Flotation Decarbonization and Desulfurization of a High-Sulfur Bauxite in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
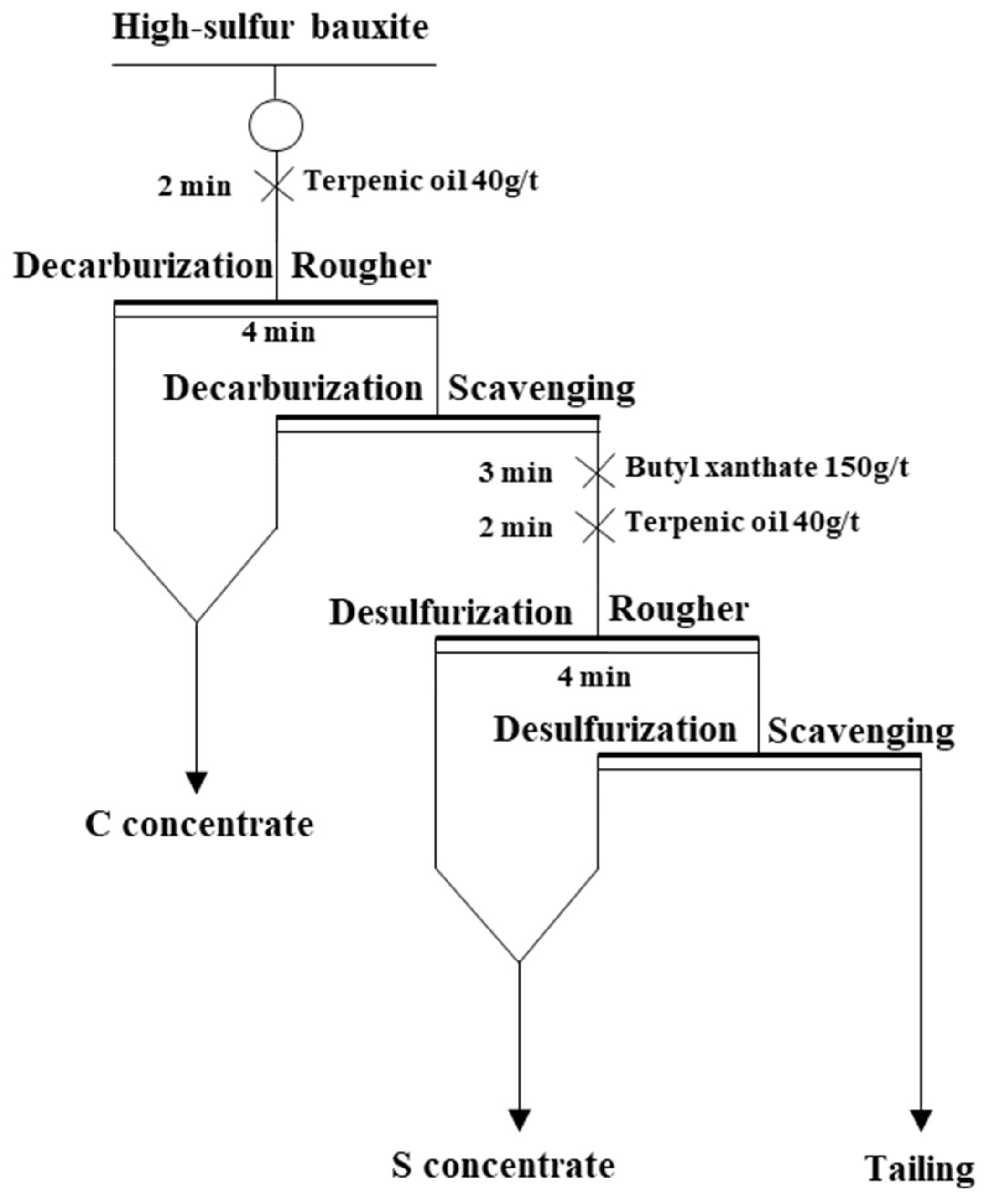
2.3. Flotation Experiments
3. Results and Discussions
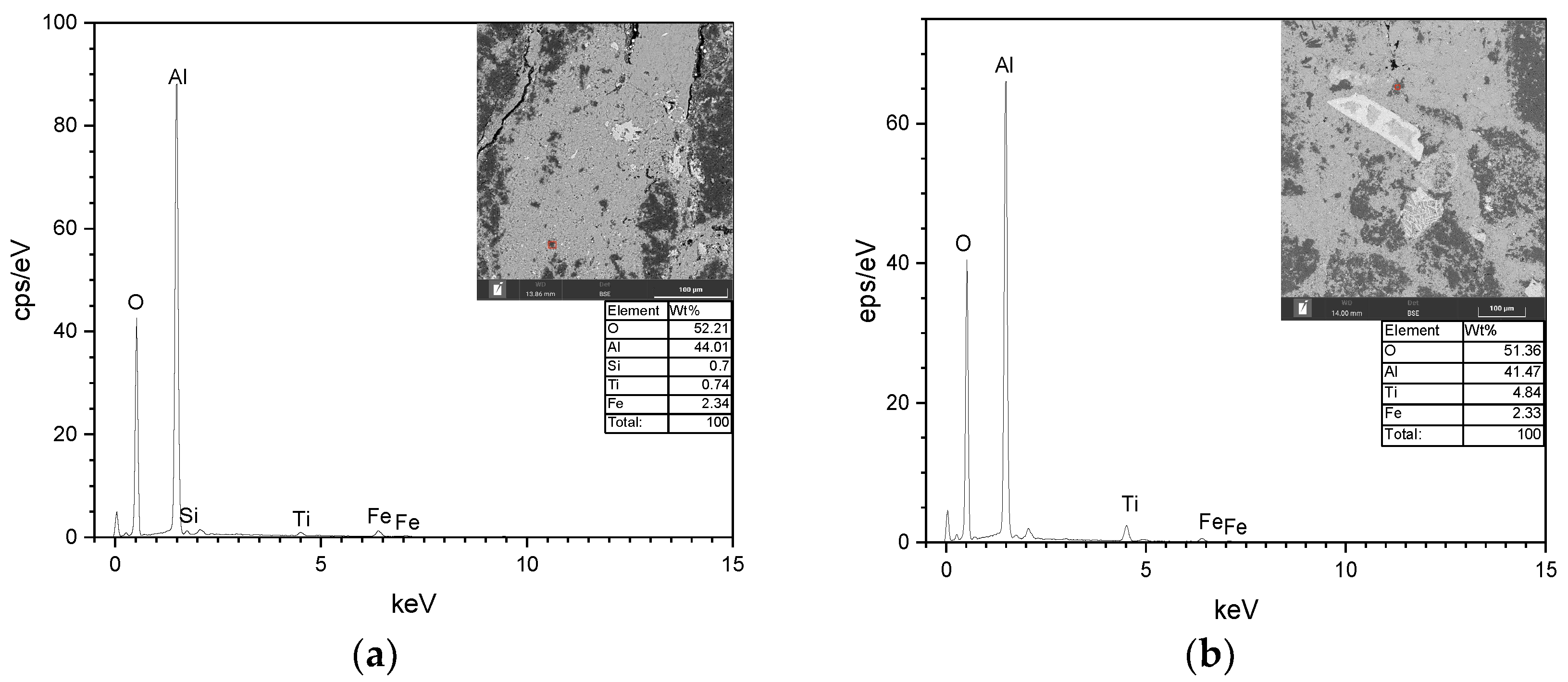
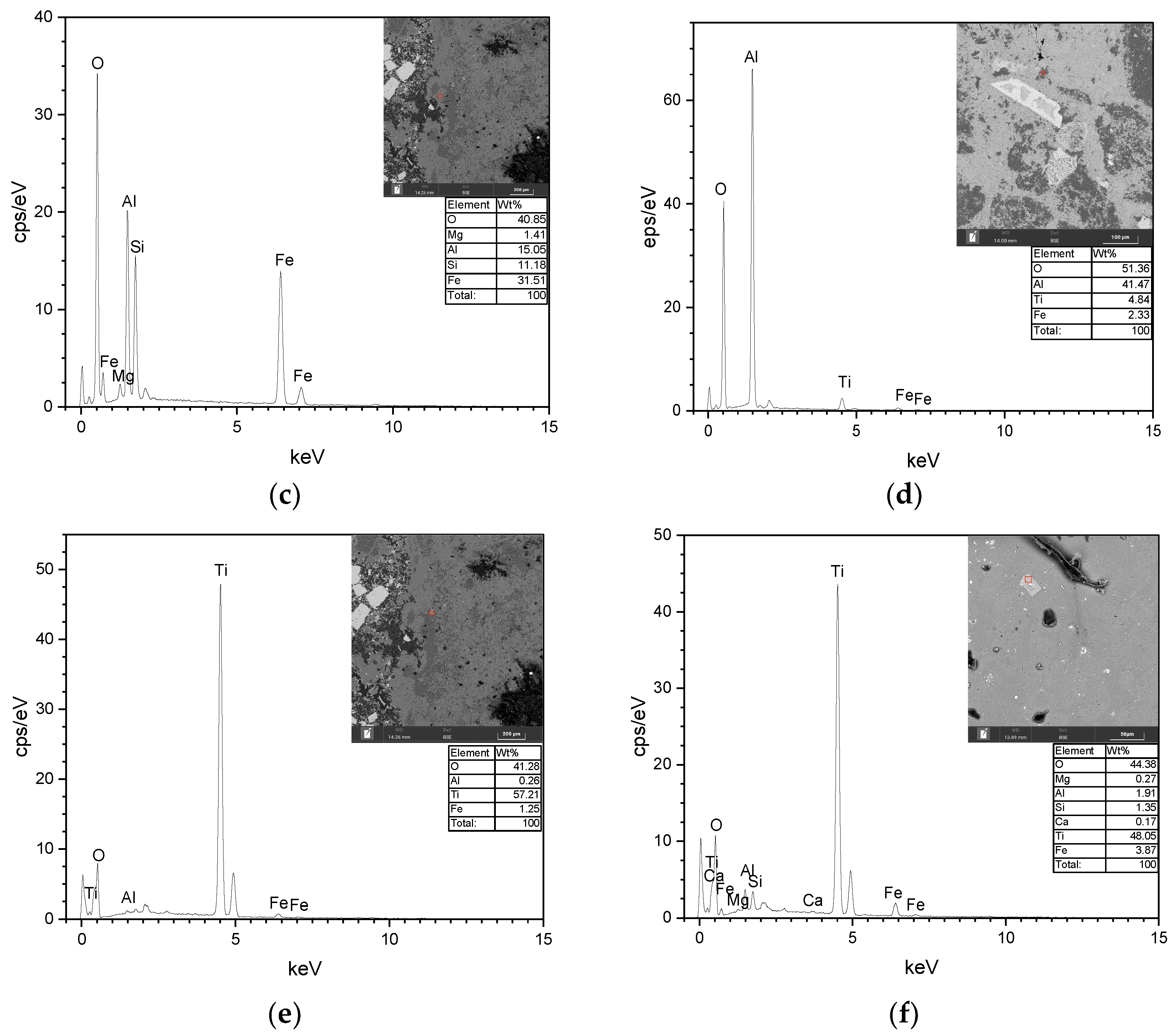
3.1. Microscopic Identification
3.2. Trial Tests
3.2.1. Batch Flotation Tests
3.2.2. Pre-Decarbonization Test
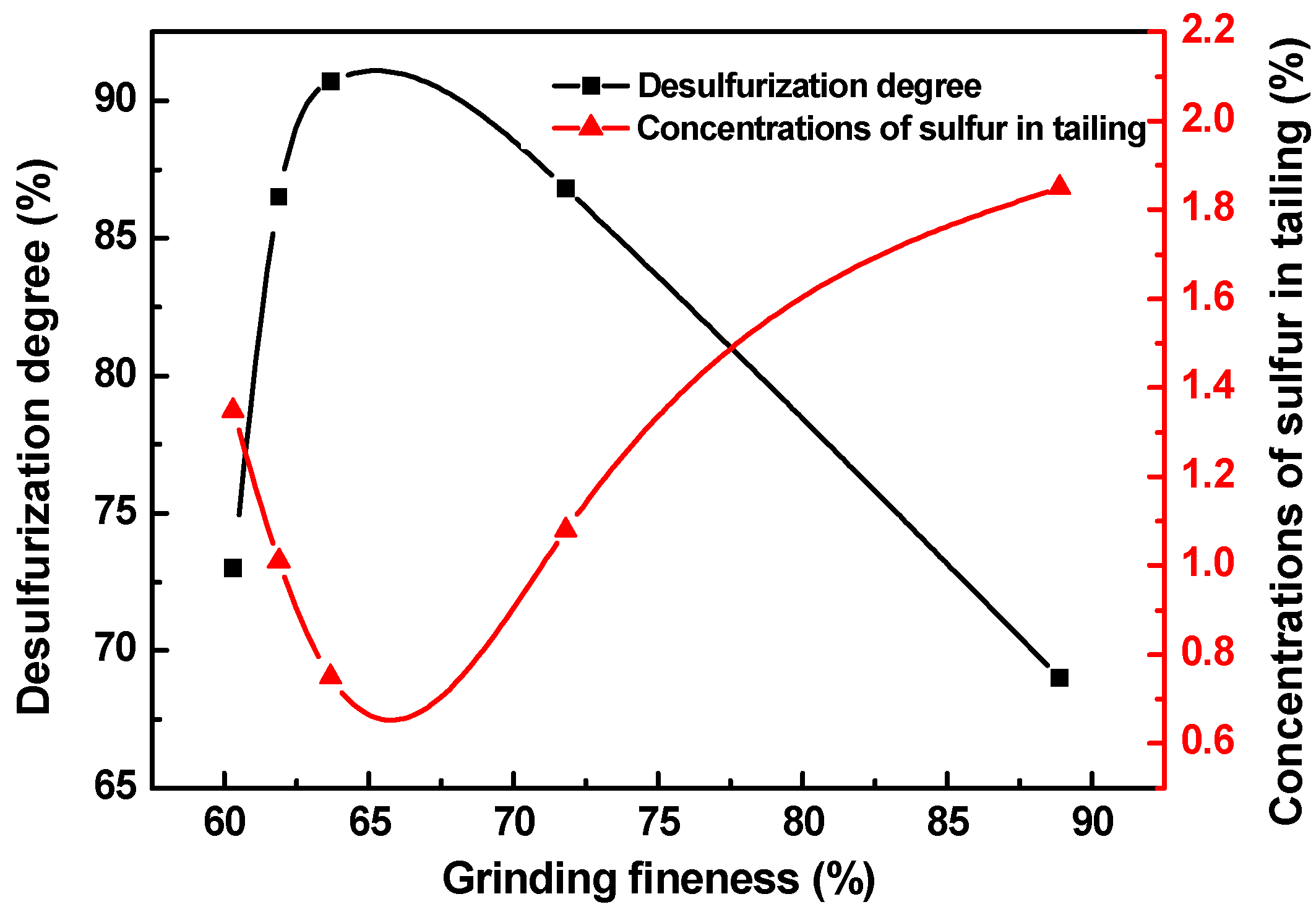
3.3. Grinding
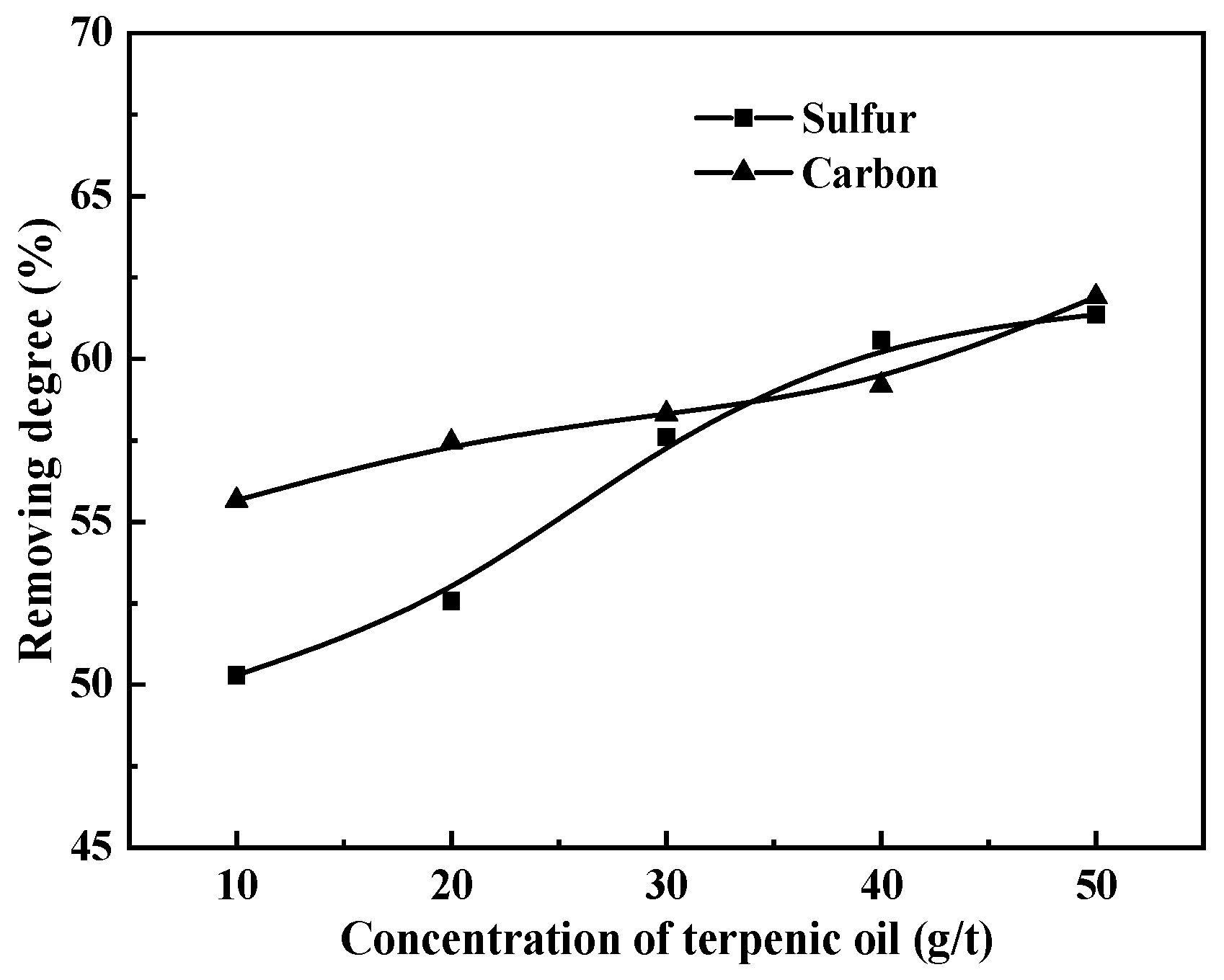
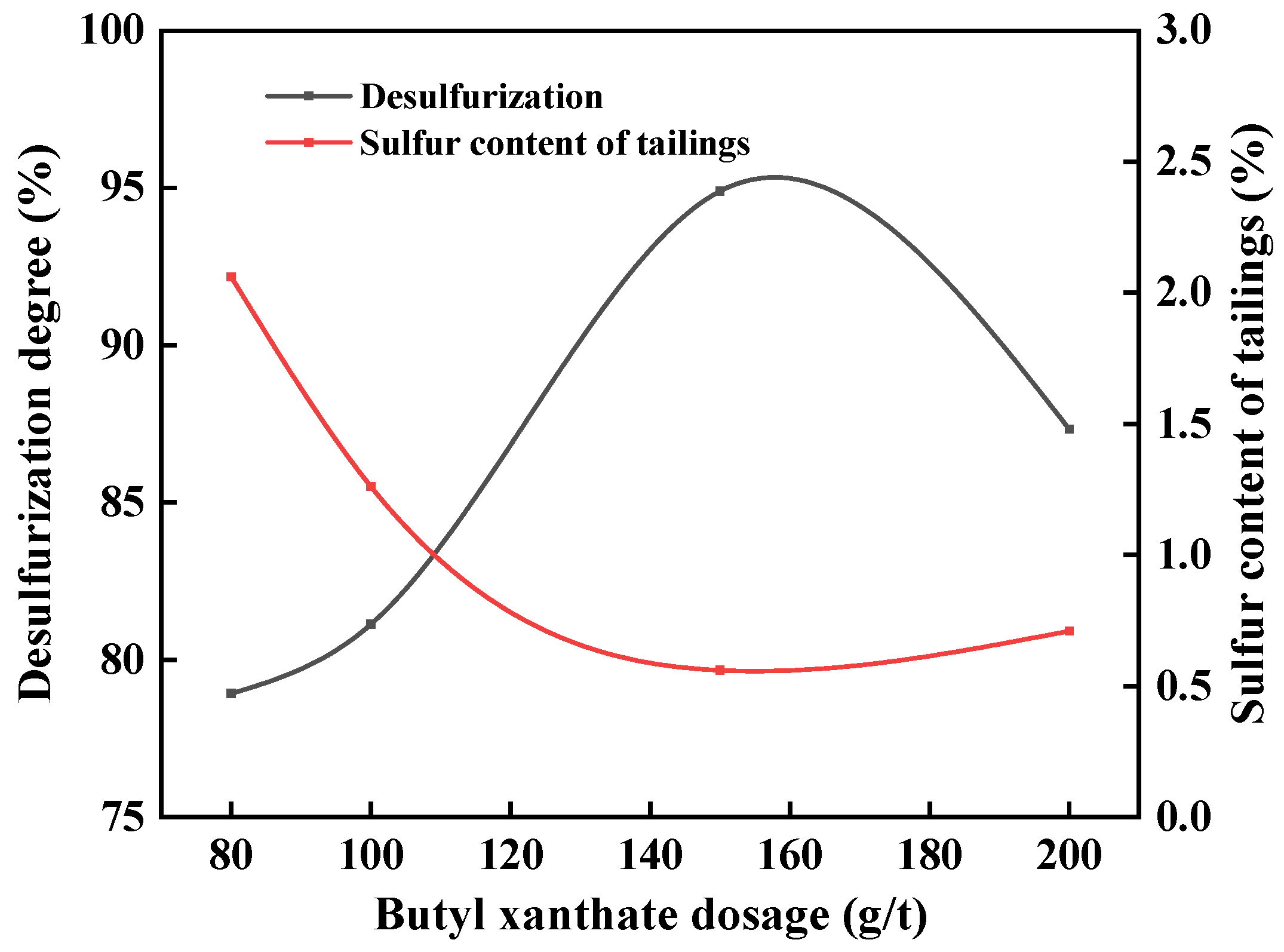
3.4. Dosage of Collector
3.5. Open-Circuit Flotation Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, G.; Zhang, J.; Su, H.; Zhang, Z. Synthesis and characterization of a novel collector for the desulfurization of fine high-sulfur bauxite via reverse flotation. Particuology 2023, 79, 64–77. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Y.-L.; Zhang, M.-N. Research progress on desulfurization technology of high-sulfur bauxite. Trans. Nonferrous Met. Soc. China 2022, 32, 3374–3387. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, J.; Su, H.; Zhang, Z. A novel collector for high-sulfur bauxite flotation desulfurization. Sep. Sci. Technol. 2023, 58, 86–100. [Google Scholar] [CrossRef]
- Chai, W.; Huang, Y.; Peng, W.; Han, G.; Cao, Y.; Liu, J. Enhanced separation of pyrite from high-sulfur bauxite using 2-mercaptobenzimidazole as chelate collector: Flotation optimization and interaction mechanisms. Miner. Eng. 2018, 129, 93–101. [Google Scholar]
- Li, X.-B.; Niu, F.; Liu, G.-H.; Qi, T.-G.; Zhou, Q.-S.; Peng, Z.-H. Effects of iron-containing phases on transformation of sulfur-bearing ions in sodium aluminate solution. Trans. Nonferrous Met. Soc. China 2017, 27, 908–916. [Google Scholar] [CrossRef]
- Xiong, D.; Ma, Z.; Peng, J.; Chen, X.; Li, Y. Present situation of research on desulphurization of high sulfur bauxite. Conserv. Utiliz. Min. Resour 2012, 32, 53–58. [Google Scholar]
- Xin, P.; Liye, J. Development and application of bauxite containing high sulfur. Light Met. 2010, 11, 14–17. [Google Scholar]
- Yin, J.; Xia, W.; Han, M. Resource Utilization of High-Sulfur Bauxite of Low-Median Grade in Chongqing China. In Light Metals 2011, Lindsay, S.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 19–22. [Google Scholar]
- Gibson, B.; Wonyen, D.G.; Chehreh Chelgani, S. A review of pretreatment of diasporic bauxite ores by flotation separation. Miner. Eng. 2017, 114, 64–73. [Google Scholar] [CrossRef]
- Abikenova, G.K.; Kovzalenko, V.A.; Ambarnikova, G.A.; Ibragimov, A.T. Investigation of the effect and behavior of sulfur compounds on the technological cycle of alumina production. Russ. J. Non-Ferr. Met. 2008, 49, 91–96. [Google Scholar] [CrossRef]
- Caldeira, C.L.; Ciminelli, V.S.T.; Dias, A.; Osseo-Asare, K. Pyrite oxidation in alkaline solutions: Nature of the product layer. Int. J. Miner. Process. 2003, 72, 373–386. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, H.; Ma, W.; Xie, K.; Xu, B.; Zheng, L. Digestion behavior and removal of sulfur in high-sulfur bauxite during bayer process. Miner. Eng. 2020, 149, 106237. [Google Scholar] [CrossRef]
- Hu, X.-L.; Chen, W.-M.; Xie, Q.-L. Sulfur phase and sulfur removal in high sulfur-containing bauxite. Trans. Nonferrous Met. Soc. China 2011, 21, 1641–1647. [Google Scholar] [CrossRef]
- Hu, X.L.; Chen, W.M.; Xie, Q.L. Desulfuration of high sulfur bauxite by oxidation roasting. J. Cent. South Univ. (Sci. Technol.) 2010, 41, 852–858. [Google Scholar]
- Lou, Z.; Xiong, Y.; Feng, X.; Shan, W.; Zhai, Y. Study on the roasting and leaching behavior of high-sulfur bauxite using ammonium bisulfate. Hydrometallurgy 2016, 165, 306–311. [Google Scholar] [CrossRef]
- Bulut, G.; Arslan, F.; Atak, S. Flotation behaviors of pyrites with different chemical compositions. Min. Metall. Explor. 2004, 21, 86–92. [Google Scholar] [CrossRef]
- Taguta, J.; O’Connor, C.T.; McFadzean, B. The effect of the alkyl chain length and ligand type of thiol collectors on the heat of adsorption and floatability of sulphide minerals. Miner. Eng. 2017, 110, 145–152. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, J.Q.; Zhang, Z.Y.; Liu, Z.Y. Experimental study on reverse flotation desulfurization of high-sulfur bauxite in Chongqing. Light Met. 2021, 509, 1002–1752. [Google Scholar]
- Blight, K.; Ralph, D.E.; Thurgate, S. Pyrite surfaces after bio-leaching: A mechanism for bio-oxidation. Hydrometallurgy 2000, 58, 227–237. [Google Scholar] [CrossRef]
- Li, S.P.; Wang, R.; Guo, Y.T.; Guo, Y.J.; Wang, G.; Liu, X.X.; Qiu, G.Z. Bio-desulfurization of high-sulfur bauxite by designed moderately thermophilic consortia. Chin. J. Nonferrous Met. 2016, 26, 2393–2402. [Google Scholar]
- Awe, S.A.; Sundkvist, J.-E.; Sandström, Å. Formation of sulphur oxyanions and their influence on antimony electrowinning from sulphide electrolytes. Miner. Eng. 2013, 53, 39–47. [Google Scholar] [CrossRef]
- Gong, X.; Zhuang, S.; Ge, L.; Wang, Z.; Wang, M. Desulfurization kinetics and mineral phase evolution of bauxite water slurry (BWS) electrolysis. Int. J. Miner. Process. 2015, 139, 17–24. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Z.; Zhuang, S.; Wang, D.; Wang, Y.; Wang, M. Roles of Electrolyte Characterization on Bauxite Electrolysis Desulfurization with Regeneration and Recycling. Metall. Mater. Trans. B 2017, 48, 726–732. [Google Scholar] [CrossRef]
- Long, H.; Dixon, D.G. Pressure oxidation of pyrite in sulfuric acid media: A kinetic study. Hydrometallurgy 2004, 73, 335–349. [Google Scholar] [CrossRef]
- He, R.; Tian, Z. Sulphur removal with barium hydroxide from industrial sodium aluminate solution. Nonferrous Met. 1996, 48, 63–66. [Google Scholar]
- He, P.; Tian, Z.; He, R. Removal sulphur during purifying industrial sodium aluminate solution with barium aluminate. Light Met. 2002, 5, 10–15. [Google Scholar]
- Li, X.-B.; Niu, F.; Tan, J.; Liu, G.-H.; Qi, T.-G.; Peng, Z.-H.; Zhou, Q.-S. Removal of S2− ion from sodium aluminate solutions with sodium ferrite. Trans. Nonferrous Met. Soc. China 2016, 26, 1419–1424. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Du, W. Experimental study on decarbonization and desulfurization of the high-sulfur bauxite in Shanxi province. Light Met. 2022, 6, 1–6. [Google Scholar]
- Wu, G.; Qi, L.; Wei, S.; Ban, S. Roasting pretreatment of high-carbon bauxite and Bayer digestion tests of the roasted ore, Nonferrous metals of the world. World Nonferrous Met. 2021, 574, 1002–5065. [Google Scholar]
- Guo, X.; Zhang, Z.; Tian, Y.; Wei, P. Experimental study on simultaneous removal of sulfur and organic matters in high sulfur and high organic bauxite by reverse flotation. Light Met. 2020, 6, 6–10. [Google Scholar]





| Element | Content (%) | Element | Content (%) |
|---|---|---|---|
| Al | 30.47 | Mg | 0.01 |
| Fe | 4.66 | Cr | 0.12 |
| Si | 3.81 | O | 47.40 |
| Ti | 2.95 | STotal l | 4.78 |
| Na | 0.031 | CTotal 1 | 3.10 |
| K | 0.083 | L.O.I. 2 | 19.13 |
| Phase | Content (%) | Distribution (%) |
|---|---|---|
| Sulfur in sulfide | 4.20 | 87.90 |
| Sulfur in sulfate | 0.57 | 11.85 |
| Elementary substance sulfur | 0.01 | 0.25 |
| STotal | 4.78 | 100.00 |
| Phase | Content (%) | Distribution (%) |
|---|---|---|
| Carbon in organic | 0.05 | 1.46 |
| Carbon in carbonate | 0.60 | 19.27 |
| Elementary substance carbon | 2.46 | 79.27 |
| CTotal | 3.10 | 100.00 |
| Product | Productivity/% | Content/% | Recovery/% |
|---|---|---|---|
| Sulfur concentrate | 16.15 | 14.63 | 49.42 |
| Tailing | 83.85 | 2.88 | 50.58 |
| Raw ore | 100.00 | 4.78 | 100.00 |
| Product | Productivity/% | S | C | Al2O3 | |||
|---|---|---|---|---|---|---|---|
| Content/% | Recovery/% | Content/% | Recovery/% | Content/% | Recovery/% | ||
| C concentrate | 26.97 | 4.68 | 26.45 | 9.69 | 84.37 | 40.38 | 19.42 |
| S concentrate | 11.34 | 27.21 | 64.60 | 1.58 | 5.78 | 19.58 | 3.96 |
| S middling 1 | 4.84 | 1.40 | 1.42 | 1.95 | 3.05 | 59.82 | 5.17 |
| S middling 2 | 5.24 | 2.55 | 2.79 | 1.13 | 1.91 | 64.02 | 5.99 |
| S middling 3 | 3.35 | 1.35 | 0.94 | 0.63 | 0.68 | 60.67 | 3.63 |
| S tailing | 48.26 | 0.38 | 3.80 | 0.27 | 4.21 | 71.85 | 61.83 |
| Raw ore | 100.00 | 4.78 | 100.00 | 3.10 | 100.00 | 56.07 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Teng, X.; Yang, Y.; Jiang, H.; Luo, J. Flotation Decarbonization and Desulfurization of a High-Sulfur Bauxite in China. Minerals 2023, 13, 1008. https://doi.org/10.3390/min13081008
Zhu Z, Teng X, Yang Y, Jiang H, Luo J. Flotation Decarbonization and Desulfurization of a High-Sulfur Bauxite in China. Minerals. 2023; 13(8):1008. https://doi.org/10.3390/min13081008
Chicago/Turabian StyleZhu, Zhongping, Xin Teng, Yang Yang, Hao Jiang, and Jun Luo. 2023. "Flotation Decarbonization and Desulfurization of a High-Sulfur Bauxite in China" Minerals 13, no. 8: 1008. https://doi.org/10.3390/min13081008
APA StyleZhu, Z., Teng, X., Yang, Y., Jiang, H., & Luo, J. (2023). Flotation Decarbonization and Desulfurization of a High-Sulfur Bauxite in China. Minerals, 13(8), 1008. https://doi.org/10.3390/min13081008





