Water Speciation and Storage Capacity of Olivine under the Reduced Fluid—Peridotite Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. High-Pressure Apparatus
2.3. Analytical Techniques
3. Results
3.1. Phase Relations
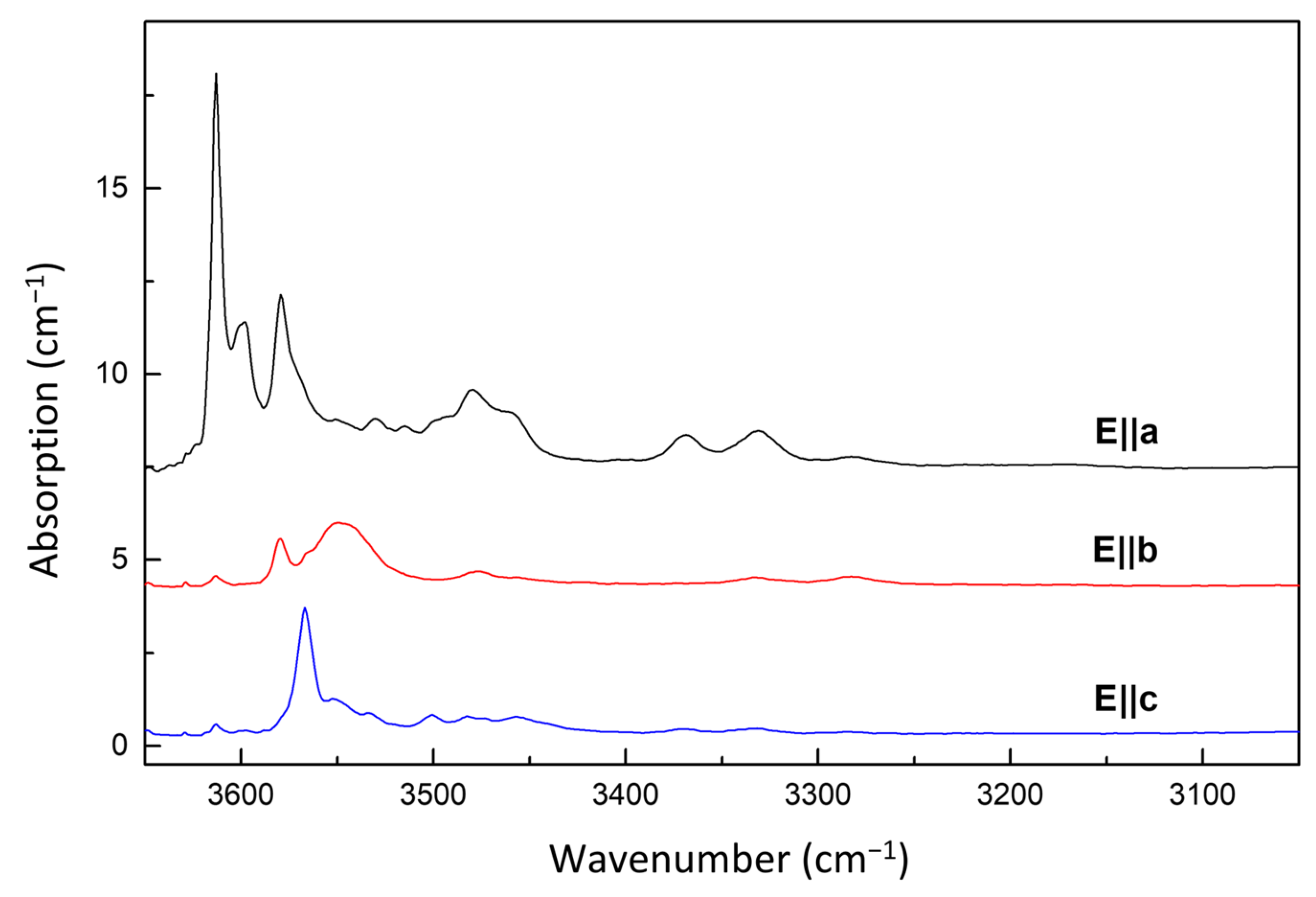
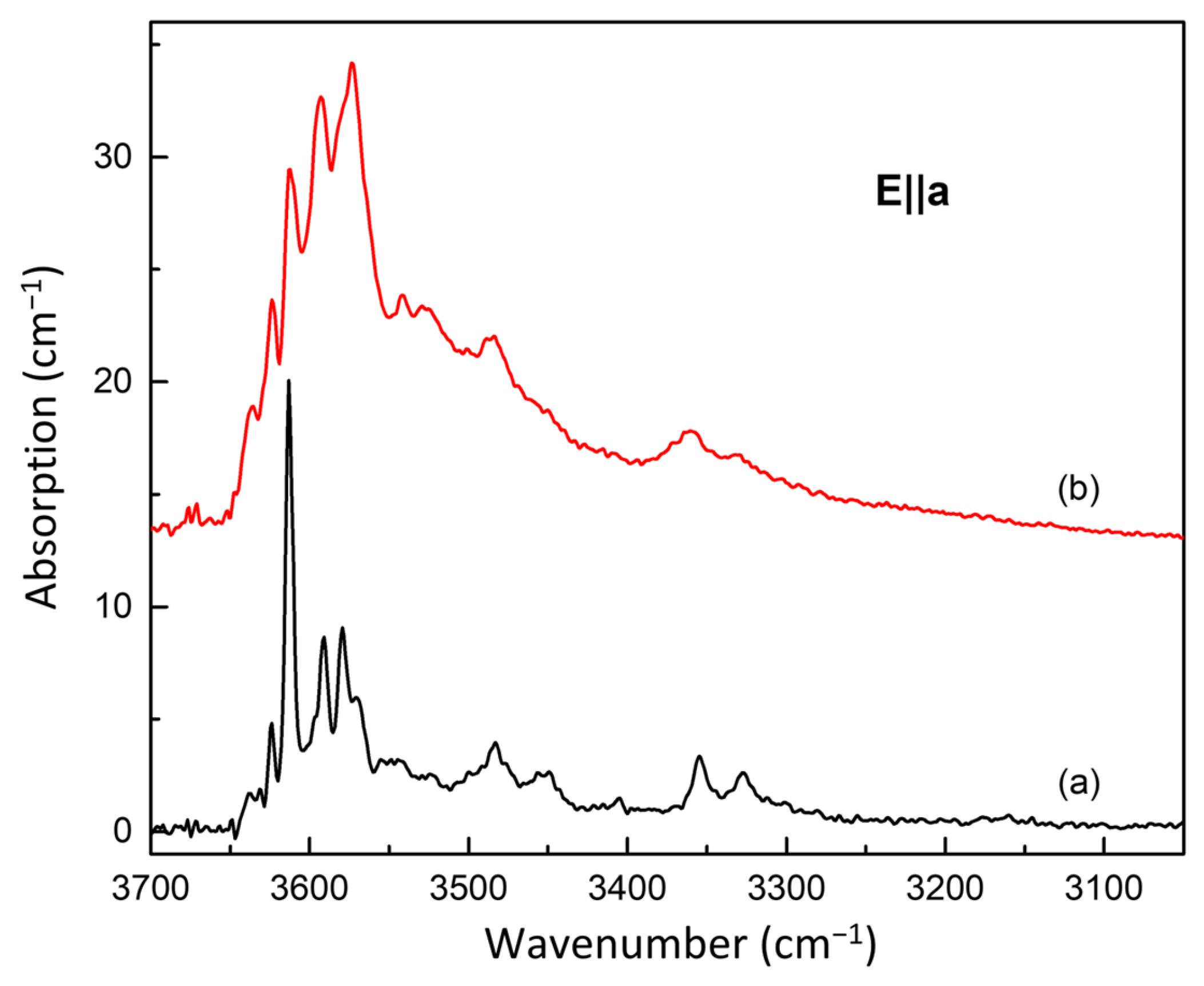
3.2. FTIR Spectroscopy
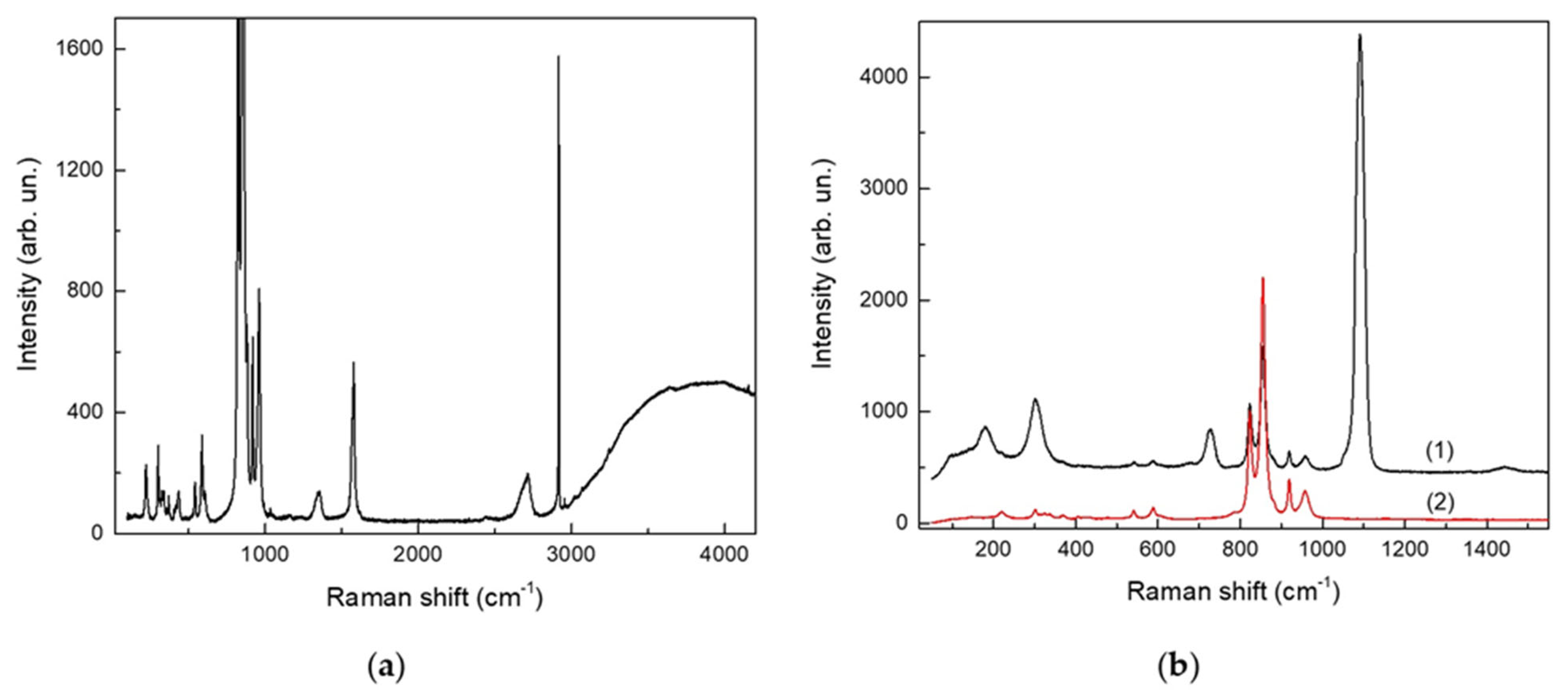
3.3. Raman Micro-Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Q.; Kohlstedt, D.L. Substantial hydrogen solubility in olivine and implications for water storage in the mantle. Nature 1992, 357, 672–674. [Google Scholar] [CrossRef]
- Kolhstedt, D.L.; Keppler, H.; Rubie, D.C. Solubility of water in the α, β and γ phases of (Mg,Fe)2SiO4. Contrib. Mineral. Petrol. 1996, 123, 345–357. [Google Scholar]
- Mosenfelder, J.L.; Deligne, N.I.; Asimow, P.D.; Rossman, G.R. Hydrogen incorporation in olivine from 2–12 GPa. Am. Mineral. 2006, 91, 285–294. [Google Scholar] [CrossRef]
- Hirschmann, M.M.; Tenner, T.; Aubaud, C.; Withers, A.C. Dehydration melting of nominally anhydrous mantle: The primacy of partitioning. Phys. Earth Planet. Inter. 2009, 176, 54–68. [Google Scholar] [CrossRef]
- Green, D.H.; Hibberson, W.O.; Kovács, I.; Rosenthal, A. Water and its influence on the lithosphere–asthenosphere boundary. Nature 2010, 467, 448. [Google Scholar] [CrossRef] [PubMed]
- Bali, E.; Bolfan-Casanova, N.; Koga, K.T. Pressure and temperature dependence of H solubility in forsterite: An implication to water activity in the Earth interior. Earth Planet. Sci. Lett. 2008, 268, 354–363. [Google Scholar] [CrossRef]
- Smyth, J.R.; Frost, D.J.; Nestola, F.; Holl, C.M.; Bromiley, G. Olivine hydration in the deep upper mantle: Effect of temperature and silica activity. Geophys. Res. Lett. 2006, 33, L153012006. [Google Scholar] [CrossRef]
- Matveev, S.; Portnyagin, M.; Ballhaus, C.; Brooker, R.A.; Geiger, C.A. FTIR spectrum of phenocryst olivine as an indicator of silica saturation in magmas. J. Petrol. 2005, 46, 603–614. [Google Scholar] [CrossRef][Green Version]
- Lemaire, C.; Kohn, S.C.; Brooker, R.A. The effect of silica activity on the incorporation mechanisms of water in synthetic forsterite: A polarized infrared spectroscopic study. Contrib. Mineral. Petrol. 2004, 147, 48–57. [Google Scholar]
- Zhao, Y.H.; Ginsberg, S.B.; Kohlstedt, D.J. Solubility of hydrogen in olivine: Dependence on temperature and iron content. Contrib. Mineral. Petrol. 2004, 147, 155–161. [Google Scholar] [CrossRef]
- Withers, A.C.; Hirschmann, M.M. Influence of temperature, composition, silica activity and oxygen fugacity on the H2O storage capacity of olivine at 8 GPa. Contrib. Mineral. Petrol. 2008, 156, 595–605. [Google Scholar] [CrossRef]
- Withers, A.C.; Hirschmann, M.M.; Tenner, T.J. The effect of Fe on olivine H2O storage capacity: Consequences for H2O in the martian mantle. Am. Mineral. 2011, 96, 1039–1053. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kupriyanov, I.N.; Palyanov, Y.N.; Kruk, A.N.; Sobolev, N.V. Melting experiments on the Udachnaya kimberlite at 6.3–7.5 GPa: Implications for the role of H2O in magma generation and formation of hydrous olivine. Geochim. Cosmochim. Acta. 2013, 101, 133–155. [Google Scholar] [CrossRef]
- Blanchard, M.; Ingrin, J.; Balan, E.; Kovacs, I.; Withers, A. Effect of iron and trivalent cations on OH defects in olivine. Am. Mineral. 2017, 102, 302–311. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Dick, H.J.B.; Quick, J.E. Formation of harzburgite by pervasive melt/rock reaction in the upper mantle. Nature 1992, 358, 635–641. [Google Scholar] [CrossRef]
- Kesson, S.E.; Ringwood, A.E. Slab-mantle interactions: 2. The formation of diamonds. Chem. Geol. 1989, 78, 97–118. [Google Scholar] [CrossRef]
- Boyd, F.R.; Pokhilenko, N.P.; Pearson, D.G.; Mertzman, S.A.; Sobolev, N.V.; Finger, L.W. Composition of the Siberian cratonic mantle: Evidence from Udachnaya peridotite xenoliths. Contrib. Mineral. Petrol. 1997, 128, 228–246. [Google Scholar] [CrossRef]
- Pearson, D.G.; Wittig, N. The Formation and Evolution of Cratonic Mantle Lithosphere—Evidence from Mantle xenoliths. Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 255–292. [Google Scholar]
- Creighton, S.; Stachel, T.; Matveev, S.; Hofer, H.; McCammon, C.; Luth, R.W. Oxidation of the Kaapvaal lithospheric mantle driven by metasomatism. Contrib. Mineral. Petrol. 2009, 157, 491–504. [Google Scholar] [CrossRef]
- Luth, R.W.; Stachel, T. The buffering capacity of lithospheric mantle: Implications for diamond formation. Contrib. Mineral. Petrol. 2014, 168, 1–12. [Google Scholar] [CrossRef]
- Stachel, T.; Luth, R.W. Diamond formation—Where, when and how? Lithos 2015, 220, 200–220. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Fedorova, E.N.; Luk’yanova, L.I.; Wirth, R.; Tomilenko, A.A.; Reutsky, V.N.; Efimova, E.S. Mineral and fluid inclusions in the diamonds from the Ural placers, Russia. In AGU Fall Meeting Abstracts; AGU: Washington, DC, USA, 2015; p. V11C–3073. [Google Scholar]
- Sobolev, N.V.; Tomilenko, A.A.; Bul’bak, T.A.; Logvinova, A.M. Composition of volatile components in the diamonds, associated garnet and olivine from diamondiferous peridotites from the Udachnaya pipe, Yakutia, Russia (by coupled gas chromatographic-mass spectrometric analysis). Engineering 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Tomilenko, A.A.; Wirth, R.; Bul’bak, T.A.; Luk’yanova, L.I.; Fedorova, E.N.; Reutsky, V.N.; Efimova, E.S. Mineral and fluid inclusions in diamonds from the Urals placers, Russia: Evidence for solid molecular N2 and hydrocarbons in fluid inclusions. Geochim. Cosmochim. Acta 2019, 266, 197–219. [Google Scholar] [CrossRef]
- Navon, O.; Wirth, R.; Schmidt, C.; Jablon, B.M.; Schreiber, A.; Emmanuel, S. Solid molecular nitrogen (δ-N2) inclusions in Juina diamonds: Exsolution at the base of the transition zone. Earth Planet. Sci. Lett. 2017, 464, 237–247. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.; Green, D.H. Measurement of reduced peridotite-COH solidus and implications for redox melting of the mantle. Nature 1988, 332, 349–352. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Ohtani, E. Melting and subsolidus phase relations in peridotite and eclogite systems with reduced C-O-H fluid at 3–16 GPa. Earth Planet. Sci. Lett. 2014, 391, 87–99. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kupriyanov, I.N.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanov, Y.N.; Sobolev, N.V. Formation of water-bearing defects in olivine in the presence of water–hydrocarbon fluid at 6.3 GPa and 1200 °C. Dokl. Earth Sci. 2018, 483, 1451–1453. [Google Scholar] [CrossRef]
- Matjuschkin, V.; Woodland, A.B.; Frost, D.J.; Yaxley, G.M. Reduced methane-bearing fluids as a source for diamond. Sci. Rep. 2020, 10, 6961. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kupriyanov, I.N.; Palyanov, Y.N. Partitioning of H2O between olivine and carbonate–silicate melts at 6.3 GPa and 1400 °C: Implications for kimberlite formation. Earth Planet. Sci. Lett. 2013, 383, 58–67. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Xia, Q. CO2-induced small water solubility in olivine and implications for properties of the shallow mantle. Earth Planet. Sci. Lett. 2014, 403, 37–47. [Google Scholar] [CrossRef]
- Doucet, L.S.; Ionov, D.A.; Golovin, A.V. The origin of coarse garnet peridotites in cratonic lithosphere: New data on xenoliths from the Udachnaya kimberlite, central Siberia. Contrib. Mineral. Petrol. 2013, 165, 1225–1242. [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.F.; Green, D.H. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: Implications for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Foley, S.F. A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J. Petrol. 2011, 52, 1363–1391. [Google Scholar] [CrossRef]
- Stagno, V.; Frost, D.J. Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth Planet. Sci. Lett. 2010, 300, 72–84. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanova, G.A.; Sokol, I.A.; Palyanov, Y.N. Carbon and nitrogen speciation in N-poor C-O-H-N fluids at 6.3 GPa and 1100–1400 °C. Sci. Rep. 2017, 7, 706. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Khokhryakov, A.F.; Borzdov, Y.M. High-pressure crystallization and properties of diamond from magnesium-based catalysts. CrystEngComm 2017, 19, 4459–4475. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high-pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Asimow, P.D.; Stein, L.C.; Mosenfelder, J.L.; Rossman, G.R. Quantitative polarized infrared analysis of trace OH in populations of randomly oriented mineral grains. Am. Miner. 2006, 91, 278–284. [Google Scholar] [CrossRef]
- Withers, A.C.; Bureau, H.; Raepsaet, C.; Hirschmann, M.M. Calibration of infrared spectroscopy by elastic recoil detection analysis of H in synthetic olivine. Chem. Geol. 2012, 334, 92–98. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Ryabchikov, I.D. Volatile components, magmas, and critical fluids in upwelling mantle. J. Petrol. 2000, 41, 1195–1206. [Google Scholar] [CrossRef]
- Huizenga, J.M.; Crossingham, A.; Viljoen, F. Diamond precipitation from ascending reduced fluids in the Kaapvaal lithosphere: Thermodynamic constraints. Compt. Rendus Geosci. 2012, 344, 67–76. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Kruk, A.N.; Sokol, I.A.; Palyanov, Y.N. Fate of fluids at the base of subcratonic lithosphere: Experimental constraints at 5.5–7.8 GPa and 1150–1350 °C. Lithos 2018, 318, 419–433. [Google Scholar] [CrossRef]
- Walker, A.M.; Hermann, J.; Berry, A.J.; O’Neill, H.S.C. Three water sites in upper mantle olivine and the role of titanium in the water weakening mechanism. J. Geophys. Res. Solid Earth 2007, 112, B052112007. [Google Scholar] [CrossRef]
- Berry, A.J.; O’Neill, H.S.C.; Hermann, J.; Scott, D.R. The infrared signature of water associated with trivalent cations in olivine. Earth Planet. Sci. Lett. 2007, 261, 134–142. [Google Scholar] [CrossRef]
- Berry, A.J.; Hermann, J.; O’Neill, H.S.; Foran, G.J. Fingerprinting the water site in mantle olivine. Geology 2005, 33, 869–872. [Google Scholar] [CrossRef]
- Bell, D.; Rossman, G.; Maldener, J.; Endisch, D.; Rauch, F. Hydroxide in olivine: A quantitative determination of the absolute amount and calibration of the IR spectrum. J. Geophys. Res. 2003, 108, 2105. [Google Scholar] [CrossRef]
- Kovacs, I.; O’Neill, H.S.; Hermann, J.; Hauri, E.H. Site-specific infrared O-H absorption coefficients for water substitution into olivine. Am. Miner. 2010, 95, 292–299. [Google Scholar] [CrossRef]
- Balan, E.; Ingrin, J.; Delattre, S.; Kovacs, I.; Blanchard, M. Theoretical infrared spectrum of OH-defects in forsterite. Eur. J. Miner. 2011, 23, 285–292. [Google Scholar] [CrossRef]
- Matveev, S.; Stachel, T. FTIR spectroscopy of OH in olivine: A new tool in kimberlite exploration. Geochim. Cosmochim. Acta 2007, 71, 5528–5543. [Google Scholar] [CrossRef]
- Burke, E.A.J. Raman microspectrometry of fluid inclusions. Lithos 2001, 55, 139–158. [Google Scholar] [CrossRef]
- Rutt, H.N.; Nicola, J.H. Raman-spectra of carbonates of calcite structure. J. Phys. C Solid State Phys. 1974, 7, 4522–4528. [Google Scholar] [CrossRef]
- Rividi, N.; van Zuilen, M.; Philippot, P.; Menez, B.; Godard, G.; Poidatz, E. Calibration of carbonate composition using micro-Raman analysis: Application to planetary surface exploration. Astrobiology 2010, 10, 293–309. [Google Scholar] [CrossRef] [PubMed]
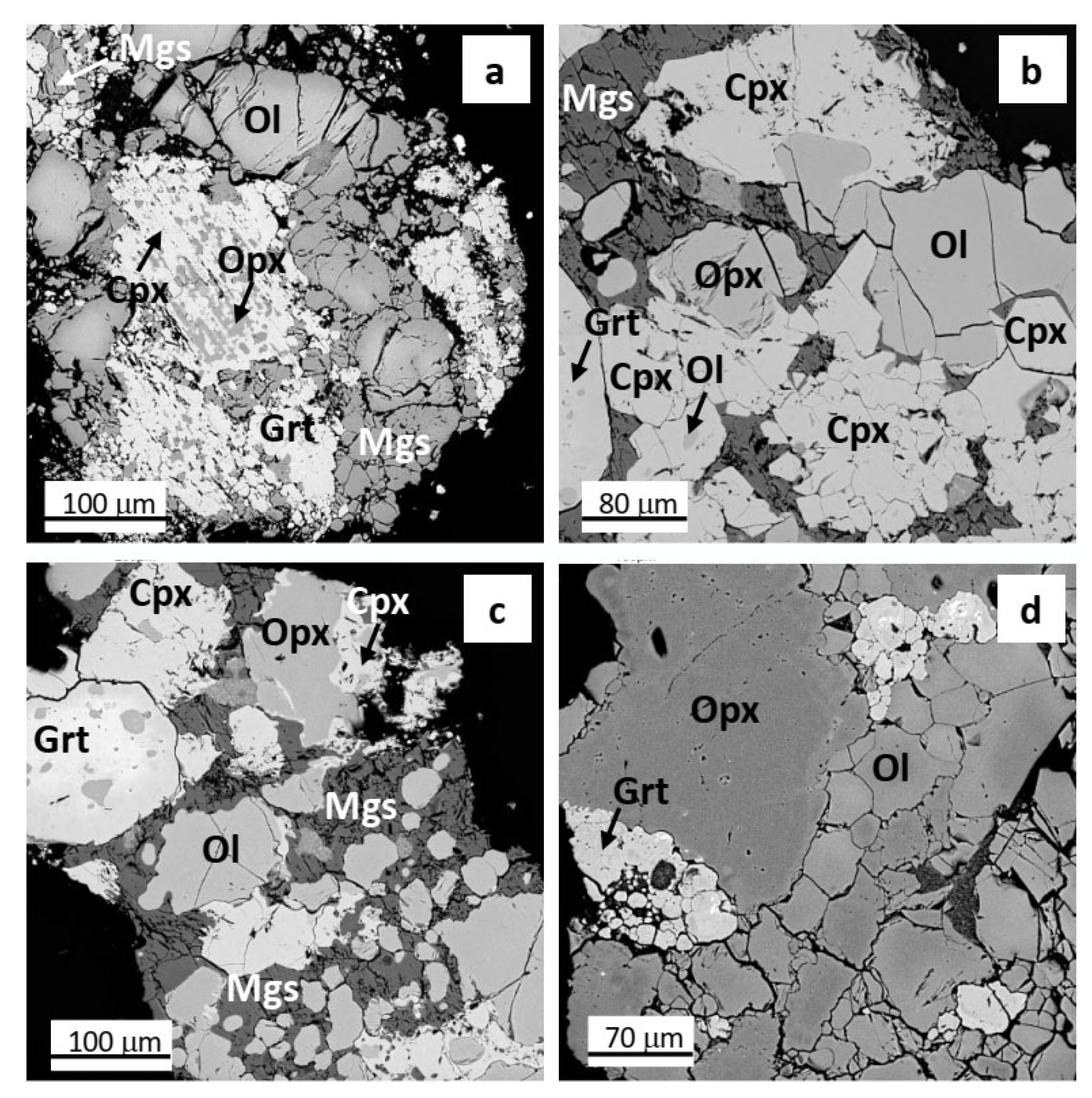


| Uv-419/09 | HC-SA 1 | HC-SA 2 | HC-SA 3 | HC-SA 4 | HC-SA 5 | HC-SA 6 | HC-SA 7 | |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 45.1 | 41.28 | 43.92 | 35.22 | 35.64 | 41.37 | 37.32 | 39.99 |
| TiO2 | 0.045 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Al2O3 | 1 | 0.92 | 0.97 | 0.78 | 0.79 | 0.92 | 0.83 | 0.89 |
| Cr2O3 | 0.409 | 0.37 | 0.4 | 0.32 | 0.32 | 0.38 | 0.34 | 0.36 |
| FeO | 6.6 | 6.04 | 6.43 | 5.15 | 5.22 | 6.05 | 5.46 | 5.85 |
| Fe2O3 | - | - | - | - | - | - | 9.48 | 7.12 |
| MnO | 0.112 | 0.1 | 0.11 | 0.09 | 0.09 | 0.1 | 0.09 | 0.1 |
| MgO | 44 | 40.27 | 42.85 | 42.02 | 42.29 | 42.14 | 36.41 | 39.02 |
| CaO | 1.37 | 1.25 | 1.33 | 2.07 | 2.06 | 1.49 | 1.13 | 1.21 |
| Na2O | 0.19 | 0.17 | 0.19 | 0.15 | 0.15 | 0.17 | 0.16 | 0.17 |
| K2O | 0.39 | 0.36 | 0.38 | 0.3 | 0.31 | 0.36 | 0.32 | 0.35 |
| NiO | 0.298 | 0.27 | 0.29 | 0.23 | 0.24 | 0.27 | 0.25 | 0.026 |
| P2O5 | 0.011 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 99.5 | 91.08 | 96.92 | 86.37 | 87.15 | 93.31 | 91.84 | 95.37 |
| C18H36O2 | - | 8.47 | 2.61 | 4.11 | 3.5 | 4.13 | 7.76 | 4.21 |
| CO2 | - | - | - | 9.15 | 8.98 | 2.12 | - | - |
| Run No. | Harzburgite, mg | Stearic Acid, mg | MgCO3, mg | Fe2O3, mg | Outer Container | Run Products * | Estimated *** Δlog(FMQ) |
|---|---|---|---|---|---|---|---|
| HC-SA 1 | 10.8 | 1.0 | - | - | - | Ol-Opx-Cpx-Grt | −4.8 |
| HC-SA 2 | 14.9 | 0.4 | - | - | HM ** | Ol-Opx-Cpx-Grt | −2.4 |
| HC-SA 3 | 22.8 | 1.2 | 5.2 | - | HM | Ol-Opx-Cpx-Grt-Mgs | −1.7 |
| HC-SA 4 | 11.3 | 0.5 | 2.5 | - | HM | Ol-Opx-Cpx-Grt-Mgs | −1.7 |
| HC-SA 5 | 11.1 | 0.5 | 0.5 | - | HM | Ol-Opx-Cpx-Grt-Mgs | −1.7 |
| HC-SA 6 | 9.6 | 0.9 | - | 1.1 | - | Ol-Opx-Cpx-Grt | −2.4 |
| HC-SA 7 | 27.4 | 1.3 | - | 2.2 | - | Ol-Opx-Grt + L | −1.5 |
| Run No. | Phase | n | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | NiO | MgO | CaO | Na2O | K2O | Total | Mg# | Ca# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uv-419/09 | Ol | 7 | 41.4 | - | - | - | 9.2 | 0.3 | 0.3 | 50.0 | - | - | - | 101.1 | 0.91 | - |
| Opx | 8 | 58.2 | - | - | 0.2 | 4.7 | - | - | 36.9 | 0.4 | - | - | 100.3 | 0.93 | 0.1 | |
| Cpx | 9 | 56.8 | 0.5 | - | 0.7 | 2.3 | - | - | 16.5 | 22.9 | 1.0 | - | 100.9 | 0.93 | 0.67 | |
| Grt | 7 | 41.4 | - | 18.4 | 7.8 | 7.8 | 0.4 | - | 18.3 | 7.0 | - | - | 101.1 | 0.81 | 0.25 | |
| Phl | 6 | 43.3 | 0.5 | 8.1 | 0.9 | 7.0 | - | - | 26.2 | - | 0.3 | 10.4 | 96.8 | 0.87 | - | |
| HC-SA 1 | Ol | 9 | 41.7 | - | - | - | 7.8 | - | 0.5 | 50.9 | - | - | - | 100.8 | 0.92 | - |
| Opx | 8 | 59.4 | - | - | 0.2 | 4.7 | - | - | 36.3 | 0.3 | - | - | 100.9 | 0.93 | 0.01 | |
| Cpx | 6 | 56.1 | 0.2 | 2.5 | 1.5 | 2.6 | 0.2 | - | 18.0 | 18.0 | 2.2 | - | 101.2 | 0.93 | 0.56 | |
| Grt | 10 | 41.7 | 0.5 | 18.1 | 5.7 | 6.9 | 0.3 | - | 22.4 | 5.6 | - | - | 100.3 | 0.85 | 0.18 | |
| HC-SA 2 | Ol | 7 | 41.1 | - | - | 0.2 | 7.7 | - | 0.5 | 51.5 | - | - | - | 101.0 | 0.92 | - |
| Opx | 9 | 59.5 | - | - | 0.2 | 4.8 | - | - | 36.4 | 0.4 | - | - | 101.3 | 0.93 | 0.01 | |
| Cpx | 8 | 55.9 | 0.4 | 2.4 | 1.3 | 2.7 | 0.2 | - | 18.4 | 17.5 | 2.0 | - | 100.9 | 0.92 | 0.54 | |
| Grt | 6 | 41.3 | 0.4 | 18.0 | 7.5 | 7.3 | 0.4 | 0.3 | 19.1 | 6.9 | - | - | 101.1 | 0.82 | 0.25 | |
| HC-SA 3 | Ol | 7 | 42.0 | - | - | - | 5.4 | 0.1 | 0.3 | 52.2 | 0.2 | - | - | 100.3 | 0.95 | - |
| Opx | 5 | 58.3 | - | 0.7 | 0.5 | 1.5 | - | - | 38.5 | 0.8 | - | - | 100.3 | 0.98 | 0.02 | |
| Cpx | 8 | 55.1 | - | 0.8 | 0.9 | 0.8 | - | - | 18.8 | 23.7 | 0.2 | - | 100.1 | 0.98 | 0.65 | |
| Grt | 9 | 42.1 | - | 18.3 | 6.8 | 2.1 | 0.2 | - | 21.6 | 8.0 | - | - | 99.2 | 0.95 | 0.28 | |
| HC-SA 4 | Ol | 7 | 41.7 | - | - | - | 7.0 | - | 0.3 | 51.3 | - | - | - | 100.2 | 0.93 | - |
| Cpx | 7 | 55.7 | - | 0.7 | 0.6 | 2.1 | - | - | 19.4 | 22.1 | 0.4 | - | 100.9 | 0.94 | 0.61 | |
| Grt | 8 | 42.5 | 0.2 | 19.6 | 5.5 | 5.6 | 0.2 | - | 21.0 | 6.6 | - | - | 101.1 | 0.87 | 0.23 | |
| Mgs | 9 | - | - | - | - | 3.9 | 0.2 | 0.3 | 41.2 | 2.3 | - | - | 47.8 | 0.95 | 0.05 | |
| HC-SA 5 | Ol | 8 | 41.6 | - | - | - | 7.1 | - | 0.3 | 51.3 | - | - | 100.3 | 0.93 | - | |
| Opx | 7 | 59.0 | - | 0.6 | 0.3 | 3.7 | - | - | 36.4 | 0.8 | - | - | 100.7 | 0.95 | 0.02 | |
| Cpx | 7 | 56.5 | - | 0.8 | 0.5 | 2.3 | - | - | 19.2 | 20.5 | 0.4 | - | 100.1 | 0.94 | 0.58 | |
| Grt | 8 | 41.1 | - | 15.9 | 10.7 | 4.9 | 0.3 | - | 18.5 | 8.8 | - | - | 100.1 | 0.87 | 0.32 | |
| Mgs | 8 | - | - | - | - | 3.5 | - | - | 42.3 | 2.9 | - | - | 48.7 | 0.96 | 0.06 | |
| HC-SA 6 | Ol (w) | 5 | 41.4 | - | - | - | 7.6 | - | 0.5 | 50.5 | - | - | - | 100.0 | 0.92 | - |
| Ol (y) | 5 | 40.8 | - | - | - | 14.5 | - | 0.4 | 44.4 | - | - | - | 100.1 | 0.85 | - | |
| Opx | 7 | 57.7 | - | 0.2 | 0.2 | 5.0 | 0.2 | - | 36.5 | 0.4 | - | - | 100.1 | 0.93 | 0.01 | |
| Cpx | 8 | 55.3 | - | 1.2 | 1.1 | 4.1 | - | - | 18.7 | 19.2 | 1.1 | - | 100.6 | 0.89 | 0.55 | |
| Grt | 9 | 40.9 | 0.3 | 18.6 | 6.4 | 8.8 | 0.3 | - | 19.0 | 6.7 | - | - | 101.0 | 0.79 | 0.23 | |
| HC-SA 7 | Ol (w) | 8 | 40.5 | - | - | - | 10.8 | 0.2 | 0.2 | 49.0 | - | - | - | 100.7 | 0.89 | - |
| Ol (y) | 6 | 40.4 | - | - | - | 14.0 | - | 0.1 | 45.4 | - | - | - | 100.0 | 0.85 | - | |
| Opx | 8 | 59.0 | - | 0.3 | 0.4 | 4.7 | - | - | 36.2 | 0.4 | - | - | 101.0 | 0.93 | 0.01 | |
| Grt | 7 | 41.4 | 0.2 | 17.4 | 7.9 | 7.3 | 0.3 | - | 19.6 | 6.2 | - | - | 100.4 | 0.83 | 0.22 | |
| L * | 4 | 4.6 | - | 0.4 | - | 5.2 | 0.1 | - | 21.4 | 15.5 | 0.5 | 1.4 | 49.1 | 0.88 | 0.31 |
| HC-SA 1 | HC-SA 2 | HC-SA 3 | HC-SA 4 | HC-SA 5 | HC-SA 6 | HC-SA 7 | |
|---|---|---|---|---|---|---|---|
| Main components | |||||||
| H2O | 55.0 | 72.4 | 84.7 | 83.4 | 73.5 | 86.1 | 86.7 |
| CO2 | 8.4 | - | 2.2 | 6.4 | 14.2 | - | 3.2 |
| CH4 | 31.1 | 0.2 | 9.8 | 1.9 | 0.2 | 12.8 | 6.4 |
| C2H6 | 5.1 | - | 1.5 | - | - | 0.9 | 1.6 |
| H2O/(H2O + CO2) | 0.87 | 0.99 | 0.97 | 0.93 | 0.84 | 0.99 | 0.96 |
| Speciation of organic components * | |||||||
| Alkanes | 36.5 | 3.3 | 11.6 | 2.9 | 1.9 | 13.8 | 8.1 |
| Olefins | - | 2.4 | 0.1 | 0.3 | 1.0 | - | 0.1 |
| Arenas | - | 0.4 | - | 0.2 | 0.2 | - | - |
| Furans | - | 0.6 | - | 0.2 | 0.4 | - | - |
| Alcohols and esters | - | 5.0 | 0.8 | 1.6 | 2.3 | - | 0.8 |
| Aldehydes | - | 7.0 | 0.3 | 1.7 | 3.0 | - | 0.7 |
| Ketones | - | 3.5 | 0.1 | 0.5 | 1.4 | - | 0.3 |
| Carboxylic acids | - | 5.4 | 0.2 | 2.7 | 2.3 | - | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupriyanov, I.N.; Sokol, A.G.; Kruk, A.N. Water Speciation and Storage Capacity of Olivine under the Reduced Fluid—Peridotite Interaction. Minerals 2024, 14, 119. https://doi.org/10.3390/min14020119
Kupriyanov IN, Sokol AG, Kruk AN. Water Speciation and Storage Capacity of Olivine under the Reduced Fluid—Peridotite Interaction. Minerals. 2024; 14(2):119. https://doi.org/10.3390/min14020119
Chicago/Turabian StyleKupriyanov, Igor N., Alexander G. Sokol, and Alexey N. Kruk. 2024. "Water Speciation and Storage Capacity of Olivine under the Reduced Fluid—Peridotite Interaction" Minerals 14, no. 2: 119. https://doi.org/10.3390/min14020119
APA StyleKupriyanov, I. N., Sokol, A. G., & Kruk, A. N. (2024). Water Speciation and Storage Capacity of Olivine under the Reduced Fluid—Peridotite Interaction. Minerals, 14(2), 119. https://doi.org/10.3390/min14020119







