Sphalerite and Pyrite Geochemistry from the Pusangguo Co-Rich Cu–Zn–Pb Skarn Deposit, Tibet: Implications for Element Occurrence and Mineralization
Abstract
:1. Introduction
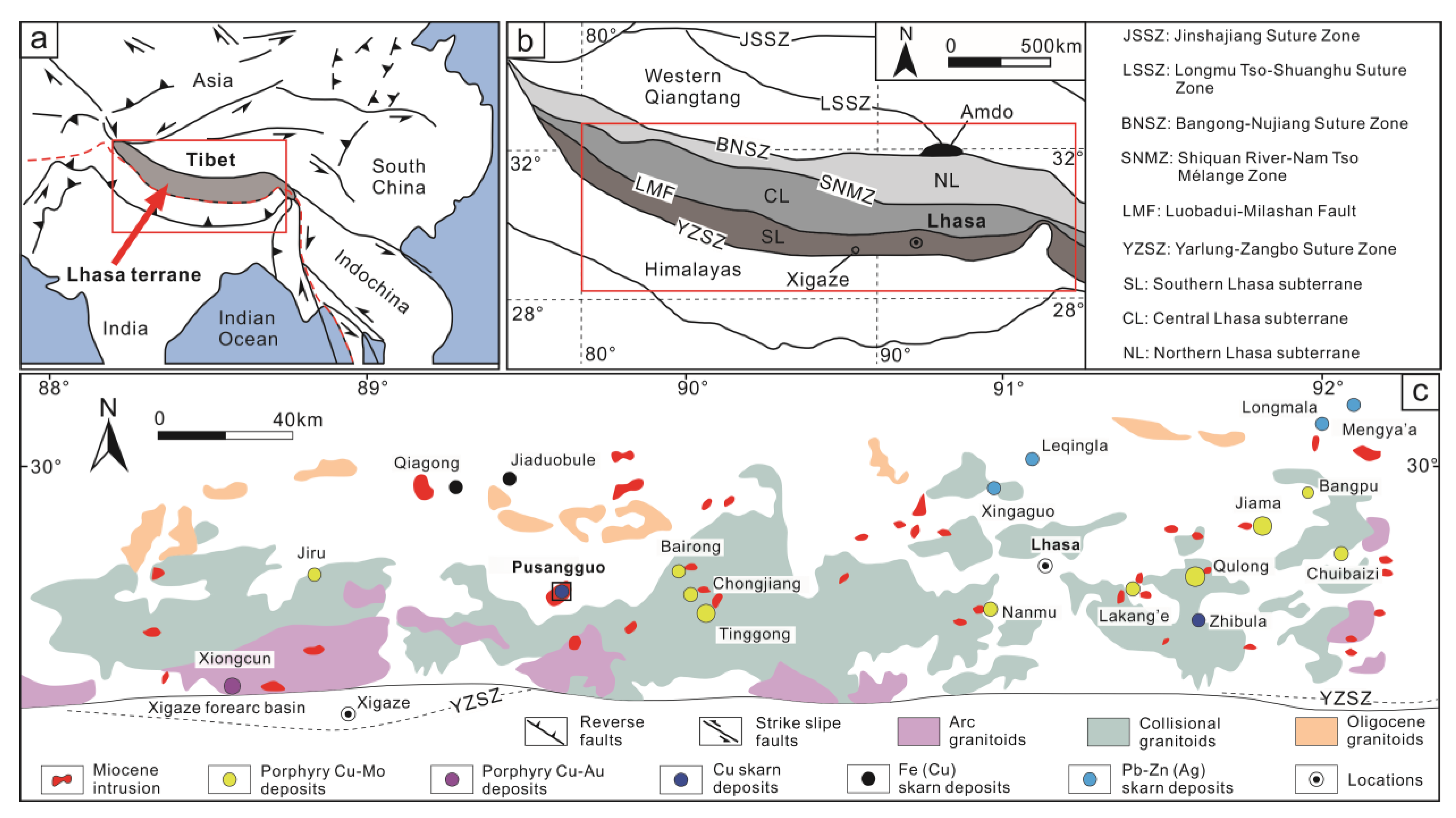
2. Regional Geology
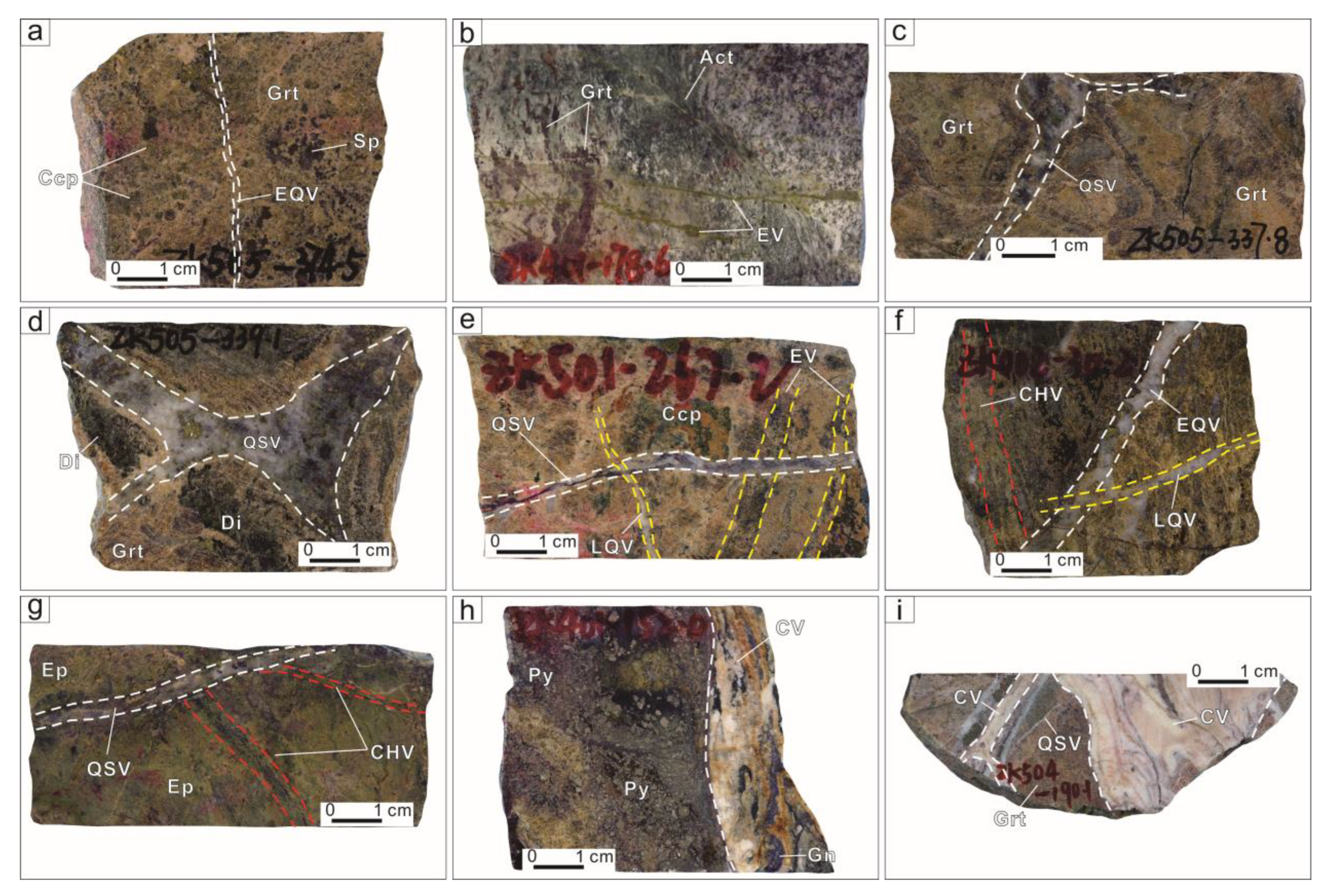
3. Deposit Geology
4. Sampling and Analytical Methods
4.1. Sampling
4.2. EPMA Major Element Analysis
4.3. LA-ICP-MS Trace Element Analysis
5. Results
5.1. Major and Trace Elements in Sphalerite
5.2. Major and Trace Elements in Pyrite
6. Discussion
6.1. Trace Element Distributions and Substitutions in Sphalerite
6.2. Trace Element Distributions and Substitutions in Pyrite
6.3. Physicochemical Condition of Mineralization
6.3.1. Temperature
6.3.2. Sulfur Fugacity (fS2)
6.3.3. Oxygen Fugacity (fO2)
6.4. Pyrite Chemistry as an Indicator of Fluid Evolution
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, J.X.; Wang, Q.; Yang, H.H.; Gao, X.; Zhang, Z.B.; Zou, B. Mineralization, Exploration and Resource Potential of Porphyry-skarn- epithermal Copper Polymetallic Deposits in Tibet. Acta Geosci. Sin. 2017, 38, 5–47, (In Chinese with English Abstract). [Google Scholar]
- Tang, J.X. Mineral resources base investigation and research status of the Tibet Plateau and its adjacent major metallogenic belts. Acta Petrol. Sinica 2019, 35, 617–624, (In Chinese with English Abstract). [Google Scholar]
- Tang, J.X.; Yang, H.H.; Song, Y.; Wang, L.Q.; Liu, Z.B.; Li, B.L.; Lin, B.; Peng, B.; Wang, G.H.; Zhong, Q.G.; et al. The copper polymetallic deposits and resource potential in the Tibet Plateau. China Geol. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Liu, Z.J.; Chen, J.; Yin, G.F.; Zhang, J.Y.; Dai, S.J. The Geological Survey Report on the Pusangguo Cu Polymetallic Deposit in Tibet; Tibet Bureau Publishing House: Tibet, China, 2012; pp. 1–152, (In Chinese with English Abstract). [Google Scholar]
- Cui, X.L. Research on Metallization of Pusangguo Polymetallic Copper Deposit in Tibet, China. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2013. (In Chinese with English Abstract). [Google Scholar]
- Yang, H.R. The Mineralogical Characteristics and Its Genetic Significance of Pusangguo Copper Poly-Metal Ore Deposit in Tibet. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2013. (In Chinese with English Abstract). [Google Scholar]
- Li, Z.; Lang, X.H.; Rickleman, D.; Duan, J.L.; Zhang, Q.Z. Age and genesis of the Pusangguo skarn Cu-dominated polymetallic deposit, Gangdese metallogenic belt, Tibet. J. Asian Earth Sci. 2019, 169, 210–227. [Google Scholar] [CrossRef]
- Li, Z.; Lang, X.H.; Zhang, Q.Z.; Liu, X.K.; Yang, X. Petrogenesis and geodynamic implications of the intrusions related to the Pusangguo skarn Cu-dominated polymetallic deposit in Tibet: Constraints from geochronology, geochemistry, and Sr-Nd-Pb-Hf isotopes. Geol. J. 2020, 55, 7659–7686. [Google Scholar] [CrossRef]
- Cao, M.J.; Qin, K.Z.; Evans, N.J.; Li, G.M.; Ling, X.X.; Mclnnes, B.I.A.; Zhao, J.X. Titanite in situ SIMS U-Pb geochronology, elemental and Nd isotopic signatures record mineralization and fluid characteristics at the Pusangguo skarn deposit, Tibet. Miner. Depos. 2021, 56, 907–916. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.Q.; Li, H.F.; Danzhen, W.X.; Shi, S. Sulfur and Lead isotopic compositions of the Pusangguo Cu–Pb–Zn polymetallic deposit in Tibet: Implications for the source of ore-forming material. Geoscience 2018, 32, 56–65, (In Chinese with English Abstract). [Google Scholar]
- Li, Z.; Tang, J.X.; Wang, L.Q.; Li, H.F.; Liu, W.Y. Mineralogical characteristics of skarn in the Pusangguo Pb–Zn polymetallic deposit of Tibet and their geological significance. Acta Petrol. Mineral. 2018, 37, 241–258, (In Chinese with English Abstract). [Google Scholar]
- Wang, S.; Cao, M.J.; Li, G.M.; Silang, W.D.; Shan, P.F.; Qin, K.Z. The occurrence of cobalt and implications for genesis of the Pusangguo cobalt-rich skarn deposit in Gangdese, Tibet. Ore Geol. Rev. 2022, 150, 105193. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.Y.; Feng, Q.L.; Xu, J.; Wu, S.; Sun, G.P. Ore genesis of skarn mineralization in continental collision orogens: A case study from the Pusangguo Co-bearing Cu–Pb–Zn deposit in Tibet. Ore Geol. Rev. 2020, 122, 103523. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Zhu, J.R.; Xu, J.Q.; Niu, X.D. Fluid origin and evolution of the Pusangguo Cu-Pb-Zn skarn deposit in Tibet: Constraints from fluid inclusions and isotope compositions. Ore Geol. Rev. 2022, 150, 105197. [Google Scholar] [CrossRef]
- Zhao, H.X.; Frimmel, H.E.; Jiang, S.Y.; Dai, B.Z. LA-ICP-MS trace element analysis of pyrite from the Xiaoqinling gold district, China: Implications for ore genesis. Ore Geol. Rev. 2011, 43, 142–153. [Google Scholar] [CrossRef]
- Lockington, J.A.; Cook, N.J.; Ciobanu, C.L. Trace and minor elements in sphalerite from metamorphosed sulphide deposits. Miner. Petrol. 2014, 108, 873–890. [Google Scholar] [CrossRef]
- Sykora, S.; Cook, D.R.; Meffre, S.; Stephanov, A.S.; Gardner, K.; Scott, R.; Selley, D.; Harris, A.C. Evolution of pyrite trace element compositions from porphyry-style and epithermal conditions at the Lihir Gold deposit: Implications for ore genesis and mineral processing. Econ. Geol. 2018, 113, 193–208. [Google Scholar] [CrossRef]
- Chen, F.C.; Deng, J.; Wang, Q.F.; Huizengga, J.M.; Li, G.J.; Gu, Y.W. LA-ICP-MS trace element analysis of magnetite and pyrite from the Hetaoping Fe-Zn-Pb skarn deposit in Baoshan block, SW China: Implications for ore-forming processes. Ore Geol. Rev. 2020, 117, 103309. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Huang, Z.; Hu, Y.; Wang, H. In situ trace elements and S isotope systematics for growth zoning in sphalerite from MVT deposits: A case study of Nayongzhi, South China. Mineral. Mag. 2021, 85, 364–378. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in cocrystallized sphalerite–galena–chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- Bauer, M.E.; Burisch, M.; Ostendorf, J.; Krause, J.; Frenzel, M.; Seifert, T.; Gutzmer, J. Trace element geochemistry of sphalerite in contrasting hydrothermal fluid systems of the Freiberg district, Germany: Insights from LA–ICP–MS analysis, near–infrared light microthermometry of sphalerite–hosted fluid inclusions, and sulfur isotope geochemistry. Miner. Depos. 2019, 54, 237–262. [Google Scholar]
- Bao, T.; Ni, P.; Dai, B.Z.; Wang, G.G.; Chen, H.; Li, S.N.; Chi, Z.; Li, W.S.; Ding, J.Y.; Chen, L.L. Pyrite Rb-Sr geochronology, LA-ICP-MS trace element and telluride mineralogy constraints on the genesis of the Shuangqishan gold deposit, Fujian, China. Ore Geol. Rev. 2021, 138, 104158. [Google Scholar] [CrossRef]
- Xu, J.; Cook, N.J.; Ciobanu, C.L.; Li, X.; Kontonikas-Charos, A.; Gilbert, S.; Lv, Y. Indium distribution in sphalerite from sulfide–oxide–silicate skarn assemblages: A case study of the Dulong Zn–Sn–In deposit, Southwest China. Miner. Depos. 2021, 56, 307–324. [Google Scholar] [CrossRef]
- Frenzel, M.; Cook, N.J.; Ciobanu, C.L.; Slattery, A.D.; Wade, B.P.; Gilbert, S.; Ehrig, K.; Burisch, M.; Verdugo-Ihl, M.R.; Voudouris, P. Halogens in hydrothermal sphalerite record origin of ore-forming fluids. Geology 2020, 48, 766–770. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Song, Y.; Liu, Y.; Fard, M.; Hou, Z. Major and trace elements and sulfur isotopes in two stages of sphalerite from the world-class Angouran Zn–Pb deposit, Iran: Implications for mineralization conditions and type. Ore Geol. Rev. 2019, 109, 184–200. [Google Scholar] [CrossRef]
- Xing, B.; Mao, J.W.; Xiao, X.N.; Liu, H.; Zhang, C.; Guo, S.; Li, H.Y.; Huang, W.Y.; Lai, C. Metallogenic discrimination by sphalerite trace element geochemistry: An example from the Fengyan Zn-Pb deposit in central Fujian, SE China. Ore Geol. Rev. 2022, 141, 104651. [Google Scholar] [CrossRef]
- Zhang, J.K.; Shao, Y.J.; Liu, Z.F.; Chen, K. Sphalerite as a record of metallogenic information using multivariate statistical analysis: Constraints from trace element geochemistry. J. Geochem. Explor. 2022, 232, 106883. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Wang, J.L.; Deng, A.P.; Zhang, Y.; Shao, Y.J.; Li, H.B. Cu–Pb–Zn and W–Mo–Pb–Zn systematic mineralization environment of Huangshaping deposit in Hunan: Indicator of sphalerite geochemistry. Miner. Res. Geol. 2020, 34, 56–63, (In Chinese with English Abstract). [Google Scholar]
- Xing, B.; Mao, J.W.; Xiao, X.N.; Liu, H.; Jia, F.D.; Wang, S.H.; Huang, W.Y.; Li, H.Y. Genetic discrimination of the Dingjiashan Pb–Zn deposit, SE China, based on sphalerite chemistry. Ore Geol. Rev. 2021, 135, 104212. [Google Scholar] [CrossRef]
- Gottesmann, W.; Kampe, A. Zn/Cd ratios in calcsilicate-hosted sphalerite ores at Tumurtijn-ovoo, Mongolia. Chem. Erde-Geochem. 2007, 67, 323–328. [Google Scholar] [CrossRef]
- Belissont, R.; Muñoz, M.; Boiron, M.; Luais, B.; Mathon, O. Distribution and oxidation state of Ge, Cu and Fe in sphalerite by µ-XRF and K-edge µ-XANES: Insights into Ge incorporation, partitioning and isotopic fractionation. Geochim. Cosmochim. Acta 2016, 177, 298–314. [Google Scholar] [CrossRef]
- Cugerone, A.; Cenki-Tok, B.; Oliot, E.; Muñoz, M.; Barou, F.; Motto-Ros, V.; Le Goff, E. Redistribution of germanium during dynamic recrystallization of sphalerite. Geology 2019, 48, 236–241. [Google Scholar] [CrossRef]
- Zhu, D.C.; Zhao, Z.D.; Niu, Y.; Mo, X.X.; Chung, S.L.; Hou, Z.Q.; Wang, L.Q.; Wu, F.Y. The Lhasa Terrane: Record of a microcontinent and its histories of drift and growth. Earth Planet. Sci. Lett. 2011, 301, 241–255. [Google Scholar] [CrossRef]
- Zhu, D.C.; Zhao, Z.D.; Niu, Y.L.; Dilek, Y.; Hou, Z.Q.; Mo, X.X. The origin and pre-Cenozoic evolution of the Tibetan Plateau. Gondwana Res. 2013, 23, 1429–1454. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Zhao, G.C.; Santosh, M.; Wang, J.L.; Dong, X.; Shen, K. Late Cretaceous charnockite with adakitic affinities from the Gangdese batholith, southeastern Tibet: Evidence for Neo-Tethyan mid-ocean ridge subduction? Gondwana Res. 2010, 17, 615–631. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Z.M.; Santosh, M.; Wang, W.; Yu, F.; Liu, F. Late Neoproterozoic thermal events in the northern Lhasa terrane, south Tibet: Zircon chronology and tectonic implications. J. Geodyn. 2011, 52, 389–405. [Google Scholar] [CrossRef]
- Lang, X.H.; Deng, Y.L.; Wang, X.H.; Tang, J.X.; Yin, Q.; Xie, F.W.; Yang, Z.Y.; Li, Z.; He, Q.; Li, L.; et al. Geochronology and geochemistry of volcanic rocks of the Bima Formation, southern Lhasa subterrane, Tibet: Implications for early Neo-Tethyan subduction. Gondwana Res. 2020, 80, 335–349. [Google Scholar] [CrossRef]
- Wang, X.H.; Lang, X.H.; Klemd, R.; Deng, Y.L.; Tang, J.X. Subduction initiation of the Neo-Tethys oceanic lithosphere by collision-induced subduction transference. Gondwana Res. 2022, 104, 54–69. [Google Scholar] [CrossRef]
- Kang, Z.Q.; Xu, J.F.; Wilde, S.A.; Feng, Z.H.; Chen, J.L.; Wang, B.D.; Wen, C.F.; Pan, H.B. Geochronology and geochemistry of the Sangri Group Volcanic Rocks, Southern Lhasa Terrane: Implications for the early subduction history of the NeoTethys and Gangdese Magmatic Arc. Lithos 2014, 200–201, 157–168. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Zhao, Z.D.; Niu, Y.L.; Zhu, D.C.; Liu, D.; Wang, Q.; Hou, Z.Q.; Mo, X.X.; Wei, J.C. Geochronology and geochemistry of the Early Jurassic Yeba Formation volcanic rocks in southern Tiebet: Initiation of back-arc rifting and crustal accretion in the southern Lhasa Terrane. Lithos 2017, 278–281, 477–490. [Google Scholar] [CrossRef]
- Mo, X.X.; Hou, Z.Q.; Niu, Y.L.; Dong, G.C.; Qu, X.M.; Zhao, Z.D.; Yang, Z.M. Mantle contributions to crustal thickening in south Tibet in response to the India–Asia collision. Lithos 2007, 96, 225–242. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chung, S.L.; Lo, C.H.; Ji, J.Q.; Lee, T.Y.; Qian, Q.; Zhang, Q. Eocene Neotethyan slab breakoff in southern Tibet inferred from the Linzizong volcanic record. Tectonophysics 2009, 477, 20–35. [Google Scholar] [CrossRef]
- Mo, X.X.; Hou, Z.Q.; Niu, Y.L.; Dong, G.C.; Qu, X.M.; Zhao, Z.D.; Hou, Z.Q.; Zhou, S.; Ke, S. Contribution of syncollisional felsic magmatism to continental crust growth: A case study of the Paleogene Linzizong volcanic Succession in southern Tibet. Chem. Geol. 2008, 250, 49–67. [Google Scholar] [CrossRef]
- Chen, X.L.; Liang, H.Y.; Zhang, J.; Sotiriou, P.; Huang, W.T.; Ren, L.; Zhang, L.; Zou, Y.Q. Geochemical characteristics and magma fertility for the Jurassic arc rocks in the Gangdese belt, Tibet. Ore Geol. Rev. 2019, 115, 103169. [Google Scholar] [CrossRef]
- Wu, F.Y.; Ji, W.Q.; Liu, C.Z.; Chung, S.L. Detrital zircon U–Pb and Hf isotopic data from the Xigaze fore-arc basin: Constraints on Transhimalayan magmatic evolution in southern Tibet. Chem. Geol. 2010, 271, 13–25. [Google Scholar] [CrossRef]
- Ji, W.Q.; Wu, F.Y.; Liu, C.Z.; Chung, S.L. Early Eocene crustal thickening in southern Tibet: New age and geochemical constraints from the Gangdese batholith. J. Asian Earth Sci. 2012, 53, 82–95. [Google Scholar] [CrossRef]
- Chu, M.F.; Chung, S.L.; O’Reilly, S.Y.; Pearson, N.J.; Wu, F.Y.; Li, X.H.; Lee, H.Y. India’s hidden inputs to Tibetan orogeny revealed by Hf isotopes of Trans Himalayan zircons and host rocks. Earth Planet. Sci. Lett. 2011, 307, 479–486. [Google Scholar] [CrossRef]
- Tang, J.X.; Lang, X.H.; Xie, F.W.; Gao, Y.M.; Li, Z.J.; Yang, H.H.; Zhang, L.; Wang, Q.; Zhou, Y. Geological characteristics and genesis of the Jurassic No. I porphyry Cu–Au deposit in the Xiongcun district, Gangdese porphyry copper belt, Tibet. Ore Geol. Rev. 2015, 70, 438–456. [Google Scholar] [CrossRef]
- Lang, X.H.; Wang, X.H.; Deng, Y.L.; Tang, J.X.; Xie, F.W.; Yang, Z.Y.; Jiang, K. Hydrothermal evolution and ore precipitation of the No. 2 porphyry Cu–Au deposit in the Xiongcun district, Tibet: Evidence from cathodoluminescence, fluid inclusions, and isotopes. Ore Geol. Rev. 2019, 114, 103141. [Google Scholar] [CrossRef]
- Zhao, J.X.; Qin, K.Z.; Li, G.M.; Li, J.X.; Xiao, B.; Chen, L.; Yang, Y.; Li, C.; Liu, Y.S. Collision-related genesis of the Sharang porphyry molybdenum deposit, Tibet: Evidence from zircon U–Pb ages, Re–Os ages and Lu–Hf isotopes. Ore Geol. Rev. 2014, 56, 312–326. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, B.; Zheng, Y.C.; Yang, Z.S.; Hou, Z.Q.; Huang, K.X.; Liu, Y.; Zhang, C.; Zhao, L. Two episodes of mineralization in the Mengya’a deposit and implications for the evolution and intensity of Pb–Zn–(Ag) mineralization in the Lhasa terrane, Tibet. Ore Geol. Rev. 2017, 90, 877–896. [Google Scholar] [CrossRef]
- Zhou, A.R.G.L.; Dai, J.G.; Li, Y.L.; Li, H.A.; Tang, J.X.; Wang, C.S. Differential exhumation histories between Qulong and Xiongcun porphyry copper deposits in the Gangdese copper metallogenic Belt: Insights from low temperature thermochronology. Ore Geol. Rev. 2019, 107, 801–819. [Google Scholar] [CrossRef]
- Sun, X.; Hollings, P.; Lu, Y.J. Geology and origin of the Zhunuo porphyry copper deposit, Gangdese belt, southern Tibet. Miner. Depos. 2021, 56, 457–480. [Google Scholar] [CrossRef]
- Zheng, W.B.; Liu, B.L.; Tang, J.X.; McKinley, J.M.; Cooper, M.R.; Tang, P.; Lin, B.; Li, C.; Wang, L.; Zhang, D. Exploration indicators of the Jiama porphyry-skarn deposit, southern Tibet, China. J. Geochem. Explor. 2022, 236, 106982. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Yang, Z.M.; Qu, X.M.; Meng, X.J.; Li, Z.Q.; Beaudoin, G.; Rui, Z.Y.; Gao, Y.F.; Zaw, K. The Miocene Gangdese porphyry copper belt generated during postcollisional extension in the Tibetan Orogen. Ore Geol. Rev. 2009, 36, 25–51. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zheng, Y.C.; Yang, Z.S.; Hu, Y. Formation and evolution of multistage ore-forming fluids in the Miocene Bangpu porphyry-skarn deposit, Southern Tibet: Insights from LA–ICP–MS trace elements of quartz and fluid inclusions. J. Asian Earth Sci. 2020, 203, 104–124. [Google Scholar] [CrossRef]
- Liu, W.Y.; Nigel, J.C.; Cristiana, L.C.; Liu, Y.; Qiu, X.P.; Chen, Y.C. Mineralogy of tin-sulfifides in the Zijinshan porphyry-epithermal system, Fujian Province, China. Ore Geol. Rev. 2016, 72, 682–698. [Google Scholar]
- Danyushevsky, L.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.; McGoldrick, P.; Shelley, M. Routine quantitative multi-element analysis of sulphide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Belissont, R.; Boiron, M.; Luais, B.; Cathelineau, M. LA-ICP-MS analyses of minor and trace elements and bulk Ge isotopes in zoned Ge-rich sphalerites from the Noailhac-Saint-Salvy deposit (France): Insights into incorporation mechanisms and ore deposition processes. Geochim. Cosmochim. Acta 2014, 126, 518–540. [Google Scholar] [CrossRef]
- Gagnevin, D.; Menuge, J.F.; Kronz, A.; Barrie, C.; Boyce, A.J. Minor elements in layered sphalerite as a record of fluid origin, mixing, and crystallization in the Navan Zn-Pb ore deposit, Ireland. Econ. Geol. 2014, 109, 1513–1528. [Google Scholar] [CrossRef]
- Bonnet, J.; Mosser-Ruck, R.; Caumon, M.C. Trace element distribution (Cu, Ga, Ge, Cd, and Fe) IN sphalerite from the tennessee MVT Deposits, USA, By combined EMPA, LA-ICP-MS, raman spectroscopy, and crystallography. Can. Mineral. 2016, 54, 1261–1284. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Sun, H.S.; Koua, K.A.D.; Lyu, C.L. LA-ICP-MS trace element analysis of sphalerite and pyrite from the Beishan Pb–Zn ore district, south China: Implications for ore genesis. Ore Geol. Rev. 2022, 150, 105128. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.J.; Ulrich, T.; Zhang, J.; Wang, J. Trace element compositions of sulfides from Pb-Zn deposits in the Northeast Yunnan and northwest Guizhou Provinces, SW China: Insights from LA-ICP-MS analyses of sphalerite and pyrite. Ore Geol. Rev. 2022, 141, 104639. [Google Scholar] [CrossRef]
- Tauson, V.L.; Chernyshev, L.V.; Makeev, A.B. Phase relations and structural peculiarities of mixed-crystals in ZnS-MnS system. Geokhimiya 1977, 5, 679–692. [Google Scholar]
- Pattrick, R.A.D.; Mosselmans, J.F.W.; Charnock, J.M. An X-ray absorption study of doped sphalerites. Eur. J. Mineral. 1998, 10, 239–249. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Li, Z.L.; Ye, L.; Hu, S.Y.; Wei, C.; Huang, Z.L.; Yang, Y.L.; Danyushevsky, L. Trace elements in sulfides from the Maozu Pb-Zn deposit, Yunnan Province, China: Implications for trace-element incorporation mechanisms and ore genesis. Am. Mineral. 2020, 105, 1734–1751. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Ai, G.L.; Xue, X.L.; Li, H.B.; Shah, S.A.; Wang, N.H.; Chen, X. LA-ICP-MS trace element geochemistry of sphalerite: Metallogenic constraints on the Qingshuitang Pb-Zn deposit in the Qinghang Ore Belt, South China. Ore Geol. Rev. 2022, 141, 104659. [Google Scholar] [CrossRef]
- Wang, K.X.; Zhai, D.G.; Liu, J.J.; Wu, H. LA-ICP-MS trace element analysis of pyrite from the Dafang gold deposit, South China: Implications for ore genesis. Ore Geol. Rev. 2021, 139, 104507. [Google Scholar] [CrossRef]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Liu, Y.P.; Zhang, Q.; Liu, T.G.; Gao, W.; Yang, Y.L.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- Hu, Y.S.; Ye, L.; Huang, Z.L.; Danyushevsky, L.; Wang, H.Y. LA-ICP-MS sphalerite and galena trace element chemistry and mineralization-style fingerprinting for carbonate-hosted Pb-Zn deposits: Perspective from early Devonian Huodehong deposit in Yunnan, South China. Ore Geol. Rev. 2021, 136, 104253. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Wei, J.H.; Liang, S.N.; Gao, T. Sulfide remobilization and trace element redistribution during metamorphism and deformation at the Xitieshan Pb-Zn deposit, NW China. Ore Geol. Rev. 2021, 136, 104170. [Google Scholar] [CrossRef]
- Wright, K. The incorporation of cadmium, manganese and ferrous iron in sphalerite: Insights from computer simulation. Can. Miner. 2009, 47, 615–623. [Google Scholar] [CrossRef]
- Murakami, H.; Ishihara, S. Trace elements of Indium-bearing sphalerite from tinpolymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, C.Q.; Yu, H.J.; Yang, Y.M.; Zhao, Y.X.; Zhu, C.C.; Ding, Q.F.; Zhou, Y.B.; Yang, J.C.; Xu, Y. Element enrichment characteristics: Insights from element geochemistry of sphalerite in Daliangzi Pb–Zn deposit, Sichuan, Southwest China. J. Geochem. Explor. 2018, 186, 187–201. [Google Scholar] [CrossRef]
- Torro, L.; Benites, D.; Vallance, J.; Laurent, O.; Ortiz-Benavente, B.A.; Chelle-Michou, C.; Proenza, J.A.; Fontboté, L. Trace element geochemistry of sphalerite and chalcopyrite in arc hosted VMS deposits. J. Geochem. Explor. 2022, 232, 106882. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.L.; de Jonge, M.D.; Ryan, C.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu–In–Fe–bearing sphalerite via–XANES spectroscopy. Am. Mineral. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Makovicky, E.; Topa, D. Crystal chemical formula for sartorite homologues. Mineral. Mag. 2018, 79, 25–31. [Google Scholar] [CrossRef]
- Thomas, H.V.; Large, R.R.; Bull, S.W.; Maslennikov, V.; Berry, R.F.; Fraser, R.; Froud, S.; Moye, R. Pyrite and pyrrhotite textures and composition in sediments, laminated quartz veins, and reefs at Bendigo gold mine, Australia: Insights for ore genesis. Econ. Geol. 2011, 106, 1–31. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Green, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Mineral. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.; Chryssoulis, S.; Li, J.W.; Ma, C.Q.; Parada, M.A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochim. Cosmochim. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Grant, H.L.J.; Hannington, M.D.; Petersen, S.; Frische, M.; Fuchs, S.H. Constraints on the behavior of trace elements in the actively-forming TAG deposit, Mid-Atlantic Ridge, based on LA-ICP-MS analyses of pyrite. Chem. Geol. 2018, 498, 45–71. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy volcanichosted massive sulfide deposit (Southern Urals, Russia) using laser ablationinductively coupled plasma mass spectrometry (LA-ICPMS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Rottier, B.; Kouzmanov, K.; Walle, M.; Bendezu, R.; Fontbote, L. Sulfide replacement processes revealed by textural and LA-ICP-MS trace element analyses: Example from the early mineralization stages at Cerro de Pasco, Peru. Econ. Geol. 2016, 111, 1347–1367. [Google Scholar] [CrossRef]
- Kelley, K.; Leach, D.; Johnson, C.; Clark, J.; Fayek, M.; Slack, J.; Anderson, V.; Ayuso, R.; Ridley, W. Textural, Compositional, and Sulfur Isotope Variations of Sulfide Minerals in the Red Dog Zn-Pb-Ag Deposits, Brooks Range, Alaska: Implications for Ore Formation. Econ. Geol. 2004, 99, 1509–1532. [Google Scholar] [CrossRef]
- Bauer, M.E.; Seifert, T.; Burisch, M.; Krause, J.; Richter, N.; Gutzmer, J. Indium bearing sulfides from the Hammerlein skarn deposit, Erzgebirge, Germany: Evidence for late-stage diffusion of indium into sphalerite. Miner. Depos. 2019, 54, 175–192. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Z.L.; Xu, D.R.; Yu, D.S.; Dong, G.J.; Ning, J.T.; Kang, B.; Peng, E.K. Trace element characteristics of sphalerite in the Lishan Pb-Zn polymetallic deposit in Hunan Province and the metallogenic implications. Earth Sci. Front. 2020, 27, 66–81. [Google Scholar]
- Ye, L.; Li, Z.L.; Hu, Y.S.; Huang, Z.L.; Zhou, J.X.; Fan, H.F.; Danyushevskiy, L. Trace elements in sulfide from the Tianbaoshan Pb-Zn deposit, Sichuan Province, China: A LA-ICPMS study. Acta Petrol. Sin. 2016, 32, 3377–3393, (In Chinese with English Abstract). [Google Scholar]
- Wu, Y.; Sun, Z.G.; Chen, M.H.; Zhang, C.Q.; Cao, L.; Tang, Y.J.; Yuan, X.; Zhang, P. Trace elements in sphalerites from the Mississippi Valley-type lead-zinc deposits around the margins of Yangtze Block and its geological implications: A LA-ICP-MS study. Acta Petrol. Sin. 2019, 35, 3443–3460. [Google Scholar]
- Wen, H.J.; Zhu, C.W.; Zhang, Y.X.; Cloquet, C.; Fan, H.F.; Fu, S.H. Zn/Cd ratios and cadmium isotope evidence for the classification of lead–zinc deposits. Sci. Rep. 2016, 6, 25273. [Google Scholar] [CrossRef]
- Keith, M.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R.; Petersen, S.; Bach, W. Effects of temperature, sulfur, and oxygen fugacity on the composition of sphalerite from submarine hydrothermal vents. Geology 2014, 42, 699–702. [Google Scholar] [CrossRef]
- Cook, N.J. Mineralogy of the sulphide deposits at Sulitjelma, Northern Norway. Ore Geol. Rev. 1996, 11, 303–338. [Google Scholar] [CrossRef]
- Maghfouri, S.; Rastad, E.; Lentz, D.R.; Mousivand, F.; Choulet, F. Mineralogy, microchemistry and fluid inclusion studies of the Besshi-type Nudeh Cu-Zn VMS deposit, Iran. Geochemistry 2018, 78, 40–57. [Google Scholar] [CrossRef]
- Li, H.B.; Ishiyama, D.Z.; Zhang, Y.; Shao, Y.J. Geology and geochemical characteristics of the Xiajinbao gold deposit in the Hebei Province, China. J. Mineral. Petrol. Sci. 2018, 113, 24–40. [Google Scholar] [CrossRef]
- Scott, S.D.; Barnes, H.L. Sphalerite geothermometry and geobarometry. Econ. Geol. 1971, 66, 653–669. [Google Scholar] [CrossRef]
- Warmada, I.W.; Lehmann, B. Polymetallic sulfides and sulfosalts of the Pongkor epithermal gold-silver deposit, west java, Indonesia. Can. Mineral. 2003, 41, 185–2003. [Google Scholar] [CrossRef]
- Bernardini, G.P.; Borgheresi, M.; Cipriani, C.; Di Benedetto, F.; Romanelli, M. Mn distribution in sphalerite: An EPR study. Phys. Chem. Miner. 2004, 31, 80–84. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Houghton, J.L.; Shanks, W.C., III; Seyfried, W.E., Jr. Massive sulfifide deposition and trace element remobilization in the Middle Valley sediment-hosted hydrothermal system, northern Juan de Fuca Rdge. Geochim. Cosmochim. Acta 2004, 68, 2863–2873. [Google Scholar] [CrossRef]
- Kampmann, T.C.; Jansson, N.F.; Stephens, M.B.; Olin, P.H.; Gilbert, S.; Wanhainen, C. Syn-tectonic sulphide remobilization and trace element redistribution at the Falun pyritic Zn-Pb-Cu-(Au-Ag) sulphide deposit, Bergslagen, Sweden. Ore Geol. Rev. 2018, 96, 48–71. [Google Scholar] [CrossRef]
- Shu, Q.H.; Chang, Z.S.; Mavrogenes, J. Fluid compositions reveal fluid nature, metal deposition mechanisms, and mineralization potential: An example at the Haobugao Zn-Pb skarn, China. Geology 2021, 49, 473–477. [Google Scholar] [CrossRef]
- Shu, Q.H.; Chang, Z.S.; Hammerli, J.; Lai, Y.; Huizenga, J.M. Composition and evolution of fluids forming the Baiyinnuo’er skarn Zn-Pb deposit, northeastern China: Insights from laser ablation ICP-MS study of fluid inclusions. Econ. Geol. 2017, 112, 1441–1460. [Google Scholar] [CrossRef]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World Skarn Deposits. In Economic Geology 100th Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 299–336. [Google Scholar]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tan, H.; Zhao, F.; Xiang, Z.; Wu, H.; Zhang, P. Sphalerite and Pyrite Geochemistry from the Pusangguo Co-Rich Cu–Zn–Pb Skarn Deposit, Tibet: Implications for Element Occurrence and Mineralization. Minerals 2023, 13, 1165. https://doi.org/10.3390/min13091165
Li Z, Tan H, Zhao F, Xiang Z, Wu H, Zhang P. Sphalerite and Pyrite Geochemistry from the Pusangguo Co-Rich Cu–Zn–Pb Skarn Deposit, Tibet: Implications for Element Occurrence and Mineralization. Minerals. 2023; 13(9):1165. https://doi.org/10.3390/min13091165
Chicago/Turabian StyleLi, Zhuang, Hao Tan, Feng Zhao, Zuopeng Xiang, Han Wu, and Peng Zhang. 2023. "Sphalerite and Pyrite Geochemistry from the Pusangguo Co-Rich Cu–Zn–Pb Skarn Deposit, Tibet: Implications for Element Occurrence and Mineralization" Minerals 13, no. 9: 1165. https://doi.org/10.3390/min13091165



