Abstract
Lithium-bearing polymetallic pegmatite ores are an important raw material for lithium extraction. They contain not only lithium but also other associated elements such as beryllium, tantalum, and niobium, with great recovery values. It is therefore often called lithium-bearing polymetallic pegmatite ore (LPPO). The recovery and utilization of Be-bearing minerals in LPPOs have yet to be further studied. This paper briefly expounds the geological aspects of LPPO deposits in China and Chinese experiences on the beneficiation of LPPOs, with special emphasis on the flotation separation of lithium-beryllium minerals. In LPPO, spodumene is the main target mineral for lithium, while beryl is the main Be-bearing mineral in a fine-grained embedded state. If the BeO grade of LPPO is greater than the industrial grade (BeO ≥ 0.04%), it will be processed for recovery. Tantalum and niobium minerals are mainly in the form of tantalite, columbite, or ferrotapiolite, which may be recovered by gravity separation or magnetic separation. Gangue minerals are mainly composed of albite and quartz. Currently, the most commonly used methods for separating the target minerals from gangue are dense medium separation and flotation. The manual sorting method has become obsolete and is expected to be replaced by machine sorting methods such as color sorters and X-ray transmission sorters. Flotation is the main method for the separation of fine-grained beryl and spodumene. The success of flotation depends on the selection of suitable pretreatment methods and appropriate flotation reagents for altering the surface properties of spodumene and beryl and for expanding the floatability differences between spodumene and beryl and between spodumene and gangue.
1. Introduction
Although the lithium resources in brine are abundant, accounting for 62.6% of the lithium resources, the extensive use of the resources is compromised by the existing impurity removal technology [1]. For this reason, hard rock lithium ore remains the main raw material for the production of high-purity lithium products [2,3]. The lithium extraction technologies from hard rock lithium mines are relatively mature, and they have helped with the rapid response to the growth of the global demand for lithium [4]. In 2019, 72% of China’s lithium salt products came from hard rock lithium ore [5]. However, with the possible breakthrough of the impurity removal technology, the current dominance by the hard rock lithium ore may be challenged in the future by possible technological breakthroughs in lithium extraction from brine [6,7,8].
Hard rock lithium ores used for lithium extraction may be grouped into two types based on geological deposits in China [9]: (1) lithium-bearing polymetallic pegmatite ores (LPPO); (2) alkaline feldspar granite clay-lithium ore. LPPO is the most important hard rock ore for extracting lithium because of its high lithium grade and large lithium reserves. It is associated with multi-metals, often containing beryllium, niobium, tantalum, etc. [10]. The main lithium-bearing minerals in this type of ore include spodumene, rare lepidolite, amblygonite, petalite, etc. Taking Xinjiang Dahongliutan lithium ore as an example, the content of spodumene in the ore is 93.32%, that of lepidolite is 6.15%, and that of amblygonite is 1.53%. Other lithium-bearing minerals have not been detected. The main Be-bearing minerals are beryl, etc. [11,12], and the main tantalum-niobium minerals include tantalite, columbite, and ferrotapiolite [13,14]. If the grades of the beryllium, niobium, and tantalum ores are greater than the industrial grade (BeO ≥ 0.04%, Ta2O5 and Nb2O5 0.022%~0.026%), it is believed that beryllium, tantalum, and niobium should be recovered from the ore. If the grade is lower than the industrial grade, it is not economical to recover them from the ore [15]. In the alkaline feldspar granite clay lithium ore, the main lithium-bearing mineral is lepidolite. The Yichun lithium deposit in Jiangxi, China is an example of such type of deposit [16].
The focus of this paper is on the review of beneficiation technologies for LPPO. The most unique feature of the ores lies in the co-association of Li, Be, Ta, and Nb minerals.
Beryllium, as an important strategic metal, is highly valued by all countries in the world. It is an indispensable precious material in the atomic energy, rocket, missile, aviation, aerospace, and metallurgical industries [17]. Beryllium is also known as the treasure of the atomic energy industry. In atomic reactors, beryllium is a neutron source that can provide a large shell of neutrons (hundreds of thousands of neutrons per second) [18,19,20].
There are more than 60 Be-bearing minerals that have been discovered in the world. About 40 of them either have industrial values or exist in most deposits. The main industrial minerals are beryl, bertrandite, xrizoberyl, phenacite, gelvin, getgelvin, danolit, etc. [15,21]. Be-bearing minerals are mainly found in co-associated deposits. According to the mineral composition and deposit origin, two distinct classes of deposits currently account for most beryllium ores; they are (a) pegmatite deposits that have an abundance of the mineral beryl and (b) volcanic and carbonate-hosted deposits that contain the mineral bertrandite [22].
Since beryl-bearing ores are non-magnetic and have a specific gravity close to that of gangue minerals, they cannot be separated by gravity or magnetic separation. Flotation is the most important means of separating beryl from the ores. Beryl is often associated with silicate minerals such as spodumene, quartz, albite, and mica. The flotation of beryl-bearing ores can be divided into the acid method and alkali method. In the acid process, the raw ore was adjusted with HCl, HF, or H2SO4, and mica was first removed by flotation using an amine collector under acidic conditions. After that, the slurry was adjusted to a basic pH to float the beryl using a petroleum sulfonic acid soap collector. In the alkaline process, the pulp was adjusted with NaOH, and then the beryl was floated with an anionic collector such as fatty acids under weak alkaline conditions [23,24].
In bertrandite-bearing ores, the main gangue minerals associated with bertrandite ore are fluorite, quartz, mica, etc. In China, the most commonly used mineral processing method of bertrandite-bearing ores is the flotation-leaching combined method. Zheng and his coworkers, in 2012, used the oxidized paraffin soap and oleic acid with a mass ratio of 3:1 as the combined collector and floated the bertrandite by a closed-circuit test of the ore with a process flow of “one roughing, two sweeping, and three cleaning after grinding”; the BeO grade of the product was improved from 0.28% to 6.5%, and the BeO recovery rate was raised to 84% [25].
Chrysoberyl is also an important beryllium resource in China, which is associated with fluorite, mica, and other minerals. When the grinding fineness was −74 μm particles accounted for 85%, the sulfide minerals and calcium-bearing minerals in the ore were removed by the flotation method, and then the chrysoberyl was floated with oxidized paraffin soap as the collector to obtain beryllium concentrate with a BeO grade of 0.4%. The concentrate then underwent roasting, sulfuric acid leaching, solvent extraction, and reverse extraction, and the recovery rate of the beryllium product reached 80% [26,27,28].
Tantalum and niobium metals show very good ductility and corrosion resistance and are widely used in national defense, aviation, aerospace, electronic computers, and high-end household appliances. For instance, tantalum and its alloys are widely used in the orthopedics and dentistry fields due to their good biocompatibility, corrosion resistance, proper elastic modulus, and high damping capacities [29]. A niobium alloy that can be used in liquid rocket thruster nozzles was used in the main engine of the Apollo lunar module [30,31,32]. Hence, the comprehensive recovery of lithium, beryllium, tantalum, and niobium from LPPO plays a crucial role in the improvement of the effective utilization of lithium, beryllium, tantalum, and niobium resources.
2. Geological Aspects of LPPO Deposits in China
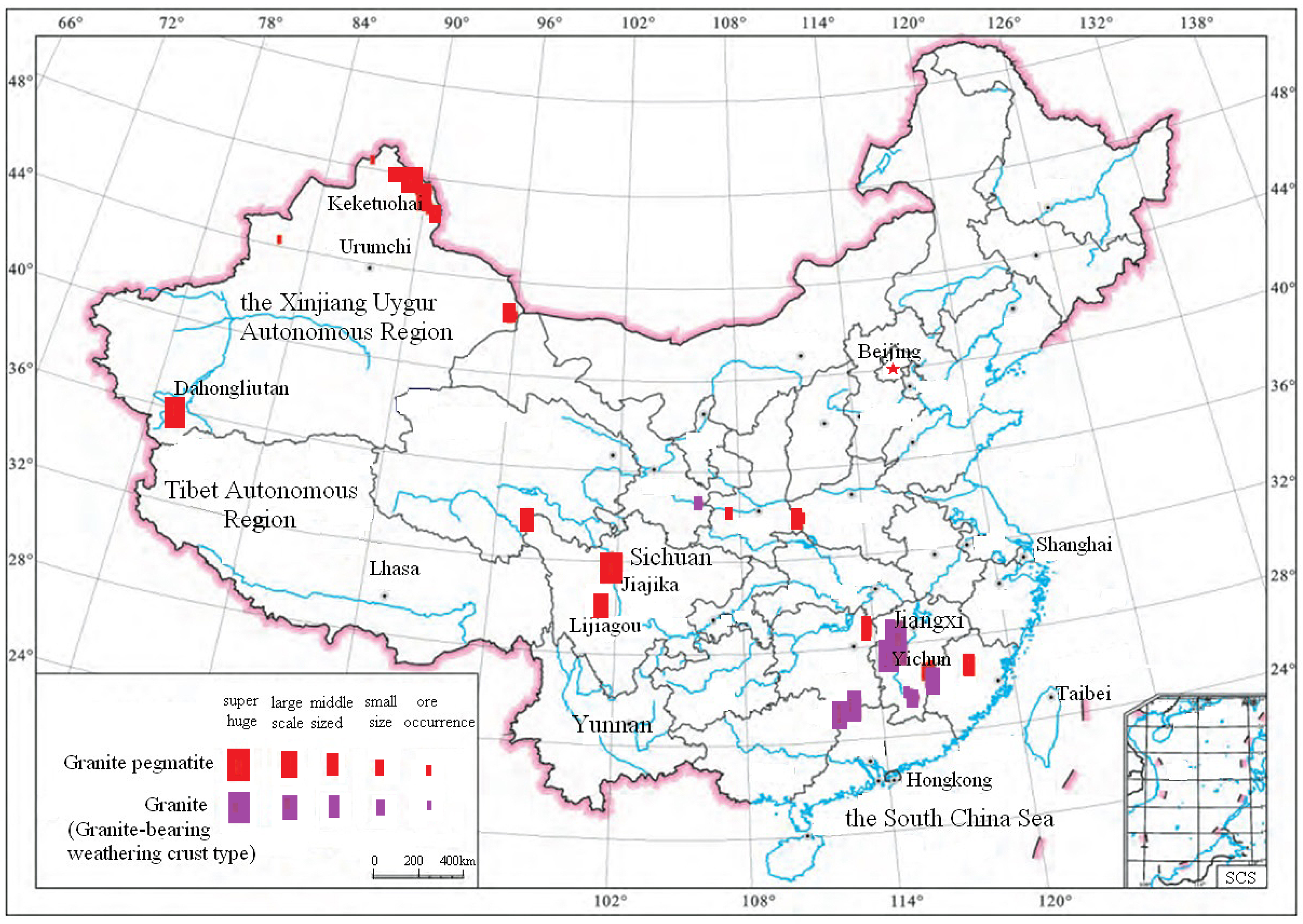
LPPO deposits in China are mainly distributed in Sichuan Province and Xinjiang Autonomous Region [33,34,35,36]. Most of the LPPO deposits are lithium and beryllium co-associated ore deposits, in which the BeO grade is relatively low, only close to the industrial grade [37,38]. Some typical LPPO deposits and their distribution map in China are shown in Table 1 and Figure 1 [39,40].

Table 1.
Typical LPPO deposits in China.
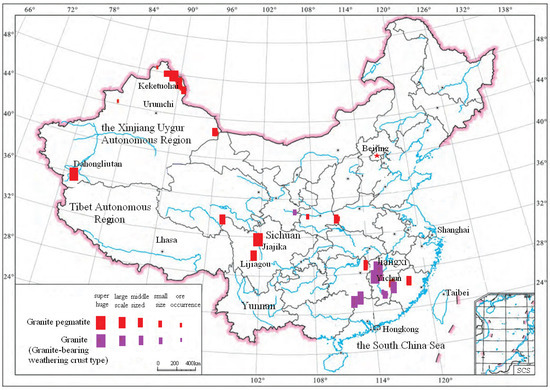
Figure 1.
The distribution map of hard rock lithium deposits in China [39].
As seen from Table 1 and Figure 1, the LPPO deposits in China include Jiajika and Lijiagou lithium deposits in Sichuan and Keketohai and Dahongliutan lithium deposits in Xinjiang.
Of all the above-mentioned deposits, the Dahongliutan lithium deposit was newly discovered in 2017 in Hetian County, Xinjiang Autonomous Region. According to the report by Wang He and his team [36] in 2021, the Dahongliutan lithium deposit has the following characteristics: (1) polymetallic co-associated; (2) coarse crystal grain size; (3) large lithium and beryllium reserves; the lithium reserve is 5.06 million tons in terms of lithium oxide, and the associated BeO reserve is 160,000 tons; the average Li2O grade is between 0.93% and 3.44%, and the BeO grade is between 0.54% and 0.63% [36].
Table 2, Table 3 and Table 4 show the mineral species, mineral composition, theoretical grade, density, and hardness of lithium-bearing minerals, beryllium-bearing minerals, tantalum or niobium minerals, and main gangue minerals in granite pegmatite lithium-beryllium polymetallic deposits [9,21].

Table 2.
Lithium-bearing minerals in LPPO deposits [9,21].

Table 3.
Beryllium-bearing minerals in LPPO deposits [9,21].

Table 4.
Tantalum or niobium minerals and main gangue minerals in LPPO deposits [9,21].
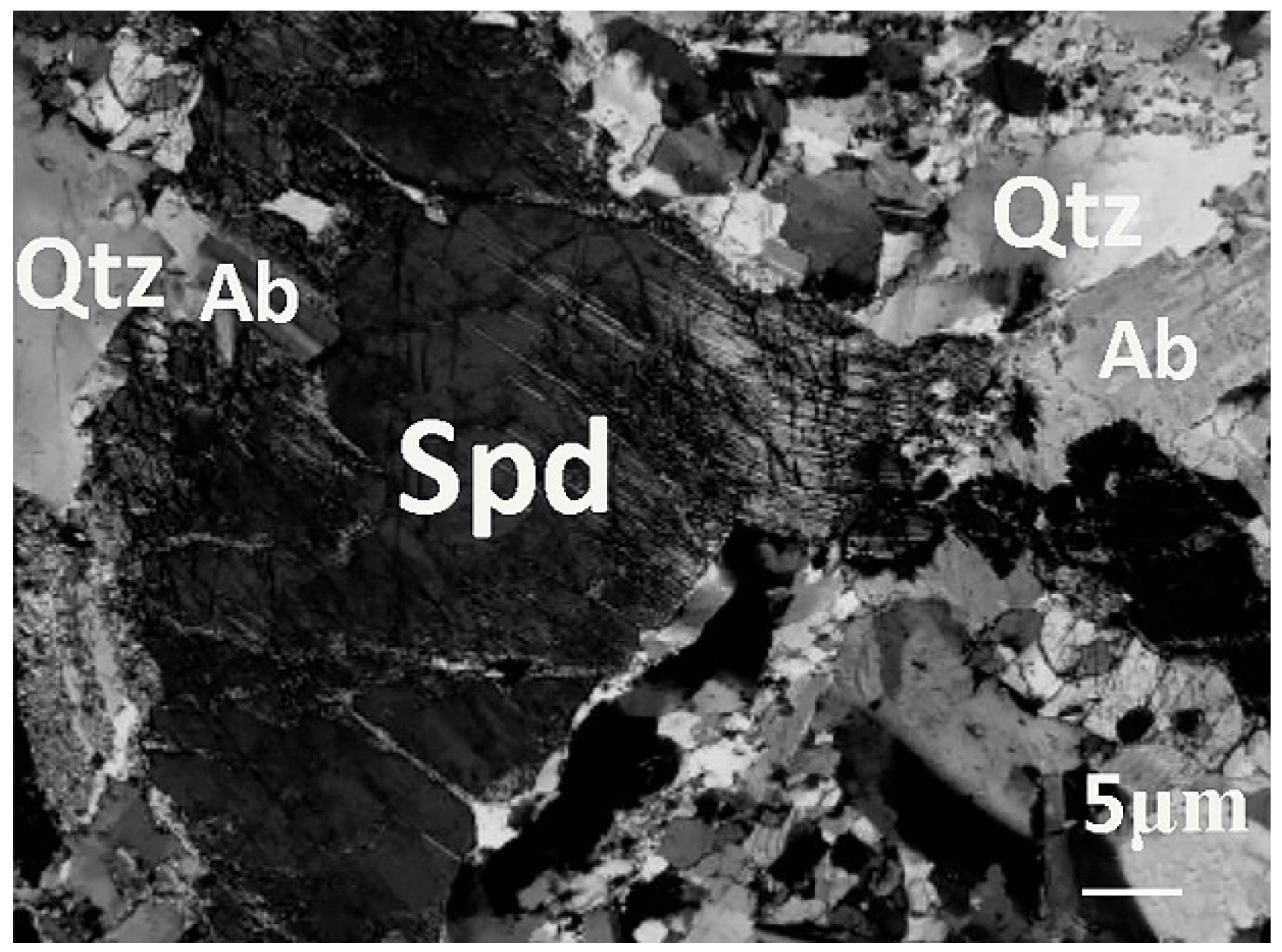
As shown in Table 2, LPPO deposits include spodumene, lepidolite, amblygonite, zinnwaldite, petalite, and eucryptite. Among them, spodumene is the main lithium-bearing mineral existing in the deposit; its Li2O theoretical grade is 8.03% [13,41]. The beryllium-bearing minerals in the granite pegmatite deposit, as shown in Table 3, are beryl, bertrandite, xrizoberyl, phenacite, etc. Among them, beryl is the main beryllium-bearing mineral [13,22,41] existing in the deposit; its BeO theoretical grade is 5.03%. It can be seen from Table 4 that the main tantalum or niobium minerals are tantalite, columbite, and ferrotapiolite. These three minerals, tantalite (6.25–7.90), columbite (5.00), and ferrotapiolite (5.00–7.90), are relatively denser compared to the density of albite (2.62) and quartz (2.65). If dissociated sufficiently, they might be separated from the gangue minerals by gravity separation. The main gangue minerals are quartz, plagioclase, albite, and muscovite. Figure 2 illustrates the morphology of an LPPO in Hetian County, Xinjiang Autonomous Region [23].
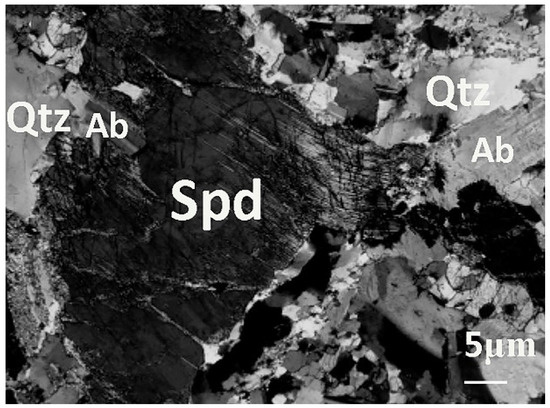
Figure 2.
Morphological picture of an LPPO in Hetian County, Xinjiang Autonomous Region [23].
Figure 2 is the microphotograph of albite-spodumene pegmatite in Xinjiang. As can be seen, spodumene, quartz, and albite all have coarse crystalline grain sizes. Quartz and albite coexist with spodumene. The dahongliutan lithium deposit belongs to the pegmatite deposit. The main mineral composition is about 20% spodumene, 44% albite, and 28% quartz. These three main minerals account for about 92%, and the remaining 8% are lepidolite, amblygonite, beryl, ferrotapiolite, muscovite, etc. [13,36]. Coarse-grained pegmatite minerals and fine-grained disseminated minerals exist simultaneously. There are two selection methods: the positive selection of spodumene and the reverse selection of quartz and albite. It is more economical to pick out those well-dissociated pure spodumene particles in the size of −10 mm + 0.5 mm in advance and dispose those well-dissociated pure quartz and albite particles in the size of −10 mm + 0.5 mm prior to the flotation process in order to reduce the amount of ore for subsequent flotation. The remaining ore then undergoes size reduction to liberate the gangue minerals, particularly quartz and albite, so that they can be removed. After this, the ore will undergo fine grinding to liberate the spodumene and will then undergo flotation to be recovered.
Although there are various forms of lithium-bearing minerals, spodumene is the most valuable lithium-bearing mineral, while other lithium minerals, such as lepidolite, amblygonite, zinnwaldite, petalite, and eucryptite, have little in terms of content, and their composition in the ore is not sufficient to warrant economic recovery. The beryllium-bearing minerals with recovery value in the deposit are mainly beryl, while other beryllium-bearing minerals such as bertrandite, xrizoberyl, phenacite, gelvin, getgelvin, and danolit have very little in terms of content, and their composition in the ore is not sufficient to warrant economic recovery.
3. Methods in Recovering Li2O and BeO Commonly Used in China
There are many methods for recovering Li2O and BeO from LPPOs, but there are few works of literature focusing on the recovery of beryllium, tantalum, and niobium minerals, especially the flotation separation between lithium and beryllium minerals.
At present, methods for recovering Li2O and BeO from LPPOs mainly include manual sorting, color sorting, X-ray transmission sorting, dense media separation, flotation, and so on. The details are shown in Table 5.

Table 5.
Eight methods for recovering Li2O and BeO commonly used in China.
As can be seen from Table 5, there are eight methods for recovering Li2O and BeO that are commonly used in China. Each method has its own scope of adaptation, but the most commonly used and most efficient methods are the dense medium method and flotation. Flotation is one of the most effective methods for separating lithium and beryllium minerals [38]. The following are detailed descriptions of each LPPO beneficiation method.
3.1. Manual and Color Sorting
The manual sorting method is usually applied based on the fact that pegmatite spodumene ores are coarse crystals. They show obvious distinctions in color and shape from gangue minerals, which makes them feasible for hand sorting. The particle size of hand-selected ore is generally between 10 mm and 25 mm. In pegmatite deposits, bulk spodumene minerals, bulk feldspar, and quartz minerals can be hand-selected, and the determination of the lower limit of the hand-selected particle size depends on economic benefits. The hand selection operation is applied in Maerkang Lithium Mine in Sichuan Province. The Keketuohai mine and Aletai mine in Xinjiang also applied manual sorting to produce spodumene concentrate [38]. When the raw ore Li2O grade was 1.5%–1.8%, a spodumene concentrate with an Li2O grade of 5%–6% and a recovery rate of 20%–30% was obtained by manual picking. The color of the gangue is black—conspicuously different from that of the spodumene—so it is easy to be picked out by hand [42].
Manual sorting is the method that has the longest history in lithium ore beneficiation. With the development of science and technology, the manual sorting method is expected to be replaced by machine selection—such as color sorters and X-ray transmission sorters. When exposed to 365 nm ultraviolet light, pure spodumene mineral has fluorescent luminescence properties, while other minerals and gangue minerals do not. Using this property, spodumene can be accurately separated from albite and quartz.
For minerals with a good crystalline state and obvious color difference, color sorting technology is used for mineral separation [43,44]. Liu and coworkers (2021) found that spodumene, plagioclase, and quartz were in light green, white, and gray respectively; some dark minerals were in black, brown, or dark gray [45]. The manual sorting and mineralogical analysis of the sorted ores showed that 74% of the dark minerals were amphibole, 8% were chlorite, 8% were mica, 5% were quartz, and 5% were plagioclase, and the overall particle sizes were relatively coarse. The LS300 crawler color sorter was used based on the color difference of the ore. The test conditions were a crawler speed of 3.2 m/s, an injection valve pressure of 0.8 MPa, and a feeder vibration frequency of 20 Hz. The final color sorting results were that the operational yield of the color sorting concentrate was 82.72%; the grade of Li2O was 6.18%; the recovery rate of Li2O was 89.23%; the yield of tailing was 17.28%; and the Li2O grade of tailing was 3.57%. Color separation technology was suitable for coarse particle beneficiation with a low production cost [45].
3.2. X-ray Transmission Sorting
As an X-ray is irradiated to a substance, the linear coefficient of the X-ray absorption of the substance will be different when its elemental composition, density, and other properties are different. Using this principle, the characteristic values of the ore can be accurately detected by an X-ray penetrating through the ore to a depth of 6–8 cm [46]. By an artificial intelligence algorithm and X-ray signal depth recognition neural network, the datum information of the target ore is recognized and processed, and the inner composition and density of the ore are detected in a 360° all-round way. The characteristic data of the ore are transmitted to the solenoid valve of the sorting system, and the automatic and intelligent injection device can accurately separate the gangue from the target minerals. An X-ray transmission sorter is used for the ore pre-selection of −60 mm + 10 mm in size. The X-ray transmission sorting machine produced by Longji Magnetoelectric Equipment Co., Ltd. in Fushun city, China has a processing capacity of up to 70 tons per machine per hour. In China, the X-ray transmission sorting method has been applied gradually in mines such as fluorite, phosphate ore, quartz, and kaolin mines with an ideal separation effect and remarkable economic benefit. However, it is still in the experimental/promotion stage for spodumene mines. The X-ray transmission sorting method has yet to be studied, and it is expected to take the place of the manual sorting method and be used in the pre-selection of coarse-grained pegmatite spodumene ore in the future [47].
3.3. Gravity Separation
Valuable elements such as tantalum and niobium are often associated with LPPO deposits. Tantalum or niobium mainly exist in the form of tantalite, columbite, or ferrotapiolite. Due to the high density of these three minerals, they could be recovered by gravity. For example, Wang and coworkers, 2021 found that, in addition to spodumene and beryl, a certain granite pegmatite lithium ore also contained tantalum or niobium minerals such as tantalite, columbite, or ferrotapiolite. The process flow of “a course grinding, a gravity pre-enrichment, a strong magnetic separation, and centrifugal separation” was determined according to the properties of the ore. The selected particle size of the gravity separation was 100% −0.35 mm, and the pre-concentration of tantalum and niobium minerals was achieved through the roughing selection process of a spiral chute-shaker [41].
3.4. Dense Medium Separation
The principle of dense medium separation is to select a dense medium whose density is between the density of spodumene and gangue minerals so that minerals with different densities float or settle in the medium to achieve effective separation. The heavier particles settle and are discharged through the underflow, while the light particles float and are discharged through the overflow. The dense medium beneficiation method is used for ores with a particle size of −10 mm + 0.5 mm. The dense medium cyclone is used as the dense medium sorting equipment; tantalite, columbite, or ferrotapiolite suspension is selected as the dense medium; the processing capacity of the dense medium cyclone can reach 50~100 tons per hour [48,49,50].
3.5. Magnetic Separation
Magnetic separation is an auxiliary method for improving the quality of lithium concentrates. It is often used to (1) remove iron-containing impurities in spodumene concentrates, such as hematite or magnetite; (2) separate tantalite, columbite, or ferrotapiolite and zinnwaldite; (3) separate Sn, Mn, and Co impurities from ores; etc.
The spodumene concentrate obtained from the flotation method sometimes contains a lot of iron-bearing minerals. Magnetic separation is used to obtain low-iron spodumene and improve the product grade of the spodumene concentrate. For instance, the granite pegmatite spodumene ore in Renli-Chuanzi, Hunan Province contains hematite. In order to ensure that the Fe2O3 content in lithium concentrate or in non-metallic gangue minerals (albite, quartz) products does not exceed the requirement (Fe2O3 content < 2.5%), it is necessary to carry out high-intensity magnetic separation for the iron removal of feed ores. The process flow of “desliming–magnetic separation–flotation” is adopted to obtain a lithium concentrate with an Li2O grade of 6.05%, Li2O recovery of 79.77%, and Fe2O3 content of 0.83% [51].
Han et al., in 2012, made a microscopic observation on a pegmatite lithium ore with a total iron content of 0.71% and found that, in addition to niobium-tantalum minerals and cassiterite, a small amount of magnetite was also found in coarse-grained lithium ore. Therefore, a low-intensity magnetic separation test was carried out on coarse-grained ores to remove magnetite, and then high = intensity magnetic separation was conducted on its tailings. The high magnetic concentrate consisted of tantalite, niobite, or ferrotapiolite, and the high magnetic tailing consisted of cassiterite. After the two-step magnetic separation, three products were obtained, which are tantalite concentrate with a Ta2O5 grade of 18.81% and Ta2O5 recovery of 66.16%, niobite concentrate with an Nb2O5 grade of 37.25% and Nb2O5 recovery of 68.95%, and cassiterite concentrate with an SnO2 grade of 44.26% and SnO2 recovery of 83.27% [52].
3.6. Flotation Method
Flotation is considered the most important method for LPPOs beneficiation [9]. If the BeO grade of LPPOs is greater than the industrial grade (0.04%), Be-bearing minerals will tend not to be discarded due to their economic value. They will be recovered, and flotation is the only way to recover Be-bearing minerals (beryl).
The key to the success of the flotation technology of the pegmatite lithium ore lies in (1) slime treatment before flotation to prevent the slimes from being adsorbed on the surface of the target minerals; (2) a significant difference in the surface properties between the target minerals and the gangue minerals; and (3) the use of selective collectors, activators and depressants [9]. The following is a detailed explanation [53].
3.6.1. Slime Treatment Method before Flotation
In a flotation system, due to the existence of viscous minerals in the pulp, it is easy to have fine mud cover the surface of the target mineral particles. Therefore, the treatment method of argillaceous minerals before flotation is also critical to successful separation [54,55,56]. There are many treatment methods for argillaceous minerals, and the following six methods are commonly used, as shown in Table 6.

Table 6.
Treatment methods of argillaceous minerals before the flotation of LPPOs.
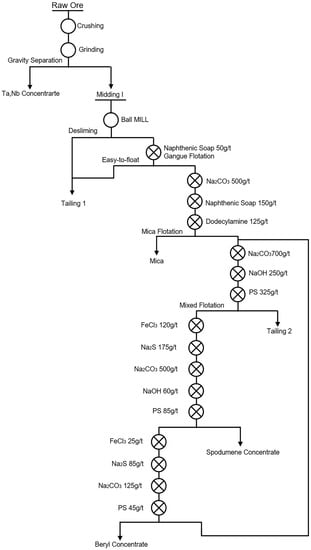
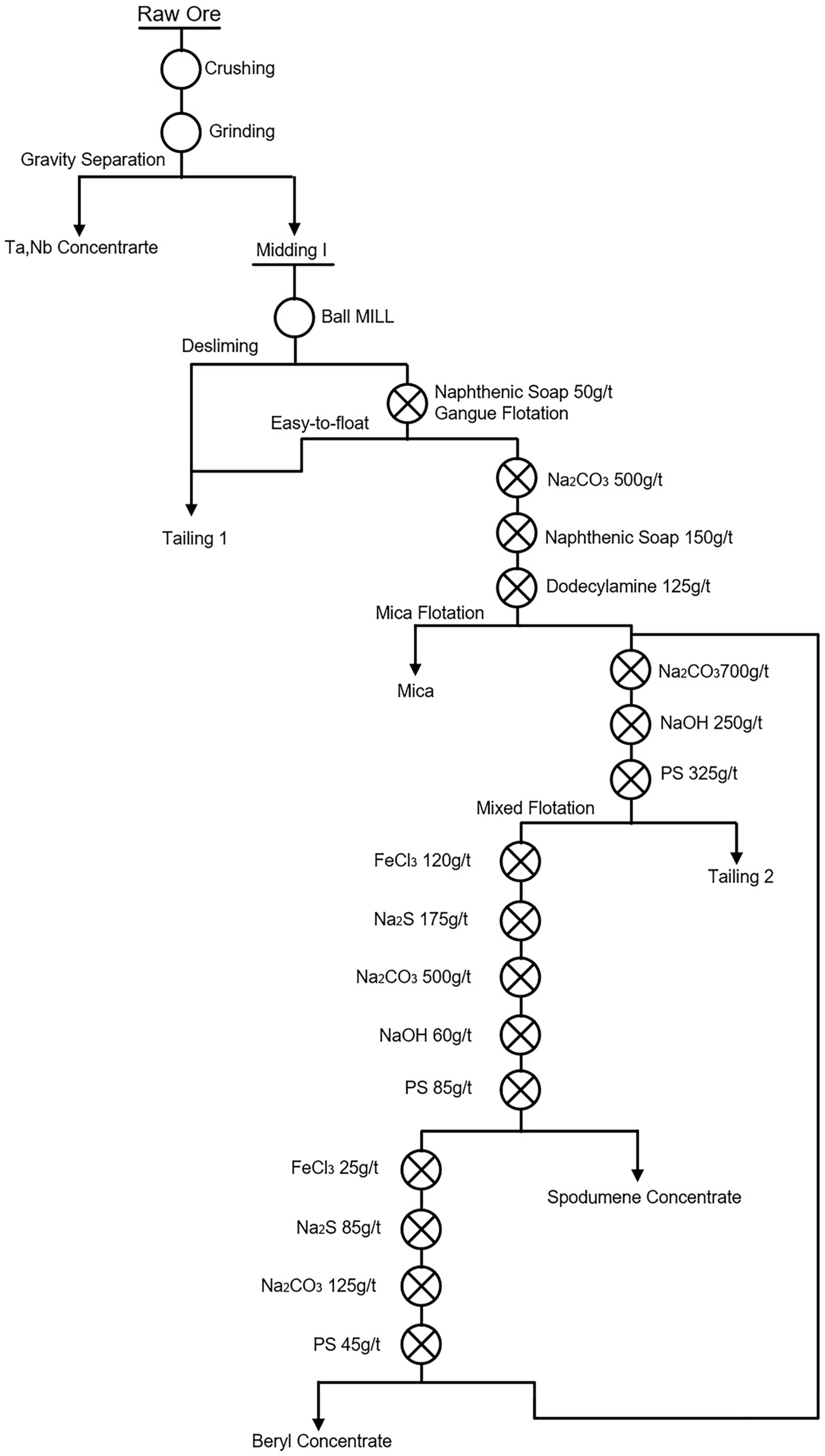
Figure 3.
Flotation process flow of Xinjiang Keketuohai Mine [9].
Table 6.
Treatment methods of argillaceous minerals before the flotation of LPPOs.
| No. | Slime Processing Method | Technological Feature | Reference |
|---|---|---|---|
| 1 | Depression method by autogenous sodium silicate at alkaline pulp |
| [55] [57] |
| 2 | Selective erosion method |
| [58] [51] |
| 3 | Gravity desliming method | Deslime by a countercurrent gravity column. | [59] |
| 4 | Ultrasonic washing method |
| [60] |
| 5 | Ore washing method using surface-cleaning agents |
| [61] |
| 6 | Flotation desliming method | The slime minerals were floated by a small amount collector. The foam products with a lower grade of lithium than the original ore were directly mixed into tailings to achieve effective desliming. An example of a flotation desliming method is shown in Figure 3. | [9] |
When the surface of a spodumene mineral is polluted by weathering or by argillaceous minerals in the pulp, its floatability will deteriorate. Some soluble salt ions (Ca2+, Fe3+, and Mg2+) in the pulp will not only activate spodumene but also activate beryl and gangue minerals at the same time, which reduces the separation efficiency. There is a consensus that pre-treatment and desliming must be carried out before the flotation of spodumene ore. Method 1 (depression method by autogenous sodium silicate at alkaline pulp) in Table 6 is a traditional desliming method which has the advantage of a low cost. Under the condition of strong alkali, sodium silicate formed by the reaction of silicate minerals with strong alkali acts as an inhibitor for separating spodumene from beryl and gangue. However, the changes in the mineral composition create limitations in its controllability, which sometimes may result in a low flotation efficiency. The methods of adding chemical agents (method 2, 5, and 6) are commonly used at present [9,52,61]. Emerging physical methods such as the gravity desliming method (method 3) and ultrasonic wave washing equipment (method 4) have shown development prospects [59,60].
3.6.2. Enhanced Differences in Mineral Surface Properties
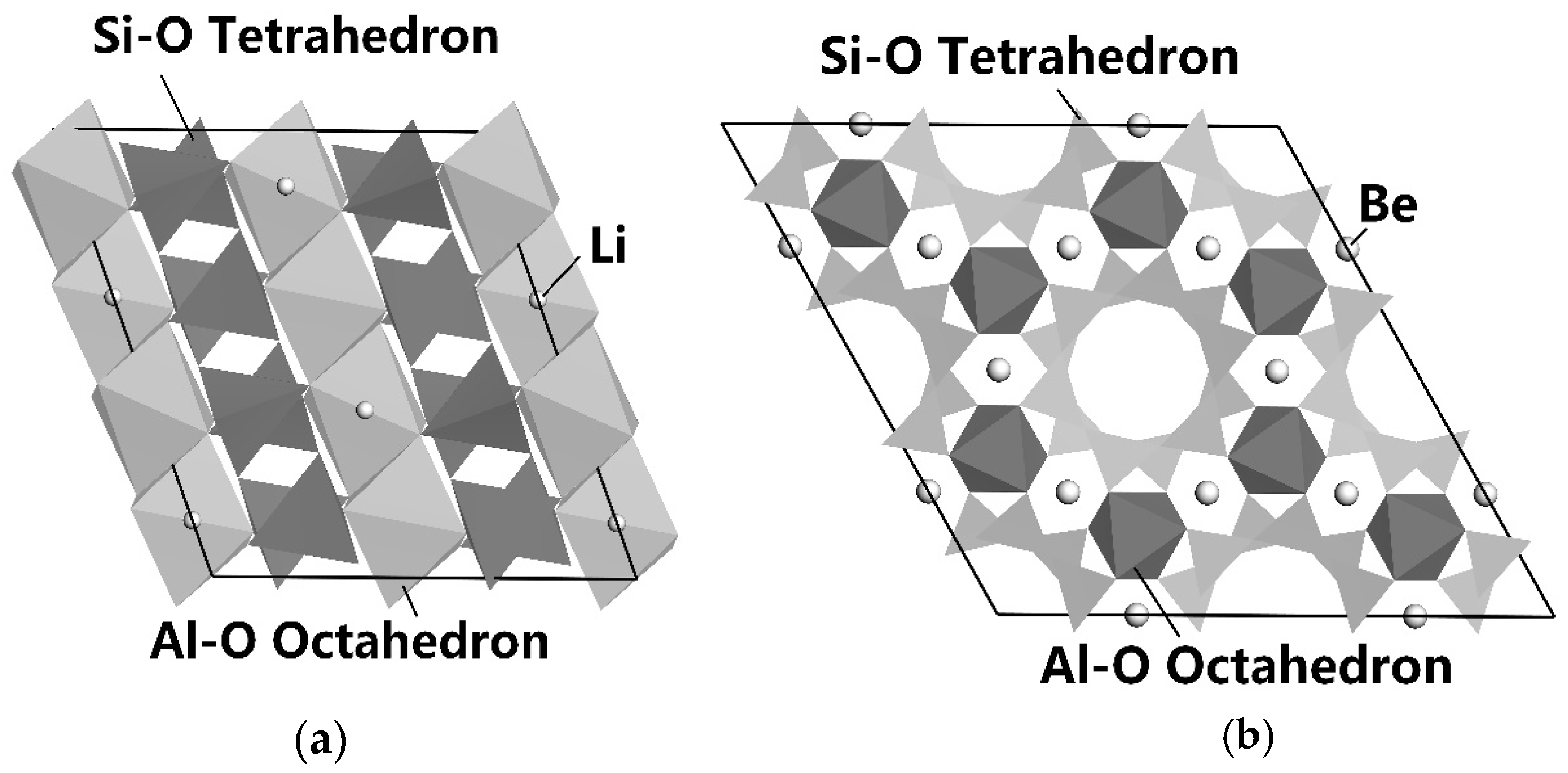
The main minerals in LPPOs are spodumene, beryl, albite, and quartz, which are aluminosilicates or silicon oxide, as shown in Figure 4 and Figure 5. The surface of these minerals has a similar chemical composition and chemical activities. It is very crucial for flotation separation to enhance the difference in the surface characteristics of these minerals and further enhance their floating difference. Figure 4a,b show the lattice structures of spodumene and beryl simulated by Materials Studio [62,63].
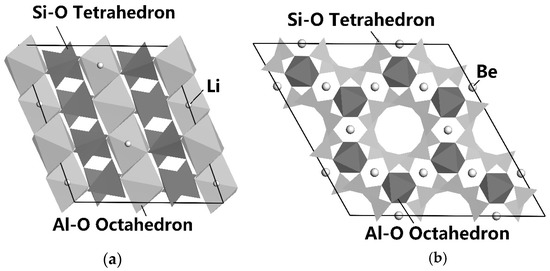
Figure 4.
The lattice structure of spodumene (a) and beryl (b) [64].
It can be seen from Figure 4 that spodumene is a chain-like lithium silicate mineral; the dark chain in the figure has a silicon-oxygen tetrahedron chain, plus a combination chain of Al-O octahedron and Li-O tetrahedron. Among them, the Li-O bond has the highest probability of bond breaking, followed by the Al-O bond; the Si-O bond has the lowest probability of breaking. Although both spodumene and beryl are aluminosilicate minerals, their structures are different, where one is chain-like (Figure 4a) and the other is ring-like (Figure 4b). After the fragmentation of spodumene and beryl, differences are shown in the distribution and quantity of the chemical active sites of Al, Si, O, Be, and Li exposed on the surface of spodumene and beryl minerals. The number of bonds per unit area and the number of dangling bonds on the surface are different, and the surface chemical properties are different as well [61].
Figure 5a,b show the lattice structures of albite and quartz simulated by Materials Studio [65].
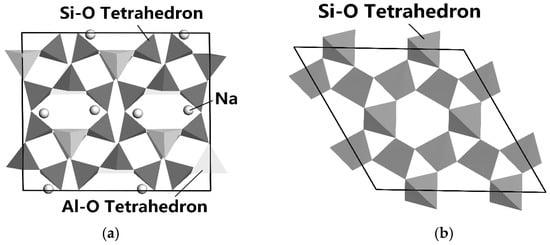
Figure 5.
The lattice structure of albite (a) and α-quartz (b) [66].
Figure 5.
The lattice structure of albite (a) and α-quartz (b) [66].
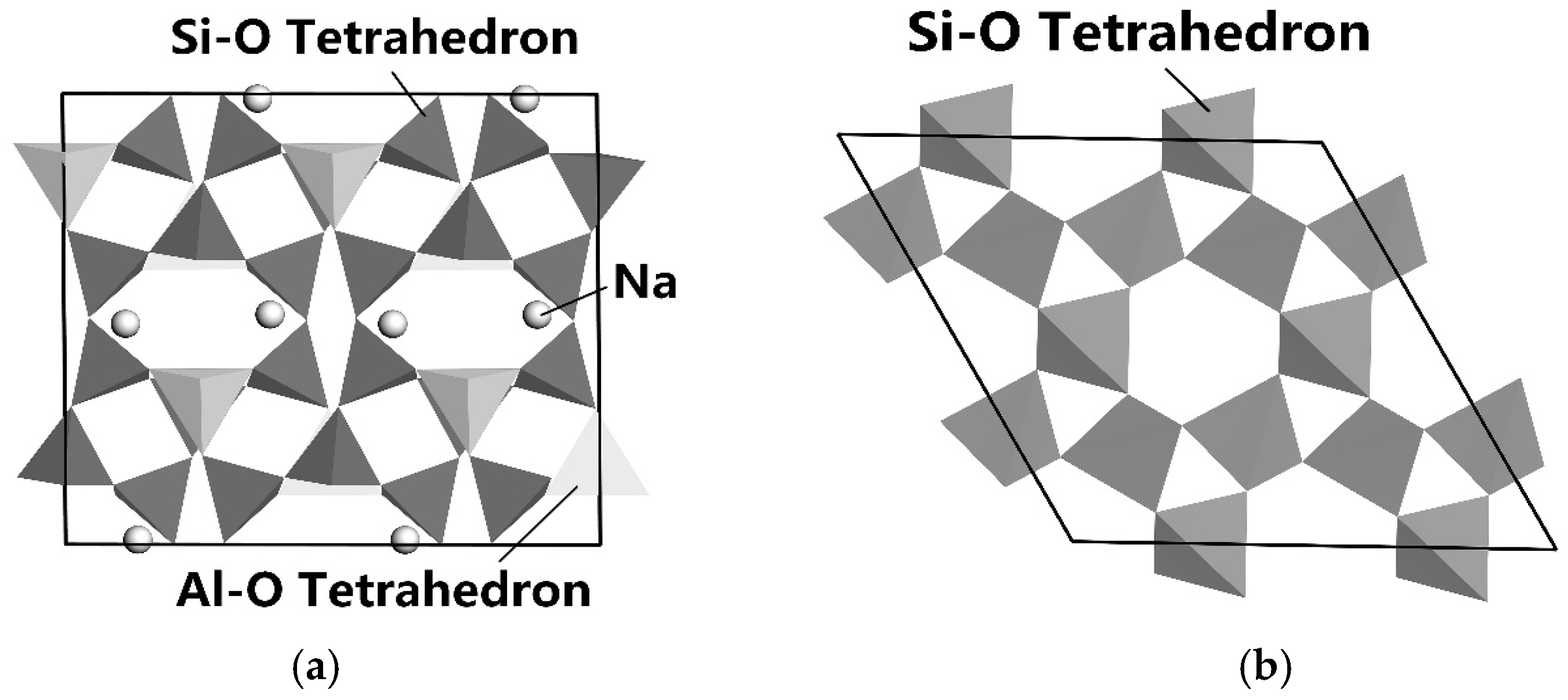
It can be seen from Figure 5a,b that the crystal structures of albite and quartz are both framework-type structures. On the surface of albite mineral, there are active sites such as Al, Si, Na, K, O, etc. [66].
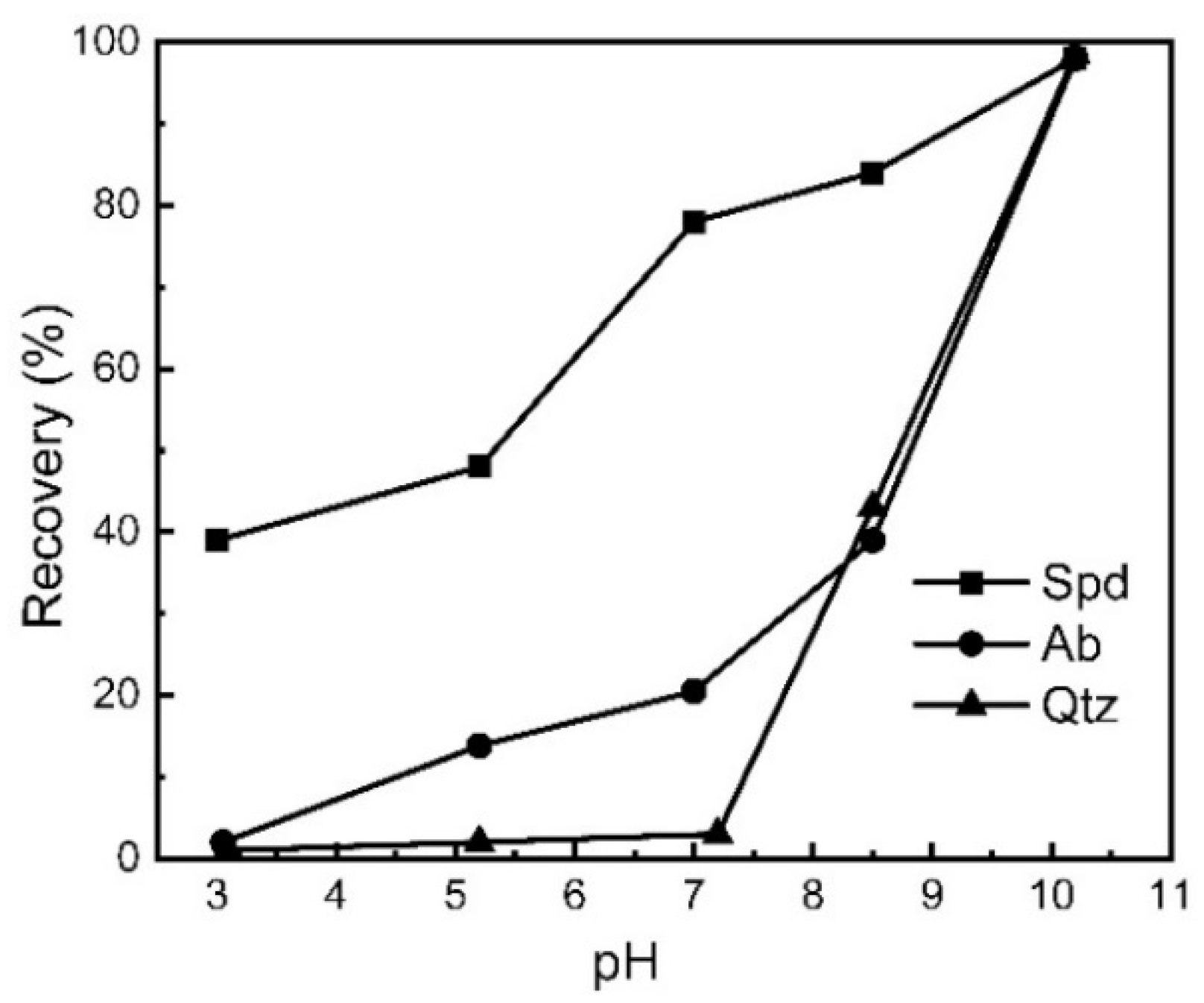
As demonstrated, the types and number of active sites per unit area on the crystal surface after fragmentation are all different; therefore, the surface properties of spodumene, albite, quartz, and beryl are different, though they are all aluminosilicates or aluminum oxides. In the flotation experiments of these four pure minerals, it was found that there is still a difference in flotation among these minerals when appropriate activators, collectors, and depressants are selected. Figure 6 shows the relationship of the recovery of spodumene, albite, and quartz to pH in a collector system of α-bromododecanoic acid (α-BDDA) [64].
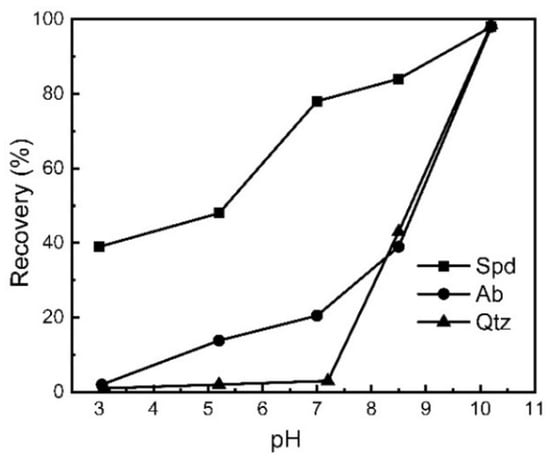
Figure 6.
The relationship of the recovery of spodumene, albite, and quartz to pH [64].
As shown in Figure 6, the recovery of spodumene (Spd), albite (Ab), or quartz (Qtz) is 79.2%, 20.3%, or 1.8%, respectively, with the following flotation parameters: pH 7.2, 15 °C pulp temperature, dosage of α-BDDA (collector) of 1.43 mmol/L, and Fe3+ concentration of 0.69 mmol/L; the maximum floating difference (68.9% and 78.4%) between spodumene (Spd) and albite (Ab) or quartz (Qtz) is obtained [64].
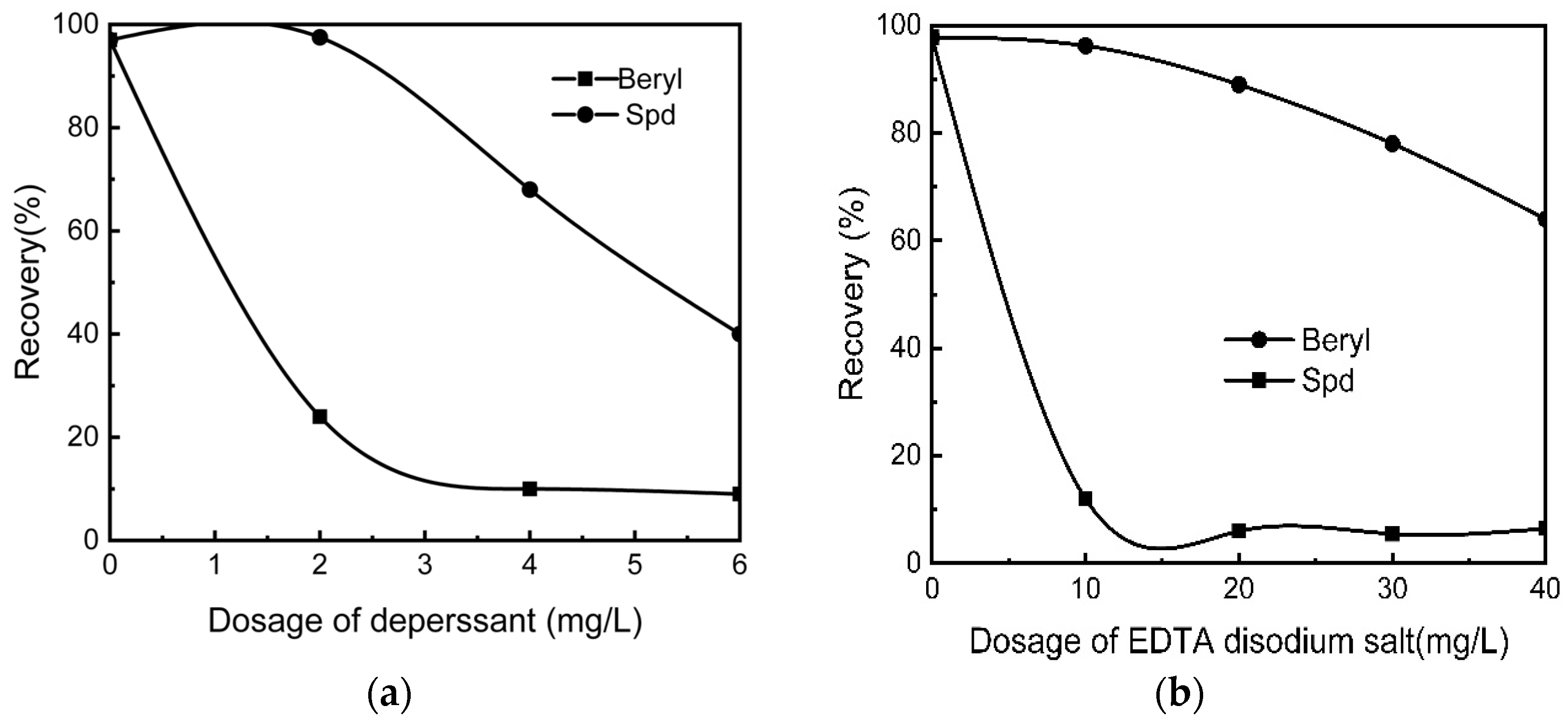
Figure 7a,b show the flotation results of spodumene and beryl under the following conditions: pH 7.5, dosage of collector at 200 mg/L, activator of FeCl3·6H2O concentration at 35 mg/L [67].
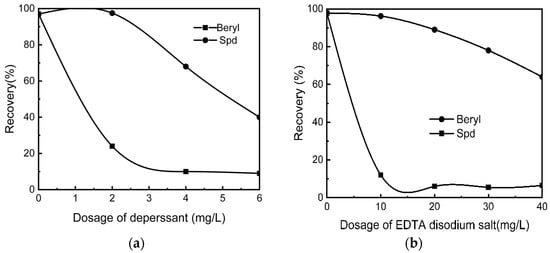
Figure 7.
Floating recovery between spodumene and beryl, (a) with sodium hexametaphosphate as the depressant; (b) with EDTA disodium salt as the depressant [67].
As observed from the results in Figure 7a,b, there is a difference in the flotation performance of these pure minerals. As long as slime minerals were processed in advance and appropriate collectors, depressants, or activators were selected, there could be a large floating difference between spodumene and gangue, spodumene, and beryl.
3.6.3. Selective Flotation Reagents for Lithium and Beryllium Mineral Separation
The function of flotation reagents is mainly to improve the surface hydrophobicity of minerals and the firmness of their adhesion on bubbles. Therefore, flotation reagents play a very important role in regulating the surface properties of minerals and improving the flotation speed and selectivity of minerals. The selection and use of flotation reagents are some of the most important steps in improving flotation efficiency. There are a large number of industrial applications and laboratory studies of various flotation reagents related to the separation of spodumene from beryl and gangue. Table 7, Table 8 and Table 9 showed the application characteristics of the single collectors, mixed collectors, and regulators used in the flotation separation of spodumene and beryl.

Table 7.
Application characteristics of single collectors used in the flotation separation of spodumene and beryl.

Table 8.
Application characteristics of mixed collectors used in the flotation separation of spodumene and beryl.

Table 9.
Application characteristics of some regulators used in the flotation separation of spodumene and beryl.
There are various collectors for the separation of spodumene from quartz and albite via flotation, but there is little research on collectors for separating spodumene and beryl. The four single collectors mentioned in Table 7 are all fatty acid-type collectors, and all of them contain COOH groups. Under appropriate conditions, they exhibit collecting properties for both spodumene and beryl, to a certain degree. Further studies on depressants need to be conducted in order to achieve the separation of spodumene and beryl [72].
The research on combined collectors has been conducted briskly. The advantages of combined collectors are that it plays the synergistic effect of various collectors in combination collectors, supplements the deficiency of a single collector, and achieves a more favorable collection effect. The development of combination collectors is an inevitable outcome of the development of flotation collectors and the roadmap of the development of LPPO flotation collectors [63,75,77].
In LPPO, spodumene and beryl are both aluminosilicate minerals with very similar properties. Another key aspect is to look for a more selective reagent. As seen from Table 8, the types of depressants for LPPO flotation separation include inorganic depressants (sodium carbonate, sodium sulfide, sodium hexametaphosphate, etc.) and organic depressants (Ligno-sulfonate, Disodium EDTA, etc.). Organic small-molecule depressants have the advantage of good selectivity, and even at a small dosage, they have been widely used in the flotation of sulfide ore separation [67,84]. Disodium EDTA has the best selective inhibition effect on spodumene and beryl but is not as selective as an inorganic depressant [71,85]. The research or development of selective depressants for the flotation of gangue minerals, beryl, and spodumene is an important pathway for further improving the flotation separation efficiency of spodumene and gangue minerals based on the regulation of mineral surface properties and the development of high-efficiency collectors [86].
3.6.4. Industrial Application Cases of Lithium and Beryllium Separation in China
There are three separation processes for lithium and beryl that have been utilized in production, as shown in Table 10.

Table 10.
Application cases of the lithium beryllium separation process in production.
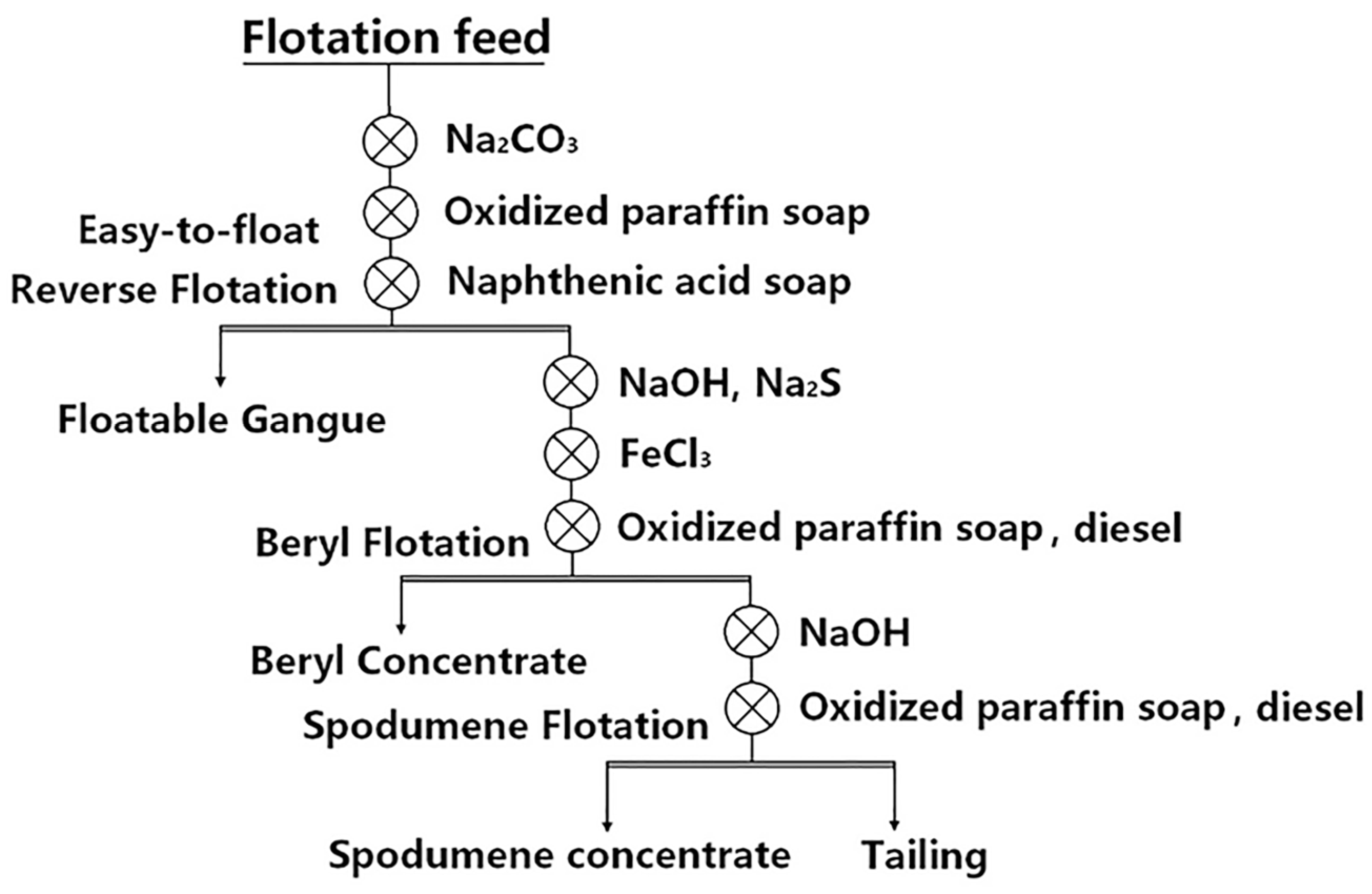
It can be seen from Table 10 that (1) the first process is used when some easy-floating spodumene exists in the ore, i.e., “Priority flotation of part of spodumene—mixed flotation of lithium beryllium—flotation separation between spodumene and beryl” [87]; (2) the second process was used for high beryllium and low lithium ores, i.e., “Priority flotation of beryl—flotation of spodumene”, as shown in Figure 8 [9]; (3) for high lithium and low beryllium ores, the process of “Priority flotation of spodumene—flotation of beryl” was adopted. Different flotation flowsheets should be used based on different ore properties [56,88].
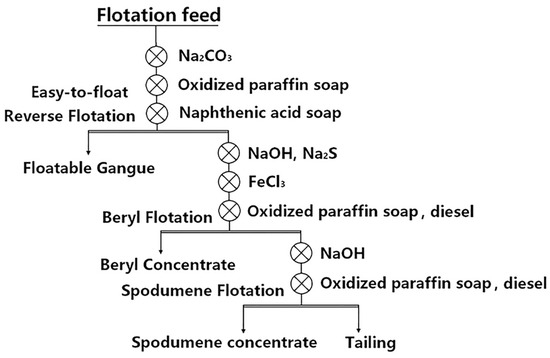
Figure 8.
Process flow for the separation of spodumene and beryl from Keketuohai stockpile ore in Xinjiang Autonomous Region [9].
Problems that exist in the field of spodumene and beryl flotation separation are (1) The lack of efficient, reasonably priced and low-temperature flotation collectors [9]; (2) Depressants commonly used in production are not sufficiently selective [74]; (3) The comprehensive recovery process of granite pegmatites polymetallic ore is complex, and the separation results are not ideal [9,75]. Moving forward, the direction is aimed at (1) research on low-temperature, easily degradable, environmentally friendly high-efficiency collectors [9]; (2) developing a new type of green dedicated spodumene and beryl inhibitors [67,74]; (3) improving the sorting process with the help of modern research methods to develop a green sorting process based on the premise of economical rationality [44].
3.7. The Leaching Method Used in the Extraction of LPPOs
In LPPOs, spodumene, beryllium-bearing minerals, niobium and tantalum minerals, quartz, albite, and mica are co-occurring with a complex occurrence. Sometimes, when the separation methods such as manual or color sorting, gravity separation, magnetic separation, or flotation cannot fully meet the mineral separation requirements, the leaching method is used instead [89,90,91]. For example, in LPPO, it is difficult to obtain a high-grade beryllium concentrate by flotation when the beryllium-bearing minerals are not purely beryl but also Bertrandite and Xrizoberyl. The combined flotation–leaching process is used to recover beryllium [92,93]. Liu Yong et al., in 2019, adopted a new process of “roasting-countercurrent leaching-acidification-secondary leaching” for beryllium concentrate with a BeO content of 1.13%, where the roasting temperature was 1050 °C and the roasting time was 2 h. The roasted products were finely ground and then leached at a liquid–solid ratio of 4:1, a 30 °C leaching temp, and a leaching time of one (1) hour. The leaching slag with a BeO content of 0.14% and a beryllium leaching rate of 88.88% was obtained [94].
There are three kinds of spodumene crystal structures: α-spodumene, β-spodumene, and γ-spodumene, of which α-spodumene is the most stable and natural in structure [95]. In the process of lithium extraction from LPPOs, it is usually necessary to convert stable natural spodumene ore (α-spodumene) into β-spodumene with high activity by roasting at a high temperature (i.e., 1050 °C) before the next lithium leaching process [96]. Therefore, roasting and leaching are the two main steps in lithium extraction from LPPOs. According to the different types of leaching reagents used in the process, lithium extraction methods from LPPOs can be divided into the sulfuric acid method, limestone sintering method, sodium carbonate pressure boiling method, etc. [97]. For example, Tian Qianqiu and co-workers, in 2011, after having investigated the roasting and sulfuric acid leaching behavior of spodumene, believed that most of α-spodumene was converted into β-spodumene when roasted at 1050 °C for 30 min. In order to improve the leaching efficiency, acidizing roasting was also carried out. The optimum acid roasting conditions are as follows: 1.4 times of the sulfuric acid theoretical amount, a 250 °C roasting temperature, and a 30 min roasting time. During the leaching process, the leaching rate reached 96.93% at 25 °C, 15 min, and a liquid–solid ratio of 1.85. Kinetics and thermodynamics theory was applied to explain why the leaching efficiency was high at a low temperature, low lithium concentration, and acidity condition. The pH value was the key influence factor [98].
Guo Hui et al., in 2019, studied Li leaching from α-spodumene using a mixture of hydrofluoric and sulfuric acid (HF/H2SO4) as the medium. Under the optimal leaching conditions, the leaching kinetics of Li were investigated in an ore/HF/H2SO4 ratio of 1:3:2 g:mL:mL, with the leaching temperature ranging from 50 to 100 °C. The results showed that the 1/3ln(1 − x) − [1 − (1 − x)−1/3] = kt model developed from the shrinking core model could describe the leaching kinetics of Li, indicating that the leaching rate of Li was controlled by a chemical reaction [99].
TU Tao et al., in 2020, investigated the kinetics of the lithium extraction of the spodumene–calcium oxide sintering process. Their experimental results indicated that the leaching rate of lithium reached 92.14% when the mixture ratio was 1:1.25 at the sintering temperature of 1150 °C and the sintering time of 60 min. The kinetic analysis showed that the spodumene–calcium oxide sintering system was controlled by the chemical reaction of spherical particles at the three-dimensional interface, and the kinetic fitting equation was 1 − (1 − x)1/3 = 0.00677t [100].
CHEN Ya et al., in 2011, studied the extraction of lithium from spodumene by the sodium carbonate autoclave process. Spodumene concentrate was roasted at 1050 °C and then treated with sodium carbonate in an autoclave. The operation conditions for the autoclave process were optimized. The results showed that the extraction can reach up to 96% under optimal conditions of a stirring speed of 300 r/min, a liquid–solid ratio of 4, and a reacting time at 225 °C for 60 min [101].
3.8. Brief Analysis of the Economic Efficiency of the Separation Methods
Eight methods of separation and extraction for LPPOs are described in the previous sections. Each has different characteristics and economic benefits. In LPPOs, the main minerals such as spodumene, quartz, and albite are present in a coarser crystal size, and the morphological differences of the minerals are large, which makes it feasible for manual sorting. Manual sorting is often carried out on a slow-moving belt, but it can also be carried out on mine piles or stopes. The selected ore size is usually greater than 10–25 mm, and the lower limit of the ore size is mainly determined by economic factors. In order to improve the efficiency of manual sorting, it is often necessary to pre-screen the ore before manual sorting and wash it, if necessary. A good working environment is required in the working area. Manual sorting has a high labor intensity and low production efficiency. With the development of color sorters and X-ray sorters, manual sorting will gradually be phased out [9,41]. The advantages of color sorting and X-ray sorting are that they can undergo direct selection or reverse selection, making use of professional image acquisition and a processing system to obtain a high-quality image processing program. They have a high accuracy and can detect some defects that are not visible to the naked eye. The downside of the two sorting methods is that they are costly and require professional expertise and special equipment [46]. Gravity separation and dense medium separation methods are known for their simple equipment structure, low mineral processing cost, and high economic efficiency. As long as there is a difference in density between useful minerals and gangue minerals in the ore, the gravity beneficiation process can be used [48,49]. Other advantages of dense medium separation include a higher separation efficiency, wider separation density adjustment range, stronger adaptability, wider separation granularity range, larger processing capacity, and easier automation of the production process. In addition, no sewage is discharged in the dense medium separation process, reducing environmental pollution [47,50]. There are weak or strong magnetic minerals in LPPOs, i.e., Ta-bearing minerals, Nb-bearing minerals, Fe-bearing minerals, Mn-bearing minerals, and Co-bearing minerals. Magnetic separation is an optimal method with a high economic efficiency and low cost in recovering Ta- and Ni-bearing minerals or removing Fe, Mn, or Co impurities from spodumene concentrate. Flotation is the most effective and commonly used method in the beneficiation of LPPOs, with a strong adaptability and high separation efficiency, which is suitable for the treatment of fine-grained materials [74,75,76,77,78,79,80,81,82,83].
The leaching process is categorized into the field of hydrometallurgy, which is a supplement to beneficiation. The leaching process can replace the lithium in the ore into a soluble lithium ion, which provides the basis for the preparation of lithium products. In order to improve the leaching efficiency, roasting pretreatment is usually added to increase the leaching efficiency. The energy consumption of the roasting–leaching process is usually higher compared with the beneficiation process, but the leaching rate is higher when roasting pretreatment is adopted [95,96,97,98,99,100,101,102,103].
4. Conclusions
Based on the previous review of the geological aspects of LPPO deposits in China and Chinese experiences regarding the beneficiation of LPPOs, the following conclusions and suggestions are drawn:
- LPPO, with large reserves and a high lithium grade, is an important lithium extraction ore. The beneficiation technologies of LPPO in China include the following: manual or color sorting, X-ray transmission sorting, dense medium separation, gravity separation, magnetic separation, flotation, and leaching.
- At present, the main industrial practice includes manual selection, dense medium beneficiation, and the flotation method. Color sorting and X-ray transmission sorting are promising alternatives to manual sorting, which are still in the research stage.
- When the ore contains magnetic substances, such as hematite, magnetite, lepidolite, tantalum-niobite, and other minerals, the magnetic separation method is used. Magnetic separation is an auxiliary method for improving the quality of lithium concentrate.
- Flotation is the main method for the separation of fine-grained intercalated lithium beryllium minerals. The key to the success of flotation lies in the treatment of argillaceous minerals and the selection of appropriate flotation reagents to expand the floatation difference between spodumene, beryl, and gangue minerals.
- Since beryllium, tantalum, niobium, etc. are all metals with important uses, beryllium, tantalum, and niobium in LPPO should be recovered. The development of lithium-beryllium separation and beneficiation technology is lagging behind and needs to be further strengthened.
- The future research on lithium-beryllium flotation separation will focus on: (1) the research and development of high-efficiency collectors, depressants, and activators; (2) the improvement of the efficiency of the separation process with the help of modern research methods and under the premise of economic sustainability to develop the green separation process.
Author Contributions
Conceptualization, S.L. and J.L.; Data curation, S.L. and S.Z.; Formal analysis, S.L.; Funding acquisition, Y.H. and J.L.; Investigation, S.L.; Project administration, Y.H.; Writing—original draft, S.L.; Writing—review & editing, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the financial funding of the National Key Research and Development Program of China (No. 2021YFC2903200) and the “Xingliao Talent Plan” Project in Liaoning Province, China (XLYC2007055).
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sahoo, S.K.; Tripathy, S.K.; Nayak, A.; Hembrom, K.C.; Dey, S.; Rath, K.R.; Mohanta, M.K. Beneficiation of lithium bearing pegmatite rock: A review. Miner. Process. Extr. Metall. Rev. 2022, 43, 1–27. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wall, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium—A Literature Review Part 1: Pretreatment of Spodumene. Miner. Process. Extr. Metall. Rev. 2020, 41, 335–348. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, L.; Kong, L.; Jiang, A.; Li, J. Overview of lithium resources and distribution at home and abroad. Nonferr. Met. Eng. 2020, 10, 95–104. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Ding, G. Status quo and development trend of global lithium resources. Conserv. Util. Miner. Resour. 2019, 39, 26–40. [Google Scholar] [CrossRef]
- Zhou, Y. Analysis on supply and demand situation and external dependence of Lithium resources in China. Resour. Ind. 2019, 21, 46–50. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, F.; Gao, X.; Li, X.; Shang, Y.; Kong, Z.; Jia, L.; Meng, J.; Guo, H.; Fang, F.; et al. Geological characteristics, metallogenic regularity, and research progress of lithium deposits in China. China Geol. 2022, 5, 734–767. [Google Scholar] [CrossRef]
- Sun, C. Mineral Processing Engineer’s Manual; Metallurgical Industry Press: Beijing, China, 2015; Volume 4, pp. 59–129. ISBN 978-7-5024-6797-5. [Google Scholar]
- Rao, N.K.; Sreenivas, T. Beryllium—Geochemistry, Mineralogy and Beneficiation. Miner. Process. Extr. Metall. Rev. 1994, 13, 19–42. [Google Scholar] [CrossRef]
- Gupta, C.K.; Saha, S. Extractive Metallurgy of Beryllium. Miner. Process. Extr. Metall. Rev. 2010, 22, 413–451. [Google Scholar] [CrossRef]
- Babu, R.S.; Gupta, C.K. Beryllium Extraction—A Review. Miner. Process. Extr. Metall. Rev. 2007, 4, 39–94. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Xu, L.; Li, K.; Li, Y.; Zheng, Y.; Wang, H.; Wang, Y. Resource potential analysis of pegmatite type lithium beryllium in Dahongliutan Xinjiang. Gold Sci. Technol. 2019, 27, 802–815. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Zhu, J. Overview of lithium resources at home and abroad and its beneficiation, metallurgy and processing technology. World Nonferr. Met. 2001, 8, 4–8. [Google Scholar]
- Editorial Board of Handbook of Mineral Resources Industry Requirements. Mineral Resources Industry Requirements Manual; 2014 Revised Edition; Geological Publishing House: Beijing, China, 2014. [Google Scholar]
- Che, X.; Wang, R.; Hu, H.; Zhang, W.; Hang, X. Beryllium mineralization in the topaze-lithium mica granite in Yichun, Jiangxi province: Beryllium phosphate mineral assemblages. Acta Petrol. Sin. 2007, 6, 1552–1560. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Rare Earth Metals and Alloys by Electrolytic Methods. Miner. Process. Extr. Metall. Rev. 2002, 22, 477–507. [Google Scholar] [CrossRef]
- Xu, X.; Jiao, Z.; Hai, G.; Yang, Y.; Li, J.; Teng, Y. Development status of beryllium industry. Xinjiang Non-Ferr. Met. 2021, 44, 4–8. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L. Analysis on supply and demand pattern and industrial policy of American beryllium industry. China Min. Mag. 2022, 31, 31–36. [Google Scholar] [CrossRef]
- Butterman, W.C. Current Status of the Specialty Metals. Miner. Process. Extr. Metall. Rev. 1988, 3, 69–86. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents (Volume III), Chemistry, Theory and Practice, Flotation of Gold PGM and Oxide Minerals; Elsevier B.V.: Amsterdam, The Netherlands, 2010; pp. 21–56. ISBN 978-7-122-19692-7. [Google Scholar]
- Foley, N.K.; Jaskula, B.W.; Piatak, N.M.; Schulte, R.F. Beryllium, Chap E of Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2017; pp. E1–E32. [CrossRef]
- Li, H.; Hong, T.; Yang, Z.; Chen, J.; Ke, Q.; Wang, X.; Niu, L.; Xu, X. Zircon dating of rare metal granitic pegmatite, Cassiterite and tantalite U-Pb dating and Muscovite -(40) Ar/-(39) Ar dating: A case study of the Tugman North Li-Be deposit in the middle Altun. Acta Petrol. Sin. 2020, 36, 2869–2892. [Google Scholar] [CrossRef]
- Yao, W. Process progress and Industrial practice of caustic flotation of beryl. Xinjiang Min. Metall. 1985, 2, 1–4. [Google Scholar]
- Zheng, Y.; Wang, G. Experimental study on flotation of Bertrandit from Yangzhuang, Xinjiang. Xinjiang Nonferr. Met. 2012, 35, 66–68. [Google Scholar]
- Li, H.; Tan, X.; Zhang, X.; Zhang, L.; Yi, Y.; Wang, W. The present situation of beryllium resources and its beneficiation technology. Nonferr. Met. Sci. Eng. 2022, 13, 44–53. [Google Scholar]
- Rui, H. Study on Extraction Technology of Beryllium from Chrysoberyl Type Beryllium Ore. Ph.D. Thesis, Xiangtan University, Xiangtan, China, 2017. [Google Scholar]
- Deng, C. A Technology of Extracting Lithium Beryllium from Li-Be-Bearing Chrysolite. Ph.D. Thesis, Xiangtan University, Xiangtan, China, 2018; pp. 48–56. [Google Scholar]
- Bordbar-Khiabani, A.; Bahrampour, S.; Mozafari, M.; Gasik, M. Surface functionalization of anodized tantalum with Mn3O4 nanoparticles for effective corrosion protection in simulated inflammatory condition. Ceram. Int. 2022, 48, 3148–3156. [Google Scholar] [CrossRef]
- Nzeh, N.S.; Adeosun, S.; Popoola, A.P.; Adeleke, A.; Okanigbe, D. Process Applications and Challenges in Mineral Beneficiation and Recovery of Niobium from Ore Deposits—A Review. Miner. Process. Extr. Metall. Rev. 2022, 43, 833–864. [Google Scholar] [CrossRef]
- Lee, H.; Mishra, B. Recovery of Copper and Precious Metals and Separation of Lead from Flue Dust of Electronic Waste Processing. Miner. Process. Extr. Metall. Rev. 2020, 41, 153–161. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, R.; Hu, H.; Zhang, H.; Zhu, J. The structure and petrological significance of niobite family mineral ring in Keketuohai No.3 Pegmatite dike, Altai. Acta Geol. Sin. 2004, 2, 181–189. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Wang, D.; Dai, H.; Ma, S.; Liu, L.; Wang, C. Discovery and significance of granite type lithium ore body in Jiajika ore field, Sichuan province. Acta Geol. Sin. 2019, 93, 1309–1320. [Google Scholar] [CrossRef]
- Fei, G.; Fang, B. Ore fabric characteristics of Lijiagou spodumene deposit in Keeryin ore field, western Sichuan. Acta Mineral. Sin. 2015, 35, 1000. [Google Scholar]
- Pei, Y. Research status of keketuohai pegmatite deposit in Altay. Xinjiang West Leather 2020, 42, 1. [Google Scholar]
- Wang, H.; Xu, Y.; Yan, Q.; Zhang, X. Research progress of pegmatite type lithium deposit in Bailongshan, Xinjiang. Acta Geol. Sin. 2021, 95, 3085–3098. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Z.; Wang, W. Research status and prospect of mineral processing and comprehensive utilization of rare lithium beryllium metals. Nonferr. Met. (Miner. Process. Sect.) 2020, 6, 24–29. [Google Scholar] [CrossRef]
- Xu, G. Enrichment of spodumene minerals in Keketuohai granitic pegmatite deposit. Xinjiang Non-Ferr. Met. 2010, 33, 7–9. [Google Scholar]
- Liu, L.; Wang, D.; Liu, X.; Li, J.; Dai, H.; Yan, W. The main types, distribution features and present situation of exploration and development for domestic and foreign lithium mine. Geol. China 2017, 44, 263–278. [Google Scholar] [CrossRef]
- Gao, Y.; Bagas, L.; Li, K.; Jin, M.; Liu, Y.; Teng, J. Newly Discovered Triassic Lithium Deposits in the Dahongliutan Area, Northwest China: A Case Study for the Detection of Lithium-Bearing Pegmatite Deposits in Rugged Terrains Using Remote Sensing Data and Images. Front. Earth Sci. 2020, 8, 591966. [Google Scholar] [CrossRef]
- Wang, T.; Li, P.; Li, H.; Zou, J.; Wang, W.; Yang, K.; Wang, C. Recovery of associated elements from a pegmatite type lithium polymetallic ore in Xinjiang. Met. Mine 2021, 11, 81–85. [Google Scholar] [CrossRef]
- Song, X. Application of hand separation in a spodumene ore. Xinjiang Non-Ferr. Met. 2012, 35, 50–51. [Google Scholar]
- Gülcan, E.; Gülsoy, Y.Ö. Evaluation of complex copper ore sorting: Effect of optical filtering on particle recognition. Miner. Eng. 2018, 127, 208–223. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y. Measurement of Gem Colour Using a Computer Vision System: A Case Study with Jadeite-Jade. Minerals 2021, 11, 791. [Google Scholar] [CrossRef]
- Liu, G.; Peng, T.; Liu, L.; Li, S. Recovery of lithium from a granite-type spodumene by mineral processing combined with gravity separation, color separation and flotation. Met. Mine 2021, 3, 124–129. [Google Scholar] [CrossRef]
- Ge, D.; Liang, D. Study on ore grade detection method based on X-ray transmission. Nonferr. Met. (Miner. Process. Sect.) 2019, 4, 87–93. [Google Scholar]
- Wu, Z. Key points of ore pre-concentration and waste disposal technology and selection of intelligent photoelectric beneficiation equipment. World Nonferr. Met. 2020, 16, 202–205. [Google Scholar] [CrossRef]
- Tao, J. Industrial test and research on dense medium beneficiation of spodumene ore. Non-Ferr. Met. (Benef. Part) 2002, 2, 13–16. [Google Scholar]
- Xian, H. Dense medium beneficiation technology and its application in spodumene beneficiation. Xinjiang Non-Ferr. Met. 2018, 41, 71–73. [Google Scholar]
- Liang, X.; Huang, J.; Wu, G.; Hang, G.; Zhang, Y.; Li, M. Extended continuous beneficiation test of dense medium in a spodumene ore. Mod. Min. 2017, 33, 132–134. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Q.; Zhao, J.; Zhou, H.; Wei, D.; Le, Y.; Liu, W. High efficiency recovery of lithium from a pegmatite spodumene ore. Met. Mine 2021, 9, 107–112. [Google Scholar] [CrossRef]
- Han, M.; Li, Z.; Sun, Z.; Sun, Y.; Liu, Y. Experimental study on comprehensive recovery of a Lithium polymetallic ore. Conserv. Util. Miner. Resour. 2012, 2, 27–31. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y.; Wang, X.; Zhang, S. Research Status of Spodumene Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2020, 42, 321–334. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, N.; Chu, H. Surface dissolution behavior and its influences on the flotation separation of spodumene from silicates. Sep. Sci. Technol. 2021, 56, 1407–1417. [Google Scholar] [CrossRef]
- Yu, F.; Jiang, M.; Wang, J.; Li, J.; An, J.; Pan, D. Study on Pre-desliming flotation tests of spodumene ore in Australia. Non-Ferr. Met. (Miner. Process. Part) 2019, 6, 69–73. [Google Scholar]
- Lv, Y.; Xing, W.; Li, J. Generalization on flotation theory and practice of spodumene and beryl. Nonferr. Met. 1965, 6, 14–19. [Google Scholar]
- Lv, Y. A new flotation separation method of spodumene and beryl—Selective desorption separation of contaminated ion Ca2+. Comprehensive utilization of mineral resources. Miner. Resour. 1980, 1, 11–19. [Google Scholar]
- Wang, Y.; Zhu, G.; Zhang, L.; Lu, D.; Wang, L.; Zhao, Y.; Zheng, H. Surface dissolution of spodumene and its role in the flotation concentration of a spodumene ore. Miner. Eng. 2018, 125, 120–125. [Google Scholar] [CrossRef]
- Gao, D.; Wang, Y.; Zheng, H.; Chu, H.; Lu, D.; Zheng, X. Effect of surface pretreatment on flotation of spodumene and silicate minerals. J. Chin. Univ. Min. Technol. 2020, 49, 991–997. [Google Scholar]
- Chu, H.; Chen, L.; Lu, D.; Wang, Y.; Zheng, X. Ultrasonic pretreatment of spodumene with different size fractions and its influence on flotation. Ultrason. Sonochem. 2022, 82, 105889. [Google Scholar] [CrossRef]
- Ji, G.; Wang, Y. Application of non-dissolution cleaning technology for Lithium beryllium minerals. Xinjiang Non-Ferr. Met. 2020, 1, 73–76. [Google Scholar]
- Xie, R.; Zhu, Y.; Li, Y.; Han, Y. Flotation behavior and mechanism of a new mixed collector on separation of spodumene from feldspar. Colloids Surf. A 2020, 599, 124932. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Han, X.; Zhang, M.; Li, Y. Study on flotation performance and action mechanism of a new spodumene collector DRQ-3. Met. Mine 2020, 6, 68–74. [Google Scholar]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. Effects of metal ions on the flotation separation of spodumene from feldspar and quartz. Miner. Eng. 2021, 168, 106931. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Xie, R.; Cheng, Z. Crystal chemical gene characteristics and floatability prediction of albite. Met. Mine 2020, 6, 81–86. [Google Scholar]
- Xu, L.; Peng, T.; Tian, J.; Lu, Z.; Hu, Y.; Sun, W. Anisotropic surface physicochemical properties of spodumene and albite crystals: Implications for flotation separation. Appl. Surf. Sci. 2017, 426, 1005–1022. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F.; Chen, X. Selective flotation between spodumene and beryl. Rare Met. 2005, 3, 320–324. [Google Scholar] [CrossRef]
- Xiang, H.; He, R.; Zhang, H. Flotation research on spodumene pure mineral. China Sci. Technol. Inf. 2015, 5, 64–66. [Google Scholar] [CrossRef]
- Liu, N. Experimental study on flotation of spodumene in V26 and V38 ore bodies of Keketuohai Rare Mine. Xinjiang Non-Ferr. Met. 2008, 5, 48–49. [Google Scholar]
- Tian, J.; Xu, L.; Wu, H.; Fang, S.; Deng, W.; Peng, T.; Sun, W.; Hu, Y. A novel approach for flotation recovery of spodumene, mica and feldspar from a lithium pegmatite ore. J. Clean. Prod. 2018, 174, 625–633. [Google Scholar] [CrossRef]
- Zhao, Y. Exploration of flotation recovery of high grade spodumene. Xinjiang Non-Ferr. Met. (S1) 2005, 37–38+41. [Google Scholar]
- He, Y.; Xie, Z. Discussion on beneficiation test of a spodumene ore in western Sichuan. Chem. Miner. Process. 2017, 46, 13–15+51. [Google Scholar]
- Zhang, J.; Wang, W.; Dong, F. Experimental study on flotation of spodumene ore. Acta Mineral. Sin. 2013, 33, 423–426. [Google Scholar]
- Wang, Y.; Yu, F. Flotation of spodumene and beryl with a new collector. J. Cent. South Univ. Sci. Technol. 2005, 5, 93–97. [Google Scholar] [CrossRef]
- He, J. Application of new collector in lithium beryllium flotation. Xinjiang Non-Ferr. Met. 2009, 32, 37–38. [Google Scholar]
- Weng, C.; Wen, S. Desliming and flotation recovery test of a low-grade spodumene in Southern Jiangxi Province. Mod. Min. 2017, 33, 126–131. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Yang, Y.; Zeng, X.; Wang, Z.; Wang, J. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner. Eng. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Wu, H.; Tian, J.; Xu, L.; Fang, S.; Zhang, Z.; Chi, R. Flotation and adsorption of a new mixed anionic/cationic collector in the spodumene-feldspar system. Miner. Eng. 2018, 127, 42–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Sun, J.; Dong, Y. Study on the action rule and mechanism of sodium carbonate, sodium fluoride and sodium sulfide on Ca2+ and Fe3+ activated beryl and spodumene. Rare Met. 1983, 4, 2–7. [Google Scholar]
- Liu, F.; Sun, C. Effect of adding sequence of metal cation and dodecylamine on flotation of silicate minerals. Non-Ferr. Met. Miner. Process. Sect. 2011, 4, 58–62. [Google Scholar] [CrossRef]
- Jiao, Y. Preparation and application of peroxide fatty acid soap. Chem. World 1988, 8, 337–341. [Google Scholar] [CrossRef]
- Luo, X.; Lv, L.; Chen, X.; Zhou, H. Direct flotation process for a low-grade refractory spodumene ore in Jiangxi Province. Nonferr. Met. Eng. 2012, 2, 36–39. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, M. Mechanism of activation and flotation separation of beryl and spodumene by grinding iron medium. Hunan Nonferr. Met. 1993, 9, 332–336. [Google Scholar]
- Piao, Z.; Wei, D.; Liu, Z. Effects of Small Molecule Organic Depressants on the Flotation Behavior of Chalcopyrite and Galena. J. Northeast. Univ. (Nat. Sci.) 2013, 34, 884–888. [Google Scholar] [CrossRef]
- Liu, F.; Sun, C. Influence of regulator and the adding order of sodium oleate to the flotation of silicate minerals. Met. Mine 2011, 3, 90–94. [Google Scholar]
- Hu, Y.; Wu, G.; Chu, H.; Wang, Y.; Sun, N.; Feng, H.; Lu, D.; Zheng, X. New Development in the flotation Theory and reagents of Spodumene Ore. Nonferr. Met. Eng. 2021, 11, 10–19. [Google Scholar] [CrossRef]
- Zhang, C. Study on flotation of Lithium beryllium from Sichuan Methyl card rare metal Ore. Sichuan Nonferr. Met. 1994, 1, 22–26. [Google Scholar]
- Ren, W. Separation of lithium beryllium from Keketuohai beryllium ore. Xinjiang Non-Ferr. Met. 2012, 5, 67–69. [Google Scholar]
- Wu, Y.; Zhang, X.; Tian, X.; Yao, Z.; Luo, Y. Recovery of Lithium from Acidic Leaching Solution of Lithium-containing Ore. Hydrometall. China 2020, 39, 182–185. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, J.; Li, H.; Guo, J. Research Status and Prospects of Lithium Extraction from Lithium Containing Resources. Multipurp. Util. Miner. Resour. 2021, 5, 32–36. [Google Scholar] [CrossRef]
- Mao, S.; Li, G.; Zhong, L.; Wen, W.; Yu, X.; He, T.; Zhang, C. Present Situation and Prospect of Mineral Processing of Beryllium. Non-Ferr. Met. (Benef. Part) 2022, 6, 17–24. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, H.; Wei, H.; Tian, Y.; Yu, J.; Zhao, Z. Present Situation and Development Trend of Lithium Resource Extraction Process. Rare Met. Cem. Carbides 2018, 46, 11–19. [Google Scholar]
- Liu, Y.; Liu, M.; Liu, Z. Experimental Study on new metallurgical treatment process of low-grade beryllium concentrate. Rare Met. Cem. Carbides 2014, 2, 13–15. [Google Scholar]
- Hu, Z. Analysis of Typical Lithium Extraction from Mines and its Economic Benefit. J. Salt Sci. Chem. Ind. 2019, 48, 5–8. [Google Scholar] [CrossRef]
- Tian, J.; Li, T.; Wang, M.; Zhao, H.; Qi, T. Research progress in lithium extraction technology for typical lithium ores. J. Hubei Univ. (Nat. Sci.) 2020, 42, 56–60. [Google Scholar]
- Su, H.; Zhu, Z.; Wang, L.; Qi, T. Research progress in the extraction and recovery of lithium from ore resources. CIESC J. 2019, 70, 10–23. [Google Scholar] [CrossRef]
- Xiao, M. Study on the process of extracting lithium salts from spodumene using sulfuric acid. Xinjiang Nonferr. Met. 1982, 2, 97–102. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, B.; Chen, Y.; Ma, L.; Shi, X. Roasting and Leaching Behavior of Spodumene in Sulphuric Acid Process. Chin. J. Rare Met. 2011, 35, 118–123. [Google Scholar] [CrossRef]
- Guo, H.; Yu, H.; Zhou, A.; Lv, M.; Wang, Q.; Kuang, G.; Wang, H. Kinetics of leaching lithium from α-spodumene in enhanced acid treatment using HF/H2SO4 as medium. Trans. Nonferr. Met. Soc. China 2019, 29, 407–415. [Google Scholar] [CrossRef]
- Tu, T.; Guo, H.; Cheng, H.; Qiu, J.; Wang, X.; Liu, Q. Phase reconstruction and kinetics of lithium extraction by spodumene calcium oxide sintering method. Chem. Ind. Eng. Prog. 2020, 39, 3478–3486. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, T.; Chen, B.; Tian, Q. Study on the extraction of lithium from spodumene by soda pressure boiling. Nonferr. Met. (Extr. Metall.) 2011, 9, 21–23+32. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Li, S.; Ma, H. Separation performance and value analysis of typical pegmatite lithium ore. Min. Metall. 2020, 29, 49–52+97. [Google Scholar]
- Peng, J. Domestic lithium carbonate production process and benefit analysis. J. Salt Sci. Chem. Ind. 2019, 48, 18–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).