Evaluation of the Gemological Properties of Datolites from the Campotrera Deposit in the Northern Apennines (Italy)
Abstract
:1. Introduction
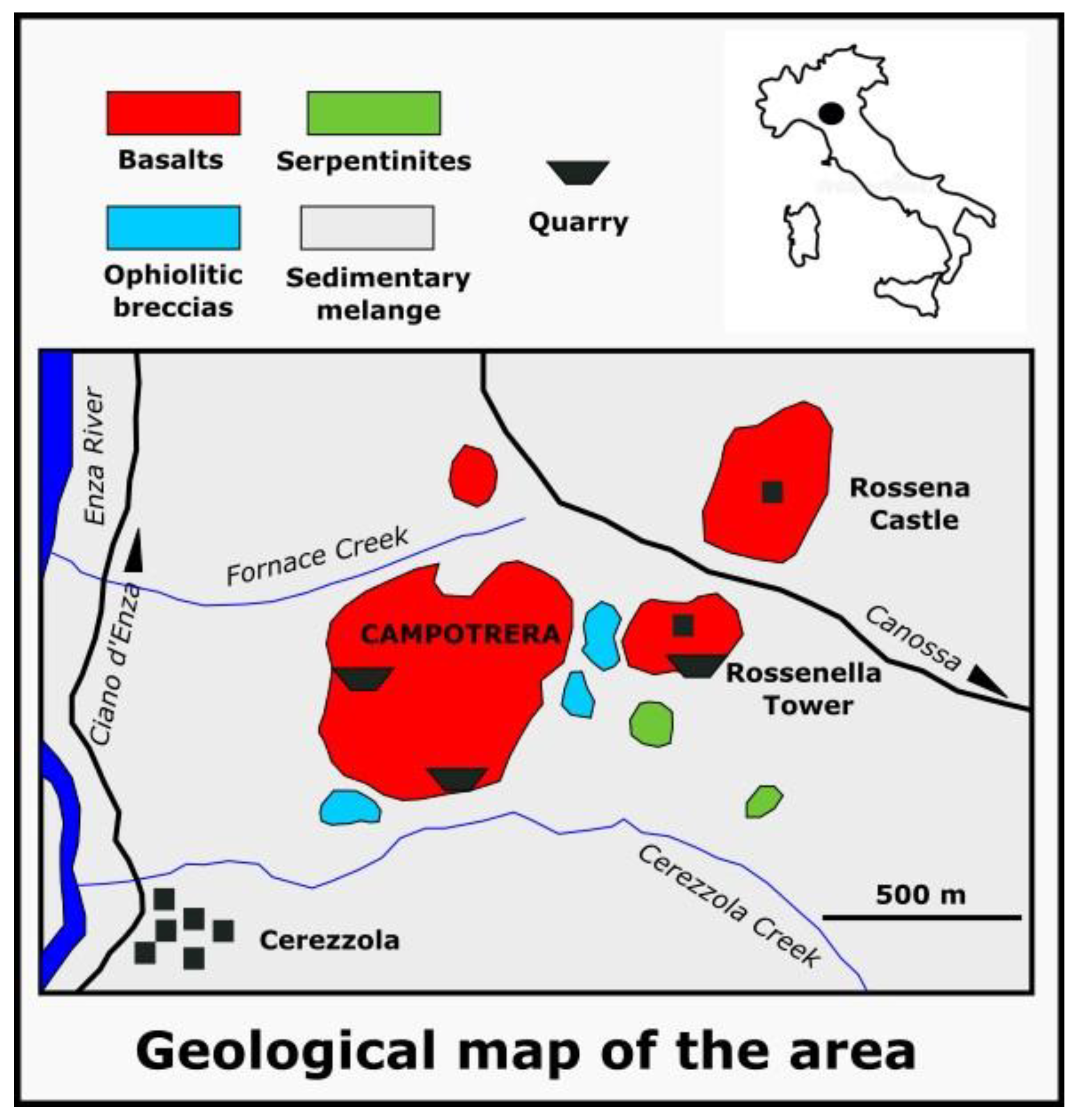
2. Geological Setting of Campotrera
3. Materials and Methods
4. Results
4.1. Gemological Analyses
4.2. Petrographic Observations
4.3. XRPD Analyses
4.4. EDS and SEM Analyses
4.5. LA-ICP-MS Analyses
4.6. Raman Spectroscopy Analyses
5. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A










References
- Borghi, E.; Scacchetti, M. L’attività estrattiva nella riserva naturale orientata Rupe di Campotrera e nella zona di Rossena. Comune Canossa 2002, 1, 2–47. [Google Scholar]
- Scacchetti, M.; Bartoli, O.; Bersani, D.; Laurora, A.; Lugli, S.; Malferrari, D.; Valeriani, L. Minerali della provincia di Reggio Emilia. AMI Ed. Cremona 2015, 93–147. [Google Scholar]
- Zaccarini, F.; Morales-Ruano, S.; Scacchetti, M.; Garuti, G.; Heide, K. Investigation of datolite (CaB[SiO4/OH]) from basalts in the Northen Apennines ophiolites (Italy): Genetic implications. Geochemistry 2008, 68, 265–277. [Google Scholar] [CrossRef]
- Foit, F.F.; Phillips, M.W.; Gibbs, G.V. A refinement of the crystal structure of datolite, CaBSiO4(OH). Am. Miner. 1973, 58, 909–914. [Google Scholar]
- Bellatreccia, F.; Camara, F.; Della Ventura, G.; Mottana, A. Datolite: A new occurence in volcanic ejecta (Pitigliano, Toscana, Italy) and crystal-structure refinement. Rend. Lincei 2006, 17, 289–298. [Google Scholar] [CrossRef]
- Bačík, P.; Fridrichova, J.; Uher, P.; Pršek, J.; Ondrejka, M. The crystal chemistry of gadolinite-datolite group silicates. Can. Miner. 2015, 51, 625–642. [Google Scholar] [CrossRef]
- BaČik, P.; Miyawaki, R.; Fridrichová, J.; Atencio, D.; Cámara, F.; Fridrichová, J. Nomenclature of the gadolinite supergroup. Eur. J. Miner. 2017, 29, 1067–1082. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, R.; Gatta, G.D.; Angel, R.J. Crystal chemistry and low-temperature behavior of datolite: A single-crystal X-ray diffraction study. Am. Miner. 2010, 95, 1413–1421. [Google Scholar] [CrossRef]
- Perchiazzi, N.; Gualtieri, A.F.; Merlino, S.; Kampf, A.R. The atomic structure of bakerite and its relationship to datolite. Am. Mineral. 2004, 89, 767–776. [Google Scholar] [CrossRef]
- Gemstones Encyclopedia. Available online: https://www.gemsociety.org/ (accessed on 2 May 2023).
- Datolite Gemstone Informations. Available online: https://www.gemdat.org/ (accessed on 26 April 2023).
- Konerskaya, L.P.; Orlova, R.G.; Bogdanis, E.P.; Konerskii, V.D.; Guseva, N.P. Using datolite and diopside raw materials in the electrical engineering industry. Glass Ceram. 1988, 45, 199–201. [Google Scholar] [CrossRef]
- Medvedovski, E. Low-temperature sintering of ceramics for the production of low-voltage insulators. Intern. Ceram. Rev. 1996, 45, 82–86. [Google Scholar]
- Bartoli, O.; Bersani, D.; Borghi, E.; Scacchetti, M. I minerali delle ofioliti: Rossena e Campotrera (RE). Riv. Miner. It. 2003, 27, 196–208. [Google Scholar]
- Bartoli, O.; Bersani, D.; Borghi, E.; Garuti, G.; Morales-Ruano, S.; Scacchetti, M.; Zaccarini, F. Datolite di Valmozzola, Parma. Un ritrovamento eccezionale. Riv. Miner. It. 2008, 32, 8–15. [Google Scholar]
- Kiss, G.; Molnar, F.; Zaccarini, F. Fluid inclusion studies in datolite of low grade metamorphic origin from a Jurassic pillow basalt series in northeastern Hungary. Cent. Eur. J. Geosci. 2012, 4, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Pezzotta, F.; Diella, V.; Guastoni, A. Chemical and paragenetic data on gadolinite-group minerals from Baveno and Cuasso al Monte, Southern Alps, Italy. Am. Miner. 1999, 84, 782–789. [Google Scholar] [CrossRef]
- Ratkin, V.V.; Eliseeva, O.A.; Pandian, M.S.; Orekhov, A.A.; Mohapatra, M.; Priya, S.K.V. Stages and formation conditions of productive mineral associations of the Dalnegorsk borosilicate deposit, Sikhote Alin. Geol. Ore Depos. 2018, 60, 672–684. [Google Scholar] [CrossRef]
- Datolite Mineral Information, Data and Localities. Available online: https://www.mindat.org/ (accessed on 26 April 2023).
- Boselli, F.; Boselli, L.; Ferretti, P.; Demartin, F. Datolite. Nuovo ritrovamento sul Buffaure (Val di Fassa, Trentino). Riv. Miner. It. 2013, 2, 108–115. [Google Scholar]
- Albertini, C. Famous mineral localities: Baveno, Italy. Miner. Rec. 1983, 14, 157–168. [Google Scholar]
- Marchesini, M.; Lunaccio, S.; Zampa, A. Il burrone di Vallegrande. Riv. Miner. It. 1988, 4, 19–24. [Google Scholar]
- Riserva Naturale Rupe di Campotrera. Available online: http://www.parchiemiliacentrale.it/riserva.rupe.campotrera/ (accessed on 1 March 2021).
- Borghi, E.; Patteri, P.; Scacchetti, M. I minerali delle ofioliti di Campotrera e Rossena. Comune Canossa 2002, 2–19. [Google Scholar]
- Marroni, M.; Molli, G.; Montanini, A.; Tribuzio, R. The association of continental crust rocks with ophiolites in the Northern Apennines (Italy): Implications for the continent–ocean transition in the Western Tethys. Tectonophysics 1998, 292, 43–66. [Google Scholar] [CrossRef]
- Marroni, M.; Molli, G.; Montanini, A.; Ottria, G.; Pandolfi, L.; Tribuzio, R. The external Liguride units (Northern Apennines, Italy): From rifting to convergence history of a fossil ocean-continent transition zone. Ofioliti 2002, 27, 119–132. [Google Scholar]
- Montanini, A.; Tribuzio, R. Gabbro-derived Granulites from the Northern Apennines (Italy): Evidence for Lower-crustal Emplacement of Tholeiitic Liquids in Post-Variscan Times. J. Petrol. 2001, 42, 2259–2277. [Google Scholar] [CrossRef]
- Montanini, A.; Tribuzio, R.; Vernia, L. Petrogenesis of basalts and gabbros from an ancient continent to ocean transition (External liguride ophiolites, Northern Italy). Lithos 2008, 101, 453–479. [Google Scholar] [CrossRef]
- Tribuzio, R.; Thirlwall, M.F.; Vannucci, R. Origin of the gabbro-peridotite association from the Northern Apennine ophiolites (Italy). J. Petrol. 2004, 45, 1109–1124. [Google Scholar] [CrossRef] [Green Version]
- Bertolani, M. La datolite della formazione ofiolitica appenninica. Pontif. Accad. Sci. 1948, 12, 305–366. [Google Scholar]
- Ferrari, M. Sulla datolite del monte Campotrera. Rend. R. Accad. Naz. Lincei 1924, 33, 439. [Google Scholar]
- Maddalena, L. Un nuovo giacimento di datolite e prehnite nell’Appennino Emiliano. Period. Miner. 1933, 3. [Google Scholar]
- X-ray Diffractometers. Available online: https://www.malvernpanalytical.com/ (accessed on 1 March 2021).
- Miller, C.; Zanetti, A.; Thoni, M.; Konzett, J.; Klotzli, U. Mafic and silica-rich glasses in mantle xenolits from Wau-ennamus, Lybia: Textural and geochemical evidence for peridotite melt reactions. Lithos 2012, 128, 11–26. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Roedder, E. Fluid Inclusions. In Reviews in Mineralogy and Geochemistry; Ribbe, P.H., Ed.; Mineralogical Society of America: Reston, VA, USA, 1984; Volume 12, p. 646. [Google Scholar]
- Goldstein, R.H. Fluid inclusions in sedimentary and diagenetic systems. Lithos 2001, 55, 159–193. [Google Scholar] [CrossRef]
- Caucia, F.; Scacchetti, M.; Marinoni, L.; Gilio, M. Black quartz from the Burano formation (Val Secchia, Italy): An unusual gem. Minerals 2022, 12, 1449. [Google Scholar] [CrossRef]
- Mernagh, T.P.; Wilde, A.R. The use of the laser Raman microprobe for the determination of salinity in fluid inclusions. Geochim. Cosmochim. Acta 1989, 53, 765–771. [Google Scholar] [CrossRef]
















| Sample | Cut | Shape | Color (RGB) | Reflection Index | Weight (ct) | Specific Gravity |
|---|---|---|---|---|---|---|
| 1 | Brilliant modified | Trapezoidal | Colorless | x = 1.62; y = 1.65; z = 1.67 | 3.12 | 3 |
| 2a | Brilliant modified | Oval | Colorless | x = 1.62; y = 1.65; z = 1.67 | 3.41 | 2.99 |
| 2b | Brilliant modified | Rectangular | Colorless | x = 1.62; y = 1.65; z = 1.67 | 4.77 | 2.99 |
| 2c | Brilliant modified | Square | Colorless | x = 1.62; y = 1.65; z = 1.67 | 3.09 | 2.99 |
| 2d | Baguette | Rectangular | Colorless | x = 1.63; y = 1.65; z = 1.67 | 0.91 | 3 |
| 3 | Brilliant modified | Pear | Colorless | x = 1.63; y = 1.64; z = 1.67 | 5.04 | 3 |
| 4a | Brilliant modified | Pear | Colorless | x = 1.62; y = 1.65; z = 1.67 | 3.04 | 3 |
| 4b | Brilliant modified | Oval | Colorless | x = 1.62; y = 1.66; z = 1.67 | 1.35 | 2.98 |
| 4c | Carré | Square | Colorless | x = 1.62; y = 1.65; z = 1.67 | 2.68 | 2.98 |
| 5d | Brilliant modified | Oval | Colorless, salmon4 * | x = 1.63. y = 1.64; z = 1.67 | 2.25 | 2.99 |
| 5e | Brilliant modified | Rectangular | White, light salmon4 | x = 1.62; y = 1.65; z = 1.67 | 2.22 | 3 |
| Wt% | Datolite 3 | Datolite 5A |
|---|---|---|
| SiO2 | 37.938 | 38.362 |
| CaO | 34.615 | 34.035 |
| Fe2O3 | 0.000 | 0.080 |
| B2O3calc | 21.802 | 21.865 |
| H2Ocalc | 5.645 | 5.658 |
| TOT | 100.000 | 100.000 |
| a.p.f.u. per 5 anions (4O2− + OH−) | ||
| Si4+ | 1.008 | 1.016 |
| Ca2+ | 0.985 | 0.966 |
| Fe3+ | 0.000 | 0.002 |
| B3+ | 1.00 | 1.000 |
| OH− | 1.000 | 1.000 |
| TOT | 3.993 | 3.984 |
| Element (ppm) | SPOT 1 | SPOT 2 | SPOT 3 | SPOT 4 | SPOT 5 |
|---|---|---|---|---|---|
| Sc | 0.98 | 0.921 | 0.936 | 0.838 | 0.746 |
| Ti | 0.6 | 0.59 | 0.82 | 0.45 | 0.7 |
| V | 1.027 | 0.986 | 0.973 | 1.018 | 1.065 |
| Mn | 0.76 | 0.7 | 0.53 | 0.36 | 0.71 |
| Fe | 19.87 | 41.54 | 34.56 | 57.81 | 25.64 |
| Co | 0.0145 | 0.0195 | 0.0186 | 0.0155 | 0.0116 |
| Ni | 0.176 | 0.222 | 0.126 | 0.192 | 0.156 |
| La | 0.0157 | 0.0058 | 0.061 | 0.105 | 0.559 |
| Sr | 1.653 | 1.514 | 1.482 | 1.637 | 1.529 |
| Rb | 0.0212 | 0.0184 | 0.0217 | 0.0160 | 0.0182 |
| Cs | 0.0158 | 0.0103 | 0.0106 | 0.0199 | 0.0127 |
| Ba | 0.036 | 0.00 | 0.00 | 0.0027 | 0.0086 |
| Cu | 0.58 | 0.082 | 0.087 | 0.093 | 0.104 |
| Zn | 0.48 | 0.34 | 0.42 | 0.5 | 0.23 |
| Elem. (ppm) | SPOT 1 * | SPOT 2 | SPOT 3 * | SPOT 4 * | SPOT 5 | SPOT 6 | SPOT 7 * | SPOT 8 | SPOT 9 * | SPOT 10 | SPOT 11 | SPOT 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc | 0.49 | 0.71 | 0.64 | 0.89 | 0.68 | 0.77 | 0.92 | 0.59 | 0.91 | 0.70 | 3.21 | 9.28 |
| Ti | 0.44 | 3.35 | 3.92 | 3.61 | 6.62 | 19.49 | 53.24 | 1.09 | 11.71 | 2.15 | 0.89 | 0.97 |
| V | 1.36 | 1.30 | 1.49 | 1.69 | 1.27 | 1.85 | 4.79 | 1.26 | 7.11 | 1.26 | 1.20 | 1.4 |
| Mn | 2.14 | 3.35 | 2.08 | 7.54 | 3.52 | 3.47 | 18.94 | 0.94 | 2.11 | 1.63 | 2.04 | 3.47 |
| Fe | 27.79 | 69.86 | 133.58 | 313.68 | 131.81 | 135.38 | 1070.8 | 23.3 | 1437.4 | 57.92 | 18.04 | 34.64 |
| Co | 0.02 | 0.07 | 0.07 | 0.25 | 0.10 | 0.08 | 0.56 | 0.02 | 0.07 | 0.03 | 0.02 | 0.03 |
| Ni | 0.19 | 0.43 | 0.29 | 0.83 | 0.87 | 0.25 | 1.35 | 0.09 | 0.27 | 0.18 | 0.20 | 0.24 |
| La | 0.34 | 0.65 | 0.62 | 3.75 | 3.19 | 0.52 | 0.69 | 0.44 | 0.61 | 0.82 | 1.81 | 1.6 |
| Ce | 0.10 | 0.24 | 0.57 | 1.86 | 2.54 | 0.57 | 0.93 | 0.90 | 1.19 | 2.15 | 5.39 | 5.69 |
| Sr | 21.05 | 13.13 | 11.07 | 25.49 | 7.75 | 5.54 | 6.36 | 5.54 | 5.64 | 5.67 | 19.58 | 41.88 |
| Rb | 0.02 | 0.04 | 0.02 | 0.07 | 0.07 | 0.09 | 0.60 | 0.01 | 0.08 | 0.02 | 0.02 | 0.02 |
| Cs | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.05 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 |
| Ba | 0.07 | 0.01 | 0.05 | 0.09 | 0.05 | 0.11 | 0.48 | 0.01 | 0.17 | 0.04 | 0.02 | 0.01 |
| Cu | 0.41 | 0.08 | 0.07 | 0.09 | 0.09 | 0.11 | 0.12 | 0.08 | 0.10 | 0.09 | 7.65 | 0.09 |
| Zn | 0.71 | 0.29 | 0.36 | 0.55 | 0.49 | 0.49 | 1.85 | 0.57 | 1.63 | 0.74 | 7.75 | 0.26 |
| Sample 3-Fluid Inclusion | Salinity (%) |
|---|---|
| 01 | 3.74 ± 0.57 |
| 03 | 3.16 ± 0.48 |
| 04 | 3.64 ± 0.55 |
| 06 | 3.37 ± 0.51 |
| Salinity (%) Campotrera [3] | Salinity (%) Hungary [15] |
| 10–16 | 1.8–2 |
| Geological Setting | Salinity |
|---|---|
| Diagenetic fluids | 9%–25% |
| Magmatic exhalative fluids | 2%–10% |
| Seafloor hydrothermal fluids | 3.50%–10% |
| Seawater evaporated with gypsum saturation | 10.50%–11.50% |
| Magmatic fluids | 28.5%–30% |
| Fluids in veins of quartz and calcite in sulfide mineralizations in the Northern Apennines basalts (Italy) | 1.5%–4% |
| Recycled seawater | 3.5% |
| Seawater | 3.2% |
| Freshwater | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinoni, L.; Caucia, F.; Gilio, M.; Scacchetti, M. Evaluation of the Gemological Properties of Datolites from the Campotrera Deposit in the Northern Apennines (Italy). Minerals 2023, 13, 1057. https://doi.org/10.3390/min13081057
Marinoni L, Caucia F, Gilio M, Scacchetti M. Evaluation of the Gemological Properties of Datolites from the Campotrera Deposit in the Northern Apennines (Italy). Minerals. 2023; 13(8):1057. https://doi.org/10.3390/min13081057
Chicago/Turabian StyleMarinoni, Luigi, Franca Caucia, Mattia Gilio, and Maurizio Scacchetti. 2023. "Evaluation of the Gemological Properties of Datolites from the Campotrera Deposit in the Northern Apennines (Italy)" Minerals 13, no. 8: 1057. https://doi.org/10.3390/min13081057





