Insights in the Physicochemical and Mechanical Properties and Characterization Methodology of Perlites
Abstract
:1. Introduction
- As frothy material consisting of tiny, thin-walled glassy bubbles, expanded perlite is friable and vulnerable to fragmentation that will alter its composition if not treated accordingly.
- It presents a very low bulk density that may reach even 30 kg/m3 and in mass-based dosages it might exceed capacity of equipment.
- Samples present non-uniform composition, since further to expanded grains of different bulk and apparent density, consist of non-expandable and unexpanded particles, and grains fragments of different size, favoring segregation [6]. Thus, further to good homogenization, a proper sampling technique should be applied to obtain representative samples.
- Each application sets a different specification, thus expanded perlite grades that are intended for different uses present radically different characteristics, mainly in terms of size, morphology, strength, density and porosity, thus the characterization strategy should be focused accordingly.
2. Materials and Methods
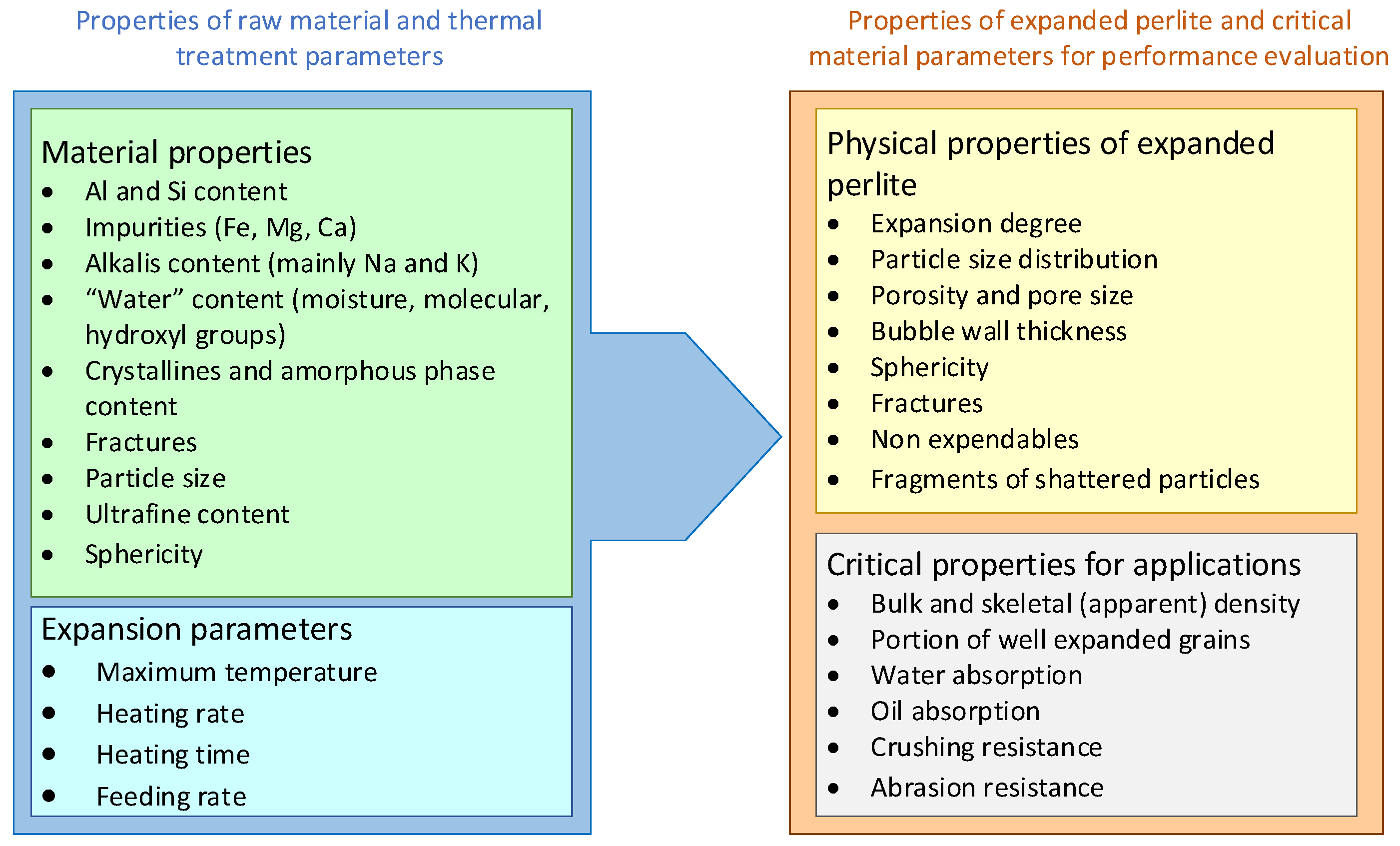
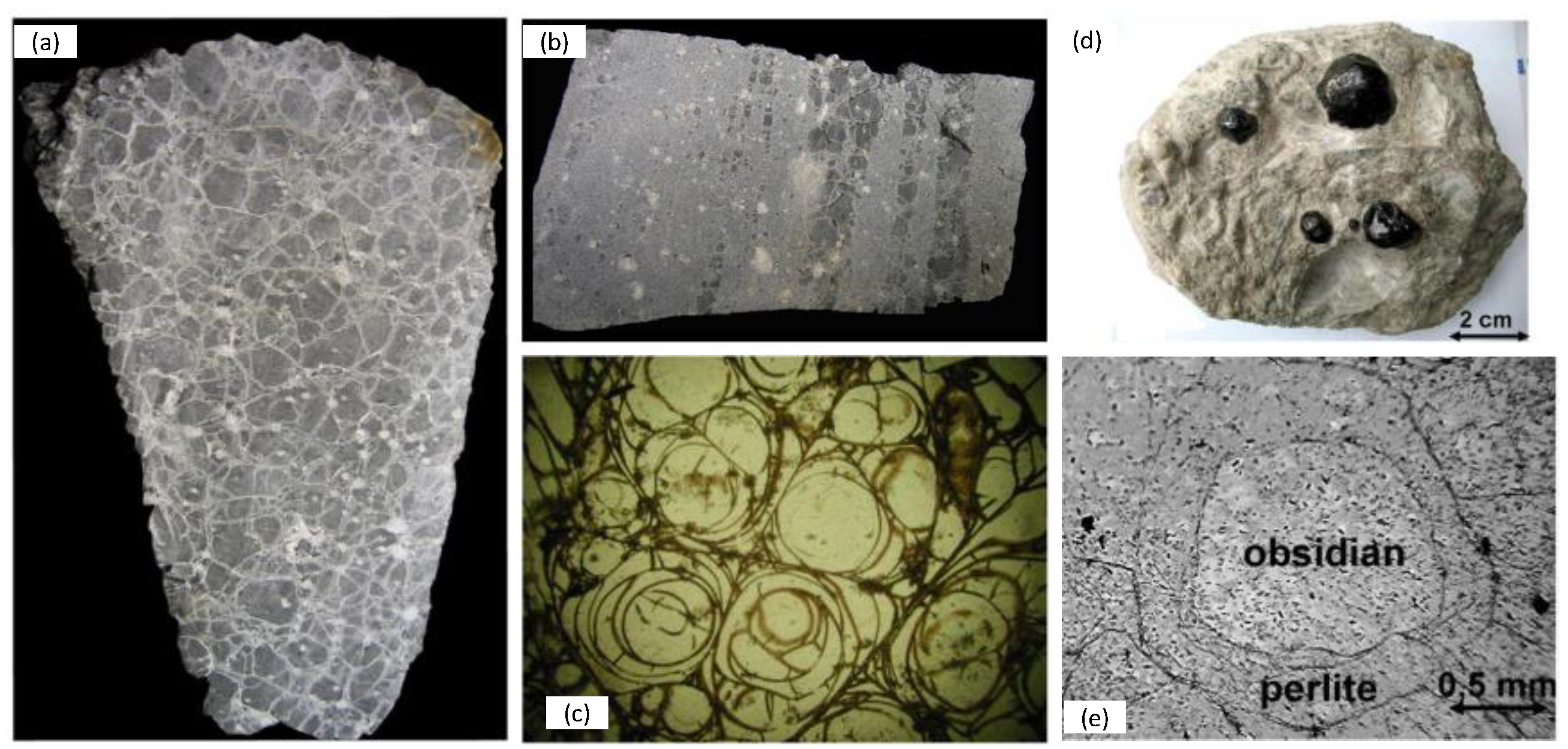
2.1. Ore Genesis, Morphology and Physical Properties
2.2. Chemical and Mineralogical Composition
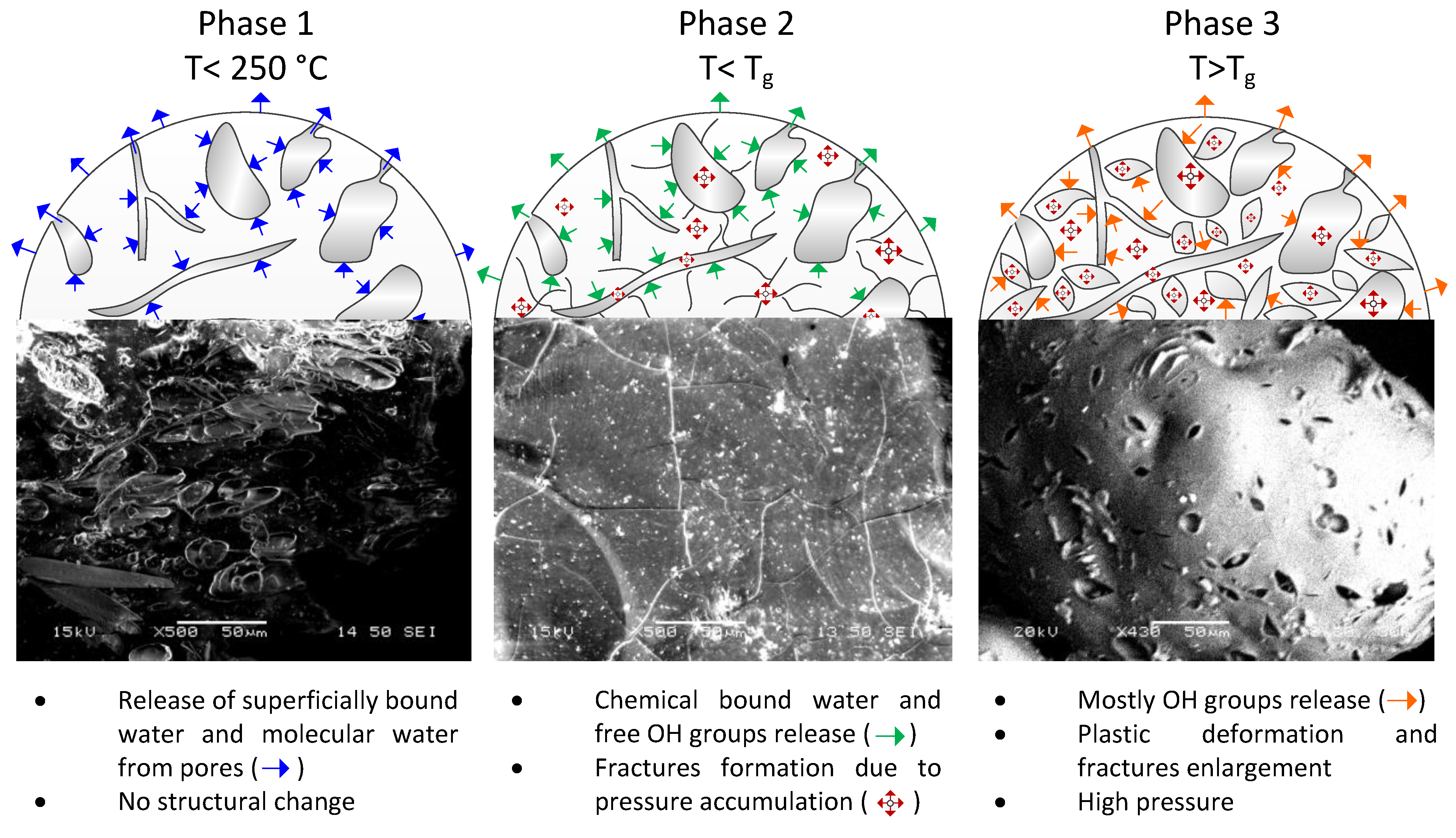
2.3. Perlite Expansion Mechanism
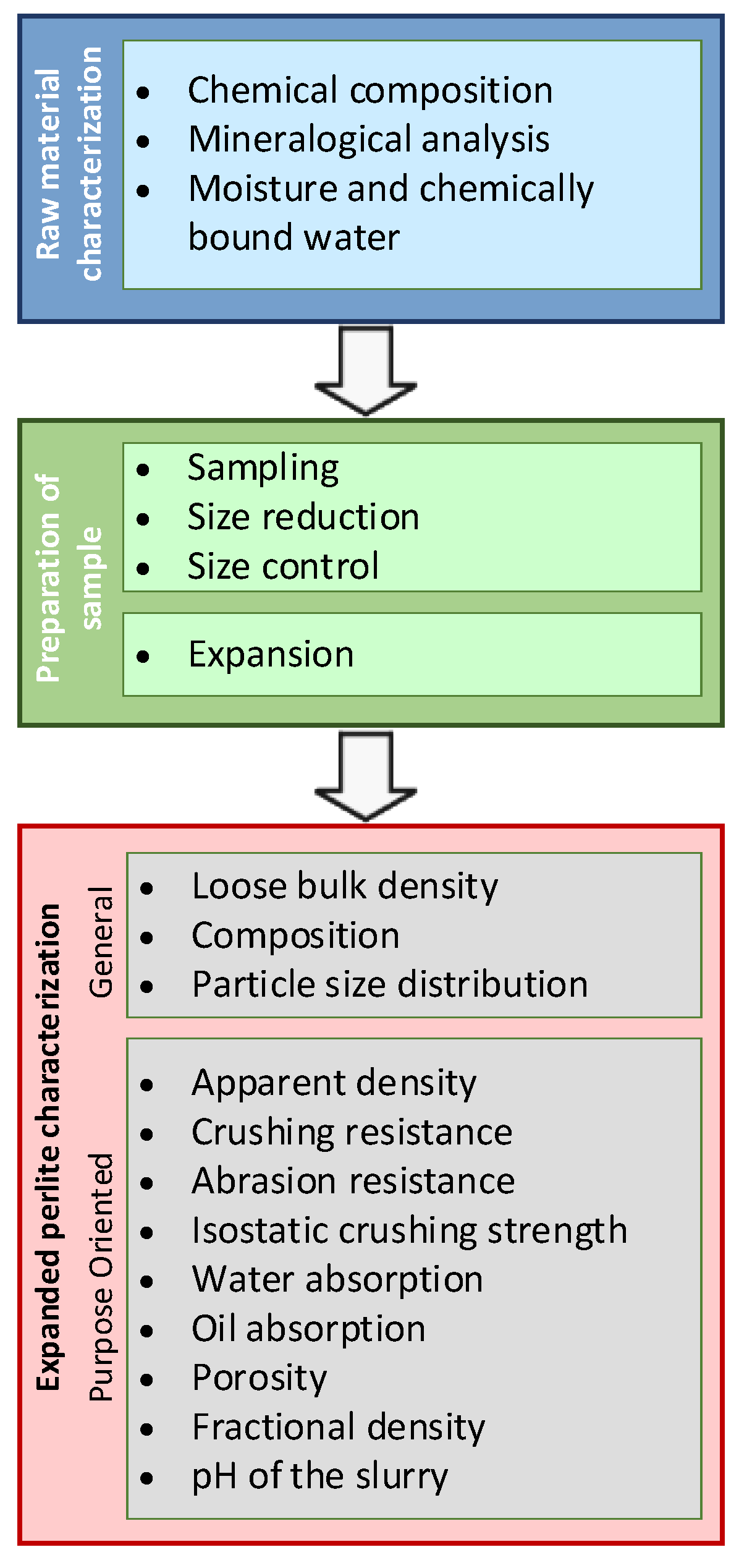
3. Methods for Characterization of Raw and Expanded Perlite
3.1. Sampling and Sample Preparation (Size Reduction)
3.2. Density and Size Distribution
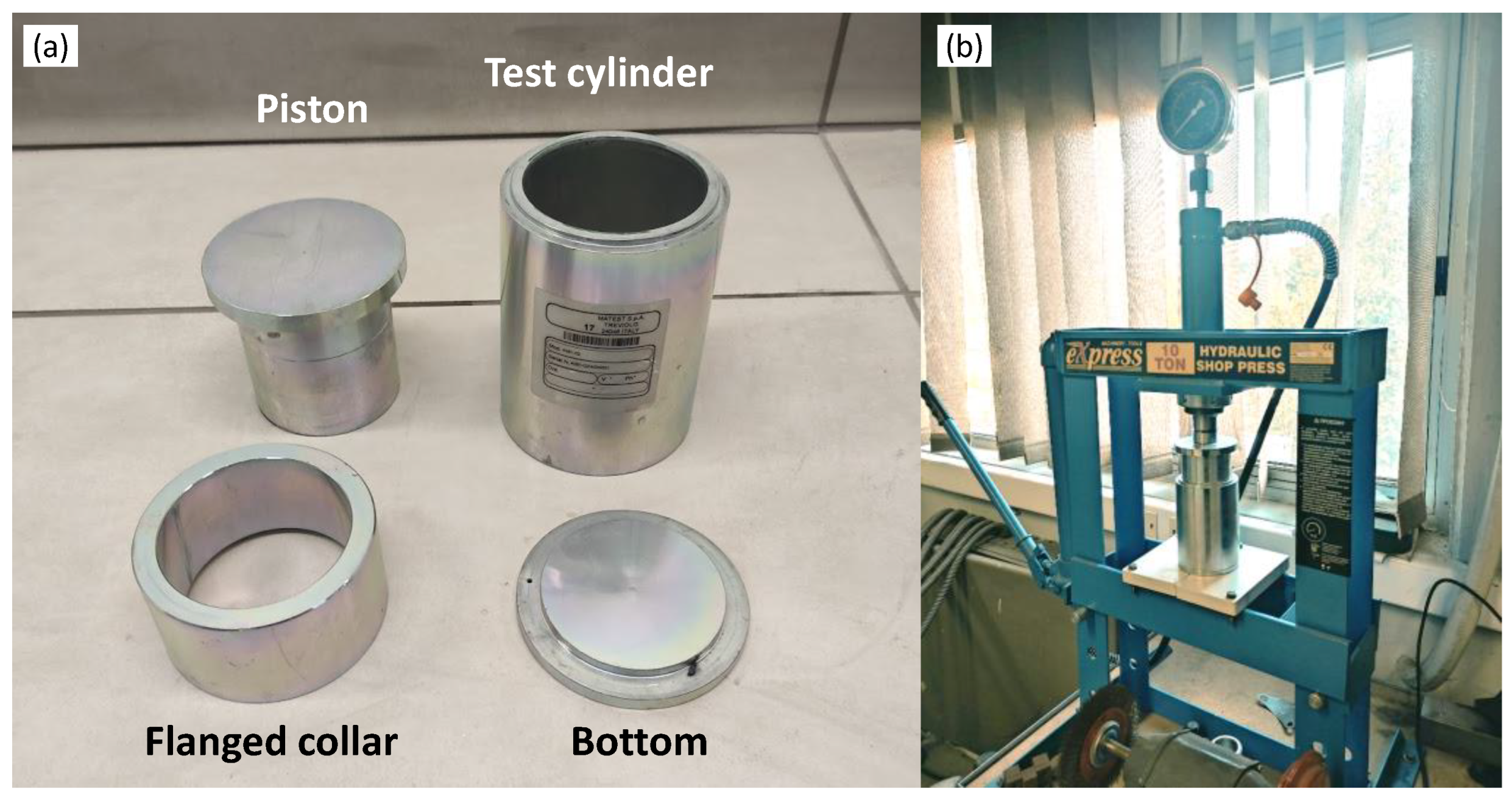
3.3. Strength-Related Testing
3.4. Water Repellency/Absorption and Oil Absorption
3.5. Microstructural Composition of the Sample
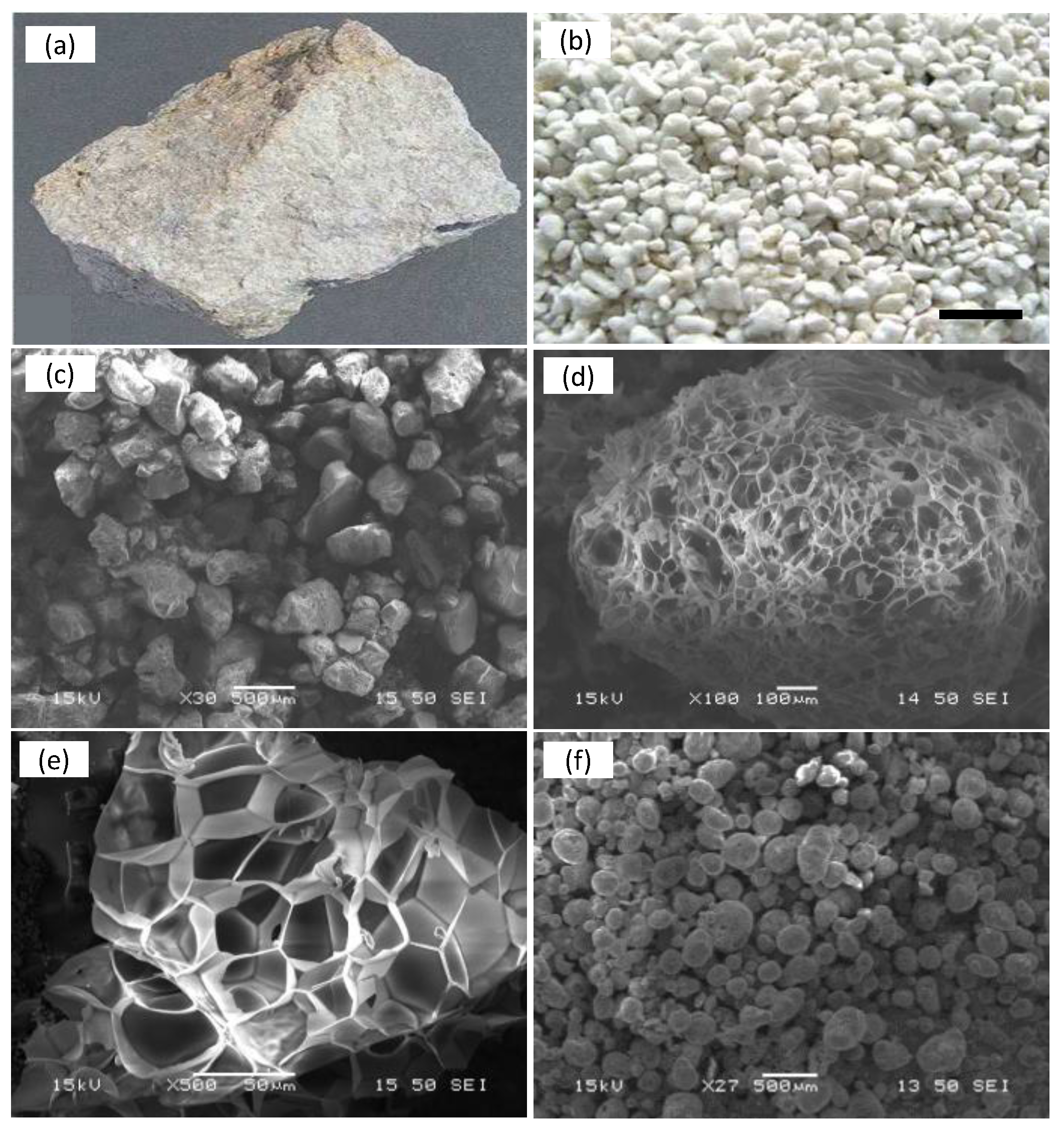
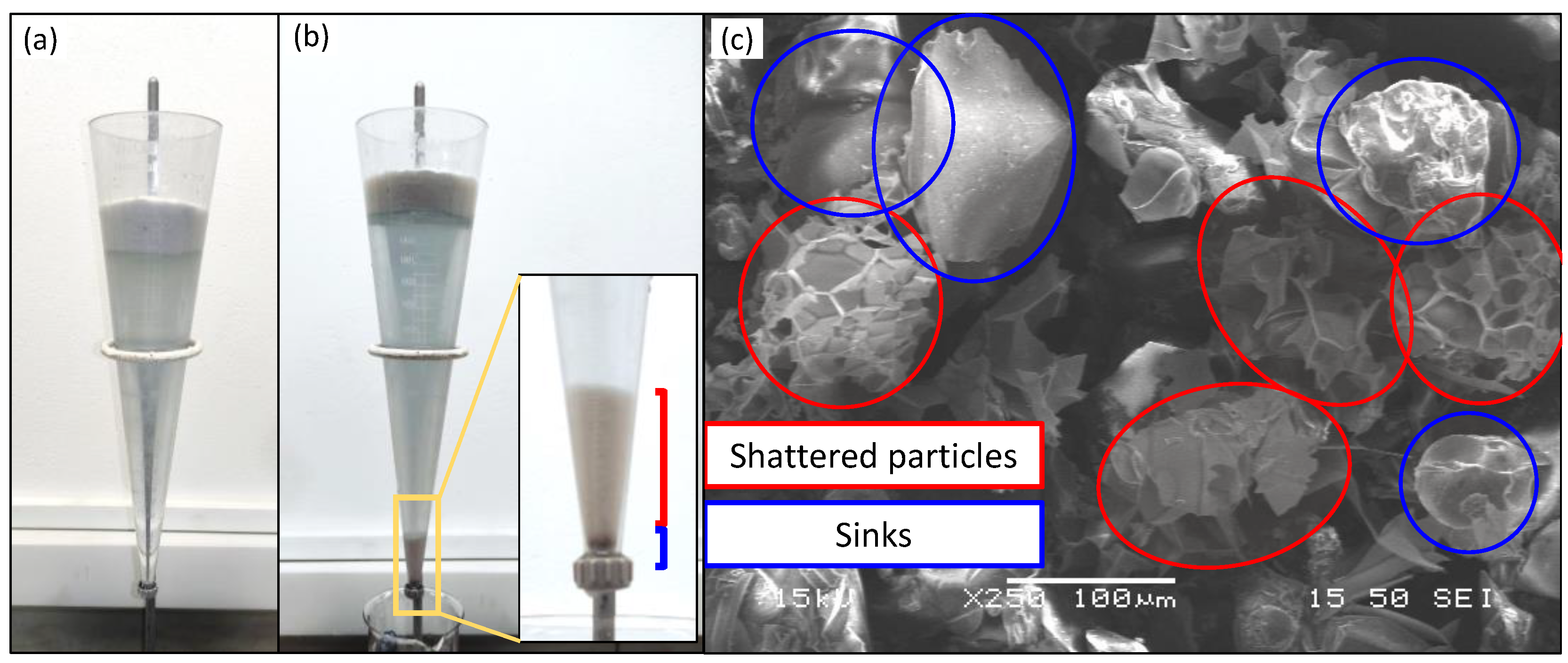
- Expanded grains: white grains with cellular structure and rounded edges, having open or closed surface porosity. In adequately expanded perlite, the majority of the perlite grains are of this category. Expanded grains float once inserted in the water due to the low density.
- Unexpanded grains: are the non-crystalline, dark colored impurities commonly present in perlite such as obsidian and non-expandable crystalline minerals (quartz, biotite, plagioclase, feldspar, mica, hematite, magnetite, ilmenite, zircon and spinel, etc.). Sometimes, small portions of the feed may remain unexpanded due to the applied expansion conditions. Due to fact that their specific gravity exceeds 1, both non-expandable and unexpanded perlite grains, sink rapidly in the water.
- Fragments of shattered grains: constitute the fragments of expanded perlite that are produced either during or after the expansion. The application of extreme heating conditions and violent expansion, or the coexistence of crystalline and non-crystalline matter in a single grain leads to grain fragmentation. Additionally, due to the fragility of expanded perlite, grain fragmentation occurs due to grain–grain or grain–surface collision inside the furnace or the exhaust system. Due to their tiny size and flattened shape, fragments suspend themselves in water, sinking slowly and finally settling above the sinks. These fragments are of light white color, similar to the expanded grains.
3.6. Moisture and Chemically Bound Water Content
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, A.; Bellil, A.; El Amrani El Hassani, I.; Fekhaoui, M.; Achab, M.; Dahrouch, A.; Benzaouak, A. Geopolymers based on natural perlite and kaolinitic clay from Morocco: Synthesis, characterization, properties, and applications. Ceram. Int. 2021, 47, 24683–24692. [Google Scholar] [CrossRef]
- Kogel, J.E. Industrial Minerals & Rocks: Commodities, Markets and Uses; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006. [Google Scholar]
- Perlite Institute Webpage. Available online: https://www.perlite.org/ (accessed on 10 June 2023).
- Truong, H.B.; Rabani, I.; Huy, B.T.; Hoa, N.; Tran, T.; Hur, J. Using Floating Photocatalyst Mpg-C3N4/Expanded Perlite to Treat Natural Organic Matter under Visible Light. Chem. Eng. J. 2023, 466, 143178. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Sun, Q.; Yuan, M.; Sun, Z.; Xia, S.; Zhao, J. Enhanced Visible Light Photo-Fenton-like Degradation of Tetracyclines by Expanded Perlite Supported FeMo3Ox/g-C3N4 Floating Z-Scheme Catalyst. J. Hazard. Mater. 2022, 424, 127387. [Google Scholar] [CrossRef]
- Gray, J.M.N.T.; Ancey, C. Particle-Size and -Density Segregation in Granular Free-Surface Flows. J. Fluid Mech. 2015, 779, 622–668. [Google Scholar] [CrossRef]
- BS EN 1097-6; Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. British Standard Institution: Milton Keynes, UK, 2022.
- ASTM C136-01; Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. ASTM Int.: West Conshohocken, PA, USA, 2006. [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2023: U.S. Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2023; ISBN 9781411345041.
- White, J.D.L. Volcanic Textures: A Guide to the Interpretation of Textures in Volcanic Rocks. Bull. Volcanol. 1994, 56, 412–413. [Google Scholar] [CrossRef]
- Rotella, B.M.; Simandl, G. Marilla Perlite-Volcanic Glass Occurrence, British Columbia, Canada. In Proceedings of the 37th Forum on the Geology of Industrial Minerals, Victoria, BC, Canada; 2001; pp. 263–272. [Google Scholar]
- Breese, R.; Barker, J. Perlite. In Industrial Minerals and Rocks; SME: Hongkong, China, 1994; pp. 735–740. [Google Scholar]
- Le Maitre, R.; Streckeisen, A.; Zanettin, B.; Le Bas, M.; Bonin, B.; Bateman, P. Igneous Rocks: A Classification and Glossary of Terms: Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521619486. [Google Scholar]
- Marshall, R. Devitrification of Natural Glass. GSA Bull. 1961, 72, 1493–1520. [Google Scholar] [CrossRef]
- Friedman, I.; Smith, R.L.; Long, W.D. Hydration of Natural Glass and Formation of Perlite. GSA Bull. 1966, 77, 323–328. [Google Scholar] [CrossRef]
- Austin-Erickson, A.; Ort, M.H.; Carrasco-Núñez, G. Rhyolitic Phreatomagmatism Explored: Tepexitl Tuff Ring (Eastern Mexican Volcanic Belt). J. Volcanol. Geotherm. Res. 2011, 201, 325–341. [Google Scholar] [CrossRef]
- Heide, K.; Heide, G. Vitreous State in Nature-Origin and Properties. Chemie der Erde 2011, 71, 305–335. [Google Scholar] [CrossRef]
- Koukouzas, N.K.; Dunham, A.C.; Scott, P.W. Suitability of Greek Perlite for Industrial Applications. Trans. Inst. Min. Metall. Sect. B Appl. Earth Sci. 2000, 109, 105–111. [Google Scholar] [CrossRef]
- Shackley, D. Characterisation and Expansion of Perlite; University of Nottingham: Nottingham, UK, 1988. [Google Scholar]
- Angelopoulos, P.M.; Maliachova, C.; Papakonstantinou, K.; Taxiarchou, M.; Diplas, S. Structural and Physical Characteristics of Fine Perlite Expanded with a Novel Method in a Vertical Electric Furnace. Miner. Process. Extr. Metall. 2016, 125, 71–80. [Google Scholar] [CrossRef]
- Klipfel, A.; Founti, M.; Zähringer, K.; Martin, J.P.; Petit, J.P. Numerical Simulation and Experimental Validation of the Turbulent Combustion and Perlite Expansion Processes in an Industrial Perlite Expansion Furnace. Flow Turbul. Combust. 1998, 60, 283–300. [Google Scholar] [CrossRef]
- Zähringer, K.; Martin, J.P.; Petit, J.P. Numerical Simulation of Bubble Growth in Expanding Perlite. J. Mater. Sci. 2001, 36, 2691–2705. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Gerogiorgis, D.I.; Paspaliaris, I. Mathematical Modeling and Process Simulation of Perlite Grain Expansion in a Vertical Electrical Furnace. Appl. Math. Model. 2014, 38, 1799–1822. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Gerogiorgis, D.I.; Paspaliaris, I. Model-Based Sensitivity Analysis and Experimental Investigation of Perlite Grain Expansion in a Vertical Electrical Furnace. Ind. Eng. Chem. Res. 2013, 52, 17953–17975. [Google Scholar] [CrossRef]
- Chatterjee, K.K. Uses of Industrial Minerals, Rocks and Freshwater; Nova Science: New York, NY, USA, 2013; Volume 53, ISBN 9788578110796. [Google Scholar]
- Roulia, M.; Chassapis, K.; Kapoutsis, J.A.; Kamitsos, E.I.; Savvidis, T. Influence of Thermal Treatment on the Water Release and the Glassy Structure of Perlite. J. Mater. Sci. 2006, 41, 5870–5881. [Google Scholar] [CrossRef]
- Perlit-92 Kft. Company Webpage. Available online: https://www.perlit92.hu/indexen.html (accessed on 1 November 2023).
- Harrati, A.; Askame, Y.; Manni, A.; Aqdim, S.; Zmemla, R.; Chari, A.; El Bouari, A.; El Amrani El Hassani, I.; Sdiri, A.; Hassani, F.O.; et al. Akermanite-based ceramics from Moroccan dolomite and perlite: Characterization and in vitro bioactivity assessment. Open Ceram. 2022, 10, 100276. [Google Scholar] [CrossRef]
- Kaufhold, S.; Reese, A.; Schwiebacher, W.; Dohrmann, R.; Grathoff, G.H.; Warr, L.N.; Halisch, M.; Müller, C.; Schwarz-Schampera, U.; Ufer, K. Porosity and Distribution of Water in Perlite from the Island of Milos, Greece. Springerplus 2014, 3, 598. [Google Scholar] [CrossRef]
- Tsikouras, B.; Passa, K.S.; Iliopoulos, I.; Katagas, C. Microstructural Control on Perlite Expansibility and Geochemical Balance with a Novel Application of Isocon Analysis: An Example from Milos Island Perlite (Greece). Minerals 2016, 6, 80. [Google Scholar] [CrossRef]
- Celik, A.G.; Kilic, A.M.; Cakal, G.O. Expanded Perlite Aggregate Characterization for Use as a Lightweight Construction Raw Material. Physicochem. Probl. Miner. Process. 2013, 49, 689–700. [Google Scholar] [CrossRef]
- Biswas, R.K.; Khan, P.; Mukherjee, S.; Mukhopadhyay, A.K.; Ghosh, J.; Muraleedharan, K. Study of Short Range Structure of Amorphous Silica from PDF Using Ag Radiation in Laboratory XRD System, RAMAN and NEXAFS. J. Non. Cryst. Solids 2018, 488, 1–9. [Google Scholar] [CrossRef]
- Sodeyama, K.; Sakka, Y.; Kamino, Y.; Seki, H. Preparation of Fine Expanded Perlite. J. Mater. Sci. 1999, 34, 2461–2468. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Manić, N.; Tsakiridis, P.; Taxiarchou, M.; Janković, B. Dehydration of Rhyolite: Activation Energy, Water Speciation and Morphological Investigation. J. Therm. Anal. Calorim. 2020, 142, 395–407. [Google Scholar] [CrossRef]
- Thomas, P.S.; Heide, K.; Földvari, M. Water and Hydrogen Release from Perlites and Opal: A Study with a Directly Coupled Evolved Gas Analyzing System (DEGAS). J. Therm. Anal. Calorim. 2015, 120, 95–101. [Google Scholar] [CrossRef]
- Stolper, E. Water in Silicate Glasses: An Infrared Spectroscopic Study. Contrib. Miner. Pet. 1982, 81, 1–17. [Google Scholar] [CrossRef]
- Varga, P.; Uhlík, P.; Lexa, J.; Šurka, J.; Bizovská, V.; Hudec, P.; Pálková, H. The Influence of Porosity on the Release of Water from Perlite Glass by Thermal Treatment. Monatshefte Chem. 2019, 150, 1025–1040. [Google Scholar] [CrossRef]
- Bagdassarov, N.; Ritter, F.; Yanev, Y. Kinetics of Perlite Glasses Degassing: TG and DSC Analysis. Glas. Sci. Technol. 1999, 72, 277–290. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Manic, N.; Jankovic, B.; Taxiarchou, M. Thermal decomposition of volcanic glass (rhyolite): Kinetic deconvolution of dehydration and dehydroxylation process. Thermochim. Acta 2022, 707, 179082. [Google Scholar] [CrossRef]
- Lehman, H.; Roosler, M. A Contribution to the Nature of Water Binding in Perlite. In Proceedings of the 4th ICTA, Budapest, Hungary, 8–13 July 1974; pp. 619–628. [Google Scholar]
- Neuville, D.R.; Courtial, P.; Dingwell, D.B.; Richet, P. Thermodynamic and Rheological Properties of Silicate Melts. Contrib. Mineral. Petrol. 1993, 113, 572–581. [Google Scholar] [CrossRef]
- Giordano, D.; Russell, J.K.; Dingwell, D.B. Viscosity of Magmatic Liquids: A Model. Earth Planet. Sci. Lett. 2008, 271, 123–134. [Google Scholar] [CrossRef]
- Allen, T. Powder Sampling and Particle Size Determination; Elsevier: Amsterdam, The Netherlands, 2013; Volume 53, ISBN 9788578110796. [Google Scholar]
- Sun, Y.; Nimbalkar, S.; Chen, C. Particle Breakage of Granular Materials during Sample Preparation. J. Rock Mech. Geotech. Eng. 2019, 11, 417–422. [Google Scholar] [CrossRef]
- Schumacher, B.A.; Shines, K.C.; Burton, J.V.; Papp, M.L. Comparison of Three Methods for Soil Homogenization. Soil Sci. Soc. Am. J. 1990, 54, 1187–1190. [Google Scholar] [CrossRef]
- Montgomery, J.R. Revolving Riffle for Subdividing Ground Magnesium Chips. Anal. Chem. 1968, 40, 1399–1400. [Google Scholar] [CrossRef]
- Hatton, T.A. Representative Sampling of Particles with a Spinning Riffler. A Stochastic Model. Powder Technol. 1978, 19, 227–233. [Google Scholar] [CrossRef]
- Allen, T.; Khan, A. Critical Evaluation of Powder Sampling Procedures. Chem. Eng. 1970, 238, 108–112. [Google Scholar]
- Jillavenkatesa, A.; Dapkunas, S.J.; Lum, L.H. Particle Size Characterization; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2001.
- Wypych, G. Handbook of Fillers, 2nd ed.; ChemTec Publishing: Toronto, QC, Canada, 2000; ISBN 0912659998. [Google Scholar]
- Angelopoulos, P.M.; Peppas, A.; Taxiarchou, M. Modelling the Thermal Treatment and Expansion of Mineral Microspheres (Perlite) in Electric Furnace Through Computational Fluid Dynamics (CFD): Effect of Process Conditions and Feed Characteristics. Miner. Process. Extr. Metall. Rev. 2023, 2023, 1–18. [Google Scholar] [CrossRef]
- Samal, S. Effect of Shape and Size of Filler Particle on the Aggregation and Sedimentation Behavior of the Polymer Composite. Powder Technol. 2020, 366, 43–51. [Google Scholar] [CrossRef]
- ASTM D5550-00; Standard Test Method for Specific Gravity of Soil Solids by Gas Pycnometer. ASTM Int.: West Conshohocken, PA, USA, 2001; Volume 4, pp. 1–4.
- Columbu, S.; Mulas, M.; Mundula, F.; Cioni, R. Strategies for Helium Pycnometry Density Measurements of Welded Ignimbritic Rocks. Meas. J. Int. Meas. Confed. 2021, 173, 108640. [Google Scholar] [CrossRef]
- Leschonski, K. Sieve Analysis, the Cinderella of Particle Size Analysis Methods? Powder Technol. 1979, 24, 115–124. [Google Scholar] [CrossRef]
- Tsakalakis, K. Some Basic Factors Affecting Screen Performance in Horizontal Vibrating Screens. Eur. J. Miner. Process. Environ. Prot. 2001, 1, 42–54. [Google Scholar]
- Liu, K.S. Some Factors Affecting Sieving Performance and Efficiency. Powder Technol. 2009, 193, 208–213. [Google Scholar] [CrossRef]
- Apling, A.C. Blinding of Screens by Sub-Sieve Sized Particles. Miner. Process. Extr. Metall. Sect. C Trans. Inst. Min. Metall. 1984, 93, 92–94. [Google Scholar]
- ASTM E11-20; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM Int.: West Conshohocken, PA, USA, 2020.
- Angelopoulos, P.M.; Kenanakis, G.; Viskadourakis, Z.; Tsakiridis, P.; Vasilopoulos, K.C.; Karakassides, M.A.; Taxiarchou, M. Manufacturing of {ABS}/Expanded Perlite Filament for 3D Printing of Lightweight Components through Fused Deposition Modeling. Mater. Today Proc. 2021, 54, 14–21. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Vrithias, N.R.; Viskadourakis, Z.; Tsakiridis, P.; Vasilopoulos, K.C.; Peppas, A.; Asimakopoulos, G.; Spyrou, A.V.; Karakassides, M.A.; Taxiarchou, M.; et al. Methods of Preparation and Performance Evaluation of ABS/Mineral Microsphere Composites Produced through FDM and Compression Molding. Materials 2022, 15, 5021. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.G.; Jandorno, J.C.; da Rocha, E.B.D.; de Sousa, A.M.F.; da Silva, A.L.N. Evaluation of Expanded Perlite Behavior in PS/Perlite Composites. Appl. Clay Sci. 2019, 181, 105223. [Google Scholar] [CrossRef]
- Spoerk, M.; Sapkota, J.; Weingrill, G.; Fischinger, T.; Arbeiter, F.; Holzer, C. Shrinkage and Warpage Optimization of Expanded-Perlite-Filled Polypropylene Composites in Extrusion-Based Additive Manufacturing. Macromol. Mater. Eng. 2017, 302, 1700143. [Google Scholar] [CrossRef]
- Akin-Öktem, G.; Tanrisinibilir, S.; Tinçer, T. Study on Mechanical Properties of Perlite-Filled Gamma-Irradiated Polypropylene. J. Appl. Polym. Sci. 2001, 81, 2670–2678. [Google Scholar] [CrossRef]
- Raji, M.; Nekhlaoui, S.; El Hassani, I.E.E.A.; Essassi, E.M.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. el kacem Utilization of Volcanic Amorphous Aluminosilicate Rocks (Perlite) as Alternative Materials in Lightweight Composites. Compos. Part B Eng. 2019, 165, 47–54. [Google Scholar] [CrossRef]
- Atagür, M.; Sarikanat, M.; Uysalman, T.; Polat, O.; Elbeyli, İ.Y.; Seki, Y.; Sever, K. Mechanical, Thermal, and Viscoelastic Investigations on Expanded Perlite–Filled High-Density Polyethylene Composite. J. Elastomers Plast. 2018, 50, 747–761. [Google Scholar] [CrossRef]
- Karakaş, H.; İlkentapar, S.; Durak, U.; Örklemez, E.; Özuzun, S.; Karahan, O.; Atiş, C.D. Properties of Fly Ash-Based Lightweight-Geopolymer Mortars Containing Perlite Aggregates: Mechanical, Microstructure, and Thermal Conductivity Coefficient. Constr. Build. Mater. 2023, 362, 27–33. [Google Scholar] [CrossRef]
- Szabó, R.; Dolgos, F.; Debreczeni, A.; Mucsi, G. Characterization of mechanically activated fly ash-based lightweight geopolymer composite prepared with ultrahigh expanded perlite content. Ceram. Int. 2022, 48, 4261–4269. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Geng, Q.; Hou, D.; Wang, M.; Li, L.; Wang, P.; Chen, D.; Sun, Z. Characterization of Sustainable Ultra-High Performance Concrete (UHPC) Including Expanded Perlite. Constr. Build. Mater. 2021, 303, 124245. [Google Scholar] [CrossRef]
- Lubin, G.; Clark, S.L. Handbook of Composites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1982; ISBN 9781461571414. [Google Scholar]
- Tajra, F.; Abd, M.; Lehmann, C.; Stephan, D. Properties of Lightweight Concrete Made with Core-Shell Structured Lightweight Aggregate. Constr. Build. Mater. 2019, 205, 39–51. [Google Scholar] [CrossRef]
- DIN EN 13055-1; 2002-08 Lightweight Aggregates, Part I: Lightweight Aggregates for Concrete, Mortar and Grout. Annex I: Determination of Crushing Resistance. German Institute of Standardization: Berlin, Germany, 2002.
- ASTM D3102-78; Practice for Determination of Isostatic Collapse Strength of Hollow Glass Microspheres (Withdrawn 1984). ASTM Int.: West Conshohocken, PA, USA, 1978.
- Scott, N.R.; Stoddard, D.L.; Nelms, M.D.; Wallace, Z.; Turner, I.; Turner, L.; Croom, M.; Franklin, K.; Sandifer, S.; Ali Al-fahdi, M.S.; et al. Experimental and Computational Characterization of Glass Microsphere-Cementitious Composites. Cem. Concr. Res. 2022, 152, 106671. [Google Scholar] [CrossRef]
- 3M Glass Bubbles. Available online: https://www.3m.com/3M/en_US/p/c/advanced-materials/glass-bubbles/i/automotive/oem-tier/ (accessed on 3 April 2023).
- Bijwe, J.; Logani, C.M.; Tewari, U.S. Influence of Fillers and Fibre Reinforcement on Abrasive Wear Resistance of Some Polymeric Composites. Wear 1990, 138, 77–92. [Google Scholar] [CrossRef]
- Bublon BmbH Company Webpage. Available online: https://www.bublon.com/en.html (accessed on 1 October 2023).
- ASTM C128-22; Standard Test Method for Relative Density (SPecific Gravity) and Absorption of Fine Aggregates. ASTM Int.: West Conshohocken, PA, USA, 2022.
- ISO 787-5; General Methods of Tests of Pigments and Extenders—Part 5: Determination of Oil Absorption Value. International Organization for Standardization: Geneva, Switzerland, 1980.
- ASTM D281-12; Standard Test Method for Oil Absorption of Pigments by Spatula Rub-Out. ASTM Int.: West Conshohocken, PA, USA, 2021.
- ASTM D2216-19; Standard Test Methods for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass. ASTM Int.: West Conshohocken, PA, USA, 2019.
- ISO 17892-1:2014; Geotechnical Investigation and Testing-Laboratory Testing of Soil—Part 1: Determination of Water Content. International Organization for Standardization: Geneva, Switzerland, 2014.
- Denton, J.S.; Tuffen, H.; Gilbert, J.S.; Odling, N. The Hydration and Alteration of Perlite and Rhyolite. J. Geol. Soc. Lond. 2009, 166, 895–904. [Google Scholar] [CrossRef]
- Ross, C.S.; Smith, R.L. Water and Other Volatiles in Volcanic Glasses*. Am. Mineral. 1955, 40, 1071–1089. [Google Scholar]
- ASTM C25-19; Standard Test Methods for Chemical Analysis of Limestone, Quickline and Hydrated Lime. ASTM Int.: West Conshohocken, PA, USA, 2019.
- Dean, W.E. Determination of Carbonate and Organic Matter in Calcareous Sediments and Sedimentary Rocks by Loss on Ignition: Comparison with Other Methods. J. Sediment. Petrol. 1974, 44, 1–9. [Google Scholar]
- ASTM C114-18; Standard Test Method for Chemical Analysis of Hydraulic Cement. ASTM Int.: West Conshohocken, PA, USA, 2018.
- ASTM D1208-96; Standard Test Methods for Common Properties of Certain Pigments. ASTM Int.: West Conshohocken, PA, USA, 2019.
- ASTM D7348-13; Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. ASTM Int.: West Conshohocken, PA, USA, 2013.









| Property | Value |
|---|---|
| Color | White to off white (expanded) Gray to brownish (unexpanded) |
| Refractive Index | 1.5 (unexpanded) |
| pH (water slurry) | 6.5–8.0 |
| Free Moisture (raw) | typical < 1% |
| Specific Gravity (raw) | 2.2–2.4 |
| Bulk Density (loose weight) | Expanded: 32–400 kg∙m−3 Crude Ore: 960–1200 kg∙m−3 |
| Softening point | >850 °C |
| Specific heat (nominal) | 837 J∙kg−1∙°K−1 |
| Thermal conductivity at 20 °C | 0.04–0.06 W∙m−1∙K−1 (expanded) |
| Solubility |
|
| Oxide | Perlite Institute [3] | Chatterjee [25] | Turkey [26] | Italy [26] | China [26] | Greece [20] | Pálháza, Hungary [27] | Morocco [28] | |
|---|---|---|---|---|---|---|---|---|---|
| Tsigrado | Trachilas | ||||||||
| SiO2 | 72.3 | 70–75 | 71.0 | 69.7 | 71.4 | 72.7 | 73.9 | 68–75 | 76.5 |
| Al2O3 | 13.6 | 12–15 | 12.8 | 14.1 | 12.4 | 14.9 | 10.3 | 10–15 | 11.8 |
| Na2O | 4.6 | 3–4 | 3.2 | 3.3 | 4.0 | 4.1 | 3.7 | 2.8–4.5 | 3.3 |
| K2O | 4.2 | 3–5 | 4.4 | 4.7 | 4.4 | 4.0 | 5.7 | 3.2–4.5 | 4.1 |
| Fe2O3 | 0.9 | 0.5–2.0 | 1.6 | 2.1 | 1.1 | 1.2 | 0.9 | 1.0–2.5 | 0.7 |
| MgO | 0.3 | 0.2–0.7 | 0.4 | 0.4 | 0.3 | 0.3 | 0.1 | 0.2–1.5 | 0.3 |
| CaO | 0.9 | 0.5–1.5 | 0.9 | 0.8 | 1.0 | 1.5 | 0.8 | 1.5–2.0 | 0.8 |
| H2O as LOI | 3.0 | 3–5 | 4.7 | 3.8 | 5.0 | 2.9 | 3.3 | 2.0–5.0 | 3.4 |
| Sampling Method | Advantages | Disadvantages | Relative Standard Deviation (%) |
|---|---|---|---|
| Cone & Quartering | Good for powders with poor flow characteristics | Operator-dependent | 6.81 |
| Chute Riffling | Ability to reduce powder samples in half after one pass | Operator bias | 1.01 |
| Application | Size Range (μm) | Loose Bulk Density (LBD) (kg∙m−3) | Appearance | Use | Key Properties |
|---|---|---|---|---|---|
| Horticulture | 500–5000 | 60–130 | Coarse Granules | Hold water and air in the soil that is rendered available in mid term | Surface porosity Water absorption |
| Construction | 63–2800 | 60–150 | Fine and Middle Granules | Reduction of static load Provide with thermal and sound insulation | Surface porosity Density Strength |
| Cryogenic | 150–1200 | 30–120 | Small Granules | Minimal thermal conductivity, used as loose fill | Density Particle size |
| Filter Aid | <200 | 50–200 | Powder | Crushed to produce tiny fragment. Used as precoat on filter septum to increase capacity, and as body feed | Size Thermal conductivity |
| Microspheres | <300 | 60–400 | Rolling Powder | Tiny expanded particles of closed porosity used as filler | Size Strength Sphericity |
| Water retentive fines for agriculture | <800 | 60–160 | Small Granules | Ultrafine highly porous particles, with great water holding capacity | Size Surface porosity |
| Sieving Parameters | Perlite Grade | |
|---|---|---|
| Coarse (>150 μm) | Fine (<150 μm) | |
| Type of sieving | Dry | Wet for 45 μm |
| Sieve size | 200/203 mm (8 in.) diameter, and 50 mm (2 in.) height | |
| Aperture size | 150 μm, 300 μm, 600 μm, 1.18 mm, 2.36 mm | 45, 75, 150 μm |
| Sample quantity | 500 mL | |
| Sieving time | 5 min | |
| Tapping frequency | 2.5 Hz (150 taps per min) | |
| Property | Value |
|---|---|
| Piston speed | 0.5 mm/s |
| Vibrating table settings | Oscillations: 3000 per min |
| Amplitude: 0.5 mm (without load) | |
| Duration: 3 s (without and with the collar) |
| Property | Value |
|---|---|
| Container dimensions | 120 mm × 300 mm |
| Sample volume | 100 mL |
| Balls | Weight: 1 kg of stainless-steel balls Diameter: 6 mm each |
| Rotation | Speed: 380 rpm Time: 15 min |
| Type | Heating Temperature, °C | Heating Time, min | Reference |
|---|---|---|---|
| Limestone | 1000 ± 20 | 20 or until constant weight | [85] |
| Calcareous sediments | 1000 | 60 | [86] |
| Portland and slag cement | 950 | 15 | [87] |
| Pigments | 900–1000 | 20 (phase 1) and 10 (phase 2) | [88] |
| Soil combustion residue | 750 (Method A) 950 (Method B) | 120 | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, P.M. Insights in the Physicochemical and Mechanical Properties and Characterization Methodology of Perlites. Minerals 2024, 14, 113. https://doi.org/10.3390/min14010113
Angelopoulos PM. Insights in the Physicochemical and Mechanical Properties and Characterization Methodology of Perlites. Minerals. 2024; 14(1):113. https://doi.org/10.3390/min14010113
Chicago/Turabian StyleAngelopoulos, Panagiotis M. 2024. "Insights in the Physicochemical and Mechanical Properties and Characterization Methodology of Perlites" Minerals 14, no. 1: 113. https://doi.org/10.3390/min14010113
APA StyleAngelopoulos, P. M. (2024). Insights in the Physicochemical and Mechanical Properties and Characterization Methodology of Perlites. Minerals, 14(1), 113. https://doi.org/10.3390/min14010113










