Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of CO2 Fluid and Garnets of Model Carbonated Eclogites under Lithospheric Mantle P,T-Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Methods and Starting Materials
2.2. Control of Fluid Composition and Redox Conditions during Experiments
2.3. Analytical Methods
3. Results
3.1. Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of ECI Garnets
3.2. Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of ECII Garnets
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luth, R.W. Carbon and carbonates in mantle. In Mantle Petrology: Field Observation and High Pressure Experimentation: A Tribute to Francis, R. (Joe) Boyd; Fei, Y., Bertka, M.C., Mysen, B.O., Eds.; The Geochemical Society: Washington, DC, USA, 1999; pp. 297–316. ISBN 0-941809-05-6. [Google Scholar]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Walter, M.J.; Kohn, S.C.; Araujo, D.; Bulanova, G.P.; Smith, C.B.; Gaillou, E.; Wang, J.; Steele, A.; Shirey, S.B. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science 2011, 334, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.A. The redox budget of subduction zones. Earth Sci. Rev. 2012, 113, 11–32. [Google Scholar] [CrossRef]
- Shirey, S.B.; Cartigny, P.; Frost, D.J.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the Geology of Mantle Carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef]
- Shirey, S.B.; Smit, K.V.; Pearson, D.G.; Walter, M.J.; Aulbach, S.; Brenker, F.E.; Bureau, H.; Burnham, A.D.; Cartigny, P.; Chacko, T.; et al. Diamonds and the mantle geodynamics of carbon: Deep Mantle Carbon Evolution from the Diamond Record. In Deep Carbon: Past to Present; Orcutt, B., Daniel, I., Dasgupta, R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 89–128. [Google Scholar]
- Stagno, V. Carbon, carbides, carbonates and carbonatitic melts in the Earth’s interior. J. Geol. Soc. 2019, 176, 375–387. [Google Scholar] [CrossRef]
- Bergman, S.C.; Dubessy, J. CO2-CO fluid inclusions in a composite peridotite xenolith: Implications for upper mantle oxygen fugacity. Contr. Mineral. Petrol. 1984, 85, 1–13. [Google Scholar] [CrossRef]
- Andersen, T.; Neumann, E.-R. Fluid inclusions in mantle xenoliths. Lithos 2001, 55, 301–320. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Andersen, T.; Neumann, E.-R.; Simonsen, S.L. Carbonatite melt–CO2 fluid inclusions in mantle xenoliths from Tenerife, Canary Islands: A story of trapping, immiscibility and fluid–rock interaction in the upper mantle. Lithos 2002, 64, 77–96. [Google Scholar] [CrossRef]
- Frezzotti, M.-L.; Peccerillo, A.; Panza, G. Carbonate metasomatism and CO2 lithosphere–asthenosphere degassing beneath the Western Mediterranean: An integrated model arising from petrological and geophysical data. Chem. Geol. 2009, 262, 102–108. [Google Scholar] [CrossRef]
- Berkesi, M.; Guzmics, T.; Szabó, C.; Dubessy, J.; Bodnar, R.J.; Hidas, K.; Ratter, K. The role of CO2-rich fluids in trace element transport and metasomatism in the lithospheric mantle beneath the Central Pannonian Basin, Hungary, based on fluid inclusions in mantle xenoliths. Earth Planet. Sci. Lett. 2012, 331–332, 8–20. [Google Scholar] [CrossRef]
- Kawamoto, T.; Yoshikawa, M.; Kumagai, M.Y.; Mirabueno, H.T.; Okuno, M.; Kobayashi, T. Mantle wedge infiltrated with saline fluids from dehydration and decarbonation of subducting slab. Proc. Natl. Acad. Sci. USA 2013, 110, 9663–9668. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, M.-L.; Touret, J.L.R. CO2, carbonate-rich melts, and brines in the mantle. Geosci. Front. 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Elazar, O.; Kessel, R.; Huang, J.-X.; Marquardt, K.; Navon, O. Silicic microinclusions in a metasomatized eclogite from Roberts Victor mine, South Africa. Lithos 2021, 388, 106057. [Google Scholar] [CrossRef]
- Navon, O. High internal pressures in diamond fluid inclusions determined by infrared absorption. Nature 1991, 353, 746–748. [Google Scholar] [CrossRef]
- Guthrie, G.D.; Veblen, D.R.; Navon, O.; Rossman, G.R. Submicrometer fluid inclusions in turbid-diamond coats. Earth Planet. Sci. Lett. 1991, 105, 1–12. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Ragozin, A.L.; Shatskii, V.S.; Shebanin, A.P. Variation in the fluid phase composition in the process of natural diamond crystallization. Dokl. Earth Sci. 2001, 379, 571–574. [Google Scholar]
- Tomlinson, E.L.; Jones, A.P.; Harris, J.W. Co-existing fluid and silicate inclusions in mantle diamond. Earth Planet. Sci. Lett. 2006, 250, 581–595. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Smith, E.M.; Kopylova, M.G.; Frezzotti, M.L.; Afanasiev, V.P. Fluid inclusions in Ebelyakh diamonds: Evidence of CO2 liberation in eclogite and the effect of H2O on diamond habit. Lithos 2015, 216, 106–117. [Google Scholar] [CrossRef]
- Weiss, Y.; Czas, J.; Navon, O. Fluid Inclusions in Fibrous Diamonds. Rev. Mineral. Geochem. 2022, 88, 475–532. [Google Scholar] [CrossRef]
- Scambelluri, M.; Philippot, P. Deep fluids in subduction zones. Lithos 2001, 55, 213–227. [Google Scholar] [CrossRef]
- Kang, N.; Schmidt, M.W.; Poli, S.; Franzolin, E.; Connolly, J.A.D. Melting of siderite to 20 GPa and thermodynamic properties of FeCO3-melt. Chem. Geol. 2015, 400, 34–43. [Google Scholar] [CrossRef]
- Tao, R.; Fei, Y.; Zhang, L. Experimental determination of siderite stability at high pressure. Am. Miner. 2013, 98, 1565–1572. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Lange, R.; Liu, J.; Militzer, B. Determination of calcium carbonate and sodium carbonate melting curves up to Earth’s transition zone pressures with implications for the deep carbon cycle. Earth Planet. Sci. Lett. 2017, 457, 395–402. [Google Scholar] [CrossRef]
- Shatskiy, A.F.; Litasov, K.D.; Palyanov, Y.N. Phase relations in carbonate systems at pressures and temperatures of lithospheric mantle: Review of experimental data. Russ. Geol. Geophys. 2015, 56, 113–142. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The equilibrium boundary between graphite and diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Luth, R.W. Experimental determination of the reaction dolomite + 2 coesite = diopside + 2 CO2 to 6 GPa. Contrib. Miner. Pet. 1995, 122, 152–158. [Google Scholar] [CrossRef]
- Eggler, D.H. The effect of CO2 upon partial melting of peridotite in the system Na2O-CaO-Al2O3-MgO-SiO2-CO2 to 35 kb, with an analysis of melting in a peridotite-H2O-CO2 system. Am. J. Sci. 1978, 278, 305–343. [Google Scholar] [CrossRef]
- Wyllie, P.J. Magmas and volatile components. Am. Mineral. 1979, 64, 469–500. [Google Scholar]
- Wyllie, P.J.; Huang, W.-L.; Otto, J.; Byrnes, A.P. Carbonation of peridotites and decarbonation of siliceous dolomites represented in the system CaO-MgO-SiO2-CO2 to 30 kbar. Tectonophysics 1983, 100, 359–388. [Google Scholar] [CrossRef]
- Newton, R.C.; Sharp, W.E. Stability of forsterite + CO2 and its bearing on the role of CO2 in the mantle. Earth Planet. Sci. Lett. 1975, 26, 239–244. [Google Scholar] [CrossRef]
- Koziol, A.M.; Newton, R.C. Experimental determination of the reaction: Magnesite + enstatite = forsterite + CO2 in the ranges 6–25 kbar and 700–1100 °C. Am. Miner. 1998, 83, 213–219. [Google Scholar] [CrossRef]
- Knoche, R.; Sweeney, R.J.; Luth, R.W. Carbonation and decarbonation of eclogites: The role of garnet. Contrib. Mineral. Petrol. 1999, 135, 332–339. [Google Scholar] [CrossRef]
- Ionov, D.A.; Dupuy, C.; O’Reilly, S.; Kopylova, M.G.; Genshaft, Y.S. Carbonated peridotite xenoliths from Spitsbergen: Implications for trace element signature of mantle carbonate metasomatism. Earth Planet. Sci. Lett. 1993, 119, 283–297. [Google Scholar] [CrossRef]
- Ionov, D.A.; Doucet, L.S.; Xu, Y.; Golovin, A.V.; Oleinikov, O.B. Reworking of Archean mantle in the NE Siberian craton by carbonatite and silicate melt metasomatism: Evidence from a carbonate-bearing, dunite-to-websterite xenolith suite from the Obnazhennaya kimberlite. Geochim. Cosmochim. Acta 2018, 224, 132–153. [Google Scholar] [CrossRef]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; Dele-Duboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Ionov, D. Trace element composition of mantle-derived carbonates and coexisting phases in peridotite xenoliths from alkali basalts. J. Petrol. 1998, 39, 1931–1941. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Taylor, L.A.; Snyder, G.A. Quantifying the effects of metasomatism in mantle xenoliths: Constraints from secondary chemistry and mineralogy in Udachnaya eclogites, Yakutia. Int. Geol. Rev. 1999, 41, 391–416. [Google Scholar] [CrossRef]
- Roedder, E. Liquid CO2 inclusions in olivine-bearing nodules and phenocrysts from basalts. The Am. Miner. 1965, 50, 1746–1782. [Google Scholar]
- Green, H.W., II. A CO2 charged asthenosphere. Nature Phys. Sci. 1972, 238, 2–5. [Google Scholar] [CrossRef]
- Eggler, D.H. Role of CO2 in Melting Processes in the Mantle: Carnegie Institution of Washington Year Book; Carnegie Institution of Washington: Washington, DC, USA, 1973; Volume 72, pp. 457–467. [Google Scholar]
- Dawson, J.B.; Hawthorne, J.B. Magmatic sedimentation and carbonatitic differentiation in kimberlite sills at Benfontein, South Africa. Quart. J. Geol. Soc. Lond. 1973, 129, 61–85. [Google Scholar] [CrossRef]
- Huang, W.-L.; Wyllie, P.J. Eutectic between wollastonite II and calcite constrained with thermal barrier in MgO-SiO2-CO2 at 30 kilobars, with applications to kimberlite-carbonatite petrogenesis. Earth Planet. Sci. Lett. 1974, 24, 305–310. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Petrogenetic grid for siliceous dolomites extended to mantle peridotite compositions and to conditions for magma generation. Am. Miner. 1976, 61, 691–698. [Google Scholar]
- Luth, R.W. Diamonds, eclogites, and the oxidation state of the Earth’s mantle. Science 1993, 261, 66–68. [Google Scholar] [CrossRef]
- Huang, W.L.; Wyllie, P.J.; Nehru, C.E. Subsolidus and liquid phase relations in the system CaO-SiO2-CO2 to 30 kbars with geological applications. Am. Miner. 1980, 65, 285–301. [Google Scholar]
- Skippen, G.B. Experimental data for reactions in siliceous marbles. J. Geol. 1971, 79, 457–481. [Google Scholar] [CrossRef]
- Käse, H.-R.; Metz, P. Experimental Investigation of the Metamorphism of Siliceous Dolomites. IV. Equilibrium Data for the Reaction: 1 Diopside + 3 Dolomite = 2 Forsterite + 4 Calcite + 2 CO2. Contrib. Mineral. Petrol. 1980, 73, 151–159. [Google Scholar] [CrossRef]
- Lüttge, A.; Metz, P. Mechanism and kinetics of the reaction: 1 dolomite + 2 quartz = 1 diopside + 2CO2: A comparison of rock-sample and of powder experiments. Contrib. Mineral. Petrol. 1993, 115, 155–164. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Peridotite, kimberlite, and carbonatite explained in the system CaO-MgO-SiO2-CO2. Geology 1975, 3, 621–624. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Carbonation and melting reactions in the system CaO-MgO-SiO2-CO2, at mantle pressures with geophysical and petrological applications. Contrib. Mineral. Petrol. 1976, 54, 79–107. [Google Scholar] [CrossRef]
- Eggler, D.H. Effect of CO2 on the Melting of Peridotite: Carnegie Institution of Washington Year Book; Carnegie Institution of Washington: Washington, DC, USA, 1974; Volume 73, pp. 215–224. [Google Scholar]
- Eggler, D.H. CO2 as a volatile component of the mantle: The system Mg2SiO4-SiO2-H2O-CO2. In Physics and Chemistry of the Earth; Ahrens, L.H., Dawson, J.B., Duncan, A.R., Erlank, A.J., Eds.; Pergamon Press: Oxford, UK, 1975; Volume 9, pp. 869–881. [Google Scholar]
- Canil, D.; Virgo, D.; Scarfe, C.M. Oxidation state of mantle xenoliths from British Columbia, Canada. Contr. Mineral. Petrol. 1990, 104, 453–462. [Google Scholar] [CrossRef]
- Katsura, T.; Ito, E. Melting and subsolidus phase relations in the MgSiO3-MgCO3 system at high pressures: Implications to evolution of the Earth’s atmosphere. Earth Planet. Sci. Lett. 1990, 99, 110–117. [Google Scholar] [CrossRef]
- Biellmann, C.; Gillet, P.; Guyot, F.; Peyronneau, J.; Reynard, B. Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet. Sci. Lett. 1993, 118, 31–41. [Google Scholar] [CrossRef]
- Haselton, H.T.; Sharp, W.E.; Newton, R.C. CO2 fugacity at high temperatures and pressures from experimental decarbonation reactions. Geophys. Res. Lett. 1978, 5, 753–756. [Google Scholar] [CrossRef]
- Eggler, D.H.; Kushiro, J.; Holloway, J.R. Free energies of decarbonation reactions at mantle pressures, I. Stability of the assemblage forsterite-enstatite-magnesite in the system MgO-SiO2-CO2-H2O to 60 kbar. Am. Mineral. 1979, 64, 288–293. [Google Scholar]
- Johannes, W. An experimental investigation of the system MgO-SiO2-H2O-CO2. Am. J. Sci. 1969, 267, 1083–1104. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Tomilenko, A.A.; Sobolev, N.V. Conditions of diamond formation through carbonate-silicate interaction. Eur. J. Miner. 2005, 17, 207–214. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Novoselov, I.D.; Kruk, A.N.; Furman, O.V.; Reutsky, V.N.; Palyanov, Y.N. Experimental modeling of decarbonation reactions resulting in the formation of Mg, Fe-garnets and CO2-fluid under mantle P,T-parameters. Russ. Geol. Geophys. 2020, 61, 650–662. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Kruk, A.N.; Novoselov, I.D.; Palyanov, Y.N. Formation of spessartine and CO2 via rhodochrosite decarbonation along a hot subduction P-T path. Minerals 2020, 10, 703. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Kruk, A.N.; Novoselov, I.D.; Furman, O.V.; Palyanov, Y.N. Decarbonation reactions involving ankerite and dolomite under upper mantle P,T-parameters: Experimental modeling. Minerals 2020, 10, 715. [Google Scholar] [CrossRef]
- Vinogradova, Y.G.; Shatskiy, A.F.; Litasov, K.D. Thermodynamic analysis of reactions of CO2 fluid with garnet and clinopyroxene at 3–6 GPa. Geochem. Int. 2021, 59, 851–857. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Shatskiy, A.; Arefiev, A.; Litasov, K. The equilibrium boundary of the reaction Mg3Al2Si3O12 + 3CO2 = Al2SiO5 + 2SiO2 + 3MgCO3 at 3-6 GPa. Am. Miner. 2023; in press. [Google Scholar] [CrossRef]
- Haggerty, S.E. Upper mantle mineralogy. J. Geodyn. 1995, 20, 331–364. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Hammouda, T. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 2003, 214, 357–368. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Withers, A.C. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 2004, 227, 73–85. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Yaxley, G.M.; Hermann, J.; Litasov, K.D.; Rosenthal, A.; Kamenetsky, V.S. An Experimental Study of Carbonated Eclogite at 3·5–5·5 GPa—Implications for Silicate and Carbonate Metasomatism in the Cratonic Mantle. J. Petrol. 2012, 53, 727–759. [Google Scholar] [CrossRef]
- Novoselov, I.D.; Palyanov, Y.N.; Bataleva, Y.V. Experimental Modeling of the Interaction between Garnets of Mantle Parageneses and CO2 Fluid at 6.3 GPa and 950–1550 °C. Russ. Geol. Geophys. 2023, 64, 379–393. [Google Scholar] [CrossRef]
- Yaxley, G.M. Phase relations of carbonated eclogite under upper mantle PT conditions—Implications for carbonatite petrogenesis. Proc. Int. Kimb. Conf. 1999, 2, 933–939. [Google Scholar]
- MacGregor, I.D.; Carter, J.L. The chemistry of clinopyroxenes and garnets of eclogite and peridotite xenoliths from the Roberts Victor mine, South Africa. Phys. Earth Planet. Inter. 1970, 3, 391–397. [Google Scholar] [CrossRef]
- McCandless, T.E.; Gurney, J.J. Sodium in garnet and potassium in clinopyroxene: Criteria for classifying mantle eclogites. In Kimberlites and Related Rocks Vol. 2: Their Crustal/Mantle Setting, Diamonds and Diamond Exploration; Ross, J.R., Ed.; Geological Society of Australia Special Publication; Blackwell: Oxford, UK, 1989; Volume 14, pp. 827–832. [Google Scholar]
- Viljoen, K.S.; Schulze, D.J.; Quadling, A.G. Contrasting Group I and Group II Eclogite Xenolith Petrogenesis: Petrological, Trace Element and Isotopic Evidence from Eclogite, Garnet-Websterite and Alkremite Xenoliths in the Kaalvallei Kimberlite, South Africa. J. Petrol. 2005, 46, 2059–2090. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Khokhryakov, A.F.; Borzdov, Y.M. High-pressure crystallization and properties of diamond from magnesium-based catalysts. Crystengcomm 2017, 19, 4459–4475. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation through carbonate-silicate interaction. Am. Mineral. 2002, 87, 1009–1013. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112S, 690–700. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Sokol, A.G.; Khokhryakov, A.F.; Palyanov, Y.N. Composition of primary kimberlite magma: Constraints from melting and diamond dissolution experiments. Contrib. Mineral. Petrol. 2015, 170, 26. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Palyanova, G.A. Conditions for the origin of oxidized carbonate-silicate melts: Implications for mantle metasomatism and diamond formation. Lithos 2012, 128–131, 113–125. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. The role of rocks saturated with metallic iron in the formation of ferric carbonate–silicate melts: Experimental modeling under PT-conditions of lithospheric mantle. Russ. Geol. Geophys. 2015, 56, 143–154. [Google Scholar] [CrossRef]
- Boettcher, A.L.; Mysen, B.O.; Allen, J.C. Techniques for the control of water fugacity and oxygen fugacity for experimentation in solid-media high-pressure apparatus. J. Geophys. Res. 1973, 78, 5898–5901. [Google Scholar] [CrossRef]
- Luth, R.W. Natural versus experimental control of oxidation state: Effects on the composition and speciation of C-O-H fluids. Am. Miner. 1989, 74, 50–57. [Google Scholar]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Sobolev, N.V. Experimental study of interaction in the CO2—C system at mantle PT parameters. Dokl. Earth Sci. 2010, 435, 1492–1495. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S.; Fischer, J.R. Geological Survey Bulletin 1452; United States Government, Printing Office: Washington, DC, USA, 1978.
- Holland, T.J.B.; Powell, L. An enlarged and updated internally consistent thermodynamic dataset with uncertainties and correlations: K2O–Na2O–CaO–MgO–FeO–Fe2O3–Al2O3–TiO2–SiO2–C–H2–O2. J. Metamorph. Geol. 1990, 8, 89–124. [Google Scholar] [CrossRef]
- Wendlandt, R.F.; Huebner, S.J.; Harrison, W.J. The redox potential of boron nitride and implications for its use as a crucible material in experimental petrology. Am. Miner. 1982, 67, 170–174. [Google Scholar]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle. Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Experimental demonstration of refractory carbonate-bearing eclogite and siliceous melt in the subduction regime. Earth Planet. Sci. Lett. 1994, 128, 313–325. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Dellas, N. The effect of bulk composition on the solidus of carbonated eclogite from partial melting experiments at 3 GPa. Contrib. Mineral. Petrol. 2005, 149, 288–305. [Google Scholar] [CrossRef]
- Wang, S.J.; Teng, F.Z.; Li, S.G. Tracing carbonate–silicate interaction during subduction using magnesium and oxygen isotopes. Nat. Commun. 2014, 5, 5328. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, L.; Lü, Z.; Bader, T. Elemental and isotopic (C, O, Sr, Nd) compositions of Late Paleozoic carbonated eclogite and marble from the SW Tianshan UHP belt, NW China: Implications for deep carbon cycle. J. Asian Earth Sci. 2018, 153, 307–324. [Google Scholar] [CrossRef]
- Ravna, E.K.; Zozulya, D.; Kullerud, K.; Corfu, F.; Nabelek, P.I.; Janák, M.; Slagstad, T.; Davidsen, B.; Selbekk, R.S.; Schertl, H.-P. Deep-seated Carbonatite Intrusion and Metasomatism in the UHP Tromsø Nappe, Northern Scandinavian Caledonides—A Natural Example of Generation of Carbonatite from Carbonated Eclogite. J. Petrol. 2017, 58, 2403–2428. [Google Scholar] [CrossRef]
- Kalugina, A.D.; Zedgenizov, D.A. Raman discrimination of garnet inclusions in Siberian diamonds. J. Raman Spectrosc. 2020, 51, 1438–1444. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Walter, M.J.; Smith, C.B.; Kohn, S.C.; Armstrong, L.S.; Blundy, J.; Gobbo, L. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: Subducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib. Mineral. Petrol. 2010, 160, 489–510. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P.; Joswig, W. Kankan diamonds (Guinea) II: Lower mantle inclusion parageneses. Contrib. Mineral. Petrol. 2000, 140, 16–27. [Google Scholar] [CrossRef]
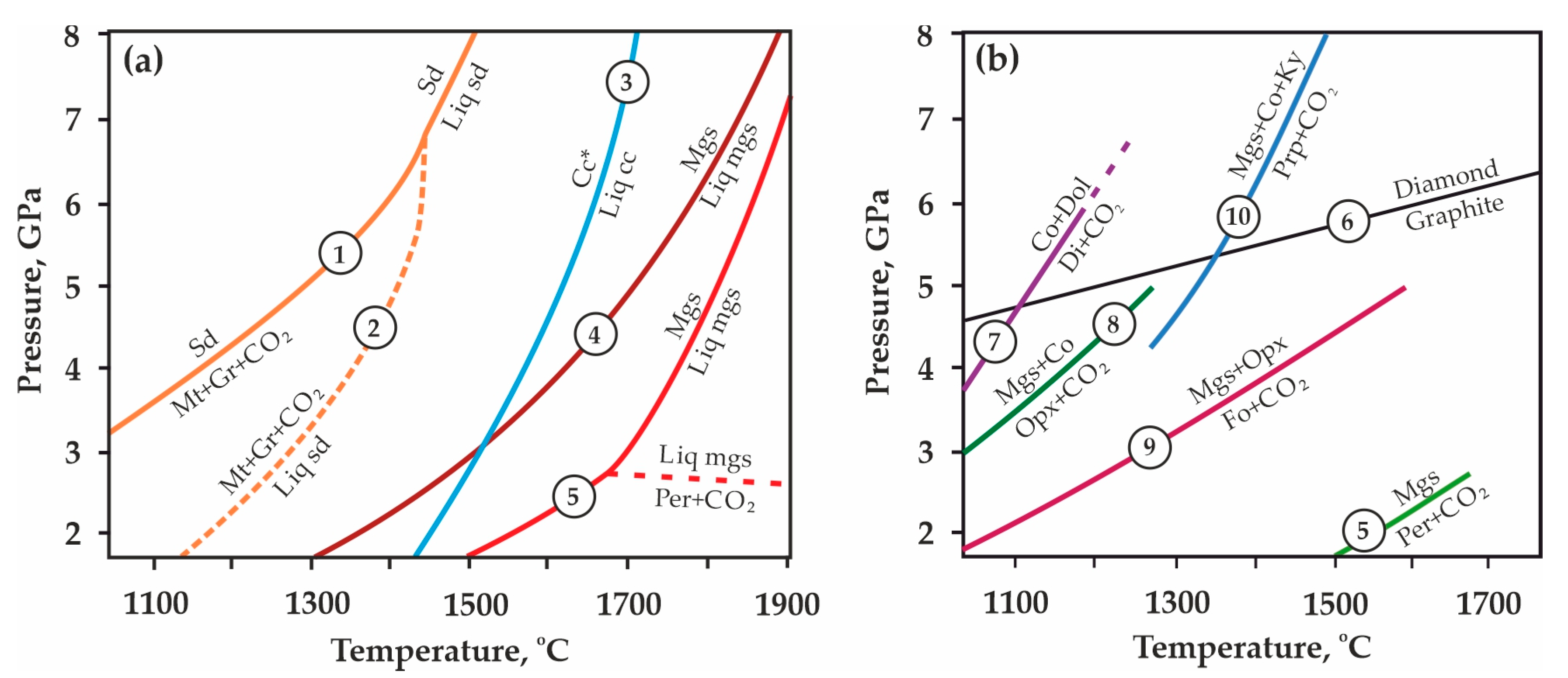

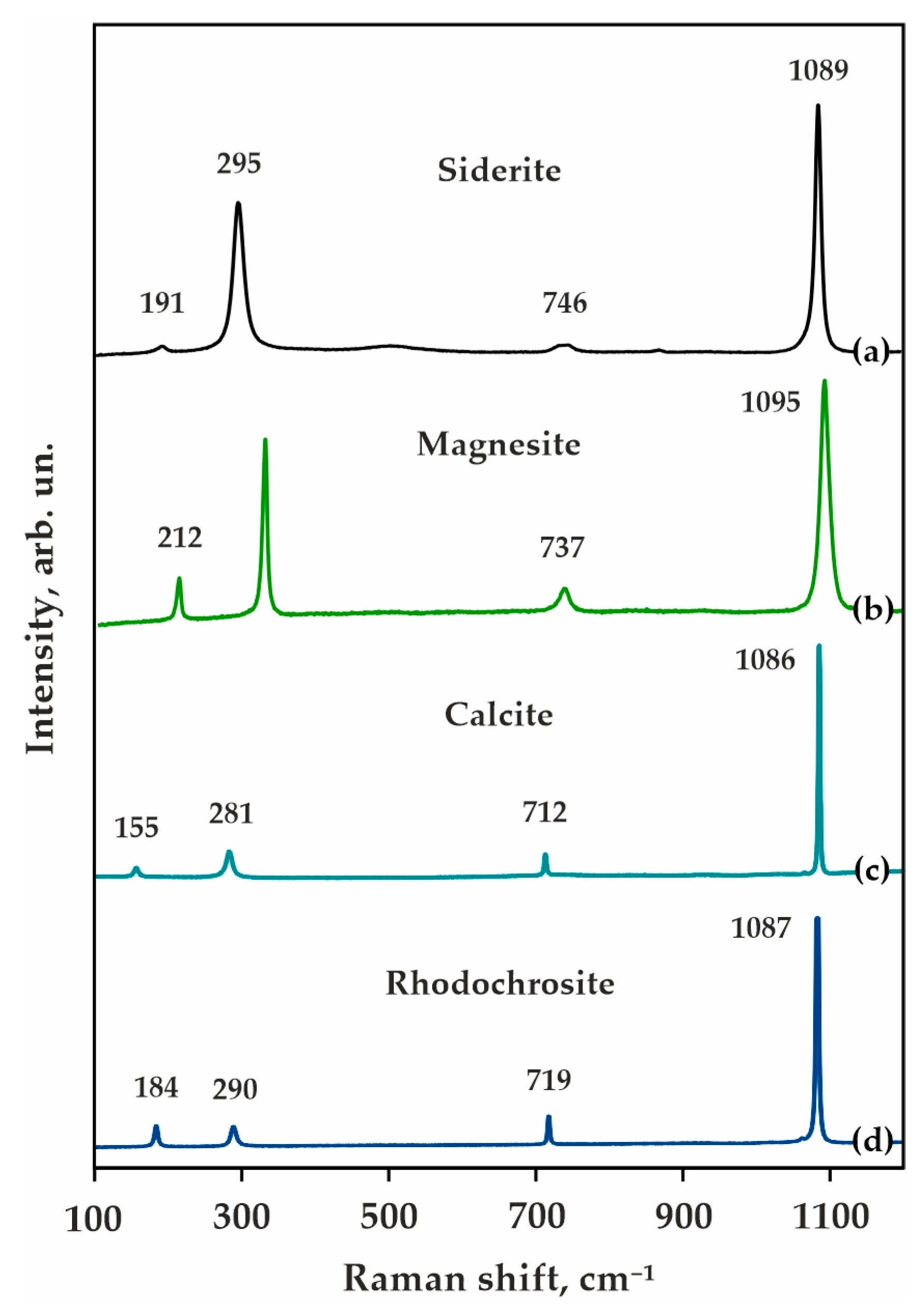
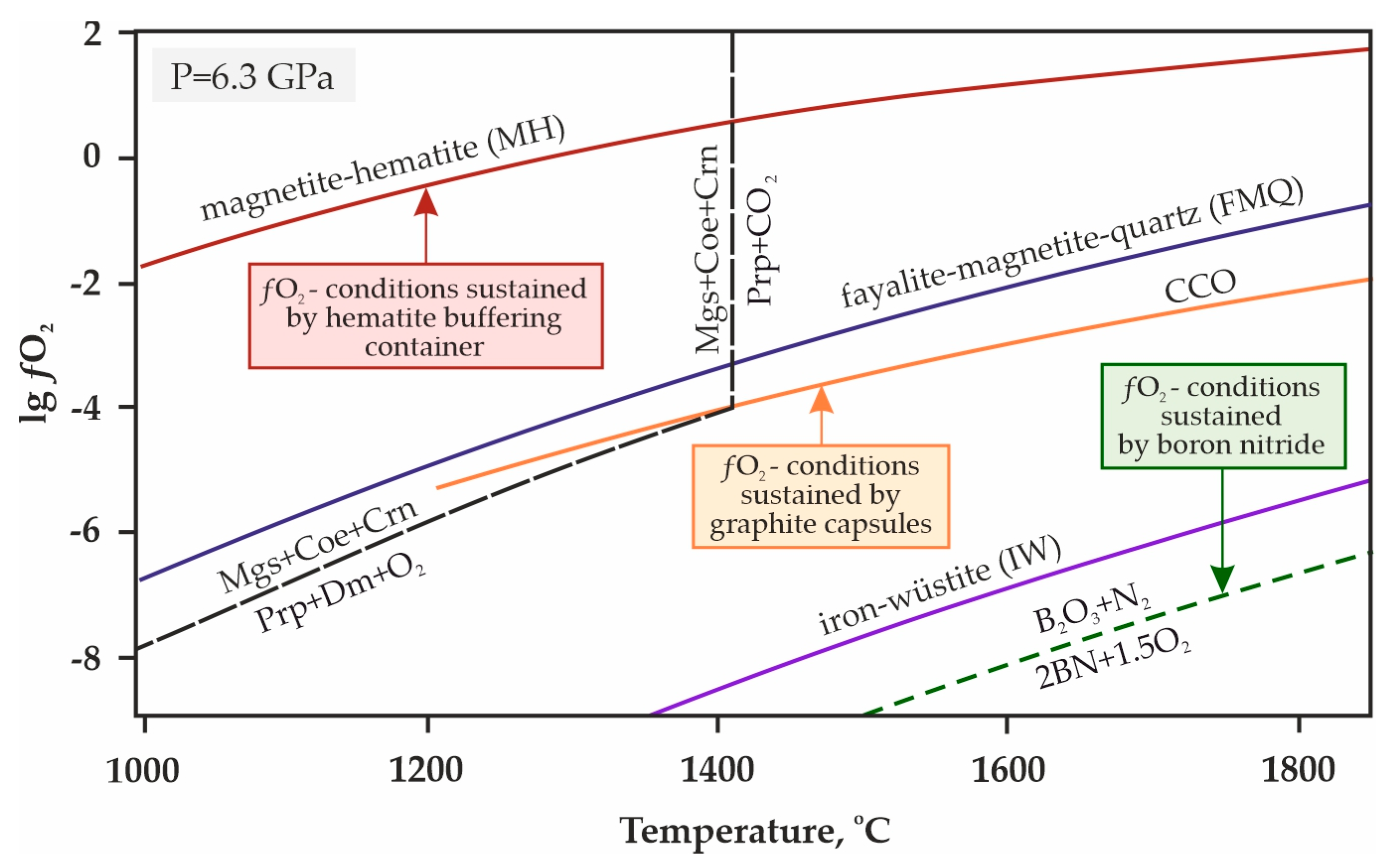
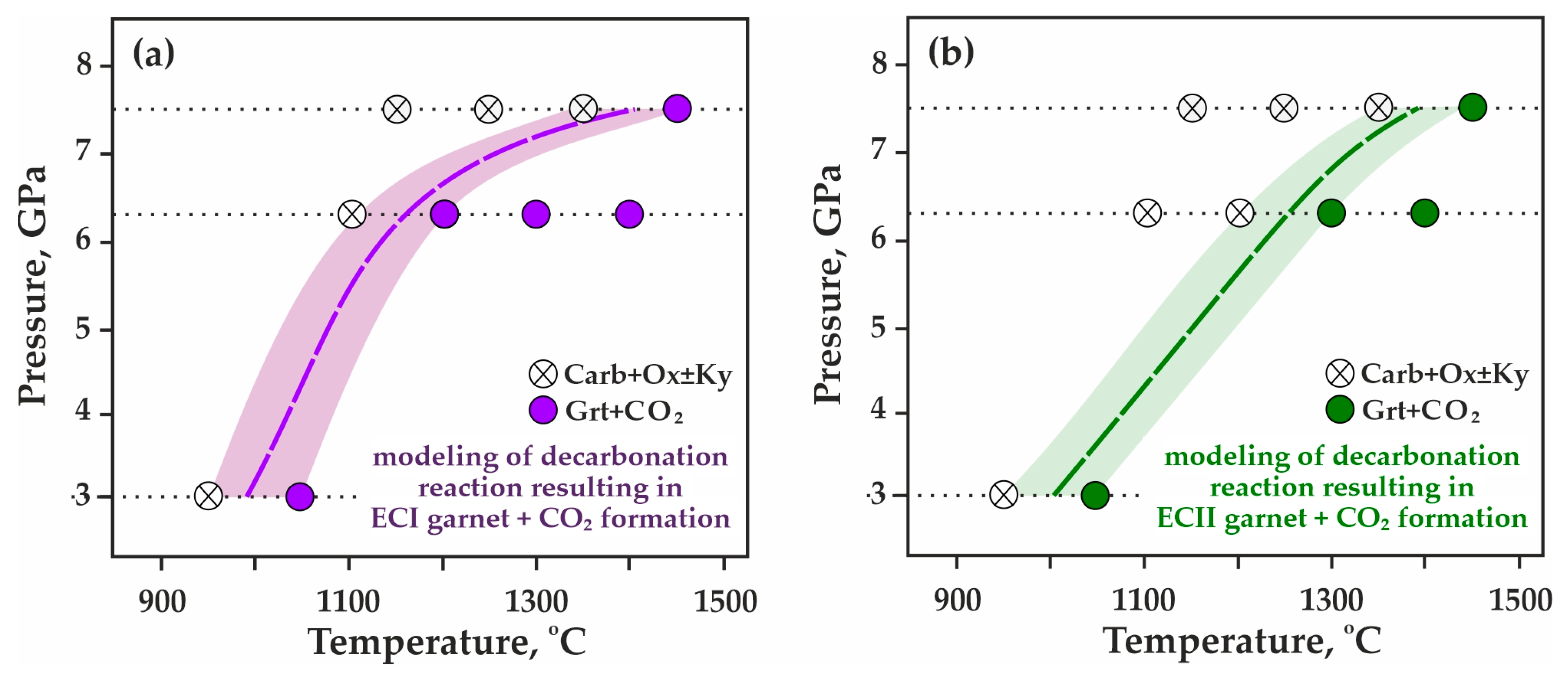

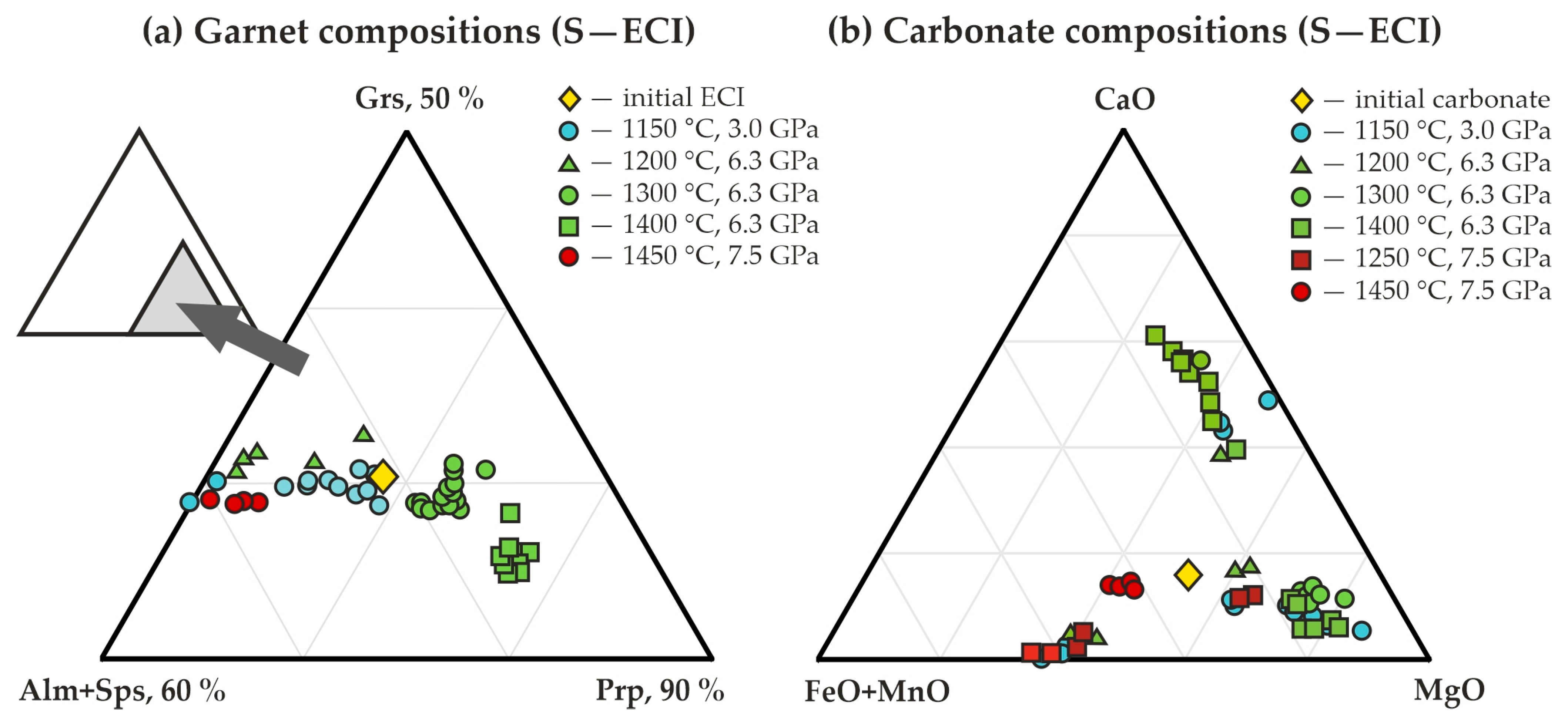
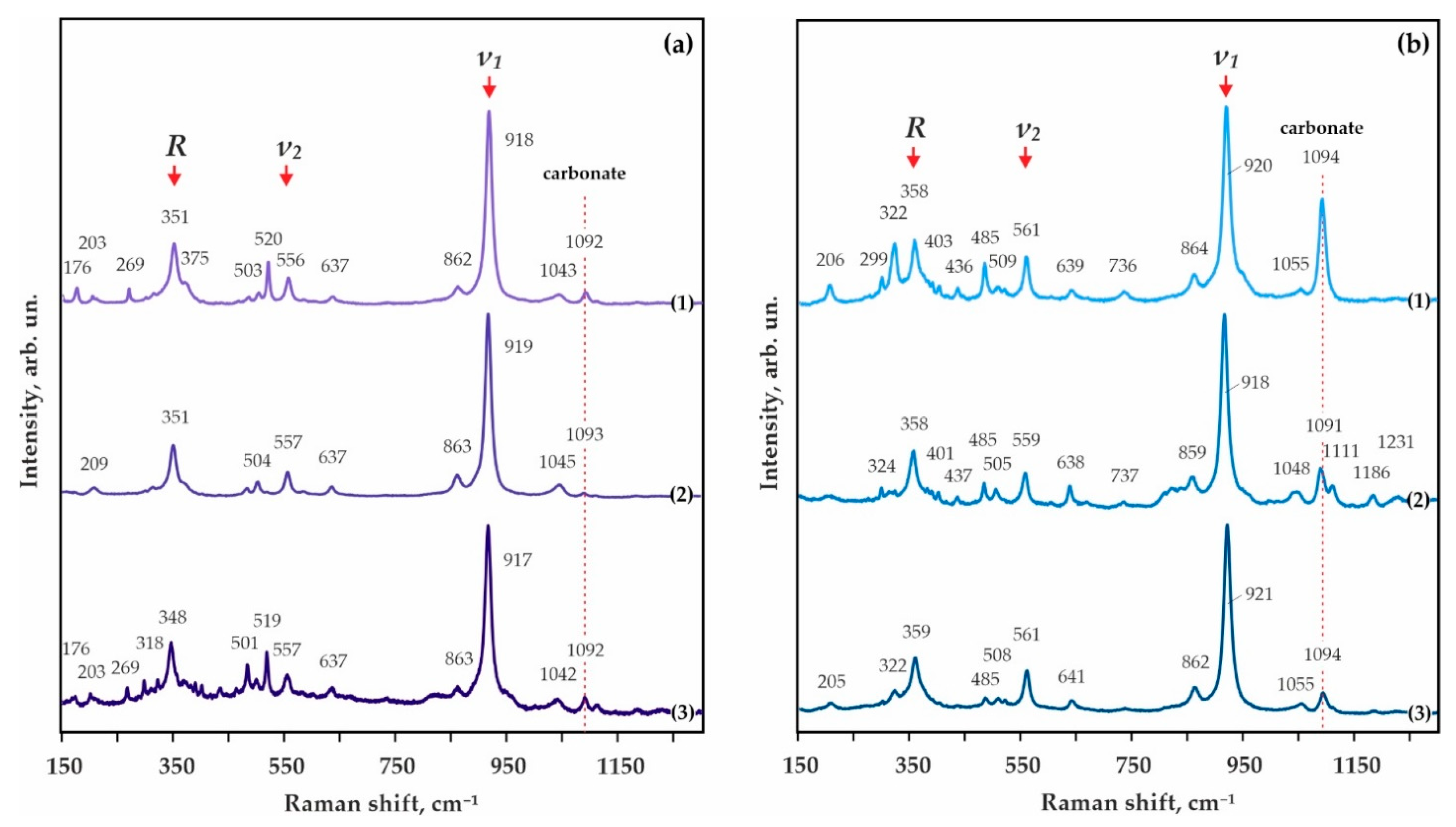

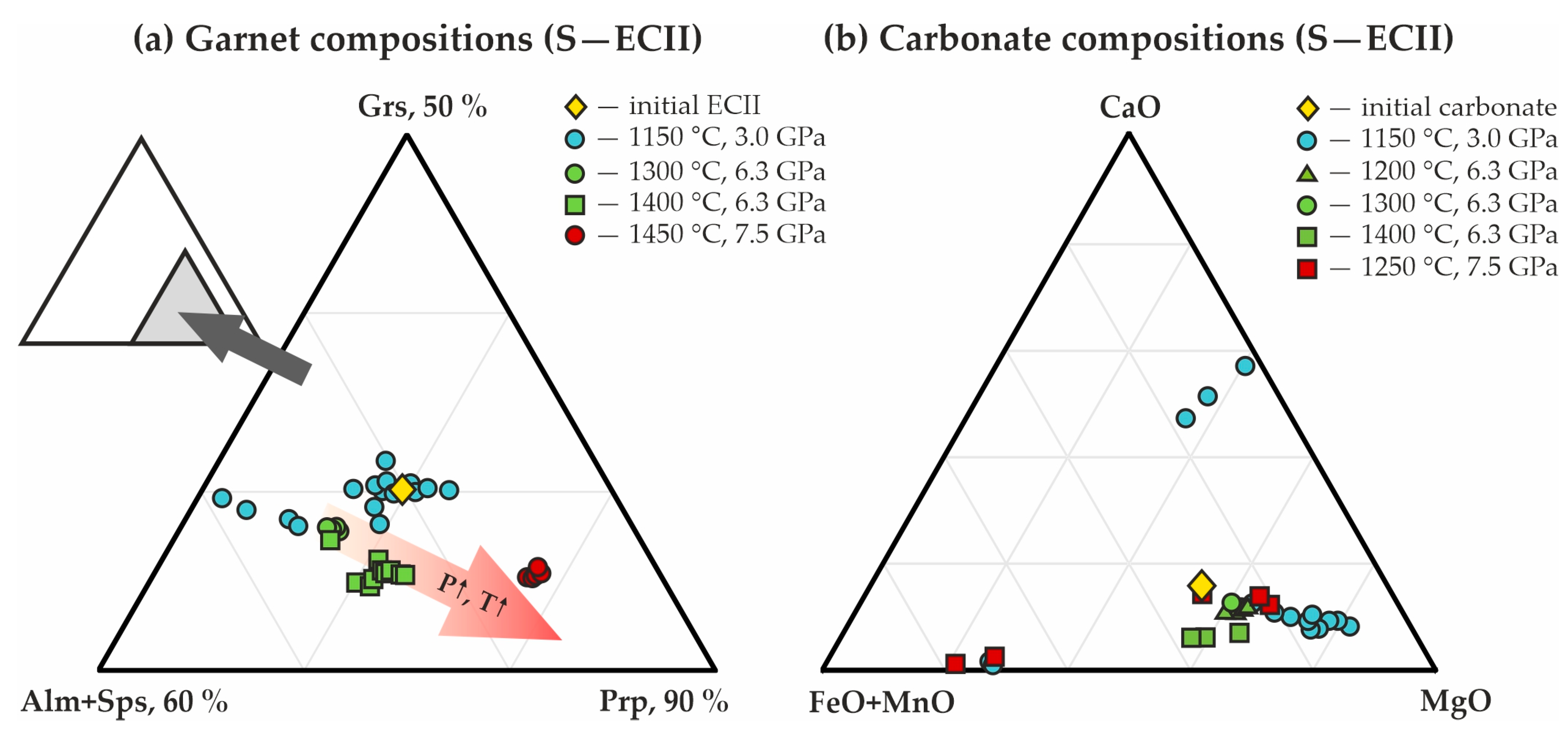

| Rock/Mineral | Mass Concentrations, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | MgO | CaO | Na2O | CO2 | Total | Mg # | |
| Garnet from ECI | 40.72 | 22.08 | 15.42 | 12.35 | 9.20 | 0.23 | - | 100.00 | 58.80 |
| Garnet from ECII | 40.36 | 22.27 | 13.74 | 12.55 | 10.80 | 0.28 | - | 100.00 | 61.96 |
| Carbonate from ECI | 1.17 | 0.59 | 5.73 | 13.36 | 32.67 | 0.22 | 46.27 | 100.00 | 80.61 |
| Carbonate from ECII | 2.01 | 0.77 | 5.73 | 13.76 | 33.78 | 0.12 | 43.83 | 100.00 | 80.58 |
| Mineral/ System | Mass Concentrations, wt.% | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | MnO | MgO | CaO | CO2 | |
| Mgs | - | - | 0.66 | 0.02 | 43.75 | 3.94 | 51.63 |
| Cc | - | - | 0.07 | 0.21 | 0.00 | 55.74 | 43.97 |
| Msd | - | - | 42.12 | 0.67 | 13.70 | 1.27 | 42.23 |
| Rds | - | - | 0.70 | 57.23 | 0.28 | 3.10 | 38.69 |
| S-ECI | 31.57 | 17.85 | 11.05 | 0.38 | 11.43 | 4.44 | 23.28 |
| S-ECII | 31.96 | 18.08 | 8.43 | 0.38 | 12.98 | 4.77 | 23.41 |
| Run # | System | P, GPa | T, °C | t, h | Phase Assemblage |
|---|---|---|---|---|---|
| 1738-I | S-ECI | 3.0 | 1050 | 60 | Crn, Dol, Mgs, Msd, Coe |
| 2122-I | S-ECI | 3.0 | 1150 | 60 | Grt, Ky, Dol, Mgs, Msd, Fms, Coe |
| 2117-I | S-ECI | 6.3 | 1100 | 40 | Ky, Msd, Dol, Coe |
| 2119-I | S-ECI | 6.3 | 1200 | 40 | Ky, Crn, Grt, Coe |
| 2115-I | S-ECI | 6.3 | 1300 | 20 | Grt, Ky, Crn, Carb |
| 2113-I | S-ECI | 6.3 | 1400 | 10 | Grt, Crn, Coe, Carb |
| 2137-I | S-ECI | 7.5 | 1150 | 60 | Ky, Coe, Fms, Cal, Msd |
| 2138-I | S-ECI | 7.5 | 1250 | 40 | Ky, Coe, Msd, Fms |
| 2142-I | S-ECI | 7.5 | 1350 | 20 | Ky, Crn, Coe, Carb |
| 2144-I | S-ECI | 7.5 | 1450 | 10 | Grt, Carb, Ky, Coe |
| 1738-II | S-ECII | 3.0 | 1050 | 60 | Crn, Fms, Msd, Coe |
| 2122-II | S-ECII | 3.0 | 1150 | 60 | Grt, Ky, Coe, Msd, Dol, Fms |
| 2117-II | S-ECII | 6.3 | 1100 | 40 | Ky, Coe, Carb |
| 2119-II | S-ECII | 6.3 | 1200 | 40 | Ky, Coe, Msd, Fms |
| 2115-II | S-ECII | 6.3 | 1300 | 20 | Grt, Ky, Fms, Coe |
| 2113-II | S-ECII | 6.3 | 1400 | 10 | Grt, Ky, Crn, Coe, Carb |
| 2135-II | S-ECII | 7.5 | 1250 | 40 | Ky, Coe, Msd, Fms |
| 2139-II | S-ECII | 7.5 | 1350 | 20 | Ky, Crn, Carb |
| 2140-II | S-ECII | 7.5 | 1450 | 10 | Grt, Carb, Ky, Coe |
| Run # | P, GPa | T, °C | Phase | Mass Concentrations, wt.% | n(O) | Cations per Formula Unit, p.f.u. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | MnO | MgO | CaO | CO2 | Σ | Si | Al | Fe | Mn | Mg | Ca | C | Σ | |||||
| 1738-I | 3.0 | 1050 | Msd | - | - | 41.2 | 0.7 | 14.4 | 0.5 | 43.2 | 100.0 | 3 | - | - | 0.59 | 0.01 | 0.37 | 0.01 | 1.01 | 1.99 |
| Mgs | - | - | 0.8 | - | 48.1 | 0.7 | 50.4 | 100.0 | 3 | - | - | 0.01 | - | 1.03 | 0.01 | 0.98 | 2.03 | |||
| Coe | 98.5 | - | 1.2 | - | - | - | - | 99.7 | 2 | 1.00 | - | 0.01 | - | - | - | - | 1.01 | |||
| 2122-I | 3.0 | 1150 | Grt | 40.6 | 22.6 | 15.1 | 0.6 | 14.4 | 6.2 | - | 99.5 | 12 | 3.00 | 1.96 | 0.93 | 0.04 | 1.60 | 0.49 | - | 8.02 |
| 40.1 | 22.1 | 17.9 | 1.1 | 12.6 | 5.7 | - | 99.6 | 12 | 3.00 | 1.95 | 1.12 | 0.07 | 1.42 | 0.46 | - | 8.02 | ||||
| 39.5 | 21.5 | 21.3 | 1.1 | 10.8 | 5.1 | - | 99.3 | 12 | 3.01 | 1.93 | 1.35 | 0.07 | 1.23 | 0.42 | - | 8.01 | ||||
| Ky | 36.1 | 58.5 | 2.6 | - | 1.2 | 1.0 | - | 99.4 | 5 | 1.00 | 1.91 | 0.06 | - | 0.05 | 0.03 | - | 3.05 | |||
| Msd | - | - | 40.5 | 0.7 | 15.5 | 0.5 | 42.7 | 100.0 | 3 | - | - | 0.58 | 0.01 | 0.40 | 0.01 | 1.00 | 2.00 | |||
| Fms | - | - | 19.8 | 0.8 | 26.7 | 6.5 | 46.2 | 100.0 | 3 | - | - | 0.26 | 0.01 | 0.63 | 0.11 | 0.99 | 2.00 | |||
| - | - | 13.4 | 0.8 | 32.8 | 5.5 | 47.6 | 100.0 | 3 | - | - | 0.17 | 0.01 | 0.75 | 0.09 | 0.99 | 2.01 | ||||
| - | - | 6.7 | - | 38.8 | 3.2 | 51.3 | 100.0 | 3 | - | - | 0.08 | - | 0.84 | 0.05 | 1.01 | 1.98 | ||||
| Dol | - | - | 8.4 | 0.7 | 19.0 | 25.4 | 46.5 | 100.0 | 6 | - | - | 0.22 | 0.02 | 0.90 | 0.86 | 2.00 | 4.00 | |||
| Coe | 99.6 | - | - | - | - | - | - | 99.6 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2117-I | 6.3 | 1100 | Ky | 35.9 | 63.6 | 0.4 | - | - | - | - | 99.9 | 5 | 0.97 | 2.02 | 0.01 | - | - | - | - | 3.00 |
| Msd | - | 1.0 | 37.1 | 0.7 | 15.0 | 2.2 | 44.0 | 100.0 | 3 | - | 0.02 | 0.52 | 0.01 | 0.38 | 0.04 | 1.01 | 1.98 | |||
| Coe | 99.6 | - | - | - | - | - | - | 99.6 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2119-I | 6.3 | 1200 | Grt | 39.7 | 22.1 | 18.6 | 1.1 | 11.4 | 6.6 | - | 99.4 | 12 | 3.00 | 1.96 | 1.17 | 0.07 | 1.29 | 0.53 | - | 8.02 |
| 40.4 | 22.6 | 14.4 | 0.8 | 13.7 | 7.4 | - | 99.4 | 12 | 2.99 | 1.97 | 0.89 | 0.05 | 1.52 | 0.59 | - | 8.01 | ||||
| Ky | 36.4 | 62.5 | 0.9 | - | - | - | - | 99.8 | 5 | 0.99 | 2.00 | 0.02 | - | - | - | - | 3.01 | |||
| Fms | - | - | 19.9 | 0.8 | 26.7 | 5.9 | 46.7 | 100.0 | 3 | - | - | 0.26 | 0.01 | 0.63 | 0.10 | 1.00 | 2.00 | |||
| Msd | - | - | 42.7 | 0.7 | 13.4 | 1.1 | 42.1 | 100.0 | 3 | - | - | 0.62 | 0.01 | 0.35 | 0.02 | 1.00 | 2.00 | |||
| Crn | - | 98.5 | 0.7 | - | - | - | - | 99.2 | 3 | - | 1.99 | 0.01 | - | - | - | - | 2.00 | |||
| 2115-I | 6.3 | 1300 | Grt | 40.9 | 22.4 | 14.0 | 0.8 | 16.0 | 5.2 | - | 99.4 | 12 | 3.01 | 1.94 | 0.86 | 0.05 | 1.76 | 0.41 | - | 8.03 |
| 41.1 | 22.5 | 10.7 | 1.3 | 17.1 | 6.5 | - | 99.3 | 12 | 3.00 | 1.93 | 0.65 | 0.08 | 1.87 | 0.51 | - | 8.04 | ||||
| Ky | 36.4 | 62.5 | 0.9 | - | - | - | - | 99.8 | 5 | 0.99 | 2.00 | 0.02 | - | - | - | - | 3.01 | |||
| Fms | - | - | 11.8 | 0.8 | 33.1 | 7.3 | 47.0 | 100.0 | 3 | - | - | 0.15 | 0.01 | 0.76 | 0.12 | 0.98 | 2.02 | |||
| Crn | 0.0 | 98.5 | 0.7 | - | - | - | - | 99.2 | 3 | - | 1.99 | 0.01 | - | - | - | - | 2.00 | |||
| 2113-I | 6.3 | 1400 | Grt | 41.9 | 22.7 | 12.7 | 0.7 | 18.4 | 3.6 | - | 100.0 | 12 | 3.02 | 1.92 | 0.76 | 0.04 | 1.99 | 0.28 | - | 8.01 |
| 41.7 | 23.0 | 11.3 | 0.7 | 18.0 | 5.0 | - | 99.7 | 12 | 3.01 | 1.95 | 0.68 | 0.04 | 1.95 | 0.39 | - | 8.02 | ||||
| Fms | - | - | 12.2 | - | 33.4 | 3.8 | 50.6 | 100.0 | 3 | - | - | 0.15 | - | 0.74 | 0.06 | 1.02 | 1.97 | |||
| - | - | 9.0 | - | 36.0 | 3.8 | 51.1 | 100.0 | 3 | - | - | 0.11 | - | 0.79 | 0.06 | 1.02 | 1.98 | ||||
| Crn | - | 98.5 | 0.7 | - | - | - | - | 99.2 | 3 | - | 1.99 | 0.01 | - | - | - | - | 2.00 | |||
| Coe | 99.6 | - | - | - | - | - | - | 99.6 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| Run # | P, GPa | T, °C | Phase | Mass Concentrations, wt.% | n(O) | Cations per Formula Unit, p.f.u. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | MnO | MgO | CaO | CO2 | Σ | Si | Al | Fe | Mn | Mg | Ca | C | Σ | |||||
| 2137-I | 7.5 | 1150 | Ky | 36.0 | 62.4 | 0.9 | - | - | - | - | 99.2 | 5 | 0.98 | 2.00 | 0.02 | - | - | - | - | 3.00 |
| Fms | - | - | 19.1 | 0.8 | 27.1 | 6.5 | 46.6 | 100.0 | 3 | - | - | 0.25 | 0.01 | 0.64 | 0.11 | 1.00 | 2.01 | |||
| Msd | - | - | 39.4 | 0.7 | 16.0 | 0.5 | 43.4 | 100.0 | 3 | - | - | 0.56 | 0.01 | 0.41 | 0.01 | 1.01 | 2.00 | |||
| Coe | 99.5 | - | - | - | - | - | - | 99.5 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2138-I | 7.5 | 1250 | Ky | 36.9 | 61.3 | 1.8 | - | - | - | - | 100.0 | 5 | 1.01 | 1.97 | 0.04 | - | - | - | - | 3.02 |
| Msd | - | - | 41.0 | 0.7 | 14.5 | 0.5 | 43.2 | 100.0 | 3 | - | - | 0.58 | 0.01 | 0.37 | 0.01 | 1.00 | 1.97 | |||
| Fms | - | - | 17.4 | 0.7 | 28.6 | 7.1 | 46.2 | 100.0 | 3 | - | - | 0.23 | 0.01 | 0.68 | 0.12 | 1.00 | 2.04 | |||
| Coe | 98.5 | - | 1.2 | - | - | - | - | 99.7 | 2 | 1.00 | - | 0.01 | - | - | - | - | 1.01 | |||
| 2142-I | 7.5 | 1350 | Ky | 35.7 | 63.1 | 0.9 | - | - | - | - | 99.7 | 5 | 0.97 | 2.02 | 0.02 | - | - | - | - | 3.01 |
| Fms | - | - | 20.8 | 0.8 | 25.3 | 6.0 | 47.1 | 100.0 | 3 | - | - | 0.27 | 0.01 | 0.59 | 0.10 | 1.00 | 1.97 | |||
| - | - | 14.9 | 0.8 | 30.1 | 6.1 | 48.0 | 100.0 | 3 | - | - | 0.19 | 0.01 | 0.69 | 0.10 | 1.00 | 1.99 | ||||
| Crn | - | 98.5 | 0.7 | - | - | - | - | 99.2 | 3 | - | 1.99 | 0.01 | - | - | - | - | 2.00 | |||
| Coe | 99.6 | - | - | - | - | - | - | 99.6 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2144-I | 7.5 | 1450 | Grt | 40.0 | 22.2 | 19.5 | 1.1 | 11.9 | 5.2 | - | 99.9 | 12 | 3.00 | 1.96 | 1.22 | 0.07 | 1.34 | 0.42 | - | 8.01 |
| Ky | 35.3 | 62.6 | 1.7 | - | - | - | - | 99.6 | 5 | 0.97 | 2.02 | 0.04 | - | - | - | - | 3.03 | |||
| Fms | - | - | 19.2 | 0.8 | 27.3 | 6.0 | 46.9 | 100.0 | 3 | - | - | 0.25 | 0.01 | 0.64 | 0.10 | 1.00 | 2.00 | |||
| Coe | 99.6 | - | - | - | - | - | - | 99.6 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| Run # | P, GPa | T, °C | Phase | Mass Concentrations, wt.% | n(O) | Cations per Formula Unit, p.f.u. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | MnO | MgO | CaO | CO2 | Σ | Si | Al | Fe | Mn | Mg | Ca | C | Σ | |||||
| 1738-II | 3.0 | 1050 | Ky | 35.9 | 63.6 | 0.4 | - | - | - | - | 99.9 | 5 | 0.97 | 2.02 | 0.01 | - | - | - | - | 3.00 |
| Msd | - | - | 41.4 | 0.7 | 15.7 | 0.5 | 41.7 | 100.0 | 3 | - | - | 0.60 | 0.01 | 0.41 | 0.01 | 0.99 | 2.02 | |||
| Fms | - | - | 8.1 | - | 40.1 | 3.8 | 48.0 | 100.0 | 3 | - | - | 0.10 | - | 0.89 | 0.06 | 0.97 | 2.02 | |||
| Coe | 98.6 | 0.0 | 1.2 | - | - | - | - | 99.8 | 2 | 1.00 | - | 0.01 | - | - | - | - | 1.01 | |||
| 2122-II | 3.0 | 1150 | Grt | 40.0 | 22.3 | 18.2 | 0.9 | 13.2 | 4.6 | - | 99.3 | 12 | 3.00 | 1.97 | 1.14 | 0.06 | 1.48 | 0.37 | - | 8.02 |
| 40.9 | 22.9 | 14.5 | 0.8 | 14.9 | 5.7 | - | 99.8 | 12 | 3.00 | 1.98 | 0.89 | 0.05 | 1.64 | 0.45 | - | 8.01 | ||||
| 41.3 | 22.7 | 12.8 | 0.6 | 16.2 | 5.9 | - | 99.6 | 12 | 3.01 | 1.95 | 0.78 | 0.04 | 1.77 | 0.46 | - | 8.01 | ||||
| Ky | 33.7 | 65.0 | 0.9 | - | - | - | - | 99.6 | 5 | 0.92 | 2.09 | 0.02 | - | - | - | - | 3.03 | |||
| Msd | - | - | 40.8 | 0.7 | 16.1 | 0.5 | 41.8 | 100.0 | 3 | - | - | 0.59 | 0.01 | 0.42 | 0.01 | 0.99 | 2.02 | |||
| Fms | - | - | 10.3 | 0.8 | 35.3 | 5.6 | 48.0 | 100.0 | 3 | - | - | 0.13 | 0.01 | 0.80 | 0.09 | 0.99 | 2.02 | |||
| - | - | 5.0 | - | 39.0 | 3.9 | 52.1 | 100.0 | 3 | - | - | 0.06 | - | 0.84 | 0.06 | 1.02 | 1.98 | ||||
| Dol | - | - | 1.6 | - | 20.9 | 29.2 | 48.3 | 100.0 | 6 | - | - | 0.04 | - | 0.96 | 0.96 | 2.02 | 3.98 | |||
| Coe | 99.5 | - | - | - | - | - | - | 99.5 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2117-II | 6.3 | 1100 | Ky | 37.0 | 62.5 | 0.4 | - | - | - | - | 99.9 | 5 | 1.00 | 1.99 | 0.01 | - | - | - | - | 3.00 |
| Fms | - | - | 11.1 | 0.8 | 33.3 | 6.2 | 48.6 | 100.0 | 3 | - | - | 0.14 | 0.01 | 0.75 | 0.10 | 1.00 | 2.00 | |||
| Msd | - | - | 40.6 | 0.7 | 14.8 | 0.5 | 43.3 | 100.0 | 3 | - | - | 0.58 | 0.01 | 0.38 | 0.01 | 1.00 | 1.98 | |||
| Coe | 100.0 | - | - | - | - | - | - | 100.0 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2119-II | 6.3 | 1200 | Ky | 36.2 | 62.5 | 0.9 | - | - | - | - | 99.5 | 5 | 0.99 | 2.01 | 0.02 | - | - | - | - | 3.02 |
| Fms | - | - | 13.4 | 0.8 | 31.6 | 5.5 | 48.7 | 100.0 | 3 | - | - | 0.17 | 0.01 | 0.72 | 0.09 | 1.01 | 2.00 | |||
| Msd | - | - | 40.5 | 0.7 | 15.5 | 0.5 | 42.7 | 100.0 | 3 | - | - | 0.58 | 0.01 | 0.40 | 0.01 | 1.00 | 2.00 | |||
| Coe | 99.1 | 0.9 | - | - | - | - | - | 99.9 | 2 | 0.99 | 0.01 | - | - | - | - | - | 1.00 | |||
| 2115-II | 6.3 | 1300 | Grt | 40.1 | 22.1 | 17.7 | 1.0 | 14.1 | 4.6 | - | 99.6 | 12 | 2.99 | 1.94 | 1.10 | 0.06 | 1.57 | 0.37 | - | 8.03 |
| Ky | 35.9 | 62.9 | 0.9 | - | - | - | - | 99.7 | 5 | 0.98 | 2.02 | 0.02 | - | - | - | - | 3.02 | |||
| Coe | 99.3 | - | - | - | - | - | - | 99.3 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| Fms | - | - | 13.3 | 0.8 | 31.8 | 6.1 | 48.0 | 100.0 | 3 | - | - | 0.17 | 0.01 | 0.73 | 0.10 | 1.00 | 2.01 | |||
| 2113-II | 6.3 | 1400 | Grt | 40.9 | 21.9 | 17.7 | 0.8 | 15.8 | 2.8 | 0.0 | 99.8 | 12 | 3.02 | 1.90 | 1.09 | 0.05 | 1.75 | 0.22 | - | 8.03 |
| Ky | 36.3 | 62.7 | 0.4 | - | - | - | - | 99.5 | 5 | 0.99 | 2.01 | 0.01 | - | - | - | - | 3.01 | |||
| Crn | 0.0 | 99.1 | 0.7 | - | - | - | - | 99.8 | 3 | - | 1.99 | 0.01 | - | - | - | - | 2.00 | |||
| Coe | 99.3 | - | - | - | - | - | - | 99.3 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| Fms | - | - | 18.0 | 0.8 | 30.4 | 3.0 | 47.8 | 100.0 | 3 | - | - | 0.23 | 0.01 | 0.70 | 0.05 | 1.00 | 1.99 | |||
| - | - | 14.2 | 0.8 | 33.7 | 3.7 | 47.7 | 100.0 | 3 | - | - | 0.18 | 0.01 | 0.77 | 0.06 | 0.99 | 2.01 | ||||
| 2135-II | 7.5 | 1250 | Msd | - | - | 40.3 | 0.7 | 15.8 | 1.1 | 42.1 | 100.0 | 3 | - | - | 0.58 | 0.01 | 0.41 | 0.02 | 0.99 | 2.01 |
| Fms | - | - | 15.6 | 0.8 | 28.6 | 7.3 | 47.7 | 100.0 | 3 | - | - | 0.20 | 0.01 | 0.66 | 0.12 | 1.00 | 1.99 | |||
| - | - | 10.3 | 0.8 | 34.0 | 6.2 | 48.6 | 100.0 | 3 | - | - | 0.13 | 0.01 | 0.77 | 0.10 | 1.00 | 2.01 | ||||
| 2139-II | 7.5 | 1350 | Ky | 32.5 | 65.8 | 1.3 | - | - | - | - | 99.6 | 5 | 0.89 | 2.12 | 0.03 | - | - | - | - | 3.04 |
| Msd | - | - | 39.3 | 0.7 | 15.7 | 2.1 | 42.2 | 100.0 | 3 | - | - | 0.57 | 0.01 | 0.41 | 0.04 | 1.00 | 2.03 | |||
| Fms | 1.9 | 5.4 | 12.2 | 0.7 | 25.0 | 8.3 | 46.5 | 100.0 | 3 | 0.03 | 0.10 | 0.16 | 0.01 | 0.59 | 0.14 | 1.00 | 2.03 | |||
| Coe | 99.8 | - | - | - | - | - | - | 99.8 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
| 2140-II | 7.5 | 1450 | Grt | 42.0 | 22.6 | 11.8 | 0.7 | 19.5 | 3.2 | - | 99.8 | 12 | 3.02 | 1.91 | 0.71 | 0.04 | 2.10 | 0.25 | - | 8.03 |
| Ky | 35.1 | 63.0 | 1.3 | - | - | - | - | 99.4 | 5 | 0.96 | 2.03 | 0.03 | - | - | - | - | 3.02 | |||
| Coe | 99.3 | - | - | - | - | - | - | 99.3 | 2 | 1.00 | - | - | - | - | - | - | 1.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataleva, Y.V.; Novoselov, I.D.; Kruk, A.N.; Furman, O.V.; Palyanov, Y.N. Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of CO2 Fluid and Garnets of Model Carbonated Eclogites under Lithospheric Mantle P,T-Parameters. Minerals 2023, 13, 859. https://doi.org/10.3390/min13070859
Bataleva YV, Novoselov ID, Kruk AN, Furman OV, Palyanov YN. Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of CO2 Fluid and Garnets of Model Carbonated Eclogites under Lithospheric Mantle P,T-Parameters. Minerals. 2023; 13(7):859. https://doi.org/10.3390/min13070859
Chicago/Turabian StyleBataleva, Yuliya V., Ivan D. Novoselov, Aleksei N. Kruk, Olga V. Furman, and Yuri N. Palyanov. 2023. "Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of CO2 Fluid and Garnets of Model Carbonated Eclogites under Lithospheric Mantle P,T-Parameters" Minerals 13, no. 7: 859. https://doi.org/10.3390/min13070859
APA StyleBataleva, Y. V., Novoselov, I. D., Kruk, A. N., Furman, O. V., & Palyanov, Y. N. (2023). Experimental Modeling of Decarbonation Reactions, Resulting in the Formation of CO2 Fluid and Garnets of Model Carbonated Eclogites under Lithospheric Mantle P,T-Parameters. Minerals, 13(7), 859. https://doi.org/10.3390/min13070859







