Insight on the Properties of Pumice Mineral for the Combined Adsorption Distillation of Membrane Reject Water
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Adsorbent Characterization
3. Results and Discussion
3.1. Water Quality of Membrane Reject and Condensed Distillate
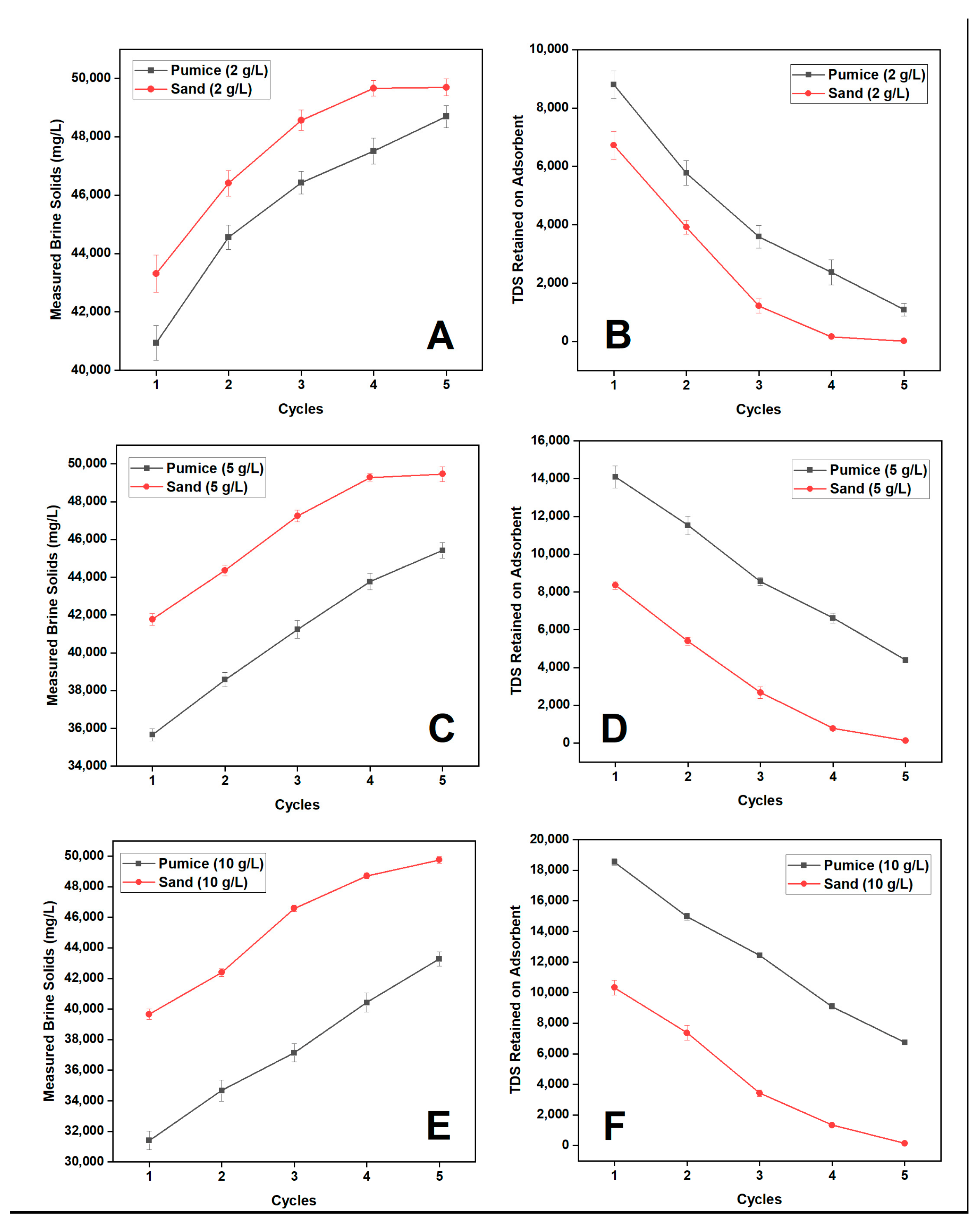
3.2. Effect of Mineral Adsorbent Dosage on CAD
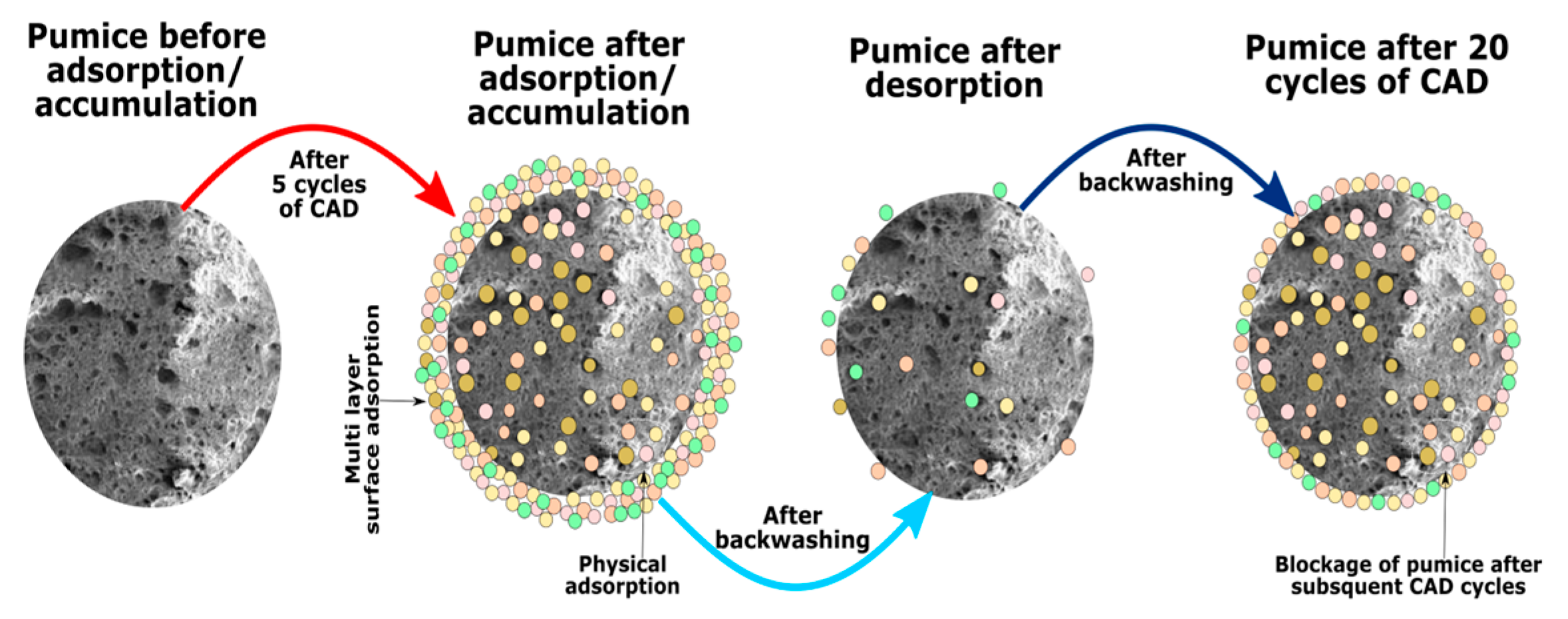
3.3. Pumice Backwashing and Long Cycles of CAD
3.4. Characterization of Adsorbents
3.4.1. Surface Characteristics and SEM Morphology
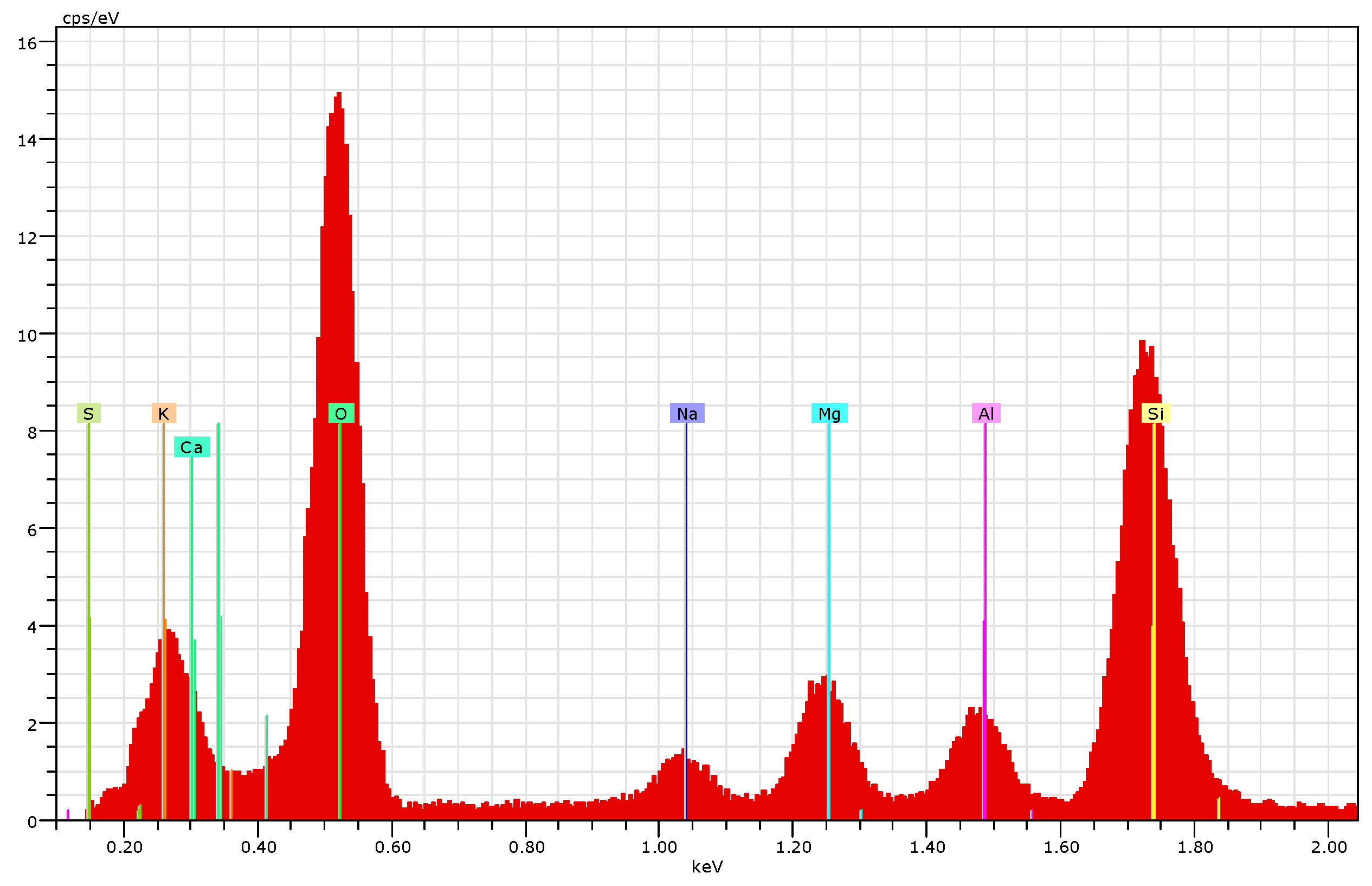
3.4.2. EDS Elemental Analysis
3.4.3. XRD Structural Analysis
3.5. Mechanism of Pumice Behavior during CAD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshameri, A.; Wei, X.; Wang, H.; Fuguo, Y.; Chen, X.; He, H.; Yan, C.; Xu, F. A review of the role of natural clay minerals as effective adsorbents and an alternative source of minerals. Minerals 2019, 3, 49–64. [Google Scholar] [CrossRef]
- Tarasevich, Y.I. Application of natural adsorbents and adsorption-active materials based thereon in the processes of water purification. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; Volume 120, pp. 659–722. [Google Scholar]
- Mustapha, S.; Tijani, J.; Ndamitso, M.; Abdulkareem, S.; Shuaib, D.; Mohammed, A.; Sumaila, A. The role of kaolin and kaolin/ZnO nanoadsorbents in adsorption studies for tannery wastewater treatment. Sci. Rep. 2020, 10, 13068. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, B.; Hong, J.; Chang, C.-T. Removal of ammonium and heavy metals by cost-effective zeolite synthesized from waste quartz sand and calcium fluoride sludge. Water Sci. Technol. 2017, 75, 587–597. [Google Scholar] [CrossRef]
- Ouyang, D.; Zhuo, Y.; Hu, L.; Zeng, Q.; Hu, Y.; He, Z. Research on the adsorption behavior of heavy metal ions by porous material prepared with silicate tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef]
- Burham, N.; Sayed, M. Adsorption behavior of Cd2+ and Zn2+ onto natural Egyptian bentonitic clay. Minerals 2016, 6, 129. [Google Scholar] [CrossRef]
- Moradi, M.; Soltanian, M.; Pirsaheb, M.; Sharafi, K.; Soltanian, S.; Mozafari, A. The efficiency study of pumice powder to lead removal from the aquatic environment: Isotherms and kinetics of the reaction. J. Maz. Univ. Med. Sci. 2013, 22, 65–75. [Google Scholar]
- Soleimani, H.; Mahvi, A.H.; Yaghmaeian, K.; Abbasnia, A.; Sharafi, K.; Alimohammadi, M.; Zamanzadeh, M. Effect of modification by five different acids on pumice stone as natural and low-cost adsorbent for removal of humic acid from aqueous solutions-Application of response surface methodology. J. Mol. Liq. 2019, 290, 111181. [Google Scholar] [CrossRef]
- Yavuz, M.; Gode, F.; Pehlivan, E.; Ozmert, S.; Sharma, Y.C. An economic removal of Cu2+ and Cr3+ on the new adsorbents: Pumice and polyacrylonitrile/pumice composite. Chem. Eng. J. 2008, 137, 453–461. [Google Scholar] [CrossRef]
- Moraci, N.; Calabrò, P.S. Heavy metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. J. Environ. Manag. 2010, 91, 2336–2341. [Google Scholar] [CrossRef]
- Catalfamo, P.; Arrigo, I.; Primerano, P.; Corigliano, F. Efficiency of a zeolitized pumice waste as a low-cost heavy metals adsorbent. J. Hazard. Mater. 2006, 134, 140–143. [Google Scholar] [CrossRef]
- Awan, M.A.; Qazi, I.A.; Khalid, I. Removal of heavy metals through adsorption using sand. J. Environ. Sci. 2003, 15, 413–416. [Google Scholar]
- Diels, L.; Spaans, P.; Van Roy, S.; Hooyberghs, L.; Ryngaert, A.; Wouters, H.; Walter, E.; Winters, J.; Macaskie, L.; Finlay, J. Heavy metals removal by sand filters inoculated with metal sorbing and precipitating bacteria. Hydrometallurgy 2003, 71, 235–241. [Google Scholar] [CrossRef]
- Abbasi, M.; Safari, E.; Baghdadi, M.; Janmohammadi, M. Enhanced adsorption of heavy metals in groundwater using sand columns enriched with graphene oxide: Lab-scale experiments and process modeling. J. Water Process Eng. 2021, 40, 101961. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, J.-C.; Li, A.W.; Wei, S. Heavy metal removal from wastewater in fluidized bed reactor. Water Res. 1999, 33, 1918–1924. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, D.; Song, H.; Sim, S.W.; Kim, D.; Yu, J.; Cho, K.H.; Oh, J.E. Recycling of reverse osmosis (RO) reject water as a mixing water of calcium sulfoaluminate (CSA) cement for brick production. Appl. Sci. 2019, 9, 5044. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies-A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Leon, F.; Ramos, A.; Vaswani, J.; Mendieta, C.; Brito, S. Climate change mitigation strategy through membranes replacement and determination methodology of carbon footprint in reverse osmosis RO desalination plants for islands and isolated territories. Water 2021, 13, 293. [Google Scholar] [CrossRef]
- Valdés, H.; Saavedra, A.; Flores, M.; Vera-Puerto, I.; Aviña, H.; Belmonte, M. Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membranes 2021, 11, 753. [Google Scholar] [CrossRef]
- Rilwani, M.L.; Agbanure, F.E. An assessment of the environmental impact of asphalt production in Nigeria. Anthropologist 2010, 12, 277–287. [Google Scholar] [CrossRef]
- Wei, C.; Lao, Y.; Ouyang, R.; Zhang, G.; Huang, G.; Deng, F.; Tan, Q.; Lin, G.; Zhou, H. Evaluation of Different Reverse Osmosis Membranes for Textile Dyeing and Finishing Wastewater Reuse. Membranes 2023, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic processes: Market overview, membrane phenomena, recent developments and sustainable strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Jia, X.; Klemeš, J.J.; Varbanov, P.S.; Wan Alwi, S.R. Analyzing the energy consumption, GHG emission, and cost of seawater desalination in China. Energies 2019, 12, 463. [Google Scholar] [CrossRef]
- Charcosset, C. Classical and Recent Developments of Membrane Processes for Desalination and Natural Water Treatment. Membranes 2022, 12, 267. [Google Scholar] [CrossRef]
- Plattner, J.; Kazner, C.; Naidu, G.; Wintgens, T.; Vigneswaran, S. Removal of selected pesticides from groundwater by membrane distillation. Environ. Sci. Pollut. Res. 2018, 25, 20336–20347. [Google Scholar] [CrossRef]
- Younos, T.; Tulou, K.E. Overview of desalination techniques. J. Contemp. Water Res. Educ. 2005, 132, 3–10. [Google Scholar] [CrossRef]
- Weber-Scannell, P.K.; Duffy, L.K.; Weber-Scannell, P.K.; Duffy, L.K. Effects of total dissolved solids on aquatic organisms: A review of literature and recommendation for salmonid species. Am. J. Environ. Sci. 2007, 3, 1–6. [Google Scholar]
- Liu, C.; Zhu, L.; Chen, L. Effect of salt and metal accumulation on performance of membrane distillation system and microbial community succession in membrane biofilms. Water Res. 2020, 177, 115805. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; Sanmartino, J.A.; Khayet, M.; García-Payo, C.; Chaouch, M. Fouling in membrane distillation, osmotic distillation and osmotic membrane distillation. Appl. Sci. 2017, 7, 334. [Google Scholar] [CrossRef]
- Al-Ahmad, M.; Aleem, F.A. Scale formation and fouling problems effect on the performance of MSF and RO desalination plants in Saudi Arabia. Desalination 1993, 93, 287–310. [Google Scholar] [CrossRef]
- Gadhi, T.; Mahar, R.; Bukhari, S. Wastewater Treatment and Reuse to Approach Zero Water Discharge in Al-Rahim Textile Industries: Substantial Increase in Water Use Efficiency in Textile Processing; US-Pakistan Center for Advanced Studies in Water, MUET: Jamshoro, Pakistan, 2019. [Google Scholar]
- Balal, S.A.; Gadhi, T.A. Treatment of RO membranes reject water using distillation technique. In Proceedings of the International Conference on Energy, Water and Environment—ICEWE-2021, Lahore, Pakistan, 31 March 2021; p. 365. [Google Scholar]
- Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Hamdhan, I.N.; Clarke, B.G. Determination of thermal conductivity of coarse and fine sand soils. In Proceedings of the Proceedings World Geothermal Congress, Bali Island, Indonesia, 25–30 April 2010. [Google Scholar]
- van Manen, S.M.; Wallin, E. Ground temperature profiles and thermal rock properties at Wairakei, New Zealand. Renew. Energy 2012, 43, 313–321. [Google Scholar] [CrossRef]
- Ren, S.; Liu, J.; Guo, A.; Zang, W.; Geng, H.; Tao, X.; Du, H. Mechanical properties and thermal conductivity of a temperature resistance hollow glass microspheres/borosilicate glass buoyance material. Mater. Sci. Eng. 2016, 674, 604–614. [Google Scholar] [CrossRef]
- Ismail, A.; El-Shafey, O.; Amr, M.; El-Maghraby, M. Pumice characteristics and their utilization on the synthesis of mesoporous minerals and on the removal of heavy metals. Int. Sch. Res. Not. 2014, 2014, 259379. [Google Scholar] [CrossRef] [PubMed]
- Mahvi, A.H.; Heibati, B.; Mesdaghinia, A.; Yari, A.R. Fluoride adsorption by pumice from aqueous solutions. E-J. Chem. 2012, 9, 1843–1853. [Google Scholar] [CrossRef]
- Guler, U.A.; Sarioglu, M. Removal of tetracycline from wastewater using pumice stone: Equilibrium, kinetic and thermodynamic studies. J. Environ. Health Sci. Eng. 2014, 12, 79. [Google Scholar] [CrossRef]
- Indah, S.; Helard, D.; Primasari, B.; Edwin, T.; Putra, R.H. Modification of natural pumice by physical and chemical treatments for removal of zinc ions from aqueous solution. MATEC Web Conf. 2019, 276, 06009. [Google Scholar] [CrossRef]
- Klug, C.; Cashman, K.; Bacon, C. Structure and physical characteristics of pumice from the climactic eruption of Mount Mazama (Crater Lake), Oregon. Bull. Volcanol. 2002, 64, 486–501. [Google Scholar]
- Fu, W.; Yu, M.; Yang, M.; Yu, H.; Yang, Y.; Qian, S.; Dong, X.; Xia, L. Preparation of Pumice Stone Morphology AgAlO2@ ZnO S-Type Heterojunction Photocatalyst with the Synergistic Effect of Photocatalytic Degradation and Hydrogen Production. Cryst. Growth Des. 2023, 23, 2331–2342. [Google Scholar] [CrossRef]
- Latif, A.; Memon, A.M.; Gadhi, T.A.; Bhurt, I.A.; Channa, N.; Mahar, R.B.; Ali, I.; Chiadò, A.; Bonelli, B. Bi2O3 immobilized 3D structured clay filters for solar photocatalytic treatment of wastewater from batch to scaleup reactors. Mater. Chem. Phys. 2022, 276, 125297. [Google Scholar] [CrossRef]
- Gadhi, T.A.; Bhurt, I.A.; Qureshi, T.A.; Ali, I.; Latif, A.; Mahar, R.B.; Channa, N.; Bonelli, B. Photocatalytic Denitrification of Nitrate Using Fe-TiO2-Coated Clay Filters. Catalysts 2023, 13, 729. [Google Scholar] [CrossRef]
- Patel, H. Review on solvent desorption study from exhausted adsorbent. J. Saudi Chem. Soc. 2021, 25, 101302. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]






| Parameter | Unit | ROR | Condensed Distillate with Sand Adsorbent (10 g) | Condensed Distillate with Pumice Adsorbent (10 g) | Condensed Distillate without Adsorbent |
|---|---|---|---|---|---|
| Avg. distillation time | 4.7 h | 2.9 h | 2.75 h | ||
| pH | ----- | 8.12 | 7.1 | 7.3 | 7.5 |
| EC | µS/cm | 8532 | 41 | 29 | 123 |
| TDS | mg/L | 5500 | 19 | 11 | 63 |
| Turbidity | NTU | 0.31 | 0 | 0 | 0 |
| Sodium | mg/L | 3112 | 7.5 | 5.4 | 12.5 |
| Calcium | mg/L | 912 | 5 | 2.1 | 6.8 |
| Magnesium | mg/L | 179 | 4.2 | 1.9 | 5.4 |
| Chloride | mg/L | 91 | 5.1 | 3.4 | 7.5 |
| Elements | Pristine Pumice % | Pumice after Five Cycles of CAD % | Pumice after Backwashing % |
|---|---|---|---|
| Oxygen | 57.92 | 54.13 | 57.21 |
| Silicon | 29.57 | 13.09 | 20.67 |
| Sodium | 2.24 | 4.02 | 1.68 |
| Aluminum | 5.43 | 1.91 | 2.80 |
| Potassium | 2.97 | 1.02 | 1.28 |
| Calcium | 1.46 | 11.39 | 10.38 |
| Magnesium | 0.41 | 2.74 | 1.63 |
| Sulfur | 0 | 4.98 | 2.27 |
| Chlorine | 0 | 3.85 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, T.A.; Gadhi, T.A.; Khokhar, D.A.; Ali, I.; Memon, N.; Channa, N.; Bakhat, S.; Rafique, T.; Mahar, R.B. Insight on the Properties of Pumice Mineral for the Combined Adsorption Distillation of Membrane Reject Water. Minerals 2023, 13, 1131. https://doi.org/10.3390/min13091131
Qureshi TA, Gadhi TA, Khokhar DA, Ali I, Memon N, Channa N, Bakhat S, Rafique T, Mahar RB. Insight on the Properties of Pumice Mineral for the Combined Adsorption Distillation of Membrane Reject Water. Minerals. 2023; 13(9):1131. https://doi.org/10.3390/min13091131
Chicago/Turabian StyleQureshi, Tayyab A., Tanveer A. Gadhi, Danish A. Khokhar, Imran Ali, Najma Memon, Najeebullah Channa, Sikandar Bakhat, Tahir Rafique, and Rasool Bux Mahar. 2023. "Insight on the Properties of Pumice Mineral for the Combined Adsorption Distillation of Membrane Reject Water" Minerals 13, no. 9: 1131. https://doi.org/10.3390/min13091131
APA StyleQureshi, T. A., Gadhi, T. A., Khokhar, D. A., Ali, I., Memon, N., Channa, N., Bakhat, S., Rafique, T., & Mahar, R. B. (2023). Insight on the Properties of Pumice Mineral for the Combined Adsorption Distillation of Membrane Reject Water. Minerals, 13(9), 1131. https://doi.org/10.3390/min13091131






