Trace Element Characteristics of Pyrite and Arsenopyrite from the Golden Ridge Gold Deposit, New Brunswick, Canada: Implications for Ore Genesis
Abstract
:1. Introduction
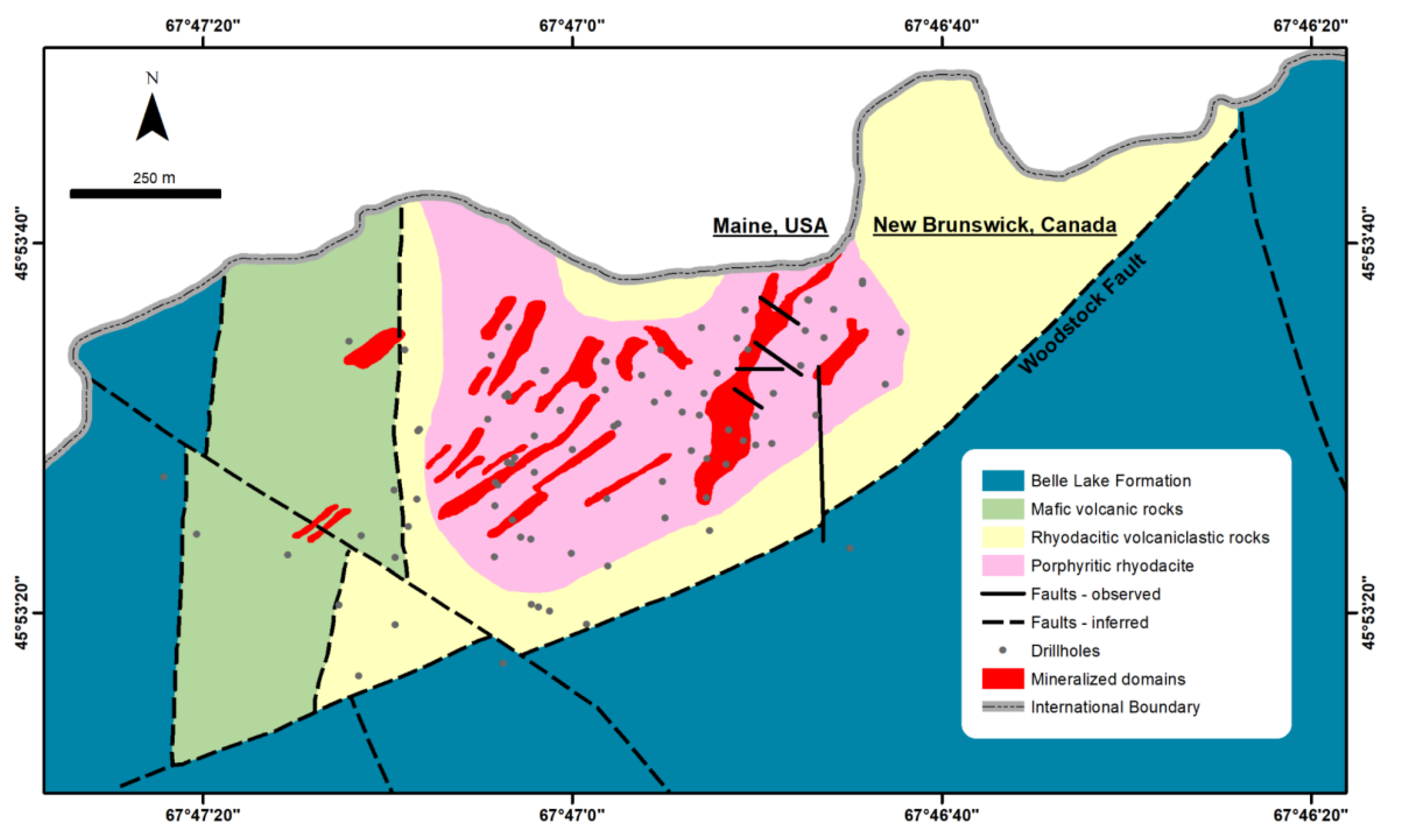
2. Geological Background
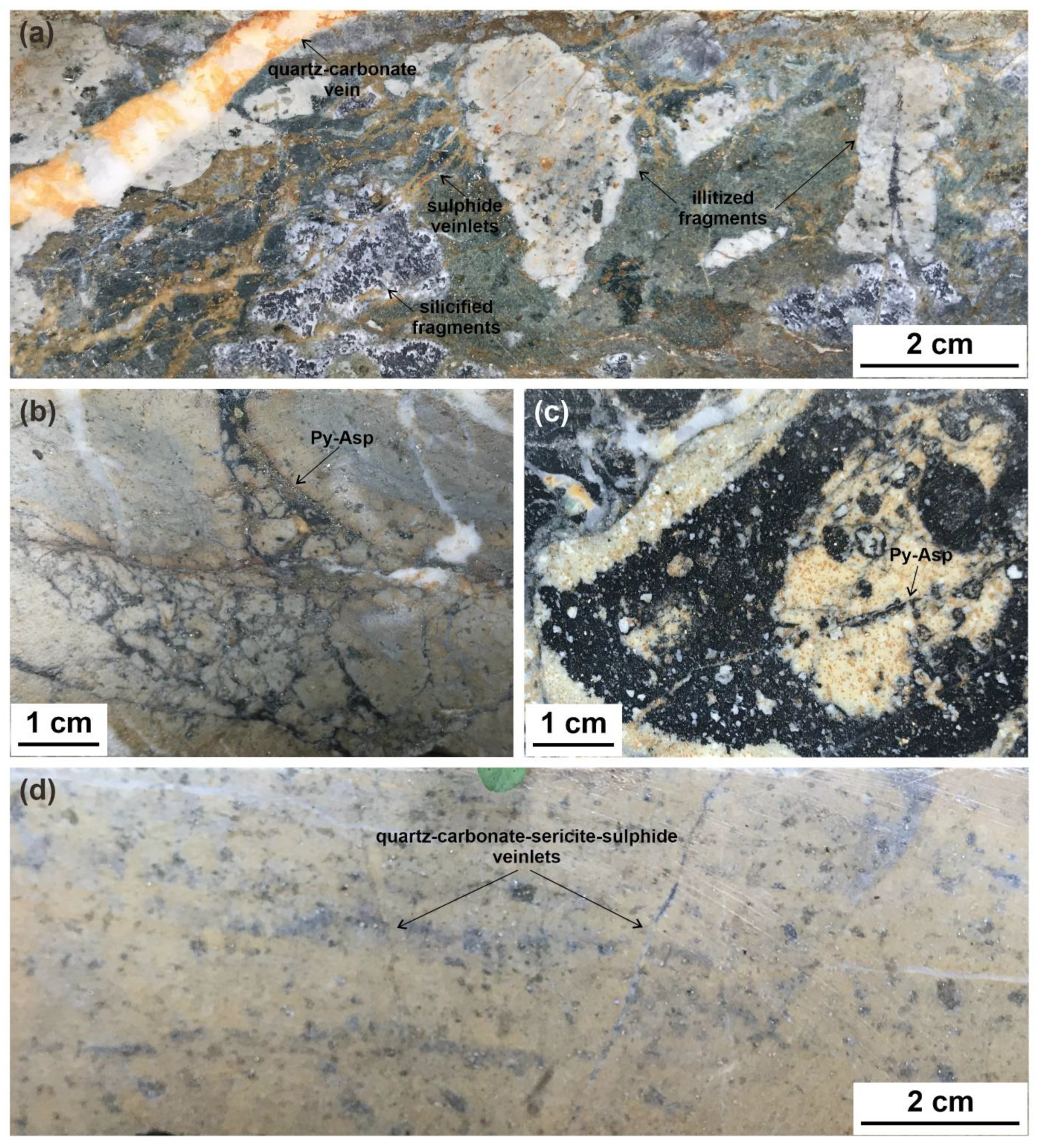
3. Ore Deposit Geology
4. Materials and Methods
4.1. Sample Selection and Bulk Analytical Methods
4.2. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry
4.3. Statistical Methods
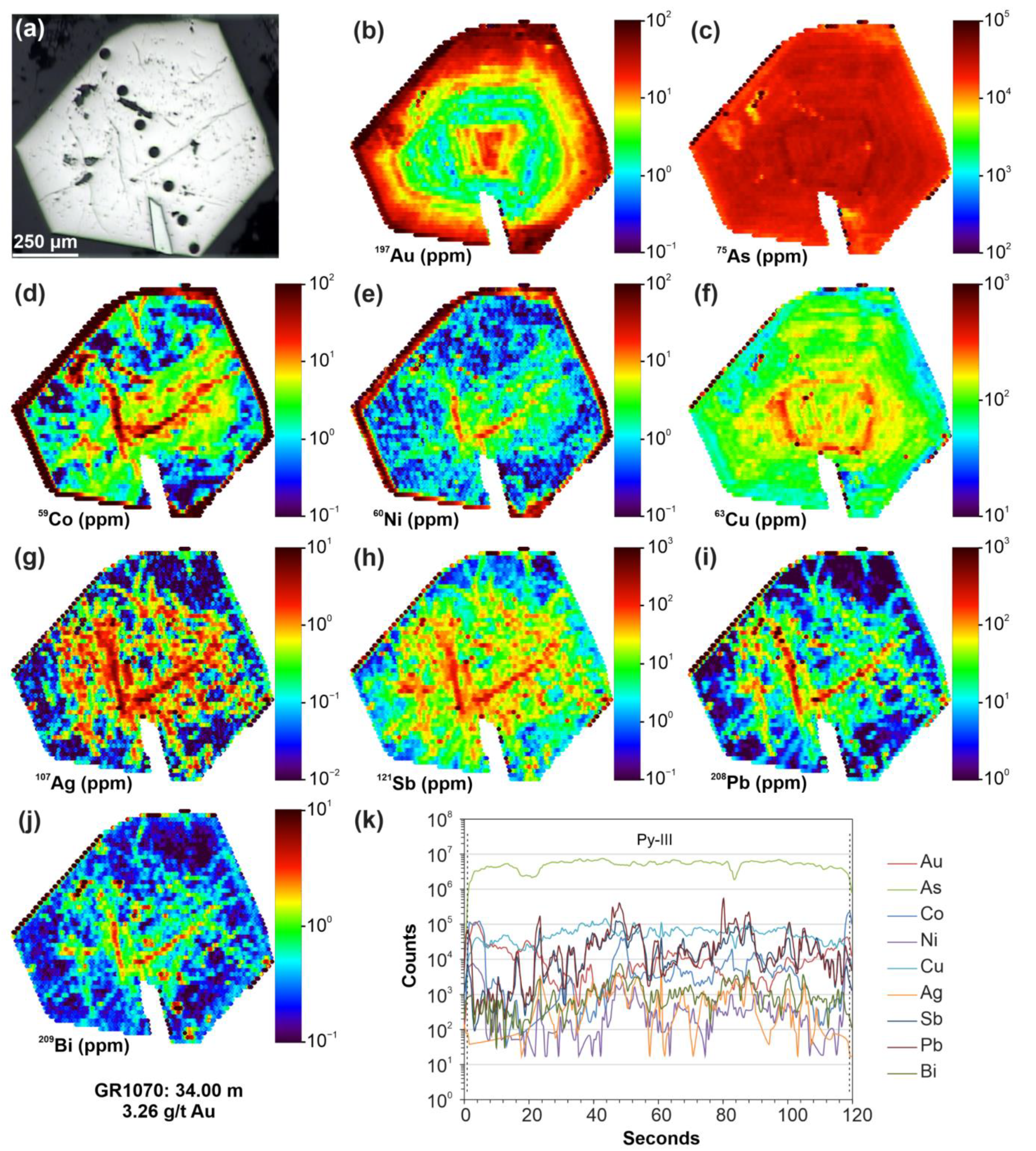
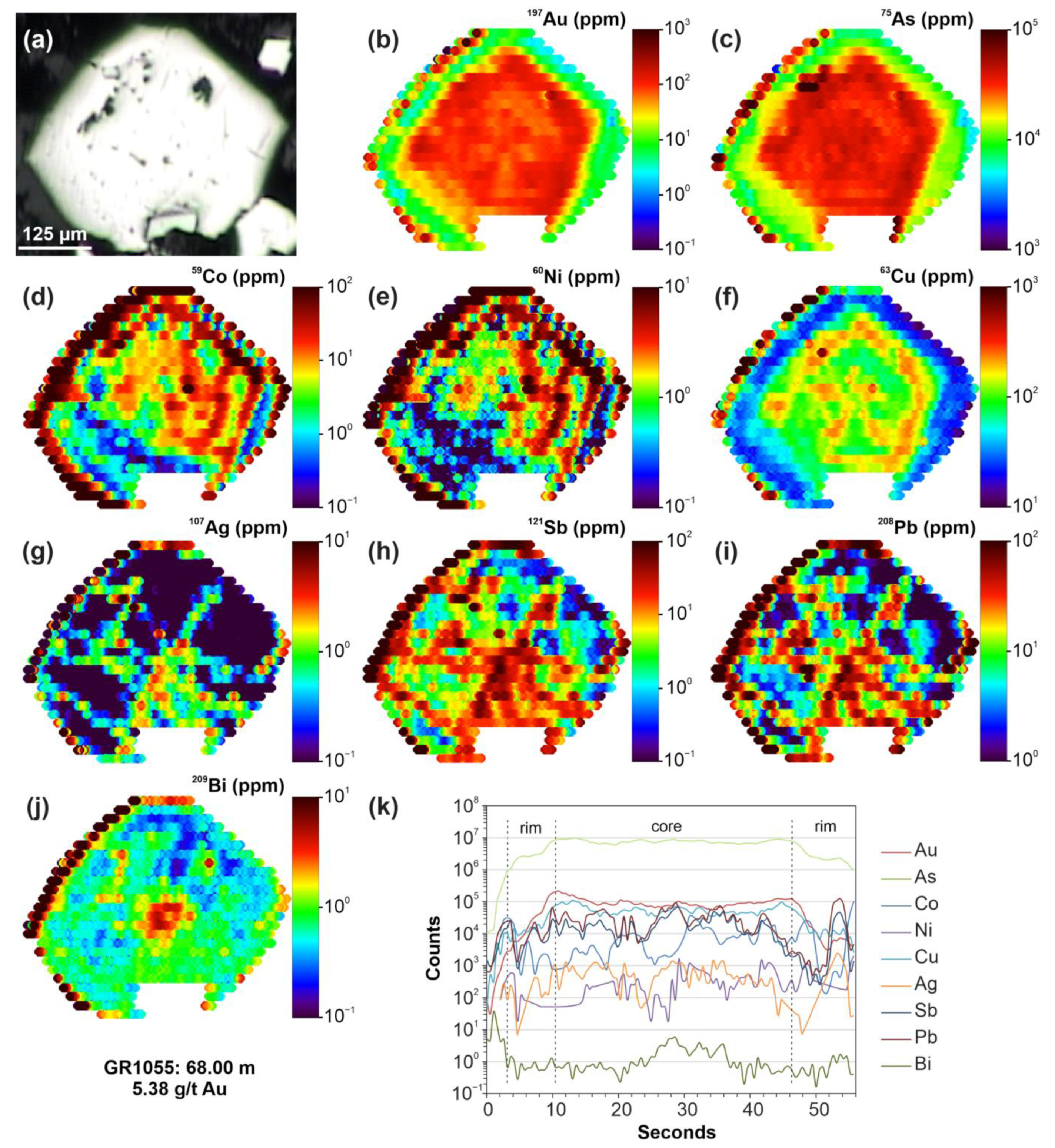
4.4. Micro-X-ray Fluorescence (μ-XRF) Elemental Mapping and BSE Imaging
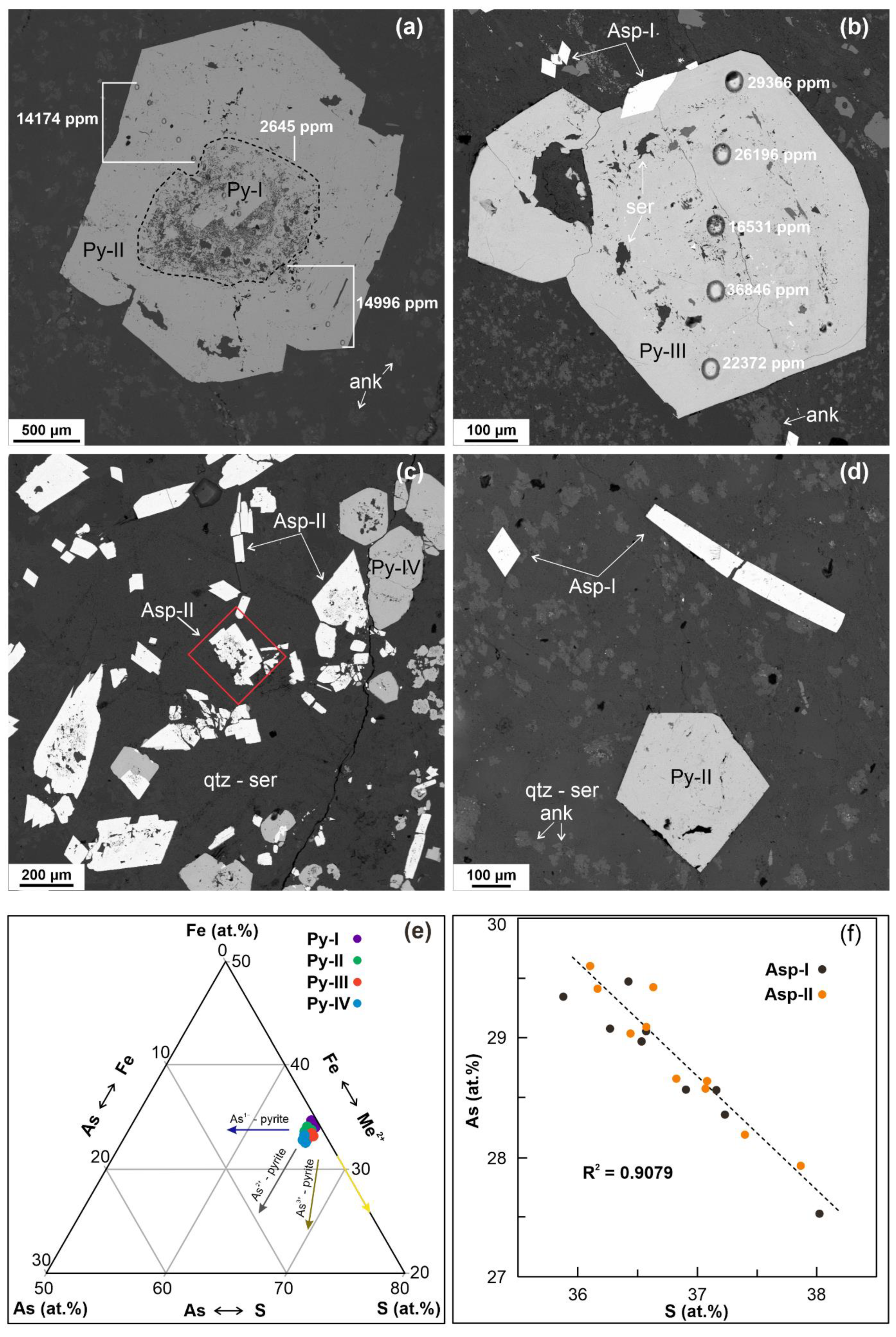
5. Results
5.1. Bulk-Rock Geochemical Analyses
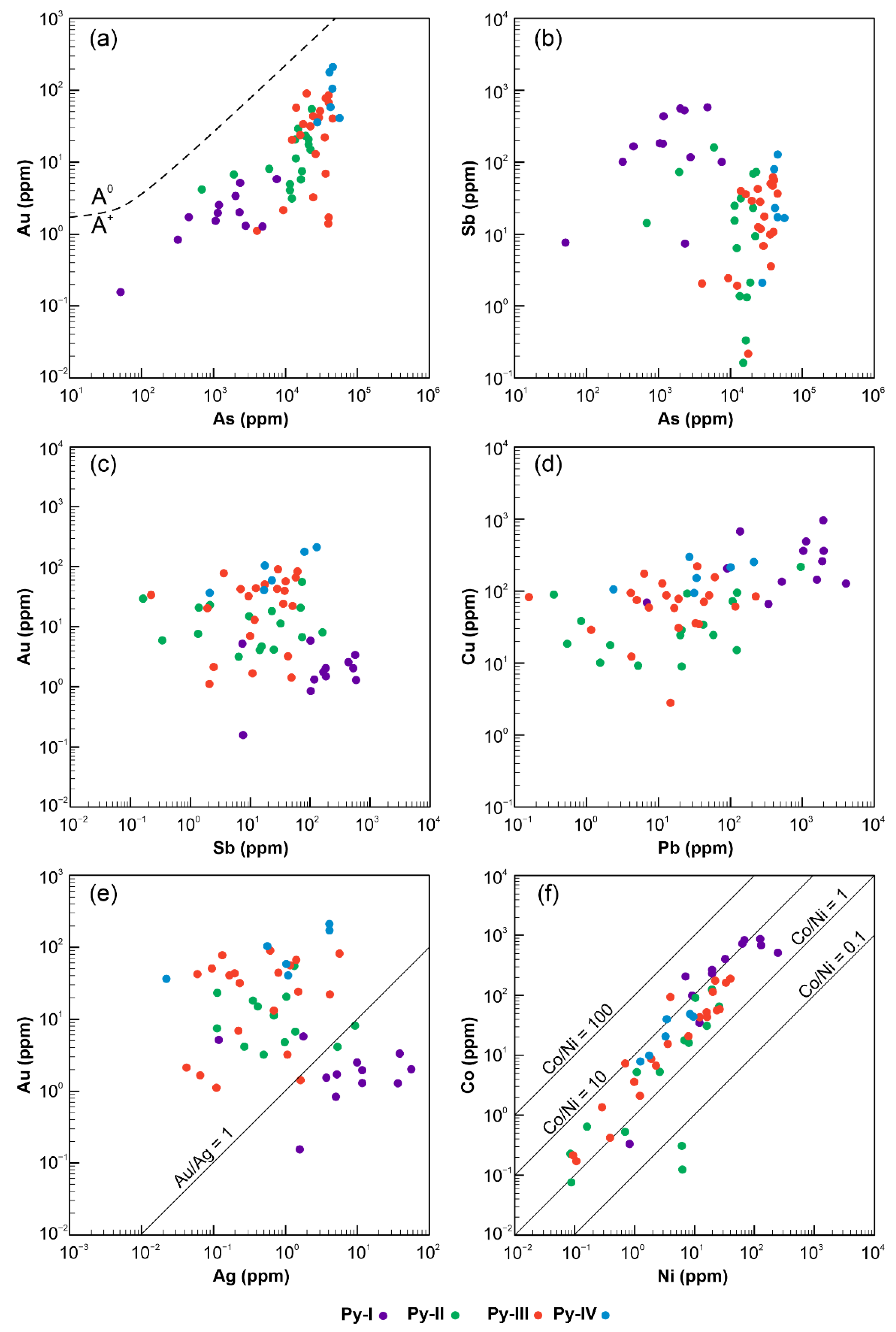
5.2. Trace Element Content of Pyrite
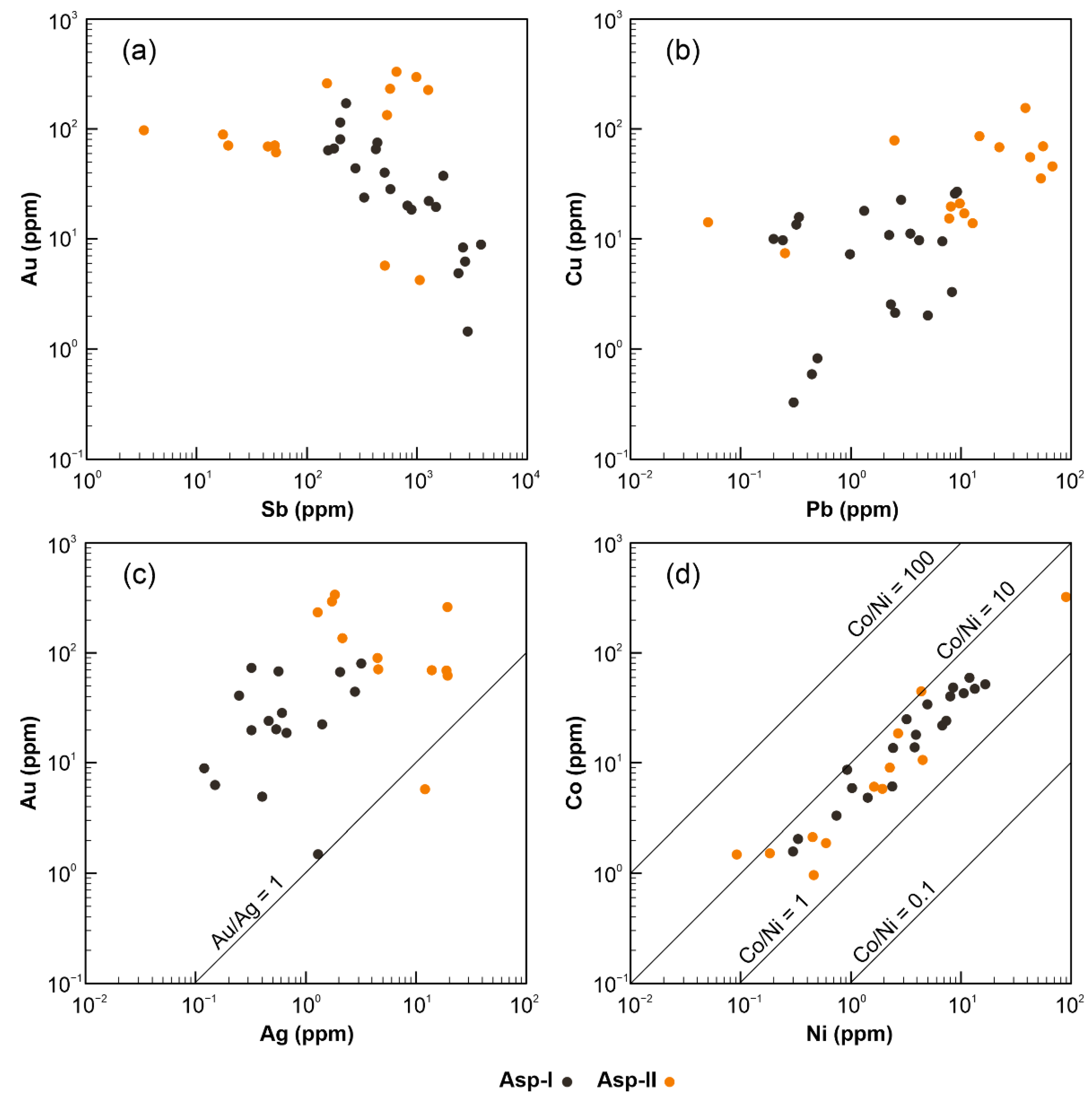
5.3. Trace Element Content of Arsenopyrite
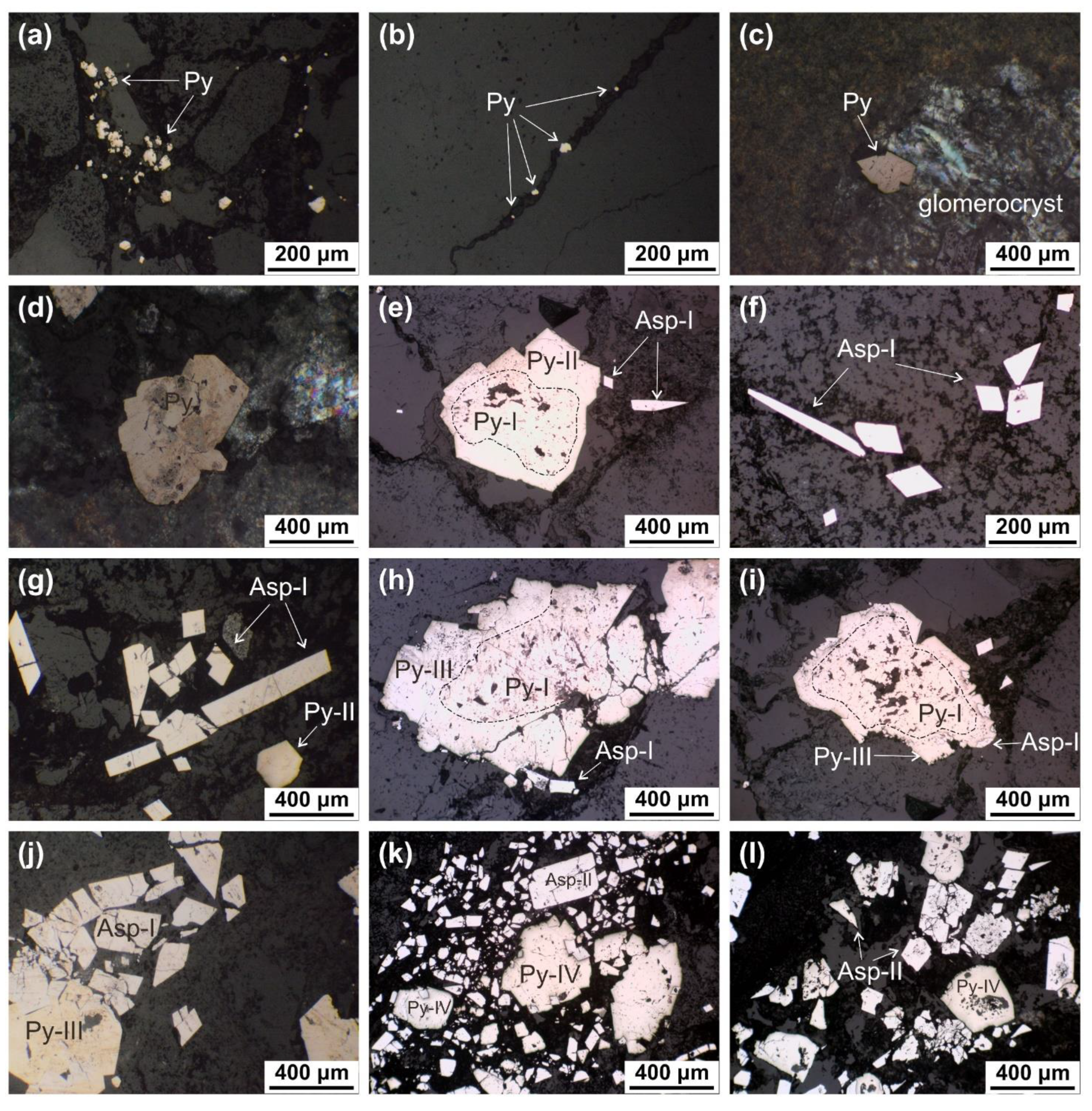
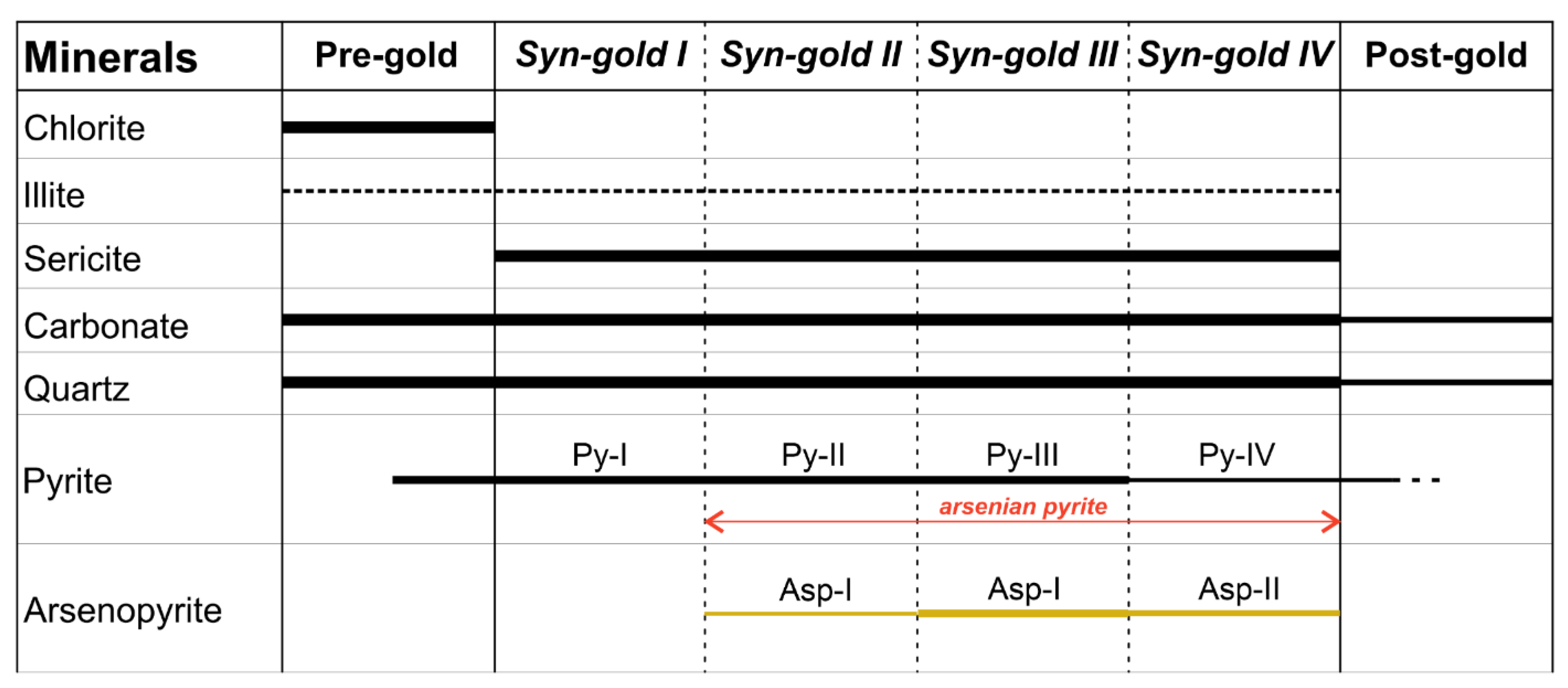
5.4. SEM-EDS Compositions of Pyrite and Arsenopyrite
6. Discussion
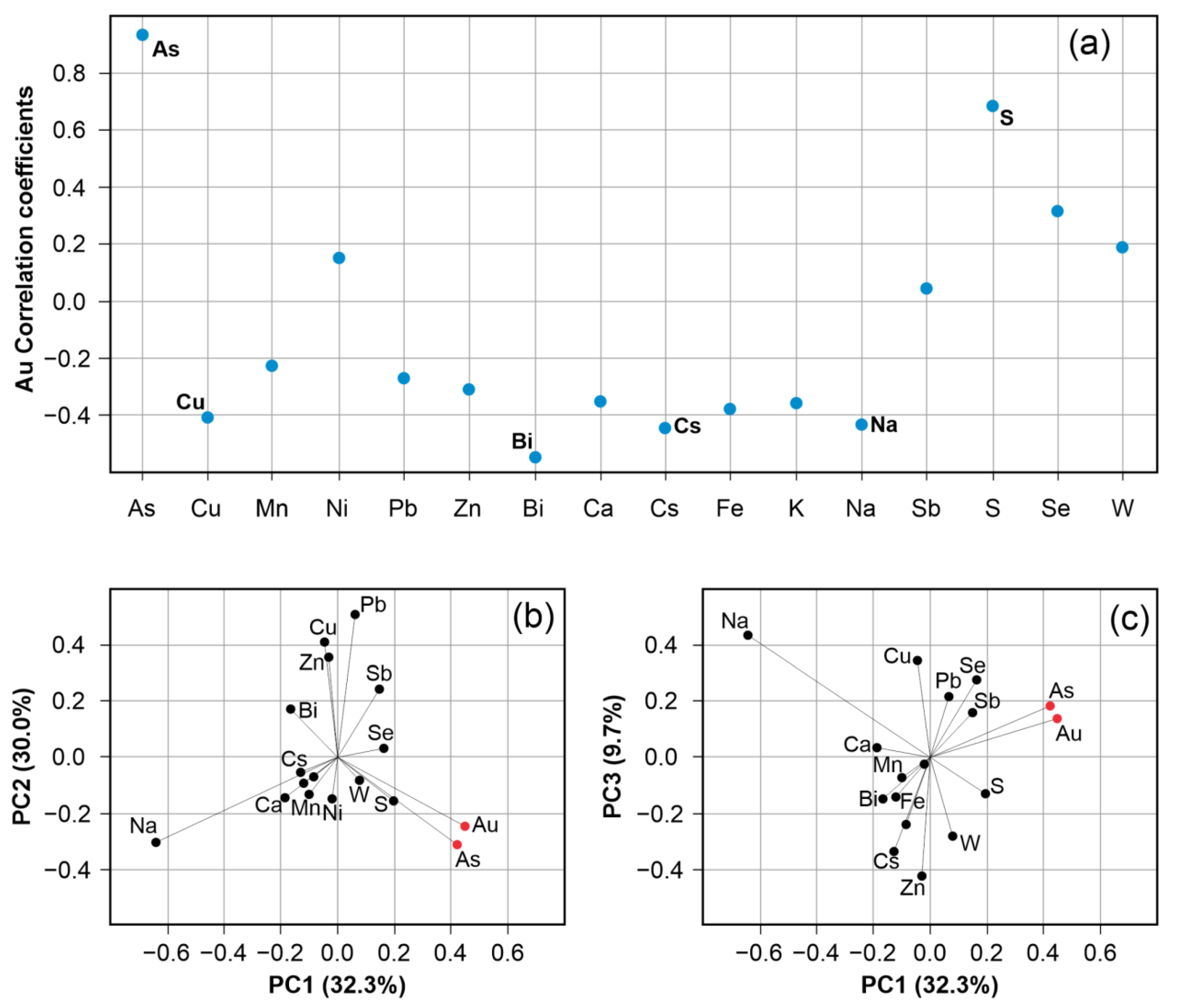
6.1. Element Association from Geochemical Data
6.2. Correlation of Trace Metals in Sulphides
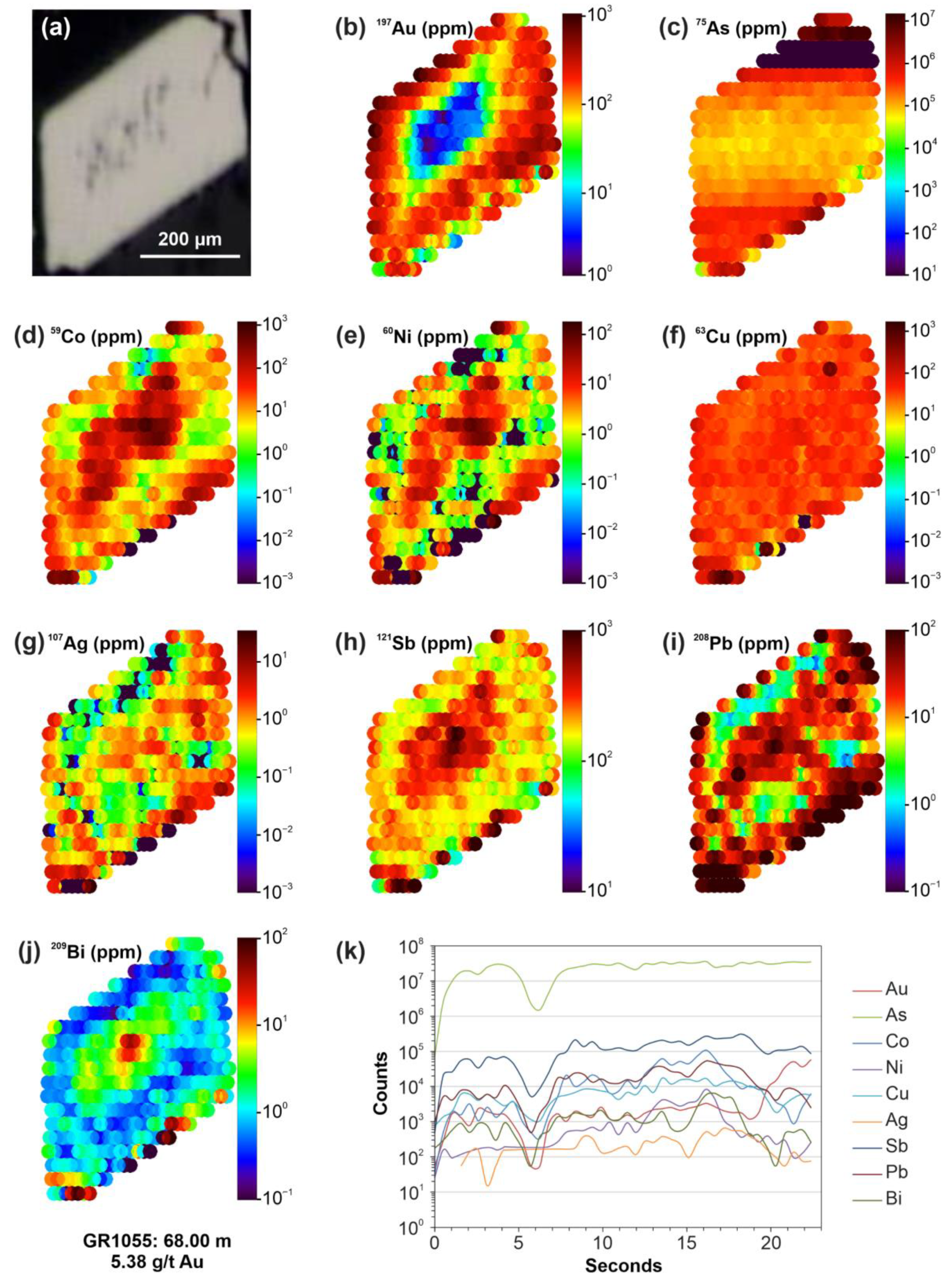
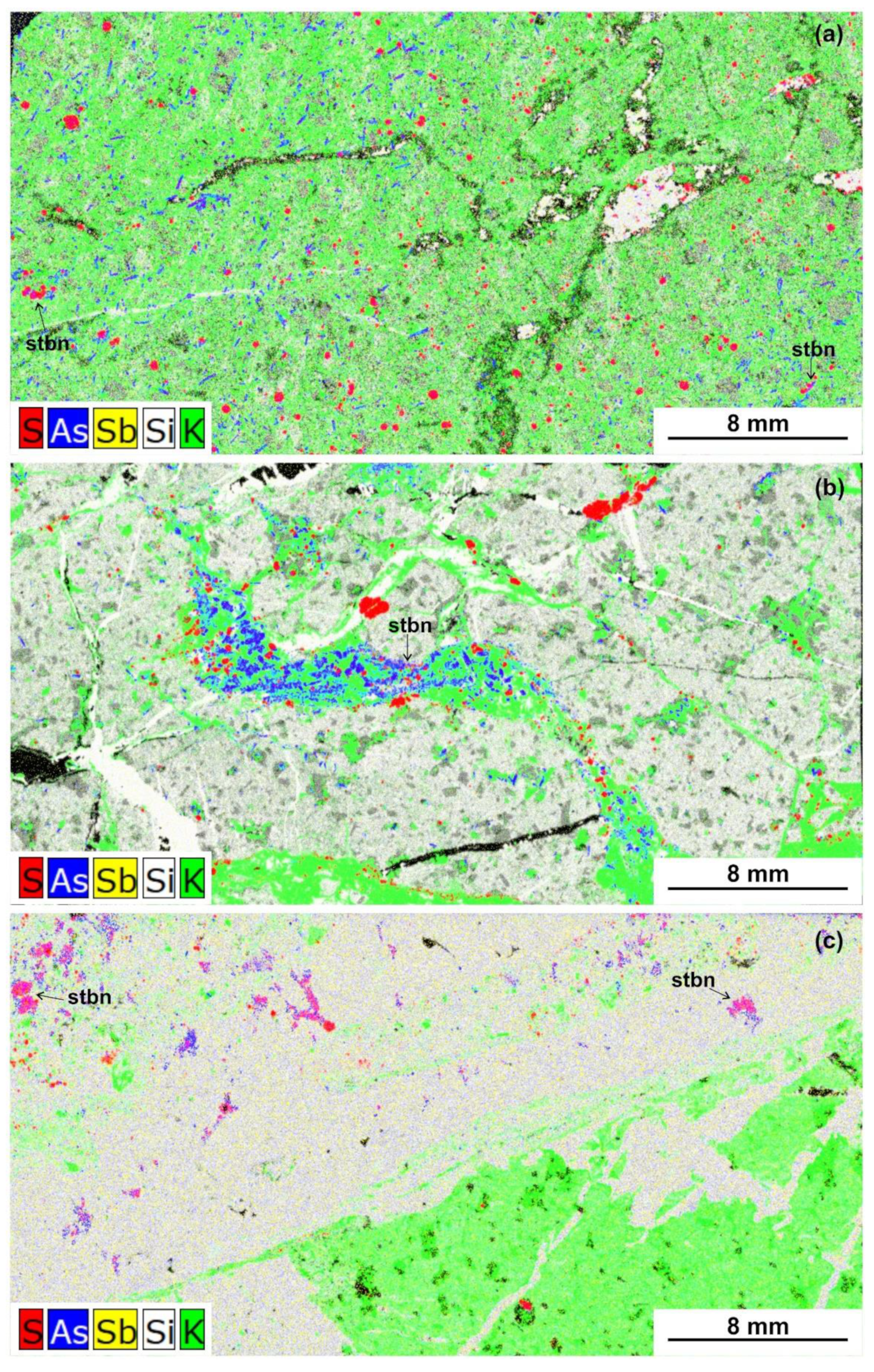
6.3. The Nature of “Invisible” Au
6.4. The Golden Ridge Gold Deposit
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Multi-Method Analysis Compositions of Rocks from Golden Ridge
| Au | Ag | As | Cd | Cu | Mn | Mo | Ni | Pb | Zn | Ba | Bi | Ca | Cs | Fe | Ga | Ge | Hg | K | Na | Sb | S | Se | Te | Tl | W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ppb | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | % | ppm | % | ppm | ppm | ppm | % | % | ppm | % | ppm | ppm | ppm | ppm |
| GR-MM-01 | 5380 | <0.2 | 7430 | <0.5 | 7 | 292 | <2 | 4 | 26 | 115 | <100 | 1.36 | 1.02 | 2.53 | 3.29 | 1 | <0.1 | <1 | 0.35 | 0.1 | 37 | 2.304 | 0.3 | <0.1 | <0.1 | 26 |
| GR-MM-02 | 4740 | 0.4 | 10,000 | <0.5 | 30 | 453 | <2 | 6 | 77 | 161 | <100 | 2.02 | 2.4 | 2.03 | 4.51 | 1 | <0.1 | <1 | 0.33 | 0.41 | 70.8 | 2.483 | 0.3 | <0.1 | 0.1 | 6 |
| GR-MM-03 | 482 | <0.2 | 464 | <0.5 | 7 | 337 | <2 | 3 | 14 | 70 | <100 | 1.11 | 2.16 | 2.19 | 3.26 | 1 | <0.1 | <1 | 0.24 | 2.61 | 10.7 | 1.421 | 0.2 | <0.1 | <0.1 | 9 |
| GR-MM-04 | 3260 | 0.3 | 4540 | <0.5 | 39 | 413 | <2 | 13 | 39 | 86 | <100 | 2.52 | 2.98 | 1 | 5.07 | 1 | <0.1 | <1 | 0.26 | 2.74 | 42.8 | 3.424 | 0.8 | <0.1 | <0.1 | 4 |
| GR-MM-05 | 39 | <0.2 | 86 | <0.5 | 8 | 407 | <2 | 1 | 11 | 40 | <100 | 2.32 | 1.85 | 2.41 | 2.26 | <1 | <0.1 | <1 | 0.41 | 0.21 | 15.6 | 0.103 | <0.1 | <0.1 | 0.1 | 5 |
| GR-MM-06 | 345 | <0.2 | 382 | 6 | 13 | 181 | <2 | <1 | 36 | 1210 | <100 | 1.06 | 1.11 | 1.08 | 1.2 | <1 | <0.1 | <1 | 0.17 | 0.04 | 27.1 | 0.762 | <0.1 | <0.1 | <0.1 | 6 |
| GR-MM-07 | 2030 | <0.2 | 8490 | <0.5 | 3 | 792 | <2 | 4 | 4 | 25 | <100 | 1.06 | 4 | 1.82 | 4.77 | <1 | <0.1 | <1 | 0.32 | 0.14 | 24.1 | 2.892 | 0.4 | <0.1 | 0.1 | <4 |
| GR-MM-08 | 4410 | <0.2 | 5750 | 0.5 | 13 | 738 | <2 | 6 | 26 | 75 | <100 | 0.91 | 1.78 | 1.9 | 3.1 | 2 | <0.1 | <1 | 0.45 | 1.13 | 31.4 | 1.686 | <0.1 | <0.1 | <0.1 | 20 |
| GR-MM-09 | 2660 | 0.2 | 1960 | 7 | 4 | 294 | <2 | 2 | 26 | 1240 | <100 | 0.92 | 0.94 | 2.05 | 5.66 | 2 | <0.1 | <1 | 0.34 | 0.1 | 16.8 | 4.969 | 0.3 | <0.1 | <0.1 | <4 |
| GR-MM-10 | 774 | <0.2 | 673 | 5.4 | 9 | 39 | <2 | 2 | 65 | 837 | <100 | 0.58 | 0.04 | 0.42 | 1.15 | <1 | <0.1 | <1 | 0.08 | 0.04 | 49.9 | 0.947 | 0.3 | <0.1 | <0.1 | 6 |
| GR-MM-11 | 2750 | <0.2 | 4770 | <0.5 | 7 | 420 | <2 | 10 | 6 | 77 | <100 | 1.78 | 2.52 | 1.03 | 3.45 | 2 | <0.1 | <1 | 0.34 | 2.55 | 20 | 1.381 | 0.5 | <0.1 | <0.1 | <4 |
| GR-MM-12 | 5390 | <0.2 | 5860 | <0.5 | 4 | 455 | <2 | 4 | 11 | 52 | <100 | 1.52 | 1.76 | 1.08 | 2.87 | <1 | <0.1 | <1 | 0.31 | 0.1 | 23.6 | 1.521 | 0.3 | <0.1 | <0.1 | 10 |
| GR-MM-13 | 4270 | 9.9 | 6320 | 1.5 | 1160 | 602 | <2 | 2 | 3500 | 343 | <100 | 2.16 | 2.02 | 0.96 | 2.66 | <1 | <0.1 | <1 | 0.26 | 0.09 | 1720 | 1.014 | 2.9 | <0.1 | <0.1 | 8 |
| GR-MM-14 | 5570 | <0.2 | 7000 | <0.5 | 9 | 579 | <2 | 2 | 19 | 58 | <100 | 2.12 | 3.1 | 2.24 | 5.72 | 1 | <0.1 | <1 | 0.33 | 1.73 | 36.5 | 3.187 | 1 | <0.1 | <0.1 | 13 |
| GR-MM-15 | 89 | <0.2 | 145 | <0.5 | 11 | 155 | <2 | <1 | 21 | 44 | <100 | 1.13 | 1.14 | 1.3 | 1.8 | <1 | <0.1 | <1 | 0.16 | 2.07 | 12.8 | 0.167 | 0.1 | <0.1 | <0.1 | <4 |
| GR-MM-16 | 723 | <0.2 | 1860 | <0.5 | 2 | 467 | <2 | 2 | 8 | 33 | <100 | 0.53 | 2.06 | 0.56 | 3.89 | 1 | <0.1 | <1 | 0.16 | 0.07 | 8.8 | 0.456 | <0.1 | <0.1 | <0.1 | <4 |
| GR-MM-17 | 57 | <0.2 | 150 | <0.5 | 9 | 341 | <2 | <1 | 8 | 74 | <100 | 0.47 | 1.28 | 1.27 | 3.66 | 1 | <0.1 | <1 | 0.19 | 4.9 | 12.6 | 0.078 | <0.1 | <0.1 | <0.1 | <4 |
| GR-MM-18 | 1940 | <0.2 | 4650 | <0.5 | 7 | 557 | <2 | 8 | 5 | 49 | <100 | 0.52 | 1.96 | 0.81 | 2.7 | <1 | <0.1 | <1 | 0.22 | 1.92 | 18.5 | 1.433 | 0.1 | <0.1 | 0.1 | 9 |
| GR-MM-19 | 905 | 0.2 | 1700 | <0.5 | 23 | 340 | <2 | 2 | 32 | 35 | <100 | 0.41 | 1.49 | 1.91 | 2.34 | 1 | <0.1 | <1 | 0.31 | 0.12 | 38.4 | 1.183 | 0.4 | <0.1 | 0.1 | 16 |
| GR-MM-20 | 680 | <0.2 | 839 | 0.9 | 3 | 358 | <2 | <1 | 5 | 34 | <100 | 0.3 | 0.22 | 1.63 | 1.46 | 1 | <0.1 | <1 | 0.33 | 0.06 | 13.5 | 0.434 | <0.1 | <0.1 | <0.1 | 11 |
| GR-MM-21 | 1550 | <0.2 | 3890 | <0.5 | 5 | 312 | <2 | 3 | 13 | 56 | <100 | 0.46 | 1.55 | 2.69 | 3.17 | 1 | <0.1 | <1 | 0.34 | 0.09 | 24.3 | 1.835 | <0.1 | <0.1 | <0.1 | 22 |
| GR-MM-22 | 435 | <0.2 | 1090 | <0.5 | 10 | 246 | 4 | 1 | 22 | 55 | <100 | 0.5 | 0.93 | 1.48 | 1.98 | <1 | <0.1 | <1 | 0.25 | 0.32 | 23.5 | 1.387 | <0.1 | <0.1 | 0.4 | 7 |
| GR-MM-23 | 1660 | <0.2 | 4260 | <0.5 | 4 | 429 | <2 | 3 | 9 | 41 | <100 | 0.31 | 1.52 | 1.4 | 2.26 | <1 | <0.1 | <1 | 0.27 | 0.08 | 29.8 | 1.173 | 0.5 | <0.1 | <0.1 | 15 |
| GR-MM-24 | 2860 | <0.2 | 8000 | <0.5 | 11 | 388 | <2 | 2 | 24 | 72 | <100 | 0.42 | 1.82 | 1.74 | 3.45 | <1 | <0.1 | <1 | 0.3 | 0.15 | 35.2 | 2.087 | 0.6 | <0.1 | <0.1 | 6 |
| GR-MM-25 | 3260 | <0.2 | 5640 | <0.5 | 5 | 495 | <2 | 5 | 14 | 69 | <100 | 0.29 | 3.02 | 2.03 | 3.26 | <1 | <0.1 | <1 | 0.3 | 0.35 | 36 | 0.992 | 0.7 | <0.1 | <0.1 | 13 |
| GR-MM-26 | 3590 | <0.2 | 5520 | <0.5 | 9 | 556 | <2 | 6 | 22 | 65 | <100 | 0.46 | 1.72 | 1.18 | 3.25 | <1 | <0.1 | <1 | 0.32 | 0.16 | 35.2 | 1.346 | 0.4 | <0.1 | <0.1 | 7 |
| GR-MM-27 | 4800 | <0.2 | 17400 | <0.5 | 4 | 522 | <2 | <1 | 9 | 34 | <100 | 0.4 | 1.5 | 1.73 | 4.47 | <1 | <0.1 | <1 | 0.34 | 0.2 | 36.8 | 2.508 | 0.7 | <0.1 | <0.1 | 27 |
| GR-MM-28 | 2620 | <0.2 | 4770 | <0.5 | 4 | 247 | <2 | <1 | 4 | 34 | <100 | 0.42 | 0.73 | 1.41 | 2.04 | <1 | <0.1 | <1 | 0.27 | 0.08 | 24 | 1.291 | 0.2 | <0.1 | <0.1 | 11 |
| GR-MM-29 | 2580 | <0.2 | 4100 | <0.5 | 6 | 740 | <2 | 3 | 14 | 113 | <100 | 0.32 | 2.46 | 1.88 | 2.76 | <1 | <0.1 | <1 | 0.33 | 0.84 | 27.9 | 1.064 | 0.3 | <0.1 | <0.1 | 14 |
| GR-MM-30 | 3880 | <0.2 | 5810 | <0.5 | 3 | 533 | <2 | 4 | 27 | 16 | <100 | 0.33 | 2.22 | 2.15 | 3.55 | 2 | <0.1 | <1 | 0.43 | 0.74 | 34.1 | 1.496 | 0.7 | <0.1 | <0.1 | 12 |
| GR-MM-31 | 2390 | <0.2 | 4490 | <0.5 | 3 | 506 | <2 | 4 | 9 | 47 | <100 | 0.37 | 2.07 | 1.9 | 3.75 | 1 | <0.1 | <1 | 0.37 | 0.81 | 22 | 2.193 | 0.6 | <0.1 | <0.1 | 6 |
| GR-MM-32 | 1930 | <0.2 | 3600 | <0.5 | 15 | 852 | <2 | 3 | 44 | 44 | <100 | 0.36 | 5.71 | 2.01 | 2.19 | <1 | <0.1 | <1 | 0.37 | 0.16 | 40.8 | 0.883 | 0.4 | <0.1 | <0.1 | 15 |
| GR-MM-33 | 2200 | <0.2 | 3150 | 0.7 | 5 | 556 | <2 | 8 | 16 | 204 | <100 | 0.34 | 3.19 | 1.61 | 2.2 | <1 | <0.1 | <1 | 0.24 | 0.09 | 30.8 | 0.978 | 0.4 | <0.1 | <0.1 | 17 |
| GR-MM-34 | 484 | 0.3 | 774 | 1.3 | 21 | 139 | <2 | <1 | 70 | 143 | <100 | 0.39 | 0.8 | 0.29 | 0.61 | <1 | <0.1 | <1 | 0.09 | 0.02 | 46.9 | 0.273 | 0.2 | <0.1 | <0.1 | 5 |
| GR-MM-35 | 10,200 | <0.2 | 13,900 | <0.5 | 7 | 539 | <2 | 2 | 13 | 57 | <100 | 0.26 | 2.04 | 1.12 | 3.74 | 1 | <0.1 | 1 | 0.32 | 1.53 | 37.3 | 2.301 | 0.7 | <0.1 | <0.1 | 25 |
| GR-MM-36 | 55 | <0.2 | 105 | <0.5 | 3 | 382 | <2 | 2 | 5 | 37 | <100 | 0.37 | 2.47 | 1.55 | 2.72 | 1 | <0.1 | <1 | 0.2 | 2.55 | 6.4 | 0.145 | <0.1 | <0.1 | <0.1 | <4 |
| GR-MM-37 | 1320 | <0.2 | 1570 | <0.5 | 2 | 264 | <2 | <1 | 5 | 31 | <100 | 0.28 | 0.78 | 0.64 | 1.55 | <1 | <0.1 | <1 | 0.29 | 0.06 | 62.5 | 0.809 | 0.4 | <0.1 | <0.1 | 9 |
| GR-MM-38 | 2440 | <0.2 | 3750 | <0.5 | 9 | 512 | <2 | 8 | 23 | 56 | <100 | 0.52 | 2.35 | 1.22 | 3.38 | 1 | <0.1 | <1 | 0.37 | 0.17 | 32 | 1.627 | 0.6 | <0.1 | <0.1 | 11 |
| GR-MM-39 | 2860 | <0.2 | 5950 | <0.5 | 5 | 323 | <2 | 5 | 8 | 67 | <100 | 0.32 | 1.07 | 1.9 | 2.51 | 1 | <0.1 | <1 | 0.37 | 0.16 | 29.9 | 1.451 | 0.1 | <0.1 | <0.1 | 28 |
| GR-MM-40 | 199 | <0.2 | 607 | <0.5 | 3 | 357 | <2 | <1 | 7 | 62 | <100 | 0.24 | 2.36 | 0.52 | 2.28 | 1 | <0.1 | <1 | 0.26 | 1.97 | 8.3 | 0.713 | <0.1 | <0.1 | <0.1 | <4 |
| GR-MM-41 | 2960 | <0.2 | 7920 | <0.5 | 8 | 790 | <2 | 6 | 7 | 56 | <100 | 0.31 | 1.69 | 1.11 | 3.3 | <1 | <0.1 | <1 | 0.34 | 1.1 | 35.1 | 1.699 | 0.1 | <0.1 | <0.1 | 15 |
| GR-MM-42 | 3470 | <0.2 | 6310 | <0.5 | 14 | 614 | <2 | 3 | 10 | 66 | <100 | 0.48 | 1.88 | 1.41 | 3.31 | <1 | <0.1 | <1 | 0.27 | 1.11 | 32.8 | 1.717 | 0.4 | <0.1 | <0.1 | 18 |
| GR-MM-43 | 2620 | <0.2 | 4700 | <0.5 | 6 | 495 | <2 | 4 | 10 | 85 | <100 | 0.27 | 2.19 | 1.75 | 2.47 | <1 | <0.1 | <1 | 0.28 | 0.94 | 37 | 1.122 | 0.2 | <0.1 | <0.1 | 20 |
| GR-MM-44 | 2500 | <0.2 | 6350 | <0.5 | 12 | 837 | <2 | 3 | 16 | 59 | <100 | 0.26 | 2.04 | 1.23 | 3.29 | <1 | <0.1 | <1 | 0.38 | 0.13 | 41.8 | 1.337 | 0.4 | <0.1 | <0.1 | 6 |
Appendix B. LA-ICP-MS Trace Element Data
| Stage | Sample | Co | Ni | Cu | As | Ag | Sb | Au | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Py-I | Gr-1070-43-1 | 0.3 | 0.8 | 68.0 | 2365.2 | 0.1 | 7.4 | 5.2 | 7.0 | 0.3 |
| Py-I | Gr-1070-43-2 | 698.8 | 128.9 | 142.8 | 455.8 | 5.2 | 166.5 | 1.7 | 1638.7 | 22.0 |
| Py-I | Gr-1070-43-3 | 97.9 | 9.3 | 202.9 | 51.2 | 1.6 | 7.7 | 0.2 | 91.8 | 1.0 |
| Py-I | Gr-1070-43-4 | 509.1 | 247.2 | 482.3 | 1194.3 | 9.9 | 435.6 | 2.5 | 1149.9 | 21.4 |
| Py-I | Gr-1070-43-5 | 204.0 | 7.2 | 132.5 | 1074.9 | 3.7 | 183.9 | 1.5 | 530.1 | 19.9 |
| Py-I | Gr-1070-43-6 | 34.6 | 12.1 | 65.9 | 7651.5 | 1.8 | 102.4 | 5.9 | 342.6 | 7.1 |
| Py-I | Gr-1070-43-7 | 841.8 | 126.0 | 125.1 | 321.5 | 5.0 | 102.2 | 0.8 | 4117.3 | 16.8 |
| Py-I | Gr-1070-38-6 | 239.7 | 19.7 | 361.3 | 1153.4 | 11.7 | 181.8 | 2.0 | 1053.5 | 13.5 |
| Py-I | Gr-1070-38-7 | 812.2 | 68.5 | 358.6 | 4861.6 | 36.3 | 583.7 | 1.3 | 2029.7 | 34.2 |
| Py-I | Gr-1070-38-8 | 253.8 | 19.7 | 664.9 | 2328.3 | 55.3 | 527.0 | 2.0 | 138.4 | 4.6 |
| Py-I | Gr-1070-38-9 | 398.9 | 32.6 | 946.4 | 2835.9 | 11.6 | 116.8 | 1.3 | 2004.5 | 24.4 |
| Py-I | Gr-1070-38-10 | 729.8 | 64.0 | 256.8 | 2049.4 | 38.6 | 562.8 | 3.4 | 1926.9 | 25.4 |
| Py-II | Gr-1070-38-1 | 17.8 | 6.7 | 88.1 | 15,133.4 | 0.0 | 0.2 | 29.4 | 0.4 | 0.0 |
| Py-II | Gr-1070-38-2 | 16.4 | 8.1 | 9.1 | 12,436.1 | 0.5 | 6.4 | 3.2 | 5.3 | 0.1 |
| Py-II | Gr-1070-38-3 | 0.2 | 0.1 | 18.2 | 16,584.1 | 0.0 | 0.3 | 5.9 | 0.5 | 0.0 |
| Py-II | Gr-1070-38-4 | 0.0 | 0.1 | 10.0 | 17,125.4 | 0.1 | 1.4 | 7.6 | 1.5 | 0.2 |
| Py-II | Gr-1070-38-5 | 91.5 | 10.4 | 214.1 | 6001.8 | 9.2 | 162.4 | 8.1 | 970.1 | 8.6 |
| Py-II | Gr-1070-38-11 | 124.5 | 19.6 | 91.5 | 22,396.7 | 0.4 | 9.6 | 15.1 | 25.5 | 1.8 |
| Py-II | Gr-1070-38-12 | 31.1 | 16.2 | 17.6 | 19,252.6 | 0.1 | 2.1 | 23.3 | 2.2 | 0.2 |
| Py-II | Gr-1070-38-13 | 5.3 | 2.6 | 24.6 | 11,608.0 | 5.3 | 25.0 | 4.2 | 20.3 | 1.0 |
| Py-II | Gr-1070-38-14 | 0.5 | 0.7 | 28.3 | 11,612.3 | 1.0 | 15.6 | 4.8 | 21.1 | 0.6 |
| Py-II | Gr-1070-38-15 | 65.1 | 25.9 | 38.0 | 13,705.0 | 0.0 | 1.4 | 20.8 | 0.8 | 0.1 |
| Py-II | GR990295-1 | 0.0 | 0.1 | 24.3 | 14,128.3 | 0.7 | 32.4 | 11.3 | 58.8 | 0.6 |
| Py-II | GR990295-2 | 0.1 | 0.1 | 33.5 | 20,987.8 | 0.4 | 23.1 | 18.3 | 42.4 | 0.5 |
| Py-II | GR990295-3 | 0.3 | 6.2 | 8.8 | 689.6 | 0.3 | 14.5 | 4.2 | 21.2 | 0.0 |
| Py-II | GR990295-4 | 0.1 | 6.3 | 14.9 | 1939.6 | 1.4 | 74.5 | 6.7 | 125.1 | 0.9 |
| Py-II | GR990295-5 | 0.6 | 0.2 | 71.7 | 21,329.3 | 1.0 | 69.8 | 20.7 | 108.3 | 1.5 |
| Py-II | GR990295-6 | 5.3 | 1.1 | 93.4 | 23,382.7 | 1.3 | 73.4 | 55.7 | 125.3 | 1.6 |
| Py-III | Gr-10-55-61-1 | 0.0 | 0.1 | 2.8 | 4083.2 | 0.1 | 2.1 | 1.1 | 14.9 | 0.0 |
| Py-III | Gr-10-55-61-2 | 0.2 | 0.1 | 35.0 | 26,430.0 | 0.7 | 11.8 | 13.1 | 33.1 | 0.1 |
| Py-III | Gr-10-55-61-3 | 15.6 | 3.6 | 90.8 | 35,543.0 | 4.1 | 51.4 | 22.4 | 131.1 | 0.6 |
| Py-III | Gr-10-55-61-4 | 8.8 | 1.9 | 83.2 | 40,146.1 | 5.6 | 61.9 | 83.2 | 229.5 | 1.2 |
| Py-III | Gr-10-55-61-5 | 0.2 | 0.1 | 12.1 | 9401.2 | 0.0 | 2.5 | 2.2 | 4.2 | 0.0 |
| Py-III | Gr-10-55-61-6 | 186.0 | 39.6 | 92.5 | 29,366.6 | 0.1 | 7.0 | 42.8 | 4.2 | 1.1 |
| Py-III | Gr-10-55-61-7 | 115.7 | 20.4 | 30.0 | 26,196.6 | 0.2 | 28.2 | 43.5 | 19.2 | 0.3 |
| Py-III | Gr-10-55-61-8 | 92.7 | 4.0 | 60.7 | 16,531.0 | 1.5 | 35.9 | 24.2 | 118.6 | 12.8 |
| Py-III | Gr-10-55-61-9 | 163.3 | 33.5 | 73.1 | 36,846.6 | 0.1 | 3.6 | 78.3 | 5.0 | 0.2 |
| Py-III | Gr-10-55-61-10 | 1.4 | 0.3 | 58.3 | 22,372.4 | 0.2 | 9.4 | 32.0 | 7.4 | 1.0 |
| Py-III | Gr-1070-34-8 | 2.1 | 1.2 | 218.1 | 39,604.5 | 1.6 | 48.6 | 1.4 | 35.1 | 0.8 |
| Py-III | Gr-1070-34-9 | 6.8 | 2.3 | 174.0 | 40,443.4 | 0.1 | 11.0 | 1.7 | 6.4 | 0.4 |
| Py-III | Gr-1070-34-10 | 3.7 | 1.0 | 125.1 | 36,642.6 | 0.2 | 10.0 | 7.0 | 11.5 | 0.1 |
| Py-III | Gr-1070-34-11 | 7.3 | 0.7 | 85.1 | 24,583.7 | 1.1 | 43.1 | 3.3 | 51.1 | 0.6 |
| Py-III | Gr-1070-34-12 | 42.2 | 12.4 | 57.1 | 24,516.9 | 0.8 | 12.5 | 44.4 | 16.7 | 0.3 |
| Py-III | Gr-1070-34-1 | 44.6 | 16.7 | 28.3 | 12,577.9 | 0.0 | 1.9 | 20.5 | 1.2 | 0.0 |
| Py-III | Gr-1070-34-2 | 56.8 | 23.7 | 69.8 | 45,753.7 | 0.2 | 37.3 | 40.6 | 42.8 | 0.4 |
| Py-III | Gr-1070-34-3 | 175.9 | 22.3 | 153.0 | 40,740.9 | 1.4 | 58.1 | 67.7 | 61.4 | 1.9 |
| Py-III | Gr-1070-34-4 | 60.8 | 26.4 | 86.2 | 30,434.8 | 0.1 | 17.7 | 51.5 | 13.0 | 0.5 |
| Py-III | Gr-1070-34-5 | 21.1 | 8.0 | 81.7 | 17,952.3 | 0.0 | 0.2 | 34.0 | 0.2 | 0.0 |
| Py-III | Gr-1070-34-6 | 52.2 | 15.9 | 76.8 | 20,107.0 | 0.6 | 29.4 | 91.0 | 19.2 | 0.3 |
| Py-III | Gr-1070-34-7 | 0.4 | 0.4 | 34.7 | 14,207.3 | 1.1 | 39.0 | 57.5 | 36.6 | 0.8 |
| Py-IV | Gr-10-55-68-1 | 20.8 | 3.3 | 103.8 | 28,196.1 | 0.0 | 2.1 | 36.3 | 2.4 | 0.3 |
| Py-IV | Gr-10-55-68-2 | 9.9 | 1.8 | 294.9 | 57,065.7 | 1.1 | 17.1 | 41.1 | 27.4 | 1.5 |
| Py-IV | Gr-10-55-68-3 | 48.0 | 8.5 | 93.7 | 45,269.8 | 0.6 | 17.8 | 104.5 | 31.7 | 1.0 |
| Py-IV | Gr-10-55-68-5 | 43.4 | 9.8 | 249.7 | 45,865.4 | 4.0 | 129.3 | 210.8 | 214.6 | 7.0 |
| Py-IV | Gr-10-55-68-6 | 39.8 | 3.5 | 212.8 | 41,399.4 | 4.1 | 80.3 | 178.6 | 103.2 | 3.6 |
| Py-IV | Gr-10-55-68-7 | 8.0 | 1.2 | 149.7 | 42,512.6 | 1.0 | 23.1 | 59.4 | 34.2 | 1.2 |
| Stage | Sample | Co | Ni | Cu | Ag | Sb | W | Au | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Asp-I | Asp_GR0414-16.35-1 | 13.8 | 3.8 | 2.6 | 0.1 | 820.0 | 1.0 | 20.1 | 2.3 | 0.1 |
| Asp-I | Asp_GR0414-16.35-2 | 23.7 | 7.4 | 11.2 | 0.1 | 573.0 | 0.6 | 28.5 | 3.5 | 0.1 |
| Asp-I | Asp_GR0414-16.35-3 | 6.1 | 2.4 | 9.8 | 0.1 | 889.0 | 0.4 | 18.8 | 4.2 | 0.3 |
| Asp-I | Asp_GR0414-16.35-4 | 21.8 | 6.8 | 27.0 | 0.2 | 176.0 | 0.8 | 66.5 | 8.9 | 0.3 |
| Asp-I | Asp_GR0414-16.35-5 | 51.8 | 16.7 | 17.9 | 0.0 | 155.8 | 0.0 | 63.3 | 1.3 | 0.0 |
| Asp-I | Asp_GR0414-16.35-6 | 46.7 | 13.5 | 26.0 | 0.3 | 201.0 | 6.1 | 80.0 | 8.9 | 0.5 |
| Asp-I | Asp_GR1057-140-20-1 | 4.8 | 1.4 | 3.3 | 0.0 | 508.0 | 0.0 | 40.7 | 8.3 | 1.0 |
| Asp-I | Asp_GR1057-140-20-2 | 17.9 | 3.9 | 9.6 | 0.1 | 1280.0 | 8.0 | 22.3 | 6.8 | 3.7 |
| Asp-I | Asp_GR1057-140-20-3 | 42.5 | 10.6 | 27.3 | 0.3 | 277.0 | 0.6 | 44.3 | 9.2 | 1.9 |
| Asp-I | Asp_GR1057-140-20-4 | 59.0 | 12.0 | 22.7 | 0.0 | 331.0 | 0.1 | 24.0 | 2.9 | 1.0 |
| Asp-I | Asp_GR1057-140-20-5 | 40.2 | 8.0 | 7.3 | 0.0 | 1478.0 | 0.0 | 19.8 | 1.0 | 3.2 |
| Asp-I | Asp_GR1057-140-20-6 | 13.6 | 2.4 | 2.1 | 0.0 | 2370.0 | 2.6 | 4.9 | 2.5 | 2.9 |
| Asp-I | Asp_GR0417-12.40-1 | 0.3 | 0.2 | 0.3 | 0.0 | 2620.0 | 0.0 | 8.4 | 0.3 | 0.7 |
| Asp-I | Asp_GR0417-12.40-2 | 1.6 | 0.3 | 2.0 | 0.1 | 2880.0 | 0.0 | 1.5 | 5.0 | 1.6 |
| Asp-I | Asp_GR0417-12.40-3 | 8.6 | 0.9 | 13.7 | 0.0 | 437.0 | 0.0 | 73.2 | 0.3 | 0.3 |
| Asp-I | Asp_GR0417-12.40-4 | 33.7 | 5.0 | 10.9 | 0.1 | 423.0 | 0.0 | 67.5 | 2.2 | 0.5 |
| Asp-I | Asp_GR0417-12.40-5 | 48.3 | 8.5 | 10.1 | 0.0 | 200.0 | 0.0 | 111.0 | 0.2 | 0.0 |
| Asp-I | Asp_GR0417-12.40-6 | 24.8 | 3.2 | 15.7 | 0.0 | 226.0 | 0.1 | 166.0 | 0.3 | 0.0 |
| Asp-I | Asp_GR0417-12.40-7 | 2.0 | 0.3 | 9.8 | 0.0 | 1730.0 | 0.0 | 37.6 | 0.2 | 1.1 |
| Asp-I | Asp_GR0417-12.40-8 | 3.3 | 0.8 | 0.8 | 0.0 | 2730.0 | 0.0 | 6.3 | 0.5 | 1.6 |
| Asp-I | Asp_GR0417-12.40-9 | 5.8 | 1.0 | 0.6 | 0.0 | 3800.0 | 0.0 | 8.9 | 0.4 | 1.4 |
| Asp-II | Asp_GR1055-68.00-1 | 44.6 | 4.4 | 14.3 | 0.0 | 0.1 | 0.0 | 17.9 | 0.1 | 0.0 |
| Asp-II | Asp_GR1055-68.00-2 | 2.1 | 0.5 | 155.0 | 1.9 | 152.0 | 0.0 | 259.0 | 38.3 | 0.9 |
| Asp-II | Asp_GR1055-68.00-3 | 18.4 | 2.7 | 86.0 | 0.5 | 17.3 | 0.0 | 88.9 | 14.7 | 0.2 |
| Asp-II | Asp_GR1055-68.00-4 | 1.5 | 0.2 | 69.9 | 1.9 | 50.8 | 4.4 | 69.2 | 55.5 | 1.7 |
| Asp-II | Asp_GR1055-68.00-5 | 1.5 | 0.1 | 45.9 | 1.9 | 52.7 | 6.3 | 62.6 | 67.3 | 1.1 |
| Asp-II | Asp_GR1055-68.00-6 | 0.3 | 0.1 | 78.7 | 0.0 | 3.3 | 3.4 | 97.2 | 2.5 | 0.1 |
| Asp-II | Asp_GR1055-68.00-7 | 0.1 | 0.1 | 68.6 | 0.5 | 19.4 | 3.6 | 70.8 | 22.2 | 0.4 |
| Asp-II | Asp_GR1055-68.00-8 | 0.1 | 0.0 | 55.7 | 1.4 | 44.1 | 5.5 | 69.5 | 42.3 | 1.3 |
| Asp-II | Asp_GR9902-95.6-1 | 1.0 | 0.5 | 7.4 | 0.0 | 1263.0 | 0.0 | 228.0 | 0.3 | 0.6 |
| Asp-II | Asp_GR9902-95.6-2 | 5.8 | 1.9 | 19.6 | 0.2 | 648.0 | 0.0 | 334.0 | 8.1 | 0.8 |
| Asp-II | Asp_GR9902-95.6-3 | 9.0 | 2.3 | 15.4 | 0.1 | 567.0 | 0.0 | 232.0 | 7.8 | 0.5 |
| Asp-II | Asp_GR9902-95.6-4 | 6.0 | 1.6 | 20.9 | 0.1 | 1050.0 | 0.0 | 4.2 | 9.7 | 1.8 |
| Asp-II | Asp_GR9902-95.6-5 | 320.0 | 90.6 | 35.6 | 1.2 | 519.0 | 0.2 | 5.7 | 52.9 | 2.5 |
| Asp-II | Asp_GR9902-95.6-6 | 1.9 | 0.6 | 14.0 | 0.2 | 535.0 | 0.0 | 135.1 | 12.7 | 1.2 |
| Asp-II | Asp_GR9902-95.6-7 | 10.5 | 4.5 | 17.2 | 0.2 | 997.0 | 0.1 | 295.0 | 10.7 | 1.1 |
Appendix C. Complementary SEM-EDS and LA-ICP-MS Analyses Data
| wt.% | at.% | |||||||
|---|---|---|---|---|---|---|---|---|
| Stage | As | S | Fe | Total | As | S | Fe | S/Fe |
| Py-I | 0.36 | 51.60 | 47.39 | 99.35 | 0.20 | 65.35 | 34.46 | 1.90 |
| Py-I | 0.36 | 51.33 | 47.83 | 99.52 | 0.20 | 65.02 | 34.79 | 1.87 |
| Py-I | 0.24 | 51.68 | 47.29 | 99.21 | 0.13 | 65.47 | 34.40 | 1.90 |
| Py-I | 0.18 | 52.31 | 46.47 | 98.96 | 0.10 | 66.16 | 33.75 | 1.96 |
| Py-II | 1.39 | 51.69 | 46.62 | 99.70 | 0.75 | 65.39 | 33.86 | 1.93 |
| Py-II | 1.28 | 51.82 | 46.12 | 99.22 | 0.69 | 65.72 | 33.58 | 1.96 |
| Py-II | 1.47 | 51.65 | 46.63 | 99.75 | 0.80 | 65.34 | 33.87 | 1.93 |
| Py-II | 1.42 | 51.72 | 46.89 | 100.03 | 0.77 | 65.26 | 33.97 | 1.92 |
| Py-III | 1.81 | 51.45 | 45.96 | 99.22 | 0.99 | 65.45 | 33.57 | 1.95 |
| Py-III | 1.69 | 52.31 | 45.39 | 99.39 | 0.91 | 66.14 | 32.95 | 2.01 |
| Py-III | 1.58 | 52.02 | 45.80 | 99.40 | 0.86 | 65.85 | 33.29 | 1.98 |
| Py-IV | 3.16 | 50.83 | 45.21 | 99.20 | 1.73 | 65.05 | 33.22 | 1.96 |
| Py-IV | 2.51 | 51.22 | 45.44 | 99.17 | 1.37 | 65.34 | 33.29 | 1.96 |
| Py-IV | 2.30 | 50.63 | 45.34 | 98.27 | 1.27 | 65.20 | 33.53 | 1.94 |
| wt.% | at.% | |||||||
| As | S | Fe | Total | As | S | Fe | As/S | |
| Asp-I | 41.96 | 22.40 | 37.27 | 101.63 | 29.07 | 36.27 | 34.65 | 0.80 |
| Asp-I | 41.20 | 22.78 | 37.12 | 101.10 | 28.56 | 36.91 | 34.52 | 0.77 |
| Asp-I | 40.15 | 23.74 | 37.46 | 101.34 | 27.52 | 38.02 | 34.45 | 0.72 |
| Asp-I | 41.00 | 23.04 | 37.08 | 101.12 | 28.36 | 37.23 | 34.41 | 0.76 |
| Asp-I | 42.05 | 22.65 | 37.06 | 101.75 | 29.06 | 36.57 | 34.37 | 0.79 |
| Asp-I | 41.42 | 23.07 | 37.06 | 101.55 | 28.56 | 37.16 | 34.28 | 0.77 |
| Asp-I | 41.78 | 22.56 | 37.09 | 101.44 | 28.96 | 36.54 | 34.50 | 0.79 |
| Asp-I | 42.19 | 22.32 | 36.38 | 100.89 | 29.47 | 36.43 | 34.10 | 0.81 |
| Asp-I | 42.24 | 22.11 | 37.30 | 101.64 | 29.35 | 35.89 | 34.77 | 0.82 |
| Asp-II | 42.25 | 22.06 | 36.49 | 100.79 | 29.60 | 36.11 | 34.30 | 0.82 |
| Asp-II | 41.13 | 22.78 | 36.70 | 100.61 | 28.64 | 37.08 | 34.28 | 0.77 |
| Asp-II | 40.52 | 23.51 | 36.99 | 101.03 | 27.93 | 37.87 | 34.21 | 0.74 |
| Asp-II | 41.71 | 22.42 | 36.97 | 101.10 | 29.03 | 36.45 | 34.52 | 0.80 |
| Asp-II | 41.64 | 22.41 | 36.65 | 100.69 | 29.08 | 36.57 | 34.35 | 0.80 |
| Asp-II | 40.81 | 23.17 | 37.14 | 101.11 | 28.19 | 37.40 | 34.41 | 0.75 |
| Asp-II | 42.09 | 22.16 | 36.72 | 100.98 | 29.41 | 36.17 | 34.42 | 0.81 |
| Asp-II | 40.86 | 22.48 | 36.68 | 100.01 | 28.66 | 36.83 | 34.51 | 0.78 |
| Asp-II | 41.13 | 22.85 | 36.87 | 100.85 | 28.57 | 37.07 | 34.36 | 0.77 |
| Asp-II | 42.14 | 22.46 | 36.25 | 100.86 | 29.42 | 36.64 | 33.95 | 0.80 |
| Sample | S | Cu | Zn | Pd | Ag | Sn | Sb | Te | W | Au | Tl | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GR1164A-unknown-1 | 468,298 | 192,471 | 121 | 4.61 | 111 | 0.39 | 260,857 | 34.4 | 56.6 | 0.01 | 1.73 | 665,040 | 272 |
| GR1164A-unknown-2 | 469,133 | 177,053 | 142 | 5.75 | 67 | 0.20 | 277,907 | 23.3 | 139.0 | 0.01 | 1.09 | 719,082 | 239 |
| GR1164A-unknown-3 | 640,517 | 300,327 | 140 | 8.40 | 91 | 0.48 | 420,674 | 66.0 | 0.20 | 0.00 | 1.55 | 1,120,807 | 349 |
| GR1164A-unknown-4 | 462,110 | 184,213 | 111 | 6.26 | 120 | 0.51 | 295,272 | 14.2 | 108.0 | 0.01 | 2.22 | 726,989 | 40 |
| GR1164A-unknown-5 | 580,348 | 272,319 | 57 | 7.71 | 95 | 0.67 | 404,485 | 68.4 | 2.5 | 0.00 | 3.47 | 1,056,271 | 199 |
| GR1164A-unknown-6 | 589,776 | 270,295 | 105 | 10.00 | 101 | 0.20 | 407,271 | 115.0 | 0.0 | 0.00 | 2.29 | 1,002,284 | 276 |
| GR1164A-unknown-7 | 710,725 | 307,788 | 130 | 7.60 | 250 | 1.90 | 513,062 | 34.0 | 0.2 | 0.00 | 4.10 | 1,299,018 | 63 |
| GR1164A-unknown-8 | 610,371 | 285,647 | 131 | 9.10 | 251 | 0.35 | 477,017 | 19.4 | 0.1 | 0.00 | 2.70 | 1,139,270 | 115 |
| GR1164A-unknown-9 | 596,101 | 258,696 | 82 | 9.30 | 215 | 0.44 | 438,638 | 22.5 | 0.0 | 0.00 | 2.93 | 998,727 | 57 |
| GR1164A-unknown-10 | 571,239 | 235,948 | 57 | 7.49 | 180 | 0.32 | 386,345 | 28.8 | 17.7 | 0.00 | 3.33 | 872,978 | 56 |
| GR1164A-unknown-11 | 495,823 | 205,861 | 74 | 7.17 | 143 | 0.34 | 314,091 | 19.6 | 83.5 | 0.00 | 3.35 | 736,430 | 44 |
Appendix D. Complementary LA-ICP-MS Map
References
- Hilchey, A.; Webster, P. Technical Report on Mineral Resource Estimate for Portage Minerals Inc. and Freewest Resources Canada Inc: Mercator Geological Services; Golden Ridge Property: York County, NB, Canada, 2011; p. 81. [Google Scholar]
- Chi, G.; Watters, S.E. Preliminary Geological and Petrographic Study of the Poplar Mountain Au Occurrence, Southwestern New Brunswick; Current Research 2002-D6; Geological Survey of Canada: Ottawa, ON, Canada, 2002; p. 11. [CrossRef]
- Chi, G.; Watters, S.; Davis, W.J.; Ni, P.; Castonguay, S.; Hoy, D. Geologic, Geochemical, and Geochronological Constraints on the Genesis of Gold Mineralization at Poplar Mountain, Western New Brunswick, Canada. Explor. Min. Geol. 2008, 17, 101–130. [Google Scholar] [CrossRef]
- Adams, M.D. Overview of the Gold Mining Industry and Major Gold Deposits. In Gold Ore Processing, 2nd ed.; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–30. [Google Scholar] [CrossRef]
- Aleksandrova, T.N.; Talovina, I.V.; Duryagina, A.M. Gold–sulphide deposits of the Russian Arctic zone: Mineralogical features and prospects of ore benefication. Geochemistry 2020, 80, 125510. [Google Scholar] [CrossRef]
- Cathelineau, M.; Boiron, M.-C.; Holliger, P.; Marion, P.; Denis, M. Gold in Arsenopyrites: Crystal Chemistry, Location and State, Physical and Chemical Conditions of Deposition. In The Geology of Gold Deposits: The Perspective in 1988; Keays, R.R., Ramsay, W.R.H., Groves, D.I., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1989; pp. 328–341. [Google Scholar] [CrossRef]
- Cook, N.; Chryssoulis, S.L. Concentrations of invisible gold in the common sulfides. Can. Mineral. 1990, 28, 1–16. [Google Scholar]
- Fleet, M.E.; Chryssoulis, S.L.; MacLean, P.J.; Davidson, R.; Weisener, C.G. Arsenian pyrite from gold deposits; Au and As distribution investigated by SIMS and EMP, and color staining and surface oxidation by XPS and LIMS. Can. Mineral. 1993, 31, 1–17. [Google Scholar]
- Genkin, A.D.; Bortnikov, N.S.; Cabri, L.J.; Wagner, F.E.; Stanley, C.J.; Safonov, Y.G.; McMahon, G.; Friedl, J.; Kerzin, A.L.; Gamyanin, G.N. A multidisciplinary study of invisible gold in arsenopyrite from four mesothermal gold deposits in Siberia, Russian Federation. Econ. Geol. 1998, 93, 463–487. [Google Scholar] [CrossRef]
- Majumdar, S.; Singh, S.; Sahoo, P.R.; Venkatesh, A.S. Trace-element systematics of pyrite and its implications for refractory gold mineralisation within the carbonaceous metasedimentary units of Palaeoproterozoic South Purulia shear zone, eastern India. J. Earth Syst. Sci. 2019, 128, 233. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.D.; Mullens, T.E. Gold-Bearing Arsenian Pyrite Determined by Microprobe Analysis, Cortez and Carlin Gold mines, Nevada. Econ. Geol. 1973, 68, 187–201. [Google Scholar] [CrossRef]
- Asamoah, R.K.; Zanin, M.; Gascooke, J.; Skinner, W.; Addai-Mensah, J. Refractory gold ores and concentrates part 1: Mineralogical and physico-chemical characteristics. Miner. Process. Extr. Metall. 2021, 130, 240–252. [Google Scholar] [CrossRef]
- Fraser, K.S.; Walton, R.H.; Wells, J.A. Processing of refractory gold ores. Miner. Eng. 1991, 4, 1029–1041. [Google Scholar] [CrossRef]
- Iglesias, N.; Carranza, F. Refractory gold-bearing ores: A review of treatment methods and recent advances in biotechnological techniques. Hydrometallurgy 1994, 34, 383–395. [Google Scholar] [CrossRef]
- Rabieh, A.; Albijanic, B.; Eksteen, J.J. A review of the effects of grinding media and chemical conditions on the flotation of pyrite in refractory gold operations. Miner. Eng. 2016, 94, 21–28. [Google Scholar] [CrossRef]
- Vaughan, J.P.; Kyin, A. Refractory gold ores in Archaean greenstones, Western Australia: Mineralogy, gold paragenesis, metallurgical characterization and classification. Mineral. Mag. 2004, 68, 255–277. [Google Scholar] [CrossRef]
- Yang, S.; Blum, N.; Rahders, E.; Zhang, Z. The nature of invisible gold in sulfides from the Xiangxi Au-Sb-W ore deposit in northwestern Hunan, People’s Republic of China. Can. Mineral. 1998, 36, 1361–1372. [Google Scholar]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Harris, D.C. The Mineralogy of gold and its relevance to gold recoveries. Miner. Deposita 1990, 25, S3–S7. [Google Scholar] [CrossRef]
- Abolins, U. National Instrument 43–101 Technical Report for First Narrow Resources: Abex Geological Services; Golden Ridge Property: York County, NB, Canada, 2003. [Google Scholar]
- McLeod, M.J.; Hoy, D.; Thorne, K.G. History, Tectonic Setting, and Models for Instrusion-Related Gold Deposits in Southwestern New Brunswick, Canada: Examples from the Clarence Stream Area. Explor. Min. Geol. 2008, 17, 1–12. [Google Scholar] [CrossRef]
- Hatcher, R.D. The Appalachian orogen: A brief summary. In From Rodinia to Pangea: The Lithotectonic Record of the Appalachian Region; Tollo, R.P., Bartholomew, M.J., Hibbard, J.P., Karabinos, P.M., Eds.; Geological Society of America: Boulder, CO, USA, 2010; Volume 206, pp. 1–19. [Google Scholar] [CrossRef]
- Van Staal, C.R. Pre-Carboniferous tectonic evolution and metallogeny of the Canadian Appalachians. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Geological Association of Canada: St. John’s, NL, Canada, 2007; Volume 5, pp. 793–818. [Google Scholar]
- van Staal, C.R.; Barr, S.M. Lithospheric architecture and tectonic evolution of the Canadian Appalachians. In Tectonic Styles in Canada: The Lithoprobe Perspective; Percival, J.A., Cook, F.A., Clowes, R.M., Eds.; Geological Association of Canada: St. John’s, NL, Canada, 2012; pp. 41–95. [Google Scholar]
- Williams, H. Appalachian Orogen in Canada. Can. J. Earth Sci. 1979, 16, 792–807. [Google Scholar] [CrossRef]
- Williams, H. Temporal and spatial divisions. In Geology of the Appalachian-Caledonian Orogen in Canada and Greenland; Williams, H., Ed.; Geological Survey of Canada: Ottawa, ON, Canada, 1995; pp. 21–44. [Google Scholar]
- van Staal, C.R.; Sullivan, R.W.; Whalen, J.B. Provenance of tectonic history of the Gander Zone in the Caledonian/Appalachian Orogen: Implications for the origin and assembly of Avalon. In Avalonian and Related Peri-Gondwanan Terranes of the Circum-North Atlantic; Nance, R.D., Thompson, M.D., Eds.; Geological Society of America: Boulder, CO, USA, 1996; Volume 304, pp. 347–367. [Google Scholar] [CrossRef]
- van Staal, C.R.; Dewey, J.F.; Niocaill, C.M.; McKerrow, W.S. The Cambrian-Silurian tectonic evolution of the northern Appalachians and British Caledonides: History of a complex, west and southwest Pacific-type segment of Iapetus. Geol. Soc. Lond. Spec. Publ. 1998, 143, 197–242. [Google Scholar] [CrossRef]
- van Staal, C.R.; Whalen, J.B.; Valverde-Vaquero, P.; Zagorevski, A.; Rogers, N. Pre-Carboniferous, episodic accretion-related, orogenesis along the Laurentian margin of the northern Appalachians. Geol. Soc. Lond. Spec. Publ. 2009, 327, 271–316. [Google Scholar] [CrossRef]
- Waldron, J.W.F.; McCausland, P.J.A.; Barr, S.M.; Schofield, D.I.; Reusch, D.; Wu, L. Terrane history of the Iapetus Ocean as preserved in the northern Appalachians and western Caledonides. Earth-Sci. Rev. 2022, 233, 104163. [Google Scholar] [CrossRef]
- Ludman, A.; McFarlane, C.; Whittaker, A.T.H. Age, chemistry, and tectonic setting of Miramichi terrane (Early Paleozoic) volcanic rocks, eastern and east-central Maine, USA. Atl. Geosci. 2021, 57, 239–273. [Google Scholar] [CrossRef]
- Romer, R.L.; Kroner, U. Paleozoic gold in the Appalachians and Variscides. Ore Geol. Rev. 2018, 92, 475–505. [Google Scholar] [CrossRef]
- Waldron, J.W.F.; Barr, S.M.; Park, A.F.; White, C.E.; Hibbard, J. Late Paleozoic strike-slip faults in Maritime Canada and their role in the reconfiguration of the northern Appalachian orogen. Tectonics 2015, 34, 1661–1684. [Google Scholar] [CrossRef]
- Waldron, J.W.F.; Schofield, D.I.; Murphy, J.B. Diachronous Paleozoic accretion of peri-Gondwanan terranes at the Laurentian margin. In Fifty Years of the Wilson Cycle Concept in Plate Tectonics; Wilson, R.W., Houseman, G.A., McCaffrey, K.J.W., Doré, A.G., Buiter, S.J.H., Eds.; Geological Society of London: London, UK, 2019; Volume 470, pp. 289–309. [Google Scholar] [CrossRef]
- van Staal, C.R.; Hatcher, R.D., Jr. Global setting of Ordovician orogenesis. In The Ordovician Earth System; Finney, S.C., Berry, W.B.N., Eds.; Geological Society of America: Boulder, CO, USA, 2010; Volume 466, pp. 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fyffe, L.R.; Johnson, S.C.; Staal, C.R. van A Review of Proterozoic to Early Paleozoic Lithotectonic Terranes in New Brunswick, Canada and their Tectonic Evolution during Penobscot, Taconic, Salinic and Acadian Orogenesis. Atl. Geosci. 2011, 47, 211–248. [Google Scholar] [CrossRef] [Green Version]
- Barr, S.M.; White, C.E. Field Relations, Petrology and Structure of Neoproterozoic Rocks in the Caledonian Highlands, Southern New Brunswick; Geological Survey of Canada Bulletin: Ottawa, ON, Canada, 1999; p. 101.
- Barr, S.M.; White, C.E. Contrasts in late Precambrian-early Paleozoic tectonothermal history between Avalon composite terrane sensu stricto and other possible peri-Gondwanan terranes in southern New Brunswick and Cape Breton Island, Canada. In Avalonian and Related peri-Gondwanan Terranes of the Circum-North Atlantic; Nance, R.D., Thompson, M.D., Eds.; Geological Society of America: Boulder, CO, USA, 1996; Volume 304, pp. 95–108. [Google Scholar] [CrossRef]
- Barr, S.M.; Johnson, S.C.; Dunning, G.R.; White, C.E.; Park, A.F.; Wälle, M.; Langille, A. New Cryogenian, Neoproterozoic, and middle Paleozoic U–Pb zircon ages from the Caledonia terrane, southern New Brunswick, Canada: Better constrained but more complex volcanic stratigraphy. Atl. Geosci. 2020, 56, 163–187. [Google Scholar] [CrossRef]
- Fyffe, L.R. Stratigraphy and geochemistry of Ordovician volcanic rocks of the Eel River area, west-central New Brunswick. Atl. Geosci. 2001, 37, 81–102. [Google Scholar] [CrossRef] [Green Version]
- Fyffe, L.R.; van Staal, C.R.; Wilson, R.A.; Johnson, S.C. An overview of Early Paleozoic arc systems in New Brunswick, Canada, and eastern Maine, USA. Atl. Geosci. 2023, 59, 01–028. [Google Scholar] [CrossRef]
- McClenaghan, S.H.; Lentz, D.R.; Fyffe, L.R. Chemostratigraphy of Volcanic Rocks Hosting Massive Sulfide Clasts Within the Meductic Group, West-Central New Brunswick. Explor. Min. Geol. 2006, 15, 241–261. [Google Scholar] [CrossRef]
- Bourque, P.-A.; Malo, M.; Kirkwood, D. Paleogeography and tectono-sedimentary history at the margin of Laurentia during Silurian to earliest Devonian time: The Gaspé Belt, Québec. GSA Bull. 2000, 112, 4–20. [Google Scholar] [CrossRef]
- Fyffe, L.R.; Fricker, A. Tectonostratigraphic terrane analysis of New Brunswick. Atl. Geosci. 1987, 23, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Malo, M. Stratigraphy of the Aroostook–Percé Anticlinorium in the Gaspé Peninsula, Quebec. Can. J. Earth Sci. 1988, 25, 893–908. [Google Scholar] [CrossRef]
- Malo, M. Late Silurian–Early Devonian tectono-sedimentary history of the Gaspé Belt in the Gaspé Peninsula: From a transtensional Salinic basin to an Acadian foreland basin. Bull. Can. Pet. Geol. 2001, 49, 202–216. [Google Scholar] [CrossRef]
- Rast, N.; Lutes, G.G.; St. Peter, C. The Geology and Deformation History of the Southern Part of the Matapedia Zone and Its Relationship to the Miramichi Zone and Canterbury Basin. In The Geology of Northeastern Maine and Neighboring New Brunswick; New England Intercollegiate Geological Excursion Conference Trips: Presque Isle, ME, USA, 1980; pp. 191–201. [Google Scholar]
- Whalen, J.B.; Jenner, G.A.; Longstaffe, F.J.; Hegner, E. Nature and evolution of the eastern margin of lapetus: Geochemical and isotopic constraints from Siluro-Devonian granitoid plutons in the New Brunswick Appalachians. Can. J. Earth Sci. 1996, 33, 140–155. [Google Scholar] [CrossRef]
- Whalen, J.B.; Rogers, N.; van Staal, C.R.; Longstaffe, F.J.; Jenner, G.A.; Winchester, J.A. Geochemical and isotopic (Nd, O) data from Ordovician felsic plutonic and volcanic rocks of the Miramichi Highlands: Petrogenetic and metallogenic implications for the Bathurst Mining Camp. Can. J. Earth Sci. 1998, 35, 237–252. [Google Scholar] [CrossRef]
- Bevier, M.L.; Whalen, J.B. Tectonic significance of Silurian magmatism in the Canadian Appalachians. Geology 1990, 18, 411–414. [Google Scholar] [CrossRef]
- Caron, A. Geology of the Pokiok Batholith Aureole, with Emphasis on the Lake George Mine, York Country, New Brunswick; Geoscience Report 94–2; New Brunswick Department of Natural Resources and Energy: Fredericton, NB, Canada, 1996; p. 91.
- Hart, C. Reduced intrusion-related gold deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Geological Association of Canada: St. John’s, NL, Canada, 2007; pp. 95–112. [Google Scholar]
- Thorne, K.G.; Lentz, D.R.; Hoy, D.; Fyffe, L.R.; Cabri, L.J. Characteristics of mineralization at the main zone of the Clarence Stream gold deposit, southwestern New Brunswick, Canada: Evidence for an intrusion-related gold system in the northern Appalachian Orogen. Explor. Min. Geol. 2008, 17, 13–49. [Google Scholar] [CrossRef]
- Taylor, B.E. Epithermal gold deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Geological Association of Canada: St. John’s, NL, Canada, 2007; pp. 113–139. [Google Scholar]
- Chi, G. Fluid Compositions and Temperature-Pressure Conditions of Intrusion-Related Gold Systems in Southwestern New Brunswick—A Fluid-Inclusion Study; Current research 2002-E13; Geological Survey of Canada: Ottawa, ON, Canada, 2002.
- Lentz, C.; Thorne, K.; McFarlane, C.R.M.; Archibald, D.A. U-Pb, Ar-Ar, and Re-Os Geochronological Constraints on Multiple Magmatic–Hydrothermal Episodes at the Lake George Mine, Central New Brunswick. Minerals 2020, 10, 566. [Google Scholar] [CrossRef]
- Hoffman, E.L. Instrumental neutron activation in geoanalysis. J. Geochem. Explor. 1992, 44, 297–319. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Ulrich, T.; Kamber, B.S.; Jugo, P.J.; Tinkham, D.K. Imaging element-distribution patterns in minerals by Laser Ablation—Inductively Coupled Plasma—Mass Spectrometry (LA–ICP–MS). Can. Mineral. 2009, 47, 1001–1012. [Google Scholar] [CrossRef]
- Paul, B.; Paton, C.; Norris, A.; Woodhead, J.; Hellstrom, J.; Hergt, J.; Greig, A. CellSpace: A module for creating spatially registered laser ablation images within the Iolite freeware environment. J. Anal. At. Spectrom. 2012, 27, 700–706. [Google Scholar] [CrossRef]
- Woodhead, J.D.; Hellstrom, J.; Hergt, J.M.; Greig, A.; Maas, R. Isotopic and Elemental Imaging of Geological Materials by Laser Ablation Inductively Coupled Plasma-Mass Spectrometry. Geostand. Geoanal. Res. 2007, 31, 331–343. [Google Scholar] [CrossRef]
- Croghan, C.W.; Egeghy, P.P. Methods of Dealing with Values below the Limit of Detection Using SAS.; United States Environmental Protection Agency: St. Petersburg, FL, USA, 2003.
- Reimann, C.; Filzmoser, P.; Hron, K.; Kynčlová, P.; Garrett, R.G. A new method for correlation analysis of compositional (environmental) data—a worked example. Sci. Total Environ. 2017, 607, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Grunsky, E.C.; Caritat, P. de State-of-the-art analysis of geochemical data for mineral exploration. Geochem. Explor. Environ. Anal. 2019, 20, 217–232. [Google Scholar] [CrossRef]
- Heidarian, H.; Lentz, D.R.; Thorne, K.; Rogers, N. Application of portable X-ray and micro-X-ray fluorescence spectrometry to characterize alteration and mineralization within various gold deposits hosted in southern New Brunswick, Canada. J. Geochem. Explor. 2021, 229, 106847. [Google Scholar] [CrossRef]
- Grunsky, E.C. The interpretation of geochemical survey data. Geochem. Explor. Environ. Anal. 2010, 10, 27–74. [Google Scholar] [CrossRef]
- Flude, S.; Haschke, M.; Storey, M. Application of benchtop micro-XRF to geological materials. Mineral. Mag. 2017, 81, 923–948. [Google Scholar] [CrossRef] [Green Version]
- Lipko, S.; Tauson, V.; Bychinskii, V. Gold Partitioning in a Model Multiphase Mineral-Hydrothermal Fluid System: Distribution Coefficients, Speciation and Segregation. Minerals 2020, 10, 890. [Google Scholar] [CrossRef]
- Levitan, D.M.; Zipper, C.E.; Donovan, P.; Schreiber, M.E.; Seal, R.R.; Engle, M.A.; Chermak, J.A.; Bodnar, R.J.; Johnson, D.K.; Aylor, J.G. Statistical analysis of soil geochemical data to identify pathfinders associated with mineral deposits: An example from the Coles Hill uranium deposit, Virginia, USA. J. Geochem. Explor. 2015, 154, 238–251. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Stepanov, A.S.; Large, R.R.; Kiseeva, E.S.; Danyushevsky, L.V.; Goemann, K.; Meffre, S.; Zhukova, I.; Belousov, I.A. Phase relations of arsenian pyrite and arsenopyrite. Ore Geol. Rev. 2021, 136, 104285. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef] [Green Version]
- Pokrovski, G.S.; Kokh, M.A.; Proux, O.; Hazemann, J.-L.; Bazarkina, E.F.; Testemale, D.; Escoda, C.; Boiron, M.-C.; Blanchard, M.; Aigouy, T.; et al. The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geol. Rev. 2019, 109, 545–563. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Frimmel, H.E.; Jiang, S.-Y.; Dai, B.-Z. LA-ICP-MS trace element analysis of pyrite from the Xiaoqinling gold district, China: Implications for ore genesis. Ore Geol. Rev. 2011, 43, 142–153. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Brugger, J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Nugus, M. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields Province, Western Australia. Miner. Deposita 2009, 44, 765–791. [Google Scholar] [CrossRef]
- Bajwah, Z.U.; Seccombe, P.K.; Offler, R. Trace element distribution, Co:Ni ratios and genesis of the big cadia iron-copper deposit, new south wales, australia. Miner. Deposita 1987, 22, 292–300. [Google Scholar] [CrossRef]
- Bralia, A.; Sabatini, G.; Troja, F. A revaluation of the Co/Ni ratio in pyrite as geochemical tool in ore genesis problems: Evidences from southern tuscany pyritic deposits. Miner. Deposita 1979, 14, 353–374. [Google Scholar] [CrossRef]
- Loftus-Hills, G.; Solomon, M. Cobalt, nickel and selenium in sulphides as indicators of ore genesis. Miner. Deposita 1967, 2, 228–242. [Google Scholar] [CrossRef]
- Thomas, H.V.; Large, R.R.; Bull, S.W.; Maslennikov, V.; Berry, R.F.; Fraser, R.; Froud, S.; Moye, R. Pyrite and Pyrrhotite Textures and Composition in Sediments, Laminated Quartz Veins, and Reefs at Bendigo Gold Mine, Australia: Insights for Ore Genesis. Econ. Geol. 2011, 106, 1–31. [Google Scholar] [CrossRef]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. Am. Mineral. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Xing, Y.; Brugger, J.; Tomkins, A.; Shvarov, Y. Arsenic evolution as a tool for understanding formation of pyritic gold ores. Geology 2019, 47, 335–338. [Google Scholar] [CrossRef]
- Tauson, V.; Lipko, S.; Kravtsova, R.; Smagunov, N.; Belozerova, O.; Voronova, I. Distribution of “Invisible” Noble Metals between Pyrite and Arsenopyrite Exemplified by Minerals Coexisting in Orogenic Au Deposits of North-Eastern Russia. Minerals 2019, 9, 660. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Di, P.; Yu, M.; Bao, J.; Zhao, Y.; Liu, D.; Wang, Y. Mineralogy and Geochemistry of Pyrite and Arsenopyrite from the Zaozigou Gold Deposit in West Qinling Orogenic Belt, Central China: Implications for Ore Genesis. Resour. Geol. 2019, 69, 314–332. [Google Scholar] [CrossRef]
- Cabri, L.J.; Newville, M.; Gordon, R.A.; Crozier, E.D.; Sutton, S.R.; McMahon, G.; Jiang, D.-T. Chemical speciation of gold in arsenopyrite. Can. Mineral. 2000, 38, 1265–1281. [Google Scholar] [CrossRef] [Green Version]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A re-examination of the arsenopyrite geothermometer; pressure considerations and applications to natural assemblages. Can. Mineral. 1985, 23, 517–534. [Google Scholar]
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe-As-S and their application. Can. Mineral. 1976, 14, 364–386. [Google Scholar]
- Chouinard, A.; Paquette, J.; Williams-Jones, A.E. Crystallographic controls on Trace-Element incorporation in auriferous pyrite from the Pascua Epithermal High-Sulfidation Deposit, Chile–Argentina. Can. Mineral. 2005, 43, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Deditius, A.P.; Utsunomiya, S.; Ewig, R.C.; Chryssoulis, S.L.; Venter, D.; Kesler, S.E. Decoupled geochemical behavior of As and Cu in hydrothermal systems. Geology 2009, 37, 707–710. [Google Scholar] [CrossRef]
- Tiller, W.A.; Ahn, K.-S. Interface field effects on solute redistribution during crystallization. J. Cryst. Growth 1980, 49, 483–501. [Google Scholar] [CrossRef]
- Watson, E.B. Surface enrichment and trace-element uptake during crystal growth. Geochim. Cosmochim. Acta 1996, 60, 5013–5020. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Simon, G.; Kesler, S.E.; Chryssoulis, S. Geochemistry and textures of gold-bearing arsenian pyrite, Twin Creeks, Nevada; implications for deposition of gold in carlin-type deposits. Econ. Geol. 1999, 94, 405–421. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Mao, J. Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chem. Geol. 2009, 264, 101–121. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and Trace Element Zonation in Pyrite Using a Laser Imaging Technique: Implications for the Timing of Gold in Orogenic and Carlin-Style Sediment-Hosted Deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Watson, E.B. A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochim. Cosmochim. Acta 2004, 68, 1473–1488. [Google Scholar] [CrossRef]
- Qian, G.; Brugger, J.; Testemale, D.; Skinner, W.; Pring, A. Formation of As(II)-pyrite during experimental replacement of magnetite under hydrothermal conditions. Geochim. Cosmochim. Acta 2013, 100, 1–10. [Google Scholar] [CrossRef]
- Simmons, S.F.; White, N.C.; John, D.A. Geological Characteristics of Epithermal Precious and Base Metal Deposits. In One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 485–522. [Google Scholar] [CrossRef]
- Lang, J.R.; Baker, T.; Hart, C.; Mortensen, J.K. An exploration model for Intrusion-Related Gold Systems. SEG Discov. 2000, 40, 1–15. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Intrusion-related gold deposits. In Gold Metallogeny and Exploration; Foster, R.P., Ed.; Springer: Boston, MA, USA, 1991; pp. 165–209. [Google Scholar] [CrossRef]
- Thompson, J.F.H.; Sillitoe, R.H.; Baker, T.; Lang, J.R.; Mortensen, J.K. Intrusion-related gold deposits associated with tungsten-tin provinces. Miner. Deposita 1999, 34, 323–334. [Google Scholar] [CrossRef]
- Lang, J.R.; Baker, T. Intrusion-related gold systems: The present level of understanding. Miner. Deposita 2001, 36, 477–489. [Google Scholar] [CrossRef]
- Cooke, D.R.; Simmons, S.F. Characteristics and Genesis of Epithermal Gold Deposits. In Gold in 2000; Hagemann, S.G., Brown, P.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000; Volume 13, pp. 221–244. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, R.A.; Gonzalez-Urien, E. Exploration for Epithermal Gold Deposits. In Gold in 2000; Hagemann, S.G., Brown, P.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000; Volume 13, pp. 245–278. [Google Scholar] [CrossRef]
- White, N.; Hedenquist, J.W. Epithermal Gold Deposits: Styles, Characteristics and Exploration. SEG Discov. 1995, 23, 1–13. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Pals, D.W.; Spry, P.G.; Chryssoulis, S. Invisible Gold and Tellurium in Arsenic-Rich Pyrite from the Emperor Gold Deposit, Fiji: Implications for Gold Distribution and Deposition. Econ. Geol. 2003, 98, 479–493. [Google Scholar] [CrossRef]
- Babedi, L.; von der Heyden, B.P.; Tadie, M.; Mayne, M. Trace elements in pyrite from five different gold ore deposit classes: A review and meta-analysis. Geol. Soc. Lond. Spec. Publ. 2022, 516, 47–83. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, X.-W.; Cui, B.; Wang, B.-C.; Yin, Y.-F.; Wang, J.-R. Re-Os isotopic and trace element compositions of pyrite and origin of the Cretaceous Jinchang porphyry Cu-Au deposit, Heilongjiang Province, NE China. J. Asian Earth Sci. 2016, 129, 67–80. [Google Scholar] [CrossRef]
- Kadel-Harder, I.M.; Spry, P.G.; Layton-Matthews, D.; Voinot, A.; von der Handt, A.; McCombs, A.L. Paragenetic relationships between low- and high-grade gold mineralization in the Cripple Creek Au-Te deposit, Colorado: Trace element studies of pyrite. Ore Geol. Rev. 2020, 127, 103847. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.T.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Doyle, K.; Holwell, D.A.; Jenkin, G.R.T.; Barry, T.L.; Becker, J.; Rampe, J. Pyrite chemistry: A new window into Au-Te ore-forming processes in alkaline epithermal districts, Cripple Creek, Colorado. Geochim. Cosmochim. Acta 2020, 274, 172–191. [Google Scholar] [CrossRef]
- Sheppard, S.M.F. Characterization and isotopic variations in natural waters. Rev. Mineral. Geochem. 1986, 16, 165–183. [Google Scholar]
- Taylor, B.E. Magmatic volatiles: Isotopic variation of C, H, and S. In Stable Isotopes in High Temperature Geological Processes; Mineralogical Society of America: Chantilly, VA, USA, 1986; pp. 185–226. [Google Scholar] [CrossRef]
- Henley, R.W.; Ellis, A.J. Geothermal systems ancient and modern: A geochemical review. Earth-Sci. Rev. 1983, 19, 1–50. [Google Scholar] [CrossRef]
- Pat Shanks, W.C. Stable Isotope Geochemistry of Mineral Deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 59–85. [Google Scholar] [CrossRef]
- Sawkins, F.J.; O’Neil, J.R.; Thompson, J.M. Fluid inclusion and geochemical studies of vein gold deposits, Baguio District, Philippines. Econ. Geol. 1979, 74, 1420–1434. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Heinrich, C.A. 100th Anniversary Special Paper: Vapor Transport of Metals and the Formation of Magmatic-Hydrothermal Ore Deposits. Econ. Geol. 2005, 100, 1287–1312. [Google Scholar] [CrossRef]
- Albinson, T.; Norman, D.I.; Cole, D.; Chomiak, B. Controls on Formation of Low-Sulfidation Epithermal Deposits in Mexico: Constraints from Fluid Inclusion and Stable Isotope Data. In New Mines and Discoveries in Mexico and Central America; Albinson, T., Nelson, C.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2001; Volume 8, pp. 1–32. [Google Scholar] [CrossRef]
- Henley, R.W. The Geothermal Framework of Epithermal Deposits. In Geology and Geochemistry of Epithermal Systems; Berger, B.R., Bethke, P.M., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1985; Volume 2, pp. 1–24. [Google Scholar] [CrossRef]
- Simmons, S.F. Hydrologic implications of alteration and fluid inclusion studies in the Fresnillo District, Mexico; evidence for a brine reservoir and a descending water table during the formation of hydrothermal Ag-Pb-Zn orebodies. Econ. Geol. 1991, 86, 1579–1601. [Google Scholar] [CrossRef]
- Camprubí, A.; Cardellach, E.; Canals, À.; Lucchini, R. The La Guitarra Ag-Au Low-Sulfidation Epithermal Deposit, Temascaltepec District, Mexico: Fluid Inclusion and Stable Isotope Data. In New Mines and Discoveries in Mexico and Central America; Albinson, T., Nelson, C.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2001; Volume 8, pp. 133–158. [Google Scholar] [CrossRef]
- Einaudi, M.T.; Hedenquist, J.W.; Inan, E.E. Sulfidation State of Fluids in Active and Extinct Hydrothermal Systems: Transitions from Porphyry to Epithermal Environments. In Volcanic, Geothermal, and Ore-Forming Fluids: Rulers and Witnesses of Processes within the Earth; Simmons, S.F., Graham, I., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2003; Volume 10, pp. 285–314. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Magma Degassing and Mineral Deposition in Hydrothermal Systems along Convergent Plate Boundaries*. In Volcanic, Geothermal, and Ore-Forming Fluids: Rulers and Witnesses of Processes within the Earth; Simmons, S.F., Graham, I., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2003; Volume 10, pp. 1–18. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Hedenquist, J.W. Linkages between Volcanotectonic Settings, Ore-Fluid Compositions, and Epithermal Precious Metal Deposits. In Volcanic, Geothermal, and Ore-Forming Fluids: Rulers and Witnesses of Processes within the Earth; Simmons, S.F., Graham, I., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2003; Volume 10, pp. 315–343. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Henley, R.W. The importance of CO2 on freezing point measurements of fluid inclusions; evidence from active geothermal systems and implications for epithermal ore deposition. Econ. Geol. 1985, 80, 1379–1406. [Google Scholar] [CrossRef]
- Lira, R.; Poklepovic, M.F.; Dorais, M.J.; Millone, H.A.; Gomez, G.M. Fluid–rock interaction at the roots of an Eopaleozoic epithermal system, Sierra Norte de Córdoba, Argentina: Paragenesis, thermometry and fluid sources. J. S. Am. Earth Sci. 2009, 28, 263–276. [Google Scholar] [CrossRef]
- Simmons, S.F.; Tutolo, B.M.; Barker, S.L.L.; Goldfarb, R.J.; Robert, F. Hydrothermal Gold Deposition in Epithermal, Carlin, and Orogenic Deposits. In Geology of the World’s Major Gold Deposits and Provinces; Sillitoe, R.H., Goldfarb, R.J., Robert, F., Simmons, S.F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2020; Volume 23, pp. 823–845. [Google Scholar] [CrossRef]
- Benning, L.G.; Seward, T.M. Hydrosulphide complexing of Au (I) in hydrothermal solutions from 150–400 °C and 500–1500 bar. Geochim. Cosmochim. Acta 1996, 60, 1849–1871. [Google Scholar] [CrossRef]
- Gammons, C.H.; Williams-Jones, A.E. Chemical mobility of gold in the porphyry-epithermal environment. Econ. Geol. 1997, 92, 45–59. [Google Scholar] [CrossRef]
- Stefánsson, A.; Seward, T.M. Gold(I) complexing in aqueous sulphide solutions to 500 °C at 500 bar. Geochim. Cosmochim. Acta 2004, 68, 4121–4143. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Herrington, R.J.; Wilkinson, J.J. Colloidal gold and silica in mesothermal vein systems. Geology 1993, 21, 539–542. [Google Scholar] [CrossRef]
- Saunders, J.A. Colloidal transport of gold and silica in epithermal precious-metal systems: Evidence from the Sleeper deposit, Nevada. Geology 1990, 18, 757–760. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Reich, M. Natural gold nanoparticles. Ore Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Wagner, F.E.; Marion, P.H.; Regnard, J.R. Moessbauer Study of the Chemical State of Gold in Gold Ores. In Proceedings of the Gold 100: Proceedings of the International Conference on Gold, Johannesburg, South Africa, 15–17 September 1986; Fivaz, C.E., King, R.P., Eds.; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1986; Volume 2, pp. 435–443. [Google Scholar]


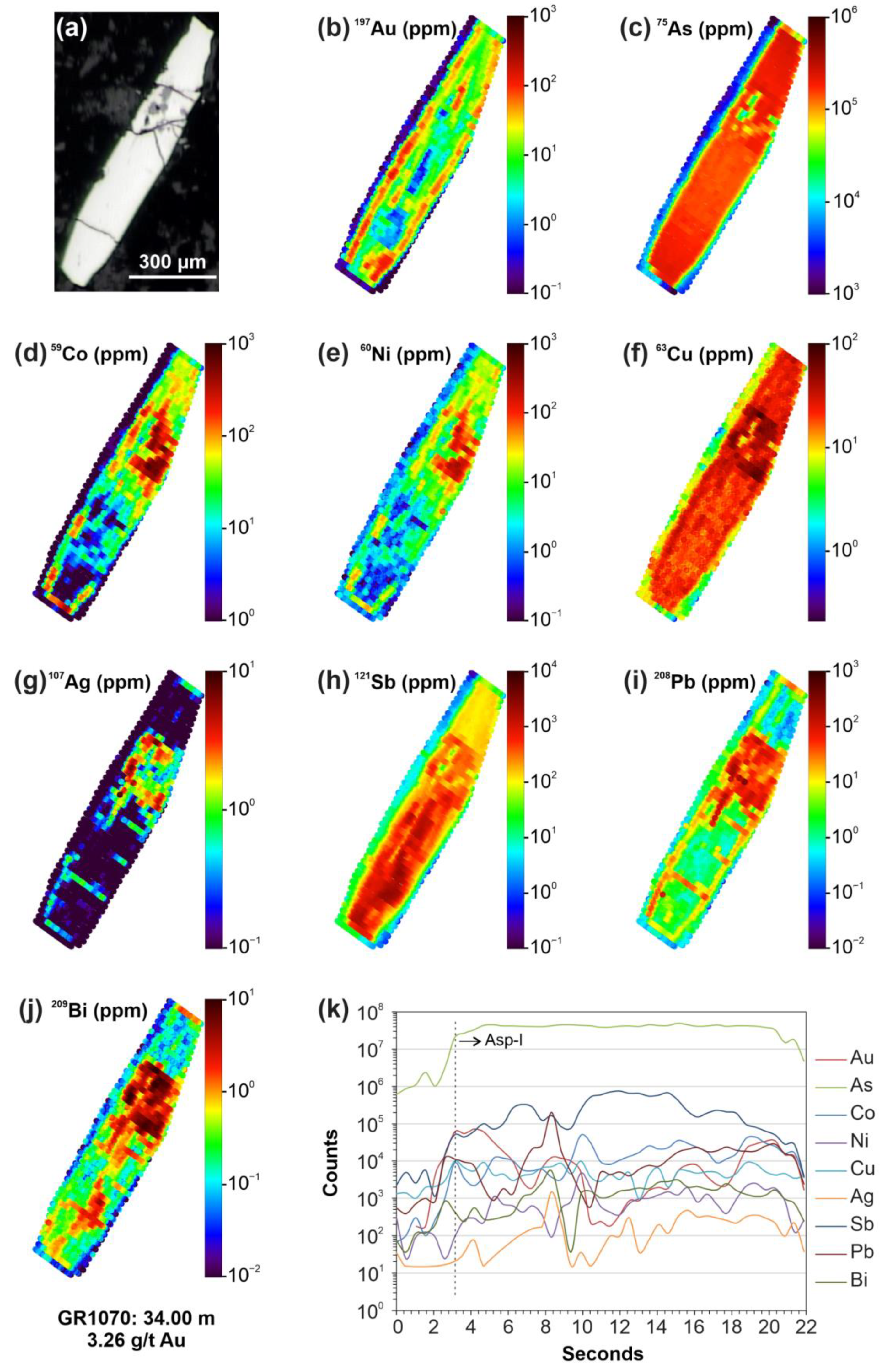
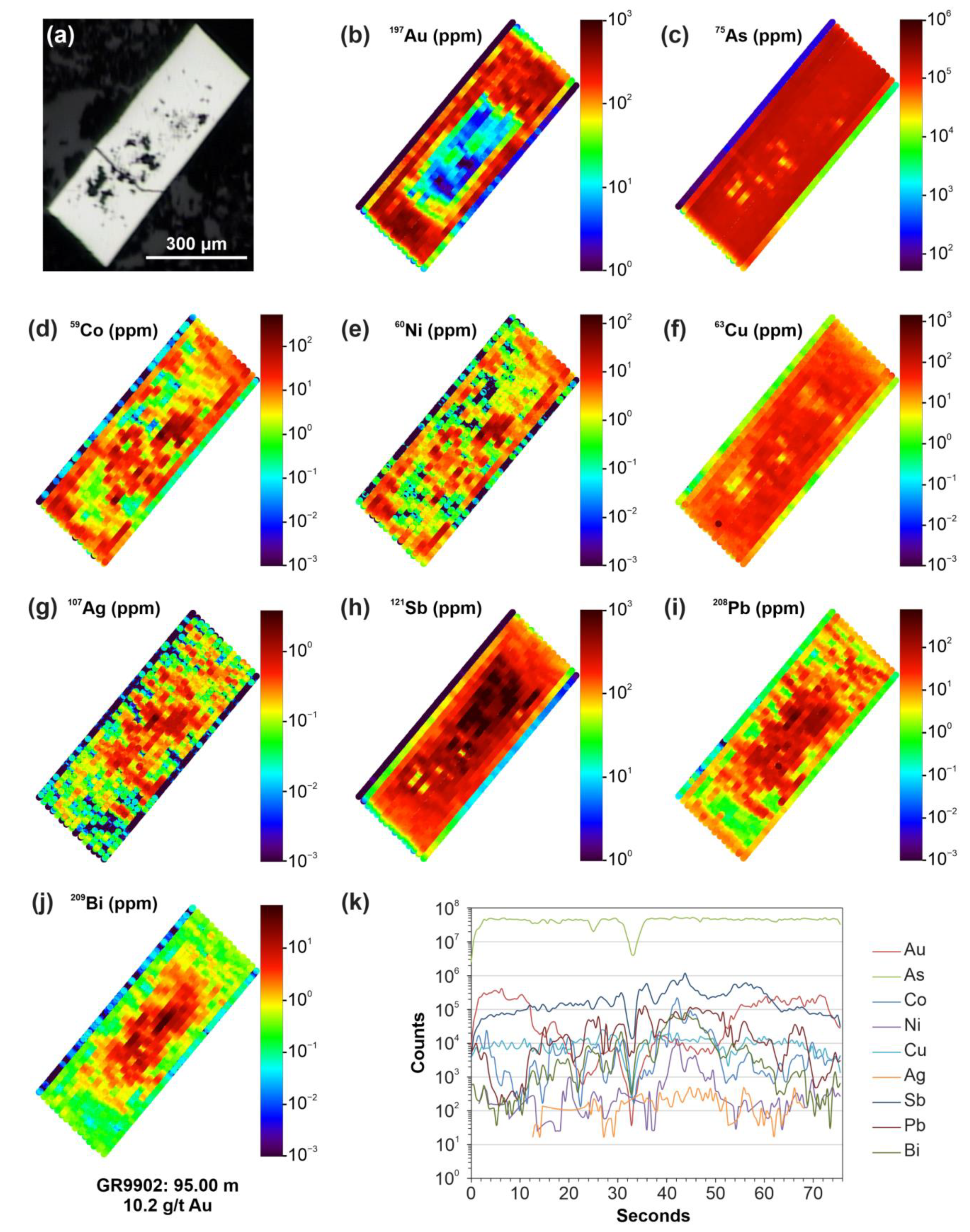
| Element * | Min. | Max. | Mean | Median | Std. Dev. | Q. 0.25 | Q. 0.75 | DL |
|---|---|---|---|---|---|---|---|---|
| Au | 0.039 | 10.20 | 2.485 | 2.470 | 1.990 | 0.761 | 3.313 | 0.005 |
| As | 86 | 17,400 | 4471 | 4515 | 3646 | 1450 | 6040 | 2 |
| Cu | 2 | 1160 | 35 | 7 | 174 | 4 | 11 | 1 |
| Mn | 39 | 852 | 451 | 441 | 189 | 333.5 | 556 | 2 |
| Ni | 0.71 | 13 | 3.5 | 3.0 | 2.8 | 1.75 | 4.25 | 1 |
| Pb | 4 | 3500 | 99 | 14 | 525 | 8 | 26 | 2 |
| Zn | 16 | 1240 | 142 | 59 | 271 | 43.25 | 79 | 1 |
| Bi | 0.24 | 2.52 | 0.76 | 0.46 | 0.65 | 0.3275 | 1.06 | 0.1 |
| Ca | 400 | 57,100 | 19,077 | 18,650 | 9912 | 12,450 | 23,525 | 100 |
| Cs | 0.29 | 2.69 | 1.50 | 1.515 | 0.58 | 1.10 | 1.90 | 0.05 |
| Fe | 6100 | 57,200 | 30,070 | 31,350 | 11,267 | 22,600 | 34,750 | 200 |
| K | 800 | 4500 | 2923 | 3100 | 814 | 2575 | 3400 | 100 |
| Na | 200 | 49,000 | 7941 | 1850 | 10,568 | 975 | 11,150 | 100 |
| Sb | 6.4 | 1720 | 68.2 | 31.1 | 255.2 | 21.5 | 37.0 | 0.2 |
| S | 780 | 49,690 | 14,607 | 13,635 | 9631 | 9310 | 17,465 | 10 |
| Se | 0.07 | 2.90 | 0.39 | 0.30 | 0.46 | 0.10 | 0.50 | 0.10 |
| W | 2.8 | 28.0 | 10.9 | 9.0 | 7.3 | 5.8 | 15.0 | 4.0 |
| Type * | Item | Co | Ni | Cu | Ag | Sb | As | Au | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Py-I (n = 12) | Minimum | 0.33 | 0.83 | 65.86 | 0.12 | 7.43 | 51.23 | 0.16 | 6.96 | 0.32 |
| Maximum | 841.8 | 247.2 | 946.4 | 55.3 | 583.7 | 7651.5 | 5.87 | 4117.3 | 34.2 | |
| Mean | 401.7 | 61.3 | 317.3 | 15.1 | 248.1 | 2195.3 | 2.32 | 1252.5 | 15.9 | |
| Median | 326.4 | 26.2 | 229.8 | 7.6 | 174.1 | 1621.9 | 1.86 | 1101.7 | 18.4 | |
| Std. Dev. | 308.2 | 73.5 | 268.2 | 18.1 | 216.4 | 2170.8 | 1.71 | 1187.8 | 10.7 | |
| Py-II (n = 16) | Minimum | 0.01 | 0.09 | 8.82 | 0.01 | 0.16 | 689.6 | 3.18 | 0.35 | 0.01 |
| Maximum | 124.5 | 26.0 | 214.1 | 9.2 | 162.4 | 23,382.7 | 55.7 | 970.1 | 8.58 | |
| Mean | 22.4 | 6.5 | 49.1 | 1.4 | 32.0 | 14,269.6 | 15.0 | 95.6 | 1.11 | |
| Median | 2.97 | 4.4 | 26.4 | 0.46 | 15.1 | 14,630.8 | 9.7 | 21.1 | 0.55 | |
| Std. Dev. | 38.0 | 7.9 | 53.5 | 2.5 | 43.7 | 6859.6 | 13.56 | 237.4 | 2.08 | |
| Py-III (n = 22) | Minimum | 0.01 | 0.10 | 2.76 | 0.01 | 0.22 | 4083.2 | 1.12 | 0.16 | 0.01 |
| Maximum | 186.0 | 39.6 | 218.2 | 5.6 | 61.9 | 45,753.7 | 91.0 | 229.5 | 12.8 | |
| Mean | 48.1 | 10.7 | 78.6 | 0.91 | 23.8 | 27,021.9 | 34.7 | 39.2 | 1.06 | |
| Median | 18.4 | 3.8 | 75.0 | 0.23 | 15.1 | 26,313.3 | 33.0 | 17.9 | 0.39 | |
| Std. Dev. | 60.8 | 12.3 | 52.1 | 1.41 | 20.1 | 11,574.1 | 28.2 | 55.1 | 2.66 | |
| Py-IV (n = 6) | Minimum | 8.04 | 1.25 | 93.7 | 0.02 | 2.13 | 28,196.1 | 36.3 | 2.39 | 0.27 |
| Maximum | 48.0 | 9.8 | 294.9 | 4.1 | 129.3 | 57,065.7 | 210.8 | 214.6 | 6.98 | |
| Mean | 28.3 | 4.7 | 184.1 | 1.8 | 44.9 | 43,384.8 | 105.1 | 68.9 | 2.43 | |
| Median | 30.3 | 3.4 | 181.2 | 1.1 | 20.4 | 43,891.2 | 82.0 | 32.9 | 1.35 | |
| Std. Dev. | 17.6 | 3.6 | 81.5 | 1.8 | 49.4 | 9,298.1 | 74.1 | 78.9 | 2.50 |
| Type * | Item | Co | Ni | Cu | Ag | Sb | W | Au | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Asp-I (n = 21) | Minimum | 0.27 | 0.18 | 0.33 | 0.003 | 155.8 | 0.004 | 1.47 | 0.2 | 0.013 |
| Maximum | 59.00 | 16.70 | 27.30 | 0.320 | 2880.0 | 8.000 | 166.0 | 9.15 | 3.66 | |
| Mean | 22.39 | 5.20 | 10.99 | 0.074 | 1015.2 | 0.972 | 43.50 | 3.294 | 1.06 | |
| Median | 17.90 | 3.81 | 9.80 | 0.040 | 540.5 | 0.036 | 28.50 | 2.31 | 0.652 | |
| Std. Dev. | 19.02 | 4.84 | 8.93 | 0.091 | 952.1 | 2.126 | 40.72 | 3.247 | 1.095 | |
| Asp-II (n = 15) | Minimum | 0.10 | 0.02 | 7.40 | 0.002 | 0.06 | 0.003 | 4.20 | 0.05 | 0.008 |
| Maximum | 320.0 | 90.60 | 155.0 | 1.94 | 1263 | 6.300 | 334.0 | 67.3 | 2.47 | |
| Mean | 28.20 | 7.33 | 46.90 | 0.67 | 394.6 | 1.570 | 131.3 | 23 | 0.94 | |
| Median | 2.10 | 0.60 | 35.60 | 0.22 | 152.0 | 0.041 | 88.90 | 12.7 | 0.86 | |
| Std. Dev. | 81.50 | 23.08 | 40.00 | 0.77 | 437.9 | 2.332 | 109.4 | 22.3 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardenas-Vera, A.; MacDonald, M.; Lentz, D.R.; Thorne, K.G. Trace Element Characteristics of Pyrite and Arsenopyrite from the Golden Ridge Gold Deposit, New Brunswick, Canada: Implications for Ore Genesis. Minerals 2023, 13, 954. https://doi.org/10.3390/min13070954
Cardenas-Vera A, MacDonald M, Lentz DR, Thorne KG. Trace Element Characteristics of Pyrite and Arsenopyrite from the Golden Ridge Gold Deposit, New Brunswick, Canada: Implications for Ore Genesis. Minerals. 2023; 13(7):954. https://doi.org/10.3390/min13070954
Chicago/Turabian StyleCardenas-Vera, Alan, Moya MacDonald, David R. Lentz, and Kathleen G. Thorne. 2023. "Trace Element Characteristics of Pyrite and Arsenopyrite from the Golden Ridge Gold Deposit, New Brunswick, Canada: Implications for Ore Genesis" Minerals 13, no. 7: 954. https://doi.org/10.3390/min13070954






