Abstract
The deeply buried Upper Ediacaran Qigebrak Formation dolostones in the Tarim Basin are promising future hydrocarbon exploration targets in China. However, the origin of these pervasive matrix dolomites is not well understood, which hampers further hydrocarbon exploration. In this study, petrographic, isotopic (C, O, and Sr), rare earth element (REE), and clumped isotope analyses were performed to unravel the mechanisms of early dolomitization. Petrographic investigations indicate that the Qigebrak Formation carbonates were completely replaced by three distinct types of dolomites: (1) dolomicrite (MD-1), (2) fabric-preserving dolomite (MD-2), and (3) fabric-destructive dolomite (MD-3). Despite different crystal textures, these three dolomite types have a narrow range of δ13C and 87Sr/86Sr values similar to those of coeval seawater. Furthermore, their seawater-normalized REE compositions display a seawater-like REE pattern with positive Ce anomalies. These findings suggest that the dolomitization fluids were seawater derived. From the clumped isotope temperature (TΔ47 ≈ 60 °C) and the δ18O water values of the dolomitization fluids, it can be inferred that the main mechanism for the formation of matrix dolomites was seepage-reflux dolomitization by mesosaline to penesaline seawater in the evaporative environment. MD-1 and MD-2 precipitated from mesosaline to penesaline seawater in slightly evaporated settings. MD-3 was likely formed via recrystallization of MD-1 and/or MD-2 at a greater depth. This study provides an insight into early dolomitization processes related to mesosaline to penesaline seawater, which may make the origins of dolomite reservoirs with similar geological backgrounds better understood.
1. Introduction
Dolomitization has continued to attract interest because dolomites comprise a large number of hydrocarbon reservoirs worldwide [1]. This diagenetic process is an important controlling factor affecting the physical properties of carbonate rock reservoirs and thus affects the development of high-quality reservoirs [2]. Dolomitization models, such as sabkha dolomitization [3], seepage-reflux dolomitization [4], hydrothermal dolomitization [5], and seawater thermal convective dolomitization [6] have been reported to interpret the formation of large-scale dolomitization in various geological circumstances. Seepage-reflux dolomitization by hypersaline brines is the main mechanism proposed for the formation of shallow marine platform dolomites, and this process usually involves the precipitation of evaporite minerals [7,8]. In contrast, dolomitization via the reflux of mesosaline to penesaline brines is generally characterized by the absence of the precipitation of evaporite minerals [9,10]. This process can also cause large-scale dolomitization, which is conducive to the formation of dolomite reservoirs. For example, the development of large-scale high-quality reservoirs in the Cambrian Longwangmiao Formation and Triassic Feixianguan Formation in the Sichuan Basin was closely related to this type of dolomitization [11,12].
Precambrian dolomite reservoirs are currently promising exploration targets for oil and gas in China. Deeper exploration in the Tarim Basin led to a significant breakthrough in 2020 (well Luntan-1) with the discovery of dolomite reservoirs in the Upper Ediacaran Qigebrak Formation, indicating a broad exploration potential [13], and the pervasive early-formed dolomites are well developed [14]. However, there are still different views on the large-scale dolomitization mechanism of the Qigebrak Formation, and the focus of the controversy is whether the formation of these matrix dolomites occurred via primary precipitation [15,16] or secondary replacement [17]. Studying the origin of these early-formed dolomites can not only provide an insight into the early dolomitization process during fluid–rock interactions but can also lead to a better understanding of the genesis of related dolomite reservoirs and hence further improve exploration strategies.
Here, based on comprehensive analysis of the petrography, rare earth elements, carbon-oxygen-strontium isotopes, and clumped isotopes, detailed analyses have been performed on the matrix dolomites in the Qigebrak Formation of the NW Tarim Basin. This study aims to (1) document the petrographic and geochemical characteristics of the matrix dolomites; (2) trace the source of dolomitization fluids; and (3) interpret the mechanisms that were responsible for the early dolomitization. The results are expected to help guide oil and gas explorations of dolomite reservoirs in the Qigebrak Formation of the study area and provide an improved understanding of the formation of such reservoirs.
2. Geological Setting
The Tarim Basin is a superimposed hydrocarbon-bearing basin, which is made of the Paleozoic Craton basin and the Mesozoic-Cenozoic foreland basin, with a rhombic shape [18] (Figure 1a). From the Cryogenian to the Early Ediacaran, due to the continuous extension process, a continental rift basin developed in the interior and margin of the Tarim Basin, which resulted in clastic deposits thousands of meters thick interbedded with volcanic rocks [19]. In the late Ediacaran, the continuous extension of the rift led to subsidence of the ocean basin, forming a depression basin characterized by the occurrence of shallow marine carbonates [20]. The Neoproterozoic strata in the NW Tarim Basin comprise the Qiaoenbrak, Youermeinake, Sugaitebrak, and Qigebrak Formations in ascending order (Figure 1b). During the depositional period of the Qigebrak Formation, the study area was the inner ramp of a carbonate platform (Figure 1c) and characterized by relatively high salinity seawater and a dry climate [21]. The main lithofacies in the Qigebrak Formation, in order of decreasing volume percentage, include dolomicrobialites, dolowackstone/dolomudstone, dolograinstone, and minor sandstone (Figure 2). By the end of the Ediacaran period and owing to either regional tectonic movement or a large-scale sea level fall [22,23], the uppermost Qigebrak Formation was substantially eroded and karstified, forming an unconformity (Figure 2) associated with karst breccias.
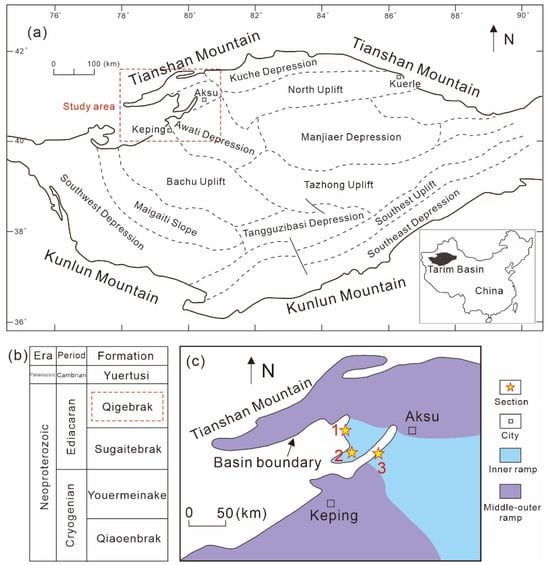
Figure 1.
Geological setting map of the study area: (a) location and structural feathers of the Tarim Basin; (b) simplified stratigraphic column of the Neoproterozoic to Early Cambrian in the Aksu area; (c) paleogeography of the study area in the late Ediacaran. The number 1, 2 and 3 show the studied outcrop sections.
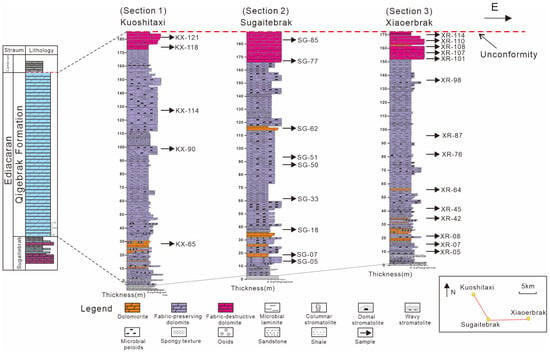
Figure 2.
The stratigraphic column shows the distribution of different matrix dolomites in the Qigebrak Formation.
3. Samples and Methods
The samples analyzed in this study were collected from three sections in the Aksu area of the NW Tarim Basin (Figure 2). Thereafter, geochemical analyses (δ13C, δ18O, 87Sr/86Sr, REE, and clumped isotopes) were conducted on part of representative samples.
Petrographical analyses were conducted at the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS). A total of 45 thin sections were prepared and examined under both a transmitted light optical microscope and a cathodoluminescence (CL) apparatus with a Reliotron, model Relion III, and voltage as well as gun current ranged 5–8 kV and 300–400 μA, respectively. Additionally, 10 samples were chosen for the examination of mineral morphological characteristics and spacing using a Nova NanoSEM 450.
Carbon and oxygen isotopic measurements were conducted at IGGCAS. For the δ13C and δ18O analyses (n = 23), 10 mg of powdered samples were obtained using a micro-drilling apparatus on the surface of polished slabs as well as hand specimens. Dolomite powders were acidified by adding H3PO4 at 25 °C and left for 72 h [24]. The analysis of the product CO2 was performed by the Finnigan MAT-252 mass spectrometer. Results were documented as a per-mil difference from the Vienna Peedee Belemnite (VPDB) international standard [24]. To monitor the precision of the measurement, the international standard NBS-19 was also utilized. The typical standard deviation yields 1σ values < 0.15‰ for δ13C and <0.20‰ for δ18O.
87Sr/86Sr measurements (n = 12) were performed at IGGCAS. A total of 70–80 mg of powdered samples was dissolved by adding 2.5 N HCl on a hotplate at 90 °C. A cation exchange resin AG50Wx12 of 200–400 mesh was used for the Sr separation from the sample matrix. The NBS-987 was used as a reference standard to correct 87Sr/86Sr ratios. The two-standard error for all 87Sr/86Sr measurements varied from 0.0000010 to 0.000013.
Furthermore, 20 samples were selected for the measurement of trace elements and REEs at the Beijing Research Institute of Uranium Geology. Detailed operating procedures to obtain solution for measurement were presented by Du’s [25] research. The results are reported with a precision of ±4%. To calculate the Ce, Eu, and Pr anomalies, respectively, the equations listed below were used: (Ce/Ce*)SN = 2 × [Ce]SN/([La]SN + [Pr]SN) [26], (Eu/Eu*)SN = [Eu]SN/(2/3 × [Sm]SN + 1/3 × [Tb]SN) [27], and (Pr/Pr*)SN = 2 × [Pr]SN/([Ce]SN + [Nd]SN) [26], SN represents seawater-normalized.
In order to accurately determine the temperature of the dolomitization fluid, clumped isotope tests were carried out on ten obtained samples. A 10–12 mg sample of dolomite powder was weighed out, an Imperial Batch Extraction (IBEX) gas automatic extraction and purification device was utilized, and the sample was reacted with 1.90 g/mL phosphoric acid to release the carbon dioxide gas. Using a MAT-253 stable isotope mass spectrometer, automatic online analysis of Δ47 was realized. For detailed instrument operation steps, please refer to Wang’s [28] research. In this test, standard ETH1-ETH4 was used to perform nonlinear correction of the original data. NB-4 (marble) and P1 (coral) were selected as standard samples to correct the related errors in the sample preparation and testing process. The sample test results were converted to the Δ47 value of the absolute reference system (carbon dioxide equilibrated scale, CDES) [29]. The Δ47 values (CDES 25 °C) of NB-4 and P1 were 0.476 (±0.002)‰ and 0.702 (±0.002)‰, respectively. For the relationship between the formation temperature of the dolomite minerals and the Δ47 value, refer to the formula: Δ47 = 0.0449 (±0.001) × 106/T2 + 0.167 (±0.01), where T is the temperature (K) [30].
4. Results
4.1. Dlomite Petrology
Following the description terminology of different dolomite textures [31,32], the matrix dolomites in the Qigebrak Formation can be divided into three types in the field: (1) dolomicrite (MD-1; Figure 3a); (2) fabric-preserving dolomite (MD-2; Figure 3b,c); and (3) fabric-destructive dolomite (MD-3; Figure 3d).

Figure 3.
Outcrop features and occurrence of the matrix dolomites from the Qigebrak Formation in the studied area: (a) thin bedded dolomicrites, Xiaoerbrak section; (b) fabric-preserving dolomites, characterized by the occurrence of stromatolites (yellow arrows), Xiaoerbrak section; (c) fabric-preserving dolomites (below dotted line) developed vertically with dolomicrites (above dottedline), Xiaoerbrak section; (d) fabric-destructive dolomite, Sugaitebrak section.
MD-1 was very rarely observed in the studied sections. Volumetrically, it accounts for less than 5% of the dolostones in the Qigebrak Formation (Figure 2). Microscopically, MD-1 dolomite crystals are less than 5 μm in size and generally show planar-s to nonplanar-a textures (Figure 4a,b). Under cathodoluminescence, this type of dolomite shows nonluminescence (Figure 4c).

Figure 4.
Thin section, CL, and SEM photomicrographs of matrix dolomites in the Qigebrak Formation of the NW Tarim Basin: (a) Dolomicrite (MD-1) with tight texture, in plane-polarized light (PPL), Xiaoerbrak section. (b) SEM image showing planar-s to nonplanar-a dolomite crystals in MD-1, Xiaoerbrak section. (c) MD-1 (PPL, left) shows nonluminescence (right), Xiaoerbrak section. (d) Very finely crystalline dolomite (MD-2), with well-preserved stromatolitic laminae, PPL, Xiaoerbrak section. (e) SEM image showing planar-e to planar-s dolomite crystals in MD-2, Xiaoerbrak section. (f) MD-2 with well-preserved spongy fabrics (PPL, left) shows very dull red luminescence (right), Xiaoerbrak section. (g) Fine to medium crystalline dolomite (MD-3) in which the primary depositional fabrics have been obliterated, PPL, Sugaitebrak section. (h) Thin section photomicrograph under cross-polarized light for (g); MD-3 shows nonplanar-a dolomite crystals with undulating extinction. (i) MD-3 with intercrystalline pores (PPL, left) shows dull red luminescence (right), Kuoshitaxi section.
MD-2 is the predominant dolomite type in volume (more than 80%) of the study area (Figure 2). In the thin sections, MD-2 dolomite crystals are 5–20 μm in size (Figure 4d). Under SEM, MD-2 includes euhedral to subhedral crystals that formed with relatively smooth but uncorroded surfaces (Figure 4e). Under CL, this type has a very dull red color (Figure 4f).
MD-3 constitutes approximately 15% of the total dolostones (Figure 2). This type is characterized by nonplanar-a dolomite crystals, and the crystal sizes vary between 50 μm and 200 μm (Figure 4g). This type is characterized by the obliteration of primary fabrics (Figure 4g), and some MD-3 dolomite crystals have undulatory extinction (Figure 4h). Under CL, this type shows dull red cathodoluminescence (Figure 4i).
4.2. Geochemical Characteristics
4.2.1. Carbon and Oxygen Isotopes
The carbon and oxygen isotope analysis results for the different carbonate minerals are presented in Figure 5 and Table 1. The δ13C values of MD-1 range from 2.79‰ to 5.16‰ VPDB (average of 3.63‰; n = 4), and the δ18O values range from −3.89‰ to −0.04‰ VPDB (average of −2.36‰). MD-2 has δ13C values of 1.79‰ to 4.79‰ VPDB (average of 2.89‰; n = 13) and δ18O values of −5.72‰ to −0.72‰ VPDB (average of −3.07‰). MD-3 has lighter δ13C and δ18O values. Its δ13C values are 1.79‰ to 3.34‰ VPDB (average of 2.37‰; n = 6), and its δ18O values are −6.78‰ to −2.35‰ VPDB (average of −3.07‰). In sum, the δ13C values of MD-1, MD-2, and MD-3 are similar to those of Ediaracan seawater range [33].
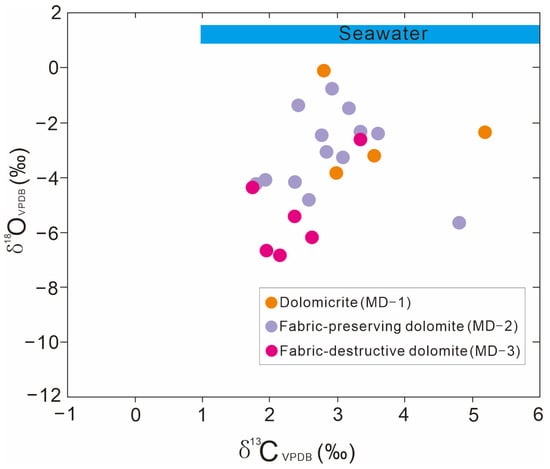
Figure 5.
Crossplot of δ13C and δ18O values for different types of matrix dolomites in the Qigrbrak Formation.

Table 1.
Carbon, oxygen, and strontium isotopic data of matrix dolomites in the Qigebrak Formation, NW Tarim Basin.
4.2.2. Strontium Isotopes
The three types of matrix dolomite have relatively narrow ranges of 87Sr/86Sr values (Figure 6, Table 1). The 87Sr/86Sr values of MD-1 are 0.708499–0.708835 (n = 3). The 87Sr/86Sr values of MD-2 are 0.708477–0.708929 (n = 5). The 87Sr/86Sr values of MD-3 are 0.708466–0.708828 (n = 4). Overall, there is no significant difference in the strontium isotope compositions of the matrix dolomites, and these 87Sr/86Sr ratios largely overlap with those of the late Ediacaran seawater range [33].
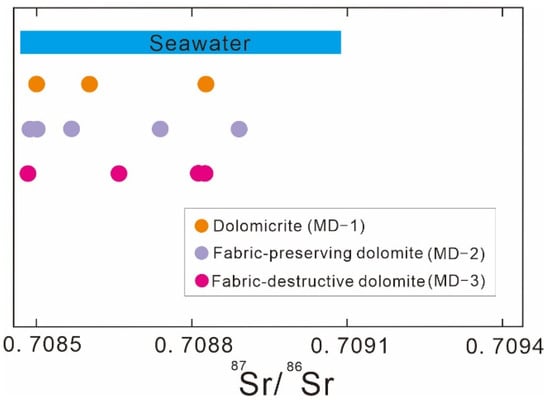
Figure 6.
The 87Sr/86Sr ratios of studied matrix dolomites.
4.2.3. REE Geochemistry
Seawater-normalized REE data for the studied samples are shown in Figure 7, and Table 2 and Table 3 display REE concentrations in the Qigebrak Formation matrix dolomites and REE concentrations normalized to seawater, respectively. Table 4 exhibits the concentrations of several trace elements for each dolomite type.
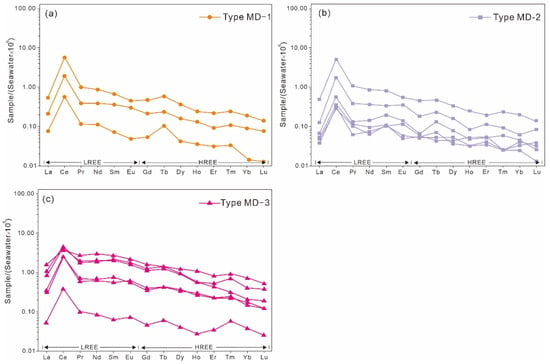
Figure 7.
Seawater-normalized REE patterns of matrix dolomites from the Qigebrak Formation in the study area. (a) REE pattern of MD-1; (b) REE pattern of MD-2; (c) REE pattern of MD-3.

Table 2.
REE concentrations of matrix dolomites from the Qigebrak Formation, NW Tarim Basin.

Table 3.
Seawater-normalized REEs of matrix dolomites from the Qigebrak Formation, NW Tarim Basin.

Table 4.
Concentrations of some trace elements of matrix dolomites from the Qigebrak Formation, NW Tarim Basin.
The total REE (ΣREE) content of the MD-1 ranges from 1.213 ppm to 33.159 ppm, with an average of 13.483 ppm, which is similar to that of MD-3 (average ΣREE = 16.985 ppm) (Table 2). In contrast, MD-2 has the lowest ΣREE, with an average of 4.696 ppm (Table 2). In general, all the studied dolomite types display right-leaning REE patterns, with light REEs (LREEs) enriched and heavy REEs (HREEs) depleted (Figure 7). In addition, the average LREE/HREE ratios of the three types of dolomite range from 5.186 to 7.531, which also indicates LREE enrichment and HREE depletion. The average (La/Sm)SN values are low, ranging from 0.474 to 0.736, displaying the low differentiation among LREEs (Table 3). The average (Gd/Yb)SN ranges from 2.151 to 3.022, showing a high degree of differentiation among HREEs (Table 3).
All of the dolomite samples exhibit positive Ce anomalies. The average (Ce/Ce*)SN values of MD-1, MD-2, and MD-3 are 4.882, 4.221, and 3.048, respectively (Table 3). In contrast, the Eu anomaly values of the three types are less than 1, with their average values ranging from 0.747 to 0.999 (Table 3).
4.2.4. Clumped Isotopes
Compared with fluid inclusion thermometry, clumped isotope thermometry could more accurately determine the temperatures of carbonate minerals in the early diagenetic stages. The clumped isotope results for the three types of matrix dolomite are presented in Figure 8 and Table 5. The Δ47 values of MD-1 and MD-2 range from 0.562‰ to 0.594‰, and the calculated clumped temperatures (TΔ47) range from 51.06 °C to 63.86 °C, with an average of 59.03 °C (n = 4) (Figure 8). The δ18Owater values of the corresponding dolomitization fluids are from 3.09‰ to 4.90‰ VSMOW. The clumped temperatures (TΔ47) of MD-1 and MD-2 are very close to those of the fibrous dolomites in the Qigebrak Formation (60.0–63.0 °C) [34]. In contrast, MD-3 display higher clumped temperatures. Its Δ47 values range from 0.533‰ to 0.566‰, and its TΔ47 values range from 62.28 °C to 75.81 °C, with an average value of 70.47 °C (n = 6). The calculated δ18Owater of the diagenetic fluids range from 0.99‰ to 2.88‰ VSMOW (Figure 8).
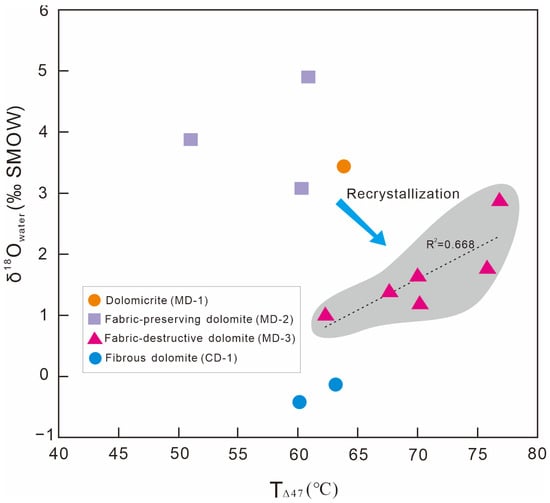
Figure 8.
Relationship between clumped temperatures and δ18Owater of the diagenetic fluids from matrix dolomites in the Qigebrak Formation.

Table 5.
Clumped isotopes and related carbon/oxygen isotopes of Sinian depositional to early-diagenetic dolomites in the NW Tarim Basin.
5. Discussion
5.1. Dolomitization Fluid Properties
5.1.1. Feasibility of Seawater-Normalized REEs
The REE concentrations of dolomites are commonly normalized to North America Shale Composite (NASC), Post Archean Australian Shale (PAAS), and C1 chondrite [35,36]. However, these dolomites do not have an inherent genetic relationship with shales or chondrites. In recent years, studies have shown that in the absence of terrestrial contaminants, the REE composition of seawater has remained relatively stable throughout geological history [37]. Moreover, numerous studies show the REE compositions of dolomites formed in marine environments are usually closely related to fluids driven by seawater, exhibiting a strong affinity [8,10,11,12,14,25]. Therefore, it seems more reasonable to utilize seawater as the standard for normalization [38], and some researchers have chosen seawater for a standardized treatment to study the genesis of marine dolomite [39,40]. In this study, the average REE concentrations of Pacific surface water were used as the standard for normalization [41]. Due to the very low content of REEs in seawater, the REE concentrations in seawater were magnified 106 times (Figure 7).
5.1.2. Validity Assessment of REE Data
Terrigenous clastic materials commonly have high REE contents, and even a small proportion (1–2%) of terrigenous clastics (such as shale) can significantly affect the REE concentrations of marine carbonates [42]. To minimize the risk of contamination by terrigenous components, samples with shale intercalations were avoided during field sampling, and a millimeter microdrill was used to extract powder from fresh hand specimens.
Some trace metals are enriched in clay minerals (e.g., Zr and Th), oxides (e.g., Ni and Cu), and sulfides (e.g., Pb and Sc). Therefore, the cross-plot between Y/Ho (as the seawater signal) and the concentrations of the above elements can assess the degree of contamination [43,44]. Figure 9 implies that none of the aforementioned elements have an anti-correlation with Y/Ho, excluding the likelihood of contamination by silicates, oxides, and sulfides. This is further supported by the 87Sr/86Sr values of the above dolomite samples (MD-1, MD-2, and MD-3), which fall within the coeval seawater range (Figure 6), suggesting that the terrigenous components were not absorbed into the dolomitization fluids. Therefore, the above data suggest that the REE patterns of the Qigebrak dolomite samples do not reflect contamination by terrigenous components and could provide useful information for tracing the source of dolomitization fluids.
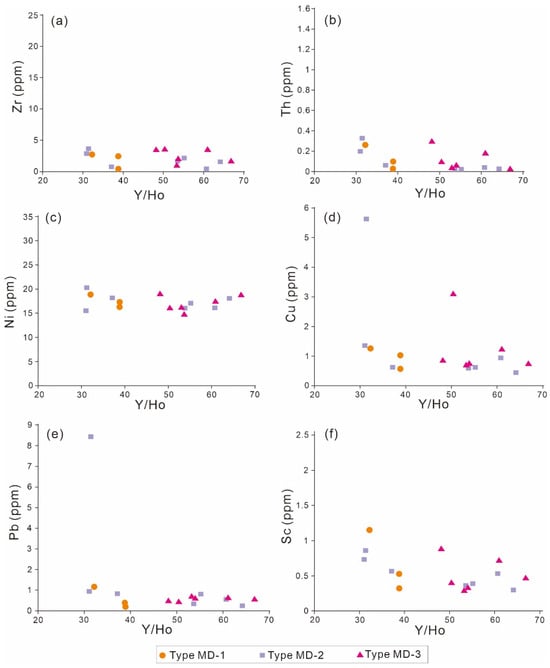
Figure 9.
Binary plots showing relationships of Y/Ho values to contents of (a) Zr, (b) Th, (c) Ni, (d) Cu, (e) Pb, and (f) Sc in matrix dolomites from the Qigebrak Formation.
5.1.3. Ce Anomaly
Cerium is very sensitive to redox conditions and can be regarded as an indicator of marine paleoredox changes [45]. In oxidizing conditions, Ce3+ in seawater can be oxidized to Ce4+, and the latter is less mobile and more easily absorbed into sediment particles [46], leading to Ce depletion of the seawater. Hence, when the REE compositions are normalized to PAAS, NASC, or C1 chondrite, seawater usually has a negative Ce anomaly [47]. However, the REE compositions of all dolomite samples in the present study demonstrate positive Ce anomalies after seawater normalization (Figure 7). The element Ce occurs as CeO2 and is easily absorbed into carbonate particles during the sedimentary differentiation process; hence, Ce is more likely to be enriched in minerals than the two adjacent rare earth elements (i.e., Pr and La) [48]. Therefore, the fact that the samples exhibited positive Ce anomalies after seawater normalization, indicates that they reflect the seawater signal.
Unfortunately, the interpretation of Ce anomalies can also be complicated by the possible anomalous abundance of La in marine precipitates. Since the concentrations of La in seawater are relatively high, the Ce anomaly values may only reflect La anomalies rather than true Ce anomalies. In comparison, the chemical properties of Pr and Nd are stable, exhibiting no obvious anomalies in seawater, and the true Ce anomalies may lead to Pr anomalies. If the (Pr/Pr*)SN value is greater than 1, it represents a negative Ce anomaly, whereas if the value is less than 1, it represents a positive Ce anomaly. Moreover, if (Ce/Ce*)SN < 1 and (Pr/Pr*)SN ≈ 1, then this would represent a positive La anomaly rather than a Ce anomaly [49]. In this study, all studied samples display (Ce/Ce*)SN > 1 and (Pr/Pr*)SN < 1, thus representing true Ce anomalies. The MD-1, MD-2, and MD-3 samples all exhibited significant positive Ce anomalies, possibly indicating that the formation of these dolomites was seawater derived.
5.1.4. Eu Anomaly
Europium usually occurs as Eu3+ in seawater, whereby fractionation is not as obvious as cerium fractionation in dissolution-precipitation processes [50]. Therefore, seawater-normalized Eu anomalies do not differ much from those resulting from PAAS, NASC, or C1 chondrite normalization. However, in a reducing environment, part of the Eu3+ will be reduced to Eu2+, causing the radius of the Eu ions to increase; thus, Eu2+ is more easily incorporated into the carbonate lattice and enriched in carbonates [51,52]. Studies have shown that the positive Eu anomaly is commonly related to reducing hydrothermal fluids with temperatures higher than 200 °C [53], so the positive Eu anomalies in marine carbonate rocks may be closely related to high-temperature hydrothermal processes [54].
The average values of Eu anomalies for MD-1, MD-2, and MD-3 are 0.747, 0.876, and 0.999, respectively (Table 3), and there were no obvious positive Eu anomalies. This shows that the three types of dolomite were not reformed by obvious high-temperature hydrothermal fluids. Although the Aksu area underwent multiple hydrothermal events [55,56], they did not have a significant impact on the REE compositions of the matrix dolomites in the Qigebrak Formation. From MD-1 to MD-3, the Eu anomaly increased gradually, which may correspond to the gradual rising temperature during dolomitization processes [40], consistent with the observed size increase of the dolomite crystals.
5.1.5. Accuracy Assessment of Clumped Temperature (TΔ47) Data
As an emerging geological thermometer, clumped isotopes have great potential for tracing the temperature of dolomitization fluids [57]. However, for ancient dolomite samples, the 13C-18O bonds within carbonate crystals may be reordered through solid-state exchange reactions during the diagenetic processes [58]. As a result, the temperature and oxygen isotope composition calculated based on the measured Δ47 values cannot represent the information when the dolomites were formed. Laboratory measurements [59] and numerical simulations [60] have shown that calcite is prone to solid-state reordering if it is buried at a temperature exceeding 100 °C. In contrast, dolomite usually does not undergo obvious solid-state reordering at temperatures below 300 °C and has a stronger resistance to reordering.
Although the Qigebrak Formation in the NW Tarim has undergone complex diagenetic phases, the primary Δ47 signatures have not been affected by significant solid-state reordering. The evidence is as follows. Firstly, the δ13C and 87Sr/86Sr values of these matrix dolomite samples are very close to those of the coeval seawater, indicating the late diagenesis did not significantly modify the original geochemical signals. Secondly, assuming that the surface temperature in the late Ediacaran was 25 °C, based on the burial history and geothermal gradient, it could be estimated that the Qigebrak Formation reached its maximum burial depth in the Triassic, and the highest burial temperature was about 192 °C [34], which is lower than the temperature (300 °C) at which dolomite undergoes obvious solid-state reordering. Thirdly, the clumped temperature ranges (TΔ47) of MD-1 and MD-2 are very close to the formation temperature of fibrous dolomite cements (Figure 8), of which the fibrous dolomites commonly indicate the paleoenvironment during the sedimentary-early diagenetic period [61]. This further indicates the original geochemical signals about MD-1 and MD-2 have not been modified. It should be noted that the clumped temperature (TΔ47) of MD-3 has a positive correlation with the δ18Owater value of the diagenetic fluid (Figure 8), which may reflect the influence of recrystallization on the TΔ47 values. Thus, the TΔ47 values of MD-3 possibly represent the lowest temperature at which MD-3 was formed via recrystallization [62,63].
5.2. Origin of the Different Types of Matrix Dolomites
5.2.1. Origin of MD-1 and MD-2
The development of MD-1 was very limited. The crystal size (<5 μm) and planar-s to nonplanar-a texture of MD-1 suggest that it was formed from a relatively low-temperature syndepositional to near-surface environment [2]. Even though the nonplanar-a texture dolomite crystals are commonly formed above the critical roughening temperature (50–60 °C), when the dolomitization fluids have high supersaturation levels, this type of dolomite can also precipitate below temperatures less than 50 °C [31,64]. This dolomite type represents the first stage of dolomitization in early diagenesis, with an associated burial depth less than 500 m [65] (Figure 10). As for MD-2, the very fine dolomite crystals (10–20 μm) with planar-e(s) textures indicate that the formation temperature was lower than the critical roughness temperature [31]. This was further supported by the TΔ47 of the MD-2 (51.06–60.29 °C). Assuming that the surface temperature was 25 °C and the average geothermal gradient was 35 °C/km [34], its formation depth can be further restricted within the range of 600–1000 m [66] (Figure 10), indicating that the dolomitization process occurred in a shallow burial environment [67].
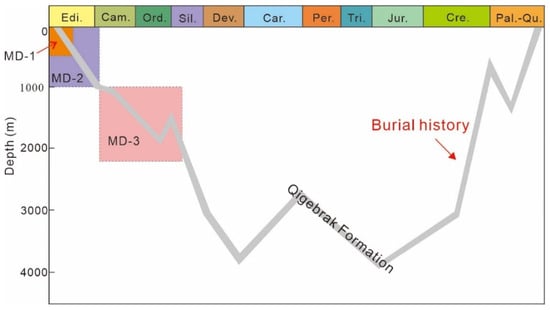
Figure 10.
Possible formation timing and depth of various matrix dolomites from the Qigebrak Formation.
The REE patterns of MD-1 and MD-2 are similar, characterized by LREE enrichment, slight HREE depletion, and positive Ce anomalies, indicating that the dolomitization fluids for the formation of MD-1/MD-2 were closely related to original seawater. This explanation is supported by the δ13C and 87Sr/86Sr values of MD-1/MD-2 lying within the scope of Ediacaran seawater (Figure 5).
The δ18O in dolomite is governed by the formation temperature and the δ18O of dolomitization fluid [68], and the oxygen isotope value of the dolomitization fluid can be calculated [69]. According to the TΔ47 values, we calculated that the δ18Owater values of the dolomitization fluid were 3.09–4.90‰ VSMOW when MD-1 and MD-2 were formed. This is about 8–10‰ VSMOW higher than the δ18O of Neoproterozoic seawater (−5‰ VSMOW) [70] and is higher than the δ18Owater (0‰ VSMOW) of current seawater. This suggests that the dolomitization fluids that formed MD-1 and MD-2 were associated with seawater subjected to evaporation.
5.2.2. Origin of MD-3
The crystal size for MD-3 is much larger (50 to 200 μm) than that of the other two types, exhibiting planar-s to nonplanar-a texture (Figure 4g,h), implying a faster growth rate and higher temperature (>50–60 °C) [31]. The cathodoluminescence color of MD-3 is dull red, and the δ18O values are depleted, further indicating that MD-3 was formed in the weakly reducing environment at higher temperatures. The similarities in MD-3’s REE patterns and Sr isotope range with the other two dolomite types indicate that its dolomitization fluid was also derived from seawater.
Chen’s [71] research showed that the late-stage hydrothermal pore-filling saddle dolomites were formed mainly between 100 °C and 130 °C. Generally, the formation temperature of MD-3 appears to be lower than that of saddle dolomite; thus, it can be assumed that the formation temperature of MD-3 was 60–100 °C. The TΔ47 range of MD-3 (62.28–75.81 °C) also supports the above inference. Similarly, according to the surface temperature and geothermal gradient, it can be estimated that the burial depth of MD-3 was 1000–2143 m (Figure 10), indicating a shallow–medium burial environment [72]. MD-3 is mostly found in intervals where hydrothermal fluids developed [56], but MD-3 does not have Eu anomalies, indicating that the hydrothermal fluids did not significantly modify the geochemical signals of MD-3. The temperature of MD-3 is about 10 °C higher than that of MD-1 and MD-2, which is the result of the greater burial depth rather than the influence of hydrothermal fluids. In addition, the calculated δ18Owater values of the MD-3 dolomitization fluid is 0.99–2.88‰ VSMOW, so the oxygen isotope composition of the dolomitization fluids still exhibited the characteristics of evaporated seawater.
5.3. Early Dolomitization Mechanism
5.3.1. Primary or Secondary Dolomitization?
In previous studies, microbial dolomitization [16] and seepage-reflux dolomitization [17] have been proposed to interpret the pervasive early-formed matrix dolomites in the Qigebrak Formation. Some researchers believe that in the late Ediacaran there existed “dolomite seas” in which primary dolomite was easily precipitated [73]. Thus, coeval seawater, microorganisms, and sedimentary organic matter jointly provided Mg2+, and large-scale dolomitization occurred via microbial induction [16]. In this study, MD-2 consists of a large number of well-preserved microbial fabrics, and its formation may have been related to the early activities of microbial mats [74]. Therefore, this study does not reject the occurrence of microbial dolomitization. However, it should be noted that how to accurately assess the scale of microbial dolomitization still deserves further research. You [75] proposed that dolomite with a spherical, dumbbell shape can be considered to be of microbial origin. Although this special crystal morphology is not the unique product of microbial activities [76,77], dolomites with the above-mentioned morphology were not found by SEM observations in the Qigebrak Formation. This suggests that the formation of large-scale matrix dolomites in the study area were not formed by microbial dolomitization. Furthermore, the gradient of the biochemical environment inside the microbial mat changes strongly, and there are dozens of corresponding mineralization products [78]. Dolomite is only one of the products induced by microorganisms, and it is still unclear whether it can be formed on a large scale. Therefore, it is more reasonable to explain the early dolomitization mechanism of the Qigebrak Formation from the view of secondary replacement.
5.3.2. Dolomitization Model
Seepage-reflux dolomitization has been considered to be the most effective hydrological model to explain the origin of pervasive early-formed dolomites [2]. Reflux of dolomitization brines is initiated by a phase of evaporation in shallow marine and/or lagoonal environments that produce a salinity gradient [1,4]. The fluids for seepage-reflux dolomitization are seawater or modified seawater, such as hypersaline seawater that results in the precipitation of evaporites (Adams and Rhodes, 1960), and mesosaline (44‰ ~ 85‰) to penesaline (72‰~199‰) seawater [10]. Because of the difference in density between fluids and the underlying pore water, aragonitic and magnesian calcite could be dolomitized by reflux in high-frequency cycles. If there is a large amount of Mg2+, then the fluids would migrate laterally toward the basin interior, resulting in massive dolomitization. Numerical simulations show that even in a carbonate platform where geothermal convection is well developed, the seepage reflux model more easily forms large-scale dolomites than the convection model does [65].
In the late Ediacaran, the study area was dominated by the inner ramp of a restricted carbonate platform (Figure 1c). In this limited evaporation setting, the restricted platform environment led to higher salinity than normal seawater. However, no evaporitic minerals (e.g., gypsum and anhydrite) were found in the field or under a microscope, suggesting that the salinity of the seawater did not reach the critical value of gypsum precipitation. Therefore, we propose that seepage-reflux dolomitization by mesosaline to penesaline seawater is a more appropriate explanation of the massive early-formed dolomites in the Qigebrak Formation (Figure 11). During the depositional period, high salinity and consequently Mg2+ concentrations occurred because of the evaporation effect. At that time, MD-1 was seemingly formed when carbonate sediments got dolomitized before consolidation by brines with high Mg/Ca (Figure 11a). Thereafter, the downward seepage reflux of brines with high Mg2+ content caused further dolomitization (Figure 11b). The growth of microbial mats may lead to the precipitation of high-Mg calcite [79], of which the high-Mg calcite could release Mg2+ to the intergranular water, providing a large amount of Mg2+ for the dolomitization of the surrounding deposits. This may explain the large volume of MD-2. When the Qigebrak Formation entered the burial realm, the high temperature led to more extensive dolomite growth over MD-1 and MD-2, thus forming the coarser and more curved crystals of MD-3 [80] (Figure 11c).
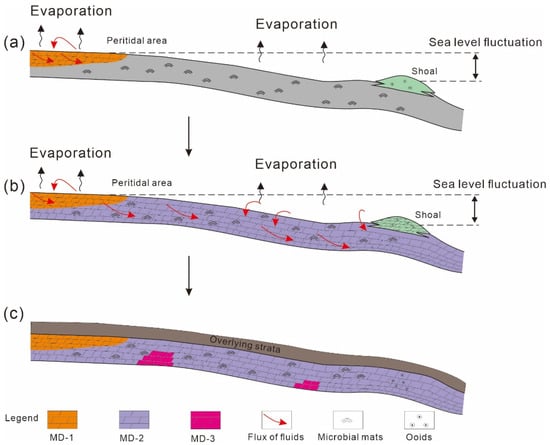
Figure 11.
Schematic model for the large-scale dolomitization from the Qigebrak Formation, Aksu area. (a) The formation processes of MD-1 during the Qigebrak depositional period; (b) Large-scale MD-2 were formed by the seepage reflux of brines with mesosaline to penesaline seawater; (c) MD-3 were formed via recrystallization of MD-1/MD-2 in the burial realm.
5.4. Implications for Hydrocarbon Exploration
The potential for pervasive early dolomitization to significantly increase porosities and permeabilities of dolostones, as compared to the precursor limestone, has been shown in several studies [81,82,83]. MD-2, in the present study, has a stable lateral distribution with porosities between 2.5% and 6.0%, which can be regarded as potential reservoir rocks [84]. As presented above, MD-2 is dominated by planar-e(s) textures associated with very finely crystalline dolomite rhombs (5–20 μm), which are beneficial for the preservation of intercrystalline pores. Numerical simulations demonstrated that mesosaline reflux without evaporite precipitation could result in a porosity increase of up to 8% by “mole for mole” replacement [85,86]. Other pervasive matrix dolomites with similar reflux dolomitization models, such as the Lower Cambrian Longwangmiao Formation of the Sichuan Basin in China [12], and the Lower Carboniferous of the Mississippian Alida Bed of Williston Basin in Canada [80] have proven to be good dolomite reservoirs. This study indicates seepage-reflux dolomitization by mesosaline to penesaline seawater is possibly the fundamental controlling factor in forming high-quality dolomite reservoirs.
6. Conclusions
Coupling petrography with geochemistry analysis, the origin of early-formed matrix dolomites in the Qigebrak Formation of the northwestern Tarim Basin was investigated. The main conclusions include the following:
- Three distinct dolomite types were recognized: (1) dolomicrite (MD-1); (2) fabric-preserving dolomite (MD-2); and (3) fabric-destructive dolomite (MD-3).
- MD-1 represents the earliest dolomitization phase in syndepositional to near-surface settings. MD-2 was probably generated by the same brine reflux with slightly elevated temperature in shallow burial settings (600–1000 m).
- Seepage-reflux dolomitization with mesosaline to penesaline seawater was probably responsible for the origin of MD-1 and MD-2. MD-3 likely resulted from the recrystallization of previously formed MD-1 and/or MD-2 dolomites and precipitated in greater depths (1000–2143 m).
Author Contributions
Conceptualization, P.T. and D.C.; methodology, P.T. and Y.W.; validation, P.T. and B.Y.; formal analysis, P.T. and D.C.; investigation, P.T. and S.L.; resources, P.T. and M.E.-S.; data curation, S.L. and B.Y.; writing—original draft preparation, P.T. and D.C.; writing—review and editing, P.T.; visualization, P.T. and S.L.; supervision, D.C.; project administration, M.E.-S. and B.Y.; funding acquisition, P.T. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (U19B6003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We are especially grateful for the assistance in geochemical analysis made by Chaofeng Li and Hongwei Li (Institute of Geology and Geophysics, CAS).
Conflicts of Interest
The authors have no conflicts of interest to declare regarding the publication of this article.
References
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and models of dolomitization: A critical reappraisal. Geol. Soc. Lond. Spec. Publ. 2004, 235, 7–63. [Google Scholar] [CrossRef]
- Friedman, G.M.; Sanders, J.E. Origin and occurrence of dolostones. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1967; Volume 9B, pp. 267–348. [Google Scholar]
- Adams, J.E.; Rhodes, M.L. Dolomitization by seepage refluxion. AAPG (Am. Assoc. Pet. Geol.) Bull. 1960, 44, 1912–1920. [Google Scholar]
- Davies, G.R.; Smith, L.B. Structurally controlled hydrothermal dolomite reservoir facies: An overview. AAPG (Am. Assoc. Pet. Geol.) Bull. 2006, 90, 1641–1690. [Google Scholar] [CrossRef]
- Vahrenkamp, V.C.; Swart, P.K. Late Cenozoic Dolomites of the Bahamas: Metastable Analogues for the Genesis of Ancient Platform Dolomites; International Association of Sedimentologists Special Publication; Blackwell Science: Oxford, UK, 1994; Volume 21, pp. 133–153. [Google Scholar]
- Shields, M.J.; Brady, P.V. Mass balance and fluid flow constraints on regional-scale dolomitization, Late Devonian, Western Canada Sedimentary Basin. Bull. Can. Pet. Geol. 1995, 43, 371–392. [Google Scholar]
- Jiang, L.; Cai, C.; Worden, R.H.; Crowley, S.F.; Jia, L.; Zhang, K.; Duncan, I.J.; Hollis, C. Multiphase dolomitization of deeply buried Cambrian petroleum reservoirs, Tarim Basin, north-west China. Sedimentology 2016, 63, 2130–2157. [Google Scholar] [CrossRef]
- Saller, A.H.; Henderson, N. Distribution of porosity and permeability in platform dolomites: Insight from the Permian of West Texas. AAPG (Am. Assoc. Pet. Geol.) Bull. 1998, 82, 1528–1550. [Google Scholar]
- Qing, H.R.; Bosence, D.W.J.; Rose, E.P.F. Dolomitization by penesaline sea water in Early Jurassic peritidal platform carbonates, Gibraltar, western Mediterranean. Sedimentology 2001, 48, 153–163. [Google Scholar] [CrossRef]
- Jiang, L.; Worden, R.H.; Cai, C. Thermochemical sulfate reduction and fluid evolution of the Lower Triassic Feixianguan Formation sour gas reservoirs, northeast Sichuan Basin, China. AAPG (Am. Assoc. Pet. Geol.) Bull. 2014, 98, 947–973. [Google Scholar] [CrossRef]
- Liu, D.; Cai, C.; Hu, Y.; Peng, Y.; Jiang, L. Multistage dolomitization and formation of ultra-deep Lower Cambrian Longwangmiao Formation reservoir in central Sichuan Basin, China. Mar. Pet. Geol. 2021, 123, 104752. [Google Scholar] [CrossRef]
- Yang, H.J.; Chen, Y.Q.; Tian, J.; Du, J.H.; Zhu, Y.F.; Li, H.H.; Pan, W.Q.; Yang, P.F.; Li, Y.; An, H.T. Great discovery and its significance of ultra-deep oil and gas exploration in well Luntan-1 of the Tarim Basin. China Pet. Explor. 2020, 25, 62–72. [Google Scholar]
- Tang, P.; Chen, D.; Wang, Y.; Ding, Y.; El-Shafeiy, M.; Yang, B. Diagenesis of microbialite-dominated carbonates in the Upper Ediacaran Qigebrak Formation, NW Tarim Basin, China: Implications for reservoir development. Mar. Pet. Geol. 2022, 136, 105476. [Google Scholar] [CrossRef]
- Bao, Z.D.; Ji, H.C.; Wang, Y.; Li, Z.F.; Liang, T.; Niu, B.; Wei, M.Y.; Lu, K.; Shi, Y.Q.; Zhang, H.; et al. The primary dolostone in the Meso-Neoproterozoic: Cases study on platforms in China. J. Palaeogeogr. 2022, 11, 151–172. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Li, X.; Li, T.T.; Zhou, L.; Wu, Y.X.; Shen, B.; Ning, M. Genesis mechanism and Mg isotope difference between the Ediacaran and Cambrian dolomites in Tarim Basin. Sci. China Earth Sci. 2023, 66, 334–357. [Google Scholar] [CrossRef]
- Zheng, J.F.; Liu, Y.; Zhu, Y.J.; Liang, F. Geochemical features and its geological significances of the Upper Ediacaran Qigeblak Formation in Wushi area, Tarim Basin. J. Palaeogeogr. 2021, 23, 983–998. [Google Scholar]
- Jia, C. Structural characteristics and oil/gas accumulative regularity in Tarim Basin. Xinjiang Pet. Geol. 1999, 20, 177–183. [Google Scholar]
- Turner, S.A. Sedimentary record of late Neoproterozoic rifting in the NW Tarim Basin, China. Precambrian Res. 2010, 181, 85–96. [Google Scholar] [CrossRef]
- Wu, L.; Guan, S.; Yang, H.; Ren, R.; Zhu, G.; Jin, J.; Zhang, C. The paleogeographic framework and hydrocarbon exporation potential of Neoproterozoic rift basin in northern Tarim Basin. Acta Pet. Sin. 2017, 38, 375–385. [Google Scholar]
- Zheng, J.F.; Shen, A.J.; Yang, H.X.; Zhu, Y.J.; Liang, F. Geochemistry and geochronology characteristics and their geological significance of microbial dolomite in Upper Sinian, NW Tarim Basin. Acta Pet. Sin. 2021, 37, 2189–2202. [Google Scholar]
- Lin, C.; Li, H.; Liu, J. Major unconformities, tectonostratigraphic frameword, and evolution of the superimposed Tarim basin, Northwest China. J. Earth Sci. 2012, 23, 395–407. [Google Scholar] [CrossRef]
- He, J.; Qing, H.; Xu, B. The unconformity-related palaeokarst in the uppermost Ediacaran carbonate rocks in the northwestern Tarim Block, NW China: Implication for sedimentary evolution during the Ediacaran–Cambrian transition. Int. Geol. Rev. 2018, 61, 839–852. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2009; p. 285. [Google Scholar]
- Du, Y.; Fan, T.; Machel, H.G.; Gao, Z. Genesis of Upper Cambrian-Lower Ordovician dolomites in the Tahe Oilfield, Tarim Basin, NW China: Several limitations from petrology, geochemistry, and fluid inclusions. Mar. Pet. Geol. 2018, 91, 43–70. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Bolhar, R.; Kamber, B.S.; Moorbath, S.; Fedo, C.M.; Whitehouse, M.J. Characterisation of early Archaean chemical sediments by trace element signatures. Earth Planet. Sci. Lett. 2004, 222, 43–60. [Google Scholar] [CrossRef]
- Wang, X.; Cui, L.L.; Li, Y.Y.; Huang, X.F.; Zhai, J.X.; Ding, Z.L. Determination of clumped isotopes in carbonate using isotope ratio mass spectrometry: Toward a systematic evaluation of a sample extraction method using a static Porapak™ Q absorbent trap. Int. J. Mass Spectrom. 2016, 403, 8–14. [Google Scholar] [CrossRef]
- Dennis, K.J.; Affek, H.P.; Passy, B.H.; Schrag, D.P.; Eiler, J.M. Defining an absolute reference frame for ‘clumped’ isotope studies of CO2. Geochim. Cosmochim. Acta 2011, 75, 7117–7131. [Google Scholar] [CrossRef]
- Bernasconi, S.M.; Mueller, I.A.; Bergmann, K.D.; Breitenbach, S.F.M.; Fernandez, A.; Hodell, D.A.; Jaggi, M.; Meckler, A.N.; Millan, I.; Ziegler, M. Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochem. Geophys. Geosyst. 2018, 19, 2895–2914. [Google Scholar] [CrossRef]
- Gregg, J.M.; Sibley, D.F. Epigenetic dolomitization and the origin of xenotopic dolomite texture. J. Sediment. Res. 1984, 54, 908–931. [Google Scholar]
- Dong, S.; Chen, D.; Qing, H.; Zhou, X.; Wang, D.; Guo, Z.; Jiang, M.; Qian, Y. Hydrothermal alteration of dolostones in the Lower Ordovician, Tarim Basin, NW China: Multiple constraints from petrology, isotope geochemistry and fluid inclusion microthermometry. Mar. Pet. Geol. 2013, 46, 270–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.; Hohl, S.V.; Zhu, B.; He, T.; Pan, W.; Chen, Y.; Yao, X.; Jiang, S. Seawater carbon and strontium isotope variations through the late Ediacaran to late Cambrian in the Tarim Basin. Precambrian Res. 2020, 345, 105769. [Google Scholar] [CrossRef]
- Shen, A.J.; Hu, A.P.; Zheng, J.F.; Liang, F.; Wang, Y.S. Reconstruction of tectonic-burial evolution based on the constraints of laser in situ U-Pb date and clumped isotopic temperature: A case study from Ediacaran Qigebrak Formation in Akesu area, Tarim Basin. Mar. Orig. Pet. Geol. 2021, 26, 200–210. [Google Scholar]
- Qing, H.R.; Mountjoy, E.W. Rare earth element geochemistry of dolomites in the Middle Devonian Presqu’ile barrier, Western Canada Sedimentary Basin: Implications for fluid-rock ratios during dolomitization. Sedimentology 1994, 41, 787–804. [Google Scholar] [CrossRef]
- Wu, S.Q.; Zhu, J.Q.; Hu, W.X.; Zhang, J.T.; Wang, X.L.; Su, Y.B. Rare earth element geochemistry characteristics of Cambrian-Ordovician dolostones in the Tarim Basin and their implications for the origin. Geoscience 2009, 23, 638–647. [Google Scholar]
- Shields, G.A.; Webb, G.E. Has the REE composition of seawater changed over geological time? Chem. Geol. 2004, 204, 103–107. [Google Scholar] [CrossRef]
- Miura, N.; Kawabe, I. Dolomitization of limestone with MgCl2 solution at 150 °C: Preserved original signatures of rare earth elements and yttrium as marine limestone. Geochem. J. 2000, 34, 223–227. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, W.X.; Wang, X.L.; Li, Q.; Hu, G.; Zhu, J.Q.; Yao, S.P.; Cao, J. Characteristics and genesis of REE patterns of Changxing and Feixianguan dolomites in Panlongdong, northeastern Sichuan. Pet. Geol. Exp. 2011, 33, 624–633+638. [Google Scholar]
- Xiang, P.; Ji, H.; Shi, Y.; Huang, Y.; Sun, Y.; Xu, X.; Zou, S. Petrographic, rare earth elements and isotope constraints on the dolomite origin of Ordovician Majiagou Formation (Jizhong Depression, North China). Mar. Pet. Geol. 2020, 117, 104374. [Google Scholar] [CrossRef]
- Kawabe, I.; Toriumi, T.; Ohta, A.; Miura, N. Monoisotopic REE abundances in seawater and the origin of seawater tetrad effect. Geochem. J. 1998, 32, 213–229. [Google Scholar] [CrossRef]
- Nothdurft, L.D.; Webb, G.E.; Kamber, B.S. Rare earth element geochemistry of Late Devonian reefal carbonates, Canning Basin, Western Australia: Confirmation of a seawater REE proxy in ancient limestones. Geochim. Cosmochim. Acta 2004, 68, 263–283. [Google Scholar] [CrossRef]
- Bolhar, R.; Van Kranendonk, M.J. A non-marine depositional setting for the northern Fortescue Group, Pilbara Craton, inferred from trace element geochemistry of stromatolitic carbonates. Precambrian Res. 2007, 155, 229–250. [Google Scholar] [CrossRef]
- Jiang, L.; Cai, C.; Worden, R.H.; Li, K.; Xiang, L.; Chu, X.; Shen, A.; Li, W. Rare earth element and yttrium (REY) geochemistry in carbonate reservoirs during deep burial diagenesis: Implications for REY mobility during thermochemical sulfate reduction. Chem. Geol. 2015, 415, 87–101. [Google Scholar] [CrossRef]
- German, C.R.; Elderfield, H. Application of the Ce anomaly as a paleoredox indicator: The ground rules. Paleoceanography 1990, 5, 823–833. [Google Scholar] [CrossRef]
- Alibo, D.S.; Nozaki, Y. Rare earth elements in seawater: Particle association, shale-normalization, and Ce oxidation. Geochim. Cosmochim. Acta 1999, 63, 363–372. [Google Scholar] [CrossRef]
- Tostevin, R.; Shields, G.A.; Tarbuck, G.M.; He, T.; Clarkson, M.O.; Wood, R.A. Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings. Chem. Geol. 2016, 438, 146–162. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Wang, X.; Cao, J.; Chen, Q. Seawater normalized REE patterns of dolomites in Geshan and Panlongdong sections, China: Implications for tracing dolomitization and diagenetic fluids. Mar. Pet. Geol. 2014, 56, 63–73. [Google Scholar] [CrossRef]
- Planavsky, N.; Bekker, A.; Rouxel, O.J.; Kamber, B.; Hofmann, A.; Knudsen, A.; Lyons, T.W. Rare earth element and yttrium compositions of Archean and Paleoproterozoic Fe formations revisited: New perspectives on the significance and mechanisms of deposition. Geochim. Cosmochim. Acta 2010, 74, 6387–6405. [Google Scholar] [CrossRef]
- Bertram, C.J.; Elderfield, H. The geochemical balance of the rare earth elements and neodymium isotopes in the oceans. Geochim. Cosmochim. Acta 1993, 57, 1957–1986. [Google Scholar] [CrossRef]
- Brookins, D.G. Aqueous geochemistry of rare earth elements. Rev. Mineral. Geochem. 1989, 21, 201–225. [Google Scholar]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Michard, A.; Albarède, F. The REE content of some hydrothermal fluids. Chem. Geol. 1986, 55, 51–60. [Google Scholar] [CrossRef]
- Bau, M.; Balan, S.; Schmidt, K.; Koschinsky, A. Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth Planet. Sci. Lett. 2010, 299, 310–316. [Google Scholar] [CrossRef]
- Chen, H.L.; Yang, S.F.; Dong, C.W.; Zhu, G.Q.; Jia, C.Z.; Wei, G.Q.; Wang, Z.G. Study on geological thermal events in Tarim Basin. Chin. Sci. Bull. 1997, 42, 1096–1099. [Google Scholar]
- Zhou, X.Q.; Chen, D.Z.; Zhang, L.Y.; Tang, D.J.; Guo, C. Silica-rich seawater in the early Cambrian: Sedimentological evidence from bedded cherts. Terra Nova 2021, 33, 494–501. [Google Scholar] [CrossRef]
- Li, P.P.; Wang, C.; Zou, H.Y.; Yu, X.Y. Application of clumped isotopes to restoration of dolomitizing fluids and its limitations. Oil Gas Geol. 2021, 42, 738–746. [Google Scholar]
- Dennis, K.J.; Schrag, D.P. Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochim. Cosmochim. Acta 2010, 74, 4110–4122. [Google Scholar]
- Lloyd, M.K.; Ryb, U.; Eiler, J.M. Experimental calibration of clumped isotope reordering in dolomite. Geochim. Cosmochim. Acta 2018, 242, 1–20. [Google Scholar] [CrossRef]
- Stolper, D.A.; Eiler, J.M. The kinetics of solid-state isotope-exchange reactions for clumped isotopes: A study of inorganic calcites and apatites from natural and experimental samples. Am. J. Sci. 2015, 315, 363. [Google Scholar] [CrossRef]
- Hood, A.v.S.; Wallace, M.W. Neoproterozoic marine carbonates and their paleoceanographic significance. Glob. Planet. Chang. 2018, 160, 28–45. [Google Scholar] [CrossRef]
- Macdonald, J.M.; John, C.M.; Girard, J.P. Testing clumped isotopes as a reservoir characterization tool: A comparison with fluid inclusions in a dolomitized sedimentary carbonate reservoir buried to 2–4 km. In From Source to Seep: Geochemical Applications in Hydrocarbon Systems; Lawson, M., Formolo, M.J., Eiler, J.M., Eds.; Geological Society Special Publication; Geological Society of London: London, UK, 2018; Volume 468, pp. 189–202. [Google Scholar]
- Lukoczki, G.; Haas, J.; Gregg, J.M.; Machel, H.G.; Kele, S.; John, C.M. Early dolomitization and partial burial recrystallization: A case study of Middle Triassic peritidal dolomites in the Villany Hills (SW Hungary) using petrography, carbon, oxygen, strontium and clumped isotope data. Int. J. Earth Sci. 2020, 109, 1051–1070. [Google Scholar] [CrossRef]
- Fu, Q.; Qing, H.; Bergman, K.M. Early dolomitization and recrystallization of carbonate in an evaporite basin: The Middle Devonian Ratner laminite in southern Saskatchewan, Canada. J. Geol. Soc. 2006, 163, 937–948. [Google Scholar] [CrossRef]
- Al-Helal, A.B.; Whitaker, F.F.; Xiao, Y. Reactive transport modeling of brine reflux: Dolomitization, anhydrite precipitation, and porosity evolution. J. Sediment. Res. 2012, 82, 196–215. [Google Scholar] [CrossRef]
- Chang, J.; Qiu, N.S.; Zuo, Y.H.; Li, C.C. The new evidence on tectonic uplift in Kepingtage area, Tarim Basin: Constraints from (U-Th)/He ages. Chin. J. Geophys. 2011, 54, 163–172. [Google Scholar]
- Guo, C.; Chen, D.; Qing, H.; Zhou, X.; Ding, Y. Early dolomitization and recrystallization of the Lower-Middle Ordovician carbonates in western Tarim Basin (NW China). Mar. Pet. Geol. 2020, 111, 332–349. [Google Scholar] [CrossRef]
- Land, L.S. The application of stable isotopes to studies of the origin of dolomite and to problems of diagenesis of clastic sediments. In Stable Isotopes in Sedimentary Geology; SEPM Short Course; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1983; Volume 10, pp. 4.1–4.22. [Google Scholar]
- Mattews, A.; Katz, A. Oxygen isotope fractionation during the dolomitization of calcium carbonate. Geochim. Cosmochim. Acta 1977, 41, 1431–1438. [Google Scholar] [CrossRef]
- Galili, N.; Shemesh, A.; Yam, R.; Brailovsky, I.; Sela-Adler, M.; Schuster, E.M.; Collom, C.; Bekker, A.; Planavsky, N.; Macdonald, F.A.; et al. The geologic history of seawater oxygen isotopes from marine iron oxides. Science 2019, 365, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.P.; Zhang, H.; Cai, Z.X.; Cong, F.Y.; Huang, S.; Tang, P. Characteristics and formation mechanisms of the unconformity-related paleokarst reservoirs in the Upper Sinian, Northwestern Tarim Basin, China. Mar. Pet. Geol. 2020, 120, 104559. [Google Scholar] [CrossRef]
- Hunt, J.M. Petroleum Geochemistry and Geology, 2nd ed.; W.H. Freeman: New York, NY, USA, 1996. [Google Scholar]
- Wang, J.; He, Z.; Zhu, D.; Liu, Q.; Ding, Q.; Li, S.; Zhang, D. Petrological and geochemical characteristics of the botryoidal dolomite of Dengying Formation in the Yangtze Craton, South China: Constraints on terminal Ediacaran “dolomite seas”. Sediment. Geol. 2020, 406, 105722. [Google Scholar] [CrossRef]
- Burns, S.J.; Mckenzie, J.A.; Vasconcelos, C. Dolomite formation and biogeochemical cycles in the Phanerozoic. Sedimentology 2000, 47, 49–61. [Google Scholar] [CrossRef]
- You, X.; Sun, S.; Zhu, J.; Li, Q.; Hu, W.; Dong, H. Microbially mediated dolomite in Cambrian stromatolites from the Tarim Basin, north-west China: Implications for the role of organic substrate on dolomite precipitation. Terra Nova 2013, 25, 387–395. [Google Scholar] [CrossRef]
- Kirkland, B.L.; Leo Lynch, F.; Rahnis, M.A.; Folk, R.L.; Molineux, I.J.; McLean, R.J.C. Alternative origins for nannobacteria-like objects in calcite. Geology 1999, 27, 347–350. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Burgos-Cara, A.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Cölfen, H.; Rodriguez-Navarro, C. A non-classical view on calcium oxalate precipitation and the role of citrate. Nat. Commun. 2017, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Pratt, B.R. Epiphyton and Renalcis; diagenetic microfossils from calcification of coccoid blue-green algae. J. Sediment. Res. 1984, 54, 948–971. [Google Scholar]
- Rott, C.M.; Qing, H.R. Early dolomitization and recrystallization in shallow marine carbonates, Mississippian Alida beds, Williston Basin (Canada): Evidence from petrography and isotope geochemistry. J. Sediment. Res. 2013, 83, 928–941. [Google Scholar] [CrossRef]
- Maliva, R.G.; Budd, D.A.; Clayton, E.A.; Missimer, T.M.; Dickson, J.A.D. Insights into the dolomitization process and porosity modification in sucrosic dolostones, Avon Park Formation (Middle Eocene), East-Central Florida, U.S.A. J. Sediment. Res. 2011, 81, 218–232. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Z.; Shi, S.; Liu, W. Multiphase dolomitization of a microbialite-dominated gas reservoir, the middle Triassic Leikoupo Formation, Sichuan Basin, China. J. Pet. Sci. Eng. 2019, 180, 820–834. [Google Scholar] [CrossRef]
- Tavakoli, V.; Jamalian, A. Porosity evolution in dolomitized Permian–Triassic strata of the Persian Gulf, insights into the porosity origin of dolomite reservoirs. J. Pet. Sci. Eng. 2019, 181, 106191. [Google Scholar] [CrossRef]
- Li, P.W.; Luo, P.; Song, J.M.; Jin, T.F.; Wang, G.Q. Characteristics of Upper Sinian dolostone reservoirs in northwestern Margin of Tarim Basin. Mar. Orig. Pet. Geol. 2015, 20, 1–12. [Google Scholar]
- Jones, G.D.; Xiao, Y. Dolomitization, anhydrite cementation, and porosity evolution in a reflux system: Insights from reactive transport models. AAPG (Am. Assoc. Pet. Geol.) Bull. 2005, 89, 577–601. [Google Scholar] [CrossRef]
- Weyl, P.K. Porosity through dolomitization: Conservation of mass requirements. J. Sediment. Res. 1960, 30, 85–90. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).