Abstract
It is well-known that the mining industry in Chile and the world is searching for eco-friendly, highly efficient mineral treatments. This is because the content of toxic elements such as arsenic, antimony, and bismuth have increased in the copper concentrates in the last years. This trend has affected the market of this metal, as well as increased the potential of producing solid wastes that represent a threat to the environment. In this paper, a review on the fundamentals of the current treatments aimed at removing arsenic, antimony, and bismuth from copper concentrates under roasting conditions is presented. The literature survey included the research conducted from 2000 until now and is focused on the different types of roasting of copper concentrates reported in the literature. A summary of the experimental conditions and major findings of each work is discussed. Depending on the type of roasting, the behavior of arsenic, antimony, and bismuth species during the experiments is analyzed.
Keywords:
arsenic; antimony; bismuth; roasting; pyrometallurgy; volatilization; encapsulation; capture 1. Introduction
Copper concentrates are currently treated by pyrometallurgy routes to obtain intermediate products with a high content of copper. Typical processes include smelting and converting to produce matte and blister copper in molten state. Although such technologies are widely used worldwide, a number of difficulties typically arise during operation. This includes the production of solid and gaseous wastes, which are potentially harmful to the environment, the high costs of energy associated with the operation of high temperature reactors, and the handling of molten materials, among others. Typical copper concentrates obtained by flotation techniques contain chalcopyrite (CuFeS2), bornite (Cu5FeS4), and chalcocite (Cu2S) as major phases. Such concentrates are generally treated by means of high-temperature technologies [1].
The chemical composition of copper concentrates in Chile varies depending on the region where it is obtained. As an example, Table 1 shows a comparison of typical compositions of concentrates from Chuquicamata [2] and of copper sulfide minerals in Northern Chile [3]. The former represented about 5% of the total Chilean copper production in 2022 [2]. Table 1 shows that the chemical composition is strongly dependent upon the region from which the material was obtained.

Table 1.
Chemical composition of Chuquicamata copper concentrate and copper sulfide minerals in Chile [3], wt%.
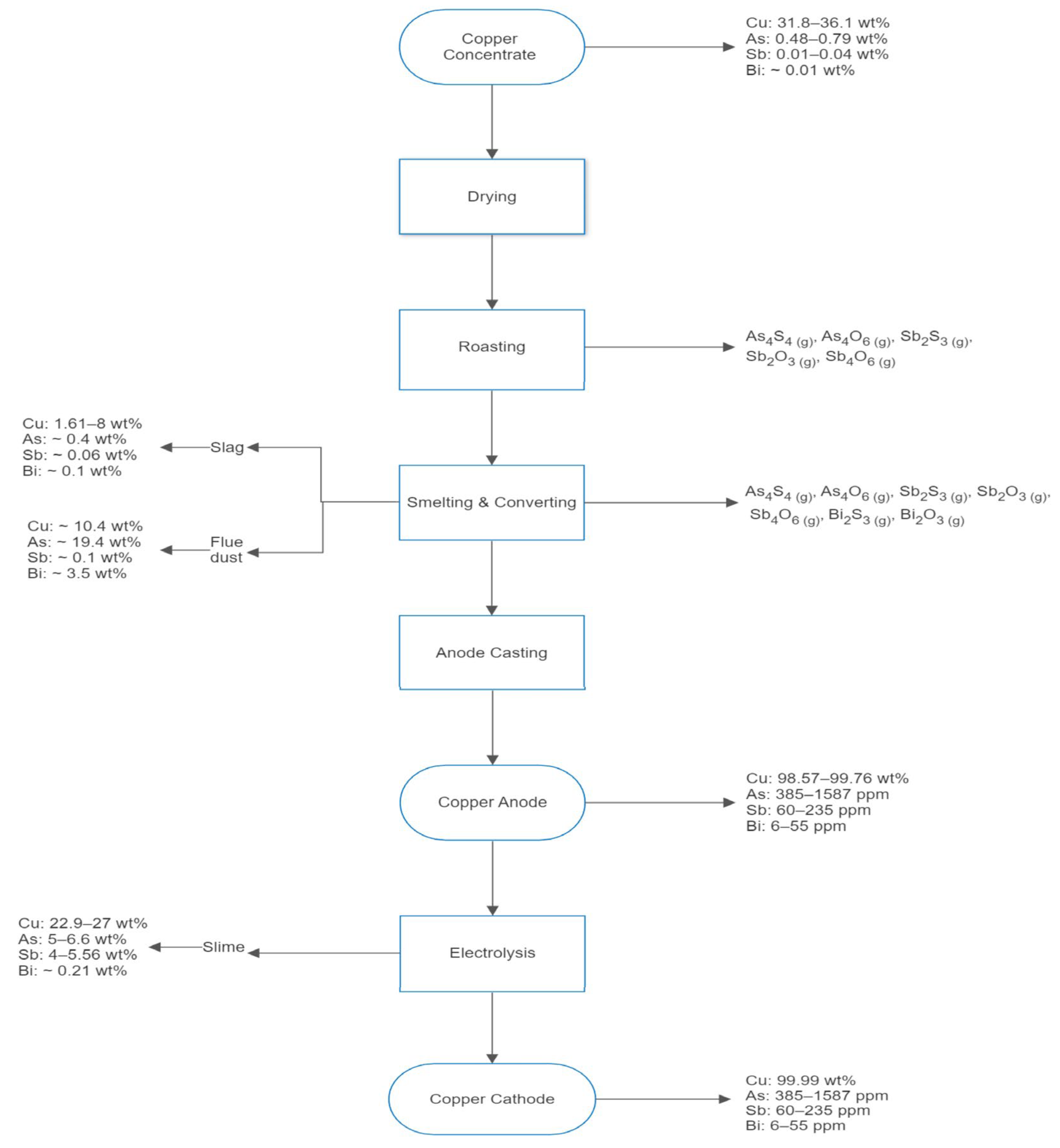
Figure 1 shows the typical pyrometallurgical route followed by Chilean copper smelters to produce cathodic copper [4,5]. Here, emphasis is made on the compositions of copper, arsenic, antimony, and bismuth in the several products and effluents obtained during the stages of roasting, smelting, converting, anode casting, and electrowinning. The information shown in Figure 1 is based on plant data collected at the Chuquicamata mine by Barros et al. [3]. It shows that the contents of arsenic, antimony, and bismuth in such streams are significant and, thus, deserve further analysis.
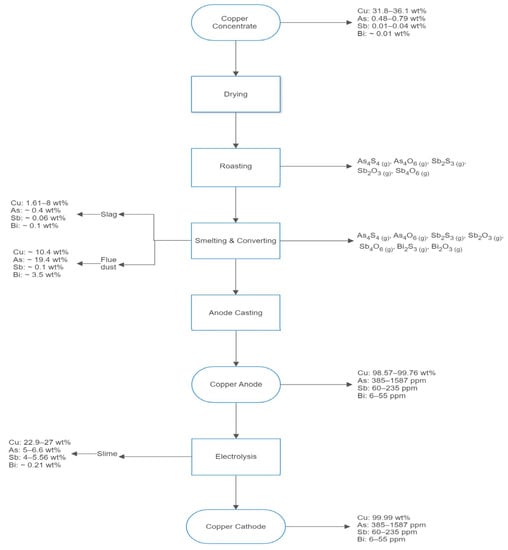
Figure 1.
Flowchart of the copper production from copper concentrate showing the concentration ranges of copper, arsenic, antimony, and bismuth in the main Chilean process and the gaseous products form on roasting and smelting/converting. Data provided by Barros et al. [3].
Arsenic is usually present as enargite (Cu3AsS4) and tennantite (Cu12As4S13) [6]; antimony is mostly present as stibnite (Sb2S3) and bismuth may be found as elemental bismuth (Bi) or bismuthine (Bi2S3). These sulfides are difficult to separate by flotation, especially enargite and tennantite. This is because the physicochemical properties of the Cu–As–S sulfides are similar to other copper sulfides present in the concentrate [7]. As a result, such species are carried towards the smelting and converting steps, where they undergo oxidation at high temperatures and distribute in both gas and molten products. The presence of such elements strongly affects the quality of the copper products, causes contamination of the flue dust, and makes further treatment difficult. It also causes operation problems in the acid plant, the collection of wastes, and decreases the efficiency of the electrorefining cells [8,9,10].
The lack of control on the rate of volatilization of arsenic, antimony, and bismuth species during the processing of copper concentrates may cause serious health problems in the human population and represent a threat to living beings in general. An example of the toxicity of these species is the use of diphenylchloroarsine (“adamsite”), a compound composed of arsenic and chloride used as a chemical weapon in World War I [11].
At the present time, few reviews on the pyrometallurgical processing of copper concentrates with high contents of arsenic, antimony, and bismuth are available in the literature. The latest work on the processing of arsenic species was reported by Castro et al. [12], who analyzed the behavior of arsenic sulfides at high temperatures. Regarding the processing of antimony, Moosavi et al. [13] carried out a review on its development through history. The authors focused on the pyrometallurgical route as well as the technologies used to obtain antimony-based products. Compared to arsenic and antimony, the literature on the processing of bismuth is scarce. The present paper is aimed at reviewing the current information on the subject. Such information is provided in a concise, compact form so that the interested reader may find it quickly in the form of tables.
2. Literature Review
Recent studies show that enargite begin oxidation at 375 °C producing tennantite (Cu12As4S13) and sulfur dioxide (SO2) according to Reaction (1):
4 Cu3AsS4 (s) + 3 O2 (g) = Cu12As4S13 (s) + 3 SO2 (g)
Both decomposition and oxidation rates of enargite were observed to be strongly dependent upon temperature and oxygen concentration. Further oxidation of tenantite produced gaseous As4O6 according to Reaction (2) [14]:
Cu12As4S13 (s) + 10 O2 (g) = 6 Cu2S (s) + As4O6 (g) + 7 SO2 (g)
These studies updated reaction mechanisms of tennantite proposed by Secco et al. [15], whose results had similar behavior to the work done by Yoshimura [16]. Padilla et al. [14] showed that under the presence of gaseous oxygen, enargite (Cu3AsS4) first oxidizes at 375 °C producing tennantite (Cu12As4S13) and sulfur dioxide gas (SO2), then tennantite further oxidizes according to Reaction (2).
Under neutral roasting conditions, enargite is assumed to undergo thermal decomposition to produce gaseous As2S3 as the major arsenic gaseous species according to Reaction (3) [15,16].
2 Cu3AsS4 (s) = 3 Cu2S (s) + As2S3 (g) + S2 (g)
In a further study, Padilla et al. [17] used a thermogravimetric analysis technique to conclude that the thermal decomposition of enargite produces As4S4, as shown in Reaction (4).
4 Cu3AsS4 (s) = 6 Cu2S (s) + As4S4 (g) + 3 S2 (g)
Regarding the behavior of antimony, this element is mostly removed in the off-gas streams of smelting and converting processes [18]. This is because antimony and its oxides are volatile at the typical temperatures under which such processes operate. Despite such behavior, during industrial operation, a significant portion of antimony is retained in the condensed phases [18,19,20]. Furthermore, the elucidation of the mechanisms of the decomposition of stibnite (Sb2S3) is relevant for the control of antimony content in both the calcine and condensed phases. It may also help to explain its distribution in subsequent stages of the production process.
Reactions (5) through (7) have been proposed for the decomposition of antimony trisulfide in molten state in a neutral atmosphere.
Sb2S3 (l) = 2 SbS (g) + ½ S2 (g)
Sb2S3 (l) = 2 Sb (l) + 3/2 S2 (g)
Sb2S3 (l) = Sb2S3 (g)
Reactions (5)–(7) were reported by Faure et al. [21], Shendiyapin et al. [22], and Komorova et al. [23], respectively. Reaction (5) indicates that molten Sb2S3 decomposes into antimony sulfide gas and sulfur gas; Reaction (6) states that decomposition occurs with the formation of metallic antimony, whereas Reaction (7) represents the volatilization of molten Sb2S3 as a physical process in which the molecular structure is conserved. The dominant step in the above set of reactions depends on the temperature of operation. Reaction (5) prevails in the range of 670 and 793 K, whereas Reaction (6) prevails between 873 and 1097 K. Finally, Reaction (7) predominates at temperatures higher than 1097 K.
Under the presence of gaseous oxygen, Sb2S3 is rapidly oxidized to produce valentinite (Sb2O3) [24,25]. These authors proposed that stibnite is oxidized to valentinite and, in a consecutive step, it is over-oxidized to antimony dioxide or cervantite (SbO2).
On the other hand, most bismuth sulfides are commonly found in association with lead, copper, and silver. Some bismuth phases found in ore deposits include bismuthine (Bi2S3), bismite (Bi2O3), and bismuth ochre (Bi2O3·3H2O).
Bismuth is found mainly around intrusive rocks, so it is paramagnetic. Bismuthine is the impurity usually found in chalcopyrite-based copper concentrates. Part of the bismuthine present in the concentrates is eliminated in the gas phase during the pyrometallurgical processes [26]. Furthermore, it was reported that 30 to 75% of bismuth in the concentrate is distributed in the matte phase, 5 to 30% in the slag phase, and 15 to 65% in the gas phase during the smelting operation [27].
It is of interest to note that of all three elements of study (As, Sb and Bi), bismuth is the element with the fewest studies reported in the literature since 2000. Furthermore, the information on the processing of bismuth species is scarce.
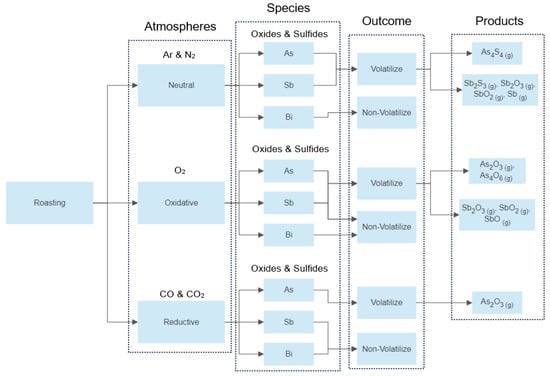
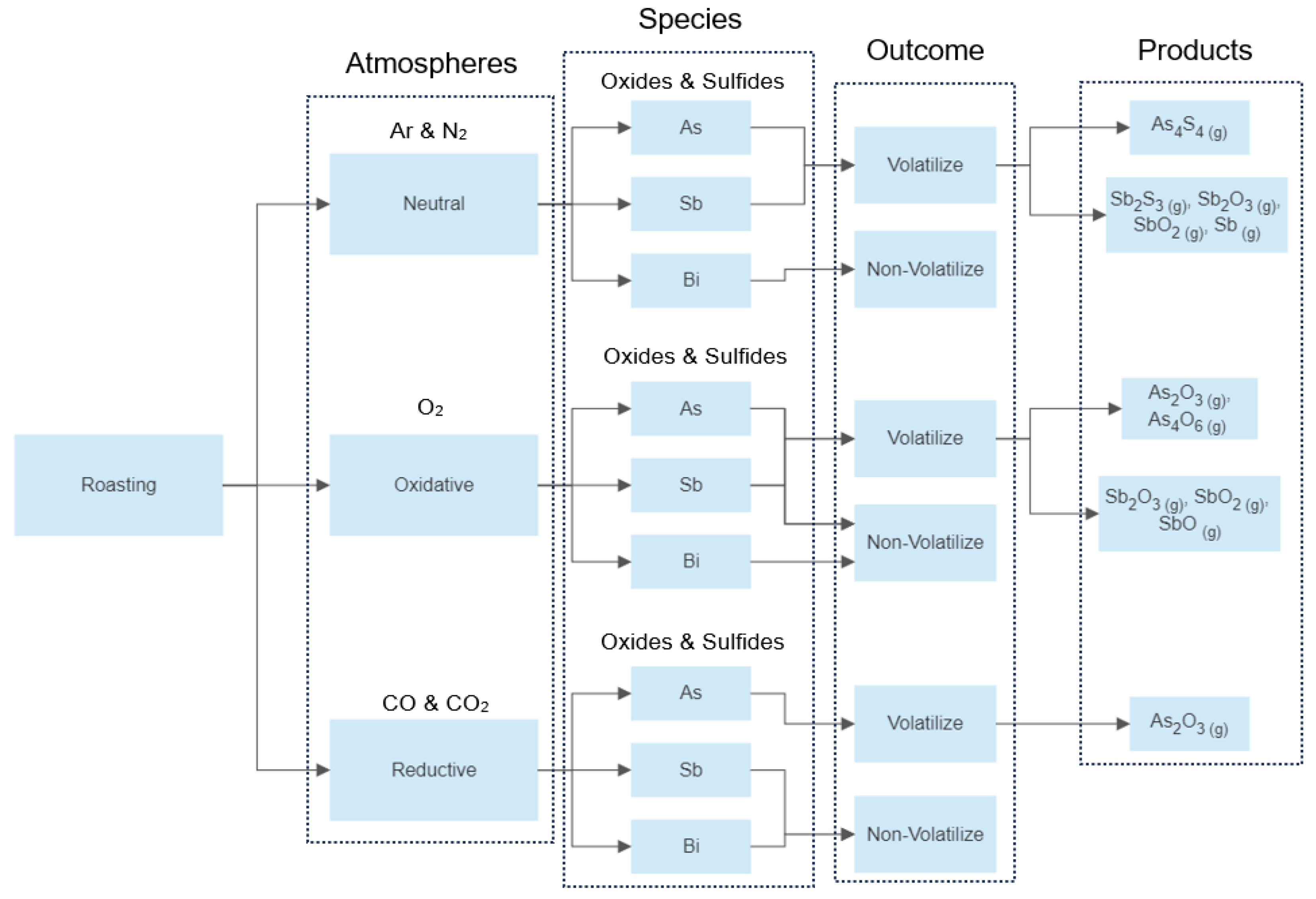
In this paper, any chemical species containing arsenic, antimony, and bismuth in its structure is considered as a toxic compound. According to the operating conditions under which such compounds are treated, the processes may be classified into three groups: thermal decomposition or inert roasting, oxidative roasting, and reductive roasting. The choice of the type of roasting to be used to treat a specific compound depends on the goal to be achieved; namely, the volatilization of the toxic compound for further cleaning of the carrying gas stream, or the capture of the toxic compound by a solid phase for a nonvolatile post-treatment.
2.1. Thermal Decomposition or Inert Roasting
In general, the concentrates containing As, Sb, and Bi are treated by roasting in a neutral environment (nitrogen, argon, etc.), which causes the volatilization of these elements. Inert roasting can achieve high efficiency removal, but the nature of the products and removal efficiency vary with the temperature of operation. As an example, the roasting of antimony trisulfide (Sb2S3) in the range of 973–1123 K proceeds according to Reaction (7) [28,29]. However, at temperatures higher than 1123 K, Reactions (5) and (6) occur simultaneously. Further, the metallic antimony produced by Reaction (6) slowly volatilizes as a result of its low equilibrium partial pressure:
Sb (l) = Sb (g)
This phenomenon was also observed with antimony (III) oxide (Sb2O3). Under a neutral environment and below 1273 K, such species volatilizes according to reaction (9) [29,30].
2 Sb2O3 (l) = Sb4O6 (g)
At temperatures higher than 1273 K, thermal decomposition produces a variety of both volatile and non-volatile compounds [28,31].
Sb2O3 (l) = SbO2 (l) + SbO (g)
2 SbO2 (l) = 2 SbO (g) + O2 (g)
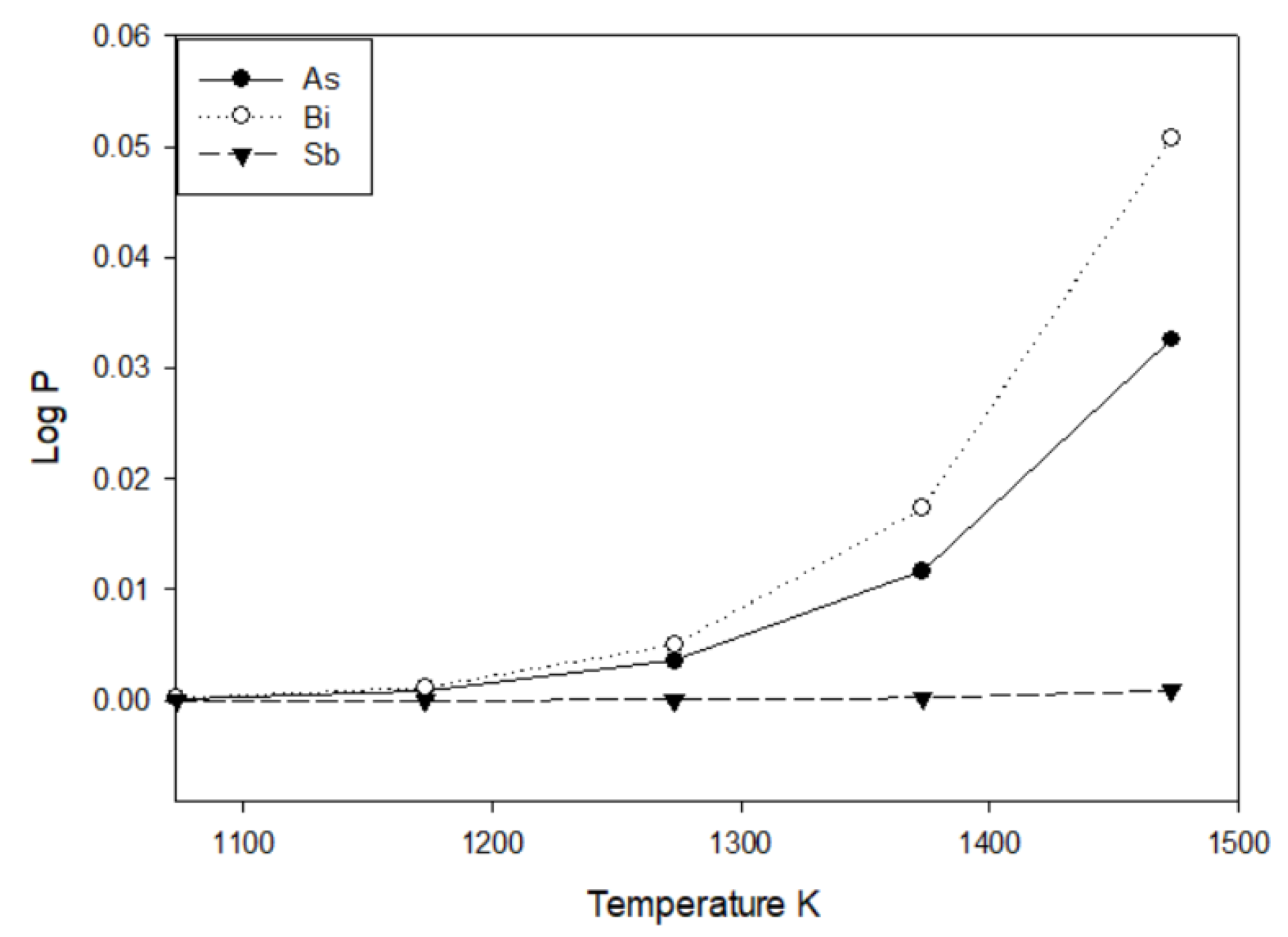
To test whether a particular species tends to volatilize, the Clausius–Clapeyron equation may be used to calculate its vapor pressure. Table 2 shows the parameters of the Clausius–Clapeyron equation for arsenic [32], antimony [33], antimony trisulfide [34], and bismuth [32]. Figure 2 shows the vapor pressure of elemental arsenic, antimony, and bismuth in the range of 1073–1473 K. As expected, the vapor pressure increases with temperature.

Table 2.
Clausius–Clapeyron equation parameters for arsenic, bismuth, and antimony trisulfides, P in Pascals.

Figure 2.
Vapor pressure of elemental arsenic, antimony and bismuth between 1073–1473 K. Pressure in Pascals.
Table 3 shows a summary of the studies on As, Sb, and Bi species of industrial interest. Relevant information regarding the experimental techniques used and the range of temperature under which volatilization was observed are also shown.

Table 3.
Compilation of inert roasting studies of arsenic, antimony, and bismuth species.
2.2. Oxidative Roasting
Unlike inert roasting, under the presence of oxygen, oxidation takes place. Furthermore, oxide species are formed. Oxidation does not necessarily produce volatile species. As an example, the oxidative roasting of antimony trisulfide in the range of 973–1273 K occurs via the following reactions [28].
Sb2S3 (s) + 4.5 O2 (g) = Sb2O3 (s) + 3 SO2 (g)
Sb2O3 (s, l) = Sb2O3 (g)
Sb2O3 (s, l) + 0.5 O2 (g) = 2 SbO2 (s)
When oxidative roasting is carried out under low oxygen partial pressure (1% to 5%), Sb2S3 produces Sb2O3 and the sulfur is volatilized as sulfur dioxide gas. Further, Sb2O3 undergoes volatilization. Reaction (14) requires a high partial pressure of oxygen to produce antimony dioxide (SbO2), also known as cervantite. This species begins to volatilize past 1373 K under the following decomposition reaction.
SbO2 (s) = SbO (g) + 0.5 O2 (g)
Table 4 summarizes the corresponding studies on the oxidative roasting of As, Sb, and Bi species of interest for this work.

Table 4.
Compilation of oxidative roasting studies of arsenic, antimony, and bismuth species.
2.3. Reductive Roasting
Reductive roasting is an efficient method to produce non-volatile products in which As, Sb, and Bi are captured. An example is the carbothermal reduction of stibine in which the production of sulfur and Sb in the gas phase is avoided [31,51].
The reduction of Sb2S3 using CaO as sulfur carrier between 973–1173 K proceeds according to the following reactions [51]. All the Gibbs free-energy data for the reduction reactions was collected from HSC database [61].
2 Sb2S3 (s) + 6 CaO (s) = Sb (l) + 3 SbO2 (s) + 6 CaS (s)
∆G 973 K = 56.450 kJ/mol
SbO2 (s) + C (s) = Sb (l) + CO2 (g)
∆G 973 K = −122.634 kJ/mol
Once CO2 (g) begins to form according to reaction (17), it reacts with carbon to form CO (g) (carbon monoxide). Further, CO is consumed according to reaction (19).
C (s) + CO2 (g) = 2 CO (g)
SbO2 (s) + 2 CO (g) = Sb (l) + 2 CO2 (g)
∆G 998 K = −122.619 kJ/mol
Reaction (19) is the predominant step, as typically solid–gas reactions, such as Reaction (18), are faster than solid–solid reactions (Reaction (17)). On the other hand, the reduction of stibine using fayalite slag (Fe3O4 and Fe2SiO4) as sulfur carrier, between 998–1173 K proceeds according to the following reactions [31].
Fe3O4 (s) + 2 C (s) = 2 FeO (s) + Fe (s) + CO2 (g)
∆G 998 K = −1.115 kJ/mol
∆G 998 K = −1.115 kJ/mol
Sb2S3 (s) + 1.5 CO2 (g) = Sb2O3 (s) + 1.5 C (s) + 1.5 S2 (g)
∆G 998 K = 309.573 kJ/mol
In this reaction scheme, magnetite is first reduced with coal according to Reaction (20) to produce wustite (FeO), metallic iron (Fe), and CO2. In the second step, Sb2S3 reacts with CO2 to produce Sb2O3, which is further encapsulated by the remaining magnetite, forming tripuhyite (FeSb2O6).
Sb2O3 (s) + 1.5 Fe3O4 (s) = FeSb2O6 (s) + 5 FeO (s)
The wustite generated in Reactions (20) and (22) also reacts with senarmontite (Sb2O3) to form tripuhyite.
Sb2O3 (s) + 3 FeO (s) = FeSb2O6 (s) + 2 Fe (s)
At temperatures higher than 1373 K, part of the senarmontite produced is decomposed to produce antimony oxide gas (SbO (g)) and cervantite, as shown in Reactions (14) and (15). Table 5 shows a summary of the reductive roasting studies of As, Sb, and Bi species so far reported in the literature.

Table 5.
Compilation of reductive roasting studies of arsenic, antimony, and bismuth species.
The overall mechanisms discussed above for neutral, oxidative, and reductive roasting of arsenic, antimony, and bismuth species are schematically summarized in Figure 3. Here, the type of atmosphere, chemical species being processed and produced, the possible outcome regarding the volatilization, and the gaseous products obtained are shown. It is noted that this a general diagram, the details concerning specific data were shown in Table 3, Table 4 and Table 5.
3. Discussion
The treatment of a specific compound by inert, oxidative, or reductive roasting does not guarantee the desired result if the experimental conditions are not established from the beginning of the experiment. This is because a number of factors affect the outcome of such experiments, such as the following.
Particle size: Relatively large particles in the treatment of these concentrates can be beneficial to maintain a control in the roasting and reduce the environmental impact. This is because fine particles may be carried away by the off-gas stream [68].
Temperature: The information shown in Table 3 through Table 5 show the temperature ranges under which volatilization and other chemical reactions of the species of interest may occur. Under such conditions, gas phase species, decomposition products, low partial pressure products, or passivating layers may be observed.
Origin of the compound: This is related to the activation energy of the chemical reactions, the reaction rate, the gas flux, and the experimental technique needed to track the evolution of the chemical processes. Based on the previous information, the following discussion addresses the main features of the processes analyzed.
3.1. Antimony
Because this element has a high vapor pressure, it tends to form volatile compounds. Depending on the experimental conditions, a variety of species can be formed.
Under a neutral atmosphere and in the absence of oxygen, antimony species are highly volatile. As an example, solid Sb2O3 under neutral atmosphere readily evaporates without passing through a liquid state. In contrast, under presence of gaseous oxygen SbO2 or SbO is formed, depending on the oxidizing conditions [51].
Finally, under reductive roasting or carbothermal reduction conditions, the volatilization of antimony species may occur as long as the temperature is high enough to volatilize the compound before it starts reacting with the reducing reactant (CO (g), CO2 (g)). This is especially relevant when a significant amount of antimony is volatilized before the reduction reactions form a stable compound. Other reducing reactants include Fe2O3 and FeO to produce stable species such as FeSb2O6 [31]. This method prevents the compound from escaping the system.
3.2. Arsenic
The behavior of As is similar to that of Sb discussed previously. Under oxidative conditions, arsenic will not volatilize unless the arsenic compound presents a high vapor pressure, which increases with the temperature as shown in Figure 2. Some authors [46,56] used metallic compounds such as Fe2O3 and CaO in addition to oxygen to form FeAsO4 and Ca3(AsO4)2, thus avoiding the volatilization of arsenic. This guaranteed the stabilization of arsenic in a solid phase.
During carbothermal reduction, solid products are formed, as the goal of such treatment is to capture and stabilize the volatile element by adding metallic compounds or salts, such as FeS, Fe2O3, or NaOH. It is noted that both arsenic and antimony behave similarly under identical process conditions. An example is the formation of volatile trioxides. The major difference between both elements is the high toxicity of arsenic. Furthermore, for the handling and processing of arsenic species, the safety regulations must be more stringent than those for antimony species. This reduces the risk of escapes to the environment, thus preventing hazards to the natural flora and fauna.
3.3. Bismuth
In contrast with the behavior of As and Sb species discussed previously, bismuth species typically show a low vapor pressure, and thus are mostly nonvolatile. Furthermore, no volatilization was observed in the studies shown in Table 3 through Table 5 under a variety of experimental conditions. As an example, Bi2S3 under a neutral atmosphere decomposes to form metallic bismuth (Bi) and gaseous sulfur at temperatures lower than 1773 K. Under oxidative roasting conditions, Bi2S3 tends to oxidize to form Bi2O3 and SO2 gas. Finally, under carbothermic reduction conditions with addition of Fe2O3 and Na2O3, the products are metallic bismuth (Bi), the sulfur species FeS and Na2S, and gaseous CO2.
4. Conclusions
A survey of the literature was conducted to summarize the characteristics of inert, oxidative, and reducing roasting to process copper concentrates with significant contents of As, Sb, and Bi.
It was shown that the selection of the type of roasting to be used depends on the goal to be achieved; namely, the separation of the metals of interest in the form of a variety of volatile species, or its capture in a solid phase for further disposal. For the former, inert and oxidative roasting may be used, whereas for the latter, reduction roasting is most appropriate. Of all three elements considered in this review, Bi was observed not to produce gaseous products under typical roasting conditions.
Because the chemistry of all three elements is complex, the selection of the experimental conditions to treat a particular material must be done with caution. This is because the products vary substantially with temperature, type of reactants, and processing time. Finally, both theoretical and experimental research are needed to further elucidate the mechanisms governing the chemistry of As, Sb, and Bi species at high temperatures.
Author Contributions
Conceptualization, A.A., O.J., E.B., M.P.-T. and M.V.; methodology, A.A. and M.V.; software, A.A., M.P.-T. and M.V.; validation, A.A., O.J., E.B., M.P.-T. and M.V.; formal analysis, A.A., O.J., E.B., M.P.-T. and M.V.; investigation, M.V.; resources, A.A.; data curation, A.A., O.J., E.B., M.P.-T. and M.V.; writing—original draft preparation, M.V.; writing—review and editing, A.A., O.J., E.B., M.P.-T. and M.V.; visualization, A.A. and M.V.; supervision, A.A., and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dimitrijević, M.; Kostov, A.; Tasić, V.; Milosević, N. Influence of Pyrometallurgical Copper Production on the Environment. J. Hazard. Mater. 2009, 164, 892–899. [Google Scholar] [CrossRef] [PubMed]
- COCHILCO Producción Minera. Available online: https://www.cochilco.cl/Paginas/Estadisticas/Bases%20de%20Datos/Producci%C3%B3n-Minera.aspx (accessed on 28 June 2023).
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Chemical Composition Data of the Main Stages of Copper Production from Sulfide Minerals in Chile: A Review to Assist Circular Economy Studies. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Aracena, A.; Fuenzalida, P. Pirometalurgia: Conceptos y Problemas; Ediciones Universitarias de la Universidad Catolica de Valparaiso: Valparaiso, Chile, 2021; pp. 161–325. [Google Scholar]
- Habashi, F. Extractive Metallurgy of Copper; Annika Parance: Montreal, QC, Canada, 2012; pp. 115–140. ISBN 9782922686197. [Google Scholar]
- Long, G.; Peng, Y.; Bradshaw, D. A Review of Copper–Arsenic Mineral Removal from Copper Concentrates. Miner. Eng. 2012, 36–38, 179–186. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsugi, K.; Kaneko, K.; Kozai, N.; Ohnuki, T.; Tuovinen, O.; Hirajima, T. Characterization of Secondary Arsenic-Bearing Precipitates Formed in the Bioleaching of Enargite by Acidithiobacillus Ferrooxidans. Hydrometallurgy 2010, 104, 424–431. [Google Scholar] [CrossRef]
- Riveros, G.; Utigard, T.A. Disposal of Arsenic in Copper Discharge Slags. J. Hazard. Mater. 2000, 77, 241–252. [Google Scholar] [CrossRef]
- Basha, C.A.; Somasundaram, M.; Kannadasan, T.; Lee, C.W. Heavy Metals Removal from Copper Smelting Effluent Using Electrochemical Filter Press Cells. Chem. Eng. J. 2011, 171, 563–571. [Google Scholar] [CrossRef]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective Leaching of Penalty Elements from Copper Concentrates: A Review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Calvo Sevillano, G. Historia del Arsenico; Almuzara: Cordoba, Spain, 2021. [Google Scholar]
- Castro, K.; Balladares, E.; Jerez, O.; Pérez-Tello, M.; Aracena, Á. Behavior of As/AsxSy in Neutral and Oxidizing Atmospheres at High Temperatures—An Overview. Metals 2022, 12, 457. [Google Scholar] [CrossRef]
- Moosavi-Khoonsari, E.; Mostaghel, S.; Siegmund, A.; Cloutier, J.P. A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes. Processes 2022, 10, 1590. [Google Scholar] [CrossRef]
- Padilla, R.; Aracena, A.; Ruiz, M.C. Reaction Mechanism and Kinetics of Enargite Oxidation at Roasting Temperatures. Metall. Mater. Trans. B 2012, 43, 1119–1126. [Google Scholar] [CrossRef]
- Secco, A.C.; Riveros, G.; Lupaschi, A. Thermal Decomposition of Enargite and Phase Relations in the System Cu-As-S; Copper 95, C. Diaz, C. Landolt and A. Lurashi, 1988; Volume 4. Available online: https://scholar.google.com/scholar_lookup?title=Thermal+decomposition+of+enargite+and+phase+relations+in+the+system+copper-arsenic-sulfur&author=Secco,+A.C.&author=Riveros,+G.A.&author=Luraschi,+A.A.&publication_year=1988&pages=225%E2%80%93238 (accessed on 1 June 2023).
- Yoshimura, Z. Bull. Inst. Min; Metall. JPN, 1962; Volume 75. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=The+Fundamental+Investigation+of+De-arsenising+Roasting+of+Copper+Concentrates+and+its+Industrial+Practices&btnG= (accessed on 1 June 2023).
- Padilla, R.; Fan, Y.; Wilkomirsky, I. Decomposition of Enargite in Nitrogen Atmosphere. Can. Metall. Q. 2001, 40, 335–342. [Google Scholar] [CrossRef]
- Davenport, W.G.; King, M.J.; Schlesinger, M.; Biswas, A.K.; Sole, K.C. Extractive Metallurgy of Copper; Pergamon Press: Londres, Inglaterra, 2002. [Google Scholar]
- Sohn, H.S.; Fukunaka, Y.; Oishi, T.; Sohn, H.Y.; Asaki, Z. Kinetics of As, Sb, Bi and Pb Volatilization from Industrial Copper Matte during Ar+O2 Bubbling. Metall. Mater. Trans. B 2004, 35, 651–661. [Google Scholar] [CrossRef]
- Arias Arce, V.; Coronado Falcón, R.; Puente Santibañez, L.; Lovera Dávila, D. Refractariedad de Concentrados Auriferos. Rev. Del Inst. De Investig. De La Fac. De Minas Metal. Y Cienc. Geográficas 2005, 8, 5–14. [Google Scholar]
- Faure, F.M.; Mitchell, M.J.; Bartlett, R.W. Vapour Pressure Study of Stibnite Sb2S3. High Tem. Sci. 1972, 4, 181–191. [Google Scholar]
- Shendiyapin, A.C.; Nestlerov, U.N.; Ibragimov, E.T. Davlenye Para Trehsernistoy Surmy. AN Kasahskoy SSR Institut Metallurgii i Obogashceniya. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Davlenye+Para+Trehsernistory+surmy+AC+Shendiyapin&btnG= (accessed on 1 June 2023).
- Komorova, L.; HolmstrÖm, A.; Imris, I. Vaporization of Antimony from Synthetic Sulphosalts. Scand. J. Metall. 1985, 14, 103–112. [Google Scholar]
- Habashi, F. Handbook of Extractive Metallurgy; Antimony; 1997; Volume 2. Available online: https://books.google.cl/books?id=wIpTAAAAMAAJ&redir_esc=y (accessed on 1 June 2023).
- Zivkovic, Z.; Strbac, N.; Zivkovic, D.; Grujicic, D.; Boyanov, B. Kinetics and Mechanism of Sb2S3 Oxidation Process. Elsevier Termochimica Acta 2002, 383, 137–143. [Google Scholar] [CrossRef]
- Steinhauser, J.; Vartiainen, A.; Wuth, W. Volatilization and Distribution of Impurities in Modern Pyrometallurgical Copper Processing from Complex Concentrates. JOM 1984, 36, 54–61. [Google Scholar] [CrossRef]
- Davenport, W.G.; Jones, D.M.; King, M.J.; Partelpoeg, E.H. Flash Smelting: Analysis, Control and Optimization, 2nd ed.; Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Padilla, R.; Ramírez, G.; Ruiz, M.C. High-Temperature Volatilization Mechanism of Stibnite in Nitrogen-Oxygen Atmospheres. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2010, 41, 1284–1292. [Google Scholar] [CrossRef]
- Qin, W.Q.; Luo, H.L.; Liu, W.; Zheng, Y.X.; Yang, K.; Han, J.W. Mechanism of Stibnite Volatilization at High Temperature. J. Cent. South Univ. 2015, 22, 868–873. [Google Scholar] [CrossRef]
- Aracena, A.; Jerez, O.; Antonucci, C. Senarmontite Volatilization Kinetics in Nitrogen Atmosphere at Roasting/Melting Temperatures. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2016, 26, 294–300. [Google Scholar] [CrossRef]
- Cabrera, E.; Aracena, A. Encapsulamiento de Antimonio En Escoria Fayalítica Mediante Reducción Carbotérmica En Ambiente Neutro; Pontificia Universidad Católica de Valparaíso: Valparaíso, Chile, 2018. [Google Scholar]
- Kim, H.G.; Sohn, H.Y. Kinetic Modeling of Minor Element Behaviour in Copper Converting. In Proceedings of the Extraction and Processing Division, New Orlands, LA, USA, 17–21 February 1991. [Google Scholar]
- Chaubal, P.C.; Sohn, H.Y.; George, D.B.; Bailey, L.K. Mathematical Modeling of Minor-Element Behavior in Flash Smelting of Copper Concentrates and Flash Converting of Copper Mattes. Metall. Trans. B 1989, 20, 39–51. [Google Scholar] [CrossRef]
- Piacente, V.; Scardala, P.; Ferro, D. Study of the Vaporization Behaviour of Sb2S3 and Sb2Te3 from Their Vapour Pressure Measurements. J. Alloys Compd. 1992, 178, 101–115. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Li, Q. Arsenic Pre-Removal from Antimony Oxide Powder by Roasting with Pyrite (FeS2) for Decreasing Arsenic Transfer and Pollution in the Followed Antimony Smelting Process. Sep. Sci. Technol. 2022, 57, 1978–1991. [Google Scholar] [CrossRef]
- Ouyang, Z.; Chen, Y.F.; Tian, S.Y.; Xiao, L.; Tang, C.B.; Hu, Y.J.; Xia, Z.M.; Chen, Y.M.; Ye, L.G. Thermodynamic and Kinetics Analysis of the Sulfur-Fixed Roasting of Antimony Sulfide Using ZnO as Sulfur-Fixing Agent. J. Min. Metall. Sect. B Metall. 2018, 54, 411–418. [Google Scholar] [CrossRef]
- Haga, K.; Altansukh, B.; Shibayama, A. Volatilization of Arsenic and Antimony from Tennantite/Tetrahedrite Ore by a Roasting Process. Mater. Trans. 2018, 59, 1396–1403. [Google Scholar] [CrossRef]
- Ye, L.; Wen, P.; Ouyang, Z.; Hu, Y.; Tang, C.; Xia, Z. Clean and SO2-Free Method for Bismuth Extraction from Bismuthinite by Multiphase Roasting: Thermodynamic Equilibria and Reaction Mechanisms. JOM 2020, 72, 3513–3520. [Google Scholar] [CrossRef]
- Jin, W.; Yang, S.; Tang, C.; Li, Y.; Chang, C.; Chen, Y. Green and Short Smelting Process of Bismuth Sulphide Concentrate with Pyrite Cinder. J. Clean. Prod. 2022, 377, 134348. [Google Scholar] [CrossRef]
- Jadhav, R.A.; Fan, L.S. Capture of Gas-Phase Arsenic Oxide by Lime: Kinetic and Mechanistic Studies. Environ. Sci. Technol. 2001, 35, 794–799. [Google Scholar] [CrossRef]
- Zhang, W.; Che, J.; Xia, L.; Wen, P.; Chen, J.; Ma, B.; Wang, C. Efficient Removal and Recovery of Arsenic from Copper Smelting Flue Dust by a Roasting Method: Process Optimization, Phase Transformation and Mechanism Investigation. J. Hazard. Mater. 2021, 412, 125232. [Google Scholar] [CrossRef]
- Tan, C.; Li, L.; Zhong, D.; Wang, H.; Li, K. Separation of Arsenic and Antimony from Dust with High Content of Arsenic by a Selective Sulfidation Roasting Process Using Sulfur. Trans. Nonferrous Met. Soc. China 2018, 28, 1027–1035. [Google Scholar] [CrossRef]
- Liu, H.; Pan, W.-P.; Wang, C.; Zhang, Y. Volatilization of Arsenic During Coal Combustion Based on Isothermal Thermogravimetric Analysis at 600–1500 °C. Energy Fuels 2016, 30, 6790–6798. [Google Scholar] [CrossRef]
- Aracena, A.; Ruiz, M.C.; Padilla, R. Oxidación de Enargita En Atmosferas de Nitrógeno-Oxígeno a Temperaturas Altas. IBEROMET XI, X CONAMET/SAM 2010, 2. [Google Scholar]
- Nakazawa, S.; Yazawa, A.; Jorgensen, F.R.A. Simulation of the Removal of Arsenic during the Roasting of Copper Concentrate. Metall. Mater. Trans. B 1999, 30, 393–401. [Google Scholar] [CrossRef]
- Fukuzawa, R.; Rao, S.R.; The Metallurgy and Materials Society Environment Section; Metals & Minerals Processing & the Environment in Memory of Dr. Ram Rao Symposium 2014.09.28-10.01 Vancouver, B.; Conference of Metallurgists 53 2014.09.28-10.01 Vancouver, B.; COM 53 2014.09.28-10.01 Vancouver, B. Metals and Mineral Processing and the Environment in Memory of Dr. Ram Rao, Proceedings of the COM 2014, Conference of Metallurgists, 28 September–1 October 2014, Hyatt Regency Hotel, Vancouver, BC, Canada; Canadian Inst. of Mining, Metallurgy and Petroleum: Vancouver, BC, Canada, 2014; ISBN 9781926872247. [Google Scholar]
- Safarzadeh, M.S.; Howard, S.M.; Miller, J.D. Analysis and Visualization of Enargite and Tennantite Roasting Using Cu-As-S-O System Predominance Volume Diagrams. Vacuum 2018, 156, 78–90. [Google Scholar] [CrossRef]
- Winkel, L.; Wochele, J.; Ludwig, C.; Alxneit, I.; Sturzenegger, M. Decomposition of Copper Concentrates at High-Temperatures: An Efficient Method to Remove Volatile Impurities. Miner. Eng. 2008, 21, 731–742. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Davey, K.J.; Jorgensen, F.R.A.; Wright, S.; Brew, D.R.M.; Haque, N.; Vance, E.R. Development and Evaluation of an Early Removal Process for the Beneficiation of Arsenic-Bearing Copper Ores. Miner. Eng. 2010, 23, 1167–1173. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, W.; Xiao, H. Arsenic Removal from Copper–Silver Ore by Roasting in Vacuum. Vacuum 2014, 101, 350–353. [Google Scholar] [CrossRef]
- Padilla, R.; Chambi, L.C.; Ruiz, M.C. Antimony Production by Carbothermic Reduction of Stibnite in the Presence of Lime. J. Min. Metall. Sect. B Metall. 2014, 50, 5–13. [Google Scholar] [CrossRef]
- Anderson, C.G. The Metallurgy of Antimony. Geochemistry 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Zhu, X.; Ma, Z.; Li, L.; Zhang, L. Direct Preparation of Micro and Nano Antimony Trioxide Using Antimony Concentrate via Microwave Roasting: Mechanism and Process. Ceram. Int. 2022, 48, 23828–23839. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, Z.J.; Zhang, C.F.; Hwang, J.Y.; Xia, C.P. Separation and Extraction of Bismuth and Manganese from Roasted Low-Grade Bismuthinite and Pyrolusite: Thermodynamic Analysis and Sulfur Fixing. JOM 2015, 67, 1114–1122. [Google Scholar] [CrossRef]
- Adham, K.; Harris, C. Two-stage fluid bed reactor for arsenic removal and fixation. In Proceedings of the Conference of Metallurgist (COM 2014), Canadian Institute of Mining, Metallurgy, and Petroleum, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Yang, Y.B.; Cui, L.N.; Li, X.S.; Li, Q.; Jiang, T.; Ge, J. Novel Technology on Preparation of Double-Layered Pellets for Sulfur and Arsenic-Bearing Gold Concentrates. J. Cent. South Univ. 2013, 20, 2967–2973. [Google Scholar] [CrossRef]
- Liu, S.; Su, Z.; Cai, Y.; Jiang, T.; Zhang, Y. An Efficient and Clean Method for the Selective Separation of Arsenic from Scrap Copper Anode Slime Containing High Arsenic and Tin. J. Clean. Prod. 2022, 354, 131640. [Google Scholar] [CrossRef]
- Mihajlovic, I.; Strbac, N.; Zivkovic, Z.; Kovacevic, R.; Stehernik, M. A Potential Method for Arsenic Removal from Copper Concentrates. Miner. Eng. 2007, 20, 26–33. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, X.; Zi, F.; Chen, Y.; Chen, S.; Li, X.; Li, J.; Jiang, Y.; Zhang, Y. Rapid Gold Cyanidation from a Sulfur-High and Arsenic-High Micro-Fine Concentrate via Facile Two-Stage Roasting Pre-Treatment. Miner. Eng. 2022, 190, 107938. [Google Scholar] [CrossRef]
- YAO, W.; MIN, X.; LI, Q.; LI, K.; WANG, Y.; WANG, Q.; LIU, H.; QU, S.; DONG, Z.; QU, C.; et al. Formation of Arsenic−copper-Containing Particles and Their Sulfation Decomposition Mechanism in Copper Smelting Flue Gas. Trans. Nonferrous Met. Soc. China 2021, 31, 2153–2164. [Google Scholar] [CrossRef]
- HSC Chemistry [Software], Metso:Outotec 2023. Available online: https://www.metso.com/portfolio/hsc-chemistry/ (accessed on 23 June 2023).
- Ouyang, Z.; Ye, L.; Tang, C.; Chen, Y. Phase and Morphology Transformations in Sulfur-Fixing and Reduction Roasting of Antimony Sulfide. Metals 2019, 9, 79. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xue, H.; Tang, C.; Yang, S.; Tang, M. One-Step Extraction of Antimony in Low Temperature from Stibnite Concentrate Using Iron Oxide as Sulfur-Fixing Agent. Metals 2016, 6, 153. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, P.; Zhu, X.; Srinivasakannan, C.; Chen, M.; Zhang, M. A Novel Production Method of Antimony Trioxide from Stibnite Concentrate and the Dielectric Properties of Antimony Sulfide with Different Desulfurizer. Miner. Eng. 2021, 171, 107097. [Google Scholar] [CrossRef]
- Li, Y.; Xue, H.; Taskinen, P.; Jokilaakso, A.; Tang, C.; Jin, W.; Rämä, M.; Chen, Y.; Yang, S. Clean Antimony Production from Stibnite Concentrate with Goethite Residue Co-Treatment for Zinc, Iron, Sulfur Conservation. J. Clean. Prod. 2021, 313, 127847. [Google Scholar] [CrossRef]
- Li, Y.; Xue, H.; Taskinen, P.; Yang, S.; Tang, C.; Jin, W.; Chen, Y.; Jokilaakso, A. Sustainable Phase-Conversion Method for Antimony Extraction and Sulfur Conservation and Waste Treatment at Low Temperature. J. Clean. Prod. 2020, 268, 121950. [Google Scholar] [CrossRef]
- Ye, L.; Tang, C.; Chen, Y.; Yang, S.; Yang, J.; Zhang, W. One-Step Extraction of Antimony from Low-Grade Stibnite in Sodium Carbonate—Sodium Chloride Binary Molten Salt. J. Clean. Prod. 2015, 93, 134–139. [Google Scholar] [CrossRef]
- Lin, W.; Yang, S.; Tang, C.; Chen, Y.; Ye, L. One-Step Extraction of Bismuth from Bismuthinite in Sodium Carbonate–Sodium Chloride Molten Salt Using Ferric Oxide as Sulfur-Fixing Agent. RSC Adv. 2016, 6, 49717–49723. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Taskinen, P.; Peng, N.; Peng, B.; Jokilaakso, A.; Liu, H.; Liang, Y.; Zhao, Z.; Wang, Z. Treatment of High-Arsenic Copper Smelting Flue Dust with High Copper Sulfate: Arsenic Separation by Low Temperature Roasting. Miner Eng. 2021, 164, 106796. [Google Scholar] [CrossRef]
- Wu, G.; Wang, T.; Chen, G.; Shen, Z.; Pan, W.-P. Coal Fly Ash Activated by NaOH Roasting: Rare Earth Elements Recovery and Harmful Trace Elements Migration. Fuel 2022, 324, 124515. [Google Scholar] [CrossRef]
- Song, B.; Song, M.; Chen, D.; Cao, Y.; Meng, F.; Wei, Y. Retention of Arsenic in Coal Combustion Flue Gas at High Temperature in the Presence of CaO. Fuel 2020, 259, 116249. [Google Scholar] [CrossRef]
- Yang, K.; Qin, W.; Liu, W. Extraction of Metal Arsenic from Waste Sodium Arsenate by Roasting with Charcoal Powder. Metals 2018, 8, 542. [Google Scholar] [CrossRef]
- Li, B.; Deng, J.; Jiang, W.; Zha, G.; Yang, B. Removal of Arsenic, Lead and Bismuth from Copper Anode Slime by a One-Step Sustainable Vacuum Carbothermal Reduction Process. Sep. Purif. Technol. 2022, 123059. [Google Scholar] [CrossRef]
- Shi, T.; He, J.; Zhu, R.; Yang, B.; Xu, B. Arsenic Removal from Arsenic–Containing Copper Dust by Vacuum Carbothermal Reduction–Vulcanization Roasting. Vacuum 2021, 189, 110213. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Xu, W.; Qin, W.; Lei, J.; Dong, Z.; Liang, Y. Arsenic Removal from Copper Slag Matrix by High Temperature Sulfide-Reduction-Volatilization. J. Hazard. Mater. 2021, 415, 125642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).