Carrier Flotation Using Coarse Pyrite for Improving the Recovery of Finely Ground Chalcopyrite: Development of Post-Process of Carrier Flotation to Separate Finely Ground Chalcopyrite Particles from Coarse Pyrite Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Materials
2.1.2. Reagents
2.2. Methods
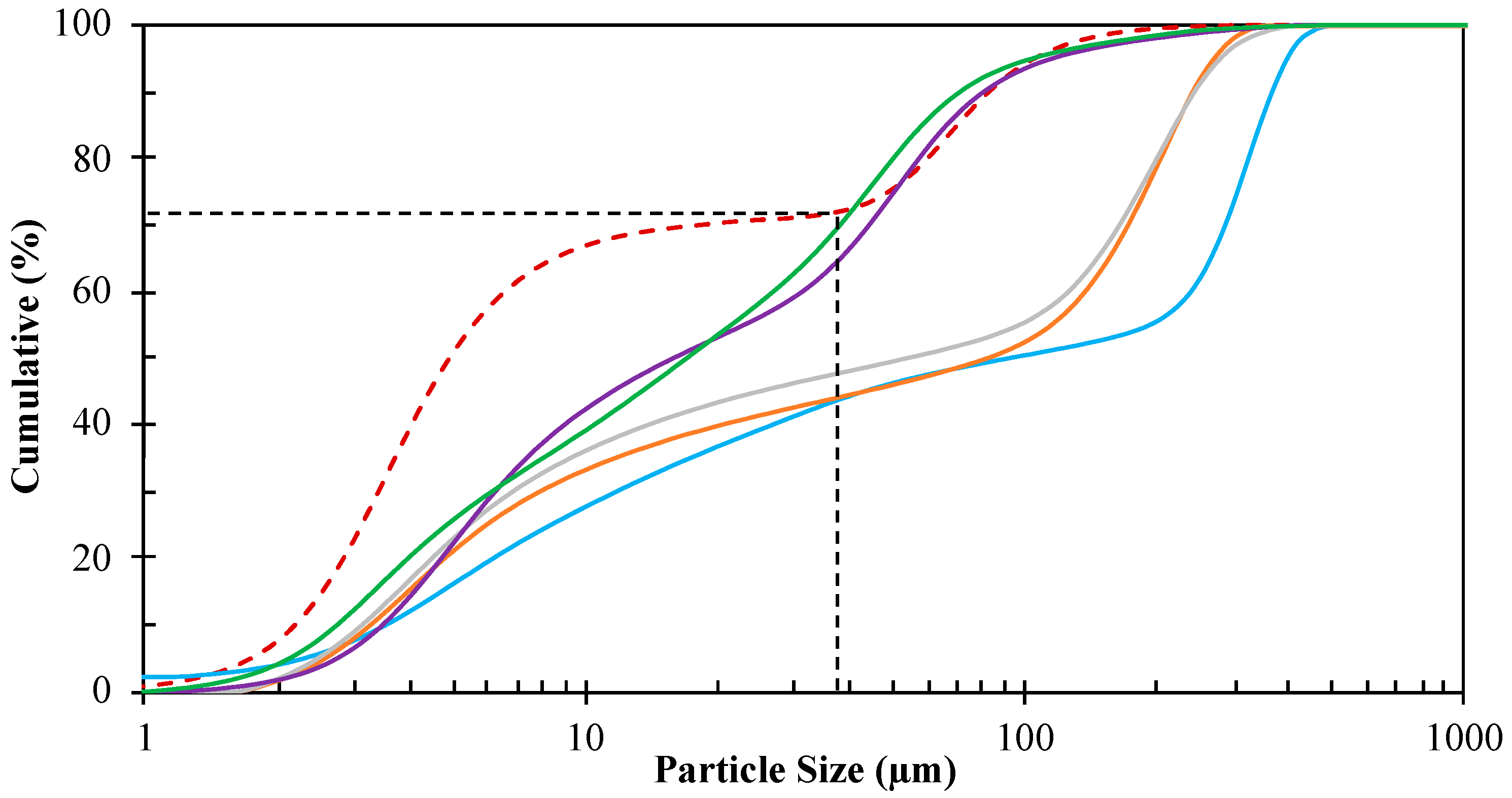
2.2.1. Sample Preparation
2.2.2. Mixed Chalcopyrite–Pyrite Flotation Tests
2.2.3. Detachment of Finely Ground Chalcopyrite from Coarse Pyrite
Ultrasonic Treatment
Acid Treatment
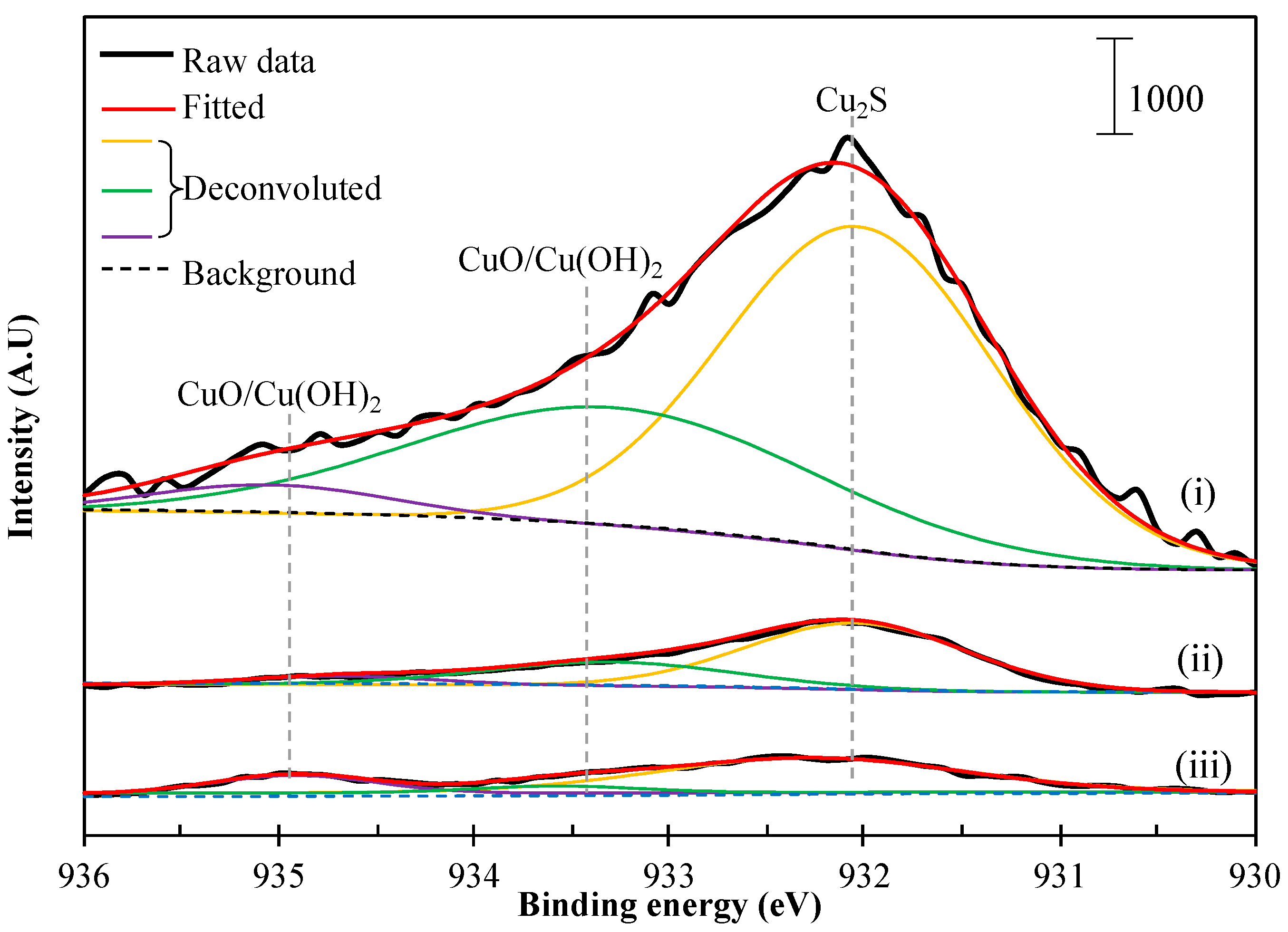
2.2.4. XPS Analysis
2.2.5. FTIR Analysis
2.2.6. Dissolution Test of Cu2+ from Cu2+-Activated Pyrite
3. Results and Discussion
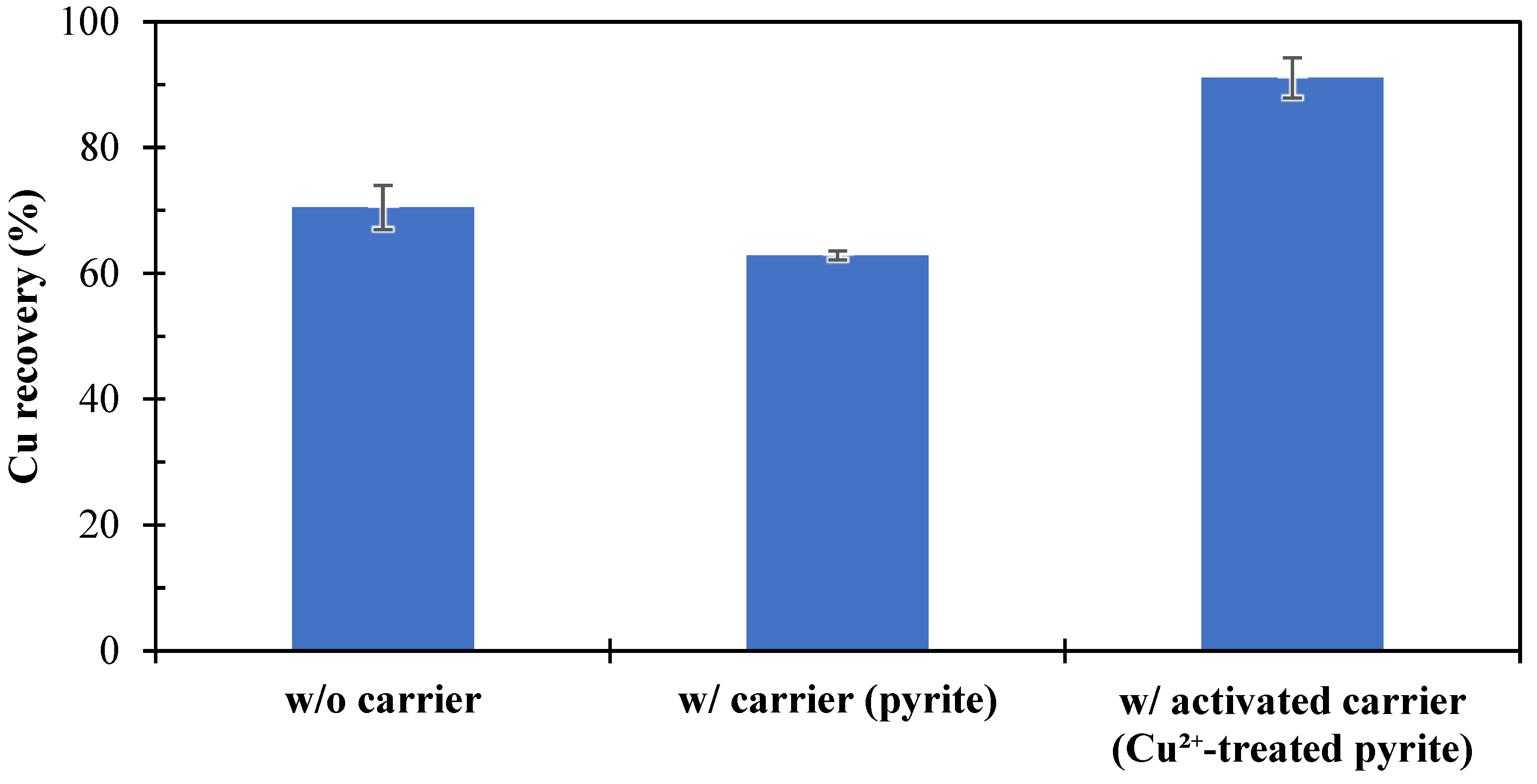
3.1. Heterogeneous Carrier Flotation of Fine Chalcopyrite Using Coarse Pyrite as a Carrier
3.2. Ultrasonic Treatment on the Detachment of Chalcopyrite from Cu2+-Treated Pyrite
3.3. Acid Treatment on the Detachment of Chalcopyrite from Cu2+-Treated Pyrite
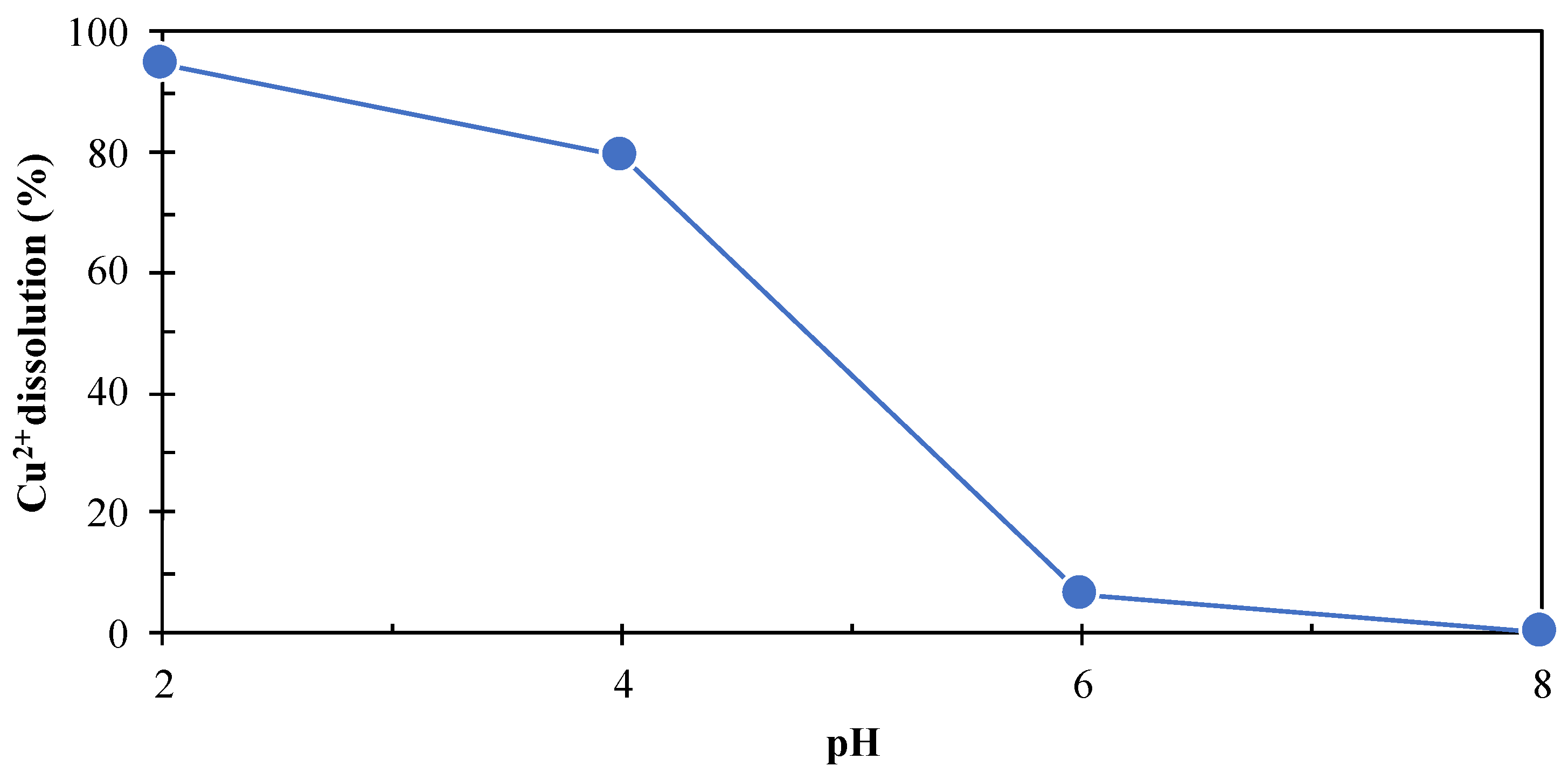
3.3.1. Dissolution of Cu Compounds Formed on the Surface of Cu2+-Treated Pyrite at Different pH Values
3.3.2. Decomposition of KAX Adsorbed on the Surface of Pyrite
3.4. Proposed Flowsheet for Heterogenous Carrier Flotation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Yin, W.; Liu, Q.; Cao, S.; Sun, Q.; Zhao, C.; Yao, J. Interactions between fine and coarse hematite particles in aqueous suspension and their implications for flotation. Miner. Eng. 2017, 114, 74–81. [Google Scholar] [CrossRef]
- Bagster, D.F.; McIlvenny, J.D. Studies in the selective flocculation of hematite from gangue using high molecular weight polymers. Part 1: Chemical factors. Int. J. Miner. Process. 1985, 14, 1–20. [Google Scholar] [CrossRef]
- Sivamohan, R. The problem of recovering very fine particles in mineral processing—A review. Int. J. Miner. Process. 1990, 28, 247–288. [Google Scholar] [CrossRef]
- Song, S.; Lu, S. Hydrophobic Flocculation of Fine Hematite, Siderite, and Rhodochrosite Particles in Aqueous Solution. J. Colloid Interface Sci. 1994, 166, 35–42. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. A Review of Recent Advances in Depression Techniques for Flotation Separation of Cu–Mo Sulfides in Porphyry Copper Deposits. Metals 2020, 10, 1269. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, C.; Bu, X.; Bilal, M.; Ni, C.; Peng, Y. Enhancement of Flotation Performance of Oxidized Coal by the Mixture of Laurylamine Dipropylene Diamine and Kerosene. Minerals 2021, 11, 1271. [Google Scholar] [CrossRef]
- Hornn, V.; Park, I.; Ito, M.; Shimada, H.; Suto, T.; Tabelin, C.B.; Jeon, S.; Hiroyoshi, N. Agglomeration-flotation of finely ground chalcopyrite using surfactant-stabilized oil emulsions: Effects of co-existing minerals and ions. Miner. Eng. 2021, 171, 107076. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of lead-activated sphalerite by pyrite via galvanic interactions: Implications to the selective flotation of complex sulfide ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Wang, X.; Bu, X.; Alheshibri, M.; Bilal, M.; Zhou, S.; Ni, C.; Peng, Y.; Xie, G. Effect of scrubbing medium’s particle size distribution and scrubbing time on scrubbing flotation performance and entrainment of microcrystalline graphite. Int. J. Coal Prep. Util. 2021, 42, 3032–3053. [Google Scholar] [CrossRef]
- Yin, W.; Xue, J.; Li, D.; Sun, Q.; Yao, J.; Huang, S. Flotation of heavily oxidized pyrite in the presence of fine digenite particles. Miner. Eng. 2018, 115, 142–149. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Koike, K.; Hornn, V.; Ul Hassan, F.; Jeon, S.; Park, I.; Hiroyoshi, N. Effects of coarse chalcopyrite on flotation behavior of fine chalcopyrite. Miner. Eng. 2021, 163, 106776. [Google Scholar] [CrossRef]
- Zhou, S.; Bu, X.; Wang, X.; Ni, C.; Ma, G.; Sun, Y.; Xie, G.; Bilal, M.; Alheshibri, M.; Hassanzadeh, A.; et al. Effects of surface roughness on the hydrophilic particles-air bubble attachment. J. Mater. Res. Technol. 2022, 18, 3884–3893. [Google Scholar] [CrossRef]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Trahar, W.J.; Warren, L.J. The flotability of very fine particles—A review. Int. J. Miner. Process. 1976, 3, 103–131. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle–bubble collision models—A review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Yamazawa, R.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Kinetic Analysis for Agglomeration-Flotation of Finely Ground Chalcopyrite: Comparison of First Order Kinetic Model and Experimental Results. Mater. Trans. 2020, 61, 1940–1948. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef] [Green Version]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.U.; Jeon, S.; Hiroyoshi, N. The Challenges and Prospects of Recovering Fine Copper Sulfides from Tailings Using Different Flotation Techniques: A Review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- Leistner, T.; Peuker, U.A.; Rudolph, M. How gangue particle size can affect the recovery of ultrafine and fine particles during froth flotation. Miner. Eng. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Akishino, R.; Bu, X.; Ul Hassan, F.; Park, I.; Jeon, S.; Aikawa, K.; Hiroyoshi, N. Heterogenous carrier flotation technique for recovering finely ground chalcopyrite particles using coarse pyrite particles as a carrier. Miner. Eng. 2022, 180, 107518. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aikawa, K.; Ito, M.; Kusano, A.; Jeon, S.; Park, I.; Hiroyoshi, N. Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe. Minerals 2022, 12, 723. [Google Scholar] [CrossRef]
- Elizondo-Álvarez, M.A.; Uribe-Salas, A.; Bello-Teodoro, S. Chemical stability of xanthates, dithiophosphinates and hydroxamic acids in aqueous solutions and their environmental implications. Ecotoxicol. Environ. Saf. 2021, 207, 111509. [Google Scholar] [CrossRef]
- Zhuge, F.; Li, X.; Gao, X.; Gan, X.; Zhou, F. Synthesis of stable amorphous Cu2S thin film by successive ion layer adsorption and reaction method. Mater. Lett. 2009, 63, 652–654. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Kusano, A.; Park, I.; Oki, T.; Takahashi, T.; Furuya, H.; Hiroyoshi, N. Flotation of Seafloor Massive Sulfide Ores: Combination of Surface Cleaning and Deactivation of Lead-Activated Sphalerite to Improve the Separation Efficiency of Chalcopyrite and Sphalerite. Metals 2021, 11, 253. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals 2020, 10, 1667. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part II. Flotation. Metals 2021, 11, 439. [Google Scholar] [CrossRef]
- Yang, B.; Tong, X.; Deng, Z.; Lv, X. The Adsorption of Cu Species onto Pyrite Surface and Its Effect on Pyrite Flotation. J. Chem. 2016, 2016, 4627929. [Google Scholar] [CrossRef] [Green Version]
- Eensalu, J.S.; Tõnsuaadu, K.; Adamson, J.; Oja Acik, I.; Krunks, M. Thermal decomposition of tris(O-ethyldithiocarbonato)-antimony(III)—A single-source precursor for antimony sulfide thin films. J. Therm. Anal. Calorim. 2022, 147, 4899–4913. [Google Scholar] [CrossRef]
- Leppinen, J.O. FTIR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated sulfide minerals. Int. J. Miner. Process. 1990, 30, 245–263. [Google Scholar] [CrossRef]
- Leppinen, J.O.; Basilio, C.I.; Yoon, R.H. In-situ FTIR study of ethyl xanthate adsorption on sulfide minerals under conditions of controlled potential. Int. J. Miner. Process. 1989, 26, 259–274. [Google Scholar] [CrossRef]
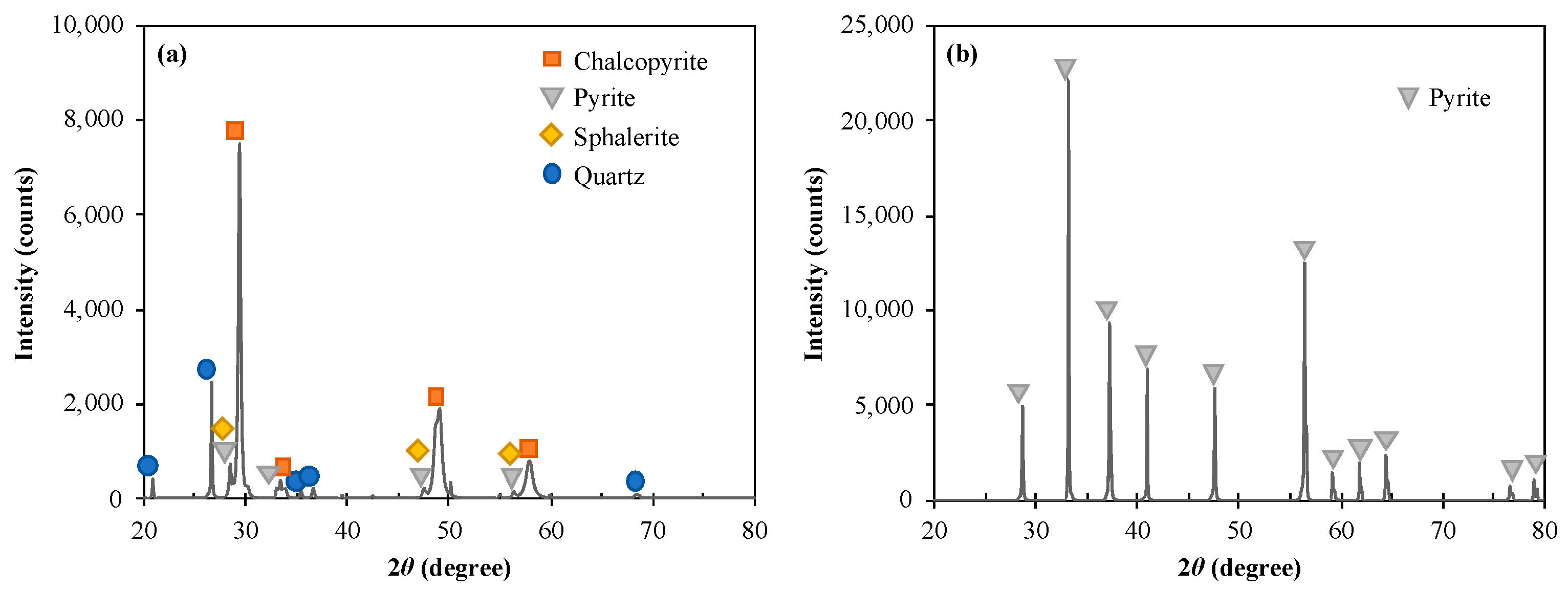







| Minerals | Mass (%) | ||||||
|---|---|---|---|---|---|---|---|
| Cu | Fe | S | Zn | Si | Ca | Others | |
| Chalcopyrite | 30.0 | 32.0 | 18.0 | 0.6 | 5.0 | 4.0 | 10.4 |
| Pyrite | 0.05 | 45.3 | 48.5 | - | 1.0 | - | 5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Park, I.; Ito, M.; Hassan, F.U.; Aikawa, K.; Jeon, S.; Hiroyoshi, N. Carrier Flotation Using Coarse Pyrite for Improving the Recovery of Finely Ground Chalcopyrite: Development of Post-Process of Carrier Flotation to Separate Finely Ground Chalcopyrite Particles from Coarse Pyrite Particles. Minerals 2023, 13, 916. https://doi.org/10.3390/min13070916
Bilal M, Park I, Ito M, Hassan FU, Aikawa K, Jeon S, Hiroyoshi N. Carrier Flotation Using Coarse Pyrite for Improving the Recovery of Finely Ground Chalcopyrite: Development of Post-Process of Carrier Flotation to Separate Finely Ground Chalcopyrite Particles from Coarse Pyrite Particles. Minerals. 2023; 13(7):916. https://doi.org/10.3390/min13070916
Chicago/Turabian StyleBilal, Muhammad, Ilhwan Park, Mayumi Ito, Fawad Ul Hassan, Kosei Aikawa, Sanghee Jeon, and Naoki Hiroyoshi. 2023. "Carrier Flotation Using Coarse Pyrite for Improving the Recovery of Finely Ground Chalcopyrite: Development of Post-Process of Carrier Flotation to Separate Finely Ground Chalcopyrite Particles from Coarse Pyrite Particles" Minerals 13, no. 7: 916. https://doi.org/10.3390/min13070916





