Occurrence Mode of Sodium in Zhundong Coal, China: Relationship to Maceral Groups
Abstract
:1. Introduction
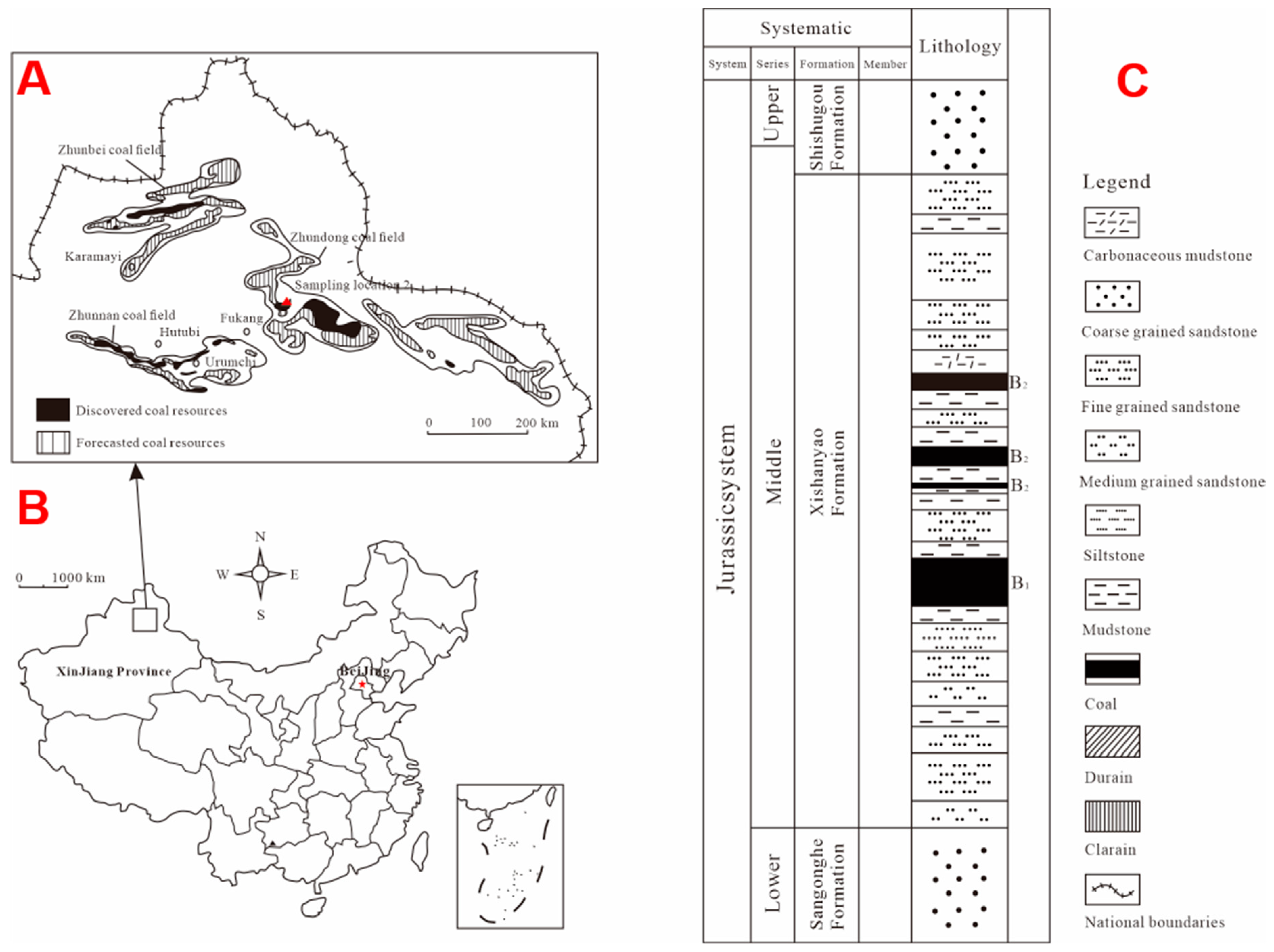
2. Geological Setting
3. Experimental Section
3.1. Sample Preparation
3.2. Sequential Extraction
3.3. Sample Characterization
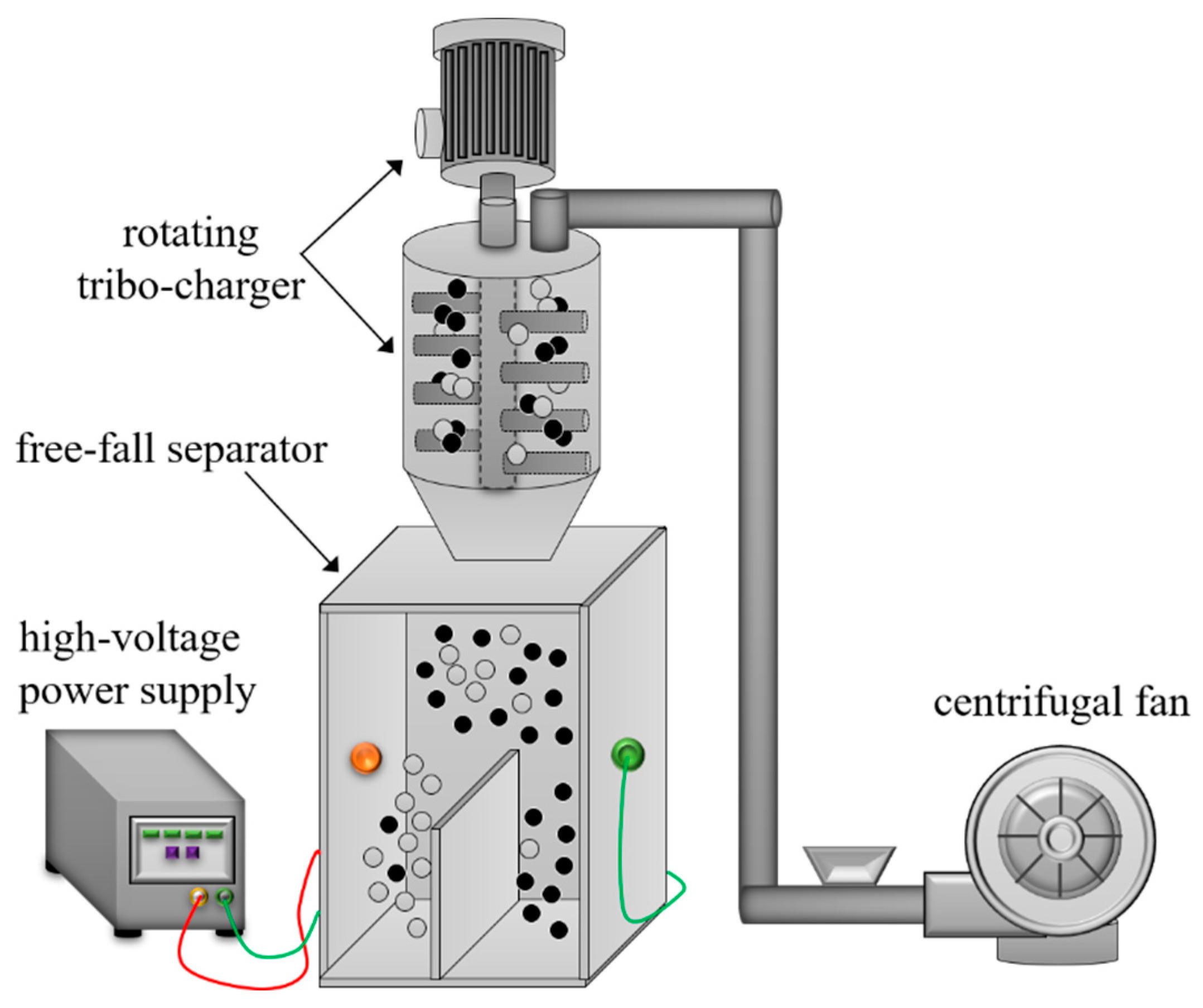
3.4. Triboelectrostatic Separation of Zhundong Coal
4. Results and Discussion
4.1. Assessment of Coal Quality
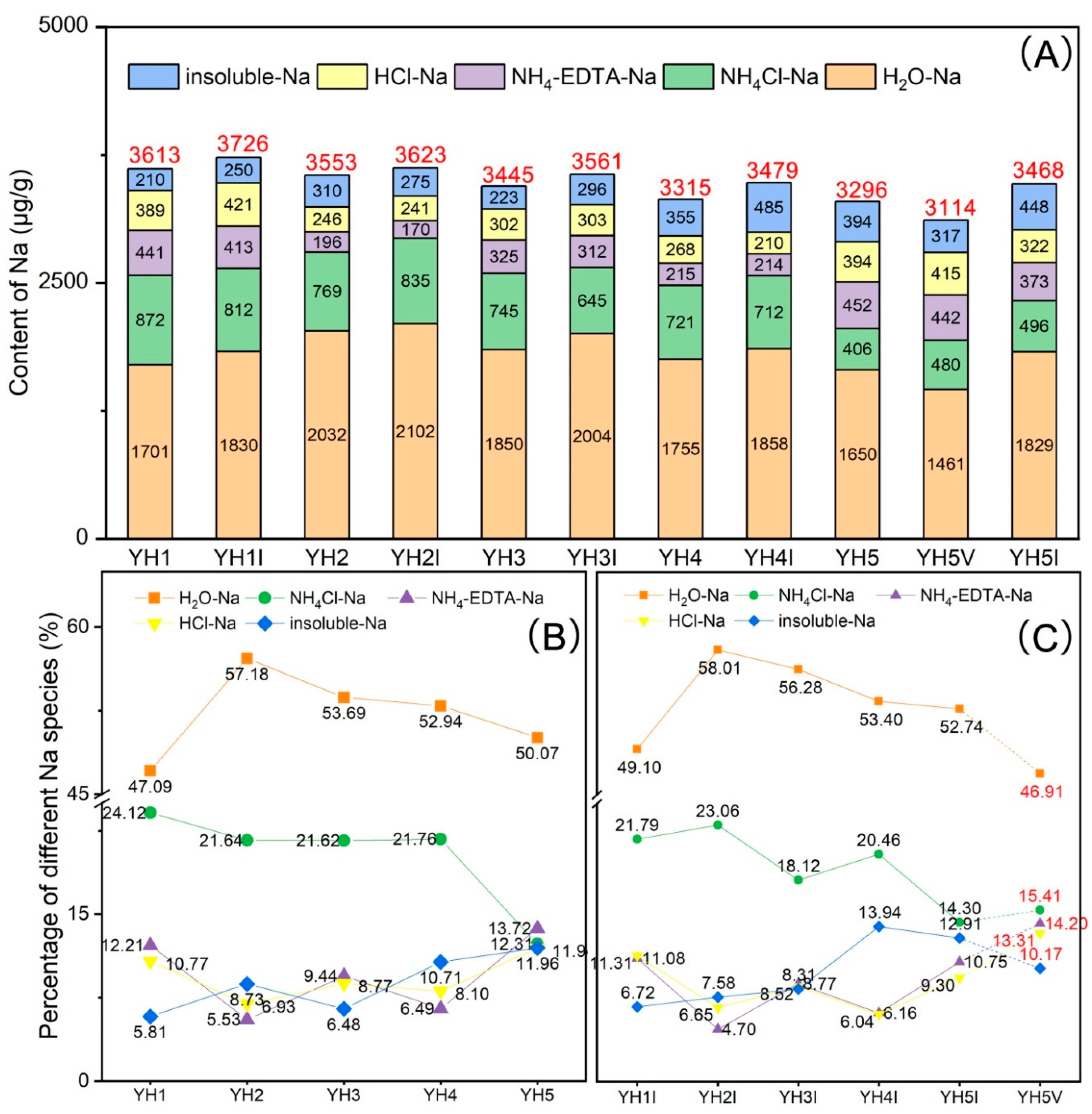
4.2. Sequential Extraction Results for Sodium
4.3. Sodium Occurrence Characteristics in Macerals
4.3.1. Sodium Occurrence Characteristics of YH1 and YH1I
4.3.2. Sodium Occurrence Characteristics of YH2 and YH2I
4.3.3. Sodium Occurrence Characteristics of YH3 and YH3I
4.3.4. Sodium Occurrence Characteristics of YH4 and YH4I
4.3.5. Sodium Occurrence Characteristics of YH5, YH5V, and YH5I
4.3.6. Sodium Occurrence Relationship with Maceral Groups
4.4. Triboelectrostatic Separation
5. Conclusions
- The total Na content of the five raw coals decreases with increasing depth, falling from 3613 μg/g in YH1 to 3296 μg/g in YH5. All raw coals and corresponding maceral-enriched samples share a similar content order of five Na occurrence modes: H2O-Na (~50%) and NH4Cl-Na (~20%) occupy the first and second positions, respectively, while NH4-EDTA-Na, HCl-Na, and insoluble Na share the rest, with no significant differences. The total Na levels in the inertinite-enriched samples are significantly higher than those in their corresponding raw coal and vitrinite-enriched sample. In addition, H2O-Na and insoluble Na show a clear enrichment trend in the inertinite-enriched samples, while NH4-EDTA-Na and HCl-Na are slightly more concentrated in the vitrinite-enriched sample.
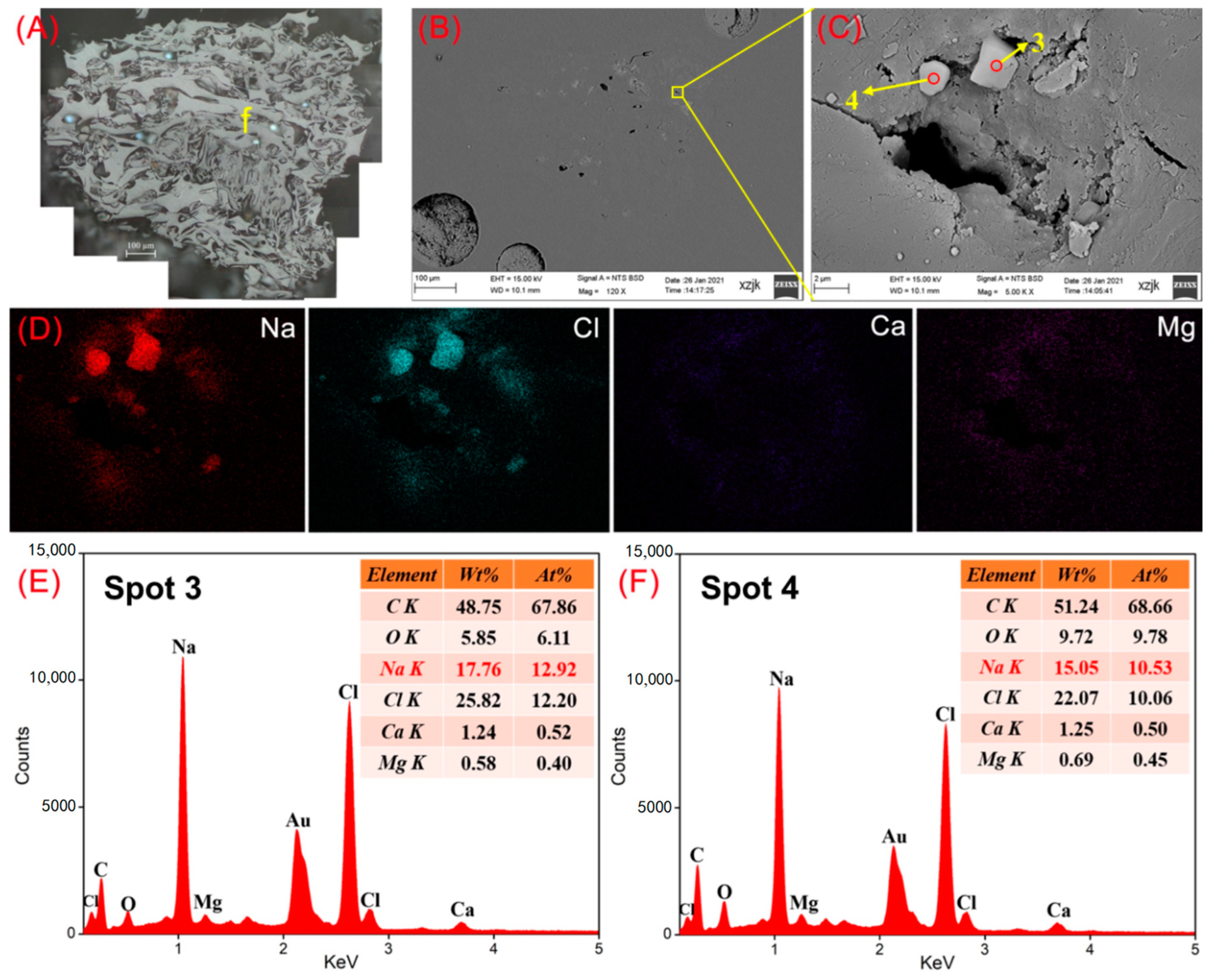
- Na was found in all the raw coal and inertinite-enriched samples except for the vitrinite-enriched one. Na was detected as H2O-Na (NaCl) and insoluble Na (associated with cell-filling minerals) in the raw coals with under 5 wt%, whereas the Na enrichments in inertinite-enriched samples mostly reached values of over 10 wt% (highest in YH4I with 18 wt%) with the uniform H2O-Na mode as NaCl. In all of the above cases, sodium was found in the cells of fusinite or semifusinite, indicating a clear positive occurrence correlation between Na-enriched minerals and the inertinite group, especially with fusinite.
- Maceral separation and Na removal were simultaneously achieved after the triboelectrostatic separation of Zhundong raw coals. Inertinite tends to be enriched in the tailings, whereas vitrinite enrichment occurs in the concentrates. The total Na level of the products rises with the inertinite content, which results in an obvious difference in the Na contents between the tailings and concentrates (up to ~1000 μg/g in YH1). In addition, the possible mixing of minerals in the tailings also contributes to the rise in Na levels.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.B.; Zhu, M.M.; Zhang, Z.Z.; Zhang, K.; Shen, G.Q.; Zhang, D.K. The mineralogy, morphology and sintering characteristics of ash deposits on a probe at different temperatures during combustion of blends of Zhundong lignite and a bituminous coal in a drop tube furnace. Fuel Process. Technol. 2016, 149, 176–186. [Google Scholar] [CrossRef]
- Yang, Y.M.; Wu, Y.X.; Zhang, H.; Zhang, M.; Liu, Q.; Yang, H.R.; Lu, J.F. Improved sequential extraction method for determination of alkali and alkaline earth metals in Zhundong coals. Fuel 2016, 181, 951–957. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.G.; Querol, X.; Font, O.; Moreno, N.; Zhou, J.B.; Lei, G.M. High quality of Jurassic coals in the southern and eastern Junggar coalfields, Xinjiang, NW China: Geochemical and mineralogical characteristics. Int. J. Coal Geol. 2012, 99, 1–15. [Google Scholar] [CrossRef]
- Ge, H.J.; Shen, L.H.; Gu, H.M.; Song, T.; Jiang, S.X. Combustion performance and sodium absorption of ZhunDong coal in a CLC process with hematite oxygen carrier. Appl. Eng. 2016, 94, 40–49. [Google Scholar] [CrossRef]
- Zhu, C.; Qu, S.J.; Zhang, J.; Wang, Y.; Zhang, Y.H. Distribution, occurrence and leaching dynamic behavior of sodium in Zhundong coal. Fuel 2017, 190, 189–197. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Zhang, H.X.; Lyu, Q.G.; Zhu, Z.P. Investigation of slagging characteristics and anti-slagging applications for Indonesian coal gasification. Fuel 2020, 267, 117285. [Google Scholar] [CrossRef]
- Liu, D.B.; Li, W.; Li, S.Y.; Song, W.H.; Liu, D.F.; Kong, R.J. Transformation characteristics of sodium, chlorine and sulfur of Zhundong coal during O2/CO2 combustion in circulating fluidized bed. Energy 2019, 185, 254–261. [Google Scholar] [CrossRef]
- Dai, S.F.; Liu, J.; Ward, C.R.; Hower, J.C.; Xie, P.; Jiang, Y.; Hood, M.M.; O’Keefe, J.M.K.; Song, H. Petrological, geochemical, and mineralogical compositions of the low-Ge coals from the Shengli Coalfield, China: A comparative study with Ge-rich coals and a formation model for coal-hosted Ge ore deposit. Ore Geol. Rev. 2015, 71, 318–349. [Google Scholar] [CrossRef]
- Li, X.; Bai, Z.Q.; Bai, J.; Han, Y.N.; Kong, L.X.; Li, W. Transformations and roles of sodium species with different occurrence modes in direct liquefaction of Zhundong coal from Xinjiang, Northwestern China. Energy Fuels 2015, 29, 5633–5639. [Google Scholar] [CrossRef]
- Liang, D.C.; Xie, Q.; Zhou, H.B.; Yang, M.S.; Cao, J.Y.; Zhang, J. Catalytic effect of alkali and alkaline earth metals in different occurrence modes in Zhundong coals. Asia-Pac. J. Chem. Eng. 2018, 13. [Google Scholar] [CrossRef]
- Feng, Z.H.; Pang, K.L.; Bai, Z.Q.; Hou, R.R.; Ye, D.H.; Guo, Z.X.; Kong, L.X.; Bai, J.; Li, W. Occurrence and transformation of sodium and calcium species in mild liquefaction solid product of hami coal during pyrolysis. Fuel 2021, 286, 119489. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.B.; Wang, Q.H.; Lu, X.F.; Zhang, Y.Y.; Zhu, M.M.; Zhang, Z.Z.; Zhang, D.K. An experimental investigation into mineral transformation, particle agglomeration and ash deposition during combustion of Zhundong lignite in a laboratory-scale circulating fluidized bed. Fuel 2019, 243, 458–468. [Google Scholar] [CrossRef]
- Song, G.L.; Yang, S.B.; Song, W.J.; Qi, X.B. Release and transformation behaviors of sodium during combustion of high alkali residual carbon. Appl. Eng 2017, 122, 285–296. [Google Scholar] [CrossRef]
- Qi, X.B.; Song, G.L.; Yang, S.B.; Yang, Z.; Lyu, Q.G. Migration and transformation of sodium and chlorine in high-sodium high-chlorine xinjiang lignite during circulating fluidized bed combustion. J. Energy Inst. 2018, 92, 673–681. [Google Scholar] [CrossRef]
- Song, G.L.; Qi, X.B.; Song, W.J.; Yang, S.B. Slagging and fouling of Zhundong coal at different air equivalence ratios in circulating fluidized bed. Fuel 2017, 205, 46–59. [Google Scholar] [CrossRef]
- Tang, C.W.; Pan, W.G.; Zhang, J.K.; Wang, W.H.; Sun, X.L. A comprehensive review on efficient utilization methods of High-alkali coals combustion in boilers. Fuel 2022, 316, 123269. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Zhu, J.W.; Sun, W.; Xu, Y.N.; Wu, S.H. Co-combustion and ash characteristics of Zhundong coal with rice husk hydrochar prepared by the hydrothermal carbonization technology for co-combustion. IET Renew. Power Gener. 2022, 16, 329–338. [Google Scholar] [CrossRef]
- Zhao, P.T.; Huang, N.; Li, J.W.; Cui, X. Fate of sodium and chlorine during the co-hydrothermal carbonization of high-alkali coal and polyvinyl chloride. Fuel Process. Technol. 2020, 199, 106277. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.L.; Wu, G.G.; Bai, Z.Q.; Li, W. Characterization of the Molecular Structural Changes Following Ion-Exchange Treatment of Zhundong Coal. Anal. Lett. 2018, 51, 2530–2540. [Google Scholar] [CrossRef]
- Ding, L.Z.; Gao, Y.X.; Li, X.; Wang, W.H.; Xue, Y.; Zhu, X.Q.; Xu, K.; Hu, H.Y.; Luo, G.Q.; Naruse, I.; et al. A novel CO2-water leaching method for AAEM removal from Zhundong coal. Fuel 2018, 237, 786–792. [Google Scholar] [CrossRef]
- Yang, Y.P.; Lin, X.C.; Chen, X.J.; Wang, Y.G.; Gao, L.; Chen, L.J. The formation of deposits and their evolutionary characteristics during pressurized gasification of Zhundong coal char. Fuel 2018, 224, 469–480. [Google Scholar] [CrossRef]
- Li, X.; Bai, Z.Q.; Li, W. Chemical transformation of sodium species during direct liquefaction of a sodium-rich Zhundong coal under different atmospheres and CO2 gasification of the direct coal liquefaction residue. Fuel 2018, 213, 144–149. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zong, Z.M.; Cao, J.P.; Ma, Y.M.; Han, L.; Liu, G.F.; Zhao, W.; Li, W.Y.; Xie, K.C.; Bai, X.F.; et al. Difference in chemical composition of carbon disulfide-extractable fraction between vitrinite and inertinite from Shenfu-Dongsheng and Pingshuo coals. Fuel 2008, 87, 565–575. [Google Scholar] [CrossRef]
- Singh, P.K. Petrological and geochemical considerations to predict oil potential of Rajpardi and Vastan Lignite Deposits of Gujarat, Western India. J. Geol. Soc. India 2012, 80, 759–770. [Google Scholar] [CrossRef]
- Lin, X.C.; Luo, M.; Li, S.Y.; Yang, Y.P.; Chen, X.J.; Tian, B.; Wang, Y.G. The evolutionary route of coal matrix during integrated cascade pyrolysis of a typical low-rank coal. Appl. Energy 2017, 199, 335–346. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.J.; Jin, J.; Li, T.Y.; Chen, Y.Y.; Ma, S.L. The Jurassic oil-source correlation of Dishuiquan Oilfield in Junggar basin, Northwest China. Pet. Sci. Technol. 2020, 38, 354–360. [Google Scholar] [CrossRef]
- Guo, H.N.; Shi, H.; Wu, Y.X.; Lyu, J.F.; Zhang, Y. Mineral transformation during rapid heating and cooling of Zhundong coal ash. Fuel 2022, 310, 122269. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Tang, M.T.; Yang, Z.Y.; Ma, J.; Liu, L.N.; Shen, B.X. SO2 and NO emissions during combustion of high-alkali coal over a wide temperature range: Effect of Na species and contents. Fuel 2022, 309, 122212. [Google Scholar] [CrossRef]
- Zhou, J.B.; Zhuang, X.G.; Alastuey, A.; Querol, X.; Li, J.H. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China. Int. J. Coal Geol. 2012, 82, 51–67. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, J.; Yi, X.; Zhao, L.; Song, Z.; Li, B. The braided river dalta-shallow lake depositional system of middle Jurassic Xishanyao formation in the Xiheishan coal district, eastern Junggar coal field, Xinjiang. Inn. Mong. Petrochem. 2009, 9, 105–108. [Google Scholar]
- He, X.; Zhang, X.X.; Jiao, Y.; Zhu, J.S.; Chen, X.W.; Li, C.Y.; Li, H.S. Complementary analyses of infrared transmission and diffuse reflection spectra of macerals in low-rank coal and application in triboelectrostatic enrichment of active maceral. Fuel 2017, 192, 93–101. [Google Scholar] [CrossRef]
- He, X.; Sun, H.; Chen, X.W.; Zhao, B.; Zhang, X.X.; Komarneni, S. Charging mechanism analysis of macerals during triboelectrostatic enrichment process: Insights from relative dielectric constant, specific resistivity and X-ray diffraction. Fuel 2018, 225, 533–541. [Google Scholar] [CrossRef]
- He, X.; Sun, H.; Zhao, B.; Chen, X.W.; Zhang, X.X.; Komarneni, S. Tribocharging of macerals with various materials: Role of surface oxygen-containing groups and potential difference of macerals. Fuel 2018, 233, 750–768. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhuang, X.G.; Zhou, J.B.; Zeng, X.J.; Amina.; Ge, D.F.; Li, X.; Yang, S. Coal quality and its distribution of the eastern Junggar coalfield in Junggar basin, Xinjiang. Xinjiang Geol. 2013, 31, 89–93. [Google Scholar]
- Benson, S.A.; Holm, P.L. Comparison of inorganics in three low-rank coals. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 145–149. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, T.; Guo, X.Y.; Tian, Q.H.; Zhong, S.P.; Dong, L.; Qin, H.; Liu, Z.W.; Makuza, B. Sustainable processing of gold cyanide tailings: Reduction roasting, mechanical activation, non-cyanide leaching, and magnetic separation. Hydrometallurgy 2023, 217, 106028. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.L.; Yan, Z.; Liu, K.L.; Xu, Y.Q.; Sheng, C.D.; Pan, W.P. The varying characterization of alkali metals (Na, K) from coal during the initial stage of coal combustion. Energy Fuels 2001, 15, 786–793. [Google Scholar] [CrossRef]
- Dai, S.F.; Li, D.H.; Ren, D.Y.; Tang, Y.G.; Shao, L.Y.; Song, H.B. Geochemistry of the late Permian No. 30 coal seam, Zhijin Coalfield of Southwest China: Influence of a siliceous low temperature hydrothermal fluid. Appl. Geochem. 2004, 19, 1315–1330. [Google Scholar] [CrossRef]
- Oskay, R.G.; Christanis, K.; Inaner, H.; Salman, M.; Taka, M. Palaeoenvironmental reconstruction of the eastern part of the Karapinar-Ayranci coal deposit (Central Turkey). Int. J. Coal Geol. 2016, 163, 100–111. [Google Scholar] [CrossRef]
- Dai, S.F.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F.H. Modes of occurrence of elements in coal: A critical evaluation. Earth-Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Li, Z.; Ward, C.R.; Gurba, L.W. Occurrence of non-mineral inorganic elements in macerals of low-rank coals. Int. J. Coal Geol. 2010, 81, 242–250. [Google Scholar] [CrossRef]
- Grigore, M.; Sakurovs, R. Inorganic matter in victorian brown coals. Int. J. Coal Geol. 2016, 154–155, 257–264. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Matsuoka, K.; Rosyadi, E.; Tomita, A. Mode of occurrence of calcium in various coals. Fuel 2002, 81, 1433–1438. [Google Scholar] [CrossRef]
- Wijaya, N.; Choo, T.K.; Zhang, L. Generation of ultra-clean coal from Victorian brown coal—sequential and single leaching at room temperature to elucidate the elution of individual inorganic elements. Fuel Process. Technol. 2011, 92, 2127–2137. [Google Scholar] [CrossRef]
- Wijaya, N.; Choo, T.K.; Zhang, L. Generation of ultra-clean coal from Victorian brown coal: Effect of hydrothermal treatment and particle size on coal demineralization and the extraction kinetic of individual metals. Energy Fuel 2012, 26, 5028–5035. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- He, X.; Wang, W.F.; Yang, Y.T.; Zhou, C.C.; He, J.F.; Duan, P.P.; Lu, Q.F. Occurrence relationship between sodium and maceral groups in subbituminous coal: A case study on Zhundong coal and Shenfu coal. Minerals 2023, 13, 122. [Google Scholar] [CrossRef]
- ICCP. The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- ICCP. The new inertinite classification (ICCP System 1994). Fuel 2001, 80, 459–471. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D. Classification of liptinite-ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- Sykorova, I.; Pickel, W.; Christanis, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite-ICCP System 1994. Int. J. Coal Geol. 2005, 62, 85–106. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An up dated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Xie, W.; Stanger, R.; Wall, T.F.; Lucas, J.A.; Mahoney, M.R. Associations of physical, chemical with thermal changes during coking as coal heats-Experiments on coal maceral concentrates. Fuel 2015, 147, 1–8. [Google Scholar] [CrossRef]


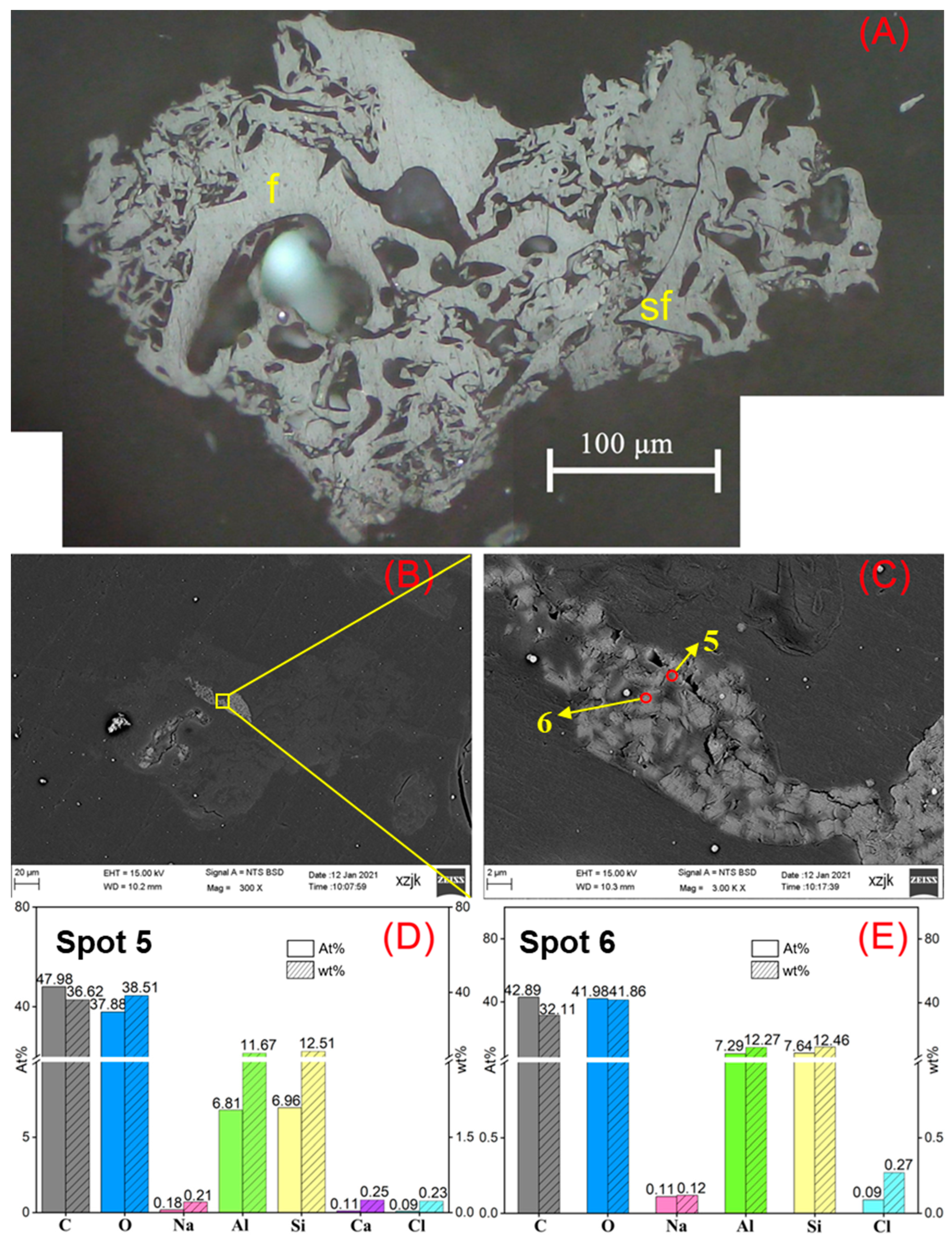
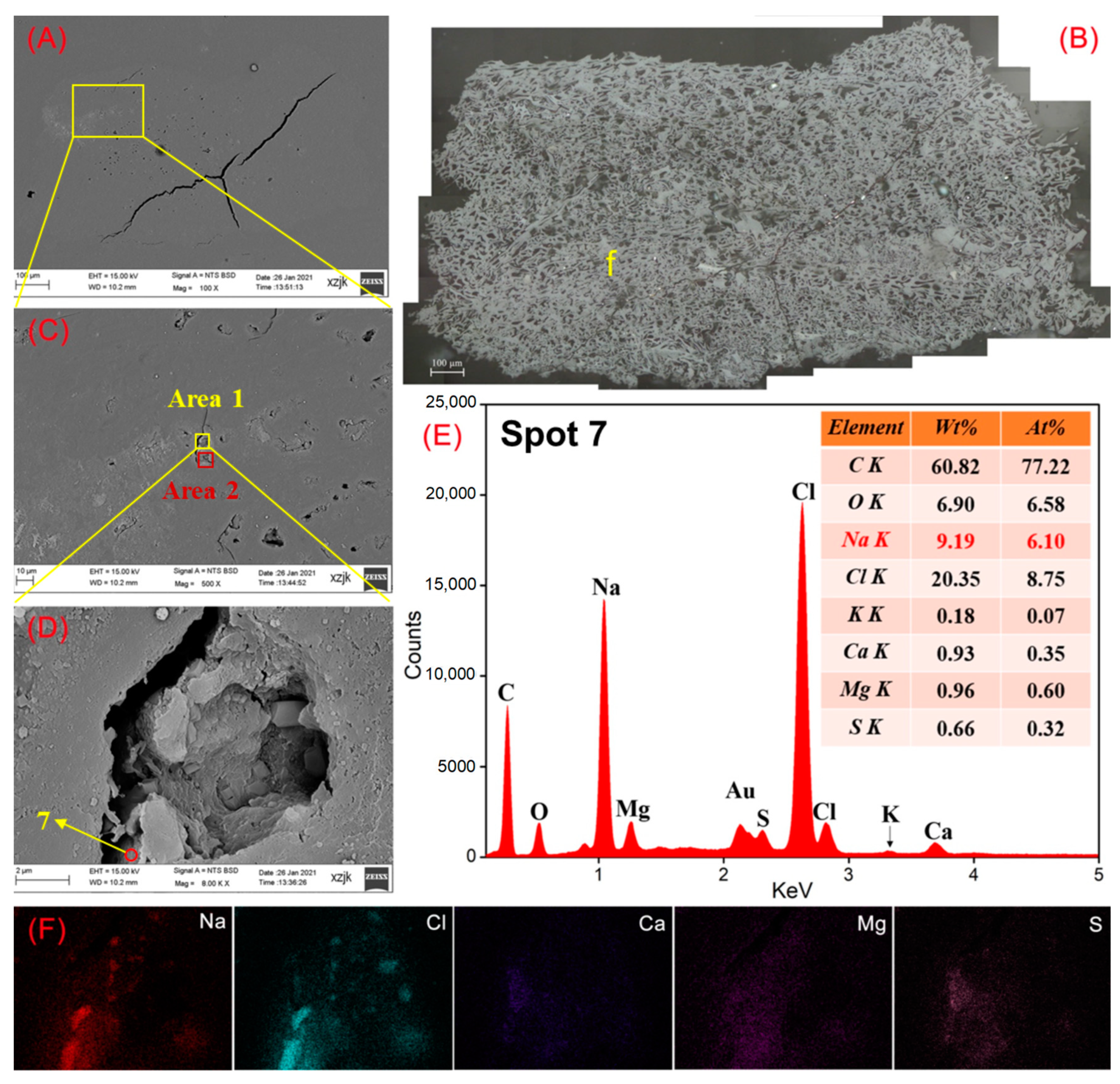
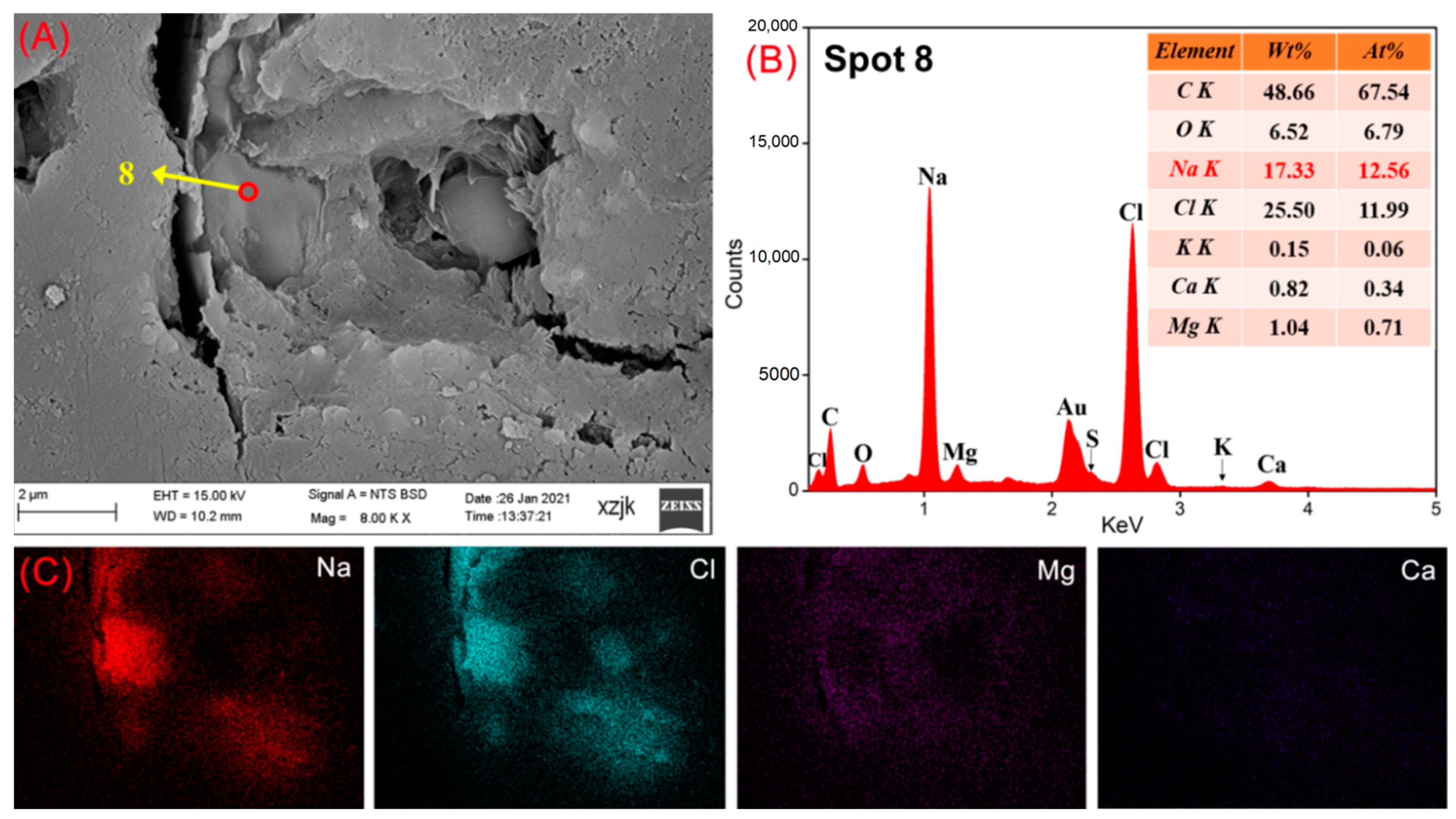
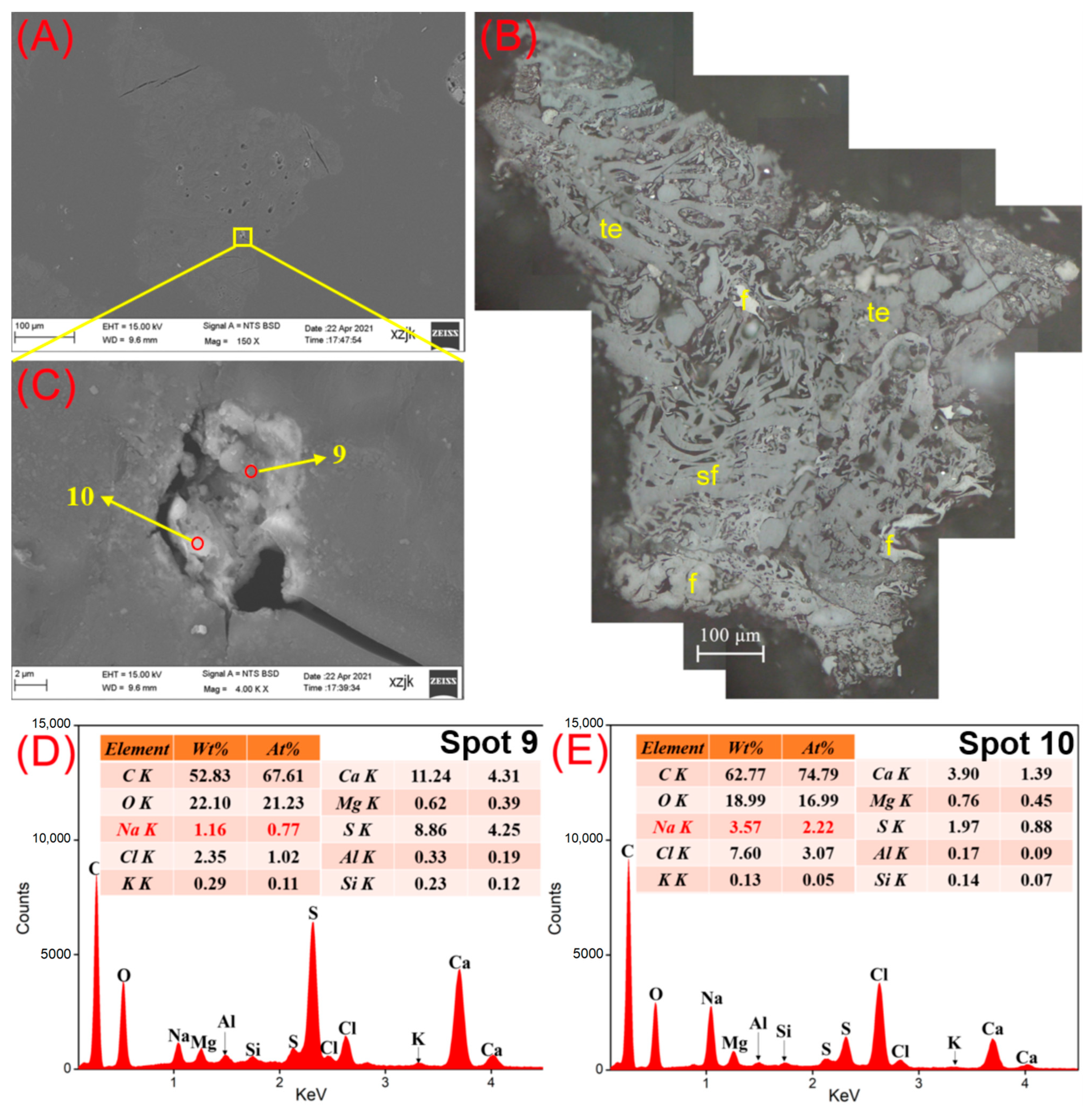
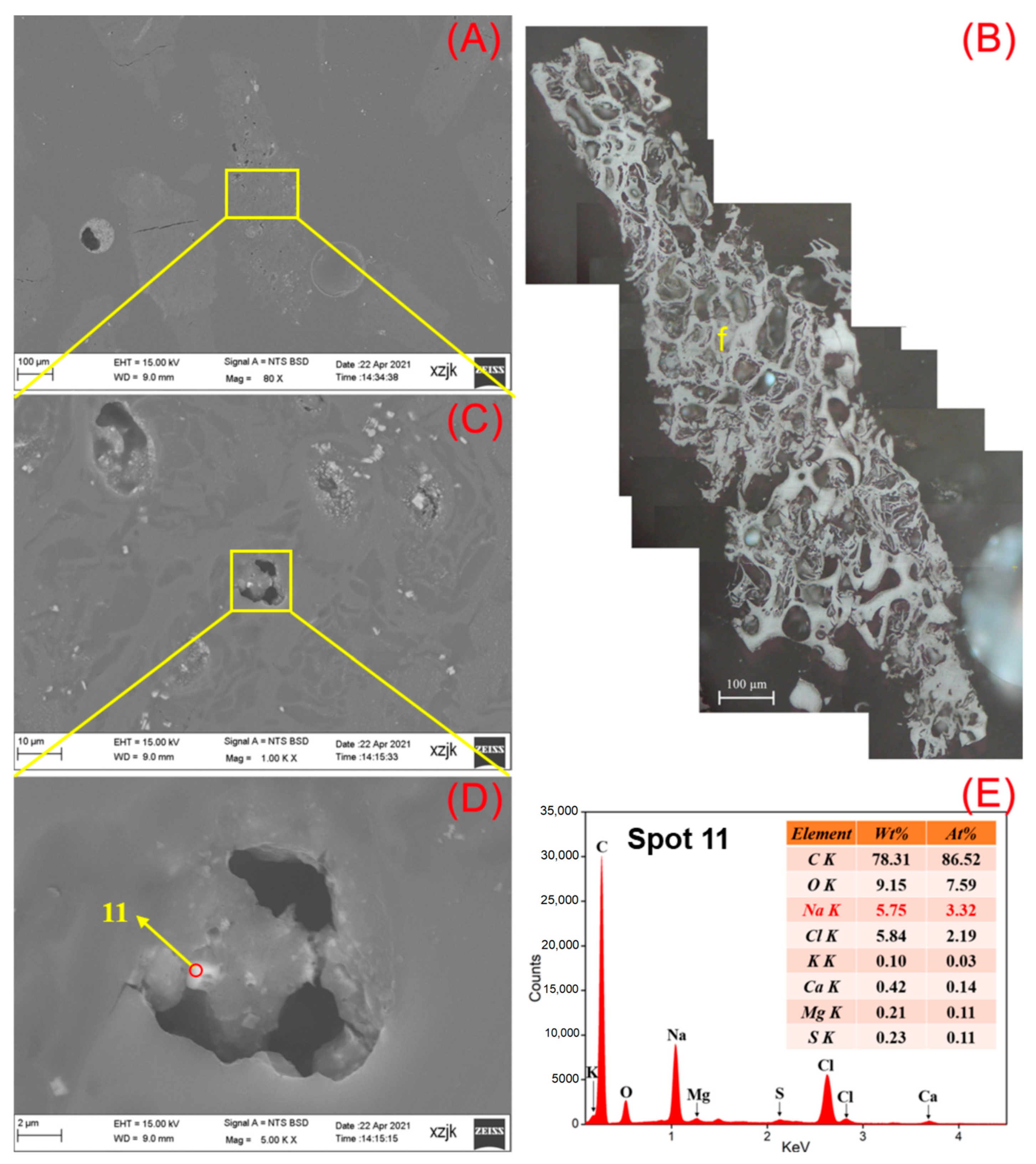
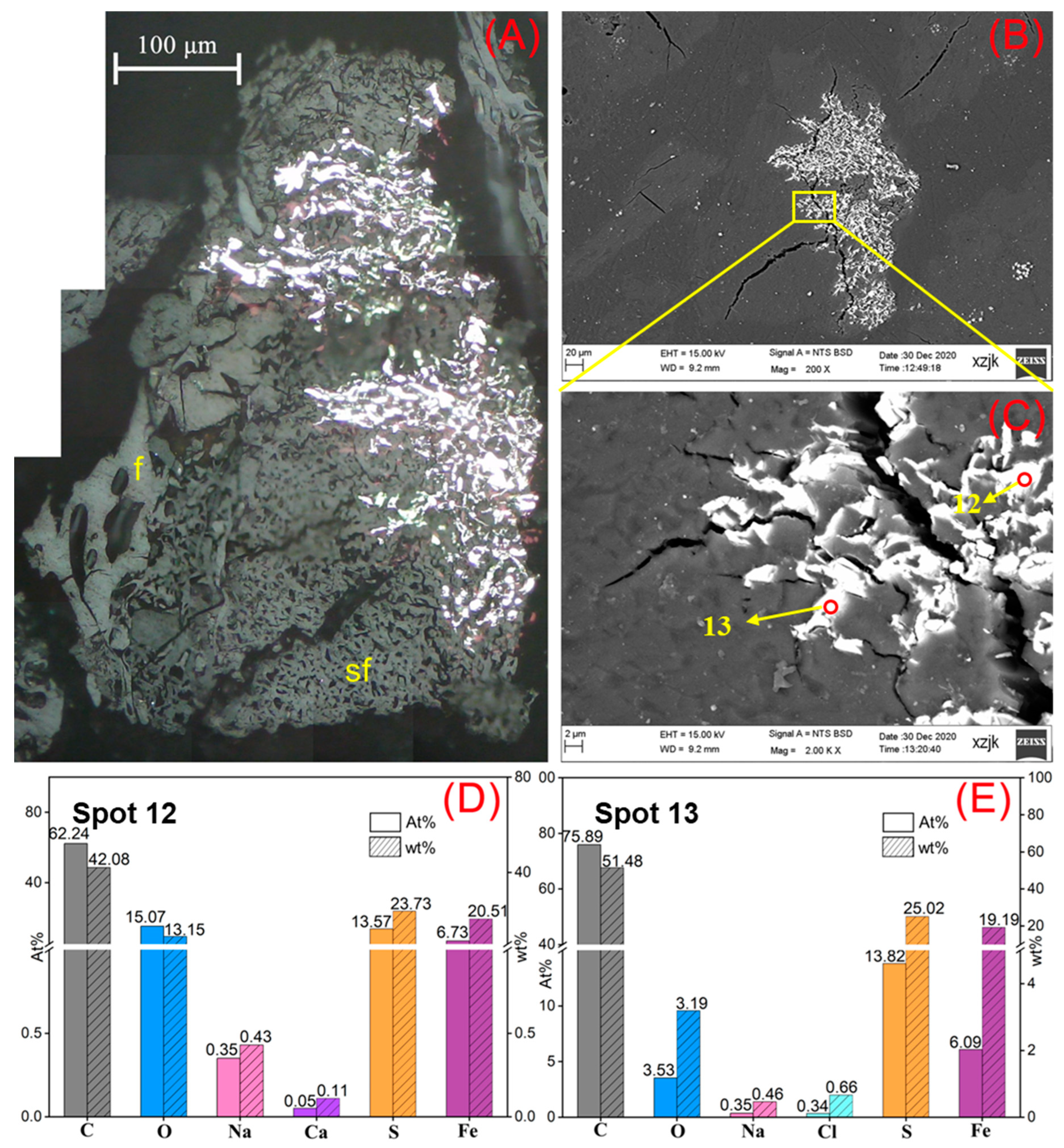
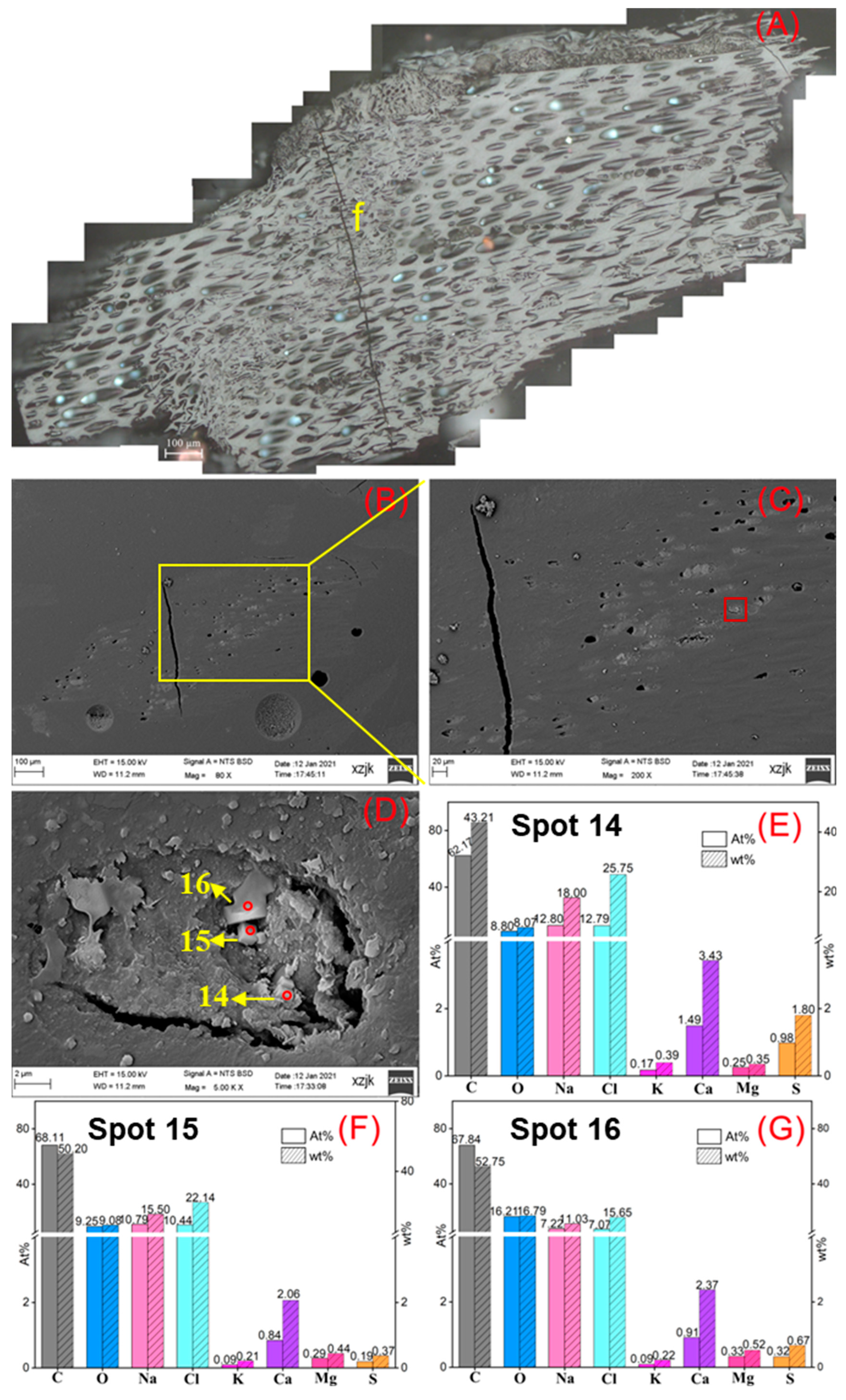
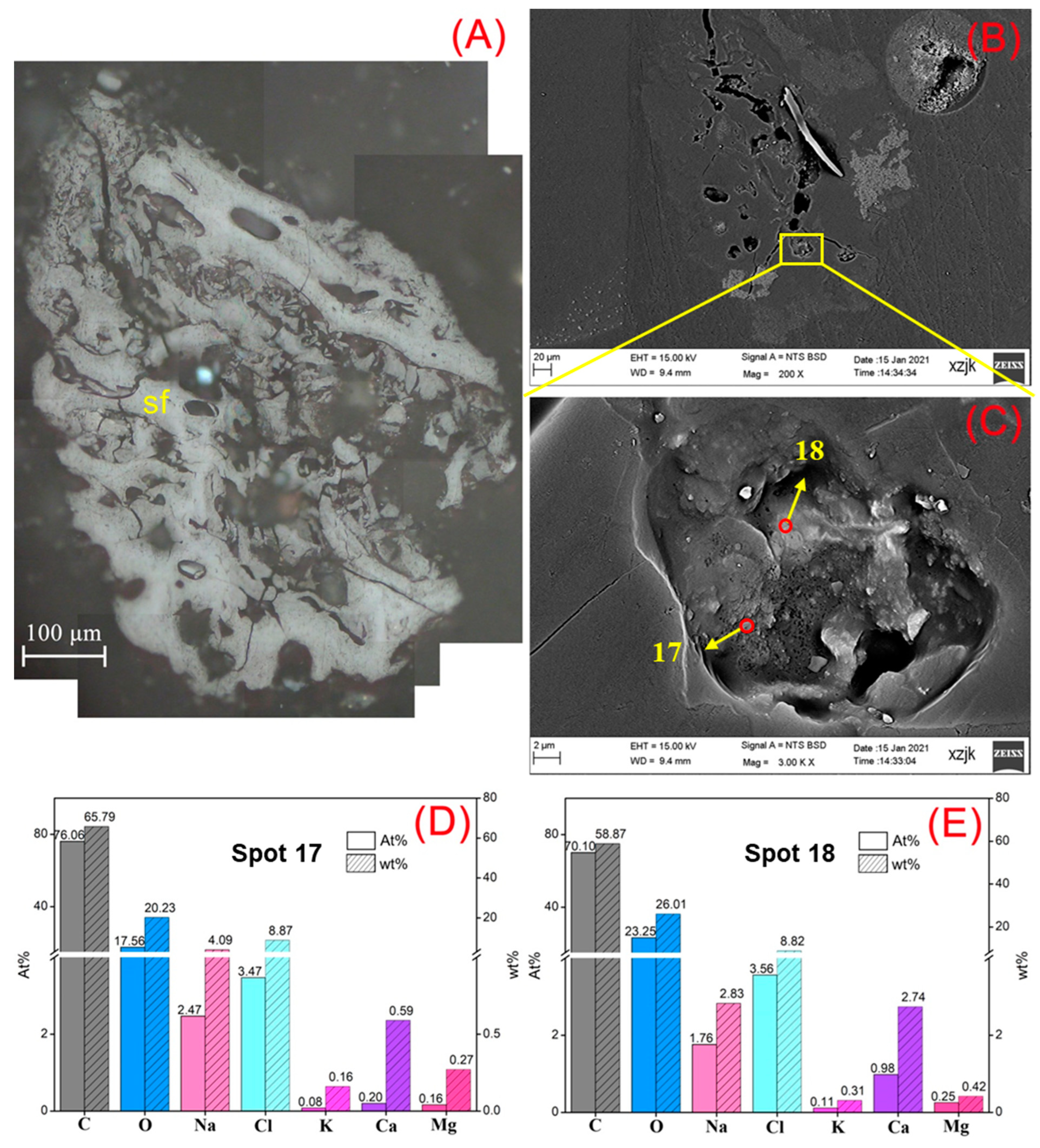
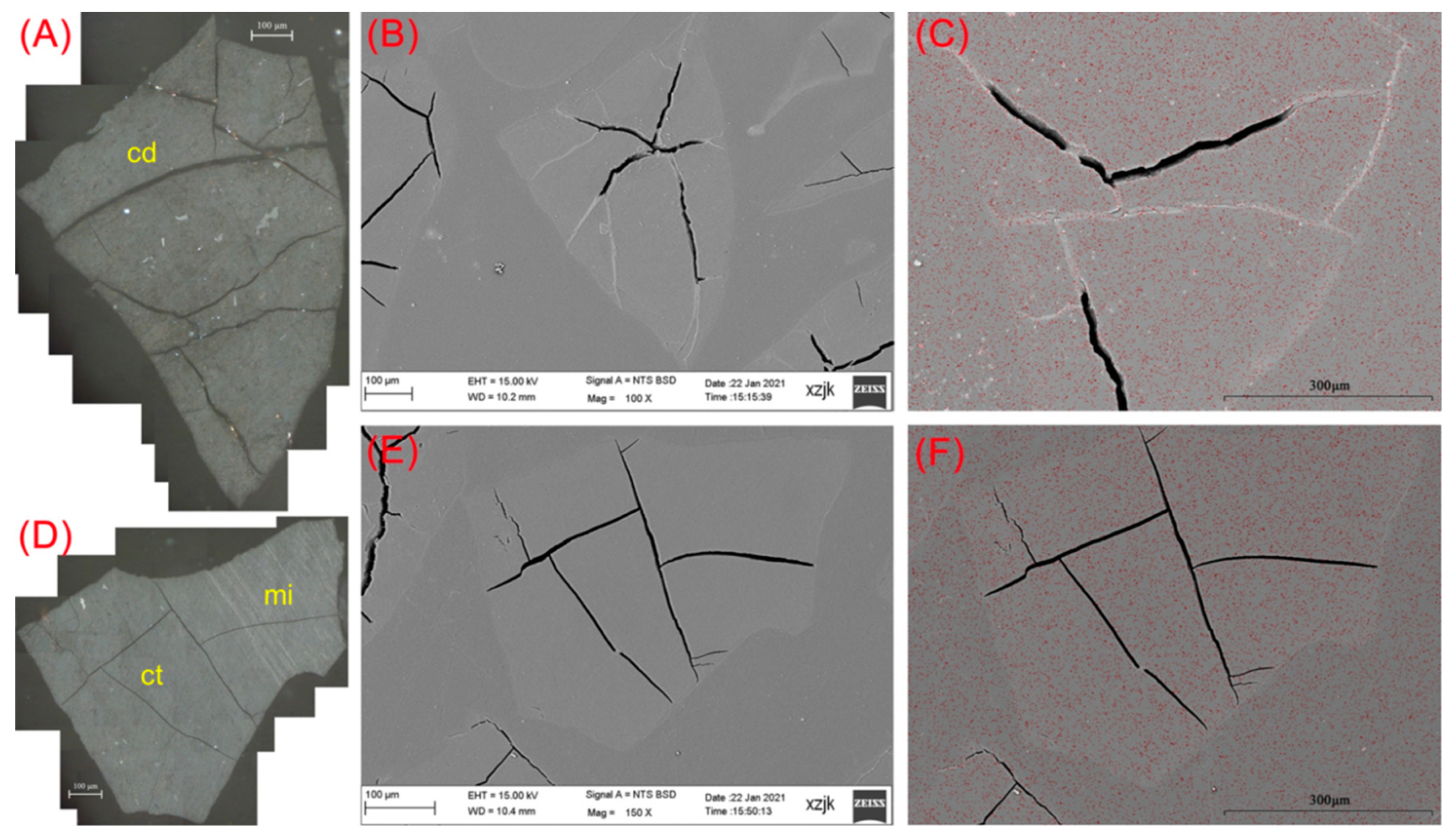
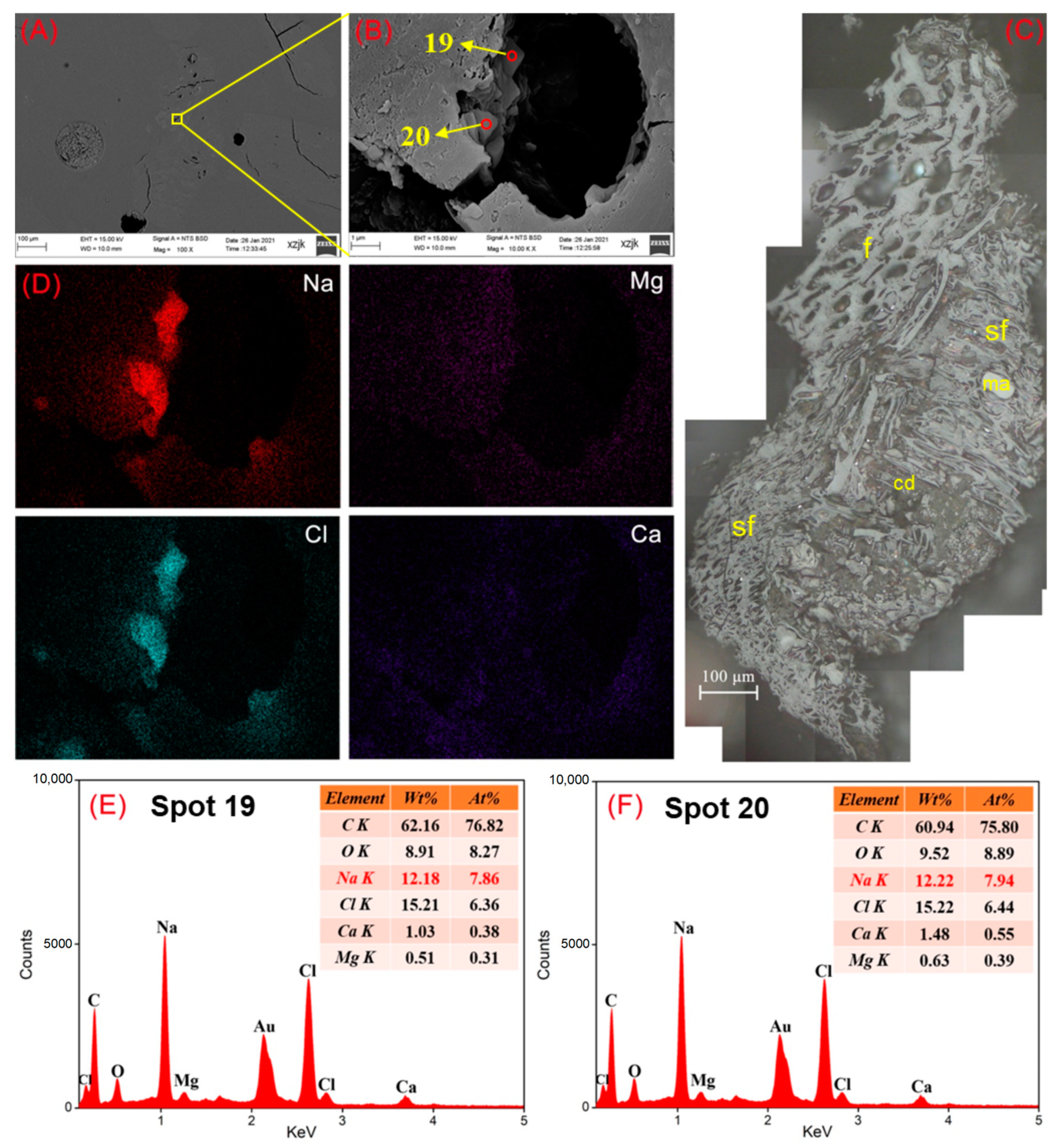
| Samples | YH1 | YH1I | YH2 | YH2I | YH3 | YH3I | YH4 | YH4I | YH5 | YH5V | YH5I |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UA (wt %) | |||||||||||
| Odaf | 18.57 | 17.92 | 17.86 | 17.39 | 16.53 | 16.64 | 16.27 | 15.79 | 17.51 | 20.28 | 16.15 |
| Cdaf | 77.27 | 78.30 | 77.88 | 78.57 | 78.97 | 79.21 | 79.59 | 80.11 | 76.58 | 74.30 | 78.90 |
| Hdaf | 3.19 | 3.07 | 3.17 | 3.30 | 3.37 | 3.25 | 2.99 | 3.33 | 3.71 | 4.45 | 3.91 |
| Ndaf | 0.77 | 0.63 | 0.65 | 0.49 | 0.68 | 0.54 | 0.65 | 0.48 | 0.72 | 0.70 | 0.60 |
| St,d | 0.18 | 0.08 | 0.22 | 0.20 | 0.45 | 0.36 | 0.34 | 0.28 | 0.50 | 0.21 | 0.33 |
| PA (wt %) | |||||||||||
| Mad | 12.32 | 12.64 | 11.10 | 10.90 | 11.90 | 11.14 | 11.12 | 10.94 | 13.31 | 12.35 | 9.86 |
| Ad | 4.30 | 3.16 | 3.46 | 3.01 | 3.64 | 3.26 | 4.38 | 2.75 | 3.39 | 2.48 | 3.04 |
| Vdaf | 30.23 | 30.57 | 30.13 | 30.79 | 30.09 | 29.50 | 29.51 | 28.08 | 37.95 | 43.00 | 35.20 |
| FCad | 66.77 | 67.24 | 67.45 | 67.13 | 67.37 | 68.20 | 67.41 | 69.94 | 59.92 | 55.54 | 62.82 |
| Oxides and Elements | YH1 | YH2 | YH3 | YH4 | YH5 | China a | World b |
|---|---|---|---|---|---|---|---|
| Na2O | 0.41 | 0.34 | 0.42 | 0.46 | 0.27 | 0.16 | nd |
| K2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.19 | nd |
| CaO | 2.36 | 1.90 | 1.86 | 2.32 | 1.74 | 1.23 | nd |
| MgO | 1.05 | 0.83 | 0.78 | 0.63 | 0.46 | 0.22 | nd |
| SiO2 | 0.49 | 0.26 | 0.23 | 0.22 | 0.17 | 8.47 | nd |
| Al2O3 | 0.33 | 0.29 | 0.29 | 0.27 | 0.43 | 5.98 | nd |
| Fe2O3 | 0.18 | 0.10 | 0.03 | 0.11 | 0.15 | 4.85 | nd |
| S | 0.30 | 0.74 | 0.83 | 0.87 | 0.72 | nd | nd |
| Cl | 0.05 | 0.07 | 0.21 | 0.26 | 0.05 | nd | nd |
| REY c | 3.24 | 19.76 | 6.22 | 13.40 | 6.17 | 138.00 | 68.40 |
| Li | 9.26 | 2.29 | 7.26 | 2.24 | 3.93 | 31.80 | 12.00 |
| Nb | 0.09 | 0.10 | 0.22 | 0.11 | 0.20 | 9.44 | 3.70 |
| Ta | 0.01 | 0.04 | 0.03 | 0.05 | 0.02 | 0.62 | 0.28 |
| Zr | 1.85 | 18.12 | 8.35 | 15.20 | 6.29 | 89.50 | 36.00 |
| Ga | 0.13 | 0.24 | 0.25 | 0.26 | 0.24 | 6.55 | 5.80 |
| In | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.05 | 0.03 |
| Ge | 0.02 | 0.10 | 0.12 | 0.14 | 0.04 | 2.78 | 2.29 |
| Cr | 2.63 | 27.85 | 3.32 | 20.76 | 6.65 | 15.40 | 16.00 |
| Co | 2.46 | 0.76 | 1.10 | 0.74 | 1.50 | 7.08 | 5.10 |
| Samples | YH1 | YH1I | YH2 | YH2I | YH3 | YH3I | YH4 | YH4I | YH5 | YH5V | YH5I |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrinite | |||||||||||
| Telinite | 15.50 | 6.00 | 12.00 | 6.5 | 19.5 | 9.0 | 11.5 | 8.0 | 2.5 | 2.0 | 3.0 |
| Collotelinite | 1.00 | 0.50 | 5.00 | 1.0 | 1.0 | 1.0 | 4.0 | 2.5 | 2.5 | 11.5 | 0.5 |
| Vitrodetrinite | 6.00 | 4.50 | 8.00 | 4.5 | 6.0 | 2.0 | 7.0 | 4.0 | 1.0 | 2.5 | |
| Collodetrinite | 8.00 | 5.00 | 5.00 | 4.0 | 17.0 | 4.5 | 8.5 | 2.0 | 43.0 | 76.5 | 12.0 |
| Corpogelinite | 0.50 | 0.50 | 1.0 | 0.5 | 0.5 | ||||||
| Gelinite | 0.50 | 0.50 | 1.0 | 1.5 | |||||||
| Total | 31.50 | 16.50 | 30.50 | 16.0 | 44.5 | 17.5 | 33.0 | 17.0 | 49.0 | 90.0 | 18.0 |
| Inertinite | |||||||||||
| Fusinite | 25.00 | 41.50 | 6.50 | 15.5 | 21.0 | 49.0 | 15.5 | 28.0 | 19.0 | 0.5 | 45.0 |
| Semifusinite | 26.00 | 35.00 | 37.50 | 54.0 | 13.0 | 23.0 | 34.5 | 39.5 | 16.5 | 1.0 | 25.5 |
| Macrinite | 7.50 | 1.50 | 11.50 | 4.0 | 3.0 | 2.5 | 4.5 | 4.0 | 5.0 | 0.5 | 1.5 |
| Micrinite | 0.50 | 1.0 | 1.0 | 4.5 | 0.5 | ||||||
| Inertodetrinite | 9.50 | 5.50 | 12.50 | 10.5 | 18.0 | 6.5 | 11.0 | 10.0 | 9.0 | 3.0 | 8.5 |
| Total | 68.50 | 83.50 | 68.00 | 84.0 | 55.0 | 82.0 | 65.5 | 81.5 | 50.5 | 9.5 | 81.0 |
| Liptinite | |||||||||||
| Cutinite | 0.50 | 0.5 | |||||||||
| Sporinite | 0.50 | 0.5 | 1.0 | 0.5 | 0.5 | ||||||
| Total | 1.00 | 0.5 | 1.0 | 0.5 | 0.5 | ||||||
| Minerals | |||||||||||
| Clay | |||||||||||
| Carbonate | |||||||||||
| Sulfide | 0.50 | 0.5 | 0.5 | 1.0 | 1.0 | ||||||
| VRC | 0.45 | 0.41 | 0.45 | 0.42 | 0.46 | 0.40 | 0.45 | 0.42 | 0.41 | 0.48 | 0.39 |
| Feed | Concentrates | Tailings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | AS | Yield | AS | VG | IG | TNa | Yield | AS | VG | IG | TNa |
| % | % | % | % | % | μg/g | % | % | % | % | μg/g | |
| YH1 | 4.30 | 40.54 | 1.92 | 56.50 | 42.50 | 2887 | 59.46 | 5.92 | 22.00 | 74.50 | 3883 |
| YH2 | 3.46 | 38.33 | 1.75 | 58.50 | 40.50 | 2768 | 61.67 | 4.52 | 26.50 | 72.00 | 3623 |
| YH3 | 3.64 | 39.40 | 1.97 | 55.50 | 44.00 | 2991 | 60.60 | 4.73 | 31.50 | 66.50 | 3561 |
| YH4 | 4.38 | 41.86 | 2.05 | 54.50 | 44.00 | 2931 | 58.14 | 6.06 | 23.50 | 72.50 | 3479 |
| YH5 | 3.39 | 38.15 | 1.52 | 59.50 | 40.00 | 2735 | 61.85 | 4.54 | 37.00 | 63.00 | 3542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Che, K.; Pan, J.; Sun, H.; Zhou, C.; Wang, W. Occurrence Mode of Sodium in Zhundong Coal, China: Relationship to Maceral Groups. Minerals 2023, 13, 1155. https://doi.org/10.3390/min13091155
He X, Che K, Pan J, Sun H, Zhou C, Wang W. Occurrence Mode of Sodium in Zhundong Coal, China: Relationship to Maceral Groups. Minerals. 2023; 13(9):1155. https://doi.org/10.3390/min13091155
Chicago/Turabian StyleHe, Xin, Kexin Che, Jinhe Pan, Hao Sun, Changchun Zhou, and Wenfeng Wang. 2023. "Occurrence Mode of Sodium in Zhundong Coal, China: Relationship to Maceral Groups" Minerals 13, no. 9: 1155. https://doi.org/10.3390/min13091155
APA StyleHe, X., Che, K., Pan, J., Sun, H., Zhou, C., & Wang, W. (2023). Occurrence Mode of Sodium in Zhundong Coal, China: Relationship to Maceral Groups. Minerals, 13(9), 1155. https://doi.org/10.3390/min13091155










