Abstract
The resource utilization of cyanide tailings has significant environmental and economic benefits. The efficient recovery of gold from low-grade cyanide tailings containing 1.71 g/t Au was performed by a chlorination roasting–flotation process. The effects of roasting temperature, calcium chloride, internal coke, external coke, copper sulfide concentrate, and kaolin on the recovery rate of concentrate, gold grade, and sorting efficiency were investigated. The optimized process parameters were as follows: 16 wt% calcium chloride dihydrate, 6 wt% internal carbon, 1 wt% external carbon, 9 wt% copper sulfide concentrate, 2 wt% kaolin, and roasting temperature of 730 °C. The sorting rate, gold grade, and recovery rate of gold concentrate can reach 88.48%, 33.46 g/t, and 76.7%, respectively, and the gold grade of tailings was as low as 0.17 g/t. In the matte phase, gold can be enriched in the form of gold grains. Therefore, through chlorination roasting, the trapped gold can be released and deposited on the surface of the matte phase due to the transformation from hematite to magnetite. The gold-deposited metal sulfide can be effectively recycled through flotation. These results have potential guiding significance for the efficient recovery of gold from cyanide tailings.
1. Introduction
In the gold smelting process, cyanide technology has the advantages of a simple process, low cost, and wide application range, and it is still the mainstream process of gold extraction [1,2,3]. However, a large amount of cyanide tailings has been produced in the past decades, which has become the main hazardous waste in the gold metallurgical industry. Cyanide tailings usually contain residual toxic cyanide and a small amount of valuable metals such as copper and gold [4,5]. Simple storage or landfill treatment of cyanide tailings not only damages the environment but also leads to resource loss. Therefore, the resource utilization of cyanide tailings has significant environmental, economic, and social benefits [6,7].
The comprehensive recovery methods of cyanide tailings include flotation enrichment–roasting, pretreatment–cyanide leaching, chlorination roasting, and so on [8]. Chlorinated roasting is a process of adding chlorination agents (Cl2, NaCl, CaCl2, etc.) to react with metals, metal oxides, and sulfides in cyanide tailings to produce metal chlorides with significantly different properties [9,10,11,12]. Because metal chloride has a low melting point, high volatility, and sound solubility for metal extraction and recovery, it can achieve metal separation, enrichment, and extraction. Due to the inert nature of precious metals, it is generally believed that the driving force of chlorination is the strong oxidation of Cl2, but chlorine gas is toxic and highly corrosive; thus, solid CaCl2 is usually used as a chlorination agent [5,13]. For instance, by adding pyrite to strengthen the chlorination roasting of cyanide tailings, Qin et al. found that the FeS2 phase can form CaSO4 and Fe2O3 by reacting with CaCl2 at 1100 °C, and the gold recovery rate can reach 98.56% [13]. For the cyanide tailings containing hematite and pyrite, Long et al. found that under the condition of 1050 °C and CaCl2 dosage of 4 wt%, the recovery rates of gold, silver, copper, and lead were 84%, 71%, 72%, and 92%, respectively [10]. Li et al. also found that a gold recovery rate can achieve close to 90 wt% by roasting cyanide tailings with 5 wt% CaCl2 at 1273 K [6]. However, this technology still suffers from high reaction temperatures, strong corrosion of volatile gas, and low recovery of precious metals.
During the chlorination roasting process, chlorination agents can form a micro-liquid phase on the surface and inside of raw materials, which provides a carrier for mass and heat transfer for the enrichment of gold and sulfide minerals [1,14]. For instance, calcium chloride can produce Cl2 or HCl gas at high temperatures, which penetrates into the solid particles and reacts with tailings to form chloride, thus achieving the separation, migration, and enrichment of gold and valuable metals by forming matte crystal nuclei in the molten salt region [15,16]. Due to the melting point of calcium chloride at 772 °C, small liquid regions can appear in the local regions of molten salt in the range of 600–800 °C, spread throughout the tailings, and distribute in an irregular network [17]. Because gold and silver have similar lattice structures and lattice radii with iron and heavy non-ferrous metals, continuous solid solutions or intermetallic compounds can be formed in a wide range of compositions [18]. Base metals and their binary or multicomponent alloys in the molten state are effective and reliable collectors of precious metals. The long-term practice of copper, nickel, lead, and zinc metallurgy shows that trace precious metals in ores can be captured into matte or final metal phases in the smelting process [19,20]. Therefore, chlorination roasting has the advantages of strong applicability of raw materials, high separation efficiency, and good comprehensive recovery efficiency for the low-grade cyanide tailings [17,21].
In this work, the recovery of gold from low-grade cyanide tailings was performed by chlorination roasting and flotation. The main factors affecting the recovery rate of gold were investigated, including roasting temperature, the amount of calcium chloride, the amount of coke, the amount of copper sulfide concentrate, and the amount of kaolin.
2. Experimental Section
2.1. Roasting Experiments
The cyanide tailings (1000 g) were mixed evenly with different additives in the mixing machine for 30 min. Especially part of the coke was used for mixing with cyanide tailings to prepare pellets (denoted as internal carbon), and part of the coke was used for mixing with the pellets for roasting (denoted as external carbon) so as to improve the roasting effect of the tailings. The mixed raw materials were added to the disk granulator (diameter = 500 mm, Zhengzhou Huazhu environmental protection and equipment Co., Ltd., Zhengzhou, China) in batches, and a certain amount of water was continuously added for pelletizing. The total water content was 15 wt% of the raw material amount, and the pellet size was about 8–10 mm. After drying in the oven at 90 °C for 4 h, the pre-dried pellets were put into the Muffle furnace and continued drying at 200 °C for 2 h. The as-completely dried pellets were roasted in the rotary kiln after mixing with external carbon (nominal diameter = 100 mm, length = 2000 mm, Yixing Shangneng Furnace Co., Ltd., Yixing, China), the rotating speed of which was 1 RPM. Unless otherwise specified, the roasting temperature was 730 °C, and the roasting time was 90 min. After roasting, the cured pellets were rapidly placed in water for quenching, cooling, and avoiding mineral oxidation. The preliminary slow cooling experiment of pellet showed that the recovery rate and grade of gold were very low due to the re-oxidation of iron-containing phases.
2.2. Flotation Experiments
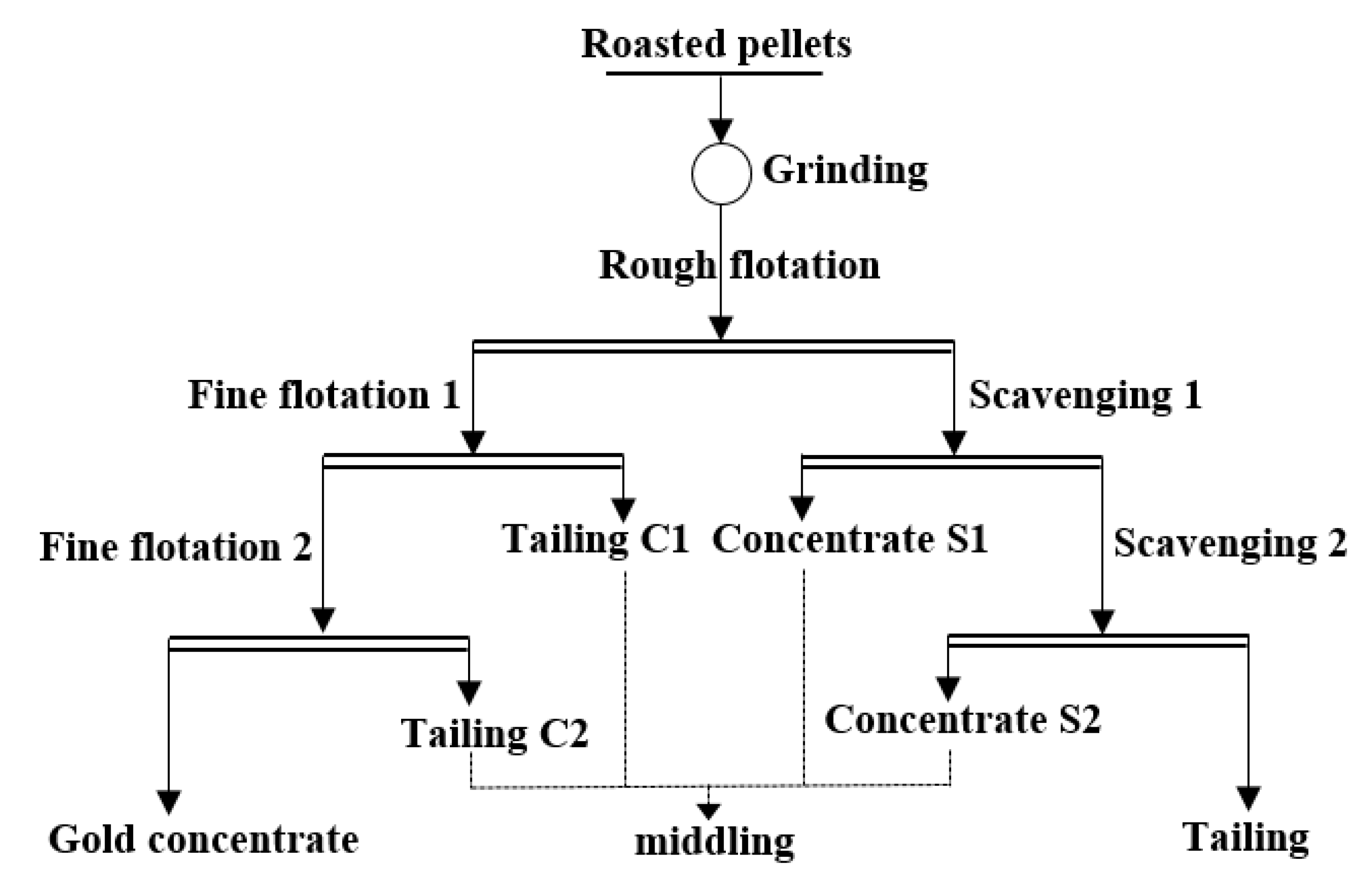
The roasted pellets (500 g) were ground for 24 min to 200 mesh with an intelligent conical ball mill (XMQ-φ 240 × 90 mm, Jilin Prospecting Machinery Factory, Jilin City, China). The obtained slurry was adjusted to an initial pulp density of 40 wt% and used for subsequent flotation. As shown in Figure 1, the process of one rough flotation, two scavengings, and two fine flotations was performed with a flotation machine (XFD-1.0 L, Jilin Prospecting Machinery Factory, China). Before flotation, the solution pH was adjusted to 7.45 with sodium carbonate, and then 7 mL of 10 g/L CuSO4 solution was added under stirring and timed. Both aerofloat (10 g/L, 4 mL) and xanthate (10 g/L, 4 mL) were added at 4.5 min, followed by adding 1 mL No. two oil (ROH, fatty alcohol) at 7.5 min. The inflation valve and scraper were opened at 9.5 min. Water was added continuously during the process, and the flotation finished at 14.5 min, where the pulp density decreased to 25%. The obtained coarse concentrate was further treated twice for 4 min without additional flotation reagent. The flotation tailing was further treated by two sweeping. At the beginning of the first sweep, sodium carbonate was added to adjust the acidity to 7.45, and then both aerofloat (10 g/L, 4 mL) and xanthate (10 g/L, 4 mL) were added and timed. The second oil was added at 2.5 min. The inflation valve and scraper were opened at 4.5 min, and the flotation finished at 8 min. During the second sweep, both aerofloat (10 g/L, 1 mL) and xanthate (10 g/L, 1 mL) were added and timed. Other procedures were the same as the first sweep. Therefore, the gold concentrate, middlings, and final tailings were obtained through the above flotation procedures.
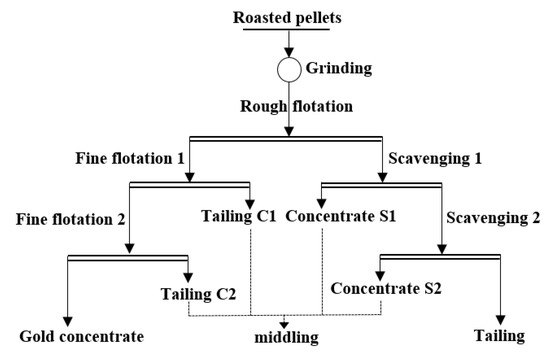
Figure 1.
Flow chart of open-circuit flotation process of roasted cyanide tailings.
2.3. Effect Evaluation
Representative parameters were selected to evaluate the recovery of gold, including gold grade of concentrate, recovery rate of concentrate, gold grade of tailings, etc. Due to the reverse trend between concentrate grade and recovery rate during the flotation process, the sorting rate was introduced as a comprehensive index to evaluate the gold recovery by following equation [22].
where E is the sorting rate, α is the gold grade in raw materials, β is the gold grade in concentrate, η is the gold grade in tailings, r is the productive rate of gold concentrate, and 55 is the assumed theoretical highest grade of gold. The sorting rate is a function of gold grade and gold recovery in the concentrate, so it can reflect the comprehensive performance of the flotation process because the quantitative and quality indicators can be integrated into this relative index.
2.4. Analysis and Characterization Methods
An inductively coupled plasma (ICP) spectrometer (Optima 8300DV, PE Company, Waltham, MA, USA) was used to analyze the content of multiple elements in cyanide tailings. X-ray diffractometer (D8 ADVANCE, Bruker Company, Bremen, Germany) was used to analyze the phase composition of the samples. The morphology and elemental distribution characteristics of the samples were analyzed by scanning electron microscope and energy dispersive spectrometer (SSX-550, Shimadzu Co., Kyoto, Japan). Since the cyanide tailings contain soluble residue, in order to focus on the morphology of the tailing itself, the cyanide tailing was washed with water before SEM analysis.
3. Results and Discussion
3.1. Composition of Cyanide Tailings
The chemical composition of cyanide tailings was analyzed. As shown in Table 1, the main element in the cyanide tailings contains Fe 32.71 wt%, Cu 0.33 wt%, Pb 0.86 wt%, Zn 0.42 wt%, S 1.38 wt%, and Si 8.28 wt%. However, the grade of silver is 54 g/t, and the grade of Au is only 1.71 g/t, which is not suitable for direct recovery by the traditional cyanide process. Therefore, the recovery of gold from these cyanide tailings is very challenging.

Table 1.
Element analysis of cyanide tailing, wt%.
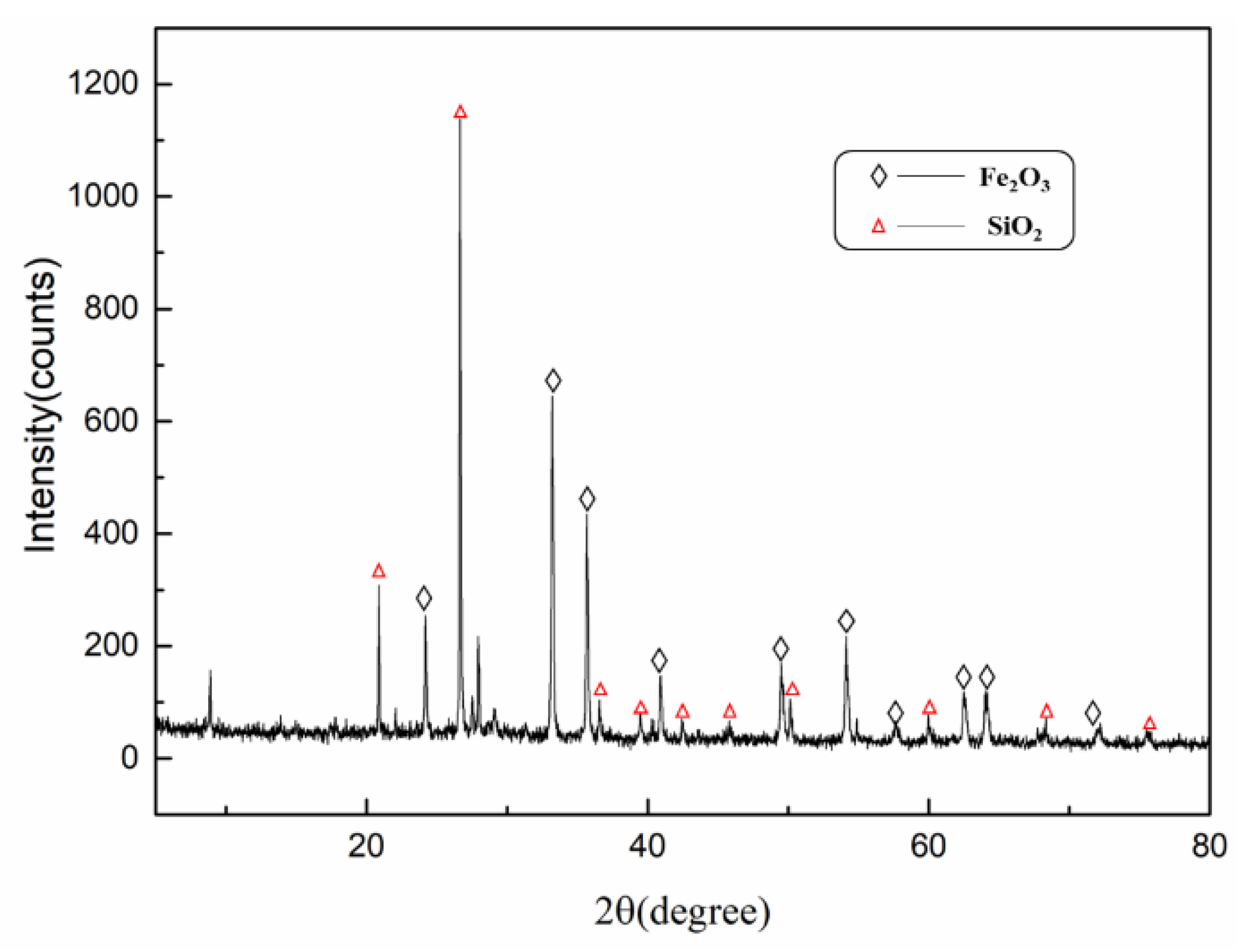
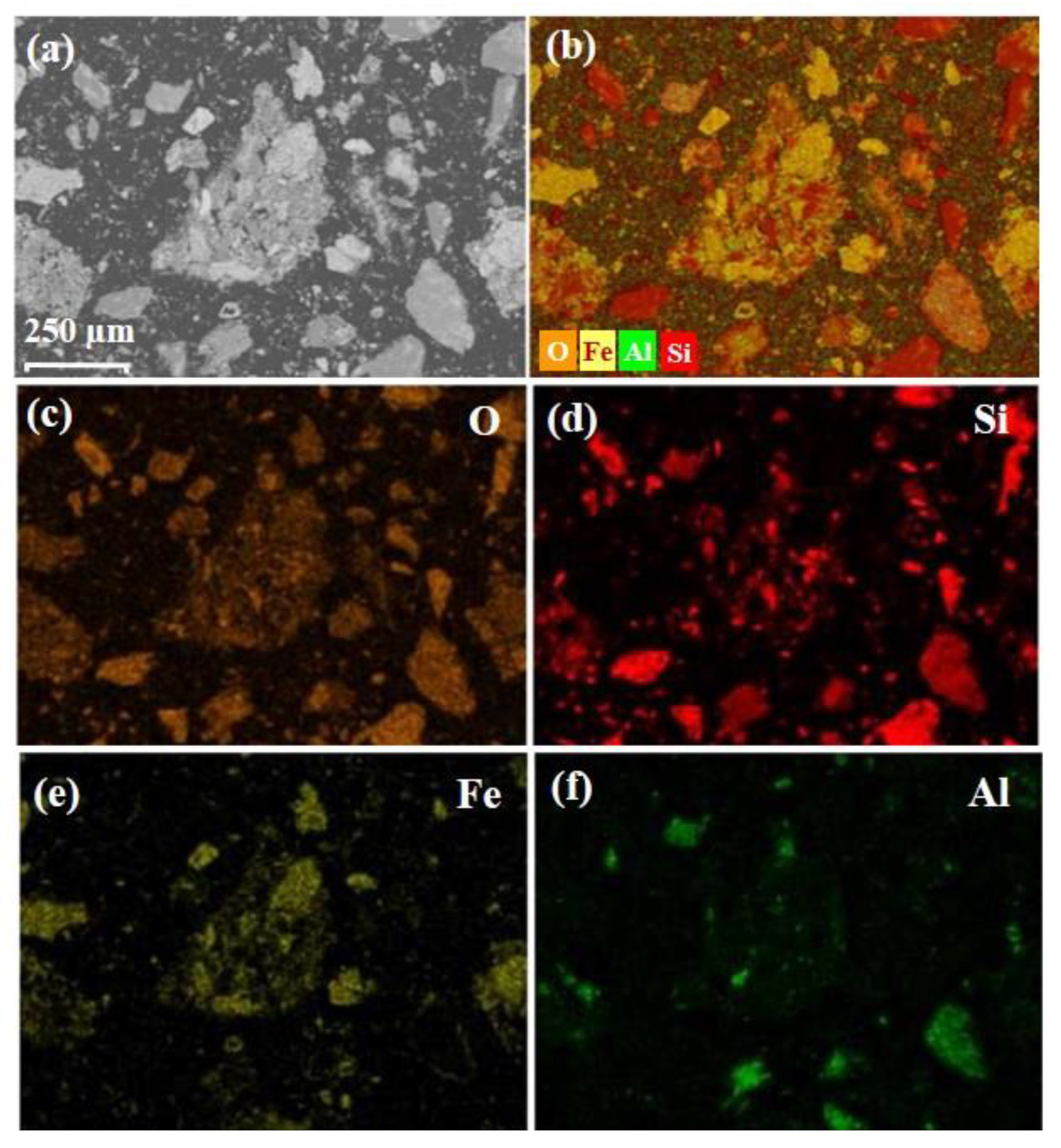
XRD results of cyanide tailings are shown in Figure 2. It can be found that iron mainly exists in the form of hematite, and gangue mainly consists of quartz. Because the content of gold, silver, and copper is much lower than the test standard of XRD, no related phase can be found. Figure 3 shows the morphology and element distribution of cyanide tailings after water washing. It can be observed from EDS that the washed tailing mainly contains Fe, O, Si, and Al elements. The bulk iron-containing phases are predominant. Part of Fe, O, and Si elements are overlapped, indicating that the hematite and quartz have a symbiosis. The cyanide tailings present mostly large clumps (0.02–0.1 mm), and the gold is not independently exposed to the surface of the tailings, which may be intermingled with other components in the tailings.
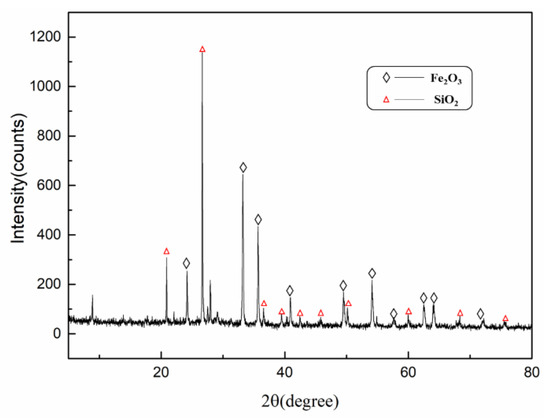
Figure 2.
XRD pattern of low-grade cyanide tailing.
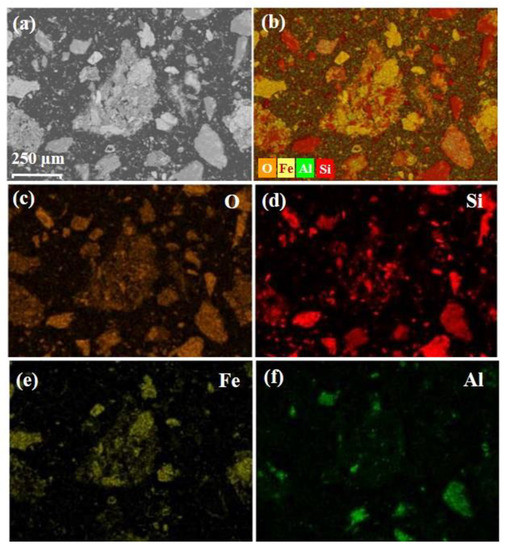
Figure 3.
SEM images (a) and mixed (b) and individual element distributions of O (c), Si (d), Fe (e), and Al (f) elements in cyanide tailing after water washing.
3.2. Influence of Roasting Temperature
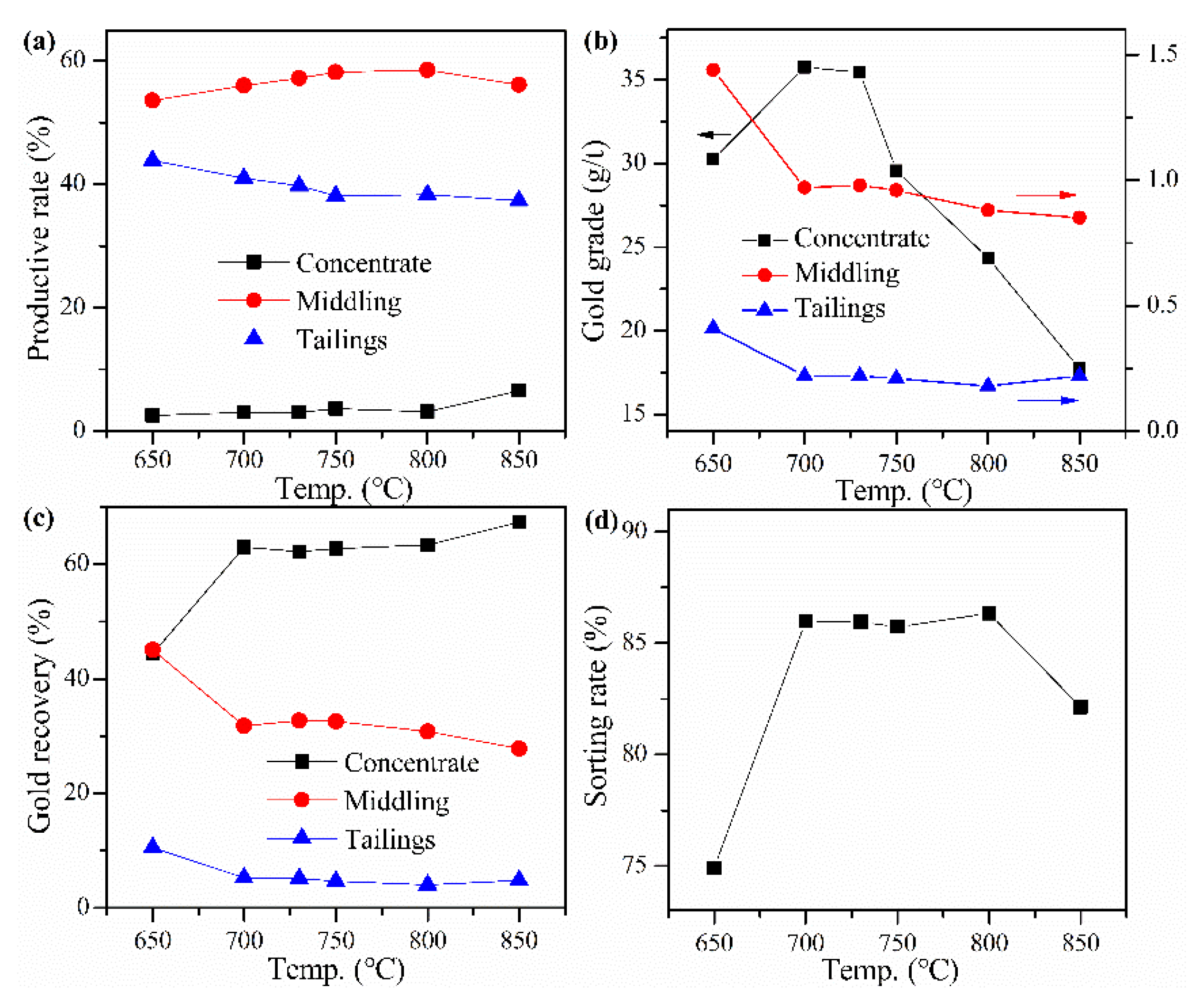
The cyanide tailings were mixed with calcium chloride 18 wt%, internal coke 8 wt%, external coke 1 wt%, copper sulfide concentrate 9 wt%, and kaolin 1 wt% for pelletizing and roasting. Roasting temperatures were 650, 700, 730, 750, 800, and 850 °C, respectively. The roasted pellets were ground after water quenching for flotation with a procedure of one rough flotation, two scavengings, and two fine flotations. The results in Figure 4 show that both the recovery rate and gold grade of concentrate are low at 650 °C, indicating that roasting at low temperatures cannot improve gold recovery. The sorting rate and recovery rate of gold concentrate can be improved by increasing temperature, but the temperature has little effect on them when the temperature is higher than 730 °C. At 730 °C, the recovery rate and gold grade of concentrate reach maximum values of about 62% and 36 g/t, respectively. When the roasting temperature is higher than 730 °C, the gold grade of the concentrate decreases rapidly because the amount of concentrate continues to increase, but the gold recovery rate does not increase significantly. Moreover, the sorting rate has no evident change at 700–800 °C, indicating that the comprehensive flotation performance cannot be further improved by increasing temperature. Therefore, the best roasting temperature is 730 °C.
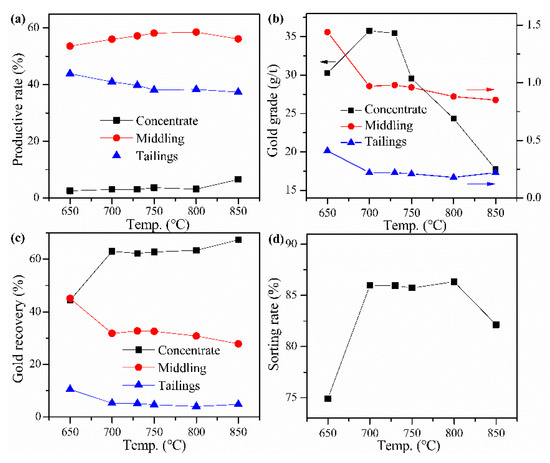
Figure 4.
Effect of roasting temperature on (a) productive rate of ore, (b) gold grade, (c) recovery rate of gold, and (d) sorting rate.
3.3. Influence of Calcium Chloride
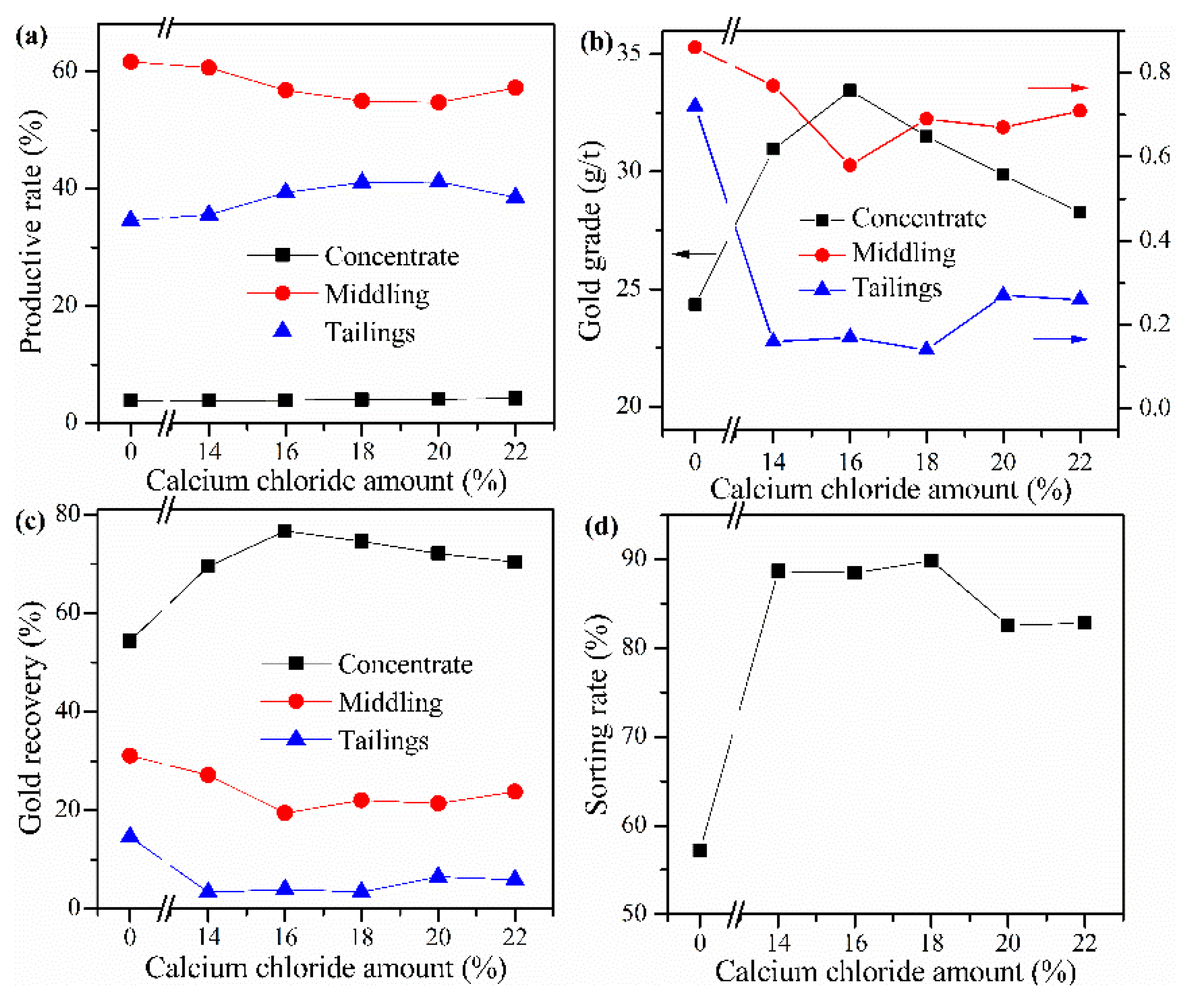
Cyanide tailing was mixed with internal coke 8 wt%, external coke 1 wt%, copper sulfide concentrate 9 wt%, and kaolin 1 wt% and different amounts of calcium chloride for pelletizing and roasting. The amounts of calcium chloride were 14 wt%, 16 wt%, 18 wt%, 20 wt%, and 22 wt%, respectively. The pellets roasted at 730 °C were ground after water quenching for flotation with the same procedure. The results in Figure 5 show that without calcium chloride, the gold recovery rate of concentrate is only 54.33%, and the loss rate of gold in tailings is 14.56%. When calcium chloride is added, the gold recovery rate and sorting rate are significantly improved. However, when the amount of calcium chloride is 14%–18%, the productive rate of gold middling gradually decreases, and the grade of concentrate increases first and then decreases. This phenomenon may be due to the dissociation of excessive calcium ions in the flotation process, and these cations will preferentially react with the anions of xanthate, thus consuming the collectors, thereby reducing the collection performance for gold concentrate. It is worth noting that the sorting rate has not changed significantly when the amount of calcium chloride is 14%–18 wt%. Therefore, the optimal amount of calcium chloride should be 16 wt%. Under this condition, the sorting rate reaches 88.48%; the gold grade in the concentrate and the recovery rate reach 33.46 g/t and 76.70%, respectively.
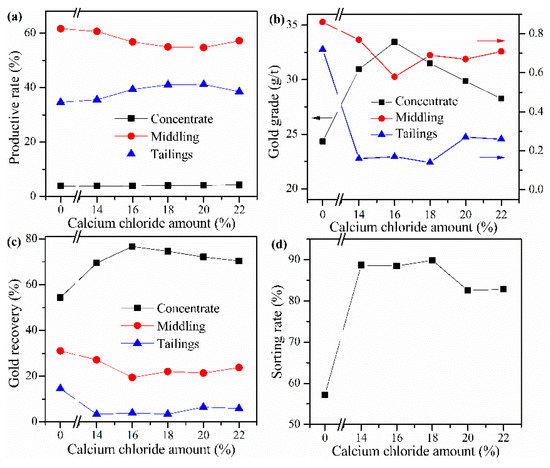
Figure 5.
Effect of CaCl2 addition on (a) productive rate of ore, (b) gold grade, (c) recovery rate of gold, and (d) sorting rate.
3.4. Effects of Copper Sulfide Concentrate
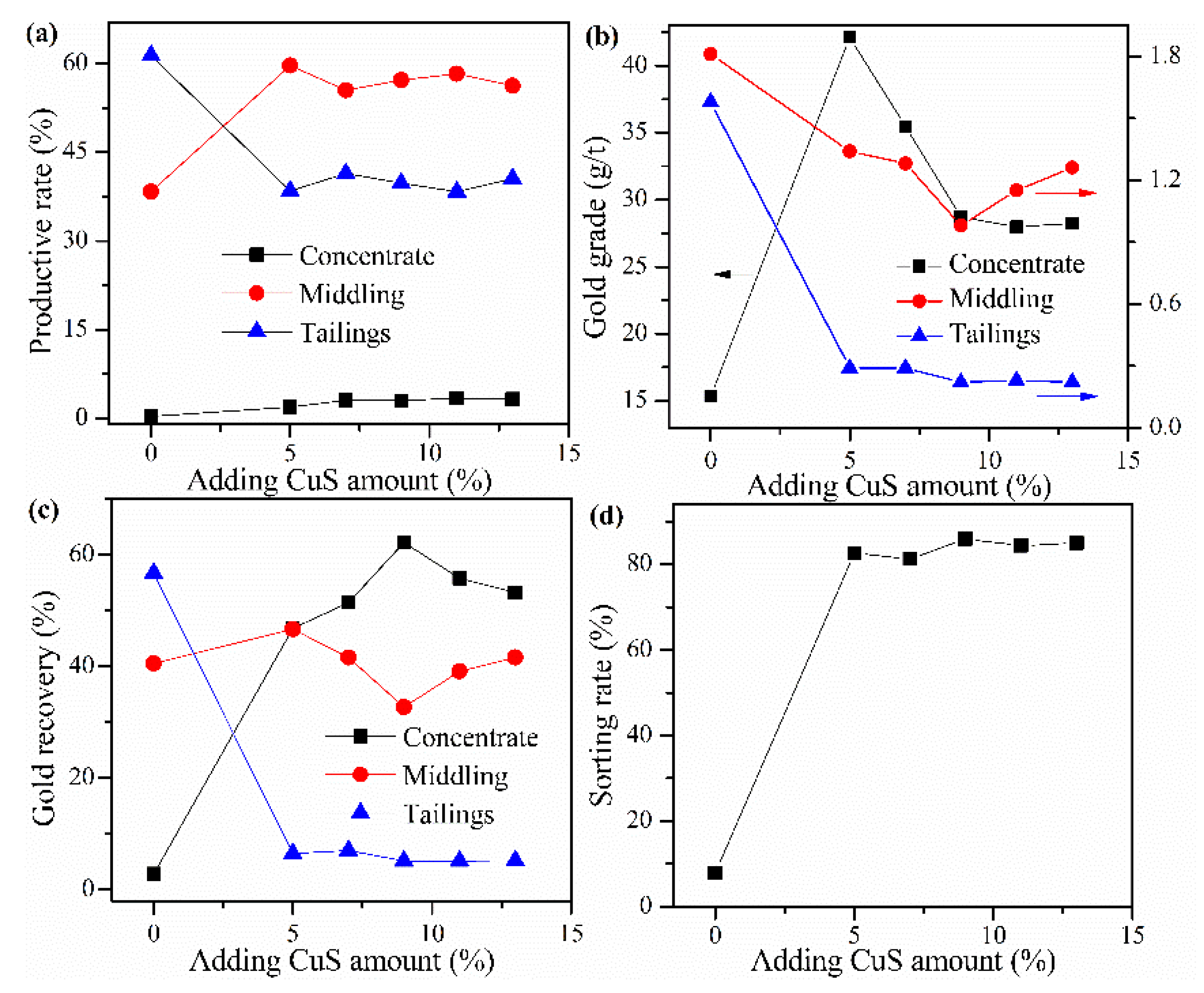
Cyanide tailing was mixed with calcium chloride 16 wt%, internal coke 8 wt%, external coke 1 wt%, kaolin 1 wt%, and different amounts of copper sulfide concentrate for pelletizing and roasting. The amounts of copper sulfide concentrate were 5 wt%, 7 wt%, 9 wt%, 11 wt%, and 13 wt%, respectively. The pellets roasted at 730 °C were ground after water quenching for flotation with the same procedure. The results in Figure 6 show that the addition of copper sulfide concentrate evidently promotes the recovery of the concentrate and improves the gold grade. Without copper sulfide concentrate, the loss rate of gold in tailings reaches 56.71%. As the amount of copper sulfide concentrate increases, the recovery rate of gold in the concentrate increases rapidly but slightly decreases above 9%, which may result from the difference in the grade of gold in concentrate and middling. Although the gold grade in the concentrate decreases rapidly when the amount of copper concentrate is greater than 5%, the overall sorting rate increases slightly. This phenomenon may be due to the increase in the number of additives, the quality of flotation concentrate and tailings have increased, but the gold recovery rate reaches the highest value, resulting in a reduction in the gold grade. Based on the sorting rate, the optimal amount of copper sulfide concentrate is 9 wt%, where the gold grade and recovery rate of concentrate reaches 35.46 g/t and 62.21%, respectively.
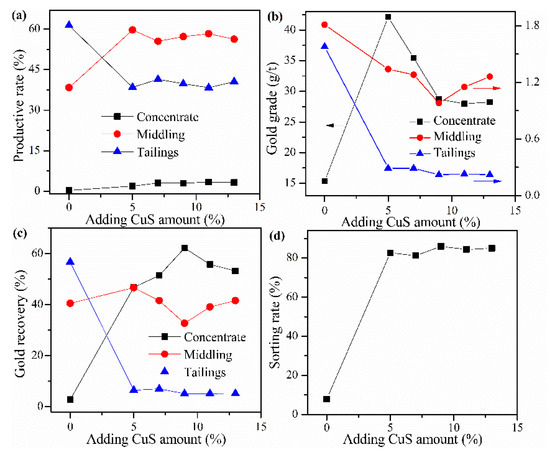
Figure 6.
Effects of CuS concentrate addition on (a) productive rate of ore, (b) gold grade, (c) recovery rate of gold, and (d) sorting rate.
3.5. Influence of Internal Coke
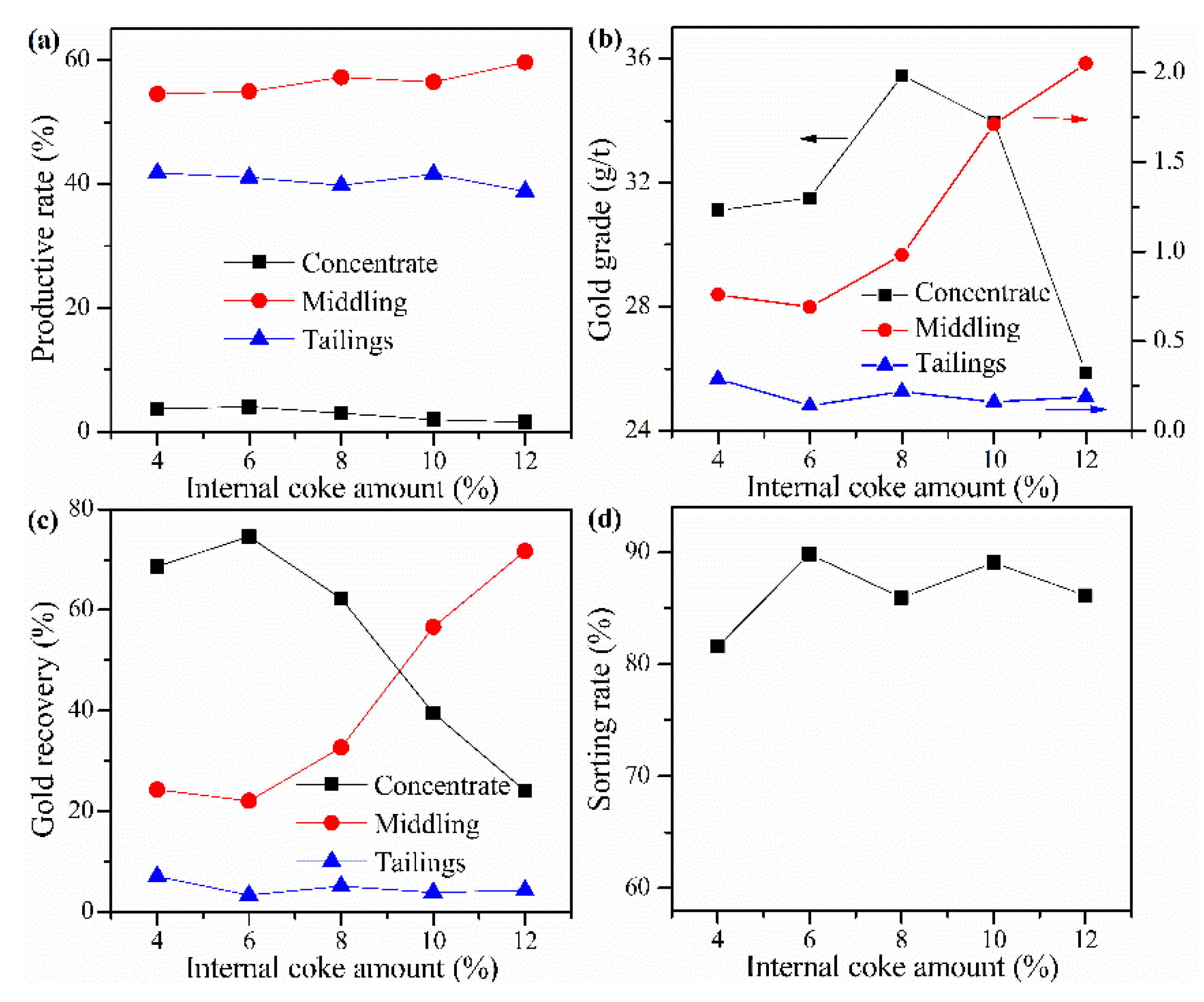
Cyanide tailing was mixed with calcium chloride 16 wt%, external coke 1 wt%, kaolin 1 wt%, copper sulfide concentrate 9 wt%, and different amounts of internal coke for pelletizing and roasting. The amounts of internal coke were 4 wt%, 6 wt%, 8 wt%, 10 wt%, and 12 wt%, respectively. The pellets roasted at 730 °C were ground after water quenching for flotation with the same procedure. The results in Figure 7 show that as the amount of internal coke increases, the recovery rate of gold in the concentrate first increases and then decreases. It can be found that the gold loss rate reaches about 7% when the amount of internal coke is 4 wt%. However, the addition of internal coke can inhibit the gold loss. Moreover, the gold grade in the concentrate also shows a trend of first increasing and then decreasing. This phenomenon shows that the amount of internal coke should not be too high because increasing internal coke may lead to a further reduction in the oxygen content of the reaction system, which is not conducive to the decomposition of chlorinating agents and the release of gold by chlorination roasting. The optimal amount of internal coke is 6 wt%, where the sorting rate, gold grade, and recovery rate of concentrate reach 89.84%, 31.5 g/t, and 74.61%, respectively.
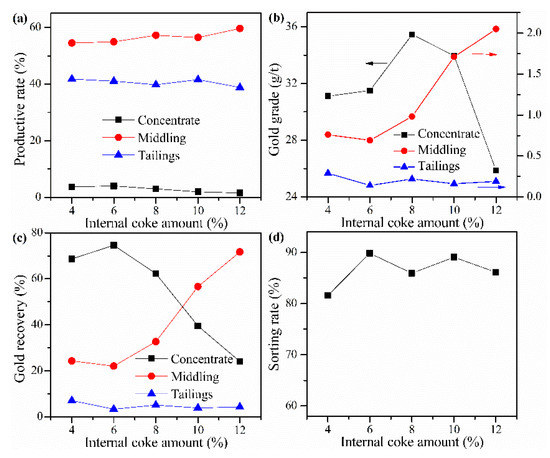
Figure 7.
Influence of internal coke amount on (a) productive rate of ore, (b) gold grade, (c) recovery rate of gold, and (d) sorting rate.
3.6. Influence of External Coke
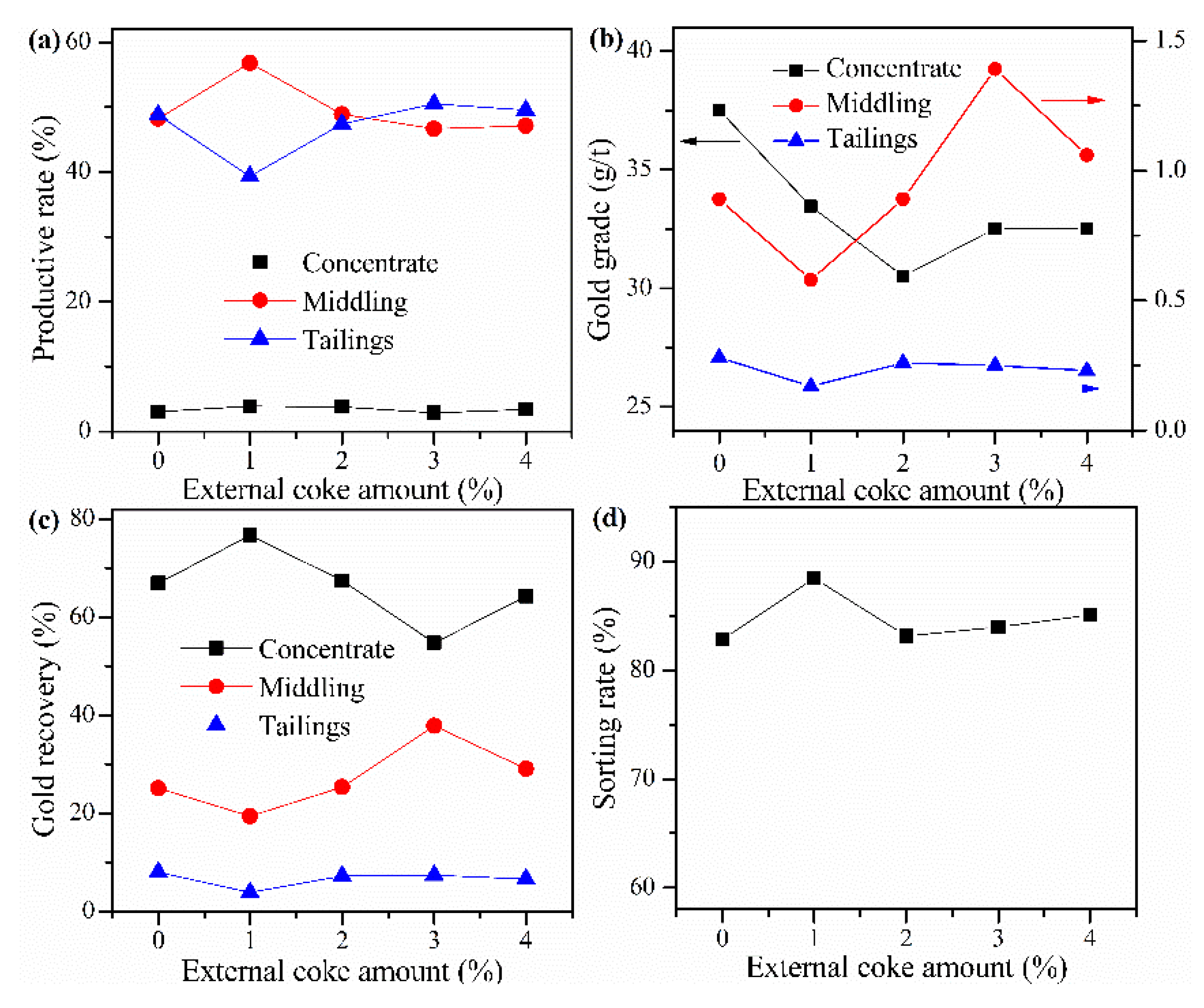
Cyanide tailing was mixed with calcium chloride 16 wt%, internal coke 6 wt%, kaolin 1 wt%, copper sulfide concentrate 9 wt%, and different amounts of external coke for pelletizing and roasting. The amounts of external coke were 0%, 1 wt%, 2 wt%, 3 wt%, and 4 wt%, respectively. The pellets roasted at 730 °C were ground after water quenching for flotation with the same procedure. The results in Figure 8 show that when no external coke is added, the gold grade of the concentrate is higher, but the gold recovery rate is lower than 70%. With the increase of the amount of external coke to 1 wt%, the gold grade in the concentrate shows a downward trend. Moreover, the recovery rate of gold in the concentrate increases, and the loss rate of gold in the tailings slightly decreases. However, both the gold recovery rate and gold grade rapidly decreased, which may be related to the rewrapping of gold due to the excess coke. Therefore, the amount of external coke should not be too much. When the amount of external coke is 1 wt%, the sorting rate, gold grade, and recovery rate of concentrate reach 88.48%, 33.46 g/t, and 76.7%, respectively. And the gold grade in the tailings has the lowest value of 0.17 g/t.
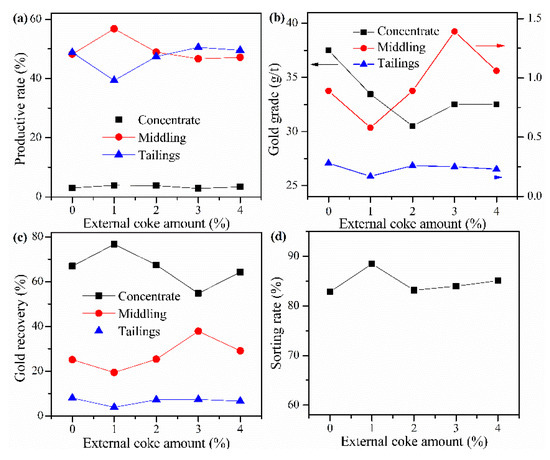
Figure 8.
Effect of external coke amount on (a) productive rate of ore, (b) gold grade, (c) recovery rate of gold, and (d) sorting rate.
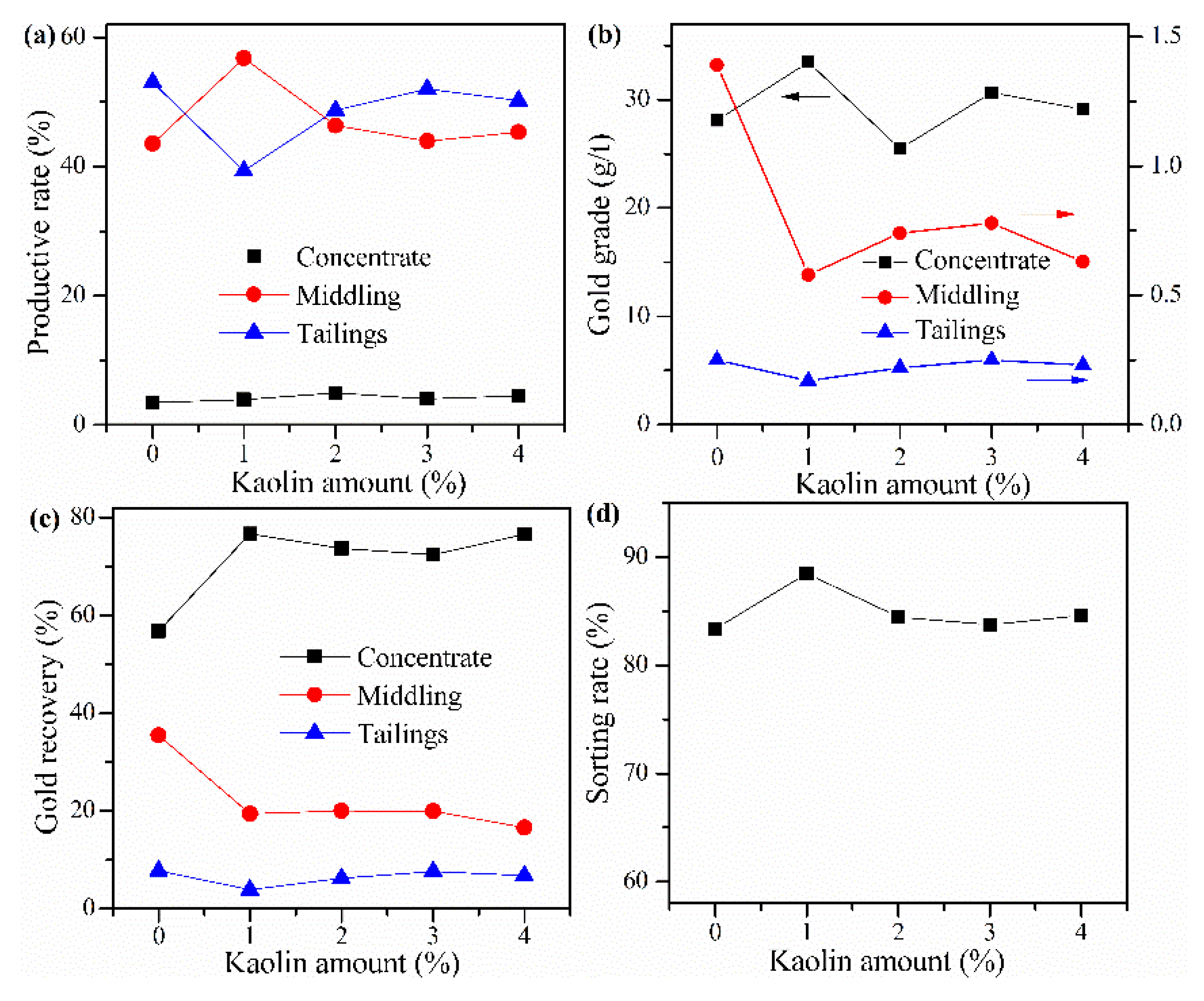
3.7. Influence of Kaolin
Cyanide tailing was mixed with calcium chloride 16 wt%, internal coke 6 wt%, external coke 1 wt%, copper sulfide concentrate 9 wt%, and different amounts of kaolin for pelletizing and roasting. The amounts of kaolin were 0 wt%, 1 wt%, 2 wt%, 3 wt%, and 4 wt%, respectively. The pellets roasted at 730 °C were ground after water quenching for flotation with the same procedure. The results in Figure 9 that when no kaolin is added, the recovery rate of gold in the concentrate is less than 60%, and the loss rate of gold in the tailings is close to 8%. With the increase in the amount of kaolin, the recovery rate of gold in the concentrate has little change, but the gold grade rapidly decreases, and the gold loss rate of tailings presents an increasing trend, indicating that the amount of kaolin should not be too large. When the amount of kaolin is 1%, the sorting rate, gold grade, and recovery rate of concentrate reach 88.48%, 33.46 g/t, and 76.7%, respectively. However, the yield of tailings increases with the increase in the amount of kaolin. When the amount of kaolin is 1 wt%, it reaches 39.33%, and the productive rate of gold concentrate is only 3.92%, which means that the proportion of middling is very large. In future industrial production, it is considered that if the yield of tailings is too low, the increase of the middling will have a negative impact on the material balance and processing capacity of the subsequent flotation process. Therefore, the amount of kaolin should be 2 wt%.
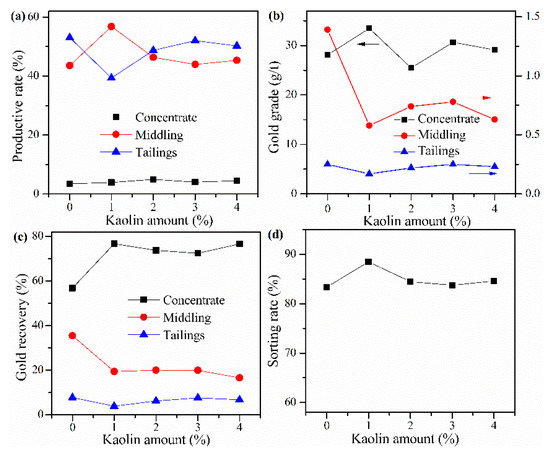
Figure 9.
Effects of kaolin amount on (a) productive rate of ore, (b) gold grade, (c) recovery rate of gold, and (d) sorting rate.
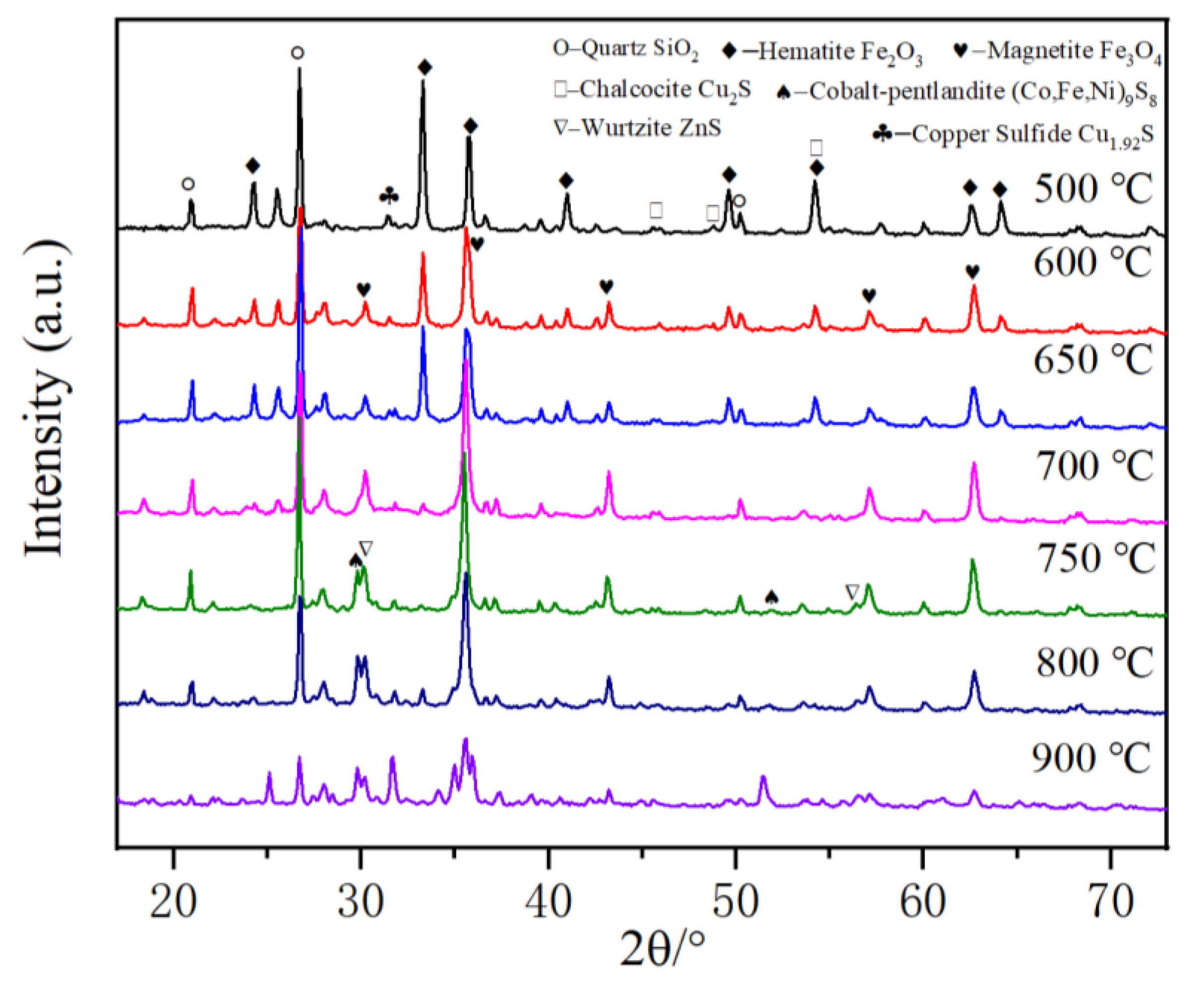
3.8. Phase Analysis of Roasted Pellets
The phase compositions of the roasted products at different temperatures were investigated by XRD. It can be seen from Figure 10 that the iron-containing phase in the cyanide tailings can transform from hematite to magnetite in the presence of calcium chloride when roasting at a low roasting temperature. At 500 °C, the main phases in the roasted pellets are calcium sulfate, hematite, and quartz, and there is no characteristic peak corresponding to magnetite, indicating that hematite has not been reduced at this temperature. At 600 °C, a new phase of magnetite appears at 30.4° due to the reduction role of carbon, indicating that the hematite phase begins to be partially reduced to magnetite, and no characteristic peak of metallic iron can be found. At 650 °C, the iron-containing phase is mainly magnetite, and the characteristic peaks of hematite disappear at 24.3, 41.1, 54.2, and 64°, indicating most of the hematite has transformed into magnetite. Within 650–900 °C, iron exists mainly in the form of magnetite. However, the contents of magnetite and quartz decrease significantly at 900 °C, and the ferroolivine phase appears, which may be because magnetite is partially reduced to ferrous oxide by coke, and even ferrous oxide reacts with quartz to form iron silicate. Therefore, the iron-containing phase in the cyanide tailings can be transformed into a ferric oxide phase at a low roasting temperature, and the iron-containing phase can be broken along with the volume expansion. The gold wrapped in the iron-containing phase can be exposed and then recovered by migration and enrichment of chlorination reaction. Meanwhile, new matte phases can be found after 700 °C, including wurtzite, chalcocite, cobalt-pentlandite, etc., which can potentially concentrate gold.
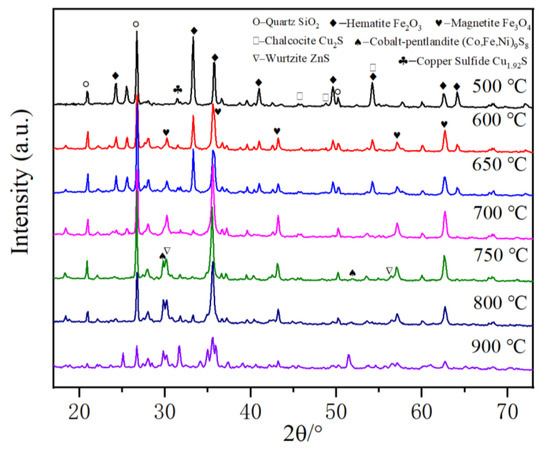
Figure 10.
XRD patterns of roasting products at different temperatures.
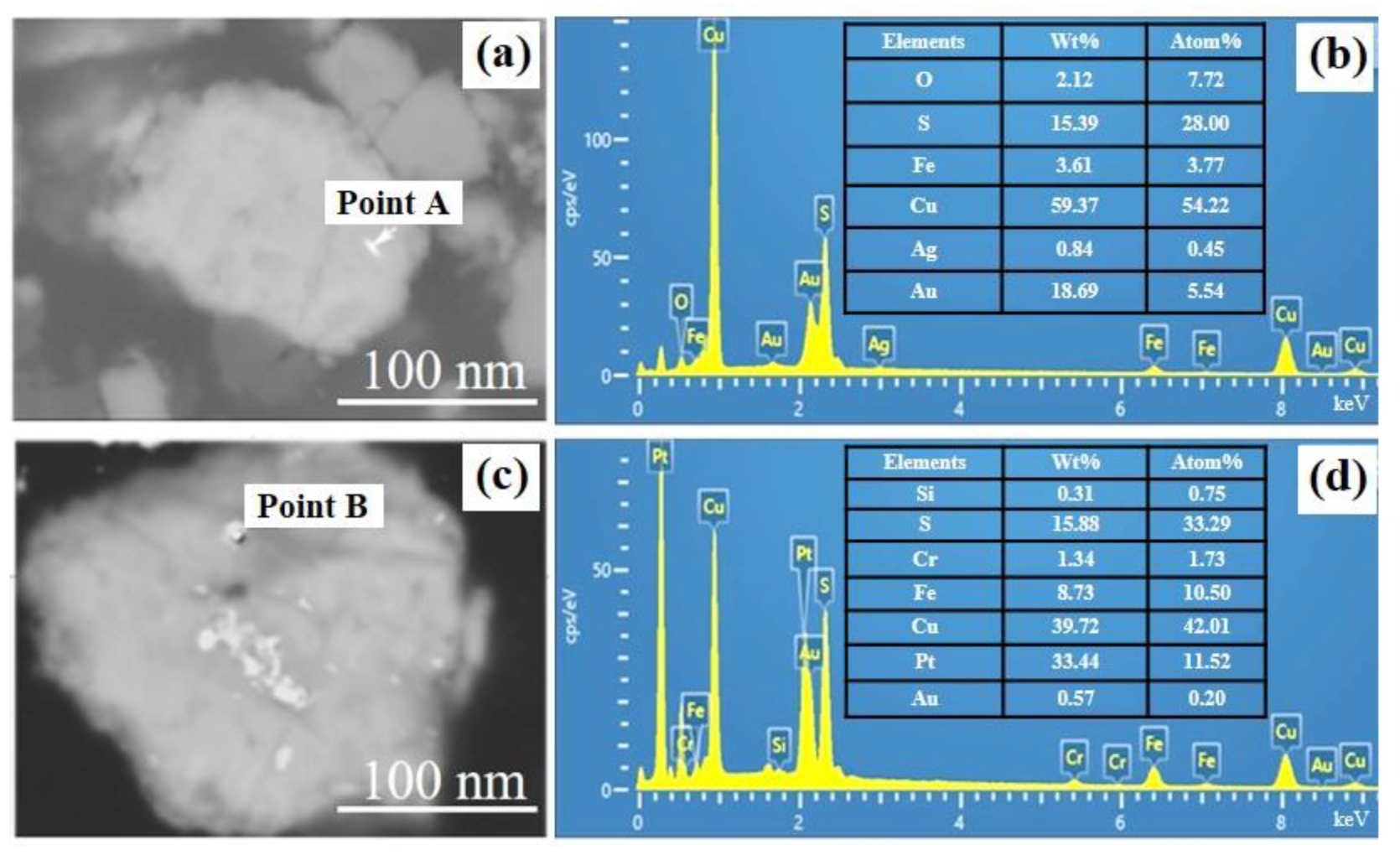
In order to determine the presence of gold in the matte phase, the roasted pellets were characterized by SEM. It can be seen from Figure 11 that gold particles can be found on the copper-containing matte phase. Intriguingly, metal Pt can also be found. Because of the inert nature of gold, it can be considered as the metallic elemental phase. Therefore, the iron-containing phase in the cyanide tailings transforms from hematite to magnetite after roasting, and the trapped gold can be released under the action of chlorination agents. The deposited gold can be captured by the metal sulfide, then migrates and enriches in the molten salt system. After flotation, these gold-deposited metal sulfides can be recovered.
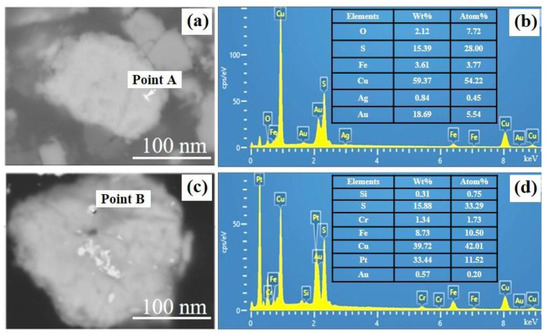
Figure 11.
SEM (a,c) and the corresponding EDX spectrum (b,d) of point A and point B on roasted pellets.
4. Conclusions
The recovery of gold from low-grade cyanide tailings by chlorination roasting and flotation was studied systematically by consideration of the effect of roasting temperature, calcium chloride, copper sulfide, kaolin, and coke. The sorting rate and recovery rate of gold concentrate can be improved by increasing temperature. The addition of copper sulfide, internal coke, and calcium chloride can increase the recovery rate and gold grade in concentrate first within a certain range, but increasing the internal coke amount can reduce the gold loss rate. The increase of external coke and kaolin leads to the decrease of gold grade and the increase of gold loss rate in tailings. The optimized composition is 16 wt% calcium chloride, 6 wt% internal coke, 1 wt% external coke, 9 wt% copper sulfide concentrate, and 2 wt% kaolin, where the sorting rate, gold grade, recovery rate of gold concentrate reaches 88.48%, 33.46 g/t, and 76.7%, respectively, and the gold grade of tailings is as low as 0.17 g/t. Therefore, through chlorination roasting, the iron-containing phase in the cyanide tailings transforms from hematite to magnetite, and the trapped gold is released. The deposited gold can be captured by the metal sulfide, then migrated and enriched in the molten salt system, and then recycled by flotation.
Author Contributions
Conceptualization, L.S. and Y.B.; methodology, K.J.; validation, F.X. and Z.Z.; formal analysis, J.T.; investigation, L.S. and J.M.; resources, L.H.; writing—original draft preparation, L.S.; writing—review and editing, Y.B.; visualization, Z.Z.; supervision, F.X.; funding acquisition, F.X. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Central Universities Basic Research Funding Project (N182506003) and BGRIMM Technology Group (04-2230).
Data Availability Statement
All data are available from the corresponding authors upon reasonable request.
Acknowledgments
All authors thank the editors and anonymous reviewers for their help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, P.; Wang, J.; Yu, D.; Guo, X.; Tian, Q. Comprehensive Reclamation of Valuable Metals from Au-Bearing Cyanide Residue by Chlorination Roasting-Carbothermic Reduction-Magnetic Separation: Recovery of Iron. J. Sustain. Metall. 2021, 7, 1748–1761. [Google Scholar] [CrossRef]
- Altinkaya, P.; Wang, Z.; Korolev, I.; Hamuyuni, J.; Haapalainen, M.; Kolehmainen, E.; Yliniemi, K.; Lundström, M. Leaching and recovery of gold from ore in cyanide-free glycine media. Miner. Eng. 2020, 158, 106610. [Google Scholar] [CrossRef]
- Aghaei, E.; Alorro, R.D.; Tadesse, B.; Browner, R. A review on current practices and emerging technologies for sustainable management, sequestration and stabilization of mercury from gold processing streams. J. Environ. Manag. 2019, 249, 109367. [Google Scholar] [CrossRef]
- Alvillo-Rivera, A.; Garrido-Hoyos, S.; Buitron, G.; Thangarasu-Sarasvathi, P.; Rosano-Ortega, G. Biological treatment for the degradation of cyanide: A review. J. Mater. Res. Technol. JmrT 2021, 12, 1418–1433. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Zhu, X.; Yang, T.; Deng, J.; Yan, B.; Mao, X.; Zhang, Y.; Li, S. Evaluation of a green-sustainable industrialized cleaner utilization for refractory cyanide tailings containing sulfur. Sci. Total Environ. 2022, 827, 154359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, S.; Ma, P.; Zhou, Z.; Long, H.; Peng, J.; Zhang, L. Evaluation of a cleaner production for cyanide tailings by chlorination thermal treatments. J. Clean. Prod. 2021, 281, 124195. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Lu, D.; Gu, X.; Chen, C. The detoxification and utilization of cyanide tailings: A critical review. J. Clean. Prod. 2021, 302, 126946. [Google Scholar]
- Li, Z.-Y.; Wang, W.-W.; Yue, K.; Chen, M.-X. High-temperature chlorination of gold with transformation of iron-containing phase. Rare Met. 2016, 35, 881–886. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Yu, D.; Zhang, L. Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction. Miner. Eng. 2021, 164, 106822. [Google Scholar] [CrossRef]
- Long, H.; Li, H.; Pei, J.; Srinivasakannan, C.; Yin, S.; Zhang, L.; Ma, A.; Li, S. Cleaner recovery of multiple valuable metals from cyanide tailings via chlorination roasting. Sep. Sci. Technol. 2021, 56, 2113–2123. [Google Scholar] [CrossRef]
- Li, H.; Peng, J.; Long, H.; Li, S.; Zhang, L. Cleaner process: Efficacy of chlorine in the recycling of gold from gold-containing tailings. J. Clean. Prod. 2021, 287, 125066. [Google Scholar] [CrossRef]
- Guo, X.; Qin, H.; Tian, Q.; Zhang, L. The efficacy of a new iodination roasting technology to recover gold and silver from refractory gold tailing. J. Clean. Prod. 2020, 261, 121147. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Zhang, L. Pyrite enhanced chlorination roasting and its efficacy in gold and silver recovery from gold tailing. Sep. Purif. Technol. 2020, 250, 117168. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, H.-W.; Li, G.-S.; Lu, C.-Y.; Lu, X.-G.; Zou, X.-L.; Xu, Q. Extraction of metals from complex sulfide nickel concentrates by low-temperature chlorination roasting and water leaching. Int. J. Miner. Metall. Mater. 2017, 24, 377–385. [Google Scholar] [CrossRef]
- Wang, W.-W.; Li, Z.-Y. Recovery and kinetics of gold and iron from cyanide tailings by one-step chlorination-reduction roasting. Miner. Eng. 2020, 155, 106453. [Google Scholar] [CrossRef]
- Liu, S.; Feng, Y.; Li, H.; Wang, H. Simultaneous Extraction of Gold and Vanadium from Vanadium and Carbon-Rich Refractory Gold Minerals by Chlorination Roasting. Metall. Mater. Trans. B 2022, 53, 3955–3966. [Google Scholar] [CrossRef]
- Ge, J.; Xiao, Y.; Kuang, J.; Liu, X. Research progress of chlorination roasting of heavy metals in solid waste. Surf. Interfaces 2022, 29, 101744. [Google Scholar] [CrossRef]
- Han, W.-W.; Yang, H.-Y.; Tong, L.-L. Cyanide removal for ultrafine gold cyanide residues by chemical oxidation methods. Trans. Nonferrous Met. Soc. China 2022, 32, 4129–4138. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Tian, R.; Ye, L.; Zhang, J.; Rao, M.; Li, G. Recycling of platinum-group metals from spent automotive catalysts by smelting. J. Environ. Chem. Eng. 2022, 10, 108709. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, T.; Guo, X.-Y.; Tian, Q.-H.; Zhong, S.-P.; Dong, L.; Qin, H.; Liu, Z.-W.; Makuza, B. Sustainable processing of gold cyanide tailings: Reduction roasting, mechanical activation, non-cyanide leaching, and magnetic separation. Hydrometallurgy 2023, 217, 106028. [Google Scholar] [CrossRef]
- Xing, Z.; Cheng, G.; Yang, H.; Xue, X.; Jiang, P. Mechanism and application of the ore with chlorination treatment: A review. Miner. Eng. 2020, 154, 106404. [Google Scholar] [CrossRef]
- Gülcan, E. A novel approach for sensor based sorting performance determination. Miner. Eng. 2020, 146, 106130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).