Closed-Loop Process of Extracting and Separating Zinc Impurities from Industrial Cobalt Products—Pilot Test Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
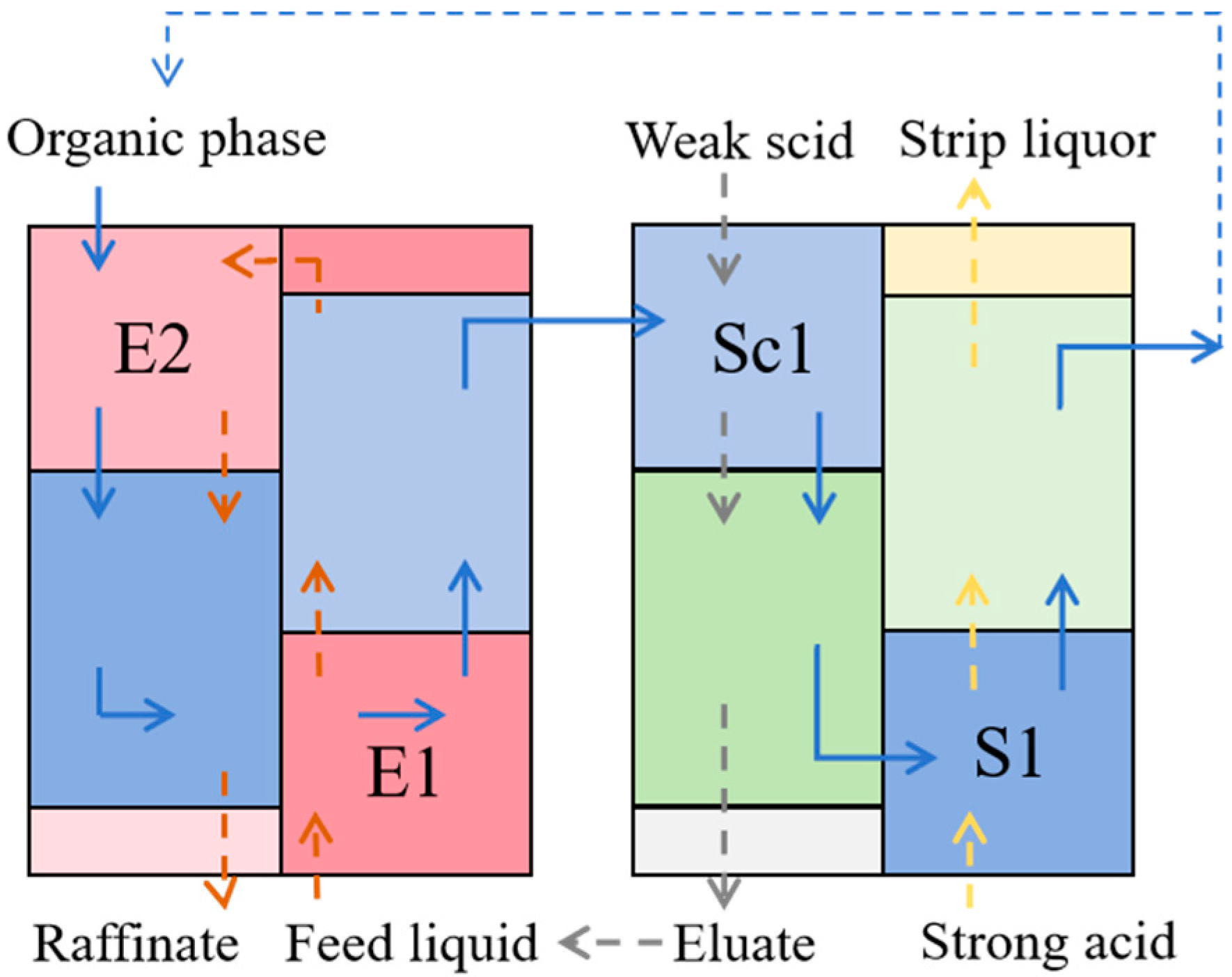
2.2. Extraction Experiment
2.3. Neutralization and Precipitation Experiment
2.4. Calculation
2.5. Data Analysis
3. Results and Discussion
3.1. Effects of Different Sulfuric Acid Addition Amounts on Zn Removal in Extraction Experiment
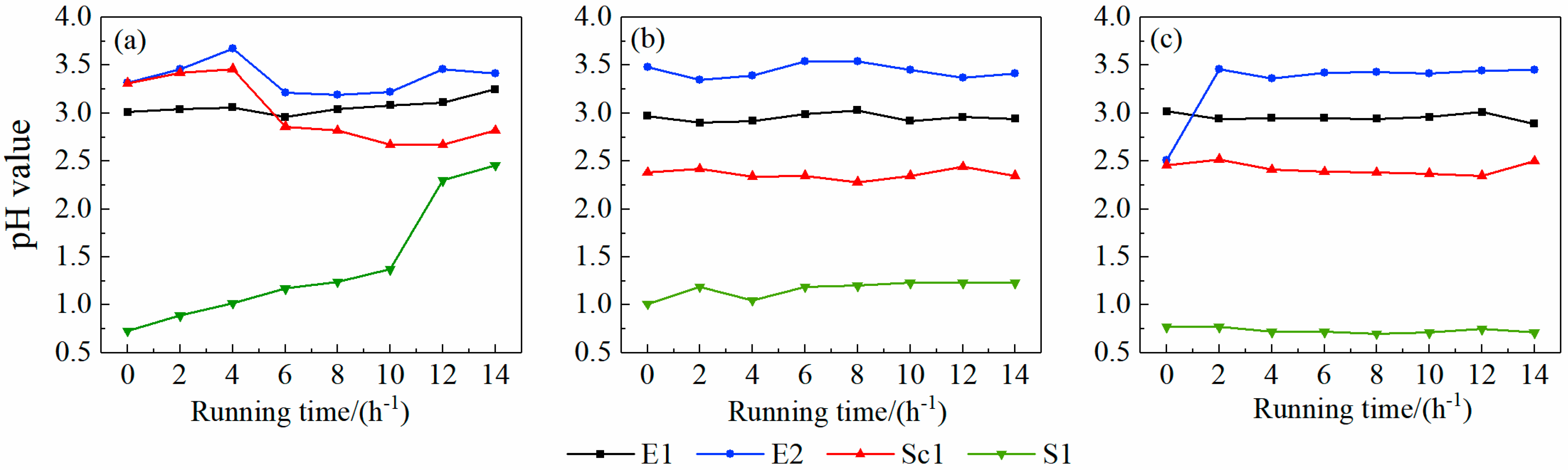
3.1.1. Effects of Different Sulfuric Acid Addition Amounts on pH
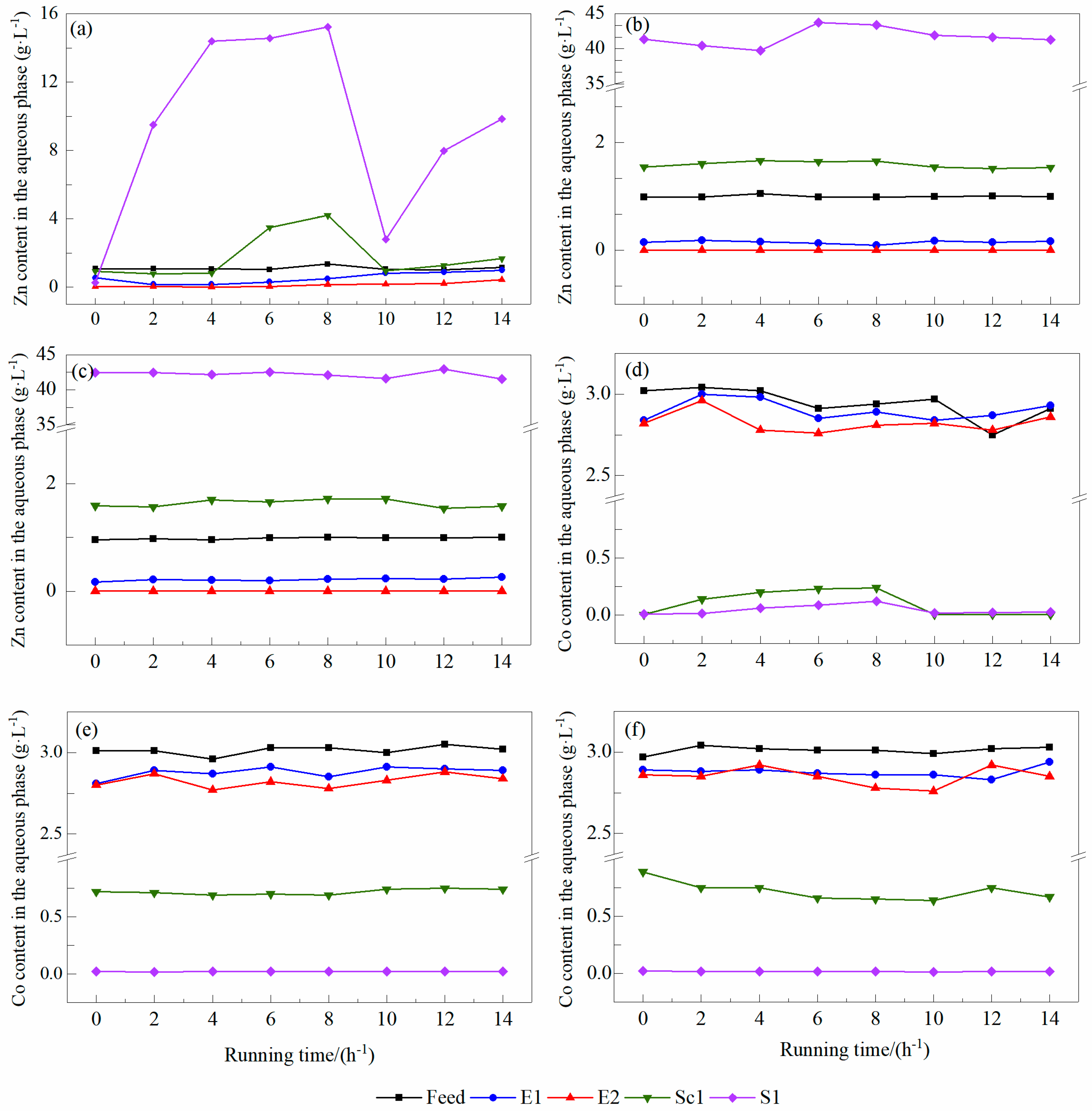
3.1.2. Effects of Different Sulfuric Acid Addition Amounts on Zn2+ and Co2+

3.1.3. Effects of Different Sulfuric Acid Addition Amounts on Recovery Rate of Each Element
3.2. Application of Simulated Deep Processing to Produce Zinc Products
3.3. Economic Cost and Benefit Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Marín, O.A.; Kraslawski, A.; Cisternas, L.A. Estimating processing cost for the recovery of valuable elements from mine tailings using dimensional analysis. Miner. Eng. 2022, 184, 107629. [Google Scholar] [CrossRef]
- Azevedo Schueler, T.; Fernandes de Aguiar, P.; Yagmurlu, B.; Goldmann, D. Removal of Base Metals from Mine Tailings in Chloride- and Seawater-Based Media Followed by Solvent Extraction. Sustainability 2023, 15, 15515. [Google Scholar] [CrossRef]
- Adewuyi, S.O.; Anani, A.; Luxbacher, K. Advancing sustainable and circular mining through solid-liquid recovery of mine tailings. Process. Saf. Environ. Prot. 2024, 189, 31–46. [Google Scholar] [CrossRef]
- Graedel, T.E.; Alessio, M.U.S. Cobalt: A Cycle of Diverse and Important Uses. Resour. Conserv. Recycl. 2022, 184, 106441. [Google Scholar] [CrossRef]
- Zhang, J.X.; Mani, R.; Louhi-Kultanen, M. Process monitoring of cobalt carbonate precipitation by reactions between cobalt sulfate and sodium carbonate solutions to control product morphology and purity. Hydrometallurgy 2023, 224, 106232. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Yang, Y.; Sun, H.; Li, Z.; Chen, Q.; Lu, S. Micro-spherical CoCO3 anode for lithium-ion batteries. Mater. Lett. 2014, 131, 236–239. [Google Scholar] [CrossRef]
- Suzuki, A.; Inui, H.; Pollock, T.M. L12-Strengthened Cobalt-Base Superalloys. Annu. Rev. Mater. Res. 2015, 45, 345–368. [Google Scholar] [CrossRef]
- Cobalt Institute, 2021a. Catalysts. Available online: https://www.cobaltinstitute.org/catalysts.html (accessed on 14 June 2021).
- Dohnalov, Z.; Sulcov, P.; Gorodylova, N. Study of ceramic pigments based on cobalt doped Y2O3–Al2O3 system. J. Therm. Anal. Calorim. 2014, 116, 647–654. [Google Scholar] [CrossRef]
- Crabtree, G.; K’ocs, E.; Trahey, L. The energy-storage frontier: Lithium-ion batteries and beyond. MRS Bull. 2015, 40, 1067–1078. [Google Scholar] [CrossRef]
- Xiong, J.C. Study on Step by Step Separation and Enrichment of Cobalt from Organic Purification Cobalt Residue of Zinc Hydrometallurgy. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2021. [Google Scholar]
- Gao, L.G. P204+P507 Collaborative Extraction Separation Process and Mechanism of Manganese, Copper, Zinc, and Magnesium from Cobalt Leaching Solution. Master’s Thesis, Guilin University of Technology, Guilin, China, 2023. [Google Scholar]
- Jin, F.F. The Degradation Behavior and Thermodynamics of P204 and P507 in Industrial Application. Master’s Thesis, Zhejiang University, Hangzhou, China, 2023. [Google Scholar]
- Tsakiridis, P.E.; Agatzini, S.L. Simultaneous solvent extraction of cobalt and nickel in the presence of manganese and magnesium from sulfate solutions by Cyanex30. Hydrometallurgy 2004, 72, 269–278. [Google Scholar] [CrossRef]
- Nayl, A.A.; Hamed, M.M.; Rizk, S.E. Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2015, 55, 119–125. [Google Scholar] [CrossRef]
- Hu, S.X.; Zhang, H.; Tan, X.Z.; Ni, S.F.; Li, S.W. Extraction of zinc from spent pickle liquor using primary amine extraction system. Hydrometallurgy 2024, 224, 106259. [Google Scholar] [CrossRef]
- Jantunen, N.; Kauppinen, T.; Salminen, J.; Virolainen, S.; Lassi, U.; Sainio, T. Separation of zinc and iron from secondary manganese sulfate leachate by solvent extraction. Miner. Eng. 2021, 173, 107200. [Google Scholar] [CrossRef]
- Ali, A.M.I.; Ahmad, I.M.; Daoud, J.A. CYANEX 272 for the extraction and recovery of zinc from aqueous waste solution using a mixer-settler unit. Sep. Purif. Technol. 2006, 47, 135–140. [Google Scholar] [CrossRef]
- Ling, J.H.; Yin, Z.L.; Hu, H.P.; Li, S.S.; Hu, J.G.; Chen, Q.Y. Leaching process of low grade zinc oxide ore of Lanping in NH3-(NH4)2SO4 system. J. Cent. South Univ. Sci. Technol. 2011, 42, 2577–2583. [Google Scholar]
- Luo, W.; Xu, Z.; Zhang, H.S.; Yang, L.M.; Li, Y. Research on zinc extraction with electric arc furnace dust. Met. Mater. Miner. 2011, 40, 153–156. [Google Scholar]
- Norul Fatiha, M.N.; Norasikin, O.; Izzat Naim, S.K.; Sazmin, S.S. Potential use of synergist D2EHPA/Cyanex 302 in kerosene system for reactive extraction: Zinc recovery and organic phase regeneration. Chem. Eng. Process. Process Intensif. 2022, 176, 108976. [Google Scholar]
- Aksamitowski, P.; Filipowiak, K.; Wieszczycka, K. Selective extraction of copper from Cu-Zn sulfate media by new generation extractants. Sep. Purif. Technol. 2019, 222, 22–29. [Google Scholar] [CrossRef]
- Meng, X.F.; Guo, J.M.; Zheng, G.D.; Yang, J.X.; Yang, J.; Chen, T.B.; He, M.K.; Li, Y.F. Combination of low-accumulation kumquat cultivars and amendments to reduce Cd and Pb accumulation in kumquat grown in contaminated soil. J. Clean. Prod. 2022, 365, 132660. [Google Scholar] [CrossRef]
- Liu, K.; Fang, L.P.; Li, F.B.; Hou, D.Y.; Liu, C.P.; Song, Y.N.; Ran, Q.W.; Pang, Y.; Du, Y.H.; Yuan, Y.Z.; et al. Sustainability assessment and carbon budget of chemical stabilization based multi-objective remediation of Cd contaminated paddy field. Sci. Total Environ. 2022, 819, 152022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Li, Q.; Sun, X.Y.; Wang, L.J. Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Sep. Sci. Technol. 2019, 54, 1302–1311. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, X.G.; Cao, H.B.; Zhang, Y. Separation of copper, iron (III), zinc and nickel from nitrate solution by solvent extraction using LK-C2. Sep. Purif. Technol. 2010, 70, 306–313. [Google Scholar] [CrossRef]
- Amirhossein, S.; Behrooz, H.K.; Fariba, F.; Armin, O. Extraction of Co, Ni and Cu by the solvent extraction method with simulation setup for control, alarm and protection system. Can. Metall. Q. 2022, 61, 33–47. [Google Scholar]
- Sarangi, K.; Reddy, B.R.; Das, R.P. Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272.: Separation of Co(II)/Ni(II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 1999, 52, 253–265. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Rashchi, F.; Ghafarizadeh, B.; Mostoufi, N. Synergistic Effect of D2EHPA and Cyanex 272 on Separation of Zinc and Manganese by Solvent Extraction. Sep. Sci. Technol. 2011, 46, 2305–2312. [Google Scholar] [CrossRef]
- Kazak, O.; Tor, A.; Akin, I.; Arslan, G. Preparation of new polysulfone capsules containing Cyanex 272 and their properties for Co(II) removal from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1654–1661. [Google Scholar] [CrossRef]
- Magdalena, R.R.; Katarzyna, S.; Karolina, W.; Anna, M. Removal of cobalt (II) and zinc (II) from sulphate solutions by means of extraction with sodium bis(2,4,4-trimethylpentyl) phosphinate (Na-Cyanex 272). Clean Technol. Environ. Policy 2016, 18, 1961–1970. [Google Scholar]
- Yao, B.; Nagaosa, Y.; Satake, Y.M.; Nomura, A.; Horita, K. Solvent extraction of metal ions and separation of nickel(II) from other metal ions by organophosphorus acids. Solvent Extr. Ion Exch. 1996, 14, 849–870. [Google Scholar]
- Kang, J.G.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
| Items | Co/g·L−1 | Zn/g·L−1 | Mn/g·L−1 | Mg/g·L−1 | Ca/g·L−1 | Cd/mg·L−1 | Fe/mg·L−1 | Al/mg·L−1 | Cu/mg·L−1 | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 3.05 | 1.07 | 0.60 | 2.04 | 0.49 | 19.21 | <0.1 | 0.20 | 5.08 | 5.01 |
| 50 g·L−1 | 80 g·L−1 | 100 g·L−1 | |
|---|---|---|---|
| Co recovery rate/% | 0.961 ± 0.034 AB | 0.981 ± 0.001 A | 0.984 ± 0.001 B |
| Items | Units | Zn LSL 1 | ||
|---|---|---|---|---|
| 50 g·L−1 | 80 g·L−1 | 100 g·L−1 | ||
| Ca | % | 24.7 | 22.95 | |
| Cd | % | <0.005 | <0.005 | |
| Co | % | <0.005 | <0.005 | |
| Na | % | - | - | |
| Zn | % | 1.19 | 1.15 | |
| Mass of precipitates | g·L−1 feed solution | 0.092 | 34.31 | 55.46 |
| Items | Units | Zn LSL 1 | ||
|---|---|---|---|---|
| 50 g·L−1 | 80 g·L−1 | 100 g·L−1 | ||
| Ca | % | 3.96 | 0.69 | 0.8 |
| Cd | % | <0.005 | <0.005 | <0.0005 |
| Co | % | 0.012 | 0.033 | 0.029 |
| Na | % | 0.18 | 5.28 | 1.98 |
| Zn | % | 53.7 | 46.74 | 50.91 |
| Mass of precipitates | g/L feed solution | 23.99 | 87.46 | 78.31 |
| Recovery of Zn | % | 108.52 | 100.29 | 96.09 |
| Input | Weight | Output | ||||
|---|---|---|---|---|---|---|
| Zn Extraction Rate/(%) | Zn Content in Raffinate/(mg·L−1) | Zn Extraction Rate | Zn Content in Raffinate | Zn Extraction Rate | Zn Content in Raffinate | |
| 50 g·L−1 | 88.394 | 0.131 | 0.5 | 0.5 | 44.31 | 1.53 |
| 80 g·L−1 | 99.562 | 0.004 | 0.5 | 0.5 | 49.90 | 50.00 |
| 100 g·L−1 | 99.755 | 0.004 | 0.5 | 0.5 | 50.00 | 50.00 |
| Input | Weight | Output | ||||
|---|---|---|---|---|---|---|
| Extraction and Production Costs/(103 RMB) | Zn Earnings (103 RMB) | Extraction and Production Costs | Zn Earnings | Extraction and Production Costs | Zn Earnings | |
| 50 g·L−1 | 6856 | 107 | 0.5 | 0.5 | 49.99 | 13.72 |
| 80 g·L−1 | 6855 | 391 | 0.5 | 0.5 | 50.00 | 50.00 |
| 100 g·L−1 | 6854 | 350 | 0.5 | 0.5 | 50.00 | 44.77 |
| Input | Weight | Output | ||||
|---|---|---|---|---|---|---|
| Co Loss Rate in Extraction | Zn Loss Rate in Precipitation/(%) | Co Loss Rate in Extraction | Zn Loss Rate in Precipitation | Co Loss Rate in Extraction | Zn Loss Rate in Precipitation | |
| 50 g·L−1 | 0.961 | 0.052% | 0.5 | 0.5 | 20.51 | 50 |
| 80 g·L−1 | 0.981 | 1.022% | 0.5 | 0.5 | 42.11 | 2.54 |
| 100 g·L−1 | 0.984 | 1.566% | 0.5 | 0.5 | 50.00 | 1.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Meng, X.; Jiang, Y.; Dong, X.; Li, S. Closed-Loop Process of Extracting and Separating Zinc Impurities from Industrial Cobalt Products—Pilot Test Study. Minerals 2024, 14, 1127. https://doi.org/10.3390/min14111127
Zou X, Meng X, Jiang Y, Dong X, Li S. Closed-Loop Process of Extracting and Separating Zinc Impurities from Industrial Cobalt Products—Pilot Test Study. Minerals. 2024; 14(11):1127. https://doi.org/10.3390/min14111127
Chicago/Turabian StyleZou, Xiaoping, Xiaofei Meng, Yingping Jiang, Xulong Dong, and Shili Li. 2024. "Closed-Loop Process of Extracting and Separating Zinc Impurities from Industrial Cobalt Products—Pilot Test Study" Minerals 14, no. 11: 1127. https://doi.org/10.3390/min14111127
APA StyleZou, X., Meng, X., Jiang, Y., Dong, X., & Li, S. (2024). Closed-Loop Process of Extracting and Separating Zinc Impurities from Industrial Cobalt Products—Pilot Test Study. Minerals, 14(11), 1127. https://doi.org/10.3390/min14111127





