Gypsum Crystals Formed by the Anhydrite–Gypsum Transformation at Low Temperatures: Implications for the Formation of the Geode of Pulpí
Abstract
1. Introduction
2. Materials and Methods
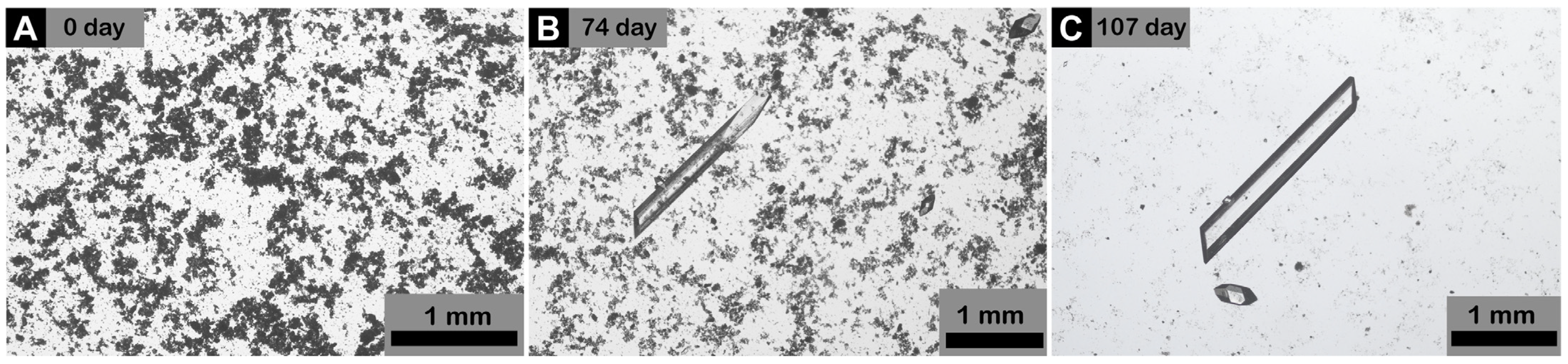
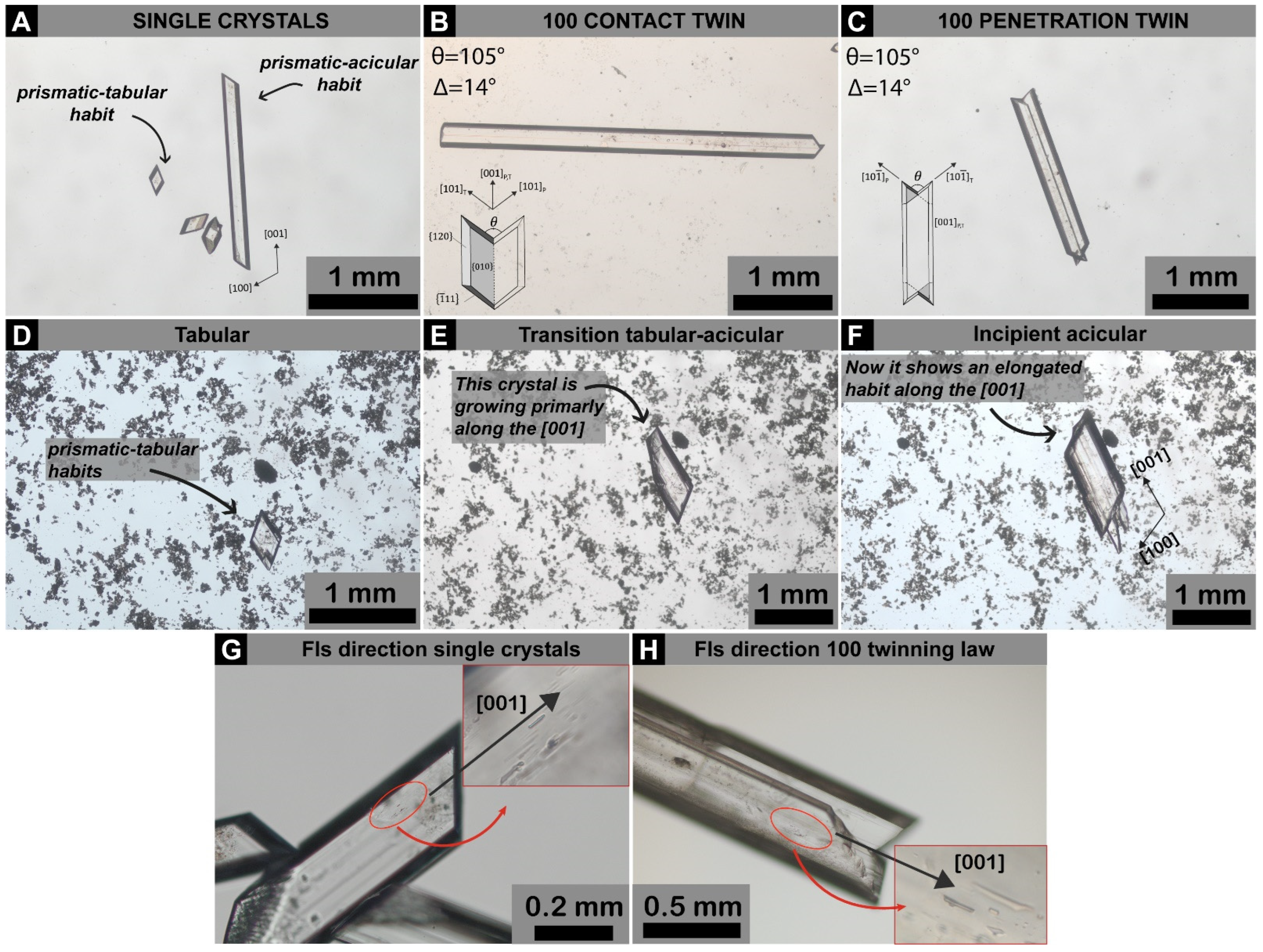
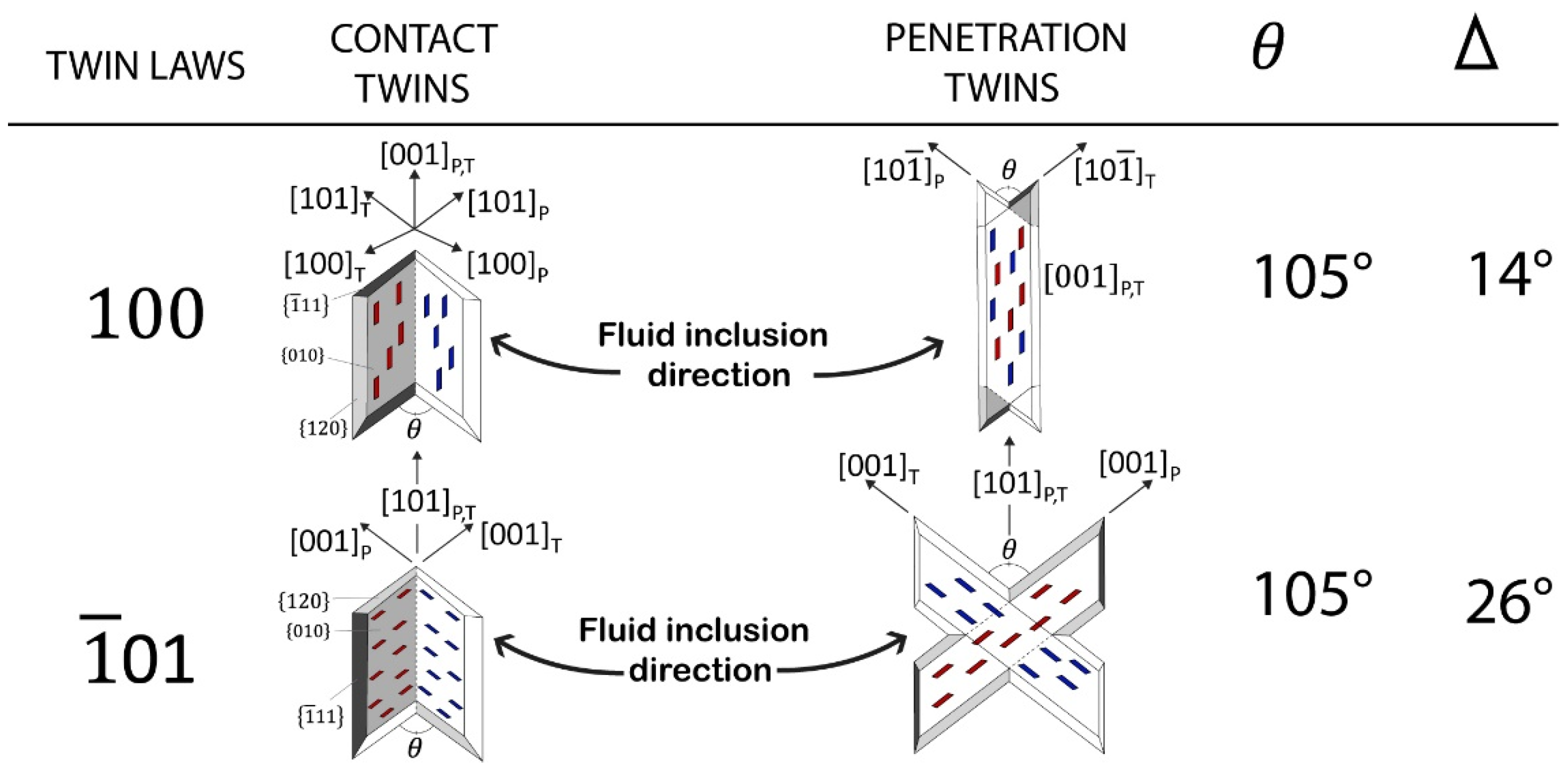
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Ruiz, J.M.; Canals, A.; Ayora, C. Gypsum Megacrystals. In McGraw-Hill Yearbook of Science and Technology; McGraw-Hill Book Co.: New York, NY, USA, 2008. [Google Scholar]
- Krüger, Y.; García-Ruiz, J.M.; Canals, À.; Marti, D.; Frenz, M.; Van Driessche, A.E.S. Determining Gypsum Growth Temperatures Using Monophase Fluid Inclusions—Application to the Giant Gypsum Crystals of Naica, Mexico. Geology 2013, 41, 119–122. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Villasuso, R.; Ayora, C.; Canals, A.; Otálora, F. Formation of Natural Gypsum Megacrystals in Naica, Mexico. Geology 2007, 35, 327–330. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; García-Ruiz, J.M.; Delgado-López, J.M.; Sazaki, G. In Situ Observation of Step Dynamics on Gypsum Crystals. Cryst. Growth Des. 2010, 10, 3909–3916. [Google Scholar] [CrossRef]
- Sanna, L.; Forti, P.; Lauritzen, S.-E. Preliminary U/Th Dating and the Evolution of Gypsum Crystals in Naica Caves (Mexico). Acta Carsologica 2011, 40, 17–28. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; García-Ruíz, J.M.; Tsukamoto, K.; Patiño-Lopez, L.D.; Satoh, H. Ultraslow Growth Rates of Giant Gypsum Crystals. Proc. Natl. Acad. Sci. USA 2011, 108, 15721–15726. [Google Scholar] [CrossRef]
- Palero, F.J.; Canals, À.; van Driessche, A.; García-Ruiz, J.M. Geology of the Fe-Pb-Ag “Mina Rica” Deposit, Pulpí (Almería, Spain). Bol. Geol. Min. 2020, 131, 495–538. [Google Scholar] [CrossRef]
- Garcia-Guinea, J.; Morales, S.; Delgado, A.; Recio, C.; Calaforra, J.M. Formation of Gigantic Gypsum Crystals. J. Geol. Soc. 2002, 159, 347–350. [Google Scholar] [CrossRef]
- Gázquez, F.; Monteserín, A.; Obert, C.; Münker, C.; Fernández-Cortés, Á.; Calaforra, J.M. The Absolute Age and Origin of the Giant Gypsum Geode of Pulpí (Almería, SE Spain). Geosciences 2022, 12, 144. [Google Scholar] [CrossRef]
- Canals, A.; Van Driessche, A.E.S.; Palero, F.; García-Ruiz, J.M. The Origin of Large Gypsum Crystals in the Geode of Pulpí (Almería, Spain). Geology 2019, 47, 1161–1165. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; García-Ruiz, J.M.; Kahr, B. Punin Ripening and the Classification of Solution-Mediated Recrystallization Mechanisms. Cryst. Growth Des. 2021, 21, 1267–1277. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In U.S. Geology Survey Techniques and Methods; USGS: Reston, VA, USA, 2013; Book 6; p. 497. [Google Scholar]
- La Bella, M.; Besselink, R.; Wright, J.P.; Van Driessche, A.E.; Fernandez-Martinez, A.; Giacobbe, C. Hierarchical synchrotron diffraction and imaging study of the calcium sulfate hemihydrate–gypsum transformation. J. Appl. Crystallogr. 2023, 56, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Cody, A.M.; Cody, R.D. SEM and Polarization Analyzes Updating Early Light Microscope Studies Related to {101} Twin Formation in Gypsum. J. Cryst. Growth 1989, 98, 731–738. [Google Scholar] [CrossRef]
- Ortí, F. Selenite Facies in Marine Evaporites: A Review. In Quaternary Carbonate and Evaporite Sedimentary Facies and Their Ancient Analogues; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 431–463. [Google Scholar] [CrossRef]
- Rubbo, M.; Bruno, M.; Massaro, F.R.; Aquilano, D. The Five Twin Laws of Gypsum (CaSO4·2H2O): A Theoretical Comparison of the Interfaces of the Contact Twins. Cryst. Growth Des. 2012, 12, 264–270. [Google Scholar] [CrossRef]
- Rubbo, M.; Bruno, M.; Massaro, F.R.; Aquilano, D. The Five Twin Laws of Gypsum (CaSO4·2H2O): A Theoretical Comparison of the Interfaces of the Penetration Twins. Cryst. Growth Des. 2012, 12, 3018–3024. [Google Scholar] [CrossRef]
- Cotellucci, A.; Otálora, F.; Canals, À.; Criado-Reyes, J.; Pellegrino, L.; Bruno, M.; Aquilano, D.; Garcia-Ruiz, J.M.; Dela Pierre, F.; Pastero, L. Contact Twins in Gypsum Experimentally Obtained from Calcium Carbonate Enriched Solutions: Mineralogical Implications for Natural Gypsum Deposits. J. Appl. Cryst. 2023, 56, 603–610. [Google Scholar] [CrossRef]
- Otálora, F.; García-Ruiz, J. Nucleation and Growth of the Naica Giant Gypsum Crystals. Chem. Soc. Rev. 2014, 43, 2013–2026. [Google Scholar] [CrossRef]
- Kern, R.; Rehn, B. Etude Experimentale de La Formation Des Macles de Croissance Du Gypse. C. R. Hebd. Seances Acad. Sci. 1960, 251, 1300–1302. [Google Scholar]
- Cody; Cody, R.D. Evidence for Micro-Biological Induction of {101} Montmartre Twinning of Gypsum (CaSO4·2H2O). J. Cryst. Growth 1989, 98, 721–730. [Google Scholar] [CrossRef]
- Cotellucci, A.; Pellegrino, L.; Costa, E.; Bruno, M.; Pierre, F.D.; Aquilano, D.; Destefanis, E.; Pastero, L. Effect of Different Evaporation Rates on Gypsum Habit: Mineralogical Implications for Natural Gypsum Deposits. Cryst. Growth Des. 2023, 23, 9094–9102. [Google Scholar] [CrossRef]
- Burgos-Ruiz, M.; Ilett, M.; Roncal-Herrero, T.; Elert, K.; Rubio-Domene, R.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Bio-Inspired Fluorescent Calcium Sulfate for the Conservation of Gypsum Plasterwork. Small 2024, 20, 2402581. [Google Scholar] [CrossRef]
- Mbogoro, M.M.; Peruffo, M.; Adobes-Vidal, M.; Field, E.L.; O’Connell, M.A. Quantitative 3d visualization of the growth of individual gypsum microcrystals: Effect of Ca2+: SO42– ratio on kinetics and crystal morphology. J. Phys. Chem. C 2017, 121, 12726–12734. [Google Scholar] [CrossRef]
- Rinaudo, C.; Robert, M.C.; Lefaucheux, F. Growth and characterization of gypsum crystals. J. Cryst. Growth 1985, 71, 803–806. [Google Scholar] [CrossRef]
- Martín, J.M.; Braga, J.C.; Betzler, C. Late Neogene–Recent Uplift of the Cabo de Gata Volcanic Province, Almería, SE Spain. Geomorphology 2003, 50, 27–42. [Google Scholar] [CrossRef]
- Dyja, V.; Hibsch, C.; Tarantola, A.; Cathelineau, M.; Boiron, M.-C.; Marignac, C.; Bartier, D.; Carrillo-Rosúa, J.; Ruano, S.M.; Boulvais, P. From Deep to Shallow Fluid Reservoirs: Evolution of Fluid Sources during Exhumation of the Sierra Almagrera, Betic Cordillera, Spain. Geofluids 2016, 16, 103–128. [Google Scholar] [CrossRef]
- Hardie, L.A. The Gypsum—Anhydrite Equilibrium at One Atmosphere Pressure1. Am. Mineral. 1967, 52, 171–200. [Google Scholar]
- Blount, C.W.; Dickson, F.W. Gypsum-Anhydrite Equilibria in Systems CaSO4-H2O and CaCO4-NaCl-H2O. Am. Mineral. 1973, 58, 323–331. [Google Scholar]
- De Jong, W.F.; Bouman, J. Das reziproke und das Bravaissche Gitter von Gips. Z. Für Krist.—Cryst. Mater. 1939, 100, 275–276. [Google Scholar]
- Aquilano, D.; Otálora, F.; Pastero, L.; García-Ruiz, J.M. Three Study Cases of Growth Morphology in Minerals: Halite, Calcite and Gypsum. Prog. Cryst. Growth Charact. Mater. 2016, 62, 227–251. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotellucci, A.; Garcia-Ruiz, J.-M.; Otálora, F.; Canals, À.; Bruno, M.; Wehrung, Q.; Pellegrino, L.; Aquilano, D.; Pastero, L. Gypsum Crystals Formed by the Anhydrite–Gypsum Transformation at Low Temperatures: Implications for the Formation of the Geode of Pulpí. Minerals 2024, 14, 1074. https://doi.org/10.3390/min14111074
Cotellucci A, Garcia-Ruiz J-M, Otálora F, Canals À, Bruno M, Wehrung Q, Pellegrino L, Aquilano D, Pastero L. Gypsum Crystals Formed by the Anhydrite–Gypsum Transformation at Low Temperatures: Implications for the Formation of the Geode of Pulpí. Minerals. 2024; 14(11):1074. https://doi.org/10.3390/min14111074
Chicago/Turabian StyleCotellucci, Andrea, Juan-Manuel Garcia-Ruiz, Fermín Otálora, Àngels Canals, Marco Bruno, Quentin Wehrung, Luca Pellegrino, Dino Aquilano, and Linda Pastero. 2024. "Gypsum Crystals Formed by the Anhydrite–Gypsum Transformation at Low Temperatures: Implications for the Formation of the Geode of Pulpí" Minerals 14, no. 11: 1074. https://doi.org/10.3390/min14111074
APA StyleCotellucci, A., Garcia-Ruiz, J.-M., Otálora, F., Canals, À., Bruno, M., Wehrung, Q., Pellegrino, L., Aquilano, D., & Pastero, L. (2024). Gypsum Crystals Formed by the Anhydrite–Gypsum Transformation at Low Temperatures: Implications for the Formation of the Geode of Pulpí. Minerals, 14(11), 1074. https://doi.org/10.3390/min14111074







