Green Biocidal Nanotechnology Use for Urban Stone-Built Heritage—Case Study from Oradea, Romania
Abstract
:1. Introduction
2. Literature Review
| Paper | Location | Methods and Tests | Results |
|---|---|---|---|
| Dei and Salvadori [65] | Limestones | NNPs calcium hydroxide treatments | Innovative and compatible with base material, consolidation process |
| D’Arienzo et al. [66] | Neapolitan yellow tuff | Nanocomposite systems based on Cloisite | Protective and consolidative |
| Pinna et al. [41] | Archaeological area of Fiesole, Italy | Tested on three stone substrates with different bioreceptivity, traditional way (tetraethylorthosilicate, methylethoxy polysiloxane, Paraloid B72, tributyltin oxide, dibutyltin dilaurate) and nanotechnology (copper nanoparticles) | Prevention in biological growth; controlling recolonisation on stone after a conservation |
| Licchelli et al. [67] | Lecce stone, Italy | Ca (OH)2 and Sr (OH)2 nanoparticles applied into the stone substrate. The chemical weathering effect of salt crystallisation of the treated samples which was evaluated through dry weight loss (DWL) test | Good results as consolidating agents |
| Aldoasri et al. [68] | Marble stone facades of historic buildings, Cairo, Egypt | Nanometric film over the stone surface, TiO2 nanoparticles, in an aqueous colloidal suspension, applied by spray-coating | Self-cleaning photo-induced effects are obvious in the experiment time and 6 months later |
| Becerra et al. [40] | Heritage stone from south of Spain | Two AgNPs syntheses have been studied; | Cleaning the limestone due to the biopatina formation reduction using (Ag/TiO2) nanocomposite treatments |
| Bruno et al. [69] | Catacombs of SS. Marcellino and Pietro (Rome, Italy) | essential oils (from L. angustifolia and T. vulgaris) biofilm photosynthetic activity on frescoes stone | Chemical modifications and discolouration, good results |
| Zarzuela et al. [70] | Cultural heritage stone | CuO/SiO2 nanocomposites: a multifunctional coating for application on building stone | |
| Ion et al. [71] | Basarabi chalk monument, Romania | Hydroxyapatite nanoparticles (HAp) | Physical–chemical and mechanical rocks properties improvements |
| Gallo et al. [72] | Stone in buildings and monuments | Disinfection procedures on natural stone using smectite and ammonium salt | Antimicrobial and long-term biostatic effects |
| Aldosari et al. [73] | Historic marble columns, Egypt | Nanoparticles of ZnO, dispersed in laboratory synthesised acrylic polymer | Biocidal against Aspergillus niger and Penicillium sp. Studies for RH/temperature, UV aging, and mechanical deterioration |
| Capitelli et al. [42] | Conservation of stone monuments of cultural heritage | Functional nano-hydroxyapatite methodological approach against acidic rain corrosion | HAp nanoparticles and their application on stony substrates has been investigated with good results |
| Caneva et al. [44] | Caestia Pyramid (Rome), Italy | Allelopathic properties of lichen use for stone restoration | Results of the tests emphasise natural product substances are a useful in control of bio-colonisation |
| Xie et al. [74] | Heritage object marble made | Colloidal protectants based on Al2O3 and SiO2 nano-powder | Self-cleaning stone effects |
| Weththimuni et al. [75] | Lecce stone, bricks, and marble | For this purpose, ZrO2-doped-ZnO-PDMS nanocomposites were synthesised by in situ reaction | Self-cleaning effects |
| Ruffolo et al. [76] | Stone heritage | NNPs calcium, magnesium hydroxide and nano-silica | Superhydrophobic properties of coatings, dirt, pollutants, and microorganisms, etc., washed out by water flowing. The combination of light and photocatalyst generated photocatalytic effects |
| Weththimuni et al. [77] | Three different stones (Lecce stone, Carrara marble, and brick) | ZnONNPs doped with ZrO2 sol–gel, to reduce the biodeterioration of cultural heritage stone buildings | Photocatalytic properties and ZnO antibacterial activity |
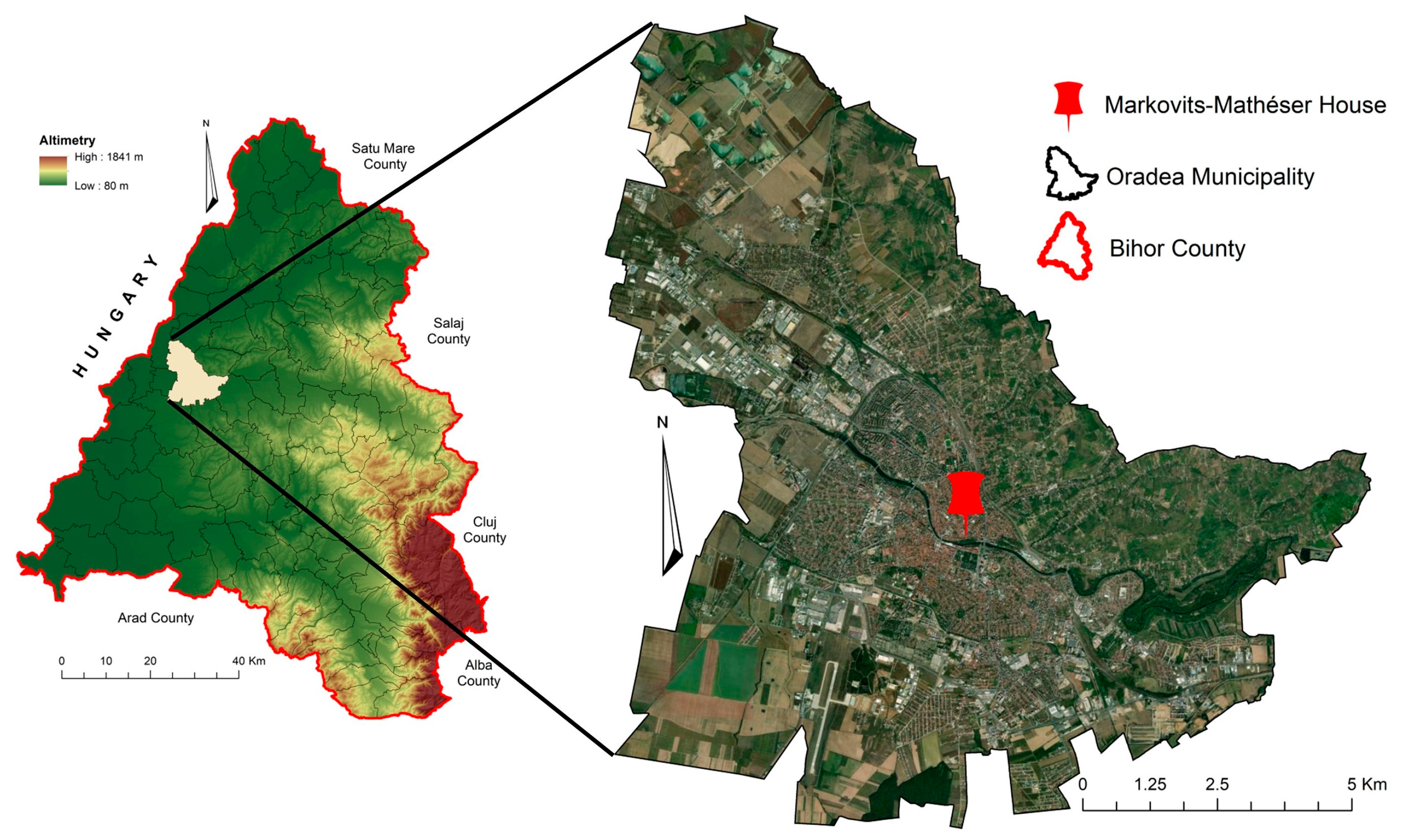
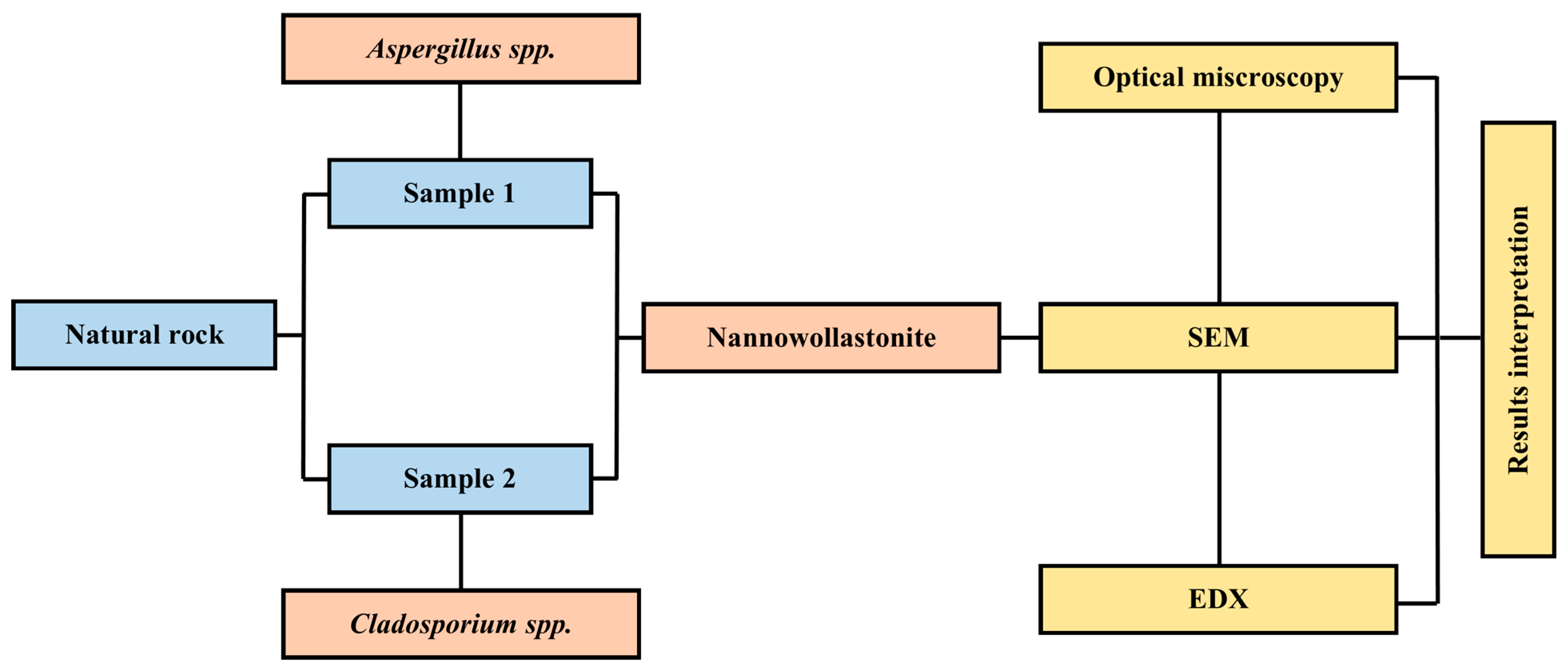
3. Materials and Methods
| Accuracy Measures from WEKA | Accuracy Measures from ENVI | Definition, Notes |
|---|---|---|
| Correctly Classified Instances (CCI) | Overall Accuracy (OA) | Proportion of pixels correctly classified [116]. |
| Kappa Coefficient (k) | Kappa Coefficient (k) | It expresses the agreement (correlation) between the performed classification (pre-dictated classes) and the dataset with the true value. It is the proportion of agreement after chance agreement is removed from consideration [117]. |
| Recall (R) | Producer Accuracy (PA) | Ratio between the number of pixels correctly identified as belonging to a class and the total number of pixels of the respective class in the training dataset [118]. |
| Precision (P) | User Accuracy (UA) | Ratio between the number of pixels correctly identified as belonging to a class and the total number of pixels labeled in the classification as belonging to the respective class [118]. |
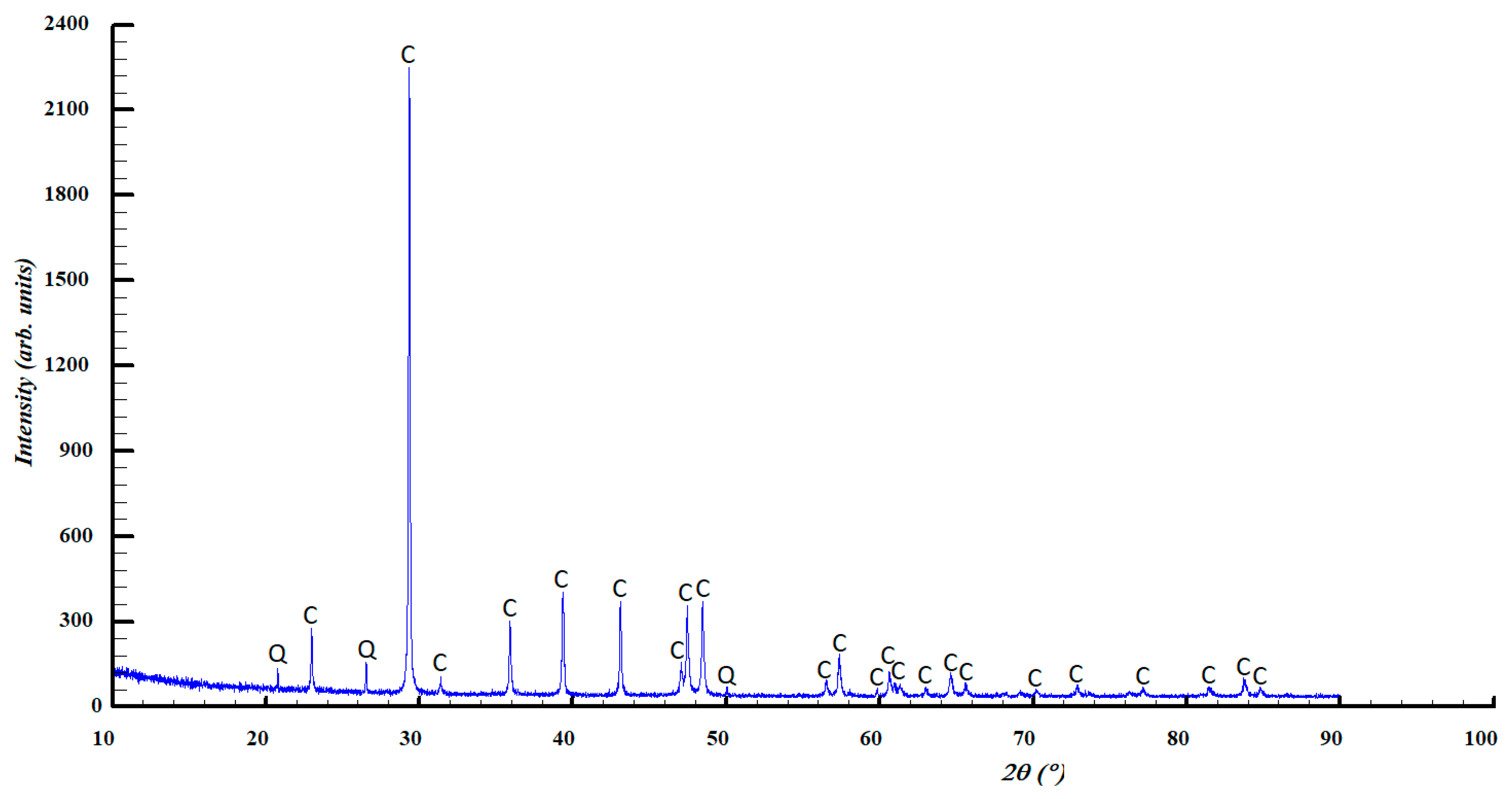
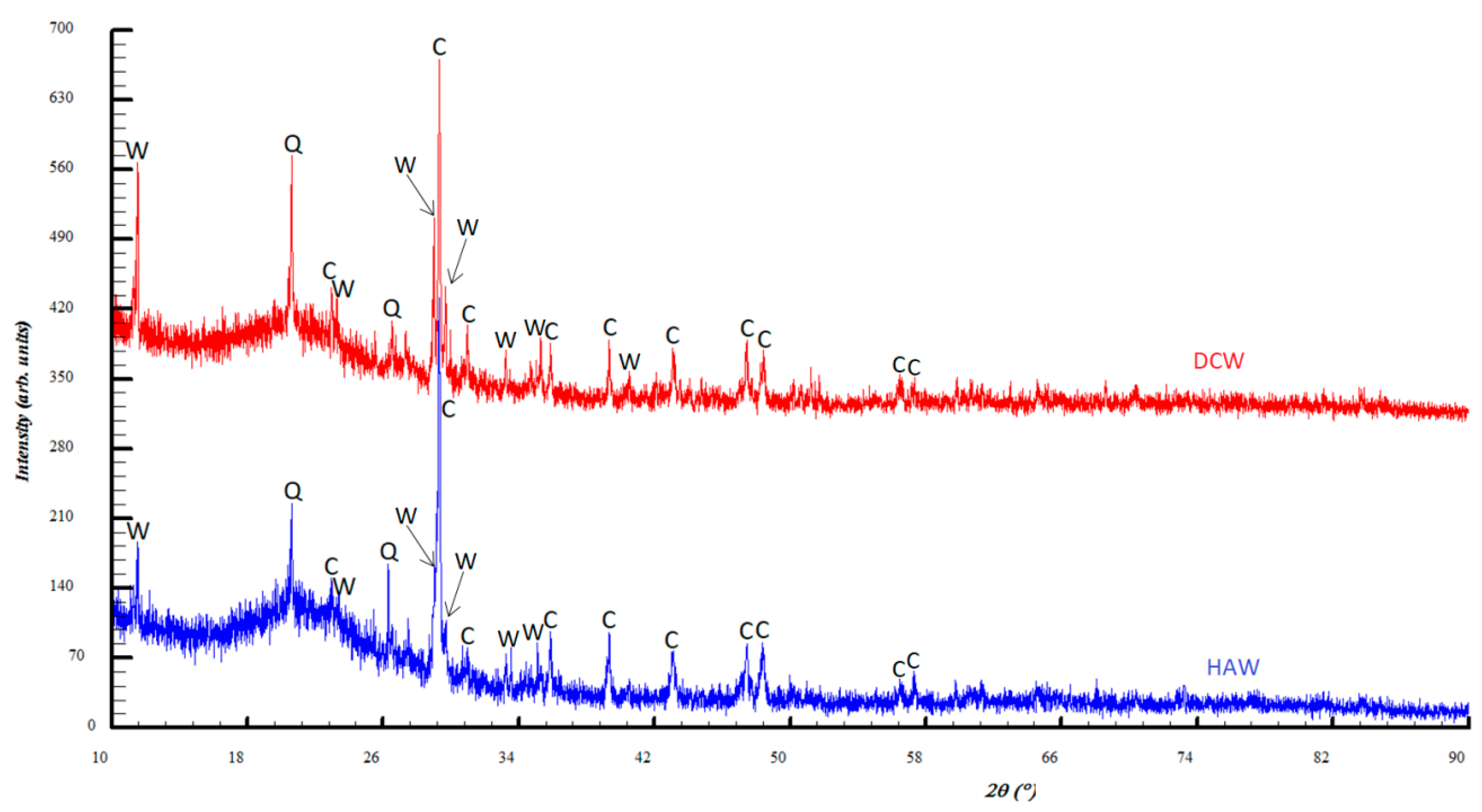
4. Results and Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in Art Conservation. Nat. Nanotechnol. 2015, 10, 287–290. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Fierascu, R.C. Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts. Nanomaterials 2022, 12, 207. [Google Scholar] [CrossRef]
- Pope, G.A.; Meierding, T.C.; Paradise, T.R. Geomorphology’s Role in the Study of Weathering of Cultural Stone. Geomorphology 2002, 47, 211–225. [Google Scholar] [CrossRef]
- Siegesmund, S.; Weiss, T.; Vollbrecht, A. Natural stone, weathering phenomena, conservation strategies and case studies: Introduction. Geol. Soc. Lond. Spec. Publ. 2002, 205, 1–7. [Google Scholar] [CrossRef]
- Huggett, R. Fundamentals of Geomorphology; Taylor & Francis: Manchester, UK, 2016. [Google Scholar]
- Zhang, G.; Gong, C.; Gu, J.; Katayama, Y.; Someya, T.; Gu, J.-D. Biochemical Reactions and Mechanisms Involved in the Biodeterioration of Stone World Cultural Heritage under the Tropical Climate Conditions. Int. Biodeterior. Biodegrad. 2019, 143, 104723. [Google Scholar] [CrossRef]
- Wild, B.; Gerrits, R.; Bonneville, S. The Contribution of Living Organisms to Rock Weathering in the Critical Zone. NPJ Mater. Degrad. 2022, 6, 98. [Google Scholar] [CrossRef]
- Sardella, A.; Palazzi, E.; von Hardenberg, J.; Del Grande, C.; De Nuntiis, P.; Sabbioni, C.; Bonazza, A. Risk Mapping for the Sustainable Protection of Cultural Heritage in Extreme Changing Environments. Atmosphere 2020, 11, 700. [Google Scholar] [CrossRef]
- Aktas, Y.D. Cities and Urban Heritage in the Face of a Changing Climate. Atmosphere 2021, 12, 1007. [Google Scholar] [CrossRef]
- Vyshkvarkova, E.; Sukhonos, O. Climate Change Impact on the Cultural Heritage Sites in the European Part of Russia over the Past 60 Years. Climate 2023, 11, 50. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Mitigation of Climate Change. In Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Dumitrescu, A.; Bojariu, R.; Birsan, M.-V.; Marin, L.; Manea, A. Recent Climatic Changes in Romania from Observational Data (1961–2013). Theor. Appl. Climatol. 2015, 122, 111–119. [Google Scholar] [CrossRef]
- Marin, L.; Birsan, M.-V.; Bojariu, R.; Dumitrescu, A.; Micu, D.M.; Manea, A. An Overview of Annual Climatic Changes in Romania: Trends in Air Temperature, Precipitation, Sunshine Hours, Cloud Cover, Relative Humidity and Wind Speed during the 1961–2013 Period. Carpathian J. Earth Environ. Sci. 2014, 9, 253–258. [Google Scholar]
- UNESCO (United Nations Educational, Scientific and Cultural Organization). The UNESCO Recommendation on the Historic Urban Landscape. Report of the Second Consultation on its Implementation by Member States. 2019. Available online: https://whc.unesco.org/document/172639 (accessed on 20 August 2023).
- Zeleňáková, M.; Purcz, P.; Hlavatá, H.; Blišťan, P. Climate Change in Urban Versus Rural Areas. Procedia Eng. 2015, 119, 1171–1180. [Google Scholar] [CrossRef]
- Masson, V.; Lemonsu, A.; Hidalgo, J.; Voogt, J. Urban Climates and Climate Change. Annu. Rev. Environ. Resour. 2020, 45, 411–444. [Google Scholar] [CrossRef]
- Hamdi, R.; Kusaka, H.; Doan QVan Cai, P.; He, H.; Luo, G.; Kuang, W.; Caluwaerts, S.; Duchêne, F.; Van Schaeybroek, B.; Termonia, P. The State-of-the-Art of Urban Climate Change Modeling and Observations. Earth Syst. Environ. 2020, 4, 631–646. [Google Scholar] [CrossRef]
- Liu, Z.; Zhan, W.; Bechtel, B.; Voogt, J.; Lai, J.; Chakraborty, T.; Wang, Z.-H.; Li, M.; Huang, F.; Lee, X. Surface Warming in Global Cities Is Substantially More Rapid than in Rural Background Areas. Commun. Earth Environ. 2022, 3, 219. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of Stone: A Review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef]
- Bungau, C.C.; Prada, I.F.; Prada, M.; Bungau, C. Design and operation of construction: A healthy living environment—Parametric studies and new solutions. Sustainability 2019, 11, 6824. [Google Scholar] [CrossRef]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for Antimicrobial Nanomaterials in Cultural Heritage Conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publications: Los Angeles, CA, USA, 2008; ISBN 9780892369393. [Google Scholar]
- Sterflinger, K. Fungi: Their Role in Deterioration of Cultural Heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomicrobiology of the Built Environment. Nat. Microbiol. 2017, 2, 16275. [Google Scholar] [CrossRef]
- De Leo, F.; Marchetta, A.; Urzì, C. Black Fungi on Stone-Built Heritage: Current Knowledge and Future Outlook. Appl. Sci. 2022, 12, 3969. [Google Scholar] [CrossRef]
- De la Torre, M.A.; Gomez-Alarcon, G.; Melgarejo, P.; Saiz-Jimenez, C. Fungi in Weathered Sandstone from Salamanca Cathedral, Spain. Sci. Total Environ. 1991, 107, 159–168. [Google Scholar] [CrossRef]
- Badalyan, A.G.; Gorbushina, A.A.; Krumbein, W.E. Physical and Microbiological Investigations of Rock Weathering at Hellenic Excavation Sites on the Crimean Peninsula. In Proceedings of the Eighth International Congress on Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996. [Google Scholar]
- Dornieden, T.; Gorbushina, A.A.; Krumbein, W.E. Patina: Physical and chemical interactions of sub-aerial biofilms with objects of art. In Of Microbes and Art—The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage; Springer: Boston, MA, USA, 2000; pp. 105–119. [Google Scholar]
- Gorbushina, A.A.; Krumbein, W.E.; Hamman, C.H.; Panina, L.; Soukharjevski, S.; Wollenzien, U. Role of black fungi in color change and biodeterioration of antique marbles. Geomicrobiol. J. 1993, 11, 205–221. [Google Scholar] [CrossRef]
- Caneva, G.; Maggi, O.; Nugari, M.P.; Pietrini, A.M.; Piervittori, V.; Ricci, S.; Roccardi, A. The biological aerosol as a factor of biodeterioration. In Cultural Heritage and Aerobiology—Methods and Measurement Techniques for Biodeterioration Monitoring; Mandrioli, P., Caneva, G., Sabbioni, C., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2003; pp. 3–29. [Google Scholar]
- Olteanu, I. Piatra în Patrimonioul Românesc: Degradări Specifice și Tratamente Adecvate; Editura ACS: București, Romania, 2015; 335p, ISBN 978-606-93583-8-2. [Google Scholar]
- Cinteză, L.O.; Tănase, M.A. Multifunctional ZnO Nanoparticle: Based Coatings for Cultural Heritage Preventive Conservation. In Thin Films; Ares, A.E., Ed.; IntechOpen: London, UK, 2020; 20p. [Google Scholar]
- Savković, Ž.; Unković, N.; Stupar, M.; Franković, M.; Jovanović, M.; Erić, S.; Šarić, K.; Stanković, S.; Dimkić, I.; Vukojević, J.; et al. Diversity and biodeteriorative potential of fungal dwellers on ancient stone stela. Int. Biodeterior. Biodegrad. 2016, 115, 212–223. [Google Scholar] [CrossRef]
- Chelazzi, D.; Camerini, R.; Giorgi, R.; Baglioni, P. Nanomaterials for the Consolidation of Stone Artifacts. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer: Cham, Switzerland, 2019; pp. 151–173. [Google Scholar] [CrossRef]
- Bogdan, A.; Chambre, D.; Copolovici, D.M.; Bungau, T.; Bungau, C.C.; Copolovici, L. Heritage Building Preservation in the Process of Sustainable Urban Development: The Case of Brasov Medieval City, Romania. Sustainability 2022, 14, 6959. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, O. Stone and related materials. In Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Nugari, M.P., Salvadori, O., Eds.; Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 128–144. [Google Scholar]
- Barakat, M.A.E.-F.; Kumar, R. Nanomaterials for Environmental Applications; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781000532852. [Google Scholar]
- Kanth, A.P.; Soni, A.K. Application of Nanocomposites for Conservation of Materials of Cultural Heritage. J. Cult. Herit. 2023, 59, 120–130. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Gómez-Morón, M.A.; Ortiz, P. Nanoparticles Applied to Stone Buildings. Int. J. Archit. Heritage Conserv. Anal. Restor. 2021, 15, 1320–1335. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Galeotti, M. Monitoring the Performance of Innovative and Traditional Biocides Mixed with Consolidants and Water-Repellents for the Prevention of Biological Growth on Stone. Sci. Total Environ. 2012, 423, 132–141. [Google Scholar] [CrossRef]
- Capitelli, F.; Dida, B.; Ventura, G.D.; Baldassarre, F.; Capelli, D.; Senesi, G.S.; Mele, A.; Siliqi, D. Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage. In Proceedings of the 2nd International Online Conference on Crystals, Bari, Italy, 10–20 November 2020; MDPI: Basel, Switzerland, 2021. [Google Scholar]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial Properties of Nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Caneva, G.; Fidanza, M.R.; Tonon, C.; Favero-Longo, S.E. Biodeterioration Patterns and Their Interpretation for Potential Applications to Stone Conservation: A Hypothesis from Allelopathic Inhibitory Effects of Lichens on the Caestia Pyramid (Rome). Sustain. Sci. Pract. Policy 2020, 12, 1132. [Google Scholar] [CrossRef]
- Jurcă, M.C.; Bembea, M.; Kozma, K.; Şandor, M.I.; Negrean, R.A.; Dobjanschi, L.; Cuc, E.A.; Petcheşi, C.D.; Jurcă, A.D. Empty sella associated with growth hormone deficiency and polydactyly. Rom. J. Morphol. Embryol. 2018, 59, 381–384. [Google Scholar] [PubMed]
- Taghiyari, H.R.; Majidinajafabadi, R.; Vahidzadeh, R. Wollastonite to Hinder Growth of Aspergillus Niger Fungus on Cotton Textile. An. Da Acad. Bras. De Cienc. 2018, 90, 2797–2804. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Kalantari, A.; Kalantari, A.; Avramidis, S. Effect of Wollastonite Nanofibers and Exposure to Aspergillus Niger Fungus on Air Flow Rate in Paper. Measurement 2019, 136, 307–313. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Enayati, A.; Gholamiyan, H. Effects of nano-silver impregnation on brittleness, physical and mechanical properties of heat-treated hardwoods. Wood Sci. Technol. 2013, 47, 467–480. [Google Scholar] [CrossRef]
- Taghiyari, H.R. Effects of heat-treatment on permeability of untreated and nanosilver-impregnated native hardwoods. MaderasCienc. Tecnol. 2013, 15, 183–194. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Samandarpour, A. Effects of nanosilver-impregnation and heat treatment on coating pull-off adhesion strength on solid wood. Drvna Ind. 2015, 66, 321–327. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Esmailpour, A.; Papadopoulos, A. Paint pull-off strength and permeability in nanosilver-impregnated and heat-treated beech wood. Coatings 2019, 9, 723. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Bayani, S.; Militz, H.; Papadopoulos, A.N. Heat treatment of pine wood: Possible effect of impregnation with silver nanosuspension. Forests 2020, 11, 466. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Esmailpour, A.; Majidi, R.; Hassani, V.; Abdolah Mirzaei, R.; Farajpour Bibalan, O.; Papadopoulos, A.N. The effect of silver and copper nanoparticles as resin fillers on less-studied properties of UF-based particleboards. Wood Mater. Sci. Eng. 2020, 17, 317–327. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Ilies, D.C.; Antov, P.; Vasile, G.; Majidinajafabadi, R.; Lee, S.H. Effects of Nanosilver and Heat Treatment on the Pull-Off Strength of Sealer-Clear Finish in Solid Wood Species. Polymers 2022, 14, 5516. [Google Scholar] [CrossRef] [PubMed]
- Terzi, E.; Kartal, S.N.; Yılgör, N.; Rautkari, L.; Yoshimura, T. Role of Various Nano-Particles in Prevention of Fungal Decay, Mold Growth and Termite Attack in Wood, and Their Effect on Weathering Properties and Water Repellency. Int. Biodeterior. Biodegrad. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Tichi, A.H.; Bari, E.; Nicholas, D.D. How Nano-Wollastonite Can Change the Fundamental Properties of a Wood Fibre and Rice Straw Composites? IET Nanobiotechnol. 2018, 12, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Weththimuni, M.L.; Capsoni, D.; Malagodi, M.; Licchelli, M. Improving Wood Resistance to Decay by Nanostructured ZnO-Based Treatments. J. Nanomater. 2019, 2019, 6715756. [Google Scholar] [CrossRef]
- Huuskonen, M.S.; Järvisalo, J.; Koskinen, H.; Nickels, J.; Räsänen, J.; Asp, S. Preliminary results from a cohort of workers exposed to wollastonite in a Finnish limestone quarry. Scand. J. Work. Environ. Health 1983, 9, 169–175. Available online: http://www.jstor.org/stable/40964396 (accessed on 1 September 2023). [CrossRef] [PubMed]
- Huuskonen, M.S.; Tossavainen, A.; Koskinen, H.; Zitting, A.; Korhonen, O.; Nickels, J.; Korhonen, K.; Vaaranen, V. Wollastonite exposure and lung fibrosis. Environ. Res. 1983, 30, 291–304. [Google Scholar] [CrossRef]
- Maxim, L.D.; McConnell, E.E. A Review of the Toxicology and Epidemiology of Wollastonite. Inhal. Toxicol. 2005, 17, 451–466. [Google Scholar] [CrossRef]
- Aitken, E. Analyses of the Effect of Silicon on Fusarium Wilt on Banana. 2010. Available online: https://espace.library.uq.edu.au/view/UQ:270994 (accessed on 1 September 2023).
- Abd Rashid, R.; Shamsudin, R.; Abdul Hamid, M.A.; Jalar, A. In-vitro bioactivity of wollastonite materials derived from limestone and silica sand. Ceram. Int. 2014, 40, 6847–6853. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage stone surfaces and frescoes: Which delivery system can be the most appropriate? Ann. Microbiol. 2014, 65, 1227–1241. [Google Scholar] [CrossRef]
- Karimi, A.; Taghiyari, H.R.; Fattahi, A.; Karimi, S.; Ebrahimi, G.; Tarmian, A. Effects of Wollastonite Nanofibers on Biological Durability of Poplar Wood (Populus nigra) against Trametes versicolor. BioResources 2013, 8, 4134–4141. [Google Scholar] [CrossRef]
- Dei, L.; Salvadori, B. Nanotechnology in Cultural Heritage Conservation: Nanometric Slaked Lime Saves Architectonic and Artistic Surfaces from Decay. J. Cult. Herit. 2006, 7, 110–115. [Google Scholar] [CrossRef]
- D’Arienzo, L.; Scarfato, P.; Incarnato, L. New Polymeric Nanocomposites for Improving the Protective and Consolidating Efficiency of Tuff Stone. J. Cult. Herit. 2008, 9, 253–260. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for Conservation of Bio-Calcarenite Stone. Appl. Phys. A Mater. Sci. Process. 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Aldoasri, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustain. Sci. Pract. Policy 2017, 9, 2002. [Google Scholar] [CrossRef]
- Bruno, L.; Rugnini, L.; Spizzichino, V.; Caneve, L.; Canini, A.; Ellwood, N.T.W. Biodeterioration of Roman Hypogea: The Case Study of the Catacombs of SS. Marcellino and Pietro (Rome, Italy). Ann. Microbiol. 2019, 69, 1023–1032. [Google Scholar] [CrossRef]
- Zarzuela, R.; Carbú, M.; Gil, M.L.A.; Cantoral, J.M.; Mosquera, M.J. CuO/SiO2 Nanocomposites: A Multifunctional Coating for Application on Building Stone. Mater. Des. 2017, 114, 364–372. [Google Scholar] [CrossRef]
- Ion, R.M.; Fierăscu, R.C.; Fierăscu, I.; Bunghez, I.R.; Ion, M.L.; Caruţiu-Turcanu, D.; Teodorescu, S.; Rădiţoiu, V. Stone Monuments Consolidation with Nanomaterials. Key Eng. Mater. 2015, 660, 383–388. [Google Scholar] [CrossRef]
- Gallo, C.; Rizzo, P.; Guerra, G. Intercalation Compounds of a Smectite Clay with an Ammonium Salt Biocide and Their Possible Use for Conservation of Cultural Heritage. Heliyon 2019, 5, e02991. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Using ZnO Nanoparticles in Fungal Inhibition and Self-Protection of Exposed Marble Columns in Historic Sites. Archaeol. Anthropol. Sci. 2019, 11, 3407–3422. [Google Scholar] [CrossRef]
- Xie, Z.; Duan, Z.; Zhao, Z.; Li, R.; Zhou, B.; Yang, D.; Hu, Y. Nano-Materials Enhanced Protectants for Natural Stone Surfaces. Herit. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Chobba, M.B.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F. Nanoparticles in the Field of Built Heritage Restoration: Challenges and Limits. In Handbook of Cultural Heritage Analysis; D’Amico, S., Venuti, V., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1033–1050. ISBN 9783030600167. [Google Scholar]
- Weththimuni, M.L.; Chobba, M.B.; Tredici, I.; Licchelli, M. ZrO2-doped ZnO-PDMS nanocomposites as protective coatings for the stone materials. Acta IMEKO 2022, 11, 5. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.M.M.; Khallaf, M.K. Biological Nanosilver Particles for the Protection of Archaeological Stones against Microbial Colonization. Int. Biodeterior. 2014, 94, 31–37. [Google Scholar] [CrossRef]
- Munafò, P.; Quagliarini, E.; Goffredo, G.B.; Bondioli, F.; Licciulli, A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014, 65, 218–231. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-Based Nanocoatings for Preserving Architectural Stone Surfaces: An Overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafò, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Quagliarini, E.; Graziani, L.; Diso, D.; Licciulli, A.; D’Orazio, M. Is Nano-TiO 2 Alone an Effective Strategy for the Maintenance of Stones in Cultural Heritage? J. Cult. Herit. 2018, 30, 81–91. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Vasanelli, E.; Calia, A.; Masieri, M.; Baldi, G. Stone Consolidation with SiO2 Nanoparticles: Effects on a High Porosity Limestone. Constr. Build. Mater. 2019, 219, 154–163. [Google Scholar] [CrossRef]
- Zornoza-Indart, A.; Lopez-Arce, P. Silica Nanoparticles (SiO2): Influence of Relative Humidity in Stone Consolidation. J. Cult. Herit. 2016, 18, 258–270. [Google Scholar] [CrossRef]
- Esmailpour, A.; Taghiyari, H.R.; Majidi, R.; Babaali, S.; Morrell, J.J.; Mohammadpanah, B. Effects of Adsorption Energy on Air and Liquid Permeability of Nanowollastonite-Treated Medium-Density Fiberboard. IEEE Trans. Instrum. Meas. 2021, 70, 1000108. [Google Scholar] [CrossRef]
- Bartoli, F.; Isola, D.; Casanova Municchia, A.; Kumbaric, A.; Caneva, G. Science for Art: Multi-Years’ Evaluations of Biocidal Efficacy in Support of Artwork Conservation. Front. Microbiol. 2023, 14, 1178900. [Google Scholar] [CrossRef] [PubMed]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Bouaziz, J.; De Leo, F.; Licchelli, M. Ag-TiO2/PDMS Nanocomposite Protective Coatings: Synthesis, Characterization, and Use as a Self-Cleaning and Antimicrobial Agent. Prog. Org. Coat. 2021, 158, 106342. [Google Scholar] [CrossRef]
- De Leo, F.; Marchetta, A.; Capillo, G.; Germanà, A.; Primerano, P.; Schiavo, S.L.; Urzì, C. Surface Active Ionic Liquids Based Coatings as Subaerial Anti-Biofilms for Stone Built Cultural Heritage. Coat. World 2020, 11, 26. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone-Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Isola, D.; Bartoli, F.; Meloni, P.; Caneva, G.; Zucconi, L. Black Fungi and Stone Heritage Conservation: Ecological and Metabolic Assays for Evaluating Colonization Potential and Responses to Traditional Biocides. Appl. Sci. 2022, 12, 2038. [Google Scholar] [CrossRef]
- Pașca, M. Oradea 1900: Un Ghid de Arhitectură, Ed. a 3-a; Editura Argonaut: Cluj-Napoca, Romania, 2019; ISBN 978-973-109-932-3. [Google Scholar]
- Pașca, M. Arhitectul Frigyes Spiegel la Oradea; Editura Arca: Oradea, Romania, 2010; ISBN 978-973-1881-49-2. [Google Scholar]
- Santo, A.P.; Cuzman, O.A.; Petrocchi, D.; Pinna, D.; Salvatici, T.; Perito, B. Black on white: Microbial growth darkens the external marble of Florence cathedral. Appl. Sci. 2021, 11, 6163. [Google Scholar] [CrossRef]
- Sert, H.B.; Sümbül, H.; Sterflinger, K. Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey). Antonie Van Leeuwenhoek J. Microb. 2007, 91, 217–227. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Zelenskaya, M.S.; Vlasov, A.D.; Bobir, S.Y.; Yakkonen, K.L.; Vlasov, D.Y. Microorganisms in superficial deposits on the stone monuments in Saint Petersburg. Microorganisms 2022, 10, 316. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E.; Uslu, H.; Ozbek, A.; Ipekoglu, B.; Ocak, I.; Hasenekoglu, I. The accelerating effects of the microorganisms on biodeterioration of stone monuments under air pollution and continental-cold climatic conditions in Erzurum, Turkey. Sci. Total Environ. 2006, 364, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Trovão, J.; Gil, F.; Catarino, L.; Soares, F.; Tiago, I.; Portugal, A. Analysis of fungal deterioration phenomena in the first Portuguese King tomb using a multianalytical approach. Int. Biodeterior. Biodegrad. 2020, 149, 104933. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Heyrman, J.; Dornieden, T.; Gonzalez-Delvalle, M.; Krumbein, W.E.; Laiz, L.; Petersen, K.; Saiz-Jimenez, C.; Swings, J. Bacterial and fungal diversity and biodeterioration problems in mural painting environments of St. Martins church (Greene–Kreiensen, Germany). Int. Biodeterior. Biodegrad. 2004, 53, 13–24. [Google Scholar] [CrossRef]
- Burford, E.P.; Kierans, M.; Gadd, G.M. Geomycology: Fungi in mineral substrata. Mycologist 2003, 17, 98–107. [Google Scholar] [CrossRef]
- Wollenzien, U.; de Hoog, G.S.; Krumbein, W.E.; Urzí, C. On the Isolation of Microcolonial Fungi Occurring on and in Marble and Other Calcareous Rocks. Sci. Total Environ. 1995, 167, 287–294. [Google Scholar] [CrossRef]
- Pangallo, D.; Chovanová, K.; Simonovicová, A.; Ferianc, P. Investigation of Microbial Community Isolated from Indoor Artworks and Air Environment: Identification, Biodegradative Abilities, and DNA Typing. Can. J. Microbiol. 2009, 55, 277–287. [Google Scholar] [CrossRef]
- Dyda, M.; Pyzik, A.; Wilkojc, E.; Kwiatkowska-Kopka, B.; Sklodowska, A. Bacterial and Fungal Diversity Inside the Medieval Building Constructed with Sandstone Plates and Lime Mortar as an Example of the Microbial Colonization of a Nutrient-Limited Extreme Environment (Wawel Royal Castle, Krakow, Poland). Microorganisms 2019, 7, 416. [Google Scholar] [CrossRef]
- Ljaljevic-Grbic, M.V.; Vukojevic, J.B. Role of Fungi in Biodeterioration Process of Stone in Historic Buildings. Zbornik Matice Srpske za Prirodne Nauke 2009, 116, 245–251. [Google Scholar] [CrossRef]
- Palla, F. Biotechnology and Cultural Heritage Conservation. In Heritage; Turcanu-Carutiu, D., Ed.; IntechOpen: London, UK, 2020; pp. 239–254. [Google Scholar]
- Hu, Z.; Zhang, R.; Zhu, K.; Li, D.; Jin, Y.; Guo, W.; Liu, X.; Zhang, X.; Zhang, Q. Probing the Pore Structure of the Berea Sandstone by Using X-Ray Micro-CT in Combination with ImageJ Software. Minerals 2023, 13, 360. [Google Scholar] [CrossRef]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the Fungus Aspergillus a Threat to Cultural Heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Vohra, S.K.; Prodanov, D. The Active Segmentation Platform for Microscopic Image Classification and Segmentation. Brain Sci. 2021, 11, 1645. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Lormand, C.; Zellmer, G.F.; Németh, K.; Kilgour, G.; Mead, S.; Palmer, A.S.; Sakamoto, N.; Yurimoto, H.; Moebis, A. Weka Trainable Segmentation Plugin in ImageJ: A Semi-Automatic Tool Applied to Crystal Size Distributions of Microlites in Volcanic Rocks. Microsc. Microanal. 2018, 24, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cairns, T.C.; Feurstein, C.; Zheng, X.; Zheng, P.; Sun, J.; Meyer, V. A Quantitative Image Analysis Pipeline for the Characterization of Filamentous Fungal Morphologies as a Tool to Uncover Targets for Morphology Engineering: A Case Study Using AplD in Aspergillus Niger. Biotechnol. Biofuels 2019, 12, 149. [Google Scholar] [CrossRef]
- Purswani, P.; Karpyn, Z.T.; Enab, K.; Xue, Y.; Huang, X. Evaluation of Image Segmentation Techniques for Image-Based Rock Property Estimation. J. Pet. Sci. Eng. 2020, 195, 107890. [Google Scholar] [CrossRef]
- Nazem-Bokaee, H.; Fallahianbijan, F.; Chen, D.; O’Donnell, S.M.; Carbrello, C.; Giglia, S.; Bell, D.; Zydney, A.L. Probing pore structure of virus filters using scanning electron microscopy with gold nanoparticles. J. Membr. Sci. 2018, 552, 144–152. [Google Scholar] [CrossRef]
- Fernandez-Carrillo, A.; Franco-Nieto, A.; Pinto-Bañuls, E.; Basarte-Mena, M.; Revilla-Romero, B. Designing a Validation Protocol for Remote Sensing Based Operational Forest Masks Applications. Comparison of Products Across Europe. Remote Sens. 2020, 12, 3159. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Congalton, R.G. Accuracy Assessment and Validation of Remotely Sensed and Other Spatial Information. Int. J. Wildl. Fire 2001, 10, 321–328. [Google Scholar] [CrossRef]
- Hesse, K.-F. Refinement of the crystal structure of wollastonite-2M (parawollastonite). Z. Fur Krist. 1984, 168, 93–98. [Google Scholar] [CrossRef]
- Szantoi, Z.; Escobedo, F.; Abd-Elrahman, A.; Smith, S.; Pearlstine, L. Analyzing Fine-Scale Wetland Composition Using High Resolution Imagery and Texture Features. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 204–212. [Google Scholar] [CrossRef]






| Weight (g) | Initial | After Wetting (Distilled Water) (Day 1) | After Fungal Inoculation (Day 1) | Before Applying Aqueous Suspension of Wollastonite (Day 4) | After Applying Nano Aqueous Suspension of Wollastonite (Damp) (Day 4) | After Gel Dried (48 h Post-Application) (Day 6) | Size L × W × H (mm) |
|---|---|---|---|---|---|---|---|
| Milestone R1 | 9.34 | - | - | - | - | - | 23/22/7 |
| Milestone R2 | 8.42 | - | - | - | - | - | 22/21/6 |
| Sample 1 A | 6.69 | 6.81 | 6.82 | 6.95 | 7.01 (+ 0.06 g gel) | 6.97 | 20/20/5 |
| Sample 2 E | 7.44 | 7.65 | 7.70 | 7.70 | 7.84 (+ 0.14 g gel) | 7.66 | 21/20/5 |
| Component | Proportion (%w/w) |
|---|---|
| SiO2 | 46.96 |
| CaO | 39.77 |
| Water | 4.67 |
| Al2O3 | 3.95 |
| Fe2O3 | 2.79 |
| MgO | 1.39 |
| TiO2 | 0.22 |
| Na2O | 0.16 |
| SO3 | 0.05 |
| K2O | 0.04 |
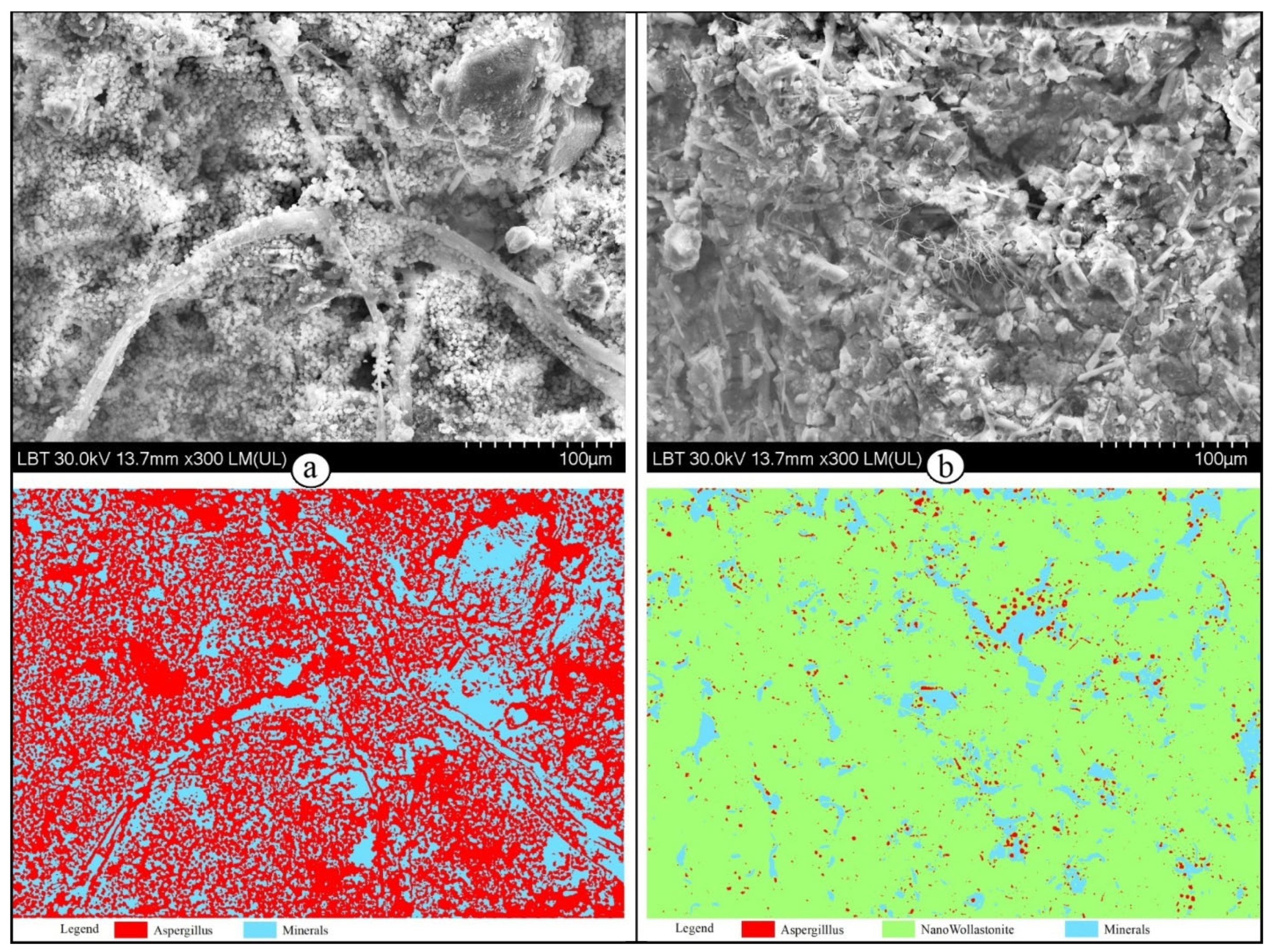
| Sample | Class | Pixel Count | Percent (%) | Total Pixel Count |
|---|---|---|---|---|
| Aspergillus spp. | Aspergillus | 679,995 | 59.33 | 1,145,984 |
| Minerals | 465,989 | 40.67 | ||
| Aspergillus spp. aqueous suspension of wollastonite | Aspergillus | 25,614 | 2.23 | 1,145,984 |
| Minerals | 111,056 | 9.69 | ||
| Aqueous suspension of wollastonite | 1,009,314 | 88.08 | ||
| Cladosporium spp. | Cladosporium | 420,198 | 36.67 | 1,145,984 |
| Class 2 (background) | 44,860 | 3.91 | ||
| Class 3 (minerals) | 36,917 | 3.22 | ||
| Class 4 (minerals) | 644,009 | 56.2 | ||
| Cladosporium spp. aqueous suspension of wollastonite | Cladosporium | 2801 | 0.25 | 1,145,984 |
| Class 2 (fractures and minerals) | 160,966 | 14.04 | ||
| Aqueous suspension of wollastonite | 982,217 | 85.71 |
| Accuracy Measures from WEKA | Accuracy Measures from ENVI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Class | R (%) | P (%) | CCI (%) | k | PA (%) | UA (%) | OA (%) | k |
| Aspergillus spp. | Aspergillus | 99.5 | 97.9 | 98.2 | 0.96 | 98.27 | 69.98 | 89.5 | 0.74 |
| Minerals | 95.8 | 98.9 | 86.77 | 99.38 | |||||
| Aspergillus spp. aqueous suspension of wollastonite | Aspergillus | 98.8 | 71.7 | 98.9 | 0.94 | 92.84 | 76.44 | 98.5 | 0.92 |
| Minerals | 99.7 | 100 | 99.61 | 93.15 | |||||
| Aqueous suspension of wollastonite | 100 | 98.9 | 98.57 | 99.78 | |||||
| Cladosporium spp. | Cladosporium | 95.6 | 93 | 94.5 | 0.91 | 94.92 | 85.16 | 87.2 | 0.79 |
| Class 2 (background) | 99.8 | 99.8 | 100 | 100 | |||||
| Class 3 (minerals) | 87.7 | 96.4 | 82.71 | 84.11 | |||||
| Class 4 (minerals) | 95.7 | 95.2 | 75.62 | 92.23 | |||||
| Cladosporium spp. aqueous suspension of wollastonite | Cladosporium | 65.9 | 82.8 | 98.6 | 0.96 | 74.27 | 94.11 | 97.1 | 0.94 |
| Minerals and fractures | 98.2 | 96.9 | 94.69 | 99.8 | |||||
| Aqueous suspension of wollastonite | 99.1 | 99.3 | 99.9 | 95.63 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilies, D.C.; Blaga, L.; Ilies, A.; Pereș, A.C.; Caciora, T.; Hassan, T.H.; Hodor, N.; Turza, A.; Taghiyari, H.R.; Barbu-Tudoran, L.; et al. Green Biocidal Nanotechnology Use for Urban Stone-Built Heritage—Case Study from Oradea, Romania. Minerals 2023, 13, 1170. https://doi.org/10.3390/min13091170
Ilies DC, Blaga L, Ilies A, Pereș AC, Caciora T, Hassan TH, Hodor N, Turza A, Taghiyari HR, Barbu-Tudoran L, et al. Green Biocidal Nanotechnology Use for Urban Stone-Built Heritage—Case Study from Oradea, Romania. Minerals. 2023; 13(9):1170. https://doi.org/10.3390/min13091170
Chicago/Turabian StyleIlies, Dorina Camelia, Lucian Blaga, Alexandru Ilies, Ana Cornelia Pereș, Tudor Caciora, Thowayeb H. Hassan, Nicolaie Hodor, Alexandru Turza, Hamid R. Taghiyari, Lucian Barbu-Tudoran, and et al. 2023. "Green Biocidal Nanotechnology Use for Urban Stone-Built Heritage—Case Study from Oradea, Romania" Minerals 13, no. 9: 1170. https://doi.org/10.3390/min13091170







