Carbonate Nanoparticles Formed by Water–Rock Reactions in Groundwater: Implication of Carbonate Rock Weathering in Carbonate Aquifers
Abstract
1. Introduction
2. Sampling and Analytical Methods
2.1. Sampling Site
2.2. Sampling Method
2.3. Analytical Methods
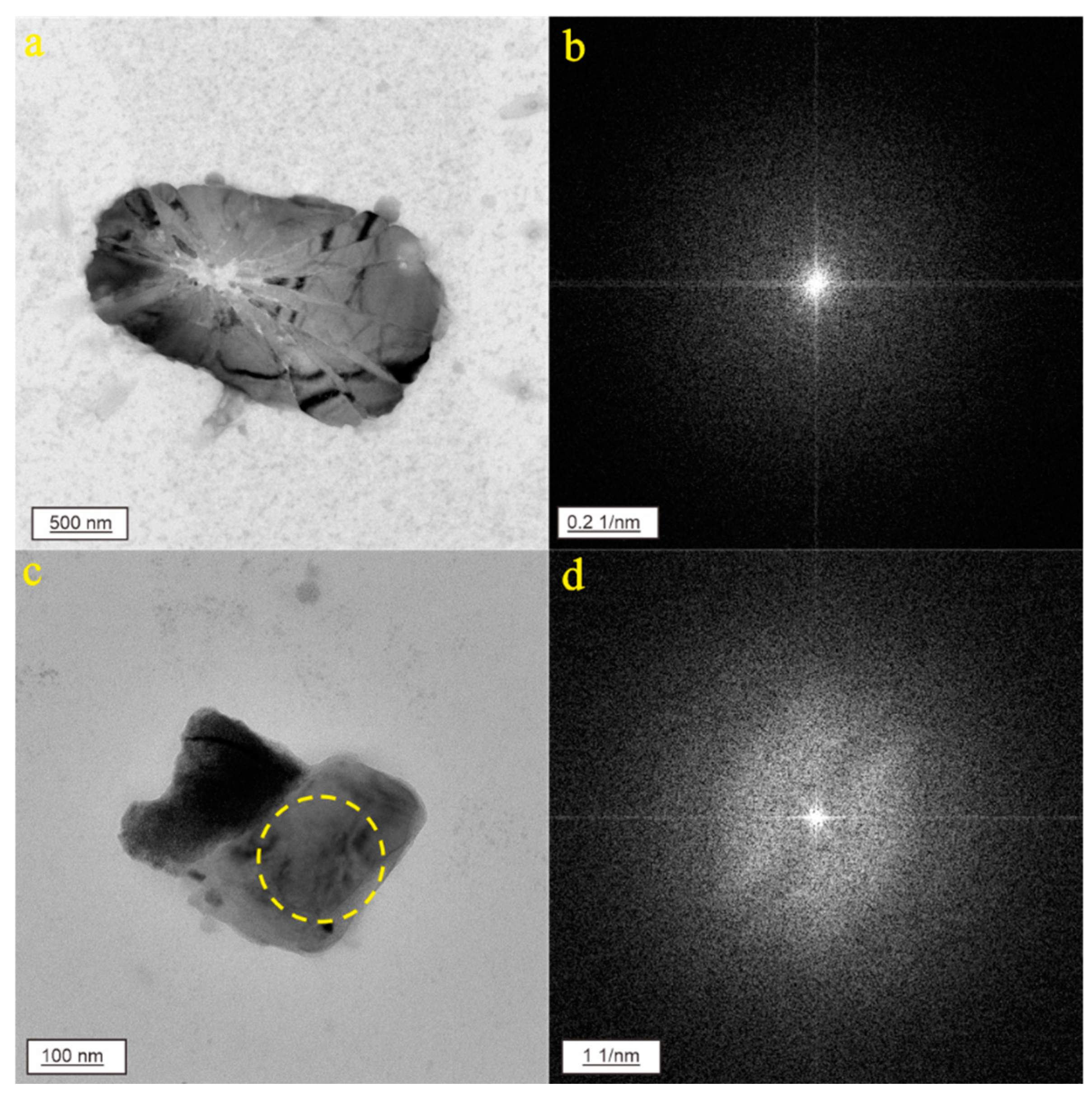
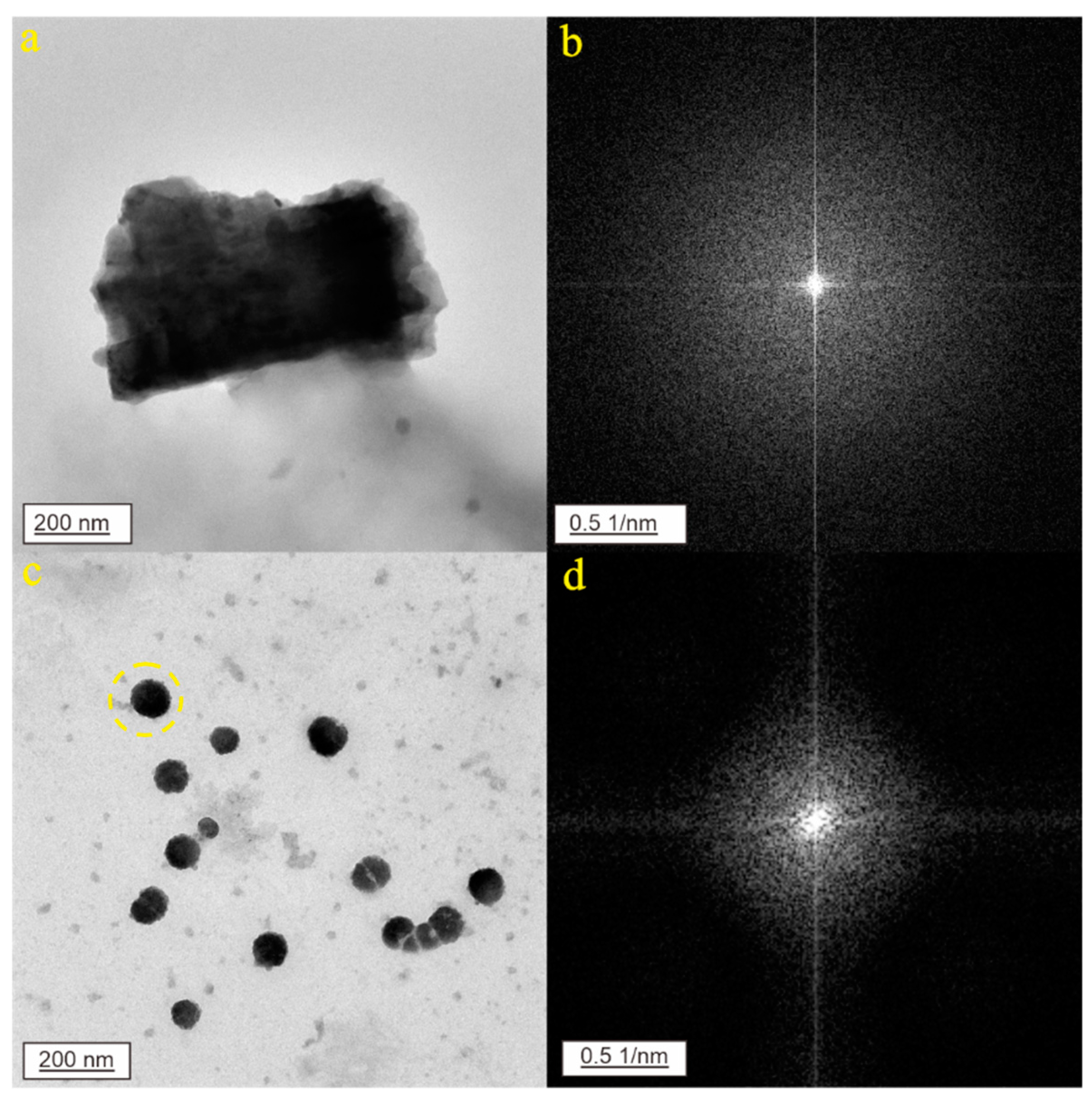
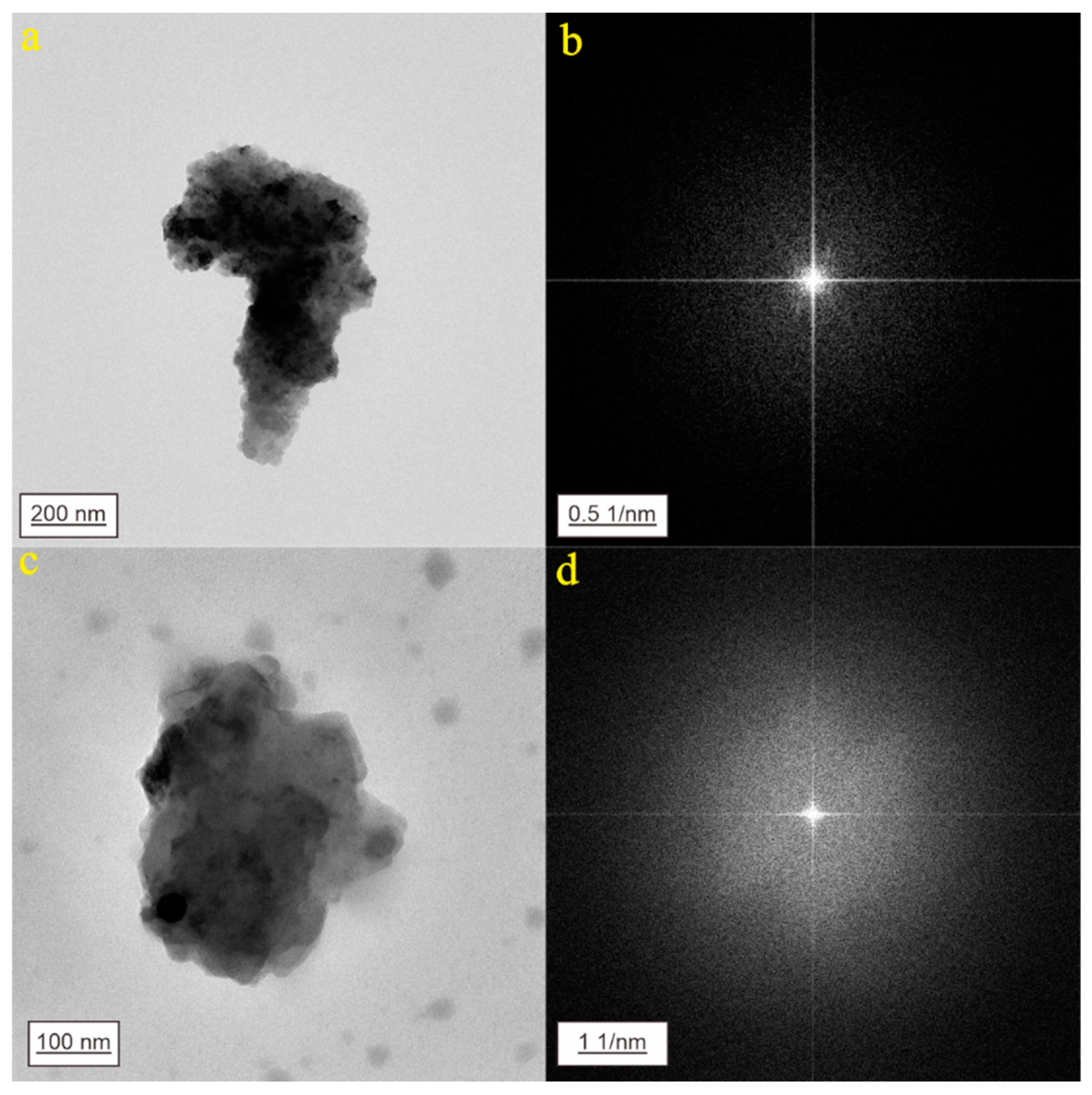
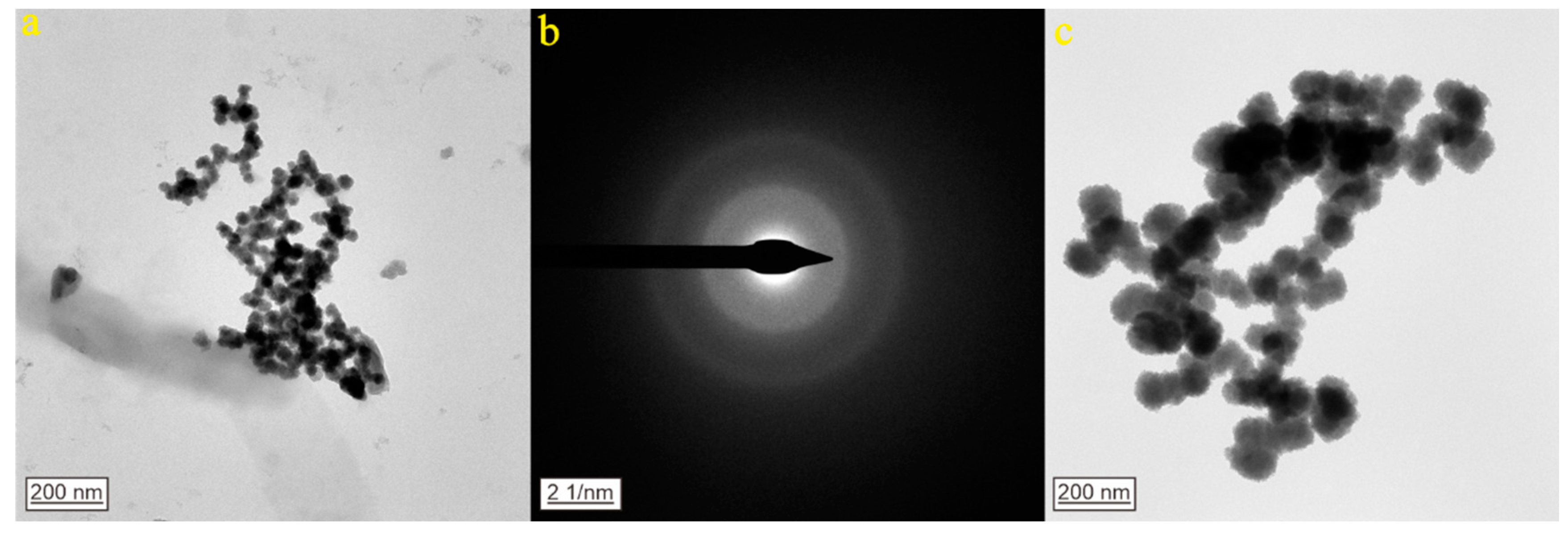
3. Results
3.1. CNPs in Karst Groundwater from Jinan City
3.2. CNPs in Geothermal Water from Jinan City
3.3. CNPs in Geothermal Water from Zibo City
4. Discussion
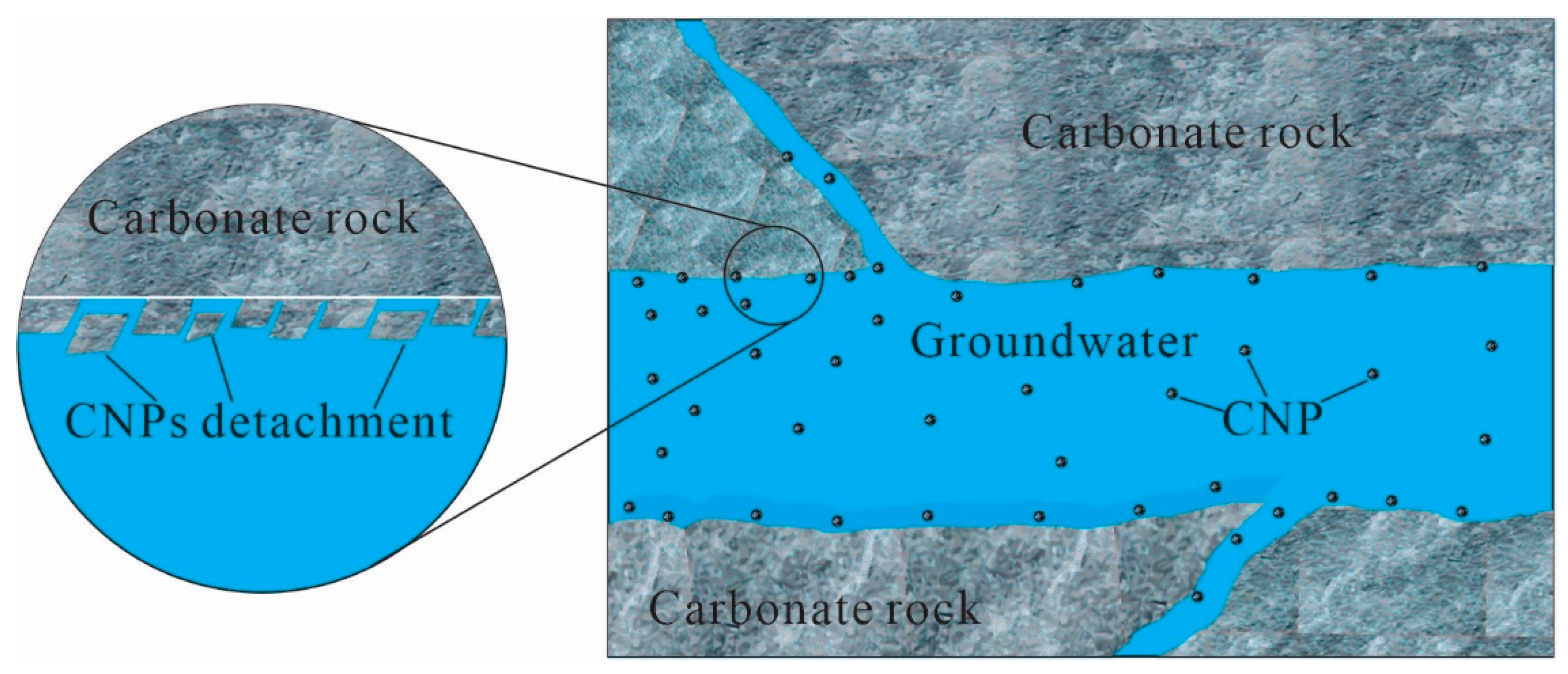
4.1. The Genesis of CNPs in Groundwater
4.2. The Role of CNPs in Carbonate Mineral Evolution and Mineralization during Weathering
4.3. Implication of CNPs for the Migration of Carbon and Associated Elements during Weathering
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural Inorganic Nanoparticles-Formation, Fate, and Toxicity in the Environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, S.; Anovitz, L.M.; Day-Stirrat, R.J. Effects of Coupled Chemo-Mechanical Processes on the Evolution of Pore-Size Distributions in Geological Media. Rev. Mineral. Geochem. 2015, 80, 45–60. [Google Scholar] [CrossRef]
- Levenson, Y.; Emmanuel, S. Quantifying Micron-Scale Grain Detachment during Weathering Experiments on Limestone. Geochim. Cosmochim. Acta 2016, 173, 86–96. [Google Scholar] [CrossRef]
- Levenson, Y.; Emmanuel, S. Repulsion between Calcite Crystals and Grain Detachment during Water-Rock Interaction. Geochem. Perspect. Lett. 2017, 3, 133–141. [Google Scholar] [CrossRef][Green Version]
- Langman, J.B.; Moberly, J.G. Weathering of a Mined Quartz-Carbonate, Galena-Sphalerite Ore and Release and Transport of Nanophase Zinc Carbonate in Circumneutral Drainage. J. Geochem. Explor. 2018, 188, 185–193. [Google Scholar] [CrossRef]
- Schindler, M.; Lussier, A.J.; Principe, E.; Mykytczuk, N. Dissolution Mechanisms of Chromitite: Understanding the Release and Fate of Chromium in the Environment. Am. Mineral. 2018, 103, 271–283. [Google Scholar] [CrossRef]
- Findlay, A.J.; Estes, E.R.; Gartman, A.; Yücel, M.; Kamyshny, A.; Luther, G.W. Iron and Sulfide Nanoparticle Formation and Transport in Nascent Hydrothermal Vent Plumes. Nat. Commun. 2019, 10, 1597. [Google Scholar] [CrossRef]
- Alekseyev, V.A. Nanoparticles and Nanofluids in Water–Rock Interactions. Geochem. Int. 2019, 57, 357–368. [Google Scholar] [CrossRef]
- Rad, S.; Louvat, P.; Gorge, C.; Gaillardet, J.; Allègre, C.J. River Dissolved and Solid Loads in the Lesser Antilles: New Insight into Basalt Weathering Processes. J. Geochem. Explor. 2006, 88, 308–312. [Google Scholar] [CrossRef]
- Bailly-Comte, V.; Martin, J.B.; Screaton, E.J. Time variant cross correlation to assess residence time of water and implication for hydraulics of a sink-rise karst system. Water Resour. Res. 2011, 47, W05547. [Google Scholar] [CrossRef]
- Lorenzi, V.; Banzato, F.; Barberio, M.D.; Goeppert, N.; Goldscheider, N.; Gori, F.; Lacchini, F.; Manetta, M.; Medici, G.; Rusi, S.; et al. Tracking flowpaths in a complex karst system through tracer test and hydrogeochemical monitoring: Implications for groundwater protection (Gran Sasso, Italy). Heliyon 2024, 10, e24663. [Google Scholar] [CrossRef] [PubMed]
- Rad, S.D.; Allègre, C.J.; Louvat, P. Hidden Erosion on Volcanic Islands. Earth Planet. Sci. Lett. 2007, 262, 109–124. [Google Scholar] [CrossRef]
- Calmels, D.; Galy, A.; Hovius, N.; Bickle, M.; West, A.J.; Chen, M.C.; Chapman, H. Contribution of Deep Groundwater to the Weathering Budget in a Rapidly Eroding Mountain Belt, Taiwan. Earth Planet. Sci. Lett. 2011, 303, 48–58. [Google Scholar] [CrossRef]
- Cai, W.Y.; Feng, L.D.; Liu, S.H.; Zhu, J.J. Hemoglobin-CdTe-CaCO3@ Polyelectrolytes 3D Architecture: Fabrication, Characterization, and Application in Biosensing. Adv. Funct. Mater. 2008, 18, 3127–3136. [Google Scholar] [CrossRef]
- Ueno, Y.; Futagawa, H.; Takagi, Y.; Ueno, A.; Mizushima, Y. Drug-Incorporating Calcium Carbonate Nanoparticles for a New Delivery System. J. Control. Release 2005, 103, 93–98. [Google Scholar] [CrossRef]
- Wang, C.Y.; He, C.Y.; Tong, Z.; Liu, X.X.; Ren, B.Y.; Zeng, F. Combination of Adsorption by Porous CaCO3 Microparticles and Encapsulation by Polyelectrolyte Multilayer Films for Sustained Drug Delivery. Int. J. Pharm. 2006, 308, 160–167. [Google Scholar] [CrossRef]
- Peng, C.Y.; Zhao, Q.H.; Gao, C.Y. Sustained Delivery of Doxorubicin by Porous CaCO3 and Chitosan/Alginate Multilayers-Coated CaCO3 Microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 132–139. [Google Scholar] [CrossRef]
- Sozer, N.; Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009, 27, 82–89. [Google Scholar] [CrossRef]
- Mcneil, S.E. Unique benefits of nanotechnology to drug delivery and diagnostics. Methods Mol. Biol. 2011, 697, 3–8. [Google Scholar] [PubMed]
- Sonkaria, S.; Ahn, S.H.; Khare, V. Nanotechnology and its impact on food and nutrition: A review. Recent Patents Food Nutr. Agric. 2012, 4, 8–18. [Google Scholar]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Arimori, A.; Isobe, M.; Tochigi, S.; Kondoh, S.; Hirai, T.; Akase, T. Size-Dependent Cytotoxic Effects of Amorphous Silica Nanoparticles on Langerhans Cells. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 199–201. [Google Scholar] [CrossRef]
- Nakayama, M.; Kajiyama, S.; Kumamoto, A.; Ikuhara, Y.; Kato, T. Bioinspired Selective Synthesis of Liquid-Crystalline Nanocomposites: Formation of Calcium Carbonate-Based Composite Nanodisks and Nanorods. Nanoscale Adv. 2020, 2, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dong, H.W.; Shi, L.T.; Tang, X.Y.; Liu, J.R.; Shi, J.H. Reproductive Toxicity of Perchlorate in Rats. Food Chem. Toxicol. 2019, 128, 212–222. [Google Scholar] [CrossRef]
- Wu, C.Y.; Martel, J.; Wong, T.Y.; Young, D.; Liu, C.C.; Lin, C.W.; Young, J.D. Formation and Characteristics of Biomimetic Mineralo-Organic Particles in Natural Surface Water. Sci. Rep. 2016, 6, 28817. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Z.Q.; Lv, M.H.; Wang, H.W.; Li, L.X.; Gang, S.T.; Zuo, L.; Zhang, P.; Wang, Y.Q.; Li, C.S.; et al. Discovery of Environmental Nanoparticles in a Mineral Water Spring from Yiyuan County, Shandong Province, Eastern China: A New Form of Elements in Mineral Water. Water 2023, 15, 3497. [Google Scholar] [CrossRef]
- Shao, G.Y.; Li, C.S.; Liu, R.; Zhang, P.; Zuo, L.; Wang, Y.Q. Silicified Microorganisms and Microorganism-like Particles in the Groundwater of an Abandoned Coal Mine. Mine Water Environ. 2023, 42, 489–499. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, P.; Wang, Y.Q.; Liu, R.; Ma, G.X. Characteristics and Research Significance of Micro-Nanoparticles in Geothermal Fluids in the Central Area of Shandong Province. Water 2023, 15, 3737. [Google Scholar] [CrossRef]
- Zuo, L.; Li, C.S.; Zhang, P.; Wang, Y.Q.; Gao, S.; Sun, B.; Liu, R. Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China. Water 2024, 16, 650. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, R.; Wang, Y.Q.; Lu, B.; Cui, Y.X.; Zuo, L.; Zhang, P.; Wang, Y.Q.; Cao, C. Biomimetic Fe-Bearing Nanoparticles in Hot Spring: Morphology, Origin and Potential Bioavailable Fe. Front. Earth Sci. 2024, 12, 1404788. [Google Scholar] [CrossRef]
- Hu, C.P.; Liu, R.; Zhang, P.; Wang, Y.Q.; Zuo, L.; Zhang, X.H.; Li, C.S. Characteristics and Significance of Natural Nanoparticles in the Groundwater of the Baotu Spring Area in Jinan, Shandong Province, Eastern China. Water 2024, 16, 1820. [Google Scholar] [CrossRef]
- Emmanuel, S.; Levenson, Y. Limestone Weathering Rates Accelerated by Micron-Scale Grain Detachment. Geology 2014, 42, 751–754. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J. Contribution of Carbonate Rock Weathering to the Atmospheric CO2 Sink. Environ. Geol. 2000, 39, 1053–1058. [Google Scholar] [CrossRef]
- Basak, C.; Martin, E.E. Antarctic Weathering and Carbonate Compensation at the Eocene–Oligocene Transition. Nat. Geosci. 2013, 6, 121–124. [Google Scholar] [CrossRef]
- Komar, N.; Zeebe, R.E.; Dickens, G.R. Understanding Long-Term Carbon Cycle Trends: The Late Paleocene through the Early Eocene. Paleoceanography 2013, 28, 650–662. [Google Scholar] [CrossRef]
- Tan, X.B.; Bo, B.Y.; Zhang, P.; Shao, G.Y.; Liu, R.; Wang, K. Carbonaceous Nanoparticles in Zibo Hot Springs: Implications for the Cycling of Carbon and Associated Elements. Environ. Chem. Lett. 2021, 19, 4009–4014. [Google Scholar] [CrossRef]
- Vleugels, G.; Van Grieken, R. Suspended Matter in Run-off Water from Limestone Exposure Setups. Sci. Total Environ. 1995, 170, 125–132. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, B.M.; Lin, X.; Xu, S.F.; Yao, W.S.; Ye, R. Geochemical Challenges of Diverse Regolith-Covered Terrains for Mineral Exploration in China. Ore Geol. Rev. 2016, 73, 417–431. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, B.M.; Ye, R. Nanogeochemistry for Mineral Exploration through Covers. Bull. Mineral. Petrol. Geochem. 2016, 35, 43–51. [Google Scholar] [CrossRef]
- Ju, Y.W.; Huang, C.; Sun, Y.; Wan, Q.; Lu, X.C.; Lu, S.F.; He, H.P.; Wang, X.Q.; Zou, C.N.; Wu, J.G.; et al. Nanogeosciences: Research History, Current Status, and Development Trends. J. Nanosci. Nanotechnol. 2017, 17, 5930–5965. [Google Scholar] [CrossRef]
- Wu, S.H.; Mao, J.W.; Yuan, S.D.; Dai, P.; Wang, X.D. Mineralogy, Fluid Inclusion Petrography, and Stable Isotope Geochemistry of Pb–Zn–Ag Veins at the Shizhuyuan Deposit, Hunan Province, Southeastern China. Miner. Depos. 2018, 53, 89–103. [Google Scholar] [CrossRef]
- Wierchowiec, J.; Mikulski, S.Z.; Zieliński, K. Supergene Gold Mineralization from Exploited Placer Deposits at Dziwiszów in the Sudetes (NE Bohemian Massif, SW Poland). Ore Geol. Rev. 2021, 131, 104049. [Google Scholar] [CrossRef]
- Cooke, D.J.; Elliott, J.A. Atomistic Simulations of Calcite Nanoparticles and Their Interaction with Water. J. Chem. Phys. 2007, 127, 104706. [Google Scholar] [CrossRef] [PubMed]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and Textural Changes Accompanying Ageing of Silica Sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Icopini, G.A.; Brantley, S.L.; Heaney, P.J. Kinetics of Silica Oligomerization and Nanocolloid Formation as a Function of PH and Ionic Strength at 25 °C. Geochim. Cosmochim. Acta 2005, 69, 293–303. [Google Scholar] [CrossRef]
- Colman, S.M. Chemical Weathering of Basalts and Andesites; Evidence from Weathering Rinds; U.S. G.P.O.: Washington, DC, USA, 1982. [CrossRef]
- Eggleton, R.A.; Foudoulis, C.; Varkevisser, D. Weathering of Basalt: Changes in Rock Chemistry and Mineralogy. Clays Clay Miner. 1987, 35, 161–169. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Wilson, R.E. Recent Chemical Weathering of Basalts. Am. J. Sci. 1992, 292, 740–777. [Google Scholar] [CrossRef]
- Gavshin, V.M.; Shcherbov, B.L.; Bobrov, V.A.; Solotchina, E.P.; Sukhorukov, F.V.; Mel’gunov, M.S. Behavior of Trace Elements in the Process of Formation of a Weathering Profile on Granites. Russ. Geol. Geophys. C/C Geol. Geofiz. 1997, 38, 1264–1276. [Google Scholar]
- Hill, I.G.; Worden, R.H.; Meighan, I.G. Yttrium: The Immobility-Mobility Transition during Basaltic Weathering. Geology 2000, 28, 923–926. [Google Scholar] [CrossRef]
- Patino, L.C.; Velbel, M.A.; Price, J.R.; Wade, J.A. Trace Element Mobility during Spheroidal Weathering of Basalts and Andesites in Hawaii and Guatemala. Chem. Geol. 2003, 202, 343–364. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice Hall: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Öztürk, H.; Hein, J.R.; Hanilçi, N. Genesis of the Doğankuzu and Mortaş Bauxite Deposits, Taurides, Turkey: Separation of Al, Fe, and Mn and Implications for Passive Margin Metallogeny. Econ. Geol. 2002, 97, 1063–1077. [Google Scholar] [CrossRef]
- Yang, S.J.; Huang, Y.X.; Wang, Q.F.; Deng, J.; Liu, X.F.; Wang, J.Q. Mineralogical and Geochemical Features of Karst Bauxites from Poci (Western Henan, China), Implications for Parental Affinity and Bauxitization. Ore Geol. Rev. 2019, 105, 295–309. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Kasama, T.; Church, N.S.; Douvalis, A.P.; Göttlicher, J.; Steininger, R.; Boubnov, A.; Pontikes, Y.; Tzamos, E.; et al. Nano-Mineralogy and -Geochemistry of High-Grade Diasporic Karst-Type Bauxite from Parnassos-Ghiona Mines, Greece. Ore Geol. Rev. 2017, 84, 228–244. [Google Scholar] [CrossRef]
- He, H.Z.; Zhang, Q.Z. Analysis on Geological Anomaly of Karst Accumulative Bauxite Deposit and Delimitation of Favorable Area for Prospecting Based on GIS in Western Guangxi. Miner. Resour. Geol. 2007, 21, 436–439. [Google Scholar]
- Perry, C.C.; Keeling-Tucker, T. Biosilicification: The Role of the Organic Matrix in Structure Control. JBIC J. Biol. Inorg. Chem. 2000, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Pancost, R.D.; Pressley, S.; Coleman, J.M.; Benning, L.G.; Mountain, B.W. Lipid Biomolecules in Silica Sinters: Indicators of Microbial Biodiversity. Environ. Microbiol. 2005, 7, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Utsunomiya, S.; Kesler, S.E.; Wang, L.; Ewing, R.C.; Becker, U. Thermal Behavior of Metal Nanoparticles in Geologic Materials. Geology 2006, 34, 1033–1036. [Google Scholar] [CrossRef]
- Tobler, D.J.; Stefánsson, A.; Benning, L.G. In-situ Grown Silica Sinters in Icelandic Geothermal Areas. Geobiology 2008, 6, 481–502. [Google Scholar] [CrossRef]
- Yi, Z.; Fu, W.; Zhao, Q.; Lu, H.; Fu, X.; Li, P.; Luo, P.; Han, Z.; Tan, Z.; Xu, C. Characterization of Nano-Minerals and Nanoparticles in Supergene Rare Earth Element Mineralization Related to Chemical Weathering of Granites. Am. Mineral. 2023, 108, 1461–1475. [Google Scholar] [CrossRef]


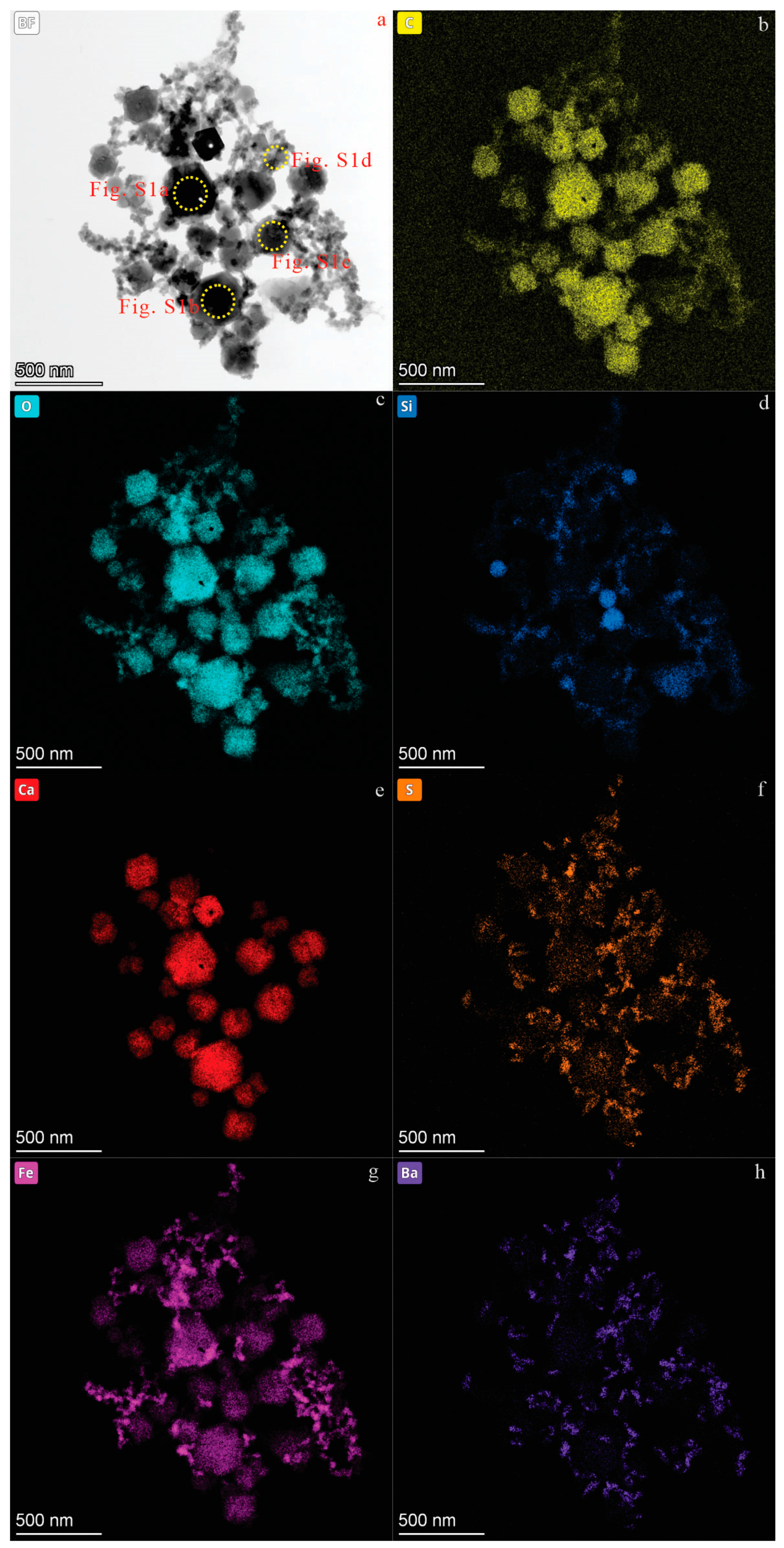
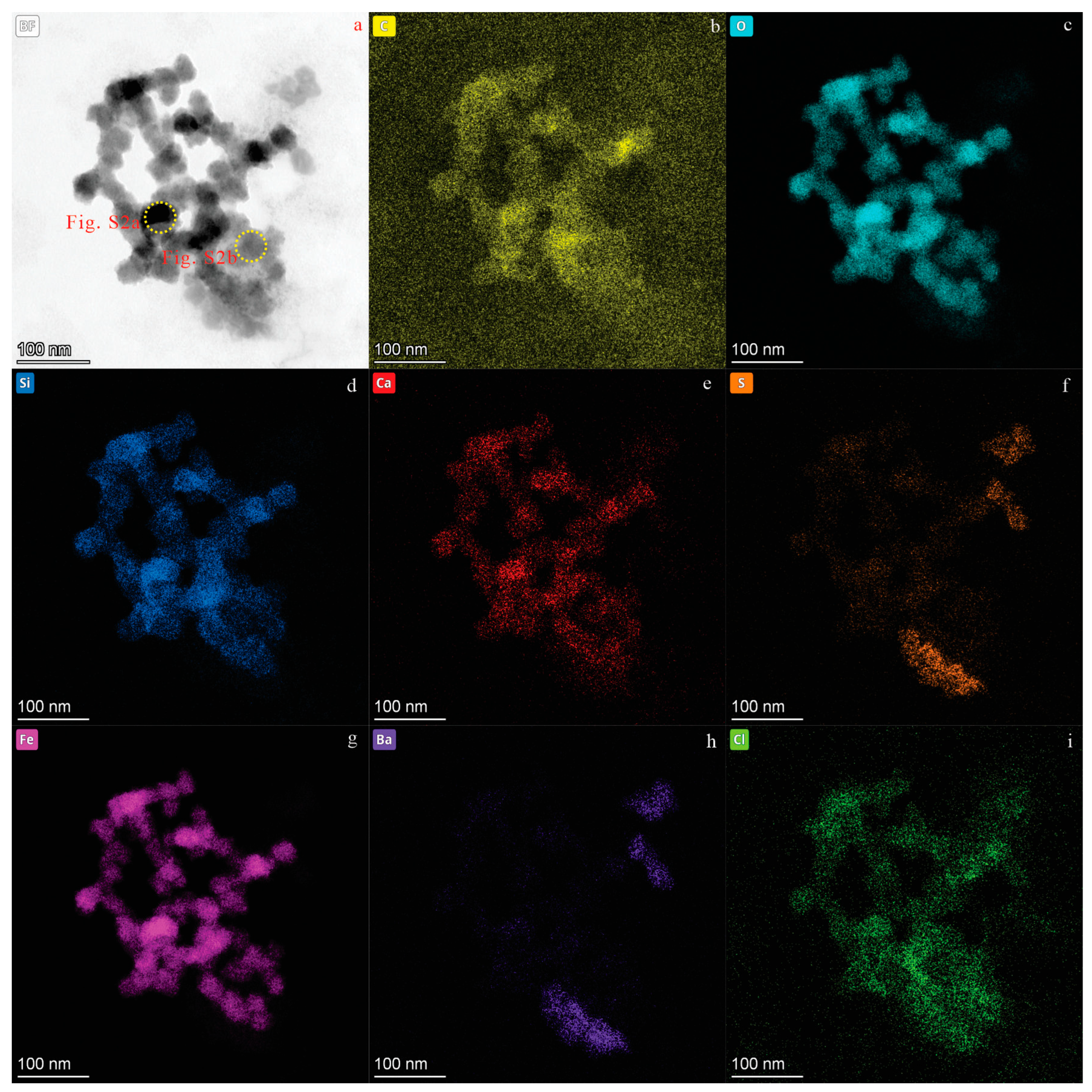
| Location | Groundwater | Samples | C | O | Ca | S | Cl | Si | Mg | Fe | Al | Na | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jinan | karst groundwater | KG-1 | 35.45 | 40.95 | 10.35 | 8.99 | 0.89 | 1.01 | 0.17 | 2.35 | |||
| KG-2 | 47.44 | 36.84 | 13.87 | 0.12 | 1.34 | 0.13 | 0.25 | ||||||
| KG-3 | 30.97 | 38.39 | 27.94 | 0.56 | 0.96 | 0.80 | |||||||
| KG-4 | 40.18 | 34.30 | 19.78 | 2.26 | 0.74 | 2.73 | |||||||
| KG-5 | 48.75 | 32.57 | 16.67 | 0.46 | 0.71 | 0.22 | 0.29 | ||||||
| KG-6 | 44.50 | 32.71 | 1.37 | 0.76 | 1.42 | 2.55 | 2.08 | 15.08 | 1.42 | 1.23 | 0.78 | ||
| geothermal water | GW-1 | 29.78 | 41.66 | 13.76 | 12.61 | ||||||||
| GW-2 | 25.75 | 46.32 | 25.60 | 0.80 | 0.36 | 0.26 | |||||||
| GW-3 | 25.94 | 38.39 | 1.51 | 0.53 | 4.11 | 0.71 | 28.80 | 0.40 | |||||
| GW-4 | 15.70 | 40.31 | 0.70 | 0.90 | 36.85 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, G.; Liu, R.; Zhang, P.; Wang, Y.; Zuo, L.; Zhang, X. Carbonate Nanoparticles Formed by Water–Rock Reactions in Groundwater: Implication of Carbonate Rock Weathering in Carbonate Aquifers. Minerals 2024, 14, 980. https://doi.org/10.3390/min14100980
Tao G, Liu R, Zhang P, Wang Y, Zuo L, Zhang X. Carbonate Nanoparticles Formed by Water–Rock Reactions in Groundwater: Implication of Carbonate Rock Weathering in Carbonate Aquifers. Minerals. 2024; 14(10):980. https://doi.org/10.3390/min14100980
Chicago/Turabian StyleTao, Gang, Rui Liu, Peng Zhang, Yaqin Wang, Lei Zuo, and Xiaoheng Zhang. 2024. "Carbonate Nanoparticles Formed by Water–Rock Reactions in Groundwater: Implication of Carbonate Rock Weathering in Carbonate Aquifers" Minerals 14, no. 10: 980. https://doi.org/10.3390/min14100980
APA StyleTao, G., Liu, R., Zhang, P., Wang, Y., Zuo, L., & Zhang, X. (2024). Carbonate Nanoparticles Formed by Water–Rock Reactions in Groundwater: Implication of Carbonate Rock Weathering in Carbonate Aquifers. Minerals, 14(10), 980. https://doi.org/10.3390/min14100980





