component, on the contrary, increases it. Correspondingly, CO2-fluid and garnets with decreased Ca# and increased Fe# are formed at temperatures below those required for complete decarbonation of the Fe,Ca,Mg,Mn-carbonate-oxide association. This process is accompanied by a change in the composition of carbonates (an increase in Ca# and a decrease in Fe# relative to the initial ones), which expands the stability field of these carbonates without a significant change in their structure.
In the present study, for both ECI and ECII series, the compositions of carbonates after experiments are represented by three trends, and the largest of them corresponds to ferromagnesite (with variable CaO content). Magnesiosiderite and ferrodolomite are less pronounced (Figure 7b and Figure 10b). The compositions of garnets correspond to a single trend with constant proportions of (Alm + Sps)/Grs, and variable concentrations of the pyrope end-member (Figure 7a and Figure 10a). Moreover, an increase in the pyrope component in garnets with increasing pressure and temperature was established.
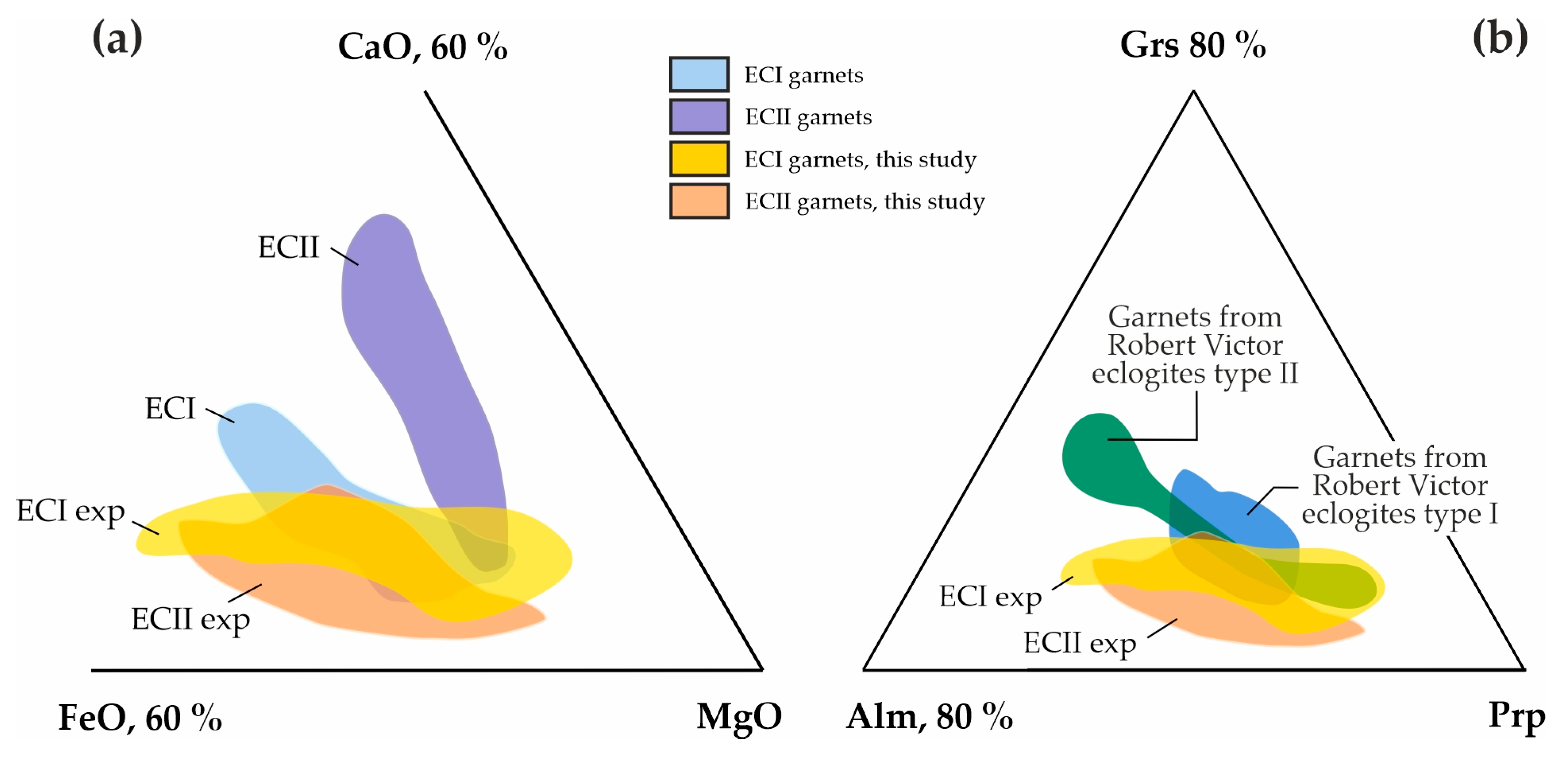
Figure S1 shows the compositional fields of natural type I and II carbonated eclogites [71,72,95,96,97], garnets from these eclogites [97,98,99], as well as bulk compositions of diamondiferous eclogites from the Robert Victor kimberlite pipe [77]. A comparison between the compositions of the garnets synthesized in the present study and natural garnets from carbonated eclogites (Figure 11a) shows high coincidence of the ECI composition fields, and partial coincidence of ECII fields (according to [79]). The composition fields of the garnets synthesized by us also demonstrate an ~50% overlap with garnet compositions from xenoliths of diamondiferous eclogites brought to the surface by the Robert Victor kimberlite pipe [77] (Figure 11b).
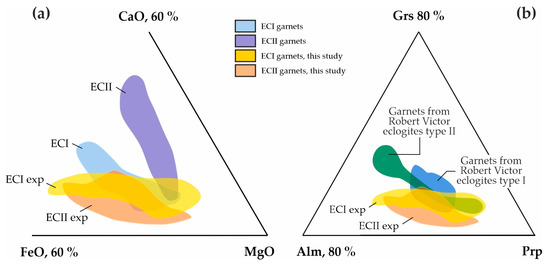
Figure 11.
Triangle diagrams of (a) the chemical compositions of natural garnets from carbonated eclogites of type I and II [79], in comparison with synthetic garnets from this study, and (b) garnets of natural diamondiferous eclogites of Robert Victor kimberlite mine [77], in comparison with synthetic garnets from this study.
In addition, it is interesting to note that when comparing the Raman characteristics of the garnets obtained in our study with data on inclusions in diamonds of various parageneses [100], the greatest similarity was found with garnets from E-type inclusions (eclogitic paragenesis). These data indicate that our studies to determine the parameters of decarbonation and assess the conditions of stability of garnets from carbonated eclogites in the presence of CO2 fluid can be considered relevant to a large extent for natural assemblages, including being used for the reconstruction of natural CO2/carbonate-related metasomatic processes associated with diamond formation. In addition, the applicability of the results obtained for the reconstruction of natural processes is also confirmed by information on inclusions of Ca,Mg,Fe-carbonates (dolomite, magnesiosiderite, ankerite) in natural diamonds from Mwadui, Tanzania [101]; Juina, Brazil [102]; and Kankan, Guinea [103].
As a result of a detailed study of the obtained experimental data, the position of the decarbonation reactions leading to the formation of CO2 fluid in association with model garnets ECI (Figure 5a) and ECII (Figure 5b) was reconstructed in the P,T field. It has been found that in the (Mg,Fe,Ca,Mn)CO3-SiO2-Al2O3 system simulating ECI compositions, the decarbonation temperatures at 3.0, 6.3, and 7.5 GPa are 1000, 1150, and 1400 °C (±20 °C), respectively. In the system simulating ECII compositions, the decarbonation temperatures at 3.0, 6.3, and 7.5 GPa are 1000, 1250, and 1400 °C (±20 °C), respectively. When transposing the experimental pressures to the natural conditions, one can suppose the correspondence of 3.0 GPa to the depth of ~90 km, 6.3 GPa to 190 km, and 7.5 GPa to ~225 km. Thus, the obtained results indicate that the lower temperature limit of stability of natural garnets from carbonated eclogites in the presence of CO2 fluid is 1000 °C at depths of ~90 km, 1150–1250 °C at 190 km, and 1400 °C at depths of about 225 km.
When comparing the obtained data with previous experimental results (Figure 2) [42,64,65,66,67], it was found that the decarbonation reaction curve designating the stability boundary of CO2 and model garnet ECI most closely matches the parameters of the magnesiosiderite + coesite + corundum = Prp30Alm70 + CO2 reaction (Figure 2a, reaction # 2). The decarbonation line resulting in the formation of model garnet ECII + CO2 association almost consists with the ankerite + coesite + corundum = Grs25Alm40Prp35 + CO2 reaction curve (Figure 2a, reaction # 3). Summarizing our systematic experimental studies, in which the reconstruction in the P,T field of seven decarbonation curves was associated with the formation of garnets of various compositions, one can build a consistent temperature series for the implementation of these reactions. At a constant pressure of 6.3 GPa and with increasing temperature, decarbonation will sequentially proceed in carbonate-oxide associations with the participation of rhodochrosite → magnesiosiderite → ECI carbonate → ECII carbonate → ankerite → magnesite → dolomite. Thus, these systematic experimental data on the modeling of decarbonation reactions with the formation of CO2 and garnets of various compositions, carried out under buffered conditions with strict control of fluid composition, provide reliable and crucial information in the framework of the complex problem of reconstructing fluid regime, the global carbon cycle, and CO2/carbonate-related metasomatism in the lithospheric mantle.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/min13070859/s1: List of reactions (1)–(16); Dissolution procedure for the carbonate preparation for the atomic absorption; Figure S1: Triangle diagrams of the chemical compositions of carbonated eclogites and garnets therein.
Author Contributions
Conceptualization, Y.V.B. and Y.N.P.; data curation, Y.V.B. and Y.N.P.; formal analysis, Y.V.B. and O.V.F.; investigation, Y.V.B., I.D.N. and O.V.F.; methodology, I.D.N. and A.N.K.; project administration, Y.V.B. and Y.N.P.; resources, Y.V.B. and Y.N.P.; software, A.N.K.; supervision, Y.N.P.; validation, Y.V.B. and Y.N.P.; visualization, Y.V.B.; writing—original draft, Y.V.B.; writing—review and editing, Y.V.B. and Y.N.P. All authors have read and agreed to the published version of the manuscript.
Funding
Work is done on state assignment of IGM SB RAS (No. 122041400159-3).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to Funder requirements.
Acknowledgments
The authors express their sincere thanks to Vadim N. Reutsky for their help in the implementation of mass spectrometry analyses, and to Yuri M. Borzdov and Alexander G. Sokol for scientific discussions at various stages of work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Luth, R.W. Carbon and carbonates in mantle. In Mantle Petrology: Field Observation and High Pressure Experimentation: A Tribute to Francis, R. (Joe) Boyd; Fei, Y., Bertka, M.C., Mysen, B.O., Eds.; The Geochemical Society: Washington, DC, USA, 1999; pp. 297–316. ISBN 0-941809-05-6. [Google Scholar]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Walter, M.J.; Kohn, S.C.; Araujo, D.; Bulanova, G.P.; Smith, C.B.; Gaillou, E.; Wang, J.; Steele, A.; Shirey, S.B. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science 2011, 334, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.A. The redox budget of subduction zones. Earth Sci. Rev. 2012, 113, 11–32. [Google Scholar] [CrossRef]
- Shirey, S.B.; Cartigny, P.; Frost, D.J.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the Geology of Mantle Carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef]
- Shirey, S.B.; Smit, K.V.; Pearson, D.G.; Walter, M.J.; Aulbach, S.; Brenker, F.E.; Bureau, H.; Burnham, A.D.; Cartigny, P.; Chacko, T.; et al. Diamonds and the mantle geodynamics of carbon: Deep Mantle Carbon Evolution from the Diamond Record. In Deep Carbon: Past to Present; Orcutt, B., Daniel, I., Dasgupta, R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 89–128. [Google Scholar]
- Stagno, V. Carbon, carbides, carbonates and carbonatitic melts in the Earth’s interior. J. Geol. Soc. 2019, 176, 375–387. [Google Scholar] [CrossRef]
- Bergman, S.C.; Dubessy, J. CO2-CO fluid inclusions in a composite peridotite xenolith: Implications for upper mantle oxygen fugacity. Contr. Mineral. Petrol. 1984, 85, 1–13. [Google Scholar] [CrossRef]
- Andersen, T.; Neumann, E.-R. Fluid inclusions in mantle xenoliths. Lithos 2001, 55, 301–320. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Andersen, T.; Neumann, E.-R.; Simonsen, S.L. Carbonatite melt–CO2 fluid inclusions in mantle xenoliths from Tenerife, Canary Islands: A story of trapping, immiscibility and fluid–rock interaction in the upper mantle. Lithos 2002, 64, 77–96. [Google Scholar] [CrossRef]
- Frezzotti, M.-L.; Peccerillo, A.; Panza, G. Carbonate metasomatism and CO2 lithosphere–asthenosphere degassing beneath the Western Mediterranean: An integrated model arising from petrological and geophysical data. Chem. Geol. 2009, 262, 102–108. [Google Scholar] [CrossRef]
- Berkesi, M.; Guzmics, T.; Szabó, C.; Dubessy, J.; Bodnar, R.J.; Hidas, K.; Ratter, K. The role of CO2-rich fluids in trace element transport and metasomatism in the lithospheric mantle beneath the Central Pannonian Basin, Hungary, based on fluid inclusions in mantle xenoliths. Earth Planet. Sci. Lett. 2012, 331–332, 8–20. [Google Scholar] [CrossRef]
- Kawamoto, T.; Yoshikawa, M.; Kumagai, M.Y.; Mirabueno, H.T.; Okuno, M.; Kobayashi, T. Mantle wedge infiltrated with saline fluids from dehydration and decarbonation of subducting slab. Proc. Natl. Acad. Sci. USA 2013, 110, 9663–9668. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, M.-L.; Touret, J.L.R. CO2, carbonate-rich melts, and brines in the mantle. Geosci. Front. 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Elazar, O.; Kessel, R.; Huang, J.-X.; Marquardt, K.; Navon, O. Silicic microinclusions in a metasomatized eclogite from Roberts Victor mine, South Africa. Lithos 2021, 388, 106057. [Google Scholar] [CrossRef]
- Navon, O. High internal pressures in diamond fluid inclusions determined by infrared absorption. Nature 1991, 353, 746–748. [Google Scholar] [CrossRef]
- Guthrie, G.D.; Veblen, D.R.; Navon, O.; Rossman, G.R. Submicrometer fluid inclusions in turbid-diamond coats. Earth Planet. Sci. Lett. 1991, 105, 1–12. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Ragozin, A.L.; Shatskii, V.S.; Shebanin, A.P. Variation in the fluid phase composition in the process of natural diamond crystallization. Dokl. Earth Sci. 2001, 379, 571–574. [Google Scholar]
- Tomlinson, E.L.; Jones, A.P.; Harris, J.W. Co-existing fluid and silicate inclusions in mantle diamond. Earth Planet. Sci. Lett. 2006, 250, 581–595. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Smith, E.M.; Kopylova, M.G.; Frezzotti, M.L.; Afanasiev, V.P. Fluid inclusions in Ebelyakh diamonds: Evidence of CO2 liberation in eclogite and the effect of H2O on diamond habit. Lithos 2015, 216, 106–117. [Google Scholar] [CrossRef]
- Weiss, Y.; Czas, J.; Navon, O. Fluid Inclusions in Fibrous Diamonds. Rev. Mineral. Geochem. 2022, 88, 475–532. [Google Scholar] [CrossRef]
- Scambelluri, M.; Philippot, P. Deep fluids in subduction zones. Lithos 2001, 55, 213–227. [Google Scholar] [CrossRef]
- Kang, N.; Schmidt, M.W.; Poli, S.; Franzolin, E.; Connolly, J.A.D. Melting of siderite to 20 GPa and thermodynamic properties of FeCO3-melt. Chem. Geol. 2015, 400, 34–43. [Google Scholar] [CrossRef]
- Tao, R.; Fei, Y.; Zhang, L. Experimental determination of siderite stability at high pressure. Am. Miner. 2013, 98, 1565–1572. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Lange, R.; Liu, J.; Militzer, B. Determination of calcium carbonate and sodium carbonate melting curves up to Earth’s transition zone pressures with implications for the deep carbon cycle. Earth Planet. Sci. Lett. 2017, 457, 395–402. [Google Scholar] [CrossRef]
- Shatskiy, A.F.; Litasov, K.D.; Palyanov, Y.N. Phase relations in carbonate systems at pressures and temperatures of lithospheric mantle: Review of experimental data. Russ. Geol. Geophys. 2015, 56, 113–142. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The equilibrium boundary between graphite and diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Luth, R.W. Experimental determination of the reaction dolomite + 2 coesite = diopside + 2 CO2 to 6 GPa. Contrib. Miner. Pet. 1995, 122, 152–158. [Google Scholar] [CrossRef]
- Eggler, D.H. The effect of CO2 upon partial melting of peridotite in the system Na2O-CaO-Al2O3-MgO-SiO2-CO2 to 35 kb, with an analysis of melting in a peridotite-H2O-CO2 system. Am. J. Sci. 1978, 278, 305–343. [Google Scholar] [CrossRef]
- Wyllie, P.J. Magmas and volatile components. Am. Mineral. 1979, 64, 469–500. [Google Scholar]
- Wyllie, P.J.; Huang, W.-L.; Otto, J.; Byrnes, A.P. Carbonation of peridotites and decarbonation of siliceous dolomites represented in the system CaO-MgO-SiO2-CO2 to 30 kbar. Tectonophysics 1983, 100, 359–388. [Google Scholar] [CrossRef]
- Newton, R.C.; Sharp, W.E. Stability of forsterite + CO2 and its bearing on the role of CO2 in the mantle. Earth Planet. Sci. Lett. 1975, 26, 239–244. [Google Scholar] [CrossRef]
- Koziol, A.M.; Newton, R.C. Experimental determination of the reaction: Magnesite + enstatite = forsterite + CO2 in the ranges 6–25 kbar and 700–1100 °C. Am. Miner. 1998, 83, 213–219. [Google Scholar] [CrossRef]
- Knoche, R.; Sweeney, R.J.; Luth, R.W. Carbonation and decarbonation of eclogites: The role of garnet. Contrib. Mineral. Petrol. 1999, 135, 332–339. [Google Scholar] [CrossRef]
- Ionov, D.A.; Dupuy, C.; O’Reilly, S.; Kopylova, M.G.; Genshaft, Y.S. Carbonated peridotite xenoliths from Spitsbergen: Implications for trace element signature of mantle carbonate metasomatism. Earth Planet. Sci. Lett. 1993, 119, 283–297. [Google Scholar] [CrossRef]
- Ionov, D.A.; Doucet, L.S.; Xu, Y.; Golovin, A.V.; Oleinikov, O.B. Reworking of Archean mantle in the NE Siberian craton by carbonatite and silicate melt metasomatism: Evidence from a carbonate-bearing, dunite-to-websterite xenolith suite from the Obnazhennaya kimberlite. Geochim. Cosmochim. Acta 2018, 224, 132–153. [Google Scholar] [CrossRef]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; Dele-Duboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Ionov, D. Trace element composition of mantle-derived carbonates and coexisting phases in peridotite xenoliths from alkali basalts. J. Petrol. 1998, 39, 1931–1941. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Taylor, L.A.; Snyder, G.A. Quantifying the effects of metasomatism in mantle xenoliths: Constraints from secondary chemistry and mineralogy in Udachnaya eclogites, Yakutia. Int. Geol. Rev. 1999, 41, 391–416. [Google Scholar] [CrossRef]
- Roedder, E. Liquid CO2 inclusions in olivine-bearing nodules and phenocrysts from basalts. The Am. Miner. 1965, 50, 1746–1782. [Google Scholar]
- Green, H.W., II. A CO2 charged asthenosphere. Nature Phys. Sci. 1972, 238, 2–5. [Google Scholar] [CrossRef]
- Eggler, D.H. Role of CO2 in Melting Processes in the Mantle: Carnegie Institution of Washington Year Book; Carnegie Institution of Washington: Washington, DC, USA, 1973; Volume 72, pp. 457–467. [Google Scholar]
- Dawson, J.B.; Hawthorne, J.B. Magmatic sedimentation and carbonatitic differentiation in kimberlite sills at Benfontein, South Africa. Quart. J. Geol. Soc. Lond. 1973, 129, 61–85. [Google Scholar] [CrossRef]
- Huang, W.-L.; Wyllie, P.J. Eutectic between wollastonite II and calcite constrained with thermal barrier in MgO-SiO2-CO2 at 30 kilobars, with applications to kimberlite-carbonatite petrogenesis. Earth Planet. Sci. Lett. 1974, 24, 305–310. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Petrogenetic grid for siliceous dolomites extended to mantle peridotite compositions and to conditions for magma generation. Am. Miner. 1976, 61, 691–698. [Google Scholar]
- Luth, R.W. Diamonds, eclogites, and the oxidation state of the Earth’s mantle. Science 1993, 261, 66–68. [Google Scholar] [CrossRef]
- Huang, W.L.; Wyllie, P.J.; Nehru, C.E. Subsolidus and liquid phase relations in the system CaO-SiO2-CO2 to 30 kbars with geological applications. Am. Miner. 1980, 65, 285–301. [Google Scholar]
- Skippen, G.B. Experimental data for reactions in siliceous marbles. J. Geol. 1971, 79, 457–481. [Google Scholar] [CrossRef]
- Käse, H.-R.; Metz, P. Experimental Investigation of the Metamorphism of Siliceous Dolomites. IV. Equilibrium Data for the Reaction: 1 Diopside + 3 Dolomite = 2 Forsterite + 4 Calcite + 2 CO2. Contrib. Mineral. Petrol. 1980, 73, 151–159. [Google Scholar] [CrossRef]
- Lüttge, A.; Metz, P. Mechanism and kinetics of the reaction: 1 dolomite + 2 quartz = 1 diopside + 2CO2: A comparison of rock-sample and of powder experiments. Contrib. Mineral. Petrol. 1993, 115, 155–164. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Peridotite, kimberlite, and carbonatite explained in the system CaO-MgO-SiO2-CO2. Geology 1975, 3, 621–624. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Carbonation and melting reactions in the system CaO-MgO-SiO2-CO2, at mantle pressures with geophysical and petrological applications. Contrib. Mineral. Petrol. 1976, 54, 79–107. [Google Scholar] [CrossRef]
- Eggler, D.H. Effect of CO2 on the Melting of Peridotite: Carnegie Institution of Washington Year Book; Carnegie Institution of Washington: Washington, DC, USA, 1974; Volume 73, pp. 215–224. [Google Scholar]
- Eggler, D.H. CO2 as a volatile component of the mantle: The system Mg2SiO4-SiO2-H2O-CO2. In Physics and Chemistry of the Earth; Ahrens, L.H., Dawson, J.B., Duncan, A.R., Erlank, A.J., Eds.; Pergamon Press: Oxford, UK, 1975; Volume 9, pp. 869–881. [Google Scholar]
- Canil, D.; Virgo, D.; Scarfe, C.M. Oxidation state of mantle xenoliths from British Columbia, Canada. Contr. Mineral. Petrol. 1990, 104, 453–462. [Google Scholar] [CrossRef]
- Katsura, T.; Ito, E. Melting and subsolidus phase relations in the MgSiO3-MgCO3 system at high pressures: Implications to evolution of the Earth’s atmosphere. Earth Planet. Sci. Lett. 1990, 99, 110–117. [Google Scholar] [CrossRef]
- Biellmann, C.; Gillet, P.; Guyot, F.; Peyronneau, J.; Reynard, B. Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet. Sci. Lett. 1993, 118, 31–41. [Google Scholar] [CrossRef]
- Haselton, H.T.; Sharp, W.E.; Newton, R.C. CO2 fugacity at high temperatures and pressures from experimental decarbonation reactions. Geophys. Res. Lett. 1978, 5, 753–756. [Google Scholar] [CrossRef]
- Eggler, D.H.; Kushiro, J.; Holloway, J.R. Free energies of decarbonation reactions at mantle pressures, I. Stability of the assemblage forsterite-enstatite-magnesite in the system MgO-SiO2-CO2-H2O to 60 kbar. Am. Mineral. 1979, 64, 288–293. [Google Scholar]
- Johannes, W. An experimental investigation of the system MgO-SiO2-H2O-CO2. Am. J. Sci. 1969, 267, 1083–1104. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Tomilenko, A.A.; Sobolev, N.V. Conditions of diamond formation through carbonate-silicate interaction. Eur. J. Miner. 2005, 17, 207–214. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Novoselov, I.D.; Kruk, A.N.; Furman, O.V.; Reutsky, V.N.; Palyanov, Y.N. Experimental modeling of decarbonation reactions resulting in the formation of Mg, Fe-garnets and CO2-fluid under mantle P,T-parameters. Russ. Geol. Geophys. 2020, 61, 650–662. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Kruk, A.N.; Novoselov, I.D.; Palyanov, Y.N. Formation of spessartine and CO2 via rhodochrosite decarbonation along a hot subduction P-T path. Minerals 2020, 10, 703. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Kruk, A.N.; Novoselov, I.D.; Furman, O.V.; Palyanov, Y.N. Decarbonation reactions involving ankerite and dolomite under upper mantle P,T-parameters: Experimental modeling. Minerals 2020, 10, 715. [Google Scholar] [CrossRef]
- Vinogradova, Y.G.; Shatskiy, A.F.; Litasov, K.D. Thermodynamic analysis of reactions of CO2 fluid with garnet and clinopyroxene at 3–6 GPa. Geochem. Int. 2021, 59, 851–857. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Shatskiy, A.; Arefiev, A.; Litasov, K. The equilibrium boundary of the reaction Mg3Al2Si3O12 + 3CO2 = Al2SiO5 + 2SiO2 + 3MgCO3 at 3-6 GPa. Am. Miner. 2023; in press. [Google Scholar] [CrossRef]
- Haggerty, S.E. Upper mantle mineralogy. J. Geodyn. 1995, 20, 331–364. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Hammouda, T. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 2003, 214, 357–368. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Withers, A.C. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 2004, 227, 73–85. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Yaxley, G.M.; Hermann, J.; Litasov, K.D.; Rosenthal, A.; Kamenetsky, V.S. An Experimental Study of Carbonated Eclogite at 3·5–5·5 GPa—Implications for Silicate and Carbonate Metasomatism in the Cratonic Mantle. J. Petrol. 2012, 53, 727–759. [Google Scholar] [CrossRef]
- Novoselov, I.D.; Palyanov, Y.N.; Bataleva, Y.V. Experimental Modeling of the Interaction between Garnets of Mantle Parageneses and CO2 Fluid at 6.3 GPa and 950–1550 °C. Russ. Geol. Geophys. 2023, 64, 379–393. [Google Scholar] [CrossRef]
- Yaxley, G.M. Phase relations of carbonated eclogite under upper mantle PT conditions—Implications for carbonatite petrogenesis. Proc. Int. Kimb. Conf. 1999, 2, 933–939. [Google Scholar]
- MacGregor, I.D.; Carter, J.L. The chemistry of clinopyroxenes and garnets of eclogite and peridotite xenoliths from the Roberts Victor mine, South Africa. Phys. Earth Planet. Inter. 1970, 3, 391–397. [Google Scholar] [CrossRef]
- McCandless, T.E.; Gurney, J.J. Sodium in garnet and potassium in clinopyroxene: Criteria for classifying mantle eclogites. In Kimberlites and Related Rocks Vol. 2: Their Crustal/Mantle Setting, Diamonds and Diamond Exploration; Ross, J.R., Ed.; Geological Society of Australia Special Publication; Blackwell: Oxford, UK, 1989; Volume 14, pp. 827–832. [Google Scholar]
- Viljoen, K.S.; Schulze, D.J.; Quadling, A.G. Contrasting Group I and Group II Eclogite Xenolith Petrogenesis: Petrological, Trace Element and Isotopic Evidence from Eclogite, Garnet-Websterite and Alkremite Xenoliths in the Kaalvallei Kimberlite, South Africa. J. Petrol. 2005, 46, 2059–2090. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Khokhryakov, A.F.; Borzdov, Y.M. High-pressure crystallization and properties of diamond from magnesium-based catalysts. Crystengcomm 2017, 19, 4459–4475. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation through carbonate-silicate interaction. Am. Mineral. 2002, 87, 1009–1013. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112S, 690–700. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Sokol, A.G.; Khokhryakov, A.F.; Palyanov, Y.N. Composition of primary kimberlite magma: Constraints from melting and diamond dissolution experiments. Contrib. Mineral. Petrol. 2015, 170, 26. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Palyanova, G.A. Conditions for the origin of oxidized carbonate-silicate melts: Implications for mantle metasomatism and diamond formation. Lithos 2012, 128–131, 113–125. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. The role of rocks saturated with metallic iron in the formation of ferric carbonate–silicate melts: Experimental modeling under PT-conditions of lithospheric mantle. Russ. Geol. Geophys. 2015, 56, 143–154. [Google Scholar] [CrossRef]
- Boettcher, A.L.; Mysen, B.O.; Allen, J.C. Techniques for the control of water fugacity and oxygen fugacity for experimentation in solid-media high-pressure apparatus. J. Geophys. Res. 1973, 78, 5898–5901. [Google Scholar] [CrossRef]
- Luth, R.W. Natural versus experimental control of oxidation state: Effects on the composition and speciation of C-O-H fluids. Am. Miner. 1989, 74, 50–57. [Google Scholar]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Sobolev, N.V. Experimental study of interaction in the CO2—C system at mantle PT parameters. Dokl. Earth Sci. 2010, 435, 1492–1495. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S.; Fischer, J.R. Geological Survey Bulletin 1452; United States Government, Printing Office: Washington, DC, USA, 1978.
- Holland, T.J.B.; Powell, L. An enlarged and updated internally consistent thermodynamic dataset with uncertainties and correlations: K2O–Na2O–CaO–MgO–FeO–Fe2O3–Al2O3–TiO2–SiO2–C–H2–O2. J. Metamorph. Geol. 1990, 8, 89–124. [Google Scholar] [CrossRef]
- Wendlandt, R.F.; Huebner, S.J.; Harrison, W.J. The redox potential of boron nitride and implications for its use as a crucible material in experimental petrology. Am. Miner. 1982, 67, 170–174. [Google Scholar]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle. Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Experimental demonstration of refractory carbonate-bearing eclogite and siliceous melt in the subduction regime. Earth Planet. Sci. Lett. 1994, 128, 313–325. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Dellas, N. The effect of bulk composition on the solidus of carbonated eclogite from partial melting experiments at 3 GPa. Contrib. Mineral. Petrol. 2005, 149, 288–305. [Google Scholar] [CrossRef]
- Wang, S.J.; Teng, F.Z.; Li, S.G. Tracing carbonate–silicate interaction during subduction using magnesium and oxygen isotopes. Nat. Commun. 2014, 5, 5328. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, L.; Lü, Z.; Bader, T. Elemental and isotopic (C, O, Sr, Nd) compositions of Late Paleozoic carbonated eclogite and marble from the SW Tianshan UHP belt, NW China: Implications for deep carbon cycle. J. Asian Earth Sci. 2018, 153, 307–324. [Google Scholar] [CrossRef]
- Ravna, E.K.; Zozulya, D.; Kullerud, K.; Corfu, F.; Nabelek, P.I.; Janák, M.; Slagstad, T.; Davidsen, B.; Selbekk, R.S.; Schertl, H.-P. Deep-seated Carbonatite Intrusion and Metasomatism in the UHP Tromsø Nappe, Northern Scandinavian Caledonides—A Natural Example of Generation of Carbonatite from Carbonated Eclogite. J. Petrol. 2017, 58, 2403–2428. [Google Scholar] [CrossRef]
- Kalugina, A.D.; Zedgenizov, D.A. Raman discrimination of garnet inclusions in Siberian diamonds. J. Raman Spectrosc. 2020, 51, 1438–1444. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Walter, M.J.; Smith, C.B.; Kohn, S.C.; Armstrong, L.S.; Blundy, J.; Gobbo, L. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: Subducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib. Mineral. Petrol. 2010, 160, 489–510. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P.; Joswig, W. Kankan diamonds (Guinea) II: Lower mantle inclusion parageneses. Contrib. Mineral. Petrol. 2000, 140, 16–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).