The Paleoproterozoic Raimunda Porphyry-Type Gold Deposit, Juruena Mineral Province, Amazonian Craton (Brazil): Constraints Based on Petrological, Fluid Inclusion and Stable Isotope Data
Abstract
1. Introduction
2. Geological Settings
2.1. Geology of the Deposit
2.1.1. Monzonite Facies
2.1.2. Monzogranite Facies
2.1.3. Syenogranite Facies
2.1.4. Granodiorite Facies
3. Materials and Methods
3.1. Fieldwork, Sampling, and Petrography
3.2. Electron Microprobe
3.3. Chlorite Geothermometry
3.4. Fluid Inclusions
3.5. Stable Isotopes
4. Results
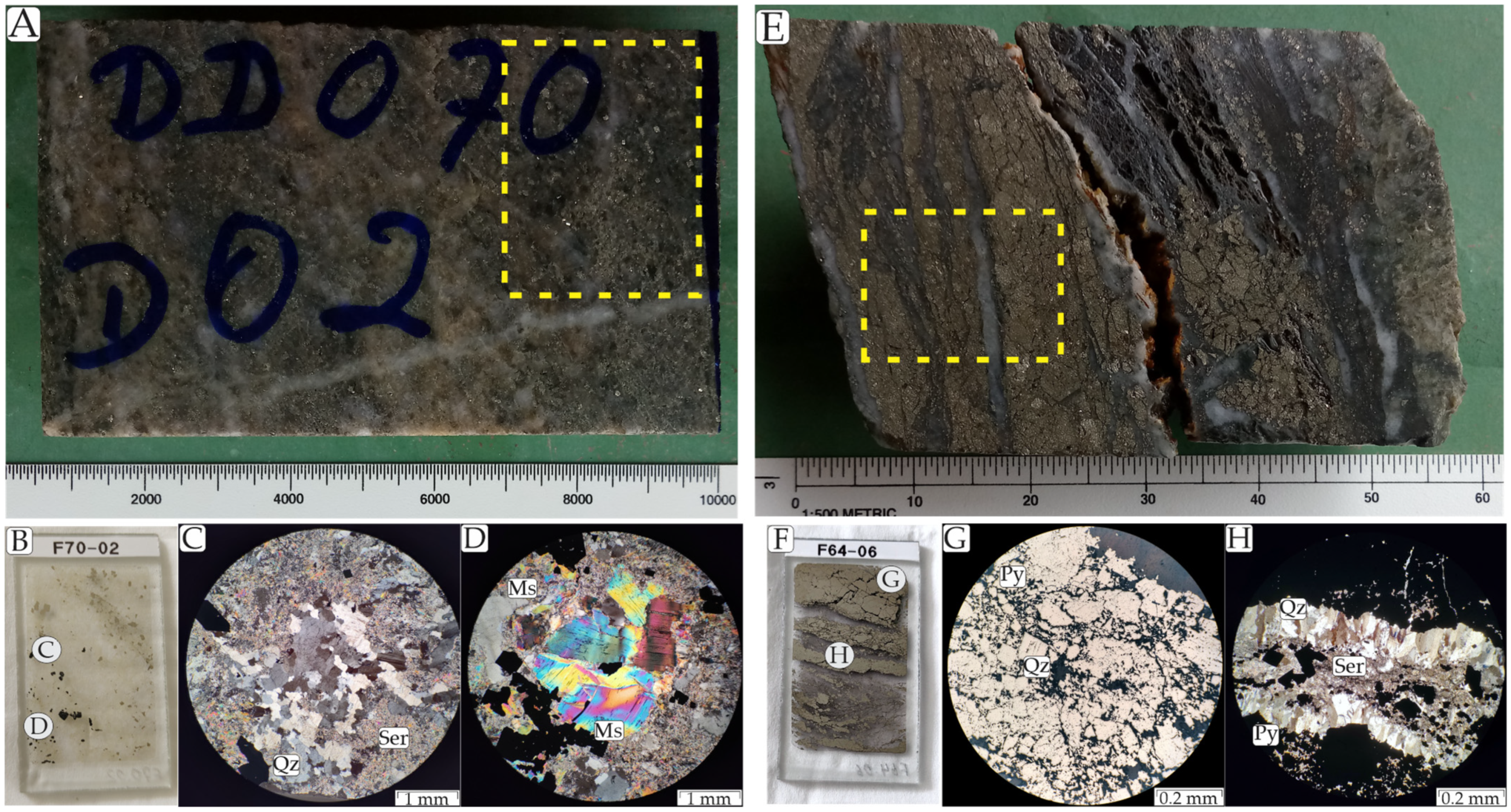
4.1. Hydrothermal Alteration and Mineralization
4.2. Ore and Hydrothermal Mineral Chemistry
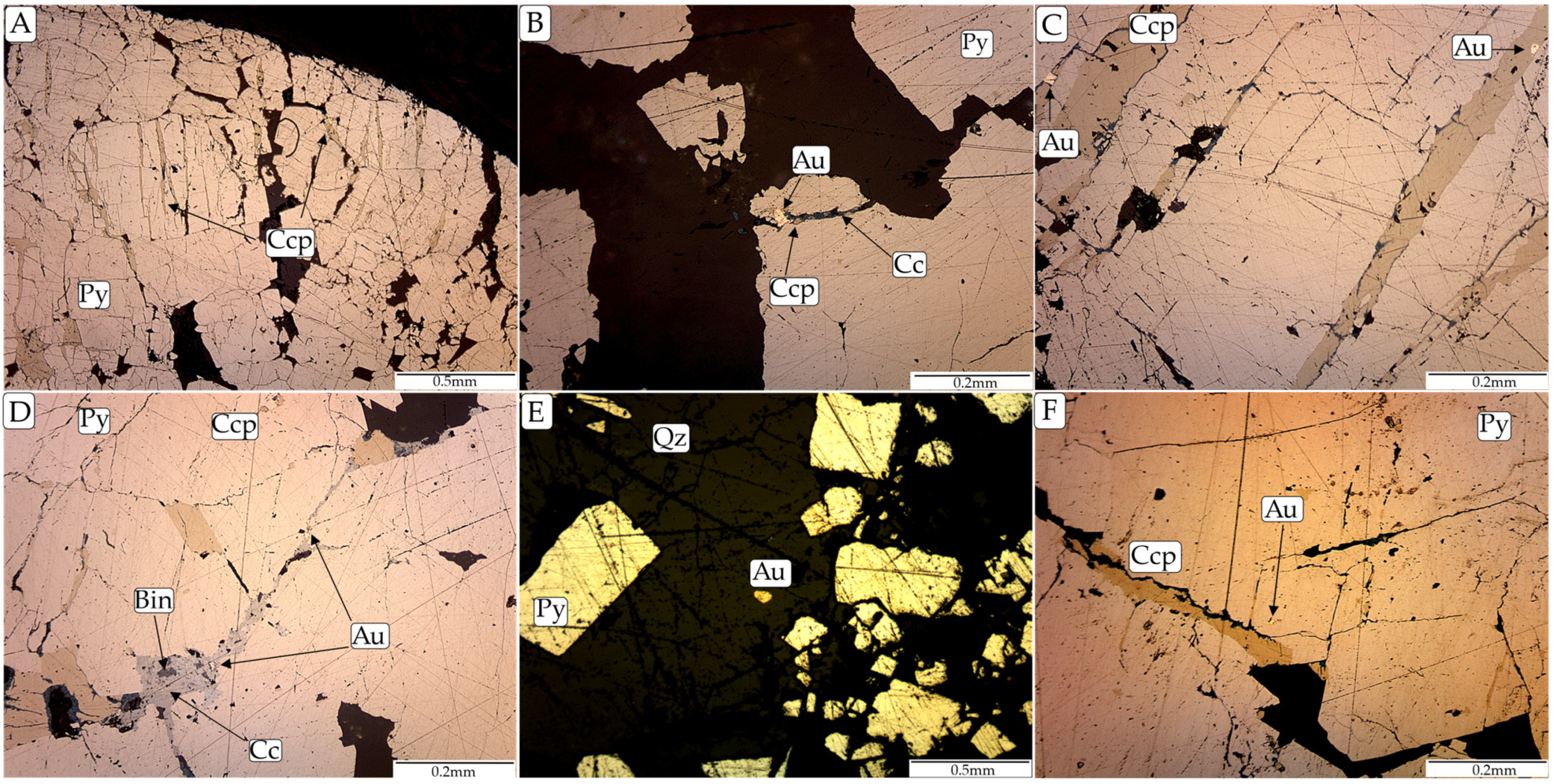
4.2.1. Sulfides and Gold
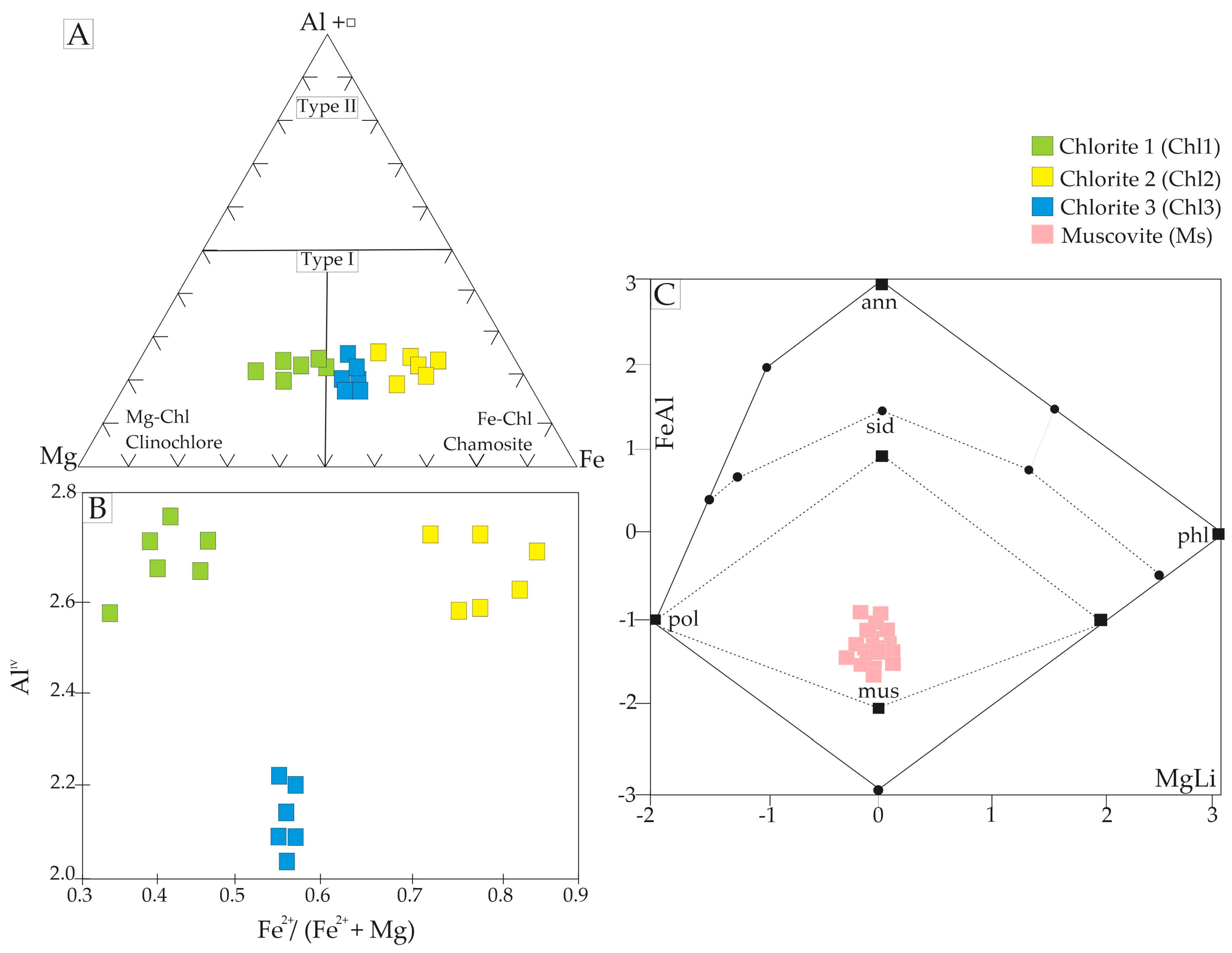
4.2.2. Chlorite Chemistry and Geothermometry
4.2.3. Muscovite
4.3. Fluid Inclusions Data
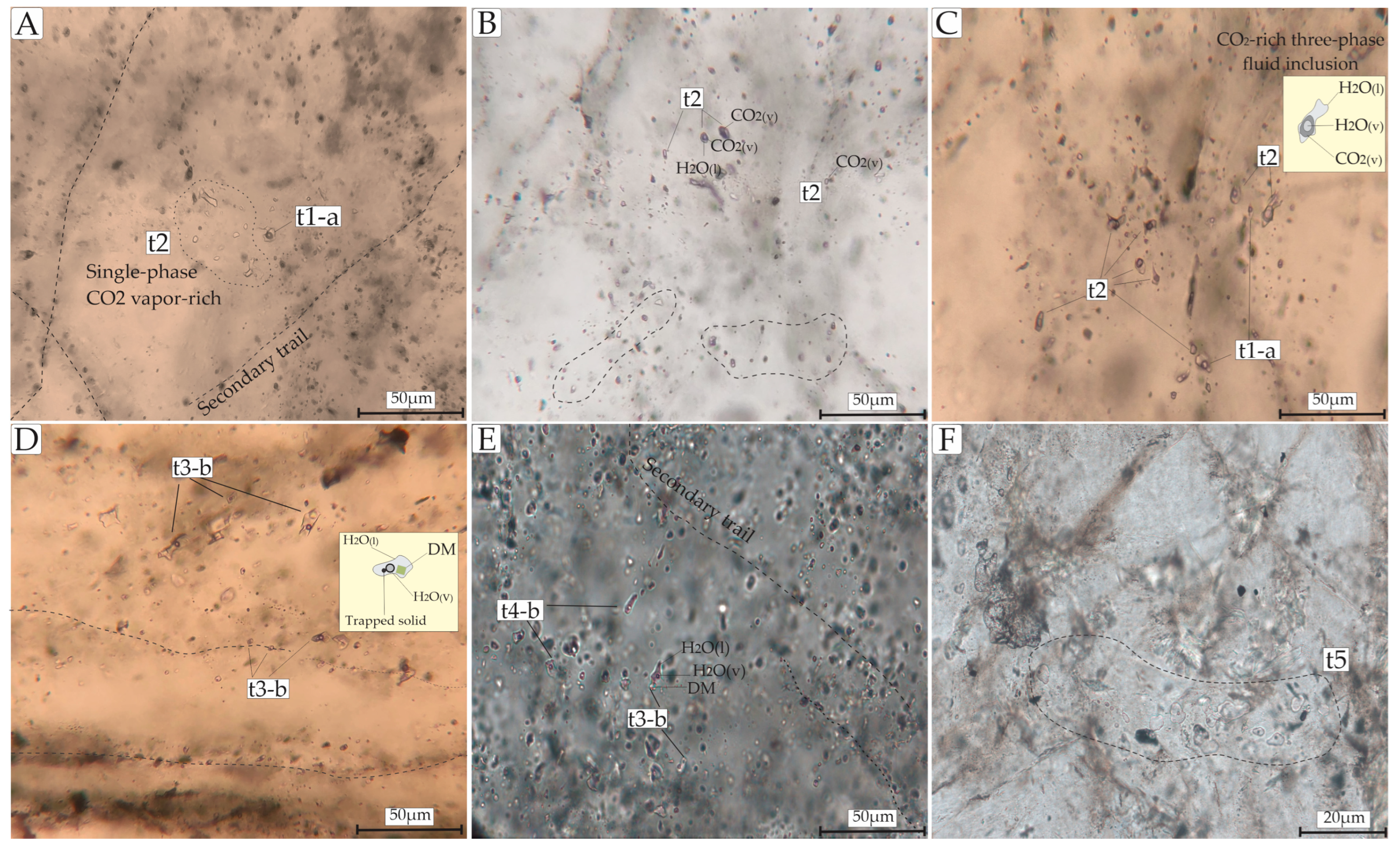
4.3.1. Petrography and Classification
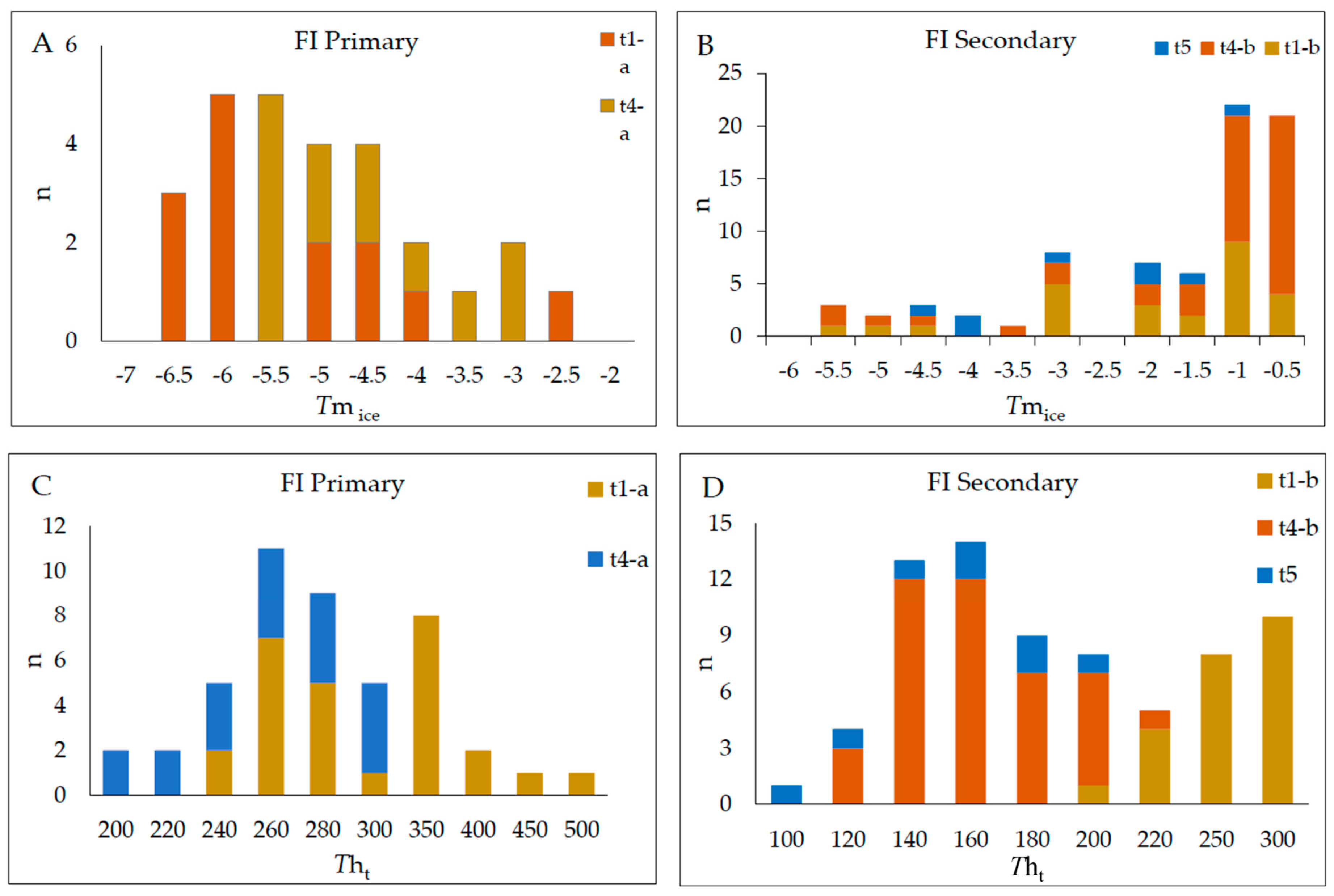
4.3.2. Microthermometric Data
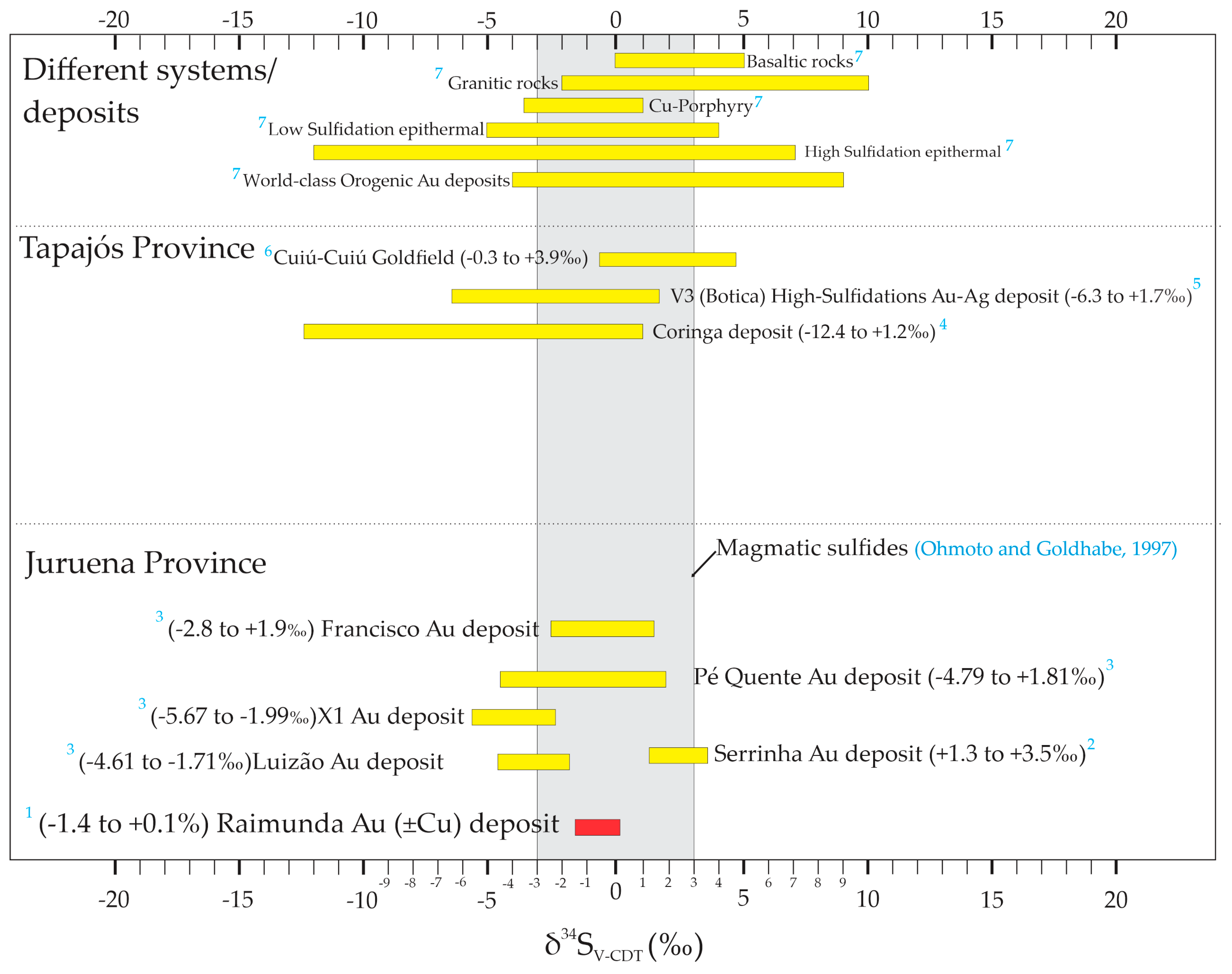
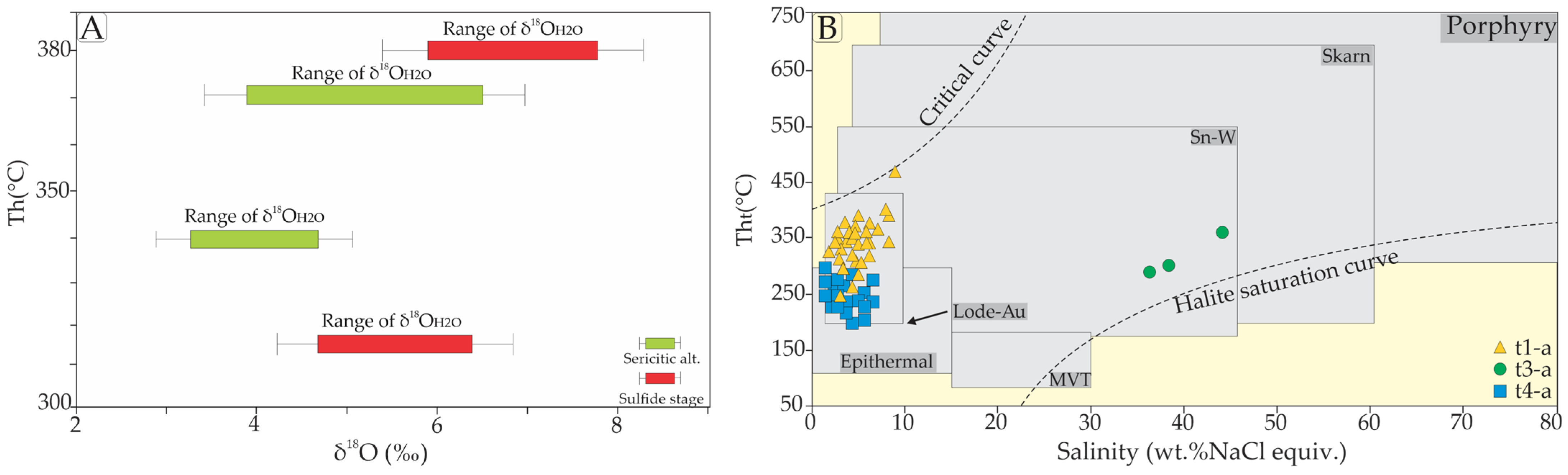
4.4. Stable Isotopes Data
5. Discussion
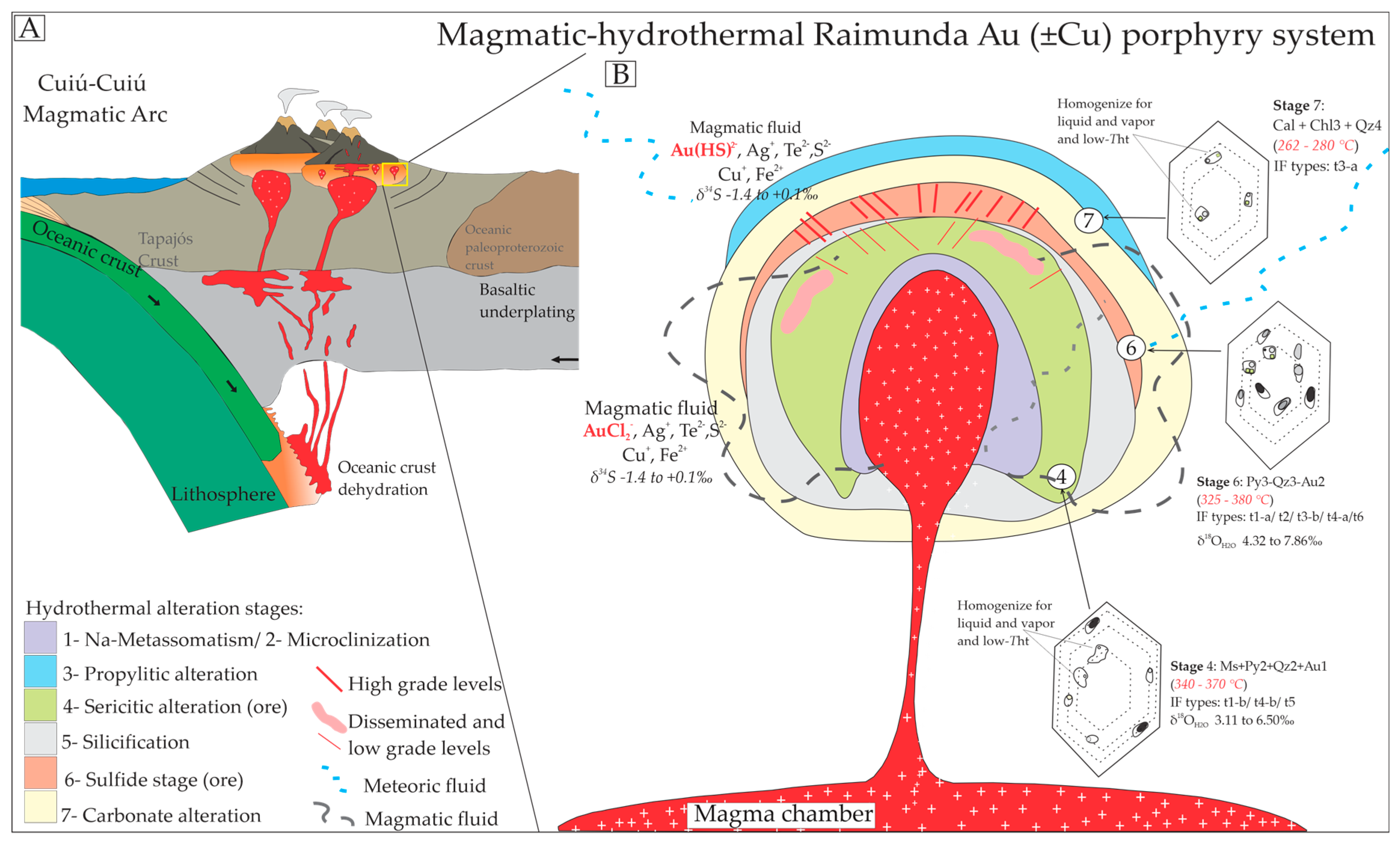
5.1. Tectonic Settings of Host Rocks, Hydrothermal Zonation and Mineralization
5.2. Origin, Hydrothermal System, and Evolution of Fluid Composition
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dardenne, M.A.; Schobbenhaus, C. Metalogênese do Brasil, 1st ed.; Universidade de Brasília: Brasília, Brazil, 2001; p. 394. [Google Scholar]
- Moura, M.A.; Botelho, N.F.; Olivo, G.R.; Kyser, T.K. Granite-related Paleoproterozoic, Serrinha gold deposit, Southern Amazonia, Brazil: Hydrothermal alteration, fluid inclusion and stable isotope constraints on genesis and evolution. Econ. Geol. 2006, 101, 585–605. [Google Scholar] [CrossRef]
- Paes de Barros, A.J. Granitos da Região de Peixoto de Azevedo—Novo Mundo e Mineralizações Auríferas Relacionadas—Província Aurífera Alta Floresta (MT). Ph.D. Thesis, Instituto de Geociências, Universidade Estadual de Campinas, Campinas, Brazil, 2007. [Google Scholar]
- Juliani, C.; Vasquez, M.L.; Klein, E.L.; Villas, R.N.; Echeverri-Misas, C.M.; Santiago, E.S.B.; Monteiro, L.V.S.; Carneiro, C.C.; Fernandes, C.M.D.; Usero, G. Metalogenia da Província Tapajós. In Metalogênese das Províncias Tectônicas Brasileiras, 1st ed.; Silva, M.G., Jost, H., Kuyumajian, R.M., Eds.; CPRM-Serviço Geológico do Brasil: Brasília, Brazil, 2014; pp. 51–90. [Google Scholar]
- Juliani, C.; Assis, R.R.; Monteiro, L.V.S.; Fernandes, C.M.D.; Martins, J.E.Z.S.; Costa, J.R.C. Gold in Paleoproterozoic (2.1 to 1.77 Ga) Continental Magmatic Arcs at the Tapajós and Juruena Mineral Provinces (Amazonian Craton. Brazil): A New Frontier for the Exploration of Epithermal-Porphyry and Related Deposits. Minerals 2021, 11, 714. [Google Scholar] [CrossRef]
- Mesquita, M.J.; Gomes, M.E.B.; Moreira, I.C.; Paes, R.A.S.; Hevelyn, E.S.M.; Matos, J.H.; Ruggiero, A.; Primo, G.; Ducart, D.F.; Poggi, L.; et al. Paleoproterozoic gold deposits at Alta Floresta Mineral Province, Brazil: Two overprinted mineralizing events? In Recent Advances in Understanding Gold Deposits: From Orogeny to Alluvium; Torvela, T., Lambert-Smith, J.S., Chapman, R.J., Eds.; Special Publications; Geological Society of London: London, UK, 2022; Volume 516, pp. 109–154. [Google Scholar] [CrossRef]
- Jamasmie, C. Anglo American ‘Encouraged’ by Drilling Results from Copper Asset in North Brazil. Available online: https://www.mining.com/anglo-american-encouraged-drilling-results-copper-asset-north-brazil/ (accessed on 18 November 2024).
- Guimarães, S.B.; Klein, E.L.; Harris, C.; Costa, I.S.L. Metallogenesis of the orosirian epithermal coringa gold-silver (Cu-Pb-Zn) deposit, southeastern Tapajós mineral Province, Amazonian craton, Brazil. Ore Geol. Rev. 2021, 128, 103908. [Google Scholar] [CrossRef]
- Poggi, L.; Ducart, D.F.; Mesquita, M.J.; Moreira, I.C.; Gomes, M.E.B.; Filho, C.R.S. New Insights into the Evolution and Footprints of the Paraíba Au-Cu-Mo Deposit, Alta Floresta Mineral Province (Brazil), through Integration of Spectral and Conventional Methods. Minerals 2022, 12, 1327. [Google Scholar] [CrossRef]
- Gaillard, N.; Williams-Jones, A.E.; Clark, J.R.; Salvi, S.; Perrouty, S.; Linnen, R.L.; Olivo, G.R. The use of lithogeochemistry in delineating hydrothermal fluid pathways and vectoring towards gold mineralization in the Malartic district, Québec. Ore Geol. Rev. 2020, 120, 103351. [Google Scholar] [CrossRef]
- Tassinari, C.C.G.; Macambira, M.J.B. A evolução tectônica do Cráton Amazônico. In Geologia do Continente Sul-Americano: Evolução da obra de Fernando Flávio Marques de Almeida; Mantesso Neto, V., Ed.; Beca: São Paulo, Brazil, 2004; pp. 471–485. [Google Scholar]
- Santos, J.O.S.; Hartmann, L.A.; Gaudette, H.E.; Groves, D.I.; McNaughto, N.; Fletcher, I.R. A new understanding of the provinces of the Amazonian Craton based on integration of sector mapping and U-Pb and Sm-Nd geochronology. Gondw. Res. 2000, 3, 453–488. [Google Scholar] [CrossRef]
- Fernandes, C.M.D.; Juliani, C.; Monteiro, L.V.S.; Lagler, B.; Echeverri Misas, C.M. High-K calc-alkaline to A-type fissure-controlled volcano-plutonism of the São Félix do Xingu region, Amazonian craton, Brazil: Exclusively crustal sources or only mixed Nd model ages? J. S. Am. Earth Sci. 2011, 32, 351–368. [Google Scholar] [CrossRef]
- Carneiro, C.D.C.; Juliani, C.; Carreiro-Araújo, S.A.; Monteiro, L.V.S.; Crósta, A.P.; Fernandes, C.M.D. New crustal framework in the Amazon Craton based on geophysical data: Evidences of deep east–west trending suture zones. IEEE Geosci. Remote Sens. Lett. 2019, 16, 20–24. [Google Scholar] [CrossRef]
- Juliani, C.; Fernandes, C.M.D.; Monteiro, L.V.S. Características da subducção, paleoclima e eventos erosivos paleoproterozoicos (2.1–1.88 Ga) e seus efeitos na estruturação da parte sul do Cráton Amazônico. In Simpósio de Geologia da Amazônia, 14th ed.; Gorayeb, P.S.S., Teixeira, S.G., Eds.; Sociedade Brasileira de Geologia–Núcleo Norte: Marabá, Brazil, 2015; p. 81. (In Portuguese) [Google Scholar]
- Fernandes, C.M.; Fontes, S.L.; La Terra, E.F.; Miquelutti, L.G.; Maurya, V.P. Electrical crustal structure of Alta Floresta Gold Province eastern sector, SW Amazon Craton, Brazil. J. S. Am. Earth Sci. 2018, 86, 244–258. [Google Scholar] [CrossRef]
- Cassini, L.V.; Moyen, J.; Juliani, C. Orosirian magmatism in the Tapajós Mineral Province (Amazonian Craton): The missing link to understand the onset of Paleoproterozoic tectonics. Lithos 2020, 356–357, 105350. [Google Scholar] [CrossRef]
- Alves, C.L.; Rizzotto, G.J.; Rios, F.S.; Barros, M.A.S. The Orosirian Cuiú-Cuiú magmatic arc in Peixoto de Azevedo domain, Southern of Amazonian Craton. J. South Am. Earth Sci. 2020, 102, 102648. [Google Scholar] [CrossRef]
- Alves, C.L.; Rizzotto, G.J.; Rios, F.S.; Gonçalves, G.F. Áreas de Relevante Interesse Mineral-Projeto Evolução Crustal e Metalogenia da Província Mineral Juruena-Teles Pires; CPRM: Goiânia, Brazil, 2019; ISBN 978-85-7499-547-2. [Google Scholar]
- Assis, R.R. Depósitos Auríferos Associados ao Magmatismo Félsico da Província de Alta Floresta (MT), Cráton Amazônico: Litogeoquímica, Idade das Mineralizações e Fonte dos Fluidos. Ph.D. Thesis, Instituto de Geociências, Universidade Estadual de Campinas, Campinas, Brazil, 2015. [Google Scholar]
- Lagler, B.; Juliani, C.; Fernandes, C.M.D.; da Cruz, R.S.; Vieira, D.A.S. Paleoproterozoic volcanic caldera in the Amazonian Craton, northern Brazil: Stratigraphy, lithofacies characterization, and lithogeochemical constraints. J. S. Am. Earth Sci. 2019, 95, 102252. [Google Scholar] [CrossRef]
- Assis, R.R.; Xavier, R.P.; Creaser, R.A. Linking the Timing of Disseminated Granite-Hosted Gold-Rich Deposits to Paleoproterozoic Felsic Magmatism at Alta Floresta Gold Province, Amazon Craton, Brazil: Insights from Pyrite and Molybdenite Re-Os Geochronology. Econ. Geol. 2017, 112, 1937–1957. [Google Scholar] [CrossRef]
- Teixeira, N.; Borda, M.; Ganade, C.; Gaspar, J.C.; Chelle-Michou, C.; Porto, F. Jaca Cu-Au (Mo) Pórfiro: Geocronologia e Isotopia do Sistema Magmático Mineralizante; Sumário Executivo; CPRM: Goiânia, Brazil, 2023; 7p. [Google Scholar]
- Testa, L.G.C. Geologia, Petrologia e Geocronologia dos Granitos da Região de Novo Mundo (MT) e Implicações para a Evolução Magmática e Regional. Master’s Thesis, University of Brasília, Brasília, Brazil, 2018. [Google Scholar]
- Miguel, E., Jr. Mineralizações Auríferas do Lineamento Peru-Trairão Província Aurífera de Alta Floresta—MT, Controle Estrutural e idade U–Pb das Rochas Hospedeiras. Master’s Thesis, Instituto de Geociências, Universidade Estadual de Campinas, Campinas, Brazil, 2011. [Google Scholar]
- Pirajno, F. Hydrothermal Processes and Mineral Systems, 1st ed.; Springer: Berlin, Germany, 2009; p. 1273. [Google Scholar]
- Hedenquist, J.W.; Arribas, A. 2022. Exploration Implications of Multiple Formation Environments of Advanced Argillic Minerals. Econ. Geol. 2022, 117, 609–643. [Google Scholar] [CrossRef]
- Cathelineau, M.; Nieva, D. A chlorite solid solution geothermometer. The Los Azufres geothermal system (Mexico). Contrib. Miner. Petrol. 1985, 91, 235–244. [Google Scholar] [CrossRef]
- Cathelineau, M. Cation site occupancy in chlorites and illites as a fuction of temperature. Clay Miner. 1988, 23, 471–485. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. In Reviews in Mineralogy, 1st ed.; Mineralogical Society of America: Blacksburg, VA, USA, 1984; Volume 12. [Google Scholar]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusions Studies; Blackie and Sons: Glasgow, UK; London, UK, 1985; p. 253. [Google Scholar]
- Van den Kerkhof, A.M.; Hein, U.F. Fluid inclusion petrography. Lithos 2001, 55, 27–47. [Google Scholar] [CrossRef]
- Chinchilla, D.; Arroyo, X.; Merinero, R.; Piña, R.; Nieto, F.; Ortega, L.; Lunas, R. Chlorite geothermometry applied to massive and oscillatory-zoned radiated Mn-rich chlorites in the Patricia Zn-Pb-Ag epithermal deposit (NE, Chile). Appl. Clay Sci. 2016, 134, 210–220. [Google Scholar] [CrossRef]
- Hey, M.H. A new review of chlorites. Miner. Mag. 1954, 30, 277–292. [Google Scholar] [CrossRef]
- Tischendorf, G.; Forster, H.-J.; Gottesmann, B.; Rieder, M. True and brittle micas: Composition and solid-solution series. Miner. Mag. 2007, 71, 285–320. [Google Scholar] [CrossRef]
- Kranidiotis, P.; MacLean, W.H. Systematics of chlorite alteration at the Phelps Dodge massive sulfide deposit, Matagami, Québec. Econ. Geol. 1987, 82, 1898–1911. [Google Scholar] [CrossRef]
- Zang, W.; Fyfe, W.S. Chloritization of the hydrothermally altered bedrock at the Igarapé Bahia gold deposit, Carajás, Brazil. Miner. Depos. 1995, 30, 30–38. [Google Scholar] [CrossRef]
- Jowett, E.C. Fitting iron and magnesium into the hydrothermal chlorite geothermometer. In Proceedings of the GAC/MAC/SEG Joint Annual Meeting, Program with Abstracts, Toronto, Canada, 27–29 May 1991; Volume 16, p. A62. [Google Scholar]
- Xie, X.; Byerly, G.R.; Ferrell, R.E. IIb trioctahedral chlorite from the Barberton greenstone belt: Crystal structure and rock composition constraints with implications for geothermometry. Contrib. Mineral. Petrol. 1997, 126, 275–291. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; SEPM (Society for Sedimentary Geology) Short Course: Tulsa, OK, USA, 1994; p. 213. [Google Scholar]
- Steele-MacInnis, M.; Bodnar, R.J.; Naden, J. Numerical model to determine the composition of H2O–NaCl–CaCl2 fluid inclusions based on microthermometric and microanalytical data. Geochim. Cosmochim. Acta 2022, 75, 21–40. [Google Scholar] [CrossRef]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Parry, W.T. Estimation of XCO2, P and fluid inclusion volume from fluid inclusion temperature measurements in the system NaCL-CO2-H2O. Econ. Geol. 1986, 81, 1009–1013. [Google Scholar] [CrossRef]
- Matsuhira, Y.; Goldsmith, J.R.; Clayton, R.N. Oxygen isotopic fractionation in the system quartz-albite-anorthite-water. Geochim. Cosmochim. Acta 1979, 43, 1131–1140. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposit, 3rd ed.; Barnes, H.L., Ed.; Wiley: Washington, DC, USA, 1979; pp. 509–561. [Google Scholar]
- Guimarães, S.B.; Klein, E.L. Geochemical and isotopic constraints on the host rocks of the magmatic-hydrothermal Coringa gold-silver (Cu-Pb-Zn) deposit of the Tapajós mineral province, Amazonia craton, Brazil. J. S. Am. Earth Sci. 2020, 103, 102726. [Google Scholar] [CrossRef]
- Monecke, T.; Monecke, J.; Reynolds, T.J.; Tsuruoka, S.; Bennett, M.M.; Skewes, W.B.; Palin, R.M. Quartz solubility in the H2O-NaCl system: A framework for understanding vein formation in porphyry copper deposits. Econ. Geol. 2018, 113, 1007–1046. [Google Scholar] [CrossRef]
- Baker, T. Emplacement depth and carbon dioxide-rich fluid inclusions in intrusion-related gold deposits. Ec. Geol. 2002, 97, 1111–1117. [Google Scholar] [CrossRef]
- Chiaradia, M. Gold endowments of porphyry deposits controlled by precipitation efficiency. Nat. Commun. 2020, 11, 248. [Google Scholar] [CrossRef]
- Reed, M.H. Hydrothermal alteration and its relationship to ore fluid composition. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; John Wiley: New York, NY, USA, 1997; pp. 303–365. [Google Scholar]
- Shanks III, W.C. Stable isotope geochemistry of mineral deposits. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 59–85. [Google Scholar] [CrossRef]
- Li, L.; Sun, F.; Bai, Y.; Wang, C.; Qian, Y.; Sun, Y.; Tan, S. Identification of hydrothermal alteration and mineralization in the Sancha magmatic Cu-Ni-Au sulfide deposit, NW China: Implications for timing and genesis of mineralization. Ore Geol. Rev. 2022, 143, 104770. [Google Scholar] [CrossRef]
- Sheppard, S.M.F. Characterization and isotopic variations in natural waters. In Stable Isotopes in High-Temperature Geological Processes; Valley, J.W., Taylor, H.P., Jr., O’Neil, J.R., Eds.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 1986; Volume 16, pp. 165–183. [Google Scholar]
- Zeng, G.; Xiang, W.; Zhang, Z.; Wang, J.; Jiang, J.; Wu, F. Fluid inclusions, H-O-S-Pb isotopes, and Rb-Sr geochronology of the Augaro gold deposit, West Eritrea, NE Africa: Implications for ore genesis. J. S. Am. Earth Sci. 2023, 206, 105029. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Liu, j.; Lin, S.; Zhang, F.; Zhang, W.; Bo, Z.; Li, H. Sulfur isotope, fluid inclusions, and quartz solubility model decipher multistage hydrothermal fluids evolution and ore precipitation mechanism at the Nantai porphyry Mo deposit, Qinling, Central China. Chem. Geol. 2023, 617, 121273. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Weis, P.; Driesner, T.; Bouvier, A.S.; Baumgartner, L.; Heinrich, C.A. Contrasting hydrological processes of meteoric water incursion during magmatic-hydrothermal ore deposition: Na oxygen isotope study by ion microprobe. Earth Planet. Sci. Lett. 2016, 451, 263–271. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Cooke, D.R.; Deyell, C.L.; Waters, P.J.; Gonzales, R.I.; Zaw, K. Evidence for magmatic-hydrothermal fluids and ore-forming processes in epithermal and porphyry deposits of the Baguio district, Philippines. Econ. Geol. 2011, 106, 1399–1424. [Google Scholar] [CrossRef]
- Xiong, S.F.; Gong, Y.J.; Jiang, S.Y.; Zhang, X.J.; Li, Q.; Zeng, G.P. Ore genesis of the Wusihe carbonate-hosted Zn-Pb deposit in the Dadu River Valley district, Yangtze Block, SW China: Evidence from ore geology, S-Pb isotopes, and sphalerite Rb-Sr dating. Miner. Deposita 2018, 53, 967–979. [Google Scholar] [CrossRef]
- Du, J.; Audétat, A. Early sulfide saturation is not detrimental to porphyry Cu-Au formation. Geology 2020, 48, 519–524. [Google Scholar] [CrossRef]
- Ohmoto, H.; Goldhabe, M. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H., Ed.; Willey: New York, NY, USA, 1997; pp. 517–612. [Google Scholar]
- Sinclair, W. Porphyry deposits. In Mineral Deposits of Canada—A Synthesis of Major Deposit Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication; Geological Association of Canada, Mineral Deposits Division: St. Johns, NL, Canada, 2007; Volume 5, pp. 223–243. ISBN 978-1-897095-24-9. [Google Scholar]
- Wilson, A.J.; Cooke, D.R.; Harper, B.J.; Deyell, C.L. Sulfur isotopic zonation in the Cadia district, southeastern Australia: Exploration significance and implications for the genesis of alkalic porphyry gold–copper deposits. Miner. Depos. 2007, 42, 465–487. [Google Scholar] [CrossRef]
- Richards, J.P. Postsubduction porphyry cu-au and epithermal Au deposits: Products of remelting of subduction-modified lithosphere. Geology 2009, 37, 247–250. [Google Scholar] [CrossRef]
- Lambert-Smith, J.S.; Allibone, A.; Treloar, P.J.; Lawrence, D.M.; Boyce, A.J.; Fanning, M. Stable C.O.; Isotope, S. Record of Magmatic-Hydrothermal Interactions Between the Faleme Fe Skarn and the Loulo Au Systems in Western Mali. Econ. Geol. 2020, 115, 1537–1558. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Juliani, C.; Rye, R.O.; Nunes, C.M.; Snee, L.W.; Silva, R.H.C.; Monteiro, L.V.; Neto, A.A. Paleoproterozoic high-sulfidation mineralization in the Tapajós gold province, Amazonian Craton, Brazil: Geology, mineralogy, alunite argon age, and stable-isotope constraints. Chem. Geol. 2005, 215, 95–125. [Google Scholar] [CrossRef]
- Hoss, A.; Haase, K.M.; Keith, M.; Klemb, R.; Melfos, V.; Gerlach, L.; Pelloth, F.; Falkenberg, J.J.; Voudouris, P.; Strauss, H.; et al. Magmatic and hydrothermal evolution of the Skouries Au-Cu porphyry deposit, northern Greece. Ore Geol. Rev. 2024, 173, 1062332024. [Google Scholar] [CrossRef]
- Audétat, A.; Simon, A.C. Magmatic controls on porphyry copper genesis. Econ. Geol. 2012, 16, 553–572. [Google Scholar] [CrossRef]
- Assunção, R.F.S.; Klein, E.L. The Moreira Gomes deposit of the Cuiú-Cuiú goldfield: Fluid inclusions and stable isotope constraints and implications for the genesis of granite-hosted gold mineralization in the Tapajós Gold Province, Brazil. J. S. Am. Earth Sci. 2014, 49, 85–105. [Google Scholar] [CrossRef]
- Groves, D.I.; Bierlein, F.P. Geodynamic setting of mineral deposit systems. J. Geol. Society 2007, 164, 19–30. [Google Scholar] [CrossRef]



| Event | Pre-Mineralization | Mineralization Stages | Post-Mineralization | |||||
|---|---|---|---|---|---|---|---|---|
| Alteration Stages | Na-Metassomatism | Microclinization | Propylitic Alteration | Sericitic Alteration (Ms-Qz2-Chl2-Py2-Au1) | Silicification (Qz3) | Sulfide Stage (Py3-Au2-Ccp-Cc-Bin) | Carbonate Alteration (Cal-Chl3-Qz4) | |
| Mineral | ||||||||
| Quartz 1 |  |  | ||||||
| Quartz 2 |  | |||||||
| Quartz 3 |  | |||||||
| Quartz 4 |  | |||||||
| K- Feldspar |  | |||||||
| Albite |  | |||||||
| Magnetite |  |  |  | |||||
| Titanite |  | |||||||
| Rutile |  |  | ||||||
| Chlorite |  |  |  |  | ||||
| Muscovite |  |  |  |  | ||||
| Calcite |  |  |  | |||||
| Epidote |  | |||||||
| Pyrite 1 |  | |||||||
| Pyrite 2 |  | |||||||
| Pyrite 3 |  | |||||||
| Chalcopyrite |  |  | ||||||
| Chalcocite |  | |||||||
| Larosite |  | |||||||
| Bismuthinite |  | |||||||
| Hodrushite/krupkaite/wittchenite |  | |||||||
| Gold |  |  | ||||||
Traces  | Minor  | Abundant  | ||||||
Temporal evolution  | ||||||||
| Fluid Inclusion Type | Host Rock | Hydrothermal Alteration Stage | Mineral Association | Petrographic Characteristics | Microthermometric Data | Model System |
|---|---|---|---|---|---|---|
| Type 1-a (L + V); n = 28 | Monzogranite; syenogranite | Sulfide stage | Py3 + Ccp + Qz3 + Au2 | Primary; random; colorless; ellipsoidal, oval to irregularly shaped; 8–20 μm; F: 0.70–0.8 | Te = −24 to −21 °C; Tmice = −6.4 to −0.8 °C; Tht = 233.5 to 471.2 °C; salinity = 1.396–9.72 wt.% NaCl eq.; density; 0.409–0.895 g/cm3 | H2O-NaCl |
| Type 1-b (L + V); n = 25 | Monzogranite; syenogranite | Sericitic alteration | Ms + Qz2 + Au1 | Secondary; planar grouping and trails; oblate; 3–12 μm; F: 0.7–0.8 | Tmice = −4.2 to −0.5 °C; Tht = 200.5 to 297 °C; salinity = 0.879–6.737 wt.% NaCl eq.; density = 0.715–0.918 g/cm3 | H2O-NaCl |
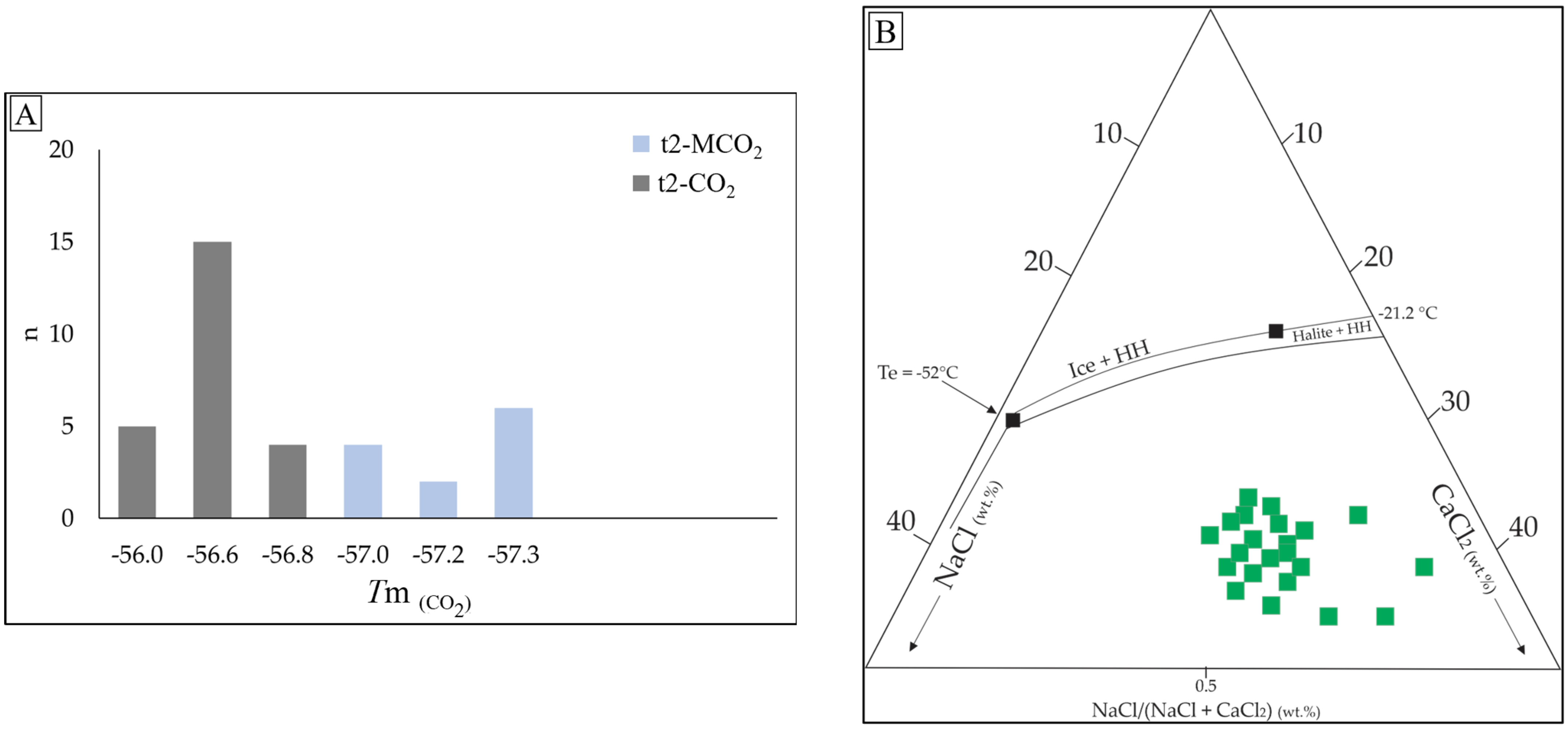
| Type 2 (V; L + V; L + L + V); n = 40 | Monzogranite | Sulfide stage | Py3 + Ccp + Qz3 + Au2 | Primary; random distribution or in clusters; grayish; dark bubble; elongated and oval; 5–20 μm; F: 30–50 | TmCO2 = −57.3 to −56°C; Tmice = −3.8 to −2.9 °C; Tmcla = 6.2–8.5 °C; ThCO2 = 19–30.1 °C; Tht = 210 to 364.7 °C; salinity = 3.00–5.94 wt.% NaCl eq.; density = 0.87–0.88 g/cm3 | H2O-NaCl-CO2 |
| Type 3-a (L + V; L + V + S); n = 3 | Monzogranite | Carbonate alteration | Cal + Chl3 + Qz4 | Primary; random distribution; square-shaped, elongated, and sub-rounded; colorless or greenish; 8–22 μm; F: 60–70; with one cubic crystal (halite daughter mineral) | Tmhh = −10.7 to −3.8 °C; Tht = 290.5 to 367.5 °C; salinity = 38.88–50.25 wt.% NaCl + CaCl2 eq.; density = 0.78–0.82 g/cm3 | H2O-NaCl-CaCl2 |
| Type 3-b (L + V; L + V + S + Ts); n = 19 | Monzogranite | Sericitic alteration | Py3 + Ccp + Qz3 + Au2 | Secondary; planar grouping and trails; oblate square-shaped, elongated, and sub-rounded; 3–12 μm; F: 60–80; with colorless cubic crystals (halite daughter mineral) and trapped solid locally | Te: −55 to −51.2 °C; Tmice = −43.2 to −37.8 °C; Tmhh = −10.6 to −1.1 °C; Tht = 100.6 to 251.5 °C; salinity = 33.02–38.21 wt.% NaCl + CaCl2 eq.; density = 0.87–0.97 g/cm3 | H2O-NaCl-CaCl2 |
| Type 4-a (L + V); n = 19 | Monzogranite; syenogranite | Silicification; Sulfide stage | Py3 + Ccp + Qz3 + Au2 | Primary; clusters and isolated; colorless; 8–20 μm; F: 0.8–0.85 | Tmice = −5.4 to −1.0 °C; Tht = 199.2 to 288 °C; salinity = 1.57–8.41 wt.% NaCl eq.; density = 0.74–0.92 g/cm3 | H2O-NaCl |
| Type 4-b (L + V); n = 41 | Monzogranite; syenogranite | Sericitic alteration | Ms + Qz2 + Au1 | Secondary; trails/aligned along micro-fractures in the quartz; 8–20 μm; F: 0.8–0.85 | Tmice = −4.7 to −0.1 °C; Tht = 101 to 195 °C; salinity = 0.53–6.59 wt.% NaCl eq.; density = 0.87–0.97 g/cm3 | H2O-NaCl |
| Type 5 (L + V); n = 8 | Monzogranite; syenogranite | Sericitic alteration | Ms + Qz2 + Au1 | Secondary; irregular to sub-rounded shaped; colorless; 15 and 50 μm; F: 0.75–0.8 | Tmice = −4.2 to −0.8 °C; Tht = 98 to 190 °C; salinity = 1.39–6.74 wt.% NaCl eq.; density = 0.92–0.97 g/cm3 | H2O-NaCl |
| Type 6 (L; V) | Monzogranite; syenogranite | All assemblages | Colorless to greenish; rounded/oval to irregular; 4 and 20 μm; it occurs in all samples studied | H2O-NaCl |
| Sample | Mineral | Hydrothermal Alteration Stage | δ34SPy (‰) | δ18OQz (‰) | δ18O H2O (‰) [44] | Estimated Isotopic Equilibrium Temperature (°C) from Fluid Inclusion Data |
|---|---|---|---|---|---|---|
| F75-05 | Qz2 | Sericitic alteration | 9 | 3.11–3.99 | 340–370 | |
| F70-02 | 9.6 | 3.72–4.59 | ||||
| F73-02 | 10.3 | 4.72–5.29 | ||||
| F70-03 | Py2 | −0.5 | ||||
| Qz2 | 11.5 | 5.62–6.50 | ||||
| F73-03 | Py3 | Sulfide stage | 0.1 | 325–380 | ||
| Qz3 | 12.6 | 6.22–7.86 | ||||
| F48-A15 | Py3 | −1 | ||||
| Qz3 | 12.2 | 5.82–7.46 | ||||
| F70-01 | Py3 | −0.1 | 11.1 | 4.73–6.36 | ||
| F78-05 | Py3 | −0.7 | ||||
| Qz3 | 11.6 | 5.23–6.85 | ||||
| F73-04 | Py3 | −1.4 | ||||
| Qz3 | 10.7 | 4.32–5.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.A.C.; Moura, M.A. The Paleoproterozoic Raimunda Porphyry-Type Gold Deposit, Juruena Mineral Province, Amazonian Craton (Brazil): Constraints Based on Petrological, Fluid Inclusion and Stable Isotope Data. Minerals 2024, 14, 1185. https://doi.org/10.3390/min14121185
Lopes AAC, Moura MA. The Paleoproterozoic Raimunda Porphyry-Type Gold Deposit, Juruena Mineral Province, Amazonian Craton (Brazil): Constraints Based on Petrological, Fluid Inclusion and Stable Isotope Data. Minerals. 2024; 14(12):1185. https://doi.org/10.3390/min14121185
Chicago/Turabian StyleLopes, Adriana Araujo Castro, and Márcia Abrahão Moura. 2024. "The Paleoproterozoic Raimunda Porphyry-Type Gold Deposit, Juruena Mineral Province, Amazonian Craton (Brazil): Constraints Based on Petrological, Fluid Inclusion and Stable Isotope Data" Minerals 14, no. 12: 1185. https://doi.org/10.3390/min14121185
APA StyleLopes, A. A. C., & Moura, M. A. (2024). The Paleoproterozoic Raimunda Porphyry-Type Gold Deposit, Juruena Mineral Province, Amazonian Craton (Brazil): Constraints Based on Petrological, Fluid Inclusion and Stable Isotope Data. Minerals, 14(12), 1185. https://doi.org/10.3390/min14121185










