Bacteria-Driven Fossil Ecosystems as Paleoindicators of Active Continental Margins and the Role of Carbonate Sediment-Hosted Vents in Geodynamic Reconstructions
Abstract
1. Introduction

2. Materials and Methods
2.1. Materials
2.2. Applied Methods
2.2.1. Optical Rock Microscopy (OM)
2.2.2. X-ray Powder Diffraction (XRD)
2.2.3. FTIR
2.2.4. SEM–EDS
3. Geological Setting
4. Studied Sections
4.1. Dezső Rezső Valley Outcrops
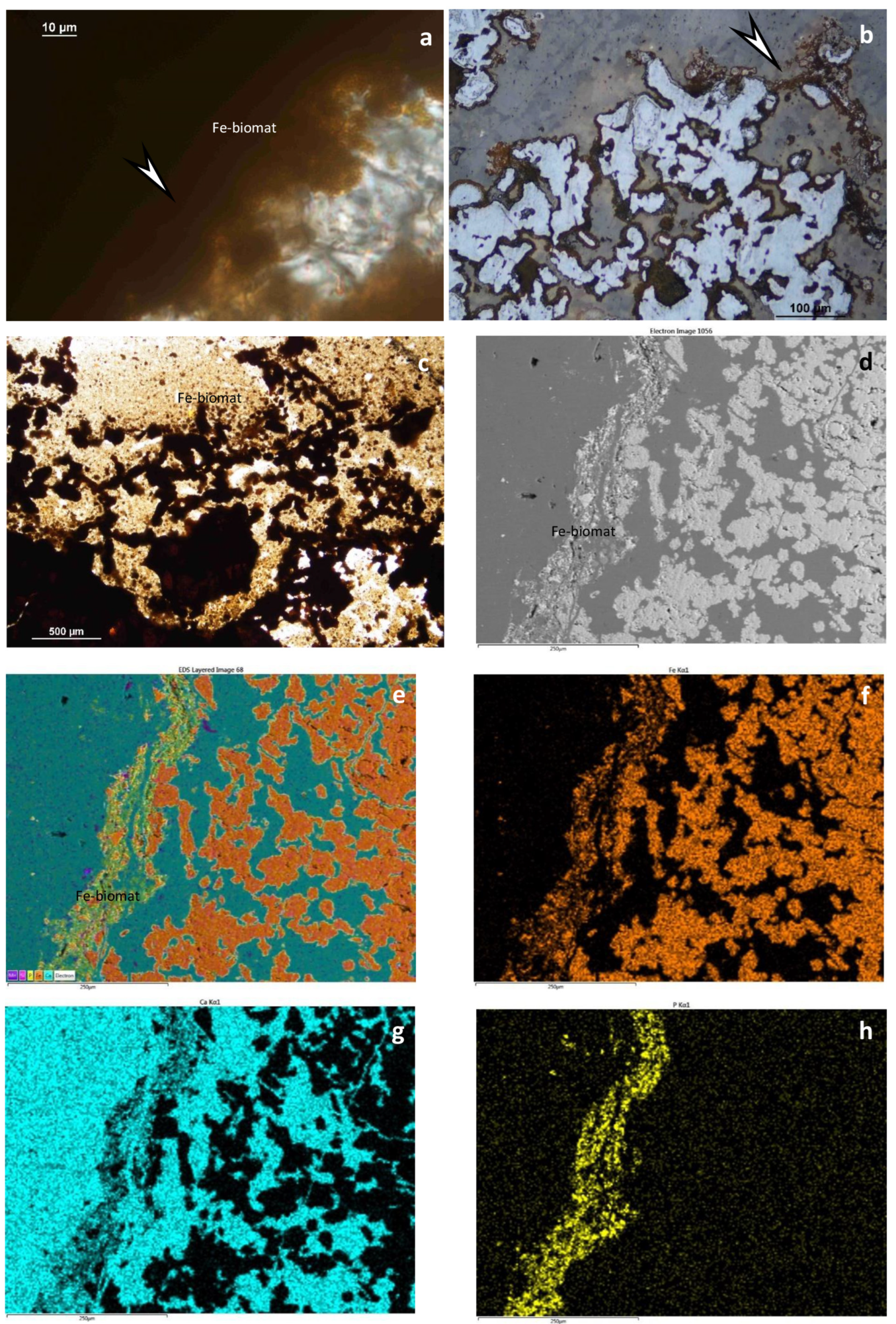
4.1.1. Hydrothermal Deposits (Figure 3 and Figure 4)
4.1.2. Limestone Beds (Apátvarasd Limestone Fm.)
4.2. Jeri Plowland (Apátvarasd Limestone, Figure 6)

5. Results
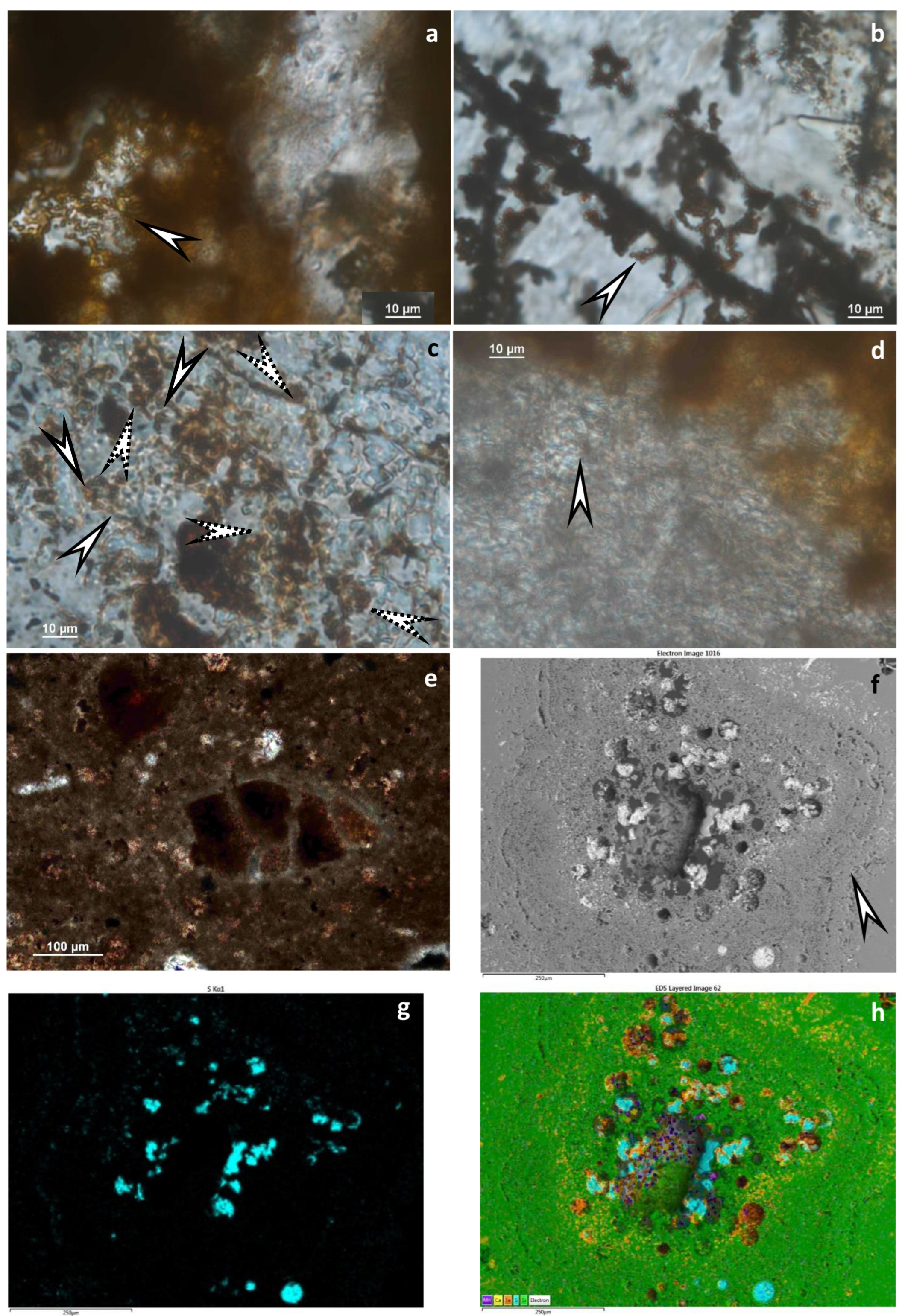
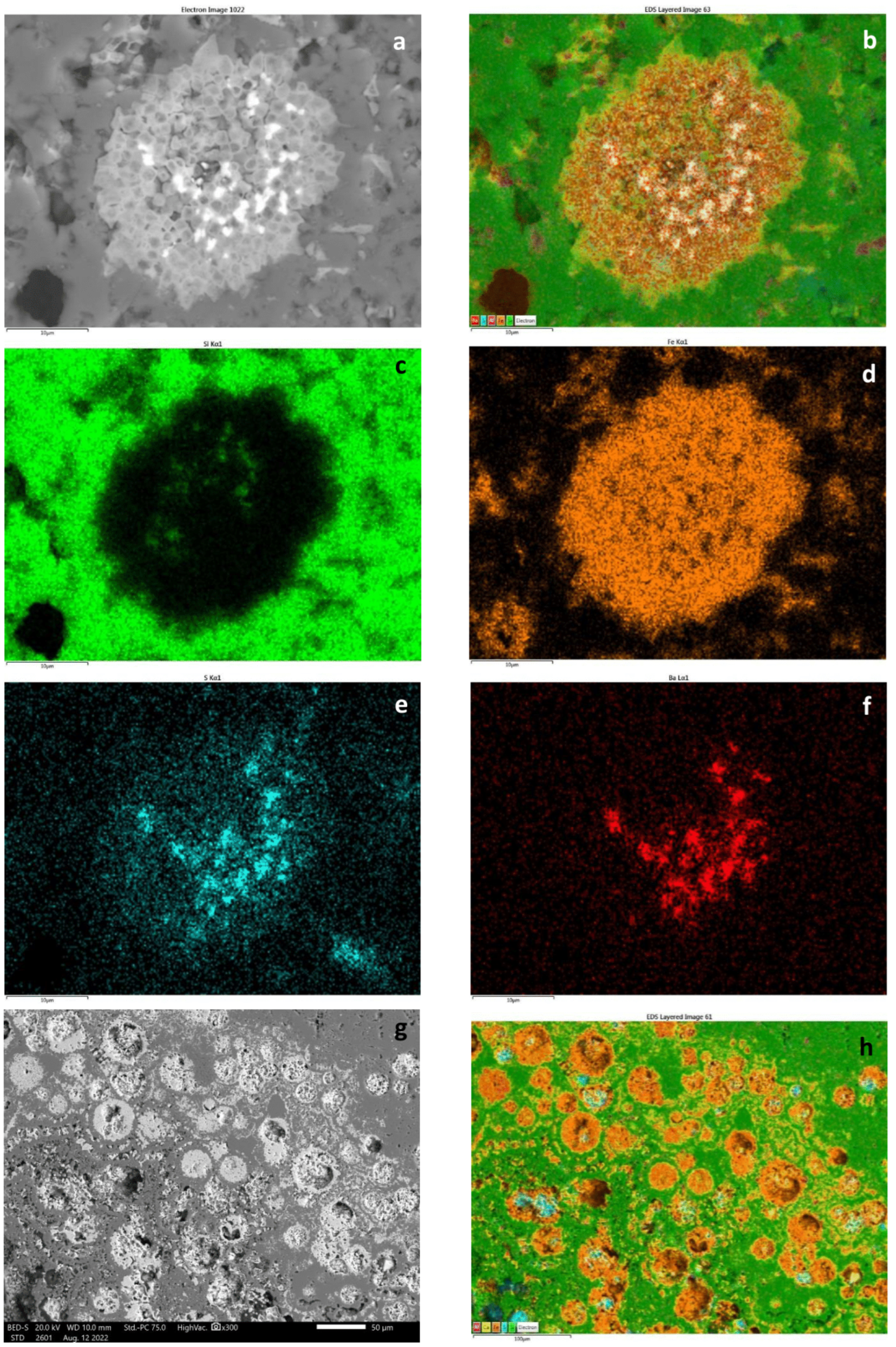
5.1. Textural and Mineralogical Observations
Optical Rock Microscopy (OM) and SEM–EDS
5.2. Mineralogical Composition
5.2.1. X-ray Powder Diffraction
5.2.2. FTIR
5.3. Microbial Activity
5.4. Fauna and Food Chain
6. Discussion
6.1. Mineralogy
6.2. Bacteria–Crustacean Interactions
6.3. Minerals–Crustacean Interactions
6.4. Ecology
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corliss, J.B.; Dymond, J.; Gordon, L.I.; Edmond, J.M.; Von Herzen, R.P.; Ballard, R.D.; Green, K.; Williams, D.; Bainbridge, A.; Crane, K.; et al. Submarine thermal springs on the Galápagos Rift. Science 1979, 203, 1073–1083. [Google Scholar] [CrossRef]
- Haymon, R.M.; Koski, R.A.; Sinclair, C. Fossils of hydrothermal vent worms from Cretaceous sulfide ores of the Samail Ophiolite, Oman. Science 1984, 223, 1407–1409. [Google Scholar] [CrossRef]
- Ramondenc, P.; Germanovich, L.N.; Von Damm, K.L.; Lowell, R.P. The first measurements of hydrothermal heat output at 9°50′N, East Pacific Rise. Earth Planet. Sci. Lett. 2006, 245, 487–497. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Szafrański, K.M. InterRidge Global Database of Active Submarine Hydrothermal Vent Fields Version 3.4. PANGAEA. 2020. Available online: https://doi.pangaea.de/10.1594/PANGAEA.917894 (accessed on 1 January 2022).
- Georgieva, M.N.; Little, C.T.S.; Maslennikov, V.V.; Glover, A.G.; Ayupova, N.R.; Herrington, R.J. The history of life at hydrothermal vents. Earth-Sci. Rev. 2021, 217, 103602. [Google Scholar] [CrossRef]
- Bemis, K.; Lowell, R.P.; Farough, A. Diffuse flow on and around hydrothermal vents at mid-ocean ridges. Oceanography 2012, 25, 182–191. [Google Scholar] [CrossRef]
- Price, R.E.; Giovannelli, D. A Review of the geochemistry and microbiology of marine shallow-water hydrothermal vents. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Procesi, M.; Ciotoli, G.; Mazzini, A.; Etiope, G. Sediment-hosted geothermal systems: Review and first global mapping. Earth-Sci. Rev. 2019, 192, 529–544. [Google Scholar] [CrossRef]
- Bell, J.B.; Woulds, C.; Brown, L.E.; Sweeting, C.J.; Reid, W.D.K.; Little, C.T.S.; Glover, A.G. Macrofaunal ecology of sedimented hydrothermal vents in the Bransfield Strait, Antarctica. Front. Mar. Sci. 2016, 3, 32. [Google Scholar] [CrossRef]
- Kamenev, G.M.; Fadeev, V.I.; Selin, N.I.; Tarasov, V.G. Composition and distribution of macro- and meiobenthos around sublittoral hydrothermal vents in the Bay of Plenty, New Zealand. N. Z. J. Mar. Freshw. Res. 1993, 27, 407–418. [Google Scholar] [CrossRef]
- Levin, L.A.; Mendoza, G.F.; Konotchick, T.; Lee, R. Macrobenthos community structure and trophic relationships within active and inactive Pacific hydrothermal sediments. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1632–1648. [Google Scholar] [CrossRef]
- Bernardino, A.F.; Levin, L.A.; Thurber, A.R.; Smith, C.R. Comparative composition, diversity and trophic ecology of sediment macrofauna at vents, seeps and organic falls. PLoS ONE 2012, 7, e33515. [Google Scholar] [CrossRef]
- Campbell, K.A. Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: Past developments and future research directions. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 232, 362–407. [Google Scholar] [CrossRef]
- Sievert, S.M.; Hügler, M.; Taylor, C.D.; Wirsen, C.O. Sulfur oxidation at deep-sea hydrothermal vents. In Microbial Sulfur Metabolism; Dahl, C., Friedrich, C.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 238–258. [Google Scholar] [CrossRef]
- Wirsen, C.O.; Brinkhoff, T.; Kuever, J.; Muyzer, G.; Molyneaux, S.; Jannasch, H.W. Comparison of a new Thiomicrospira strain from the Mid-Atlantic Ridge with known hydrothermal vent isolates. Appl. Environ. Microbiol. 1998, 64, 4057–4059. [Google Scholar] [CrossRef] [PubMed]
- Detmers, J.; Brüchert, V.; Habicht, K.S.; Kuever, J. Diversity of sulfur isotope fractionations by sulfate-reducing prokaryotes. Appl. Environ. Microbiol. 2001, 67, 888–894. [Google Scholar] [CrossRef]
- Pellerin, A.; Antler, G.; Holm, S.A.; Findlay, A.J.; Crockford, P.W.; Turchyn, A.W.; Jørgensen, B.B.; Finster, K. Large sulfur isotope fractionation by bacterial sulfide oxidation. Sci. Adv. 2019, 5, eaaw1480. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J. A zengővárkonyi vasérckutatás. Bányászati Lapok 1961, 94, 187–194. [Google Scholar]
- Sztrókay, K.I. Mecseki vasércképződés. Magy. Tud. Akad. Műszaki Oszt. Közl 1952, 3, 11–23. [Google Scholar]
- Pantó, G.; Varrók, K.; Kopek, G. Nouvelles contributions à la géologie du gisement de minerai de fer de Zengővárkony. Földtani Közlöny 1955, 85, 125–144. [Google Scholar]
- Palik, P. Remains of crustacean excrement from the Lower Cretaceous of Hungary. Micropaleontology 1965, 11, 98–104. [Google Scholar] [CrossRef]
- Pantó, G. Mezozóos magmatizmus Magyarországon. Magy. Áll Földt Int. Évkönyve 1961, 49, 785–799. [Google Scholar]
- Hetényi, R.; Hámor, G.; Nagy, I. Magyarázó a Mecsek Hegység Földtani Térképéhez, 10,000-es Sorozat; Geological Institute of Hungary: Budapest, Hungary, 1968. [Google Scholar]
- Császár, G. Urgonian facies of the Tisza Unit. Acta Geol. Hung. 1992, 35, 263–285. [Google Scholar]
- Császár, G. Urgon Formations in Hungary with special reference to the Eastern Alps, the Western Carpathians and the Apuseni Mountains. Geol. Hung. Ser. Geol. 2002, 25, 1–209. [Google Scholar]
- Bércziné, M.A.; Császár, G.; Nusszer, A. Stratigraphy and geological evolution of the Mesozoic basement of the Mecsek Zone in the Central Part of the Great Hungarian Plain (East-Central Hungary). Földtani Közlöny 1996, 126, 185–207. [Google Scholar]
- Bujtor, L.; Janssen, N.M.; Verreussel, R.M. Early Cretaceous (Valanginian and Hauterivian) belemnites and organic-walled dinoflagellate cysts from a marine hydrothermal vent site and adjacent facies of the Mecsek Mts., Hungary. Neues Jahrb. Geol. Paläontologie 2013, 269, 135–148. [Google Scholar] [CrossRef]
- László, B. Early Valanginian brachiopods from the Mecsek Mts. (southern Hungary) and their palaeobiogeographical significance. Neues Jahrb. Geol. Paläontologie Abh. 2006, 241, 111–152. [Google Scholar] [CrossRef]
- Bujtor, L. A unique Valanginian paleoenvironment at an iron ore deposit near Zengõvárkony (Mecsek Mts, South Hungary), and a possible genetic model. Cent. Eur. Geol. 2007, 50, 183–198. [Google Scholar] [CrossRef]
- Jáger, V.; Molnár, F. Lower Cretaceous continental rift-type black smoker system in the East Mecsek Mts. Mitteil Österr Mineralog. Ges. 2009, 155, 70. [Google Scholar]
- Jáger, V.; Molnár, F.; Buchs, D.; Koděra, P. The connection between iron ore formations and “mud-shrimp” colonizations around sunken wood debris and hydrothermal sediments in a Lower Cretaceous continental rift basin, Mecsek Mts., Hungary. Earth-Sci. Rev. 2012, 114, 250–278. [Google Scholar] [CrossRef]
- Bujtor, L.; Nagy, J. Fauna, palaeoecology and ecotypes of the Early Cretaceous sediment hosted hydrothermal vent environment of Zengővárkony (Mecsek Mountains, Hungary). Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 564, 110179. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Baker, E.T.; German, C.R.; Maffei, A. An authoritative global database for active submarine hydrothermal vent fields. Geochem. Geophys. Geosyst. 2013, 14, 4892–4905. [Google Scholar] [CrossRef]
- Cohen, K.; Finney, S.; Gibbard, P.; Fan, J.-X. The ICS International Chronostratigraphic Chart. Episodes 2013, 36, 199–204. [Google Scholar] [CrossRef]
- Ferretti, A.; Messori, F.; Di Bella, M.; Sabatino, G.; Quartieri, S.; Cavalazzi, B.; Italiano, F.; Barbieri, R. Armoured sponge spicules from Panarea Island (Italy): Implications for their fossil preservation. Palaeogeogr. Palaeoclim. Palaeoecol. 2019, 536, 109379. [Google Scholar] [CrossRef]
- Di Bella, M.; Sabatino, G.; Quartieri, S.; Ferretti, A.; Cavalazzi, B.; Barbieri, R.; Foucher, F.; Messori, F.; Italiano, F. Modern Iron Ooids of Hydrothermal Origin as a Proxy for Ancient Deposits. Sci. Rep. 2019, 9, 7107. [Google Scholar] [CrossRef] [PubMed]
- Van Dover, C.L. The Ecology of Deep-Sea Hydrothermal Vents; Princeton University: Press, Princeton, NJ, USA, 2000. [Google Scholar]
- Sievert, S.M.; Brinkhoff, T.; Muyzer, G.; Ziebis, W.; Kuever, J. Spatial heterogeneity of bacterial populations along an environmental gradient at a shallow submarine hydrothermal vent near Milos Island (Greece). Appl. Environ. Microbiol. 1999, 65, 3834–3842. [Google Scholar] [CrossRef]
- Pohl, M.E. Ecological observations on Callianassa major Say at Beaufort, North Carolina. Ecology 1946, 27, 71–80. [Google Scholar] [CrossRef]
- Felgenhauer, B.E. Internal anatomy of the Decapoda: An overview. In Microscopic Anatomy of Invertebrates, Decapod Crustacea; Harrison, F.W., Humes, A.G., Eds.; Wiley-Liss Inc.: New York, NY, USA, 1992; Volume 10, pp. 45–75. [Google Scholar]
- Schweigert, G.; Seegis, D.B.; Fels, A.; Leinfelder, R.R. New internally structured decapod microcoprolites from Germany (Late Triassic/Early Miocene), Southern Spain (Early/Middle Jurassic) and Portugal (Late Jurassic): Taxonomy, paleoecology, and evolutionary implications. Pal. Zeitschr. 1997, 71, 51–69. [Google Scholar] [CrossRef]
- Aliani, S.; Bianchi, N.C.; Cocito, S.; Dando, P.R.; Meloni, R.; Morri, C.; NieMeyer, A.; Peirano, A.; Ziebis, W. A map of seagrass meadows in Paleochori Bay (Milos Island, Greece), a marine area with hydrothermal activity. Rapp. Comm. Int. Mer. Médit. 1998, 35, 512–513. [Google Scholar]
- Madejová, J. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Parikh, S.J.; Chorover, J. ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide. Langmuir 2006, 22, 8492–8500. [Google Scholar] [CrossRef]
- Glotch, T.D.; Rossman, G.R. Mid-infrared reflectance spectra and optical constants of six iron oxide/oxyhydroxide phases. Icarus 2009, 204, 663–671. [Google Scholar] [CrossRef]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Müller, C.M.; Pejcic, B.; Esteban, L.; Piane, C.D.; Raven, M.; Mizaikoff, B. Infrared Attenuated Total Reflectance Spectroscopy: An Innovative Strategy for Analyzing Mineral Components in Energy Relevant Systems. Sci. Rep. 2014, 4, 6764. [Google Scholar] [CrossRef] [PubMed]
- Zviagina, B.B.; Drits, V.A.; Dorzhieva, O.V. Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data. Minerals 2020, 10, 153. [Google Scholar] [CrossRef]
- Polgári, M.; Gyollai, I. Comparative study of formation conditions of Fe-Mn Ore Microbialites based on mineral assemblages: A critical self-overview. Minerals 2022, 12, 1273. [Google Scholar] [CrossRef]
- Cady, S.L.; Farmer, J.D.; Grotzinger, J.P.; Schopf, J.W.; Steele, A. Morphological Biosignatures and the Search for Life on Mars. Astrobiology 2003, 3, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Polgári, M.; Gyollai, I.; Fintor, K.; Horváth, H.; Pál-Molnár, E.; Biondi, J.C. Microbially Mediated ore-forming processes and cell mineralization. Front. Microbiol. 2019, 10, 2731. [Google Scholar] [CrossRef]
- Knoll, A.H.; Canfield, D.E.; Konhauser, K.O. Fundamentals of Geobiology; Wiley-Blackwell: Oxford, UK, 2012; p. 456. [Google Scholar]
- Csontos, L.; Vörös, A. Mesozoic plate tectonic reconstruction of the Carpathian region. Palaeogeogr. Palaeoclim. Palaeoecol. 2004, 210, 1–56. [Google Scholar] [CrossRef]
- Haas, J.; Péró, C. Mesozoic evolution of the Tisza Mega-unit. Int. J. Earth Sci. 2004, 93, 297–313. [Google Scholar] [CrossRef]
- Hofmann, K. A Vihorlat-Guttin-hegység némely quarctartalmu trachytjának plagioklas-kristályairól (1873). In Hungarian Geological Bulletin (Földtani Közlöny); Geological Institute of Hungary: Budapest, Hungary, 1873; p. 100. (In Hungarian) [Google Scholar]
- Viczián, I. Submarine ausbruch- und Gesteinzersetzungserscheinungen im unterkrtetazischen Diabaskomplex der Bohrung Kisbattyán Nr. 1. Ann. Rep. Geol. Inst. Hung. 1966, 1964, 75–92. [Google Scholar]
- Harangi, S.; Árva-Soós, G. Early Cretaceous volcanic rocks of the Mecsek Mountains (South Hungary) I. Mineralogy and petrology. Földtani Közlöny 1993, 123, 129–165. [Google Scholar]
- Bujtor, L. The Early Valanginian ammonite, brachiopod and crustacean fauna of the Mecsek Mts. and its relationships with the embryonic shallow water hydrothermal vent at Zengővárkony (Mecsek Mts., South Hungary). Cretac. Res. 2011, 32, 565–574. [Google Scholar] [CrossRef]
- Bujtor, L. A Valanginian crustacean microcoprolite ichnofauna from the shallow-marine hydrothermal vent site of Zengővárkony (Mecsek Mts., Hungary). Facies 2012, 58, 249–260. [Google Scholar] [CrossRef]
- Bujtor, L. The paleontological character of the Lower Cretaceous (Valanginian) hydrothermal vent filling of the Mecsek Mts, Hungary. Földtani Közlöny 2012, 142, 137–148. [Google Scholar]
- Bujtor, L.; Szinger, B. Micropaleontological observations on the Lower Cretaceous iron ore-related formations of the Mecsek Mts. (Upper Valanginian–Lower Hauterivian, South Hungary). Cent. Eur. Geol. 2018, 61, 136–159. [Google Scholar] [CrossRef]
- Lee, W.-K.; Juniper, S.K.; Perez, M.; Ju, S.-J.; Kim, S.-J. Diversity and characterization of bacterial communities of five co-occurring species at a hydrothermal vent on the Tonga Arc. Ecol. Evol. 2021, 11, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Versteegh, E.A.; Van Dover, C.L.; Van Audenhaege, L.; Coleman, M. Multiple nutritional strategies of hydrothermal vent shrimp (Rimicaris hybisae) assemblages at the Mid-Cayman Rise. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2023, 192, 103915. [Google Scholar] [CrossRef]
- Hessler, R.R.; Smithey, W.M. The distribution and community structure of megafauna at the Galapagos Rift hydrothermal vents. In Hydrothermal Processes at Seafloor Spreading Centers; Rona, P.A., Bostrom, K., Laubier, L., Smith, K., Jr., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 735–770. [Google Scholar]
- Bujtor, L.; Vörös, A. New kingenoid (Terebratellidina) brachiopods with larger body sizes from the Early Cretaceous of Zengővárkony (Mecsek Mountains, Hungary). J. Paléontol. 2019, 94, 475–488. [Google Scholar] [CrossRef]
- Fautin, D.G.; Barber, B.R. Maractis rimicarivora, a new genus and species of sea anemone (Cnidaria: Anthozoa: Actiniaria: Actinostolidae) from an Atlantic hydrothermal vent. Proc. Biol. Soc. Wash. 1999, 112, 624–631. [Google Scholar]
- Kolosváry, G. Les coralliaires du Crétacé de la Hongrie. Magy. Áll Földt Int. Évkönyve 1954, 42, 67–163. [Google Scholar]
- Kolosváry, G. Über erste Korallenfunde aus der Jura-Zeit des Mecsek-Gebirges in Süd-Ungarn. Acta Univ. Szeged. Acta Biol. 1956, 2, 199–207. [Google Scholar]
- Kolosváry, G. Korallen aus der Unterkreide des Mecsek-Gebirges. Acta Univ. Szeged. Acta Biol. 1959, 5, 125–128. [Google Scholar]
- Voight, V.R. A review of predators and predation at deep-sea hydrothermal vents. Cah. Biol. Mar. 2000, 41, 155–166. [Google Scholar]
- Van Dover, C.L.; Desbruyères, D.; Segonzac, M.; Comtet, T.; Saldanha, L.; Fiala-Médioni, A.; Langmuir, C. Biology of the Lucky Strike hydrothermal field. Deep. Sea Res. I 1996, 43, 1509–1529. [Google Scholar] [CrossRef]
- Bujtor, L. Cretaceous echinoid (Plegiocidaris) from the Mecsek Mts, Hungary. Földtani Közlöny 2013, 143, 321–326. [Google Scholar]
- González, A.F.; Guerra, A.; Pascual, S.; Briand, P. Vulcanoctopus hydrothermalis gen. et sp. nov. (Molluska, Cephalopoda): An octopod from a deep-sea hydrothermal vent site. Cah. Biol. Mar. 1998, 39, 169–184. [Google Scholar]
- Strugnell, J.; Voight, J.R.; Collins, P.C.; Allcock, A.L. Molecular phylogenetic analysis of a known and a new hydrothermal vent octopod: Their relationships with the genus Benthoctopus (Cephalopoda: Octopodidae). Zootaxa 2009, 2096, 442–459. [Google Scholar] [CrossRef]
- Kniesz, K.; Jażdżewska, A.M.; Arbizu, P.M.; Kihara, T.C. DNA Barcoding of Scavenging Amphipod Communities at Active and Inactive Hydrothermal Vents in the Indian Ocean. Front. Mar. Sci. 2022, 8, 752360. [Google Scholar] [CrossRef]
- Ivarson, K.C. Microbiological formation of basic ferric sulfates. Can. J. Soil Sci. 1973, 53, 315–323. [Google Scholar] [CrossRef]
- Tazaki, K.; Mori, T.; Nonaka, T. Microbial jarosite and gypsum from corrosion of Portland cement concrete. Can. Miner. 1992, 30, 431–444. [Google Scholar]
- Farkas, I.M. Environmental Mineralogical and Geochemical Studies on the Bányabérc Waste Dump in the Gyöngyösoroszi Mining Area, Hungary. Ph.D. Dissertation, Faculty of Science, Eötvös Loránd University, Budapest, Hungary, 2012. [Google Scholar]
- Labrado, A.L.; Brunner, B.; Bernasconi, S.M.; Peckmann, J. Formation of Large Native Sulfur Deposits Does Not Require Molecular Oxygen. Front. Microbiol. 2019, 10, 24. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lack, J.G.; Coates, J.D. Biogenic Magnetite Formation through Anaerobic Biooxidation of Fe(II). Appl. Environ. Microbiol. 2001, 67, 2844–2848. [Google Scholar] [CrossRef]
- Piepenbrock, A.; Dippon, U.; Porsch, K.; Appel, E.; Kappler, A. Dependence of microbial magnetite formation on humic substance and ferrihydrite concentrations. Geochim. Cosmochim. Acta 2011, 75, 6844–6858. [Google Scholar] [CrossRef]
- Polgári, M.; Hein, J.; Vigh, T.; Szabó-Drubina, M.; Fórizs, I.; Bíró, L.; Müller, A.; Tóth, A. Microbial processes and the origin of the Úrkút manganese deposit, Hungary. Ore Geol. Rev. 2012, 47, 87–109. [Google Scholar] [CrossRef]
- Baele, J.M.; Bouvain, F.; De Jong, J.; Matielli, N.; Papier, S.; Préat, A. Iron microbial mats in Modern and Phanerozoic environments. In Instruments, Methods, and Missions for Astrobiology XI; International Society for Optics and Photonics: Washington, DC, USA, 2008; Volume 7097, p. 70970N12. [Google Scholar]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth-Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- McGaw, I.J.; Curtis, D.L. A review of gastric processing in decapod crustaceans. J. Comp. Physiol. B 2012, 183, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, M.; Gallet, A.; Szafranski, K.M.; Machon, J.; Ravaux, J.; Léger, N.; Duperron, S. Blow Your Nose, Shrimp! Unexpectedly dense bacterial communities occur on the antennae and antennules of hydrothermal vent shrimp. Front. Mar. Sci. 2018, 5, 357. [Google Scholar] [CrossRef]
- Zbinden, M.; Cambon-Bonavita, M. Rimicaris exoculata: Biology and ecology of a shrimp from deep-sea hydrothermal vents associated with ectosymbiotic bacteria. Mar. Ecol. Prog. Ser. 2020, 652, 187–222. [Google Scholar] [CrossRef]
- Guri, M.; Durand, L.; Cueff-Gauchard, V.; Zbinden, M.; Crassous, P.; Shillito, B.; Cambon-Bonavita, M.-A. Acquisition of epibiotic bacteria along the life cycle of the hydrothermal shrimp Rimicaris exoculate. ISME J. 2012, 6, 597–609. [Google Scholar] [CrossRef]
- Apremont, V.; Cambon-Bonavita, M.-A.; Cueff-Gauchard, V.; François, D.; Pradillon, F.; Corbari, L.; Zbinden, M. Gill chamber and gut microbial communities of the hydrothermal shrimp Rimicaris chacei Williams and Rona 1986: A possible symbiosis. PLoS ONE 2018, 13, e0206084. [Google Scholar] [CrossRef]
- Vermeij, G.J. The Mesozoic marine revolution: Evidence from snails, predators and grazers. Paleobiology 1977, 3, 245–258. [Google Scholar] [CrossRef]






| Sample No. | ID Number | Sampling Site | Description | Used Methods | |||
|---|---|---|---|---|---|---|---|
| XRD | OM | FTIR | SEM (EDS) | ||||
| 1 | 1 | SE, Dezső Rezső Valley, Zengővárkony, Mecsek Mountains | Weathered sample fragments from hydrothermal channels through the nonconsolidated lime mud during venting. | 1 | 44 | 64 | 26 (107) 4 maps |
| 2 | 4 | SE, Dezső Rezső Valley, Zengővárkony, Mecsek Mountains | Packet of hydrothermal channels nested in and among calcite-quartz mineral nests developed on the volcanite surface. | 1 | 25 | 34 | 6 (40) |
| 3 | 9 | Jeri plowland S of Dezső Rezső Valley, Zengővárkony, Mecsek | Peperite sample block from the plowland revealing purple-colored micrite, biogenic fragments (mainly echinoderms) and altered volcanite grains with a greater fragment of a bivalve shell. | 1 | 20 | 25 | 5 (28) |
| 4 | 12 | NW Dezső Rezső Valley, Zengővárkony, Mecsek Mountains | Metasomatized limestone block. | 1 | 21 | 26 | 6 (28) |
| 5 | 13 | NW Dezső Rezső Valley, Zengővárkony, Mecsek Mountains | Sample of a partly metasomatized limestone block revealed purple-colored micrite with mudstone texture. | 1 | 37 | 23 | 10 (28) 4 maps |
| Total | 5 | 5 | 147 | 172 | 53 (231) 8 maps | ||
| Sample No. | Calcite | Quartz | Hematite | Goethite | Jarosite | Siderite | Pyrite |
|---|---|---|---|---|---|---|---|
| 1 | - | 52 | - | 30 | 5 | - | 13 |
| 4 | 2 | 98 | - | - | - | - | |
| 9 | 92 | 7 | - | 1 | - | - | |
| 12 | 74 | 1 | 2 | 21 | - | 2 | |
| 13 | 67 | 2 | 1 | 30 | - | - |
| Minerals/ Processes | Chemical Formula | Eh | pH | Microbially Mediated | S | D | EPS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxic | Suboxic | Anoxic | Acidic | Neutral-Slightly Alkaline | Alkaline | ||||||
| Native element | |||||||||||
| Native sulfur | * | * | * | ||||||||
| Oxides and hydroxides | |||||||||||
| Quartz | SiO2 | * | * | * | |||||||
| Ferrihydrite | FeOOH | * | * | * | * | ||||||
| Hematite | Fe2O3 | * | * | * | * | ||||||
| Goethite | FeOOH | * | * | * | * | * | |||||
| Magnetite | Fe3O4 | * | * | * | * | ||||||
| Anatase | TiO2–FexTi(1−x)O(2−x)OHx | * | * | * | * | * | |||||
| Ilmenite | FeTiO3 | * | |||||||||
| Romanèchite psilomelane | [(Ba,H2O,Mn5O10, Ba(Mn4+, Mn3+)O10·1.4H2O)] | * | * | * | * | ||||||
| Carbonates | |||||||||||
| Calcite | Ca (CO3) | * | |||||||||
| Dolomite | CaMg(CO3)2 | * | * | * | * | * | * | ||||
| Rhodochrosite | MnCO3 | * | * | * | * | ||||||
| Kutnohorite | (Ca,Mn)(CO3)2 | * | * | ||||||||
| Siderite | FeCO3 | * | * | * | * | ||||||
| Sulphides | |||||||||||
| Pyrite | FeS2 | * | * | * | * | * | |||||
| Silicates | |||||||||||
| Albite (feldspar) | NaAlSi3O8 | * | * | * | |||||||
| Orthoclase (feldspar) | KAlSi3O8 | * | * | * | |||||||
| Ferrierite (zeolite) | [Mg2(K,Na)2Ca0.5](Si29Al7)O72·18H2O | * | * | * | * | ||||||
| Celadonite | KMg0.8Fe2+0.2Fe3+0.9Al0.1Si4O10(OH)2 | * | * | * | * | * | |||||
| Phlogopyte | KMg3AlSi3O10(F,OH)2 (Fe substitution) | * | |||||||||
| Phosphates | |||||||||||
| Apatite | [(Ca10(PO4)6(OH, F, Cl)2] | * | * | * | * | * | * | ||||
| Sulfates | |||||||||||
| Barite | Ba(SO4) | * | * | * | * | * | |||||
| Gypsum | CaSO4·2H2O | * | * | * | * | * | |||||
| Jarosite | Fe3+3(SO4)2(OH)6 | * | * | * | * | * | |||||
| Organic material | * | * | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujtor, L.; Gyollai, I.; Szabó, M.; Kovács, I.; Polgári, M. Bacteria-Driven Fossil Ecosystems as Paleoindicators of Active Continental Margins and the Role of Carbonate Sediment-Hosted Vents in Geodynamic Reconstructions. Minerals 2024, 14, 125. https://doi.org/10.3390/min14020125
Bujtor L, Gyollai I, Szabó M, Kovács I, Polgári M. Bacteria-Driven Fossil Ecosystems as Paleoindicators of Active Continental Margins and the Role of Carbonate Sediment-Hosted Vents in Geodynamic Reconstructions. Minerals. 2024; 14(2):125. https://doi.org/10.3390/min14020125
Chicago/Turabian StyleBujtor, László, Ildikó Gyollai, Máté Szabó, Ivett Kovács, and Márta Polgári. 2024. "Bacteria-Driven Fossil Ecosystems as Paleoindicators of Active Continental Margins and the Role of Carbonate Sediment-Hosted Vents in Geodynamic Reconstructions" Minerals 14, no. 2: 125. https://doi.org/10.3390/min14020125
APA StyleBujtor, L., Gyollai, I., Szabó, M., Kovács, I., & Polgári, M. (2024). Bacteria-Driven Fossil Ecosystems as Paleoindicators of Active Continental Margins and the Role of Carbonate Sediment-Hosted Vents in Geodynamic Reconstructions. Minerals, 14(2), 125. https://doi.org/10.3390/min14020125







