Pyrochlore-Supergroup Minerals and Their Relation to Columbite-Group Minerals in Peralkaline to Subaluminous A-Type Rare-Metal Granites with Special Emphasis on the Madeira Pluton, Amazonas, Brazil
Abstract
1. Introduction
2. Geology of the Studied Plutons and Samples
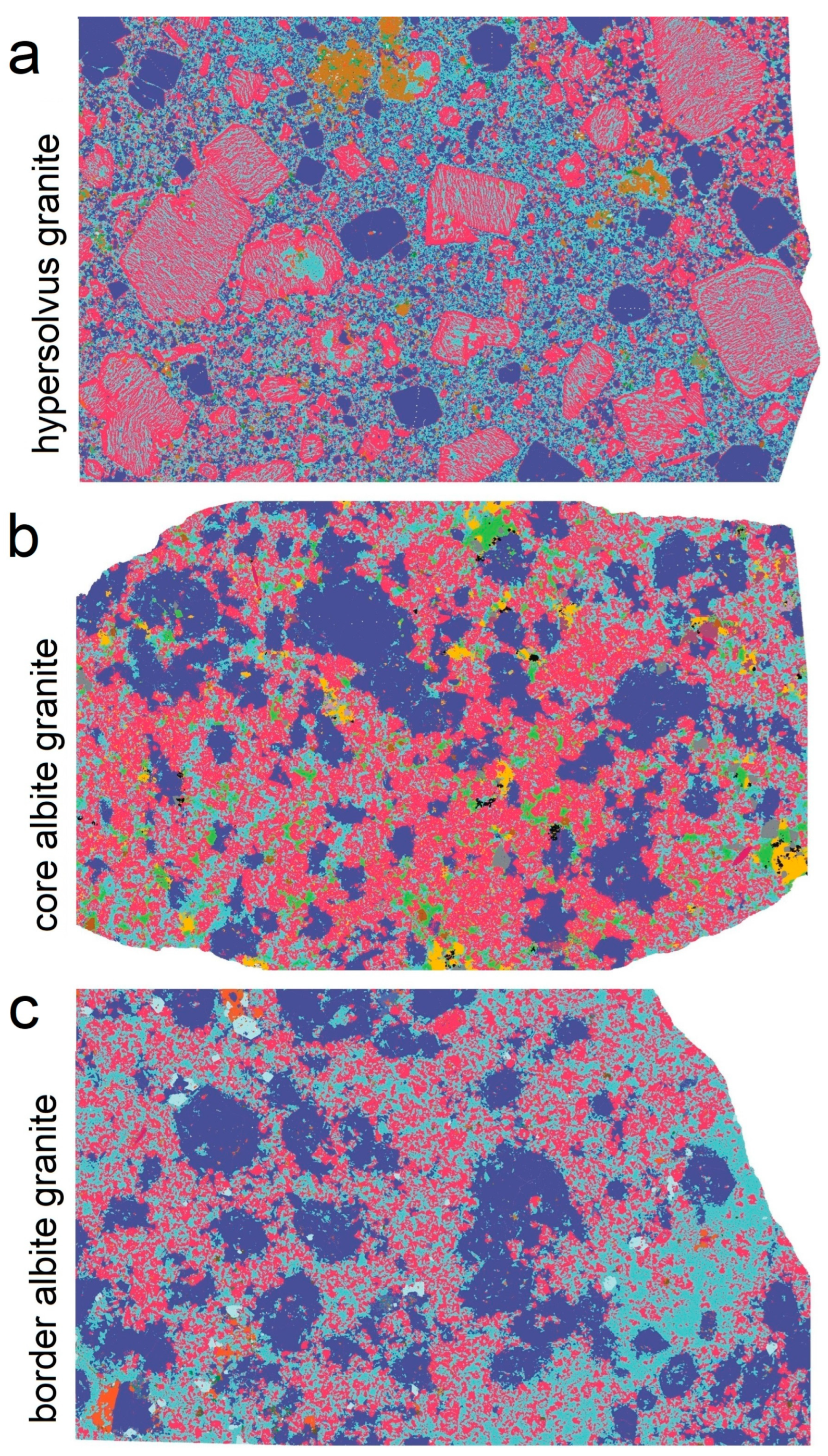
3. Methods
3.1. SEM Microscopy
3.2. Automated Mineralogy (TIMA)
3.3. Electron Probe Microanalysis (EPMA)
3.4. Remark on Mineral Names
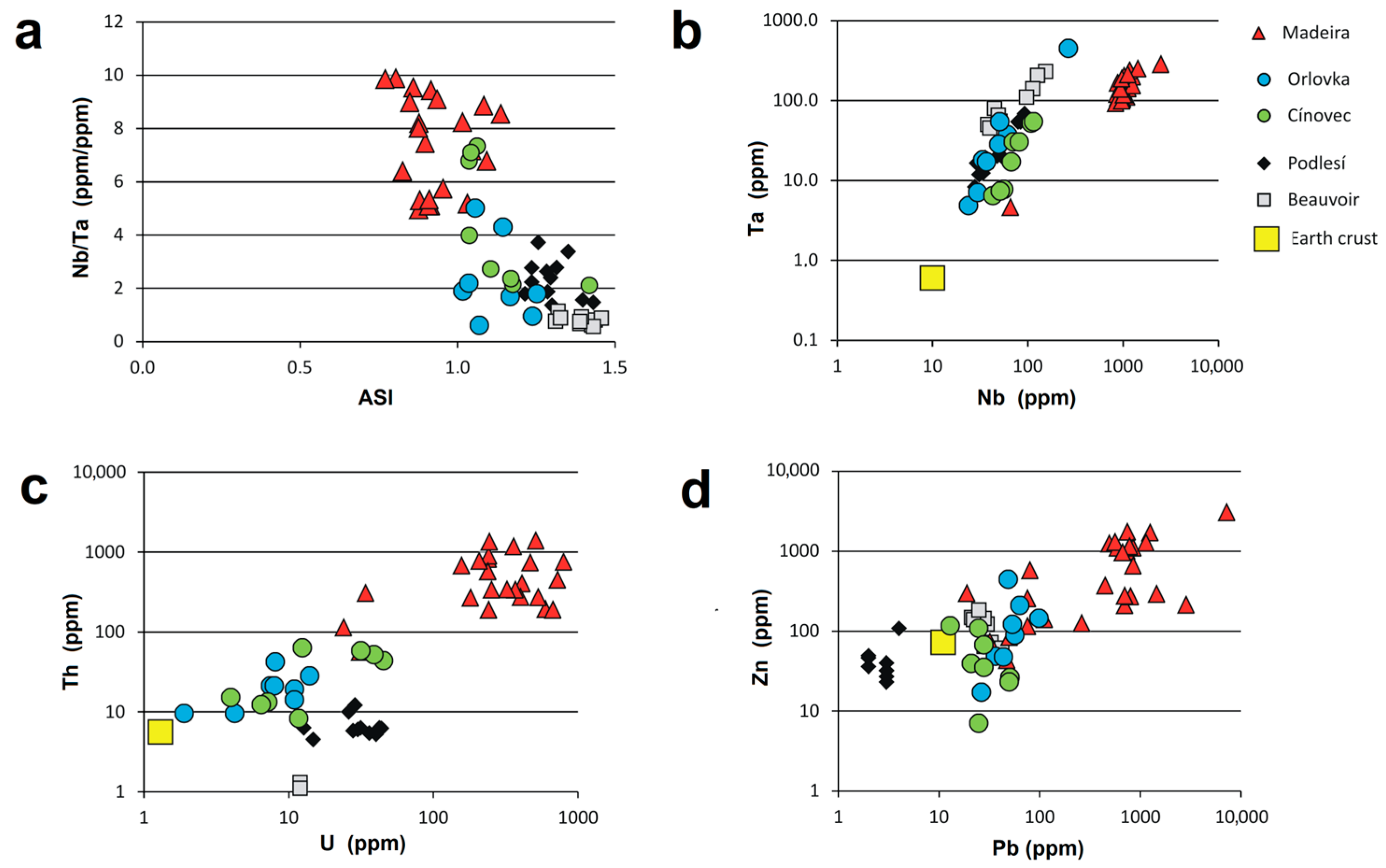
4. Results
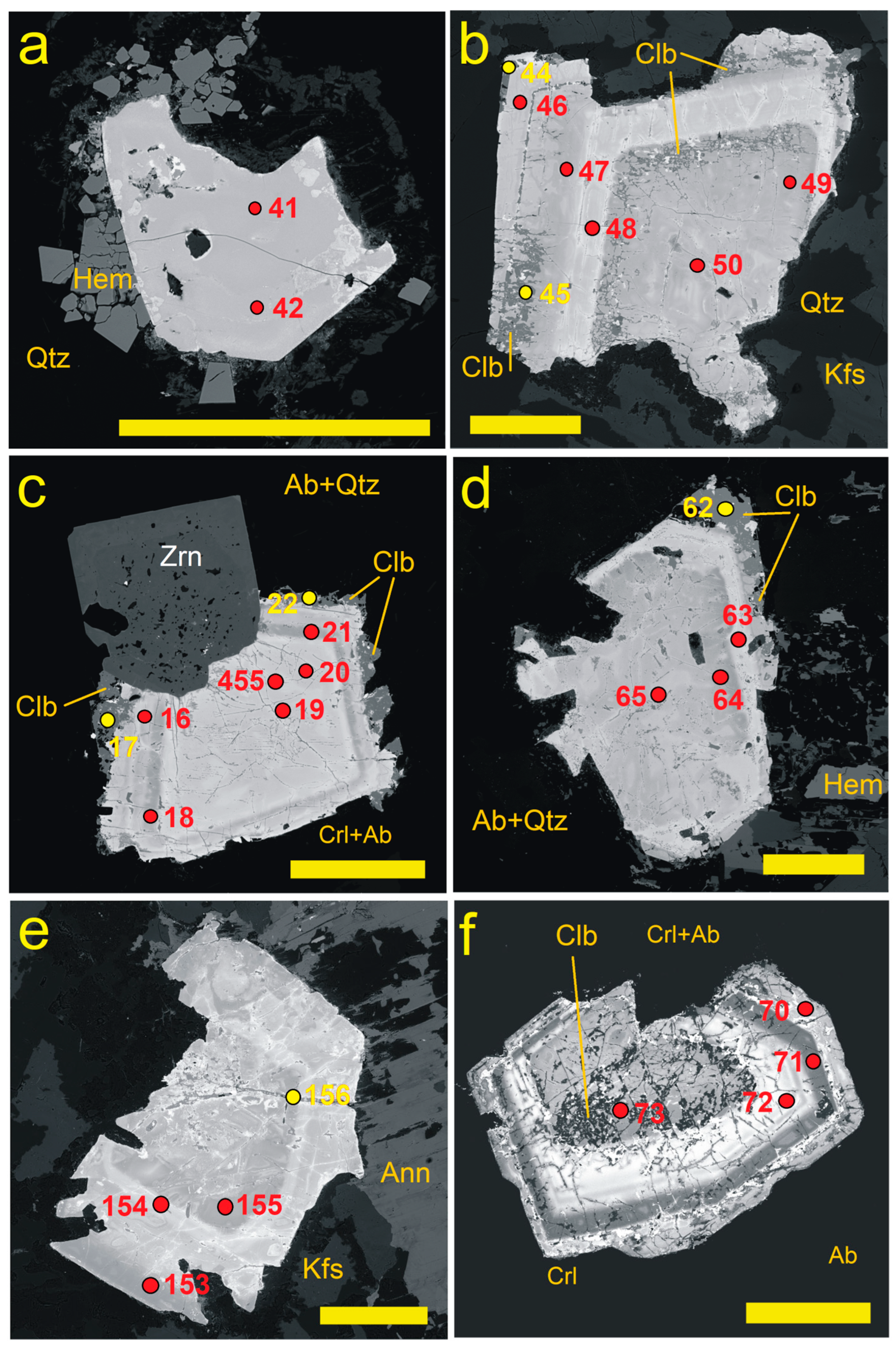
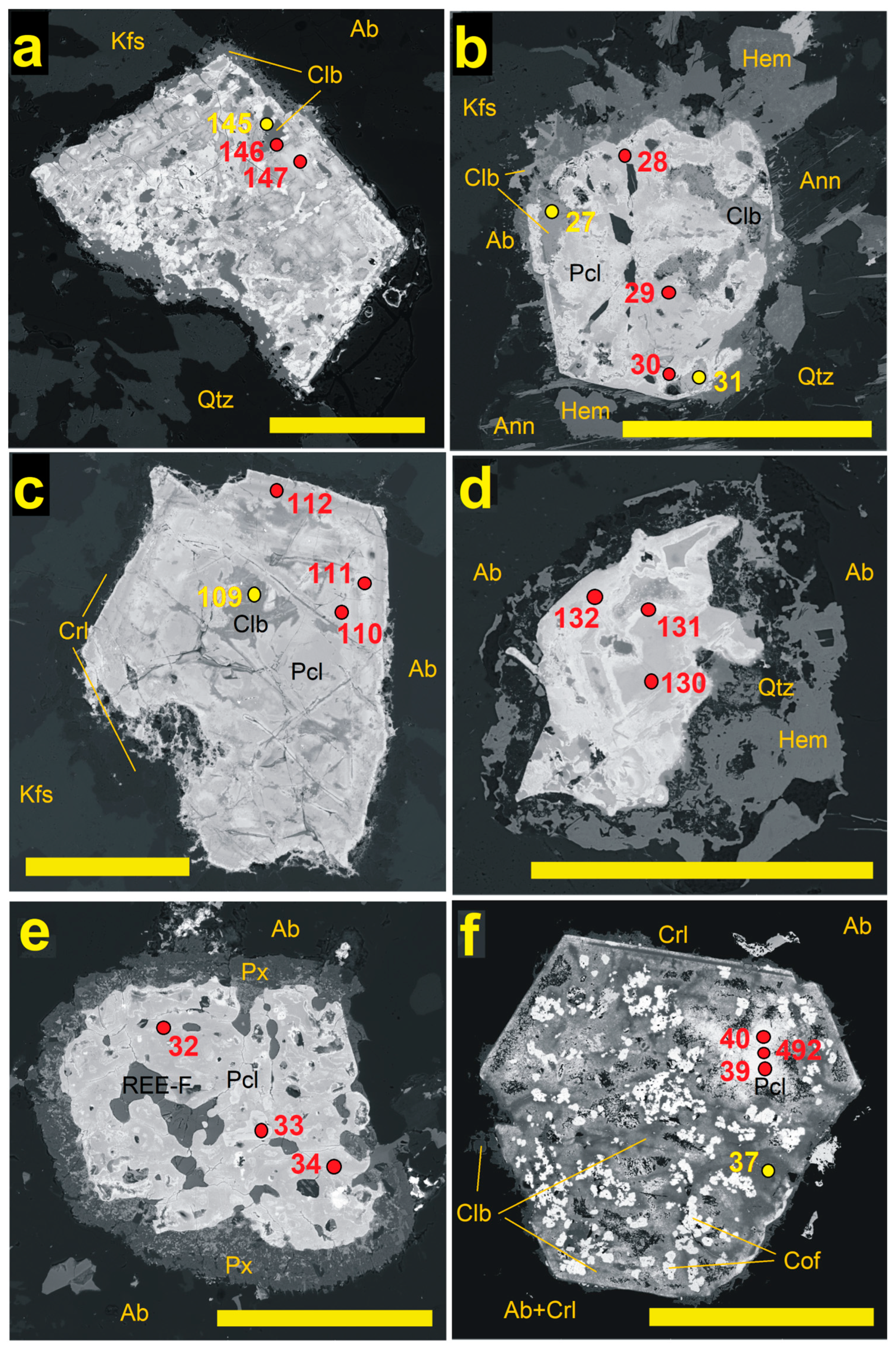
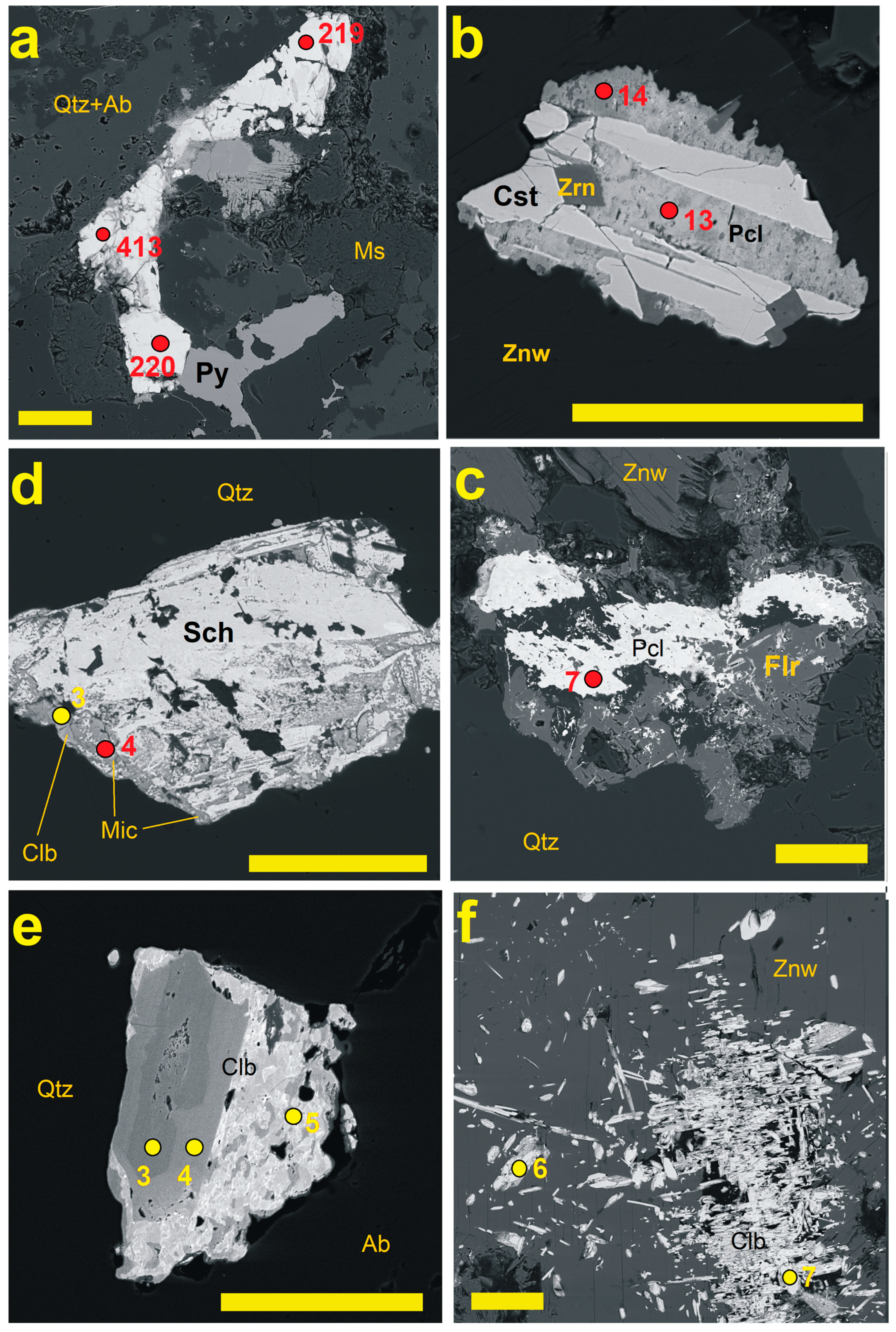
4.1. Pyrochlore and CGM from the Madeira Albite Granite
4.2. The Distribution of PSGM Across the Madeira Pluton
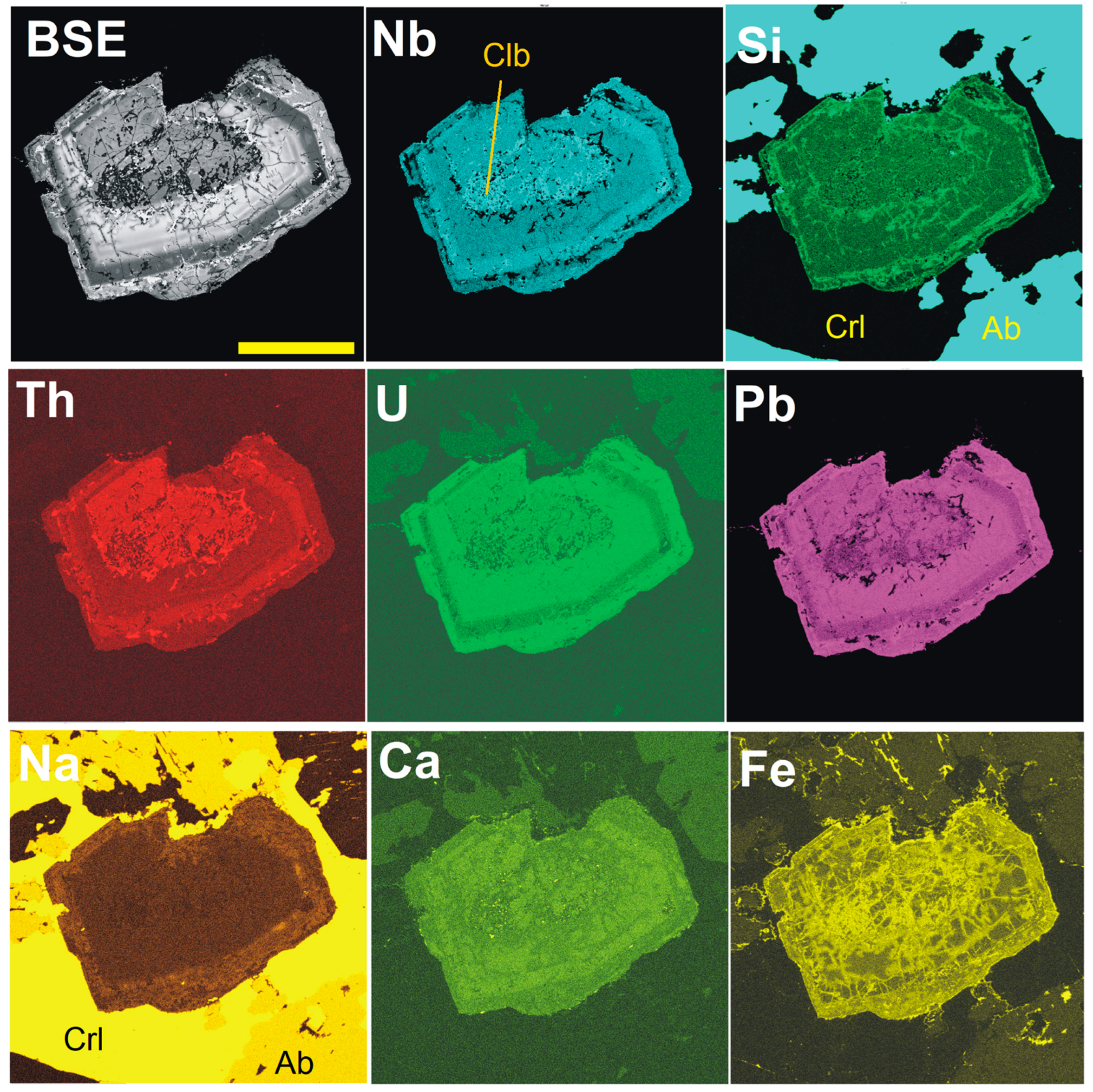
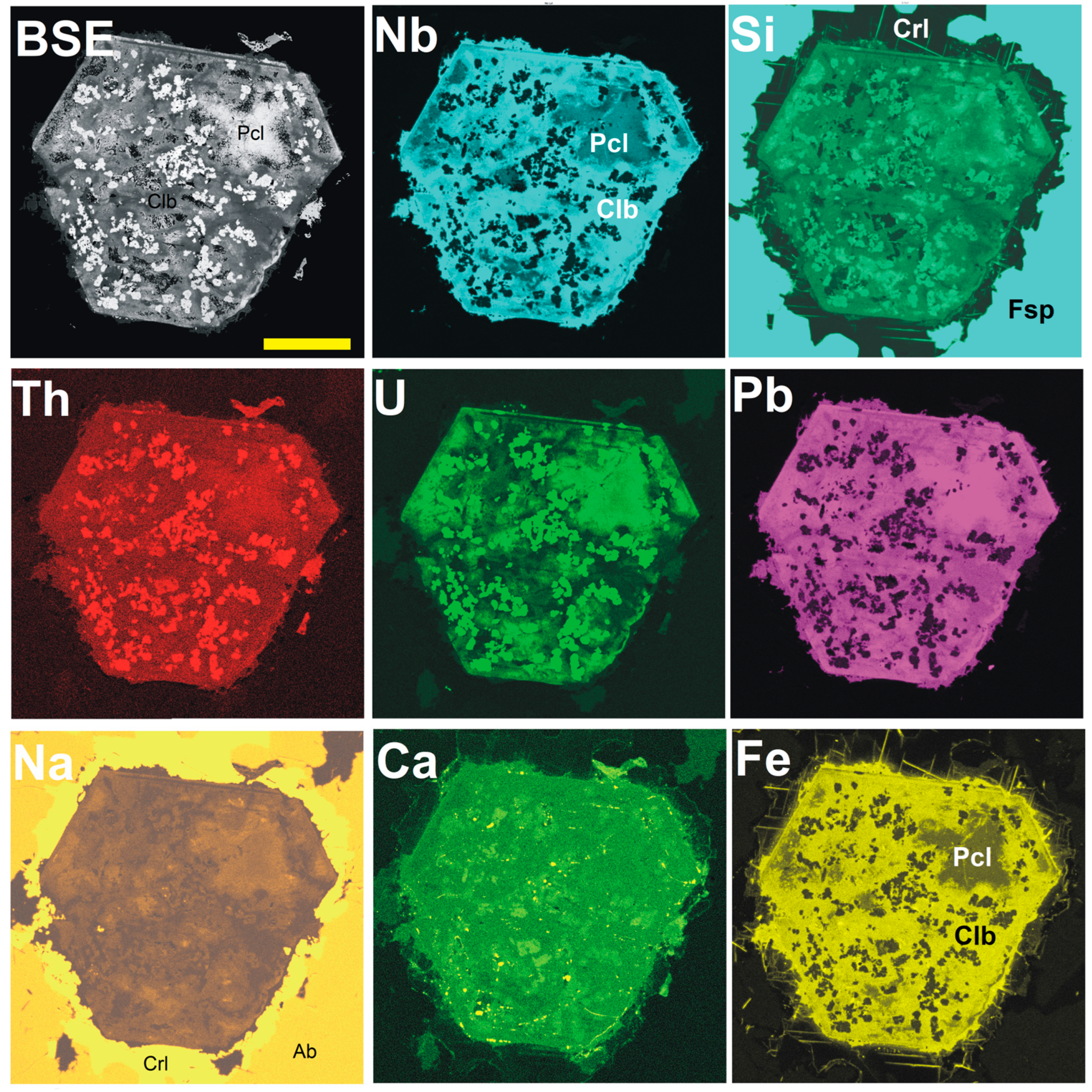
4.3. Chemical Zoning of Pyrochlore Crystals at Madeira
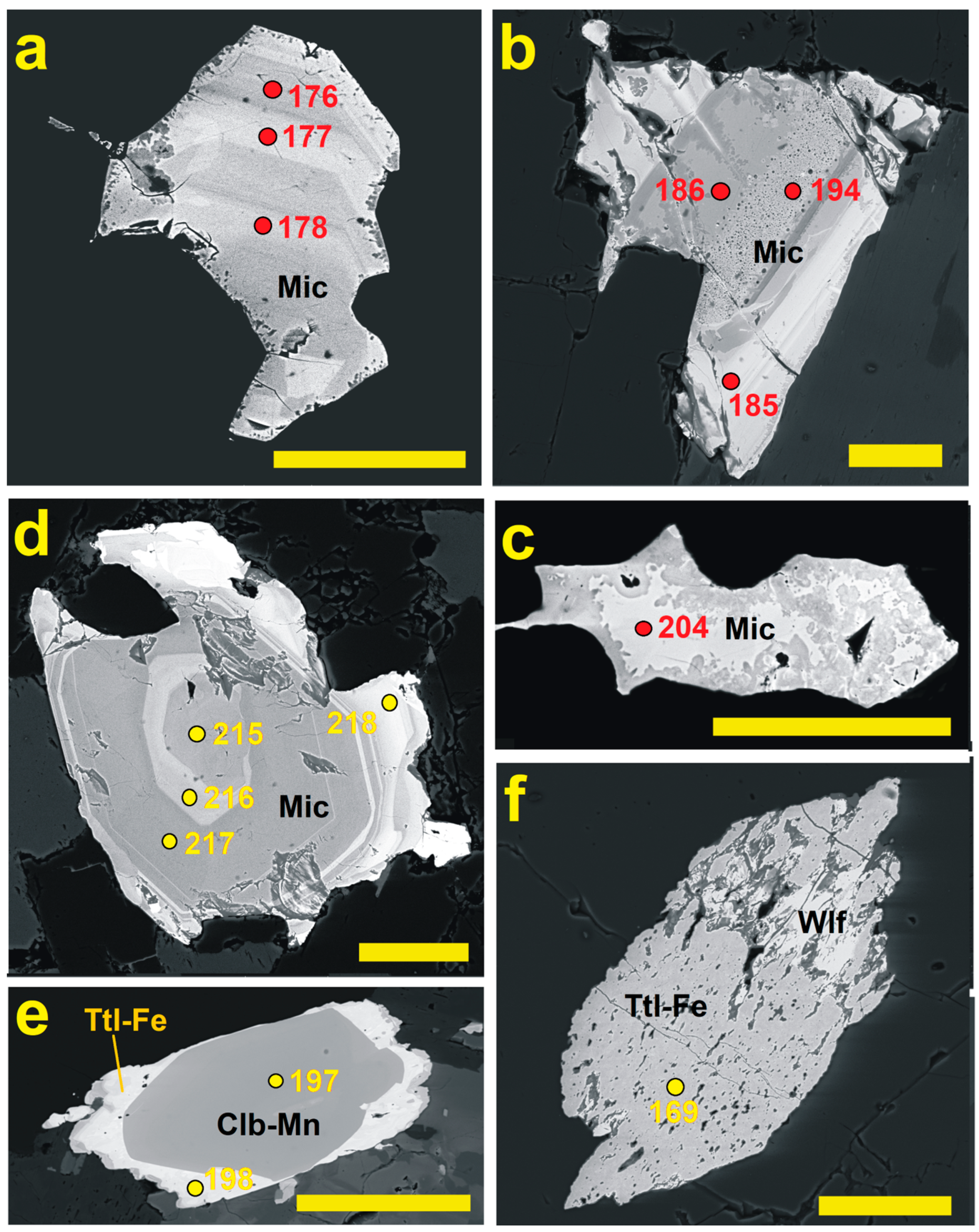
4.4. Microlite and CGM from Orlovka
4.5. Microlite, Pyrochlore and CGM from Cínovec
5. Discussion
5.1. Chemical Changes During PSGM Alteration: Generally vs. Locally Typical Elements
5.2. Time Relation Between PSGM and CGM
5.3. Chemistry of PSGM vs. Associated CGM
5.4. Silica in PSGM
5.5. Pb-Dominant Pyrochlore/Microlite
5.6. Comparison of Pyrochlore Supergroup Minerals from Different Igneous Lithologies
6. Conclusions
- -
- In a trend from peralkaline to peraluminous granitoids and from NYF to LCT pegmatites, with increasing aluminosity, dominant pyrochlore is replaced with microlite;
- -
- In pegmatites, columbite is usually the prime crystallized Nb,Ta oxide, being later replaced with late pyrochlore/microlite to a variable degree. In other lithologies, including carbonatites, alkali syenites, and peralkaline to peraluminous granites, pyrochlore/microlite is dominantly a primary magmatic phase; pyrochlore may be later partly replaced with columbite. Microlite does not undergo columbitization;
- -
- In the Madeira albite granite, pyrochlore is the sole primary Nb oxide in the core albite granite and the hypersolvus granite. Minor primary columbite occurs only in the border albite granite;
- -
- Pyrochlore from Madeira can be chemically characterized as Na, Ca-poor, U- and Pb-dominant, and Sn- and Zn-enriched. The contents of Ta and Ti are always low; REEs are enriched only during alteration;
- -
- Two stages of pyrochlore alteration are present at Madeira: (i) the introduction of Fe + Mn to the system, with the majority of these elements consumed by columbitization; (ii) the introduction of Si and Fe, in lesser amounts also Pb and U: Si, Pb, and U were incorporated into pyrochlore, while iron formed Fe-oxide halos around pyrochlore. During both stages, the contents of F and Na decreased. In the case of a (nearly) complete pyrochlore columbitization, U and Th were exsolved to form inclusions of a thorite/coffinite-like phase. In contrast to altered pyrochlores from other localities, pyrochlore from Madeira shows a relatively high occupancy of the A-site;
- -
- Although Madeira melt was Na-, F-rich, contemporaneous crystallization of cryolite consumed both elements, and pyrochlore was relatively Na-, F-poor from the beginning.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Critical Raw Materials Resilience: Charting a Path Towards Greater Security and Sustainability; European Commission: Bruxeles, Belgium, 2020. [Google Scholar]
- USGS 2020, MINERAL COMODITY SUMMARIES 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020.pdf (accessed on 13 December 2024).
- Williams-Jones, A.E.; Vasyukova, O.V. Niobium, critical metal, and progeny of the mantle. Econ. Geol. 2023, 118, 837–855. [Google Scholar] [CrossRef]
- Cordeiro, P.F.O.; Brod, J.A.; Palmieri, M.; Oliveira, C.G.; Barbosa, E.S.R.; Santos, R.V.; Gaspar, J.C.; Assis, L.C. The catalao I niobium deposit, central Brazil: Resources, geology and pyrochlore chemistry. Ore Geol. Rev. 2011, 41, 112–121. [Google Scholar] [CrossRef]
- Guarino, V.; Wu, F.Y.; Melluso, L.; Gomes, C.B.; Tassinari, C.C.G.; Roberti, E.; Brilli, M. U-Pb ages, geochemistry, C-O-Nd-Sr-Hf isotopes and petrogenesis of the Catalao II carbonatitic complex (Alto Paranaíba igneous province, Brazil): Implications for regional-scale heterogeneities in the Brazilian carbonatite associations. Int. J. Earth Sci. 2017, 106, 1963–1989. [Google Scholar] [CrossRef]
- Atencio, D.; Andrade, M.B.; Christy, A.D.; Gieré, R.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Canad. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Atencio, D. Pyrochlore-supergroup minerals nomenclature: An update. Front. Chem. 2021, 9, 713368. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Microlite subgroup. Amer. Mineral. 1992, 77, 7179–7188. [Google Scholar]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Pyrochlore subgroup. Amer. Mineral. 1995, 80, 732–743. [Google Scholar] [CrossRef]
- Ohnenstetter, D.; Piantone, P. Pyrochlore-group minerals in the Beauvoir peraluminous leucogranite, Massif Central, France. Canad. Mineral. 1992, 30, 771–784. [Google Scholar]
- Novák, M.; Černý, P. Niobium–tantalum oxide minerals from complex granitic pegmatites in the Moldanubicum, Czech Republic: Primary versus secondary compositional trends. Canad. Mineral. 1998, 36, 659–672. [Google Scholar]
- Minuzzi, O.R.R.; Bastos Neto, A.C.; Pereira, V.P.; Nunes, L. A columbitização do pirocloro do albita granito na mina Pitinga (AM): Relações com a mineralização de criolita. Rev. Bras. Geociências 2006, 36, 124–137, (In Portuguese, with English abstract). [Google Scholar] [CrossRef]
- Pršek, J.; Majka, J.; Uher, P.; Chudík, P. Niobium-tantalum minerals in the Skoddefjellet NYF granitic pegmatite, Svalbard Archipelago, Norway: Primary versus secondary assemblage. Neues Jb. Miner. Abh. 2010, 187, 235–248. [Google Scholar] [CrossRef]
- Tremblay, J.; Bédard, L.P.; Matton, G. Columbitization of fluorcalciopyrochlore by hydrothermalism at the Saint-Honoré alkaline complex, Québec /Canada): New insights on halite in carbonatites. Ore Geol. Rev. 2017, 91, 695–707. [Google Scholar] [CrossRef]
- Starikova, A.E.; Bazarova, E.P.; Savel´eva, V.B.; Sklyarov, E.V.; Khromova, E.A.; Kanakin, S.V. Pyrochlore-group minerals in the granite-hosted Katugin rare-metal deposit, Transbaikalia, Russia. Minerals 2019, 9, 490. [Google Scholar] [CrossRef]
- Galliski, M.A.; Márquez-Zavalía, M.F.; Roquet, M.B. Los minerales de Nb-Ta-U de la pegmatita María Elena, Sierra de San Luis, Argentina. Revista Asoc. Geol. Argentina 2021, 78, 355–367, (In Spanish with English Abstract). [Google Scholar]
- Uher, P.; Števko, M.; Kurylo, S. Minerals of columbite and microlite groups in granitic pegmatite near Liešťany: The first occurence of rare-element Nb-Ta mineralization in the Stražovské vrchy Mts. (Slovak Republic). Bull. Mineral. Petrolog. 2020, 28, 347–352, (In Slovak with English Abstract). [Google Scholar] [CrossRef]
- Wu, B.; Hu, Y.-Q.; Bonetti, C.; Xu, C.; Wang, R.-C.; Zhang, Z.-S.; Li, Z.-Y.; Yin, R. Hydrothermal alteration of pyrochlore group minerals from the Miaoya carbonatite complex, central China and its implications for Nb mineralization. Ore Geol. Rev. 2021, 132, 104059. [Google Scholar] [CrossRef]
- Sun, Z.; Qin, K.; Mao, Y.; Tang, D.; Wang, F.; Evans, N.J.; Zhou, Q. Mineral chemistry of pyrochlore supergroup minerals from the Boziguoer Nb-Ta-Zr-Rb-REE deposit, NW China: Implications for Nb enrichment by alkaline magma differentiation. Minerals 2022, 12, 785. [Google Scholar] [CrossRef]
- Yin, R.; Sun, X.; Wang, S.; Wu, B. Mineral chemistry of pyrochlore supergroup minerals as records of Nb mineralization processes in NYF-type pegmatites: A case study of the Emeishan large igneous province, SW China. Minerals 2024, 14, 13. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W. Oxide minerals of the Separation Rapids rare-element granitic pegmatite group, Northwestern Ontario. Canad. Mineral. 1998, 36, 609–635. [Google Scholar]
- Llorens, T.; Moro, M.C. Microlite and tantalite in the LCT granitic pegmatites of La Canalita, Navasfrías Sn-W district, Salamanca, Spain. Canad. Mineral. 2010, 48, 375–390. [Google Scholar] [CrossRef]
- Chládek, Š.; Uher, P.; Novák, M.; Bačík, P.; Opletal, T. Microlite-group minerals: Tracers of complex post-magmatic evolution in beryl-columbite granitic pegmatites, Maršíkov district, Bohemian Massif, Czech Republic. Mineral. Mag. 2021, 85, 725–743. [Google Scholar] [CrossRef]
- Ballouard, C.; Elburg, M.A.; Tappe, S.; Reinke, C.; Ueckermann, H.; Doggart, S. Magmatic-hydrothermal evolution of rare metal pegmatites from the mesoproterozoic Orange River pegmatite belt (Namaqualand, South Africa). Ore Geol. Rew. 2020, 116, 103252. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Bukharova, O.V.; Nebera, T.S.; Sveshnikova, V.L. Composition and origin of rare-metal (Nb-Ta, REE) and sulfide mineralization in magnesiocarbonatites from the Yenisei Ridge, Central Siberia. Ore Geol. Rev. 2019, 111, 102949. [Google Scholar] [CrossRef]
- Hadlich, I.W.; Bastos Neto, A.C.; Pereira, V.P.; Dill, H.G.; Botelho, N.F. The Radioactive Rare Metal Mineralization in theWorld-Class Sn-Nb-Ta-U-Th-REE-Deposit Madeira (Pitinga, Amazonas State, Brazil): With Special Reference to the Complex Alteration of Pyrochlore-Group minerals. Minerals 2024, 14, 895. [Google Scholar] [CrossRef]
- Černý, P.; Goad, B.E.; Hawthorne, F.C.; Chapman, R. Fractionation trends of the Nb- and Ta-bearing oxide minerals in the Greer Lake pegmatitic granite and its pegmatite aureole, southeastern Manitoba. Amer. Mineral. 1986, 71, 501–517. [Google Scholar]
- Černý, P.; Chapman, R.; Ferreira, K.; Smeds, S.A. Geochemistry of oxide minerals of Nb, Ta, Sn, and Sb in the Varutrask granitic pegmatite, Sweden: The case of an “anomalous” columbite-tantalite trend. Amer. Mineral. 2004, 89, 505–518. [Google Scholar] [CrossRef]
- Bastos Neto, A.C.; Pereira, V.P.; De Lima, E.F.; Ferron, J.M.; Minuzzi, O.; Prado, M.; Ronchi, L.H.; Flores, J.A.A.; Frantz, J.C.; Pires, A.C.; et al. A jazida de criolita da mina Pitinga (Amazonas). In Caracterizacao de Depósitos Minerais em Distritos Mineiros da Amazónia; Marini, O.J., Queiroz, E.T., Ramos, B.W., Eds.; DNPM-CT/Mineral-ADIMB: Brasília, Brazil, 2005; pp. 481–552. [Google Scholar]
- Bastos Neto, A.C.; Pereira, V.P.; Ronchi, L.H.; Lima, E.F.; Frantz, J.C. The world-class Sn, Nb, Ta, F (Y, REE, Li) deposit and the massive cryolite associated with the albite-enriched facies of the Madeira A-type granite, Pitinga mining district, Amazonas State, Brazil. Canad. Miner. 2009, 47, 1329–1357. [Google Scholar] [CrossRef]
- Bollaert, Q.; Chasse, M.; Bastos Neto, A.; Horbe, A.; Allard, T.; Menguy, N.; Le Guillou, C.; Courtin, A.; Quantin, C.; Vantelon, D.; et al. Hydrothermal niobium (Nb) mineralization in the world-class Madeira Sn-Nb-Ta granitic deposit (Amazonas, Brazil). Ore Geol. Rev. 2024, 174, 106321. [Google Scholar] [CrossRef]
- Lenharo, S.L.; Moura, M.A.; Botelho, N.F. Petrogenetic and mineralization processes in Paleo- to Mesoproterozoic rapakivi granites: Examples from Pitinga and Goiás, Brazil. Precambrian Res. 2002, 119, 277–299. [Google Scholar] [CrossRef]
- Costi, H.T.; Dall’Agnol, R.; Pichavant, M.; Ramo, O.T. The peralkaline tin-mineralized Madeira cryolite albite-rich granite of Pitinga, Amazonian craton, Brazil: Petrography, mineralogy and crystallization processes. Canad. Miner. 2009, 47, 1301–1327. [Google Scholar] [CrossRef]
- Costi, H.T.; Dall’Agnol, R.; Moura, C.A.V. Geology and Pb-Pb geochronology of Paleoproterozoic volcanic and granitic rocks of Pitinga province, Amazonian craton, northern Brazil. Int. Geol. Rev. 2000, 42, 832–849. [Google Scholar] [CrossRef]
- Paludo, C.M.; Bastos Neto, A.C.; Pereira, V.P.; Botelho, N.F. Mineralogia e geoquímica de pegmatitos ricos em ETR, F e metais alcalinos associados a facies albita granito no depósito de Sn-Nb-Ta- (F, ETR, U, Th) Madeira (mina Pitinga, AM, Brasil). Pesqui. Geociências 2018, 45, e0747, (In Portuguese, with English Abstract). [Google Scholar] [CrossRef]
- Badanina, E.V.; Veksler, I.V.; Thomas, R.; Syritso, L.F.; Trumbull, R.B. Magmatic evolution of Li-F rare-metal granites: A case study of melt inclusions in the Khangilay complex, Eastern Transbaikalia (Russia). Chem. Geol. 2004, 210, 113–133. [Google Scholar] [CrossRef]
- Badanina, E.V.; Syritso, L.F.; Volkova, E.V.; Thomas, R.; Trumbull, R.B. Composition of Li-F granite melt and its evolution during the formation of the ore-bearing Orlovka massif in Eastern Transbaikalia (Russia). Petrology 2010, 18, 139–167. [Google Scholar] [CrossRef]
- Breiter, K. Teplice rhyolite (Krušné hory Mts., Czech Republic): Chemical evidence of a multiply exhausted stratified magma chamber. Věst. Čes. Geol. Úst. 1997, 72, 205–213. [Google Scholar]
- Tomek, F.; Žák, J.; Svojtka, M.; Finger, F.; Waitzinger, M. Emplacement dynamics of syn-collapse ring dikes: An example from the Altenberg-Teplice caldera, Bohemian Massif. GSA Bull. 2019, 131, 997–1016. [Google Scholar] [CrossRef]
- Tichomirowa, M.; Käßner, A.; Repstock, A.; Gerdes, A.; Whitehouse, M. New CA-ID-TIMS U–Pb zircon ages for the Altenberg–Teplice Volcanic Complex (ATVC) document discrete and coeval pulses of Variscan magmatic activity in the Eastern Erzgebirge (Eastern Variscan Belt). Int. J. Earth Sci. 2022, 111, 1885–1908. [Google Scholar] [CrossRef]
- Štemprok, M.; Šulcek, Z. Geochemical profile through an ore-bearing lithium granite. Econ. Geol. 1969, 64, 392–404. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Hrstka, T.; Korbelová, Z.; Hložková Vaňková, M.; Vašinová Galiová, M.; Kanický, V.; Rambousek, P.; Knésl, I.; Dobeš, P.; et al. Assessment of magmatic vs, metasomatic processes in rare-metal granites: A case study of the Cínovec-Zinnwald Sn–W–Li deposit, Central Europe. Lithos 2017, 292, 198–217. [Google Scholar] [CrossRef]
- Rub, A.K.; Štemprok, M.; Rub, M.G. Tantalum mineralization in the apical part of the Cínovec (Zinnwald) granite stock. Mineral. Petrol. 1998, 63, 199–222. [Google Scholar] [CrossRef]
- Johan, V.; Johan, Z. Accessory minerals of the Cínovec (Zinnwald) granite cupola, Czech Republic Part 1: Nb-, Ta- and Ti-bearing oxides. Mineral. Petrol. 1994, 51, 323–343. [Google Scholar] [CrossRef]
- Förster, H.-J. Composition and origin of intermediate solid solutions in the system thorite-xenotime-zircon-coffinite. Lithos 2006, 88, 35–55. [Google Scholar] [CrossRef]
- Breiter, K.; Korbelová, Z.; Chládek, Š.; Uher, P.; Knesl, I.; Rambousek, P.; Honig, S.; Šešulka, V. Diversity of Ti–Sn–W–Nb–Ta oxide minerals in the classic granite-related magmatic–hydrothermal Cínovec/Zinnwald Sn–W–Li deposit (Czech Republic). Eur. J. Mineral. 2017, 29, 727–738. [Google Scholar] [CrossRef]
- Cuney, M.; Marignac, C.; Weisbrod, A. The Beauvoir topaz-lepidolite albite granite (Massif Central, France): The disseminated magmatic Sn-Li-Ta-Nb-Be mineralization. Econ. Geol. 1992, 87, 1766–1794. [Google Scholar] [CrossRef]
- Raimbault, L.; Cuney, M.; Azencott, C.; Duthou, J.L.; Joron, J.L. Geochemical evidence for a multistage magmatic genesis of Ta–Sn–Li mineralization in the granite at Beauvoir, French Massif Central. Econ. Geol. 1995, 90, 548–596. [Google Scholar] [CrossRef]
- Monnier, L. Utilisation de la Signature LA-ICPMS des Quartz et des Micas Pour la Reconstitution du Fonctionnement D’un Système Magmatique et Hydrothermal Polyphasé, Application au Complexe Sn-W d’Echassières (Massif Central, France). Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2018; 370p. [Google Scholar]
- Breiter, K.; Müller, A.; Leichmann, J.; Gabašová, A. Textural and chemical evolution of a fractionated granitic sytem: The Podlesí stock, Czech Republic. Lithos 2005, 80, 323–345. [Google Scholar] [CrossRef]
- Breiter, K.; Škoda, R.; Uher, P. Nb-Ta-Ti-W-Sn-oxide minerals as indicator of a peraluminous P- and F-rich granitic systém evolution: Podlesí, Czech Republic. Mineral. Petrol. 2007, 91, 225–248. [Google Scholar] [CrossRef]
- Chakmouradian, A.R.; Mitchell, R.H. New data on pyrochlore- and perovskite-group minerals from the Lovozero alkaline complex, Russia. Eur. J. Mineral. 2002, 14, 821–836. [Google Scholar] [CrossRef]
- Dumaňska-Slowik, M.; Pieczka, A.; Tempesta, G.; Olejniczak, Z.; Heflik, W. “Silicified” pyrochlore from nepheline syenite (mariupolite) of the Mariupol Massif, SE Ukraine: A new insight into the role of silicon in the pyrochlore structure. Amer. Mineral. 2014, 99, 2008–2014. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Sobolev, N.V.; Channer, D.M.D. Oscillatory-zoned crystals of pyrochlore-group minerals from the Guaniamo kimberlites, Venezuela. Lithos 2009, 112S, 976–985. [Google Scholar] [CrossRef]
- Pieczka, A. Primary Nb-Ta minerals in the Szklary pegmatite, Poland: New insights into controls of crystal chemistry and crystallization sequences. Amer. Mineral. 2010, 95, 1478–1492. [Google Scholar] [CrossRef]
- Chudík, P.; Uher, P. Pyrochlore group minerals from the granitic pegmatites of the Western Carpathians: Compositional variation and substitution mechanisms. Mineralia Slovaca 2009, 41, 159–198, (In Slovak with English Abstract). [Google Scholar]
- Bindi, L.; Zoppi, M.; Bonazzi, P. Plumbomicrolite from the Ploskaya Mountain, Keivy massif, Kola peninsula, Russia: Composition and crystal structure. Period. Mineral. 2006, 75, 51–58. [Google Scholar]
- Gladkochub, D.P.; Donskaya, T.V.; Sklayrov, E.V.; Kotov, A.B. and 15 others. The unique Katugin rare-metal deposit (southern Siberia): Constraints on age and genesis. Ore Geol. Rev. 2017, 91, 246–263. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Hrstka, T.; Gottlieb, P.; Skála, R.; Breiter, K.; Motl, D. Automated mineralogy and petrology—applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar] [CrossRef]
- Ritchie, N.W. Spectrum Simulation in DTSA-II. Microsc. Microanal. 2009, 15, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V.; Pasero, M.; Aksenov, M.P.; Britvin, S.N.; Zubkova, N.V.; Yike, L.; Witzke, T. Columbite supergroup of minerals: Nomenclature and classification. Mineral. Mag. 2023, 87, 18–33. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Dey, M.; Chakrabarty, A.; Mitchell, R.H.; Ren, R. Zero-valent-dominant pyrochlores: Endmember formula calculation and petrogenetic significance. Canad. Mineral. 2022, 60, 469–484. [Google Scholar] [CrossRef]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Ercit, T.S.; Černý, P.; Hawthorne, F.C. Cesstibtantite—A geological introduction to the inverse pyrochlores. Mineral. Petrol. 1993, 48, 235–255. [Google Scholar] [CrossRef]
- Kasatkin, A.V.; Britvin, S.N.; Peretyazhko, I.S.; Chukanov, N.V.; Škoda, R. Oxybismutomicrolite, a new pyrochlore-supergroup mineral from the Malkhan pegmatite field, Central Trabsbaikalia, Russia. Mineral. Mag. 2020, 84, 444–454. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Hreus, S.; Výravský, J.; Cempírek, J.; Breiter, K.; Vašinová Galiová, M.; Krátký, O.; Šešulka, V.; Škoda, R. Scandium distribution in the world-class Li-Sn-W Cínovec greisen-type deposit: Result of a complex magmatic to hydrothermal evolution, implications for scandium valorization. Ore Geol. Rev. 2021, 139, 104433. [Google Scholar] [CrossRef]
- McNeil, A.G.; Linnen, R.L.; Flemming, R.L. Solubility of wodginite, microlite, pyrochlore, columbite-(Mn) and tantalite-(Mn) in flux-rich haplogranitic melts between 700° and 850 °C and 200 MPa. Lithos 2020, 352–353, 105239. [Google Scholar] [CrossRef]
- Černý, P.; Meintzner, R.E.; Anderson, A.J. Extreme fractionation in rare-element granitic pegmatites: Selected examples of data and mechanisms. Canad. Miner. 1985, 23, 381–421. [Google Scholar]
- Novák, M.; Černý, P. Scandium in columbite-group minerals from LCT pegmatites in the Moldanubicum, Czech Republic. Krystalinikum 1998, 24, 73–89. [Google Scholar]
- Atencio, D.; Andrade, M.B.; Bindi, L.; Bonazzi, P.; Zoppi, M.; Stanley, C.J.; Kristiansen, R. Kenoplumbomicrolite, (Pb,□)2Ta2O6[□, (OH), O], a new mineral from Ploskaya, Kola Peninsula, Russia. Mineral. Mag. 2018, 82, 1049–1055. [Google Scholar] [CrossRef]
- Kartashov, P.M.; Voloshin, A.V.; Pakhomovskiy, Y.A.; Kovaleno, V.I. Plumbopyrochlore from western Mongolia. Doklady Earth Sci. Sect. 1994, 323, 142–146. [Google Scholar]
- Timofeev, A.; Williams-Jones, A.E. The origin of niobium and tantalum mineralization in the Nechalacho REE deposit, NWT Canada. Econ. Geol. 2015, 110, 1719–1735. [Google Scholar] [CrossRef]
- Llorens Gonzáles, T.; García Polonio, F.; López Moro, F.J.; Fernández Fernández, A.; Sanz Contreras, M.C.; Moro Benito, M.C. Tin-tantálum-niobium mineralization in the Penouta deposit (NW Spain): Textural features and mineral chemistry to unravel the genesis and evolution of cassiterite and columbite group minerals in a peraluminous system. Ore Geol. Rev. 2017, 81, 79–95. [Google Scholar] [CrossRef]
- Linnen, R.L. The solubility of Nb-Ta-Zr-Hf-W in granitic melt with Li and Li+F: Constraints for mineralization in rare metal granites and pegmatites. Econ. Geol. 1998, 93, 1013–1025. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Holtz, F.; Hanchar, J.M. Solubility of manganotantalite, zircon and hafnon in highly fluxed peralkaline to peraluminous pegmatitic melts. Contrib. Mineral. Petrol. 2010, 160, 17–32. [Google Scholar] [CrossRef]
- Aseri, A.A.; Linnen, R.L.; Che, X.D.; Thibault, Y.; Holtz, F. Effect of fluorine on the colubilities of Nb, Ta, Zr and Hf minerals in highly fluxed water-saturated haplogranitic melts. Ore Geol. Rew. 2015, 64, 736–746. [Google Scholar] [CrossRef]
- Fiege, A.; Kirchner, C.; Holtz, F.; Linnen, R.L.; Dziony, W. Influence of fluorine on the solubility of manganotantalite (MnTa2O6) and manganocolumbite (MnNb2O6) in granitic melts—An experimental study. Lithos 2011, 122, 165–174. [Google Scholar] [CrossRef]
- Ballouard, C.; Massuyaeu, M.; Elburg, M.A.; Tappe, S.; Viljoen, F.; Brandenburg, J.T. The magmatic and magmatic-hydrothermal evolution of felsic igneous rocks as seen through Nb-Ta geochemical fractionation, with implications for the origin of rare-metal mineralization. Earth-Sci. Rev. 2020, 203, 103115. [Google Scholar] [CrossRef]

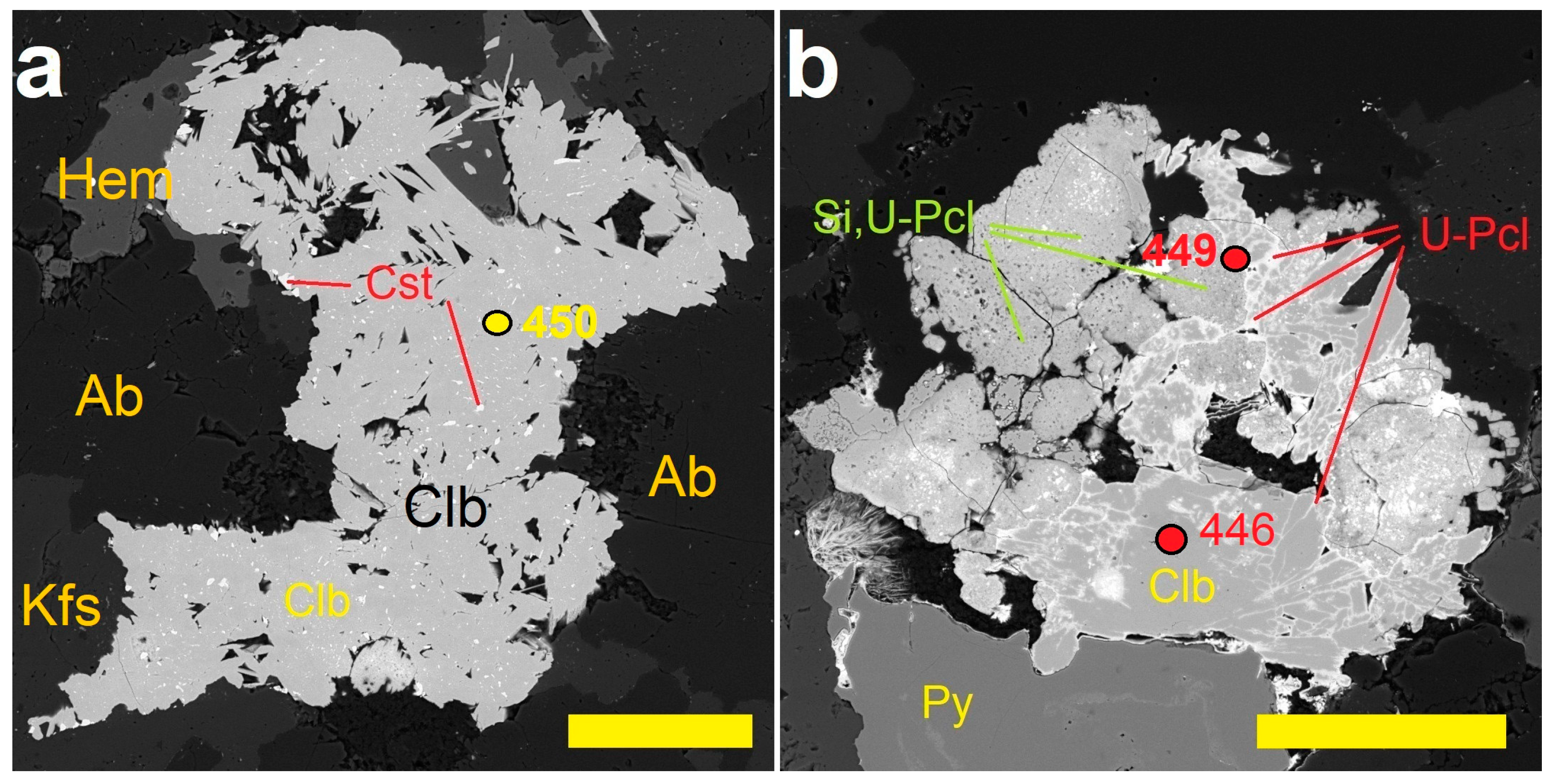
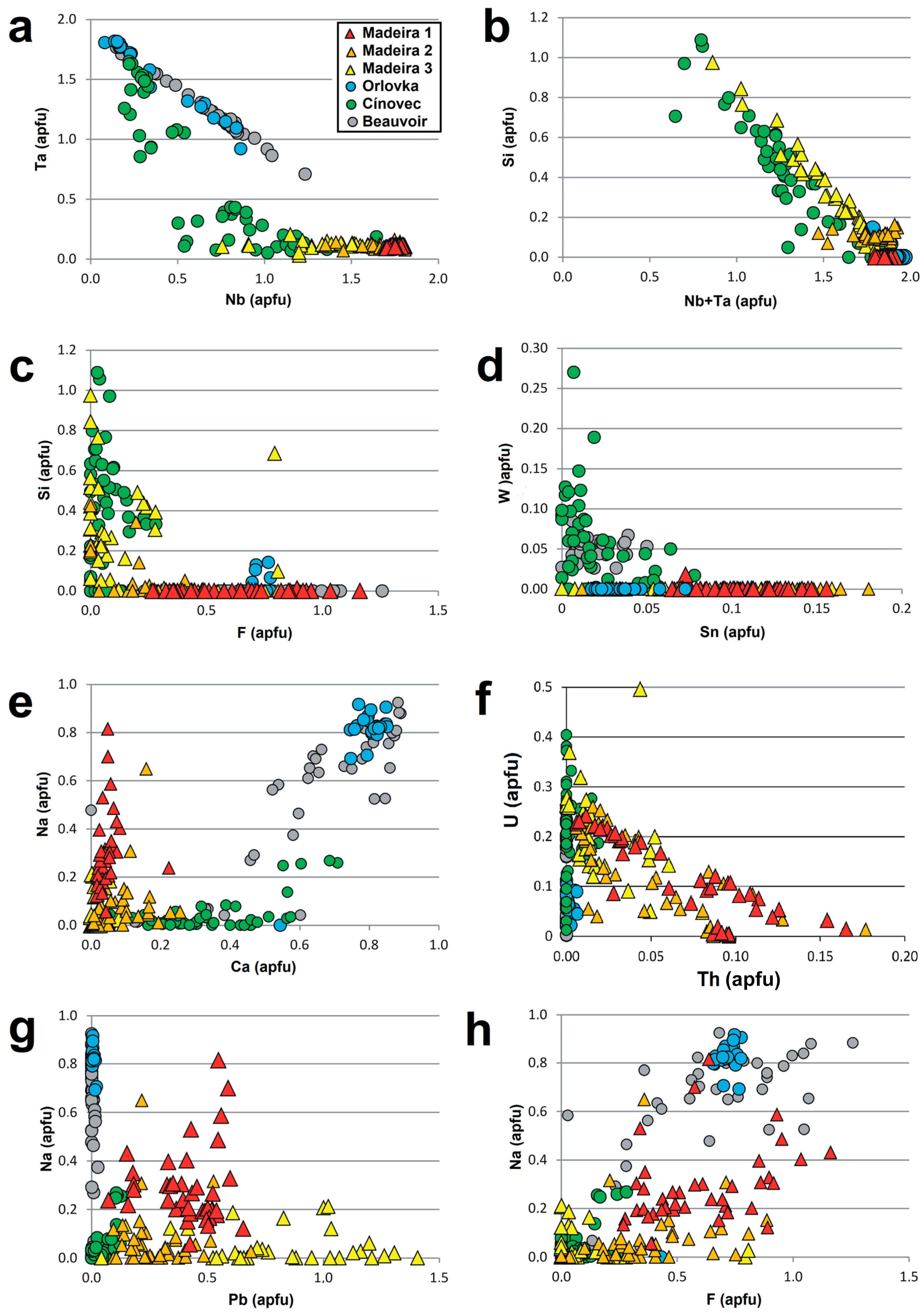
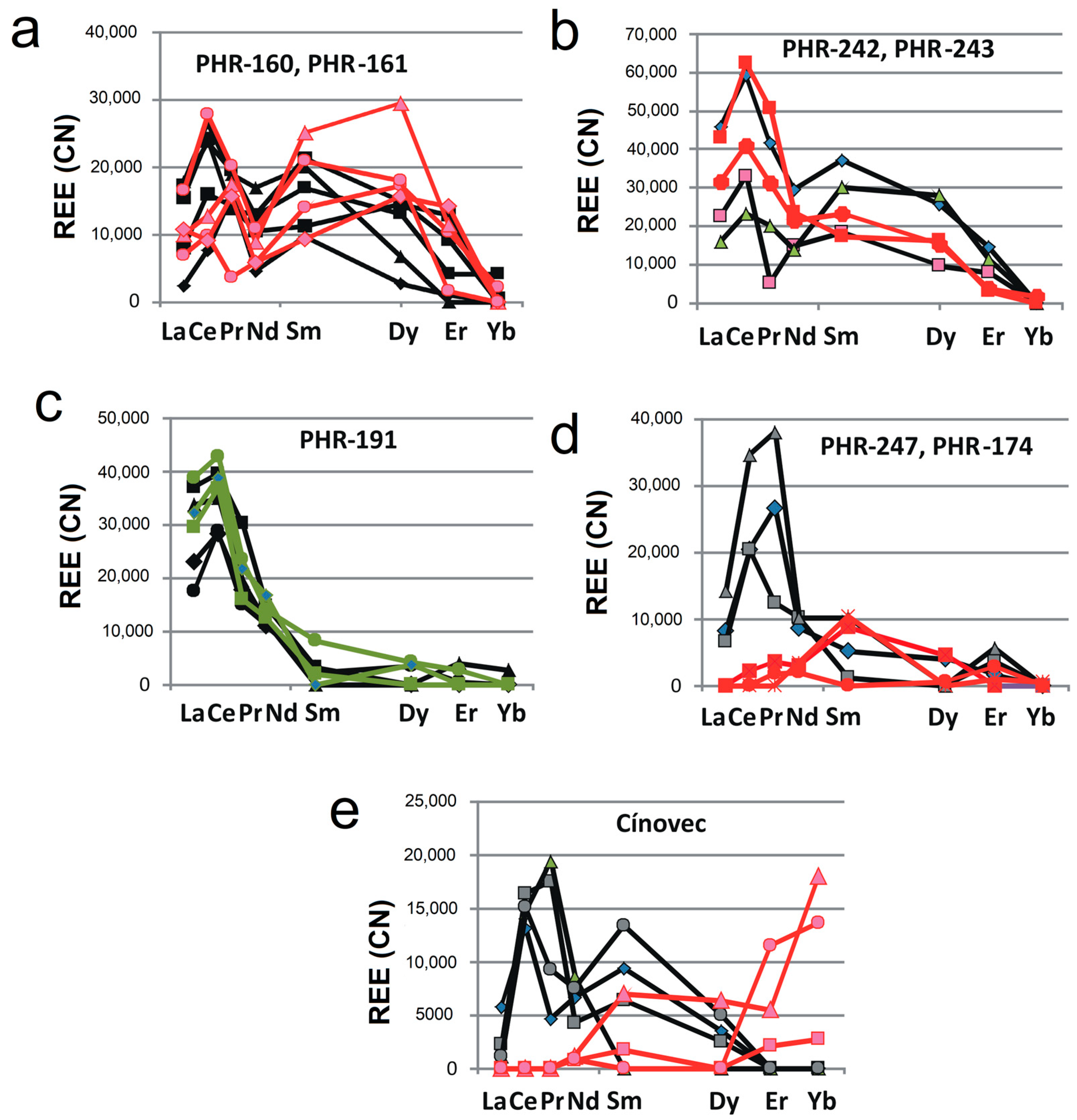
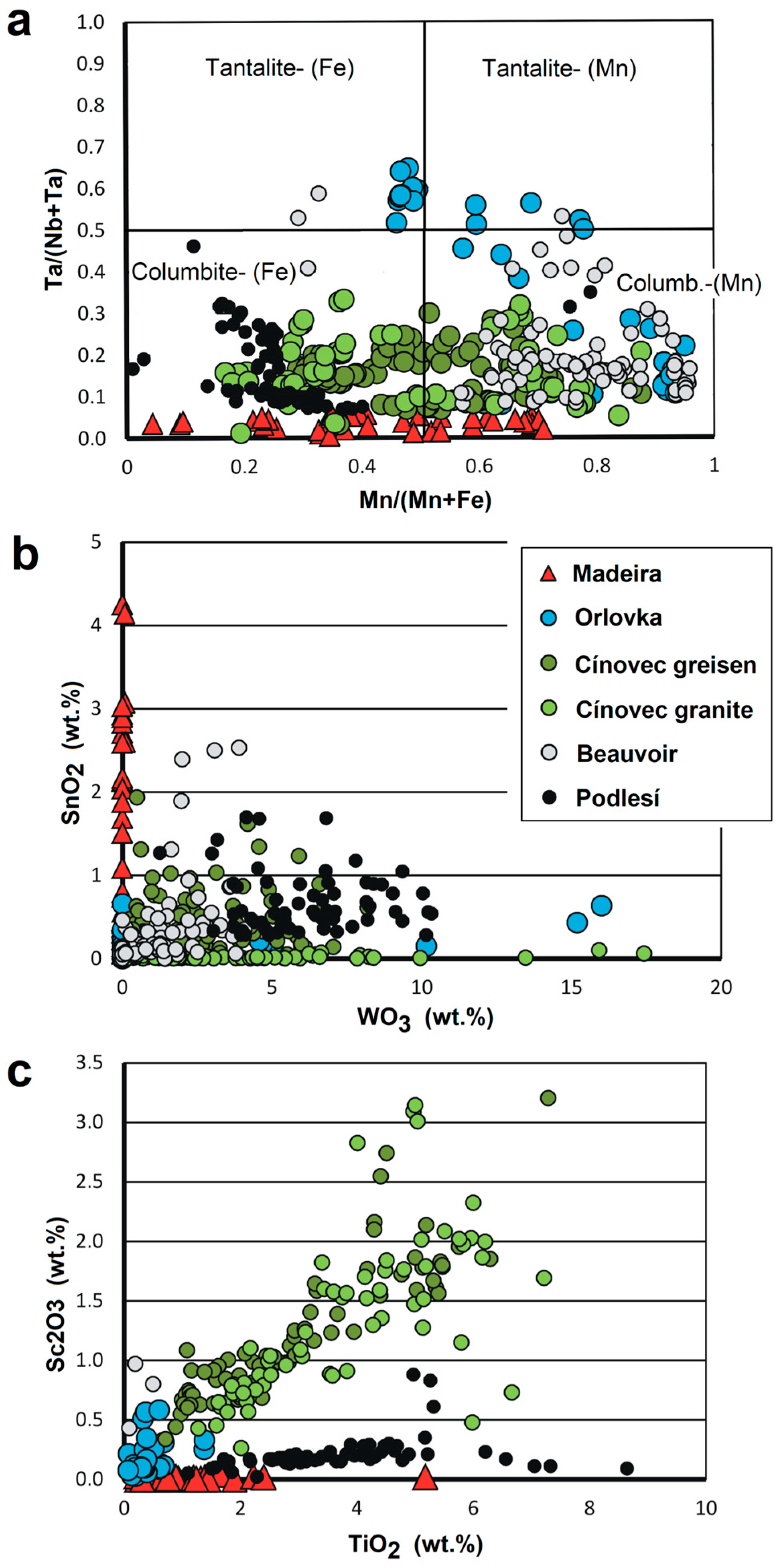

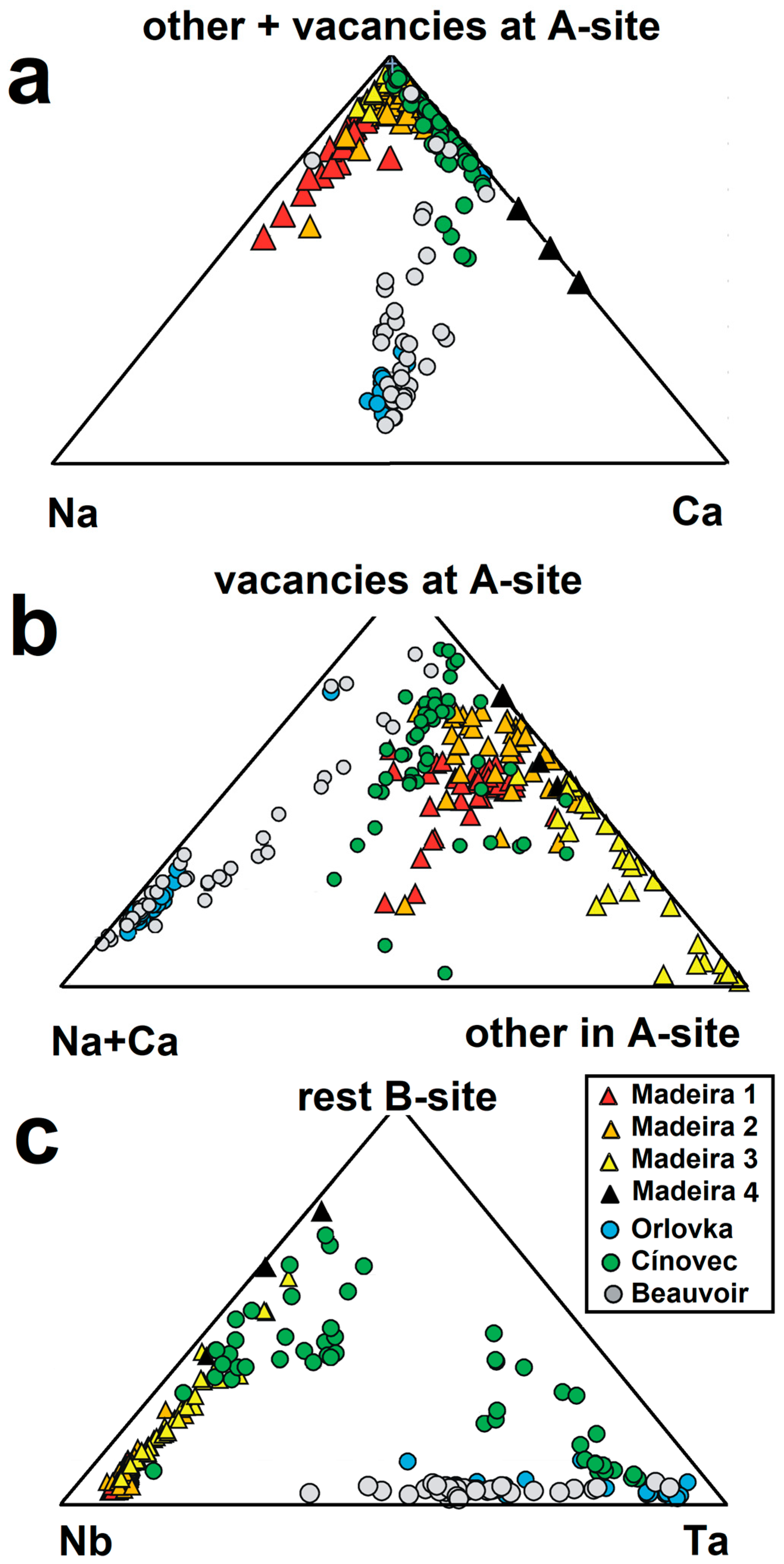
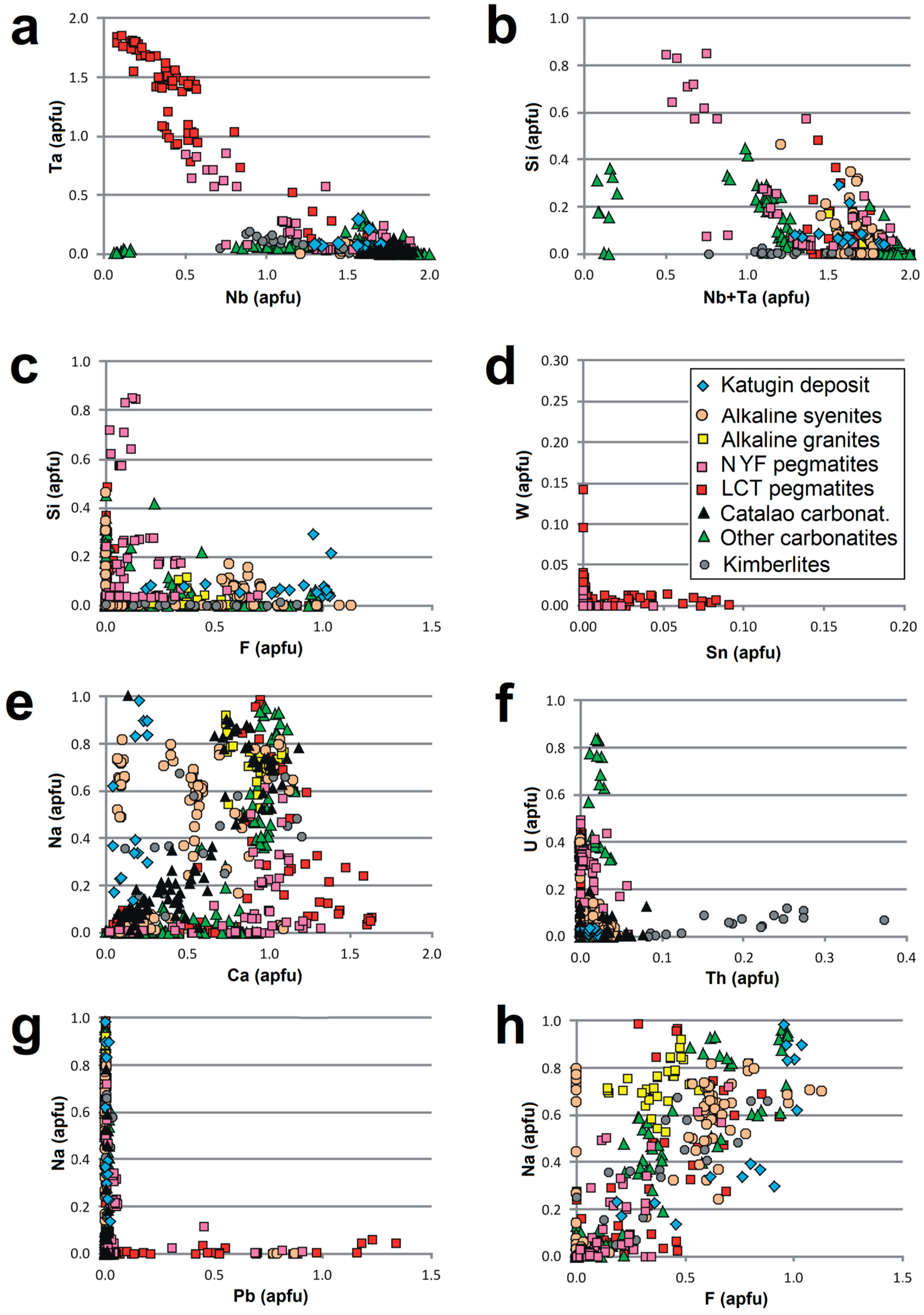
| Sample | PHR160 | PHR160 | PHR163 | PHR163 | PHR128 | PHR171 | PHR245 | PHR191 | PHR191 | PHR247 | PHR174 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | 3 | 3 | 3 | 3 | 3 | 5 | 6 | 1 | 1 | 2 | 5 |
| Spot | 20 | 455 | 63 | 64 | 138 | 479 | 124 | 30 | 31 | 492 | 449 |
| Type | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 |
| Nb2O5 | 44.23 | 46.49 | 45.95 | 48.21 | 47.84 | 49.06 | 53.45 | 35.53 | 29.7 | 31.72 | 33.77 |
| Ta2O5 | 4.31 | 4.12 | 5.15 | 4.71 | 5.38 | 5.22 | 5.48 | 4.26 | 6.77 | 3.88 | 4.28 |
| As2O5 | b.d.l. | n.a. | 0.04 | b.d.l. | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | n.a. |
| ZrO2 | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | 0.44 | 0.76 | 0.64 | n.a. |
| SnO2 | 3.51 | 3.81 | 3.38 | 4.18 | 3.45 | 2.68 | 3.52 | 1.82 | 0.28 | 0.42 | b.d.l. |
| TiO2 | 0.70 | 0.54 | 0.50 | 0.33 | 0.35 | 0.33 | 0.23 | 0.71 | 1.78 | b.d.l. | 1.11 |
| MgO | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | 0.01 | 0.01 | n.a. | n.a. |
| Al2O3 | b.d.l. | n.a. | b.d.l. | b.d.l. | 0.01 | n.a. | b.d.l. | 0.13 | 0.97 | n.a. | n.a. |
| SiO2 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 2.56 | 11.31 | 1.76 | 7.36 |
| Na2O | 0.99 | 2.15 | 1.80 | 1.98 | b.d.l. | 0.48 | 1.67 | 1.03 | 0.99 | 0.98 | b.d.l. |
| CaO | 0.18 | 0.17 | 0.28 | 0.33 | 0.57 | 0.67 | 2.84 | 0.52 | 0.21 | b.d.l. | 0.01 |
| ThO2 | 0.59 | 1.92 | 1.39 | 5.32 | 2.88 | 3.50 | 9.89 | 0.46 | 0.12 | 0.05 | 9.48 |
| UO2 | 12.55 | 10.50 | 10.89 | 5.98 | 6.55 | 3.64 | 0.86 | 11.96 | 24.51 | 10.86 | 22.75 |
| Bi2O3 | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | n.a. |
| La2O3 | n.a. | 0.46 | n.a. | n.a. | n.a. | 0.50 | n.a. | n.a. | n.a. | 0.22 | b.d.l. |
| Ce2O3 | 0.97 | 1.91 | 0.85 | 1.43 | 1.74 | 2.09 | 1.42 | 2.42 | 1.18 | 1.46 | b.d.l. |
| Pr2O3 | n.a. | 0.21 | n.a. | n.a. | n.a. | 0.19 | n.a. | n.a. | n.a. | 0.29 | 0.02 |
| Nd2O3 | n.a. | 0.90 | n.a. | n.a. | n.a. | 0.80 | n.a. | n.a. | n.a. | 0.46 | 0.11 |
| Sm2O3 | n.a. | 0.34 | n.a. | n.a. | n.a. | 0.34 | n.a. | n.a. | n.a. | 0.09 | b.d.l. |
| Dy2O3 | n.a. | 0.19 | n.a. | n.a. | n.a. | 0.69 | n.a. | n.a. | n.a. | 0.11 | 0.02 |
| Er2O3 | n.a. | b.d.l. | n.a. | n.a. | n.a. | 0.10 | n.a. | n.a. | n.a. | 0.03 | 0.05 |
| Yb2O3 | n.a. | b.d.l. | n.a. | n.a. | n.a. | 0.10 | n.a. | n.a. | n.a. | b.d.l. | b.d.l. |
| Y2O3 | 0.38 | 0.41 | 0.49 | 1.29 | 1.01 | 0.98 | 1.37 | 0.04 | 0.05 | 0.19 | 0.07 |
| Sc2O3 | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | n.a. |
| PbO | 21.79 | 17.85 | 20.2 | 16.29 | 17.28 | 15.37 | 3.67 | 24.24 | 8.06 | 35.32 | 2.48 |
| BaO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.03 | b.d.l. | 0.75 | 0.36 | b.d.l. | b.d.l. |
| SrO | b.d.l. | n.a. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | 0.26 | 0.08 | n.a. | n.a. |
| MnO | 0.16 | 0.17 | 0.15 | 0.04 | 0.18 | 0.39 | 0.13 | 0.29 | 0.16 | 0.02 | 3.13 |
| FeO | 0.23 | 0.74 | 0.20 | 0.26 | 0.98 | 0.33 | 0.34 | 4.60 | 4.32 | 4.61 | 2.72 |
| ZnO | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. | 0.05 | 0.07 | b.d.l. | b.d.l. |
| F | 1.37 | 0.51 | 2.79 | 3.40 | 0.71 | b.d.l. | 2.79 | 0.11 | 0.15 | b.d.l. | b.d.l. |
| Feq. | −0.58 | −0.24 | −1.17 | −1.43 | −0.30 | −1.17 | −0.05 | −0.06 | 0.00 | ||
| Total | 91.42 | 93.76 | 92.89 | 92.32 | 88.63 | 87.50 | 86.49 | 92.14 | 91.78 | 93.09 | 87.37 |
| Nb | 1.732 | 1.747 | 1.735 | 1.744 | 1.748 | 1.780 | 1.775 | 1.5 | 0.908 | 1.627 | 1.240 |
| Ta | 0.102 | 0.093 | 0.117 | 0.103 | 0.118 | 0.014 | 0.109 | 0.108 | 0.125 | 0.120 | 0.095 |
| As | 0.002 | ||||||||||
| Zr | 0.020 | 0.025 | 0.035 | ||||||||
| Sn | 0.121 | 0.126 | 0.113 | 0.133 | 0.111 | 0.086 | 0.103 | 0.068 | 0.008 | 0.019 | |
| Ti | 0.046 | 0.034 | 0.031 | 0.02 | 0.021 | 0.020 | 0.012 | 0.050 | 0.091 | 0.068 | |
| Mg | 0.001 | 0.001 | |||||||||
| Al | 0.001 | 0.014 | 0.077 | ||||||||
| Si | 0.239 | 0.765 | 0.199 | 0.548 | |||||||
| Sum B | 2.001 | 2.000 | 1.998 | 2.000 | 1.999 | 2.000 | 1.999 | 2.000 | 2.000 | 2.000 | 2.000 |
| Na | 0.166 | 0.346 | 0.292 | 0.307 | 0.075 | 0.238 | 0.186 | 0.130 | 0.215 | ||
| Ca | 0.017 | 0.015 | 0.025 | 0.029 | 0.049 | 0.057 | 0.224 | 0.052 | 0.016 | 0.001 | |
| Th | 0.012 | 0.036 | 0.026 | 0.097 | 0.053 | 0.064 | 0.165 | 0.010 | 0.002 | 0.001 | 0.175 |
| U | 0.242 | 0.194 | 0.202 | 0.106 | 0.118 | 0.065 | 0.014 | 0.248 | 0.369 | 0.274 | 0.411 |
| Bi | |||||||||||
| La | 0.014 | 0.015 | 0.009 | ||||||||
| Ce | 0.031 | 0.058 | 0.026 | 0.042 | 0.051 | 0.061 | 0.038 | 0.083 | 0.029 | 0.061 | |
| Pr | 0.006 | 0.006 | 0.012 | ||||||||
| Nd | 0.027 | 0.023 | 0.019 | ||||||||
| Sm | 0.010 | 0.009 | 0.004 | ||||||||
| Dy | 0.005 | 0.018 | 0.004 | ||||||||
| Er | 0.003 | 0.001 | |||||||||
| Yb | 0.003 | ||||||||||
| Y | 0.018 | 0.018 | 0.022 | 0.055 | 0.043 | 0.042 | 0.054 | 0.002 | 0.002 | 0.012 | 0.003 |
| Sc | |||||||||||
| Pb | 0.508 | 0.399 | 0.454 | 0.351 | 0.376 | 0.332 | 0.073 | 0.609 | 0.147 | 1.079 | 0.054 |
| Ba | 0.001 | 0.027 | 0.01 | ||||||||
| Sr | 0.014 | 0.003 | |||||||||
| Mn | 0.012 | 0.012 | 0.011 | 0.003 | 0.012 | 0.027 | 0.008 | 0.023 | 0.009 | 0.002 | 0.215 |
| FeO | 0.017 | 0.052 | 0.014 | 0.018 | 0.066 | 0.022 | 0.021 | 0.359 | 0.244 | 0.437 | 0.185 |
| ZnO | 0.003 | 0.004 | 0.004 | ||||||||
| Vacancy A | 2.974 | 0.869 | 0.928 | 0.992 | 1.232 | 1.253 | 1.165 | 0.383 | 1.035 | 0.000 | 0.955 |
| Sum A | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.081 | 2.000 |
| F | 0.375 | 0.133 | 0.737 | 0.861 | 0.182 | 0.648 | 0.032 | 0.031 | |||
| O | 6.134 | 6.147 | 6.102 | 6.029 | 5.920 | 5.837 | 5.883 | 6.620 | 5.762 | 7.158 | 6.300 |
| Nb + Ta | 1.833 | 1.840 | 1.852 | 1.847 | 1.866 | 1.894 | 1.884 | 1.608 | 1.033 | 1.747 | 1.334 |
| Mn/(Fe + Mn) | 0.411 | 0.191 | 0.438 | 0.142 | 0.158 | 0.551 | 0.280 | 0.059 | 0.036 | 0.005 | 0.538 |
| Ta/(Nb + Ta) | 0.055 | 0.051 | 0.063 | 0.056 | 0.063 | 0.060 | 0.058 | 0.067 | 0.121 | 0.069 | 0.071 |
| Sample | PHR128 | PHR128 | PHR159 | PHR159 | PHR160 | PHR163 | PHR191 | PHR242 | PHR245 | PHR245 | PHR247 | PHR174 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | 3 | 1 | 1 | 1 | 1 | 3 | 1 | 7 | 1 | 1 | 2 | 6 |
| Spot | 139 | 144 | 44 | 45 | 10 | 62 | 27 | 156 | 120 | 122 | 37 | 446 |
| WO3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.52 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Nb2O5 | 73.47 | 70.28 | 76.62 | 71.68 | 73.71 | 73.63 | 61.06 | 75.37 | 67.83 | 67.05 | 66.96 | 70.89 |
| Ta2O5 | 4.39 | 4.56 | 1.83 | 6.46 | 4.74 | 5.94 | 5.16 | 0.62 | 6.19 | 6.51 | 5.16 | 6.99 |
| As2O5 | b.d.l. | b.d.l. | b.d.l. | 0.07 | 0.04 | 0.03 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | n.a. |
| SnO2 | 0.46 | 2.13 | 0.23 | 0.43 | 0.47 | 0.15 | 1.69 | 0.56 | 2.17 | 2.04 | 2.59 | 0.13 |
| TiO2 | 1.32 | 0.48 | 0.80 | 0.39 | 0.22 | 0.34 | 5.18 | 1.65 | 1.38 | 1.39 | 2.22 | 1.12 |
| Al2O3 | 0.03 | 0.01 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.12 | b.d.l. | 0.02 | 0.04 | 0.05 | n.a. |
| SiO2 | b.d.l. | 0.28 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.20 | 0.01 | 0.15 | 0.22 | 0.10 | b.d.l |
| CaO | 0.01 | b.d.l. | b.d.l. | 0.02 | 0.05 | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.05 | b.d.l. | b.d.l. |
| ThO2 | 0.03 | 0.08 | b.d.l. | 0.07 | 0.42 | 0.18 | b.d.l. | b.d.l. | 0.10 | 0.07 | 0.01 | 0.03 |
| UO2 | b.d.l. | 0.25 | 0.05 | 0.09 | b.d.l. | 0.04 | 2.01 | 0.05 | 0.19 | 0.77 | 1.03 | b.d.l. |
| Y2O3 | b.d.l. | b.d.l. | 0.02 | b.d.l. | 0.12 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.02 | 0.02 |
| Sc2O3 | b.d.l. | 0.01 | 0.01 | 0.01 | 0.03 | b.d.l. | 0.02 | 0.04 | 0.01 | b.d.l. | 0.03 | n.a. |
| PbO | b.d.l. | 0.30 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.18 | 0.02 | 0.34 | 0.41 | 0.16 | b.d.l. |
| BaO | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.04 | 0.02 | 0.01 | 0.02 | b.d.l. | b.d.l. | b.d.l. |
| MnO | 0.90 | 9.82 | 10.66 | 13.79 | 13.73 | 11.91 | 12.23 | 7.14 | 7.95 | 7.04 | 4.88 | 11.05 |
| FeO | 19.53 | 11.17 | 10.03 | 6.27 | 6.63 | 8.41 | 10.71 | 13.68 | 13.02 | 13.48 | 15.53 | 9.40 |
| ZnO | 0.12 | 0.46 | 0.44 | 0.26 | 0.17 | 0.30 | 0.14 | 0.14 | 0.11 | 0.11 | 0.15 | b.d.l. |
| F | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.45 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Feq. | −0.19 | |||||||||||
| Total | 100.25 | 99.83 | 100.71 | 99.54 | 100.31 | 101.52 | 98.74 | 99.81 | 99.48 | 99.18 | 98.87 | 99.78 |
| W | 0.008 | |||||||||||
| Nb | 1.887 | 1.837 | 1.942 | 1.876 | 1.905 | 1.894 | 1.613 | 1.917 | 1.787 | 1.778 | 1.769 | 1.850 |
| Ta | 0.068 | 0.072 | 0.028 | 0.102 | 0.074 | 0.092 | 0.082 | 0.009 | 0.098 | 0.104 | 0.082 | 0.110 |
| As | 0.002 | 0.001 | 0.001 | |||||||||
| Sn | 0.010 | 0.049 | 0.005 | 0.010 | 0.011 | 0.003 | 0.039 | 0.013 | 0.050 | 0.048 | 0.060 | 0.003 |
| Ti | 0.056 | 0.021 | 0.034 | 0.017 | 0.009 | 0.015 | 0.228 | 0.070 | 0.060 | 0.061 | 0.098 | 0.049 |
| Al | 0.002 | 0.001 | 0.008 | 0.002 | 0.003 | 0.003 | ||||||
| Si | 0.016 | 0.011 | 0.001 | 0.009 | 0.013 | 0.006 | ||||||
| Ca | 0.001 | 0.003 | 0.001 | 0.003 | ||||||||
| Th | 0.001 | 0.001 | 0.005 | 0.002 | 0.001 | 0.001 | ||||||
| U | 0.003 | 0.001 | 0.001 | 0.026 | 0.001 | 0.002 | 0.010 | 0.013 | ||||
| Y | 0.001 | 0.004 | 0.001 | 0.001 | 0.001 | |||||||
| Sc | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | |||||||
| Pb | 0.005 | 0.005 | 0.003 | 0.005 | 0.006 | 0.002 | ||||||
| Ba | 0.001 | |||||||||||
| Mn | 0.043 | 0.481 | 0.506 | 0.676 | 0.665 | 0.574 | 0.605 | 0.340 | 0.392 | 0.350 | 0.242 | 0.540 |
| FeO | 0.928 | 0.540 | 0.470 | 0.304 | 0.317 | 0.400 | 0.523 | 0.644 | 0.635 | 0.661 | 0.759 | 0.454 |
| ZnO | 0.005 | 0.020 | 0.018 | 0.011 | 0.007 | 0.012 | 0.006 | 0.006 | 0.005 | 0.005 | 0.006 | |
| F | 0.081 | |||||||||||
| Ta/(Nb + Ta) | 0.035 | 0.038 | 0.014 | 0.051 | 0.037 | 0.046 | 0.048 | 0.005 | 0.052 | 0.055 | 0.044 | 0.056 |
| Mn/(Fe + Mn) | 0.044 | 0.471 | 0.518 | 0.690 | 0.677 | 0.589 | 0.536 | 0.346 | 0.382 | 0.346 | 0.241 | 0.544 |
| Locality | Orlovka | Cínovec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | O253 | O369 | O369 | O369 | 5423 | 5423 | 5427 | 5436 | 4689 | 4690B |
| Crystal | 3 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 6 | 1 |
| Spot | 204 | 185 | 186 | 194 | 413 | 220 | 4 | 13 | 406 | 7 |
| WO3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 8.39 | 4.02 | 2.58 | 4.28 |
| Nb2O5 | 4.39 | 17.69 | 22.85 | 19.92 | 8.00 | 7.89 | 14.02 | 23.62 | 33.37 | 36.88 |
| Ta2O5 | 72.44 | 58.28 | 49.65 | 55.12 | 59.16 | 61.93 | 6.19 | 14.72 | 7.10 | 6.23 |
| As2O5 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | 0.32 | 0.59 | n.a. | b.d.l. |
| SnO2 | 1.30 | 1.31 | 1.78 | 0.75 | 0.96 | 1.33 | 0.55 | 0.39 | b.d.l. | 0.10 |
| TiO2 | 0.06 | 0.07 | 0.05 | b.d.l. | 1.84 | 1.57 | 0.93 | 1.22 | 2.22 | 3.16 |
| MgO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | n.a. | b.d.l. | 0.07 | b.d.l. | n.a. | 0.04 |
| Al2O3 | 0.02 | b.d.l. | 0.04 | 0.28 | n.a. | 0.10 | 0.24 | 0.47 | n.a. | 1.04 |
| SiO2 | b.d.l. | b.d.l. | b.d.l. | 0.83 | b.d.l. | b.d.l. | 11.13 | 5.91 | 5.39 | 8.13 |
| Na2O | 4.58 | 5.26 | 5.18 | 5.37 | 0.07 | 0.03 | 0.09 | 0.35 | 0.12 | n.a. |
| CaO | 8.39 | 9.68 | 8.72 | 9.82 | 1.45 | 2.31 | 3.26 | 2.71 | 3.35 | 6.31 |
| ThO2 | b.d.l. | 0.06 | 0.27 | 0.15 | 0.55 | 0.58 | b.d.l. | b.d.l. | 0.08 | b.d.l. |
| UO2 | 5.31 | 2.86 | 5.10 | 1.25 | 11.39 | 10.85 | 38.21 | 3.71 | 23.14 | 12.60 |
| Bi2O3 | b.d.l. | b.d.l. | b.d.l. | 0.01 | n.a. | 0.14 | b.d.l. | 19.16 | n.a. | b.d.l. |
| La2O3 | n.a. | n.a. | n.a. | n.a. | 0.03 | n.a. | n.a. | n.a. | b.d.l. | n.a. |
| Ce2O3 | b.d.l. | b.d.l. | 0.19 | 0.12 | 1.05 | 1.41 | n.a. | n.a. | b.d.l. | n.a. |
| Pr2O3 | n.a. | n.a. | n.a. | n.a. | 0.21 | n.a. | n.a. | n.a. | b.d.l. | n.a. |
| Nd2O3 | n.a. | n.a. | n.a. | n.a. | 0.46 | n.a. | n.a. | n.a. | 0.05 | n.a. |
| Sm2O3 | n.a. | n.a. | n.a. | n.a. | b.d.l. | n.a. | n.a. | n.a. | b.d.l. | n.a. |
| Dy2O3 | n.a. | n.a. | n.a. | n.a. | b.d.l. | n.a. | n.a. | n.a. | b.d.l. | n.a. |
| Er2O3 | n.a. | n.a. | n.a. | n.a. | b.d.l. | n.a. | n.a. | n.a. | 0.21 | n.a. |
| Yb2O3 | n.a. | n.a. | n.a. | n.a. | b.d.l. | n.a. | n.a. | n.a. | 0.25 | n.a. |
| Y2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.32 | b.d.l. |
| Sc2O3 | 0.05 | 0.02 | 0.02 | 0.03 | n.a. | 0.03 | 0.15 | 0.01 | n.a. | 0.03 |
| PbO | 0.14 | 0.49 | 0.72 | 0.43 | 2.79 | 2.13 | 0.49 | 5.46 | b.d.l. | 4.59 |
| BaO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.22 | 0.03 | 0.62 | 2.57 | 1.06 | 5.53 |
| MnO | 0.01 | 0.10 | 0.04 | 0.02 | 0.19 | 0.06 | 0.15 | 0.15 | 4.15 | 0.69 |
| FeO | 0.05 | b.d.l. | b.d.l. | 0.03 | 0.71 | 0.79 | 0.88 | 2.97 | 3.75 | 0.68 |
| F | 2.31 | 2.93 | 2.59 | 3.12 | b.d.l. | b.d.l. | 0.30 | b.d.l. | b.d.l. | 0.32 |
| Feq. | −0.97 | −1.23 | −1.09 | −1.31 | −0.13 | −0.14 | ||||
| Total | 98.08 | 97.52 | 96.21 | 95.94 | 89.09 | 91.17 | 85.84 | 88.04 | 87.13 | 90.44 |
| W | 0.189 | 0.087 | 0.054 | 0.085 | ||||||
| Nb | 0.178 | 0.655 | 0.837 | 0.708 | 0.337 | 0.321 | 0.552 | 0.894 | 1.219 | 1.360 |
| Ta | 1.769 | 1.298 | 1.094 | 1.178 | 1.499 | 1.515 | 0.147 | 0.335 | 0.156 | 0.081 |
| As | 0.014 | 0.026 | 0.012 | |||||||
| Sn | 0.047 | 0.043 | 0.058 | 0.023 | 0.036 | 0.048 | 0.019 | 0.013 | 0.014 | |
| Ti | 0.004 | 0.005 | 0.003 | 0.000 | 0.129 | 0.106 | 0.061 | 0.077 | 0.135 | 0.118 |
| Mg | 0.009 | 0.000 | 0.001 | |||||||
| Al | 0.002 | 0.004 | 0.026 | 0.011 | 0.024 | 0.046 | 0.096 | |||
| Si | 0.065 | 0.970 | 0.495 | 0.436 | 0.222 | |||||
| Sum B | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Na | 0.798 | 0.835 | 0.814 | 0.818 | 0.013 | 0.006 | 0.015 | 0.057 | 0.019 | 0.014 |
| Ca | 0.807 | 0.849 | 0.757 | 0.827 | 0.145 | 0.223 | 0.304 | 0.243 | 0.290 | 0.051 |
| Th | 0.001 | 0.005 | 0.003 | 0.012 | 0.012 | 0.001 | ||||
| U | 0.106 | 0.052 | 0.092 | 0.022 | 0.236 | 0.217 | 0.741 | 0.069 | 0.416 | 0.012 |
| Bi | 0.003 | 0.414 | 0.053 | |||||||
| La | 0.001 | |||||||||
| Ce | 0.006 | 0.003 | 0.036 | 0.046 | ||||||
| Pr | 0.007 | |||||||||
| Nd | 0.015 | 0.001 | ||||||||
| Sm | ||||||||||
| Dy | ||||||||||
| Er | 0.005 | |||||||||
| Yb | 0.006 | |||||||||
| Y | 0.014 | |||||||||
| Sc | 0.004 | 0.002 | 0.001 | 0.002 | 0.003 | 0.011 | 0.001 | 0.020 | ||
| Pb | 0.003 | 0.011 | 0.016 | 0.009 | 0.070 | 0.052 | 0.012 | 0.123 | 0.011 | |
| Ba | 0.008 | 0.001 | 0.021 | 0.084 | 0.034 | 0.016 | ||||
| Mn | 0.001 | 0.007 | 0.003 | 0.001 | 0.015 | 0.005 | 0.153 | 0.109 | 0.284 | 0.622 |
| Fe | 0.002 | 0.055 | 0.059 | 0.064 | 0.208 | 0.253 | 0.290 | |||
| vacancy A | 0.277 | 0.243 | 0.306 | 0.313 | 1.411 | 1.373 | 0.679 | 0.692 | 0.689 | 0.911 |
| Sum A | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| F | 0.656 | 0.759 | 0.664 | 0.775 | 0.082 | |||||
| O | 6.405 | 6.370 | 6.352 | 6.237 | 5.766 | 5.790 | 6.556 | 6.198 | 6.468 | 5.871 |
| Nb + Ta | 1.947 | 1.953 | 1.932 | 1.886 | 1.836 | 1.836 | 0.699 | 1.229 | 1.375 | 1.441 |
| Mn/(Fe + Mn) | 0.139 | 1.000 | 1.000 | 0.357 | 0.213 | 0.078 | 0.705 | 0.344 | 0.528 | 0.682 |
| Ta/(Nb + Ta) | 0.908 | 0.665 | 0.567 | 0.625 | 0.816 | 0.825 | 0.210 | 0.273 | 0.113 | 0.056 |
| Locality | Orlovka | Cínovec | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | O-222 | O-222 | O-353 | 13 | 13 | 13 | 4972A | 4972A | 5427 |
| Crystal | 1 | 1 | 7 | 2 | 2 | 2 | 1 | 1 | 2 |
| Spot | 197 | 198 | 169 | 3 | 4 | 5 | 6 | 7 | 3 |
| WO3 | b.d.l. | b.d.l. | 10.15 | 1.34 | 0.35 | 8.20 | 4.00 | 3.96 | 2.66 |
| Nb2O5 | 69.96 | 40.29 | 19.86 | 64.87 | 60.25 | 44.13 | 55.93 | 56.36 | 51.94 |
| Ta2O5 | 10.09 | 41.37 | 45.28 | 10.26 | 16.04 | 23.58 | 16.95 | 16.82 | 25.28 |
| SnO2 | 0.03 | b.d.l. | 0.15 | 0.39 | 0.35 | 0.61 | 0.33 | 0.25 | 0.16 |
| TiO2 | 0.19 | 0.17 | 0.63 | 2.08 | 2.33 | 3.20 | 1.75 | 1.86 | 1.14 |
| MgO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Al2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.01 | b.d.l. | 0.03 | b.d.l. |
| CaO | 0.01 | 0.07 | 0.00 | 0.04 | 0.04 | 0.01 | b.d.l. | b.d.l. | 0.02 |
| ThO2 | b.d.l. | b.d.l. | 0.15 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| UO2 | 0.05 | 0.11 | 0.09 | 0.08 | 0.09 | 0.14 | 0.02 | b.d.l. | 0.05 |
| Sc2O3 | 0.03 | 0.06 | 0.13 | 0.68 | 0.95 | 1.41 | 0.84 | 0.77 | 0.63 |
| BaO | b.d.l. | b.d.l. | 0.02 | 0.09 | 0.13 | 0.20 | 0.07 | 0.13 | 0.11 |
| MnO | 13.07 | 11.70 | 7.93 | 12.70 | 14.08 | 10.20 | 7.61 | 7.47 | 9.91 |
| FeO | 7.48 | 5.87 | 9.21 | 7.93 | 5.38 | 8.44 | 12.01 | 11.97 | 8.57 |
| ZnO | 0.04 | b.d.l. | b.d.l. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| F | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.05 | b.d.l. |
| Feq. | −0.02 | ||||||||
| Total | 100.98 | 99.64 | 93.60 | 100.45 | 100.01 | 100.12 | 99.52 | 99.64 | 100.46 |
| W | 0.205 | 0.020 | 0.005 | 0.134 | 0.063 | 0.062 | 0.043 | ||
| Nb | 1.830 | 1.229 | 0.699 | 1.706 | 1.620 | 1.256 | 1.542 | 1.550 | 1.464 |
| Ta | 0.159 | 0.759 | 0.959 | 0.162 | 0.259 | 0.404 | 0.281 | 0.278 | 0.428 |
| Sn | 0.001 | 0.005 | 0.009 | 0.008 | 0.015 | 0.008 | 0.006 | 0.004 | |
| Ti | 0.008 | 0.008 | 0.037 | 0.091 | 0.104 | 0.152 | 0.080 | 0.085 | 0.053 |
| Mg | 0.001 | ||||||||
| Al | 0.001 | 0.001 | |||||||
| Ca | 0.001 | 0.005 | 0.002 | 0.003 | 0.001 | 0.002 | |||
| Th | 0.003 | ||||||||
| U | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | ||
| Sc | 0.001 | 0.004 | 0.009 | 0.034 | 0.049 | 0.077 | 0.045 | 0.041 | 0.034 |
| Ba | 0.001 | 0.002 | 0.003 | 0.005 | 0.002 | 0.003 | 0.003 | ||
| Mn | 0.641 | 0.669 | 0.523 | 0.626 | 0.709 | 0.544 | 0.393 | 0.385 | 0.523 |
| FeO | 0.362 | 0.331 | 0.600 | 0.386 | 0.267 | 0.444 | 0.613 | 0.609 | 0.447 |
| ZnO | 0.002 | ||||||||
| F | 0.010 | ||||||||
| Ta/(Nb + Ta) | 0.080 | 0.382 | 0.578 | 0.087 | 0.138 | 0.243 | 0.154 | 0.152 | 0.226 |
| Mn/(Fe + Mn) | 0.639 | 0.669 | 0.466 | 0.619 | 0.726 | 0.550 | 0.391 | 0.387 | 0.539 |
| Type | Madeira Pcl 1 | Madeira Pcl 2 | Madeira Pcl 3 | Madeira Pcl 4 | Cínovec Mic | Cínovec Pcl | Orlovka Mic |
|---|---|---|---|---|---|---|---|
| n | 56 | 54 | 69 | 4 | 8 | 14 | 21 |
| WO3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 3.20 | b.d.l. |
| Ta2O5 | 4.86 | 4.93 | 4.80 | 2.96 | 56.51 | 9.94 | 65.29 |
| Nb2O5 | 47.66 | 48.69 | 36.64 | 20.09 | 6.91 | 30.16 | 9.71 |
| SnO2 | 2.89 | 3.34 | 1.67 | b.d.l. | 0.86 | 0.13 | 1.00 |
| TiO2 | 0.39 | 0.55 | 0.89 | 1.02 | 1.83 | 4.32 | 0.22 |
| Al2O3 | b.d.l. | 0.02 | 0.24 | b.d.l. | 0.30 | 0.44 | 0.13 |
| SiO2 | 0.11 | 0.14 | 3.23 | 15.81 | 1.06 | 6.86 | 0.27 |
| Na2O | 1.93 | 0.55 | 0.43 | 0.00 | 0.05 | 0.69 | 4.68 |
| CaO | 0.44 | 0.86 | 0.42 | 0.06 | 2.34 | 4.04 | 8.53 |
| ThO2 | 3.60 | 3.03 | 1.37 | 16.36 | 0.39 | 0.50 | 0.08 |
| UO2 | 6.42 | 7.09 | 10.30 | 20.95 | 11.72 | 15.31 | 3.56 |
| Ce2O3 | 1.38 | 2.23 | 1.36 | 0.05 | 0.97 | 0.01 | 0.04 |
| Y2O3 | 0.75 | 0.97 | 0.22 | 0.27 | 0.00 | 0.65 | b.d.l. |
| PbO | 19.22 | 13.10 | 25.84 | 1.66 | 2.49 | 1.08 | 0.34 |
| BaO | b.d.l. | b.d.l. | 0.08 | b.d.l. | 0.85 | 4.53 | b.d.l. |
| SrO | 0.03 | 0.04 | 0.07 | b.d.l. | b.d.l. | 0.37 | 0.36 |
| MnO | 0.13 | 0.25 | 0.37 | 0.80 | 0.14 | 0.77 | 0.03 |
| FeO | 0.42 | 1.16 | 3.05 | 0.87 | 0.77 | 3.60 | 0.04 |
| ZnO | 0.03 | 0.16 | 0.39 | 0.04 | b.d.l. | 0.01 | b.d.l. |
| F | 1.67 | 1.26 | 0.34 | 0.70 | 0.02 | 0.09 | 2.59 |
| Total | 92.63 | 89.38 | 92.17 | 82.27 | 87.88 | 87.21 | 96.96 |
| A site | 1.07 | 0.84 | 1.35 | 0.81 | 0.70 | 1.17 | 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breiter, K.; Costi, H.T.; Korbelová, Z. Pyrochlore-Supergroup Minerals and Their Relation to Columbite-Group Minerals in Peralkaline to Subaluminous A-Type Rare-Metal Granites with Special Emphasis on the Madeira Pluton, Amazonas, Brazil. Minerals 2024, 14, 1302. https://doi.org/10.3390/min14121302
Breiter K, Costi HT, Korbelová Z. Pyrochlore-Supergroup Minerals and Their Relation to Columbite-Group Minerals in Peralkaline to Subaluminous A-Type Rare-Metal Granites with Special Emphasis on the Madeira Pluton, Amazonas, Brazil. Minerals. 2024; 14(12):1302. https://doi.org/10.3390/min14121302
Chicago/Turabian StyleBreiter, Karel, Hilton Tulio Costi, and Zuzana Korbelová. 2024. "Pyrochlore-Supergroup Minerals and Their Relation to Columbite-Group Minerals in Peralkaline to Subaluminous A-Type Rare-Metal Granites with Special Emphasis on the Madeira Pluton, Amazonas, Brazil" Minerals 14, no. 12: 1302. https://doi.org/10.3390/min14121302
APA StyleBreiter, K., Costi, H. T., & Korbelová, Z. (2024). Pyrochlore-Supergroup Minerals and Their Relation to Columbite-Group Minerals in Peralkaline to Subaluminous A-Type Rare-Metal Granites with Special Emphasis on the Madeira Pluton, Amazonas, Brazil. Minerals, 14(12), 1302. https://doi.org/10.3390/min14121302








