Investigation of Appropriate Collector Selection for Hematite Removal from Pyrolusite and the Adsorption Mechanism on the Crystal Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mineral Samples
2.2. Flotation Tests Method
2.3. Simulation Methods and Parameters
3. Results and Discussion
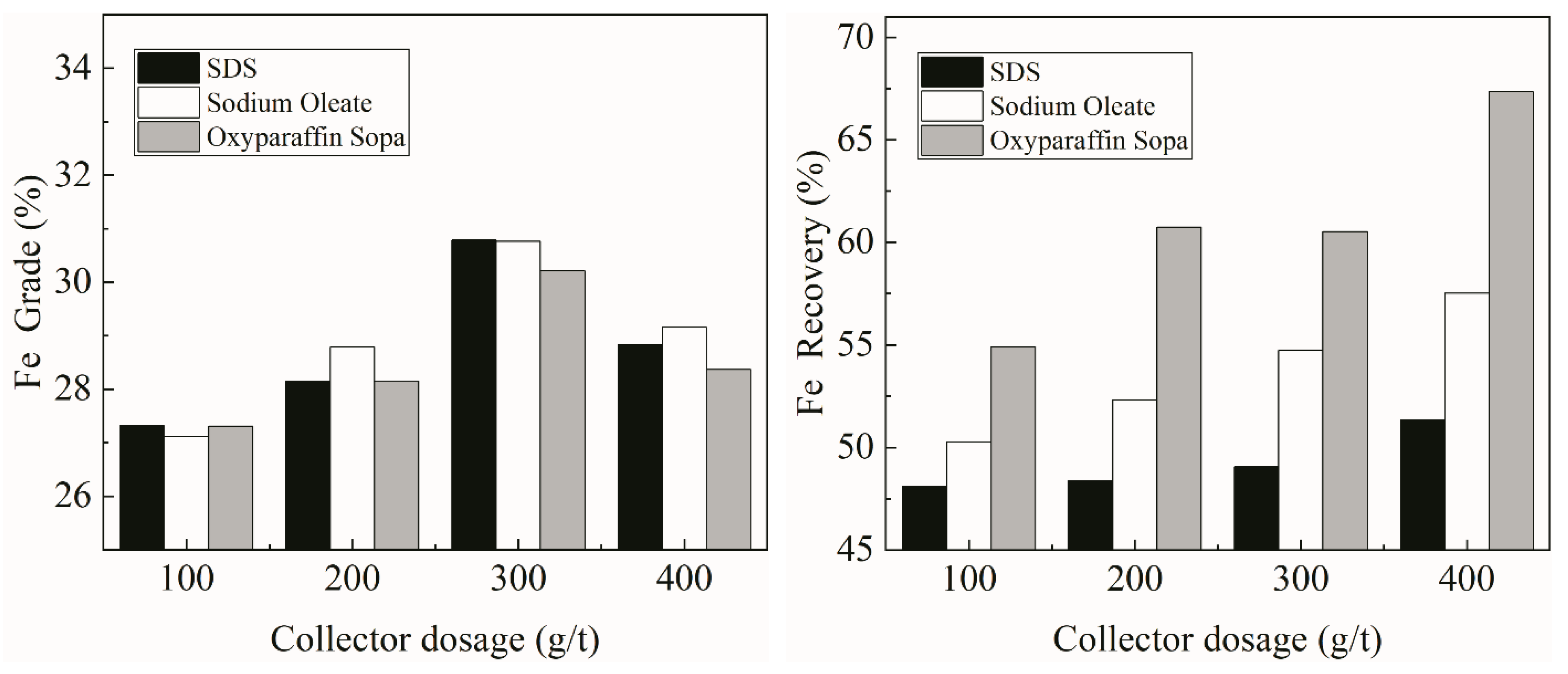
3.1. Flotation Test Results
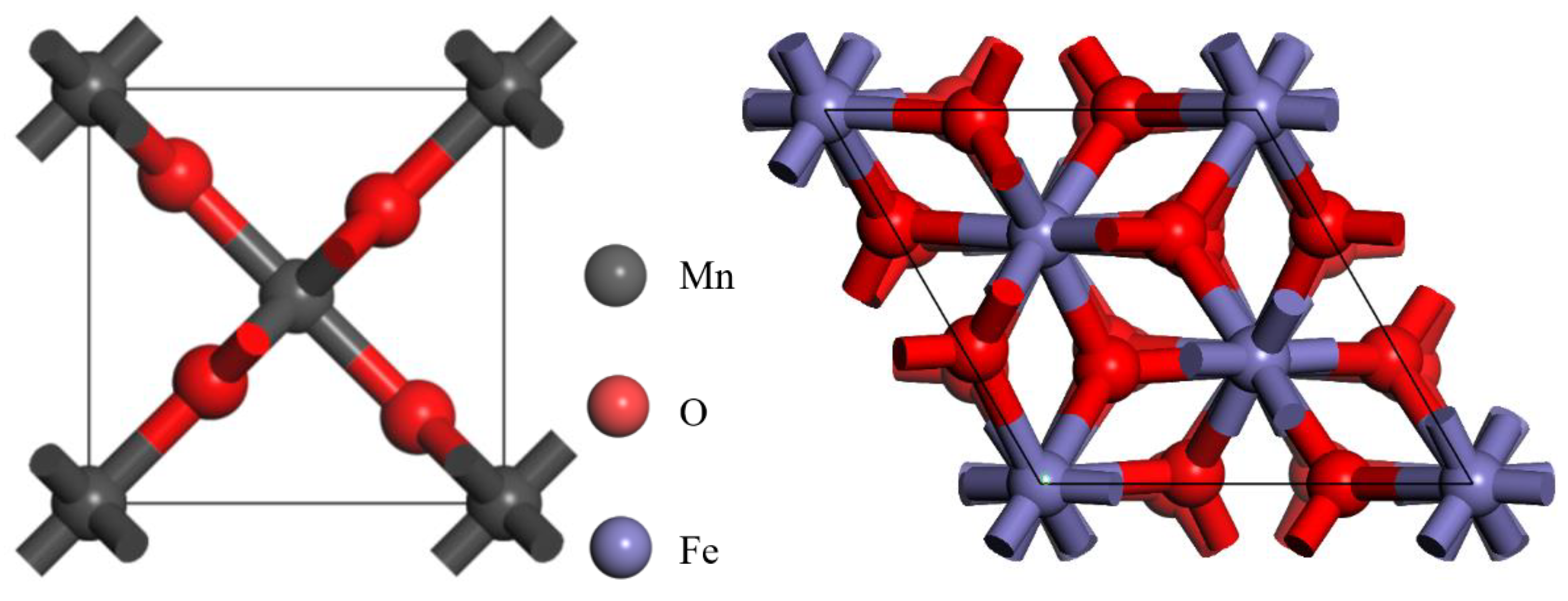
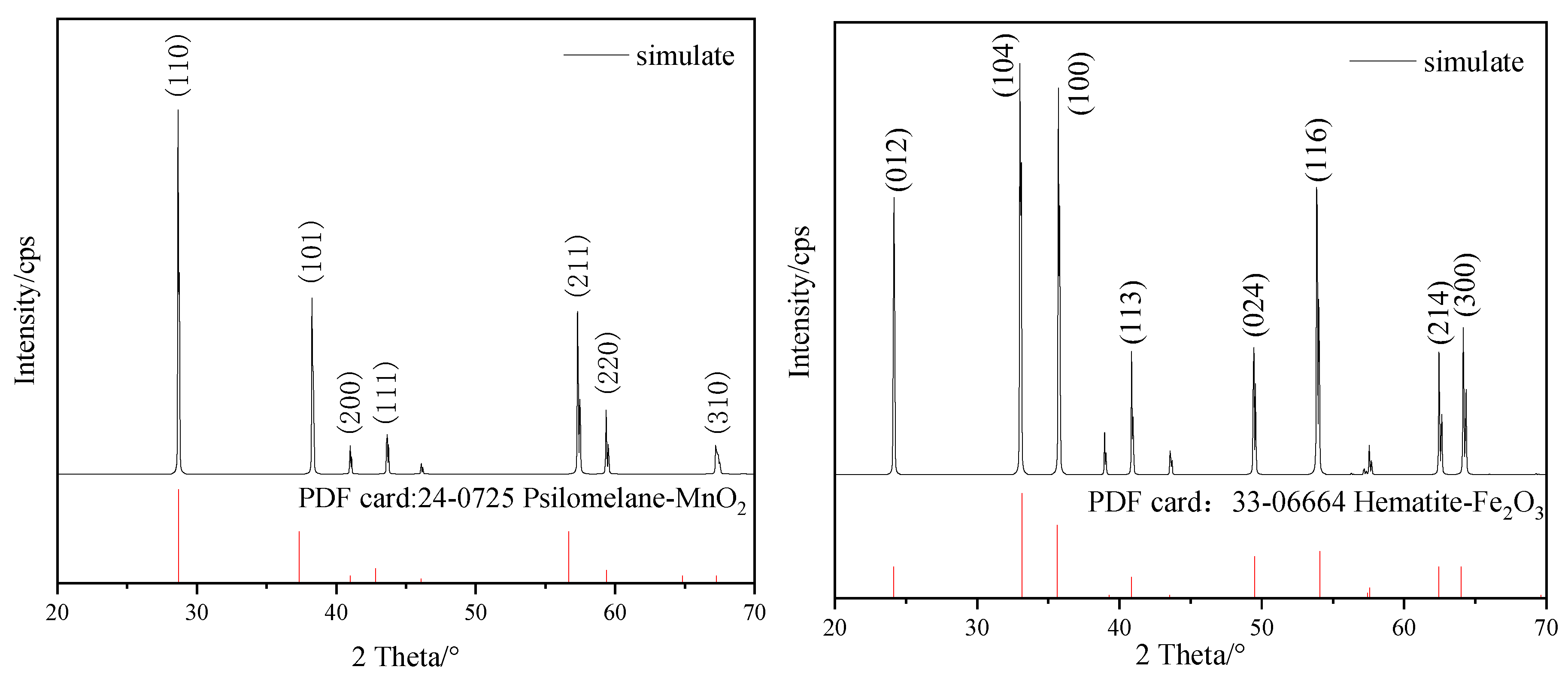
3.2. Construction and Analysis of Pyrolusite and Hematite Crystals
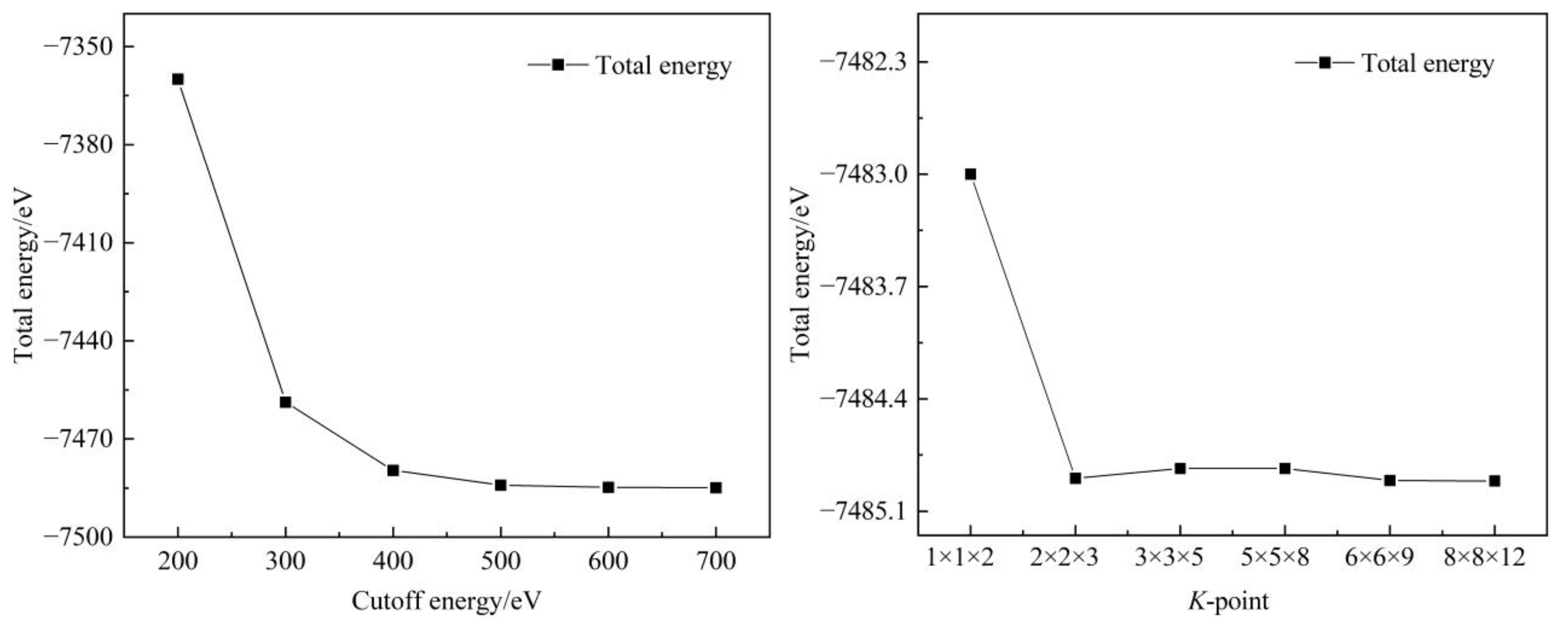
3.2.1. Structure Optimization of Unit Cell
3.2.2. Crystal Properties of Pyrolusite and Hematite
3.2.3. Distribution Analysis of Pyrolusite and Hematite
3.2.4. Frontier Molecular Orbital (FMO) Analysis
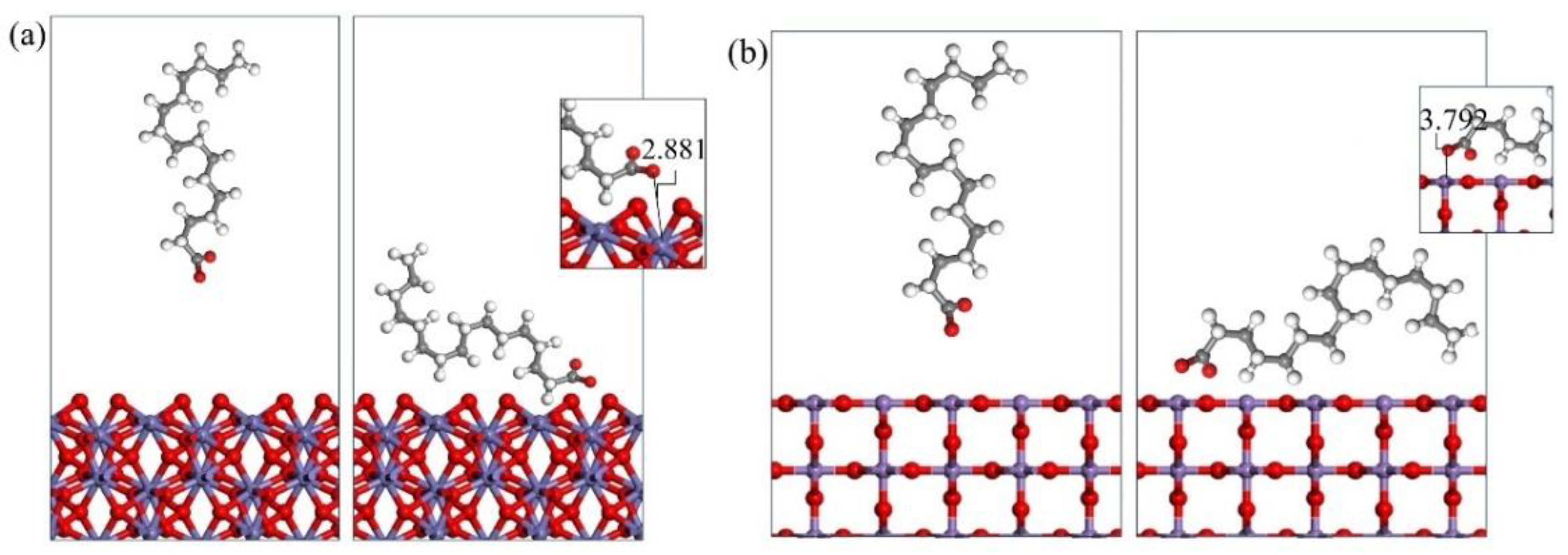
3.3. Molecular Dynamics Simulation of Collector–Mineral Interaction
4. Conclusions
- (1)
- Flotation experiments have shown that, compared with sodium oleate and dodecyl sulfonic acid, oxidized paraffin soap has better flotation separation effects on pyrolusite and hematite.
- (2)
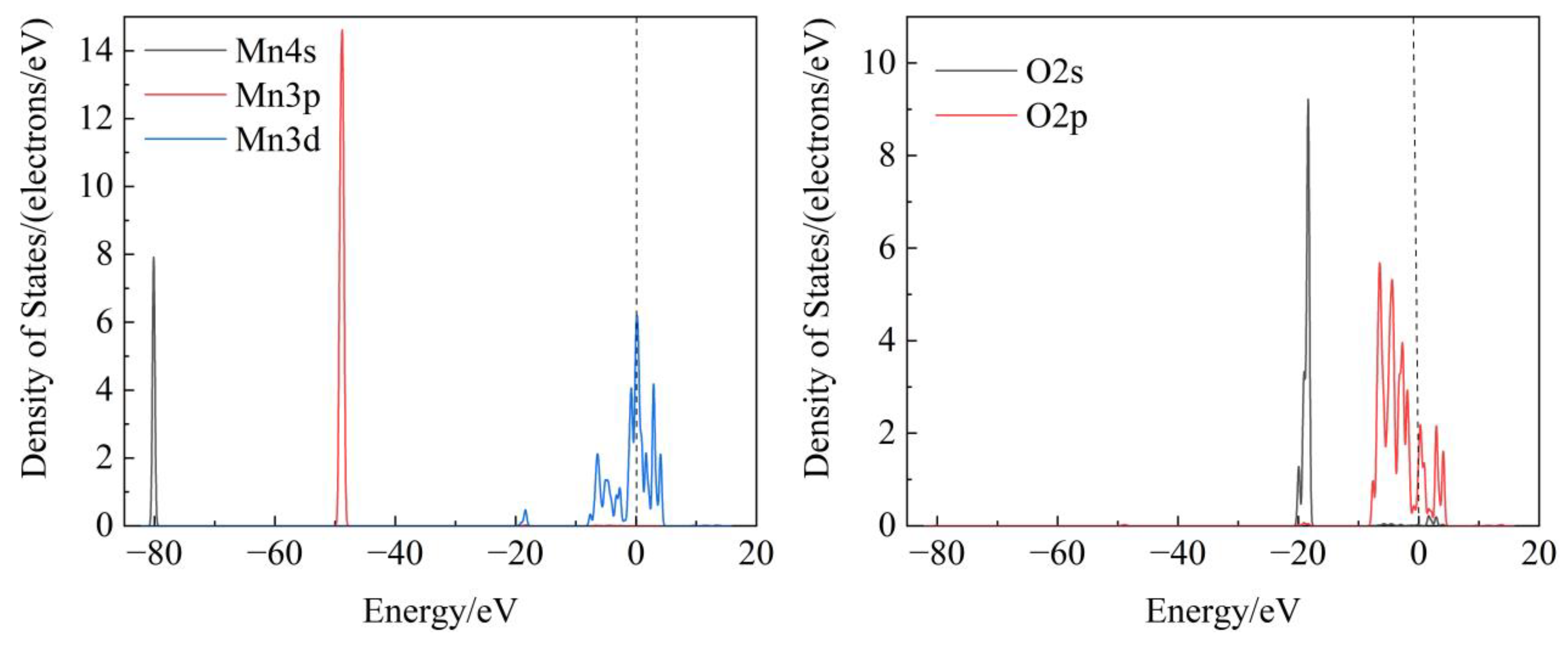
- Based on the density of state findings, the Mn atoms in pyrolusite crystals are identified as the active sites, whereas the Fe atoms in hematite crystals are identified as the active sites.
- (3)
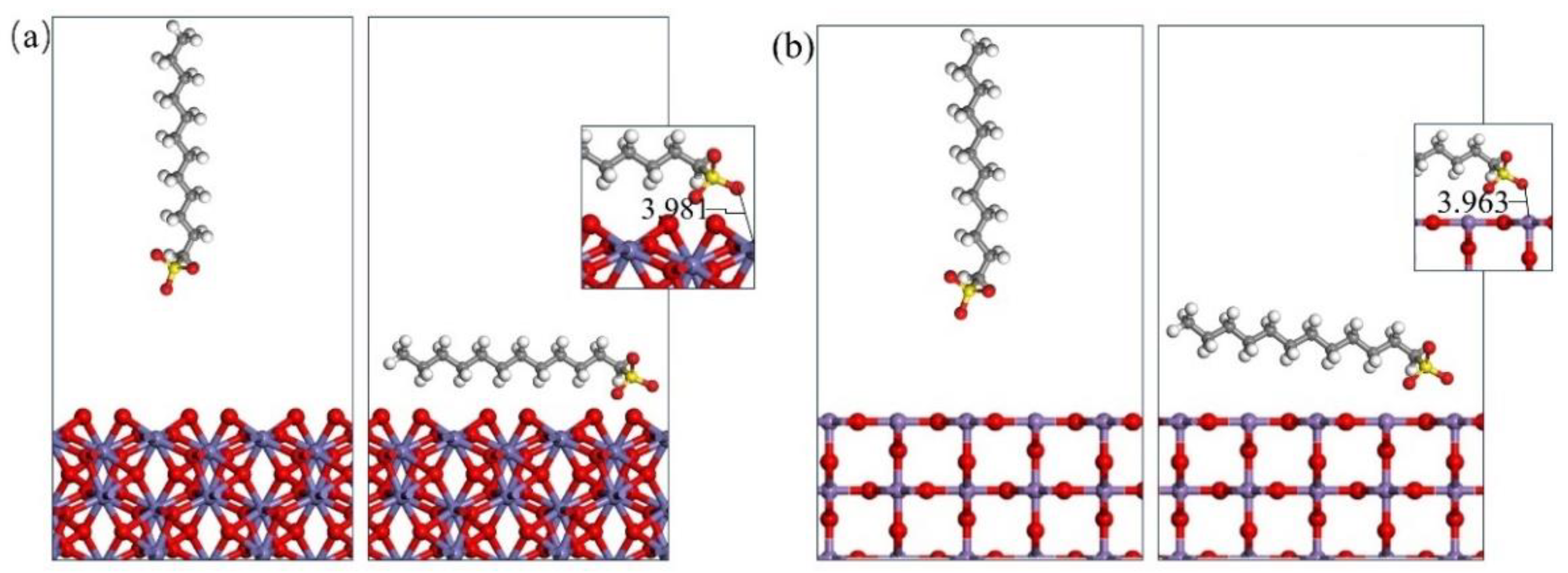
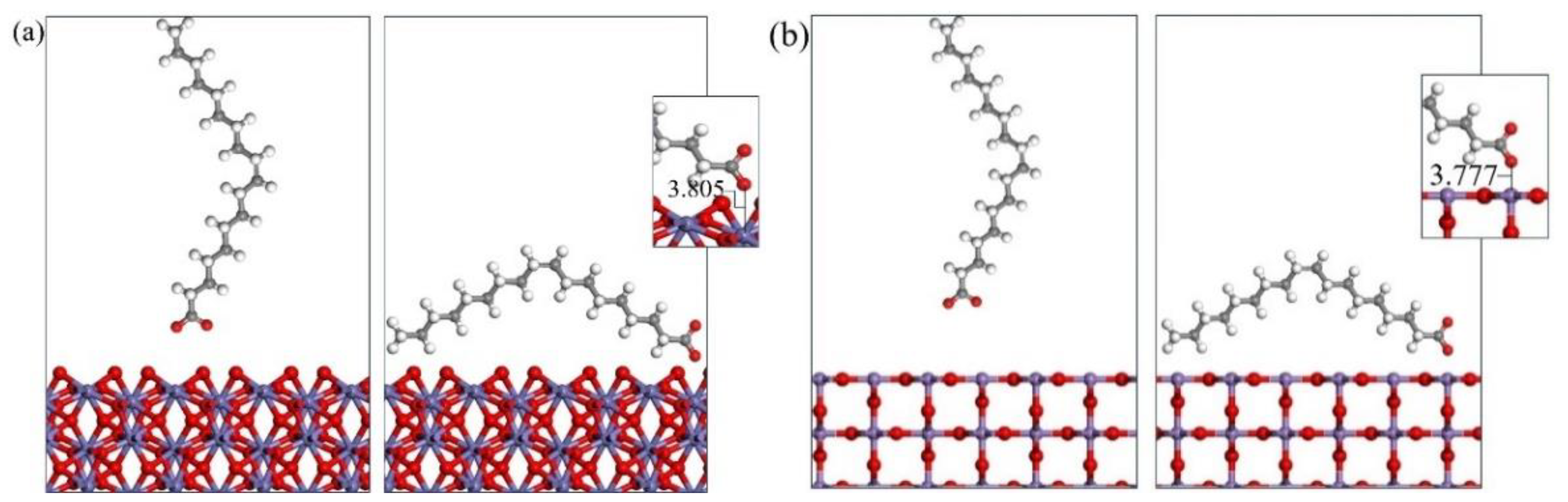
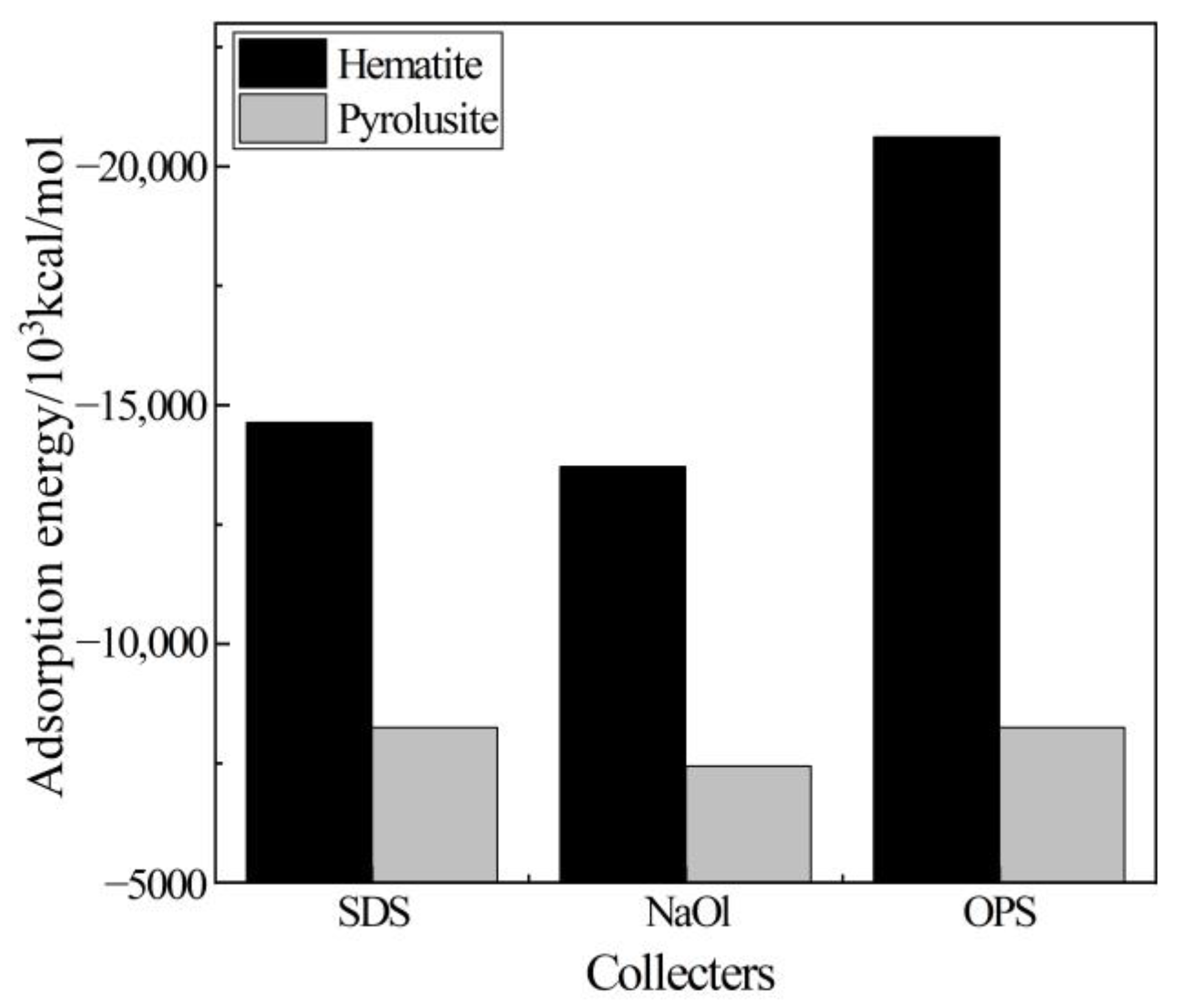
- Upon investigating the energy calculation results of the frontline orbit and the adsorption energies, it was shown that the three collectors (OPS, NaOl, and SDS) have a much stronger interaction with hematite than with pyrolusite. Therefore, it is possible to separate pyrolusite and hematite through flotation.
- (4)
- The simulation results also show that among the three collectors, OPS has the highest adsorption strength and selectivity for hematite. This characteristic makes OPS an excellent collector for effectively removing hematite from pyrolusite in the reverse flotation process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.C.; Feng, Y.L.; Li, H.R.; Wang, W.D.; Teng, Q. Effect of biological pretreatment on flotation recovery of pyrolusite. Trans. Nonferrous Met. Soc. China 2014, 24, 1571–1577. [Google Scholar] [CrossRef]
- Rahimi, S.; Irannajad, M.; Mehdilo, A. Comparative studies of two cationic collectors in the flotation of pyrolusite and calcite. Int. J. Miner. Process. 2017, 167, 103–112. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M. Evaluation of pyrolusite flotation behavior using a cationic collector. J. Min. Sci. 2014, 50, 982–993. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Wang, H.; Sun, Q.; Wang, Q.; Alshameri, A. Flotation behavior of four C18 hydroxamic acids as collectors of rhodochrosite. Miner. Eng. 2015, 78, 15–20. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, T.; Yan, C.; Liang, H.; Chen, T.; Li, D.; Wang, Q. The flotation of low-grade manganese ore using a novel linoleate hydroxamic acid. Colloids Surf. A-Physicochem. Eng. Asp. 2015, 466, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, H.; Feng, Q.; Ou, L.; Zhang, G. Magnetic-hydrophobic agglomeration of fine pyrolusite. J. Cent. S. Univ. Sci. Technol. 2012, 43, 4595–4599. [Google Scholar]
- Zhengguang, T.; Wenju, J. Process and Mechanism of Catalyzed Oxidation of SO2 with Pyrolusite Slurry. Enuivonmental Sci. Technol. 2008, 31, 13–15,37. [Google Scholar]
- Zhu, X.; Jiang, W.; Su, S.; Jin, Y.; Liu, X. The study of reaction mechanism of desulfurization in flue gas with pyrolusite pulp. Tech. Equip. Environ. Pollut. Control 2002, 3, 44–46. [Google Scholar]
- Fu-Zhong, W.U.; Jun-Qi, L.I.; Hui-Xin, J.I.N.; Jiu-Ju, C.A.I. Study on Reaction Mechanism of Flue Gas Desulfurization in Sintering With Pyrolusite. Iron Steel 2009, 44, 87–91. [Google Scholar]
- Yang, Z.-C.; Feng, Y.-L.; Li, H.-R.; Wang, W.-D.; Teng, Q.; Zhang, X. Effect of Mn (II) on quartz flotation using dodecylamine as collector. J. Cent. South Univ. 2014, 21, 3603–3609. [Google Scholar] [CrossRef]
- Rahimi, S.; Irannajad, M.; Mehdilo, A. Effects of sodium carbonate and calcium chloride on calcite depression in cationic flotation of pyrolusite. Trans. Nonferrous Met. Soc. China 2017, 27, 1831–1840. [Google Scholar] [CrossRef]
- Farghaly, M.G.; Abdel-Khalek, N.A.; Abdel-Khalek, M.A.; Selim, K.A.; Abdullah, S.S. Physicochemical study and application for pyrolusite separation from high manganese-iron ore in the presence of microorganisms. Physicochem. Probl. Miner. Process. 2021, 57, 273–283. [Google Scholar] [CrossRef]
- Singh, V.; Chakraborty, T.; Tripathy, S.K. A Review of Low Grade Manganese Ore Upgradation Processes. Miner. Process. Extr. Metall. Rev. 2020, 41, 417–438. [Google Scholar] [CrossRef]
- Long, H.-Z.; Chai, L.-Y.; Qin, W.-Q. Galena-pyrolusite co-extraction in sodium chloride solution and its electrochemical analysis. Trans. Nonferrous Met. Soc. China 2010, 20, 897–902. [Google Scholar] [CrossRef]
- Wang, R.; Gao, P.; Yuan, S.; Li, Y.; Liu, Y.; Huang, C. Precise regulation of the phase transformation for pyrolusite during the reduction roasting process. Int. J. Miner. Metall. Mater. 2024, 31, 81–90. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, W.; Liu, J.; Zhou, C.; Xie, T.; Zhang, Y. Simultaneous Leaching of Sulphureous Manganese Carbonate Ore and Pyrolusite by Novel Two-ore Method. Min. Metall. Eng. 2015, 35, 95–97. [Google Scholar]



| Element | Mn | Fe | S | P | SiO2 | Al2O3 |
|---|---|---|---|---|---|---|
| Content/% | 27.36 | 11.60 | 0.084 | 0.07 | 18.26 | 2.23 |
| Mineral | Content/% | Mineral | Content/% |
|---|---|---|---|
| Pyrolusite | 10.698 | Rutile | 0.108 |
| Titanomagnetite | 0.015 | Zoisite | 0.003 |
| Hematite | 6.215 | Fluorite | 0.002 |
| Pyrite | 0.004 | Calcite | 0.004 |
| Kaolin | 34.830 | Dolomite | 0.004 |
| Quartz | 45.398 | Apatite | 0.001 |
| Feldspar | 1.83 | Sphene | 0.025 |
| Sericite | 0.121 | Zircon | 0.003 |
| Flogopite | 0.221 | Other | 0.094 |
| Tourmaline | 0.424 | Total | 100 |
| Species | s | p | d | Total | Charge/e |
|---|---|---|---|---|---|
| Mn | 0.18 | 0.22 | 4.82 | 5.22 | 0.98 |
| Mn | 1.72 | 0.81 | 0.00 | 1.79 | |
| O | 0.96 | 2.56 | 0.00 | 3.52 | −0.66 |
| O | 0.93 | 2.21 | 0.00 | 3.14 |
| Bond Species | Overlap Population | Bond Length/Å |
|---|---|---|
| Mn-O | 0.29 | 1.98 |
| Mn-O | 0.19 | 2.07 |
| Mn-Mn | −0.47 | 2.71 |
| O-O | −0.07 | 2.72 |
| O-O | −0.06 | 2.80 |
| O-O | −0.05 | 2.89 |
| Mn-Mn | −0.63 | 2.93 |
| Species | s | p | d | Total | Charge/e |
|---|---|---|---|---|---|
| Fe | 0.20 | 0.24 | 4.79 | 5.23 | 1.02 |
| O | 0.95 | 2.56 | 0.00 | 3.51 | −0.68 |
| Bond Species | Overlap Population | Bond Length/Å |
|---|---|---|
| Fe-O | 0.29 | 1.95 |
| Fe-O | 0.22 | 2.05 |
| Fe-Fe | −0.16 | 2.66 |
| Fe-Fe | −0.68 | 2.89 |
| O-O | −0.08 | 2.68 |
| O-O | −0.03 | 2.77 |
| Mineral/Collector | EHOMO/eV | ELUMO/eV | |ΔE1|/eV | |ΔE2|/eV | |ΔE3|/eV |
|---|---|---|---|---|---|
| Pyrolusite | −7.368 | −5.546 | 1.126 | 1.142 | 0.175 |
| Hematite | −5.002 | −4.682 | 0.262 | 0.278 | 0.690 |
| OPS | −4.420 | −1.233 | |||
| NaOl | −4.404 | −1.204 | |||
| SDS | −5.372 | −1.979 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Nan, N.; Song, B.; Ma, F.; Han, J.; Huang, E.; Wang, S.; Yang, G.; Zhou, L. Investigation of Appropriate Collector Selection for Hematite Removal from Pyrolusite and the Adsorption Mechanism on the Crystal Surface. Minerals 2024, 14, 1300. https://doi.org/10.3390/min14121300
Shi Y, Nan N, Song B, Ma F, Han J, Huang E, Wang S, Yang G, Zhou L. Investigation of Appropriate Collector Selection for Hematite Removal from Pyrolusite and the Adsorption Mechanism on the Crystal Surface. Minerals. 2024; 14(12):1300. https://doi.org/10.3390/min14121300
Chicago/Turabian StyleShi, Yuhang, Nan Nan, Baoxu Song, Fangyuan Ma, Jiquan Han, Enming Huang, Shuai Wang, Guang Yang, and Lan Zhou. 2024. "Investigation of Appropriate Collector Selection for Hematite Removal from Pyrolusite and the Adsorption Mechanism on the Crystal Surface" Minerals 14, no. 12: 1300. https://doi.org/10.3390/min14121300
APA StyleShi, Y., Nan, N., Song, B., Ma, F., Han, J., Huang, E., Wang, S., Yang, G., & Zhou, L. (2024). Investigation of Appropriate Collector Selection for Hematite Removal from Pyrolusite and the Adsorption Mechanism on the Crystal Surface. Minerals, 14(12), 1300. https://doi.org/10.3390/min14121300






