Multistage Diagenetic Fluid Shaping Miocene Island Dolostones on One Isolated Atoll in the South China Sea: Insights from LA-ICP-MS U–Pb Dating and Geochemical Characterization
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
3.1. Sampling
3.2. Petrologic Features and XRD Analysis
3.3. Carbon and Oxygen Isotopes and Mg-Ca Content Analysis
3.4. In Situ Elemental Analysis
3.5. Sr Isotopes and In Situ LA-ICP-MS U–Pb Dating Analysis
4. Results
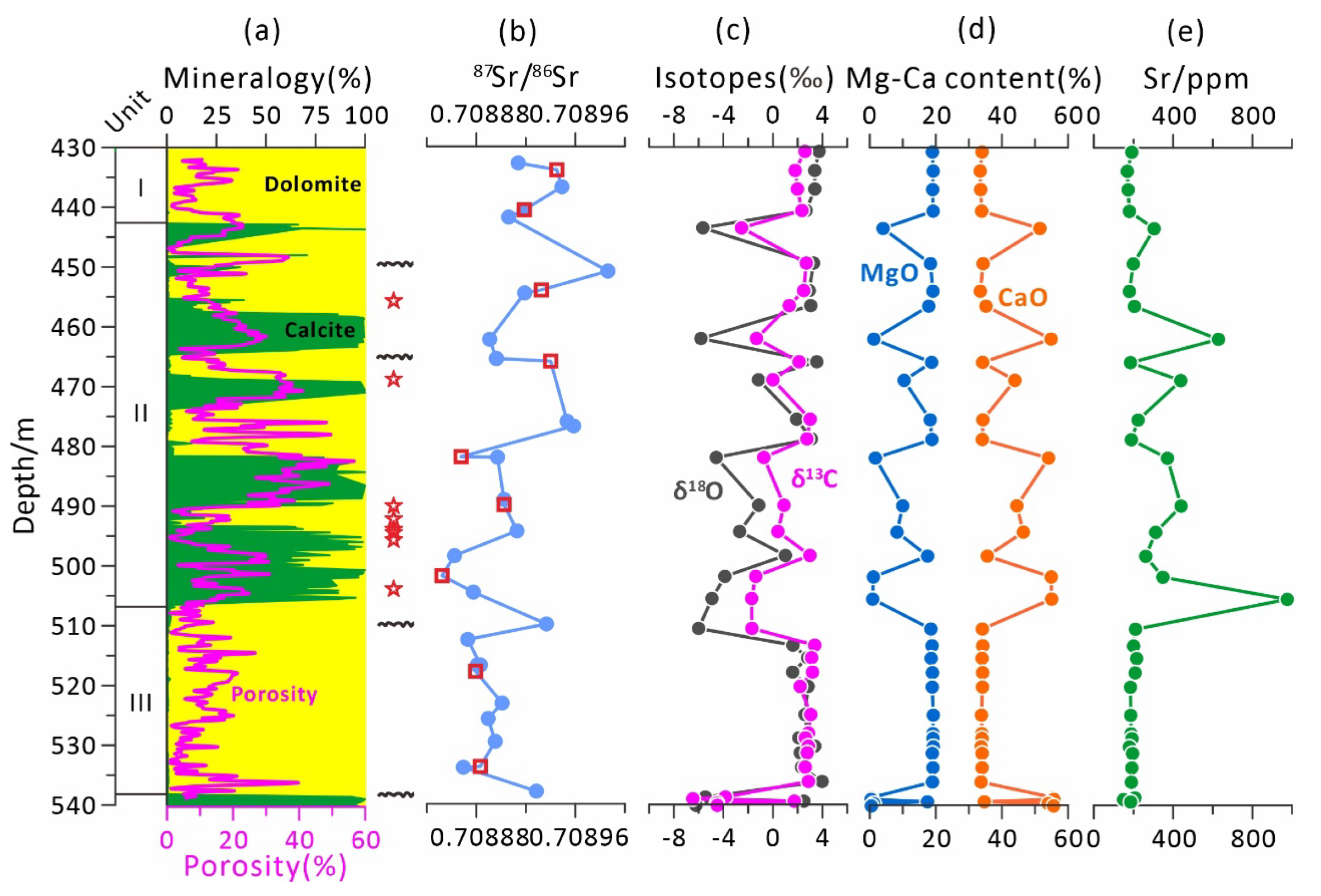
4.1. Lithology and Mineralogy of the Lower Nanwan Formation
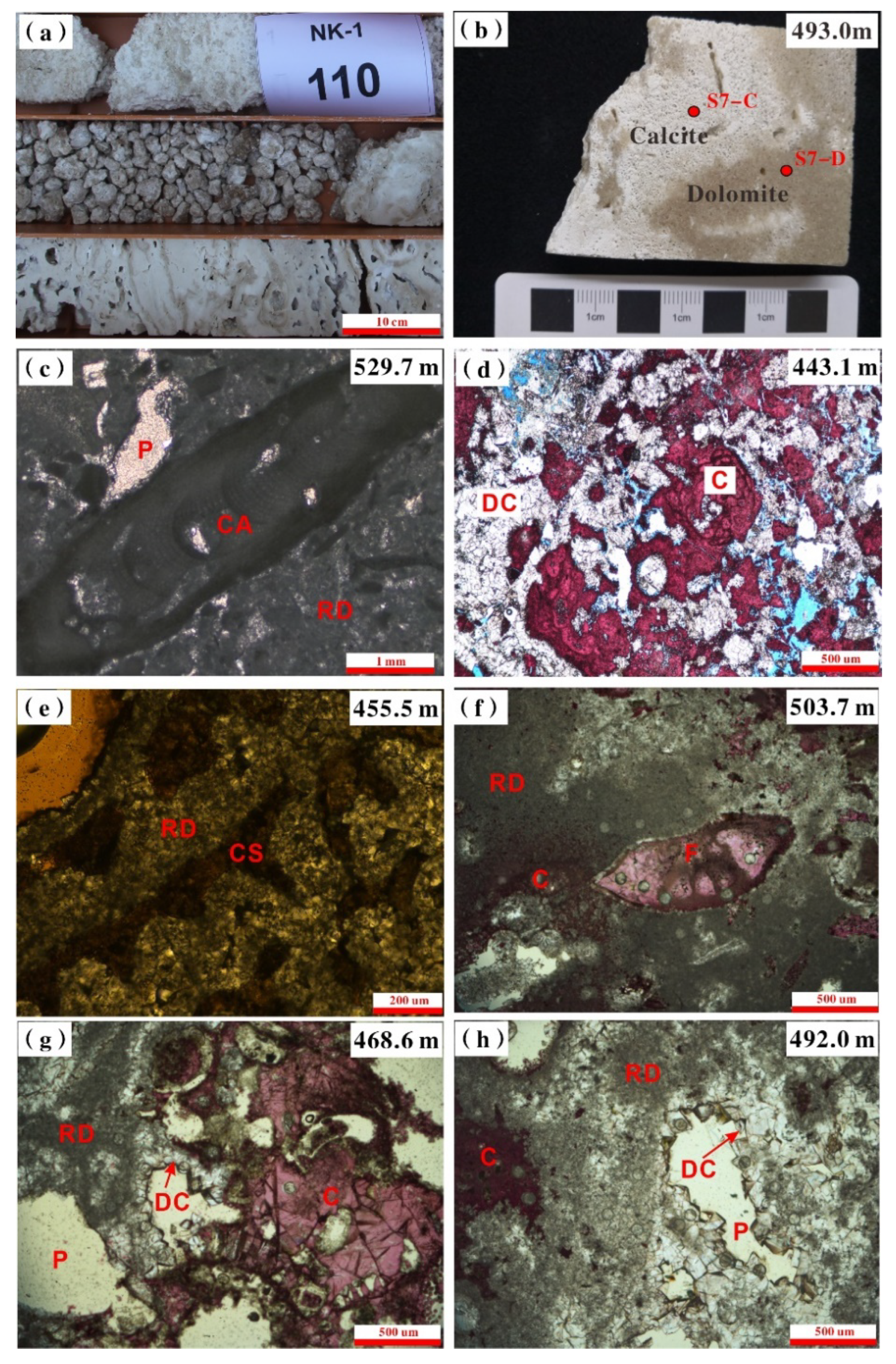
4.2. Dolomite Types
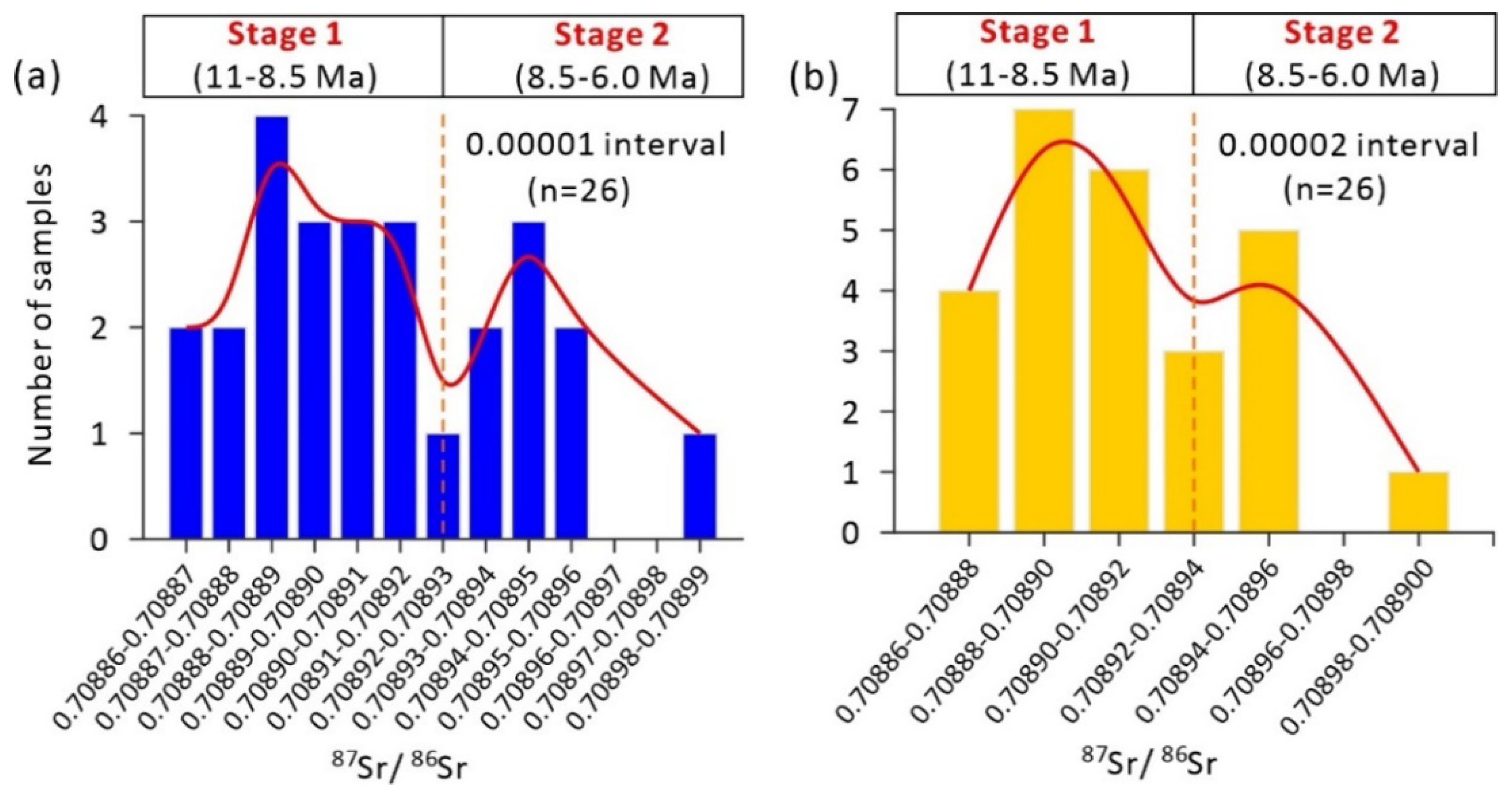
4.3. Sr Isotopic Ages and U–Pb Ages
4.4. Carbon and Oxygen Isotopes
4.5. Elemental Geochemistry
4.5.1. MgO, CaO and Sr Contents of the Bulk Rock
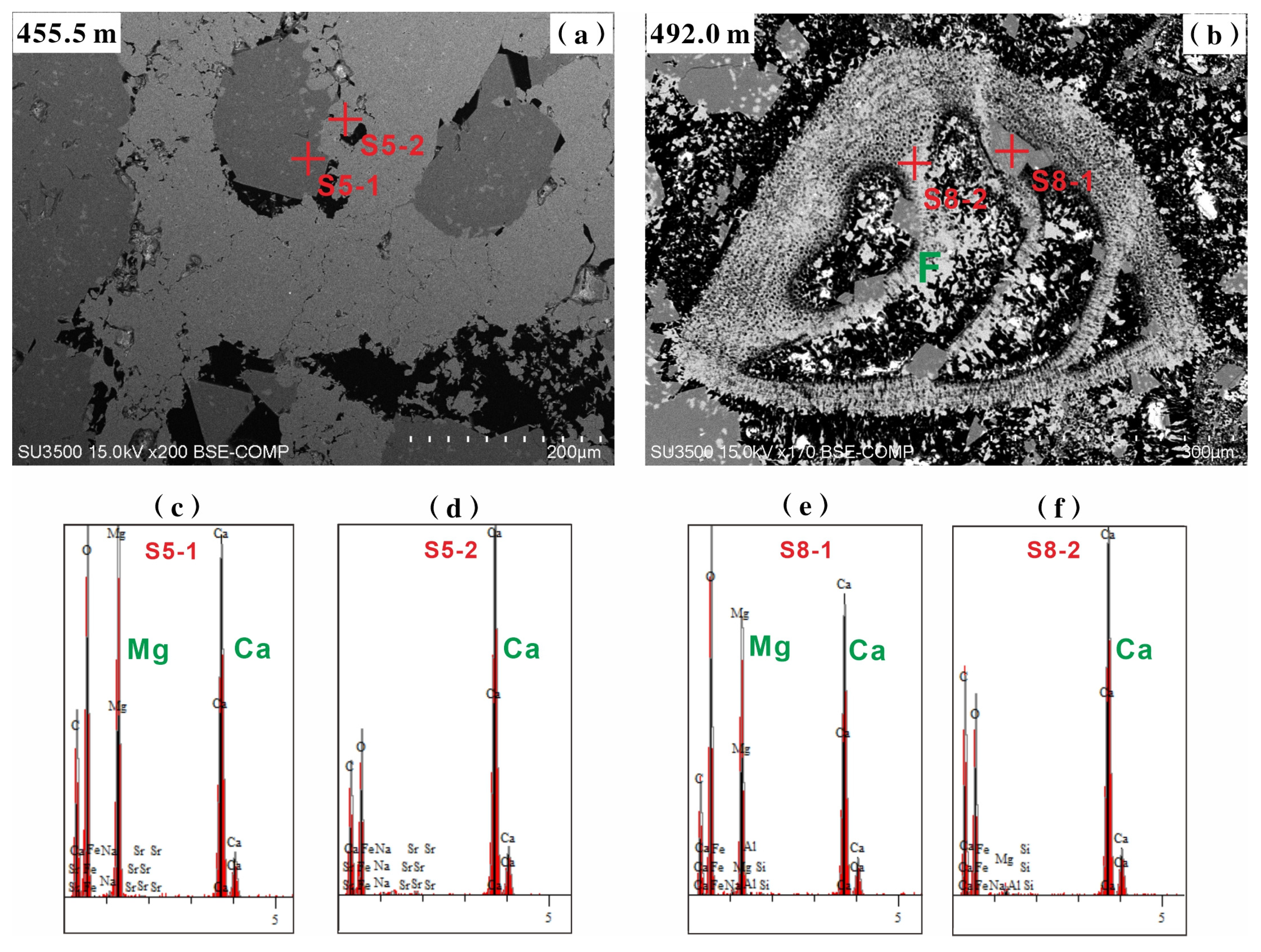
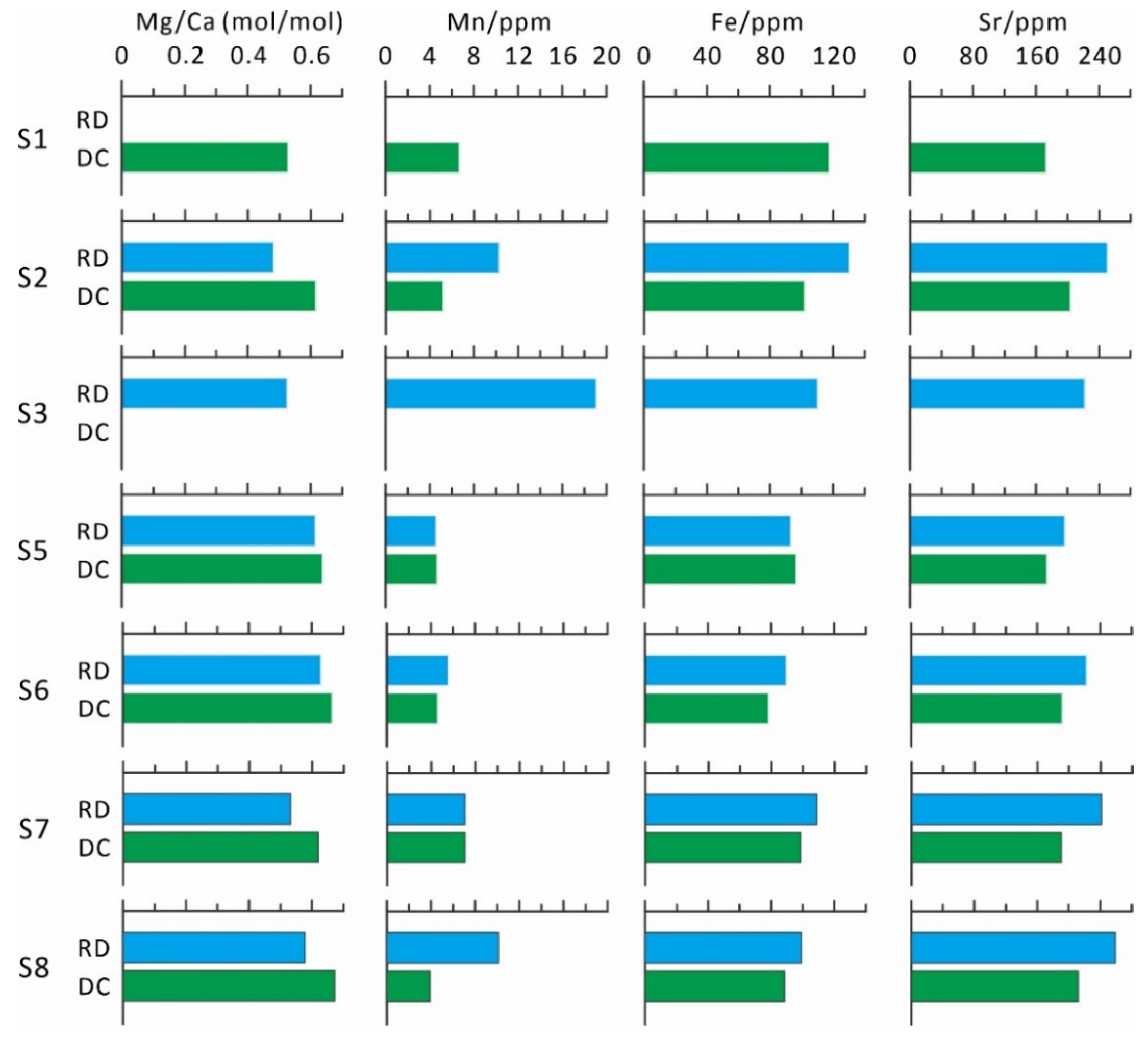
4.5.2. In Situ Fe, Mn, and Sr Concentrations and Mg/Ca Ratio
4.5.3. REY Characteristics
5. Discussion
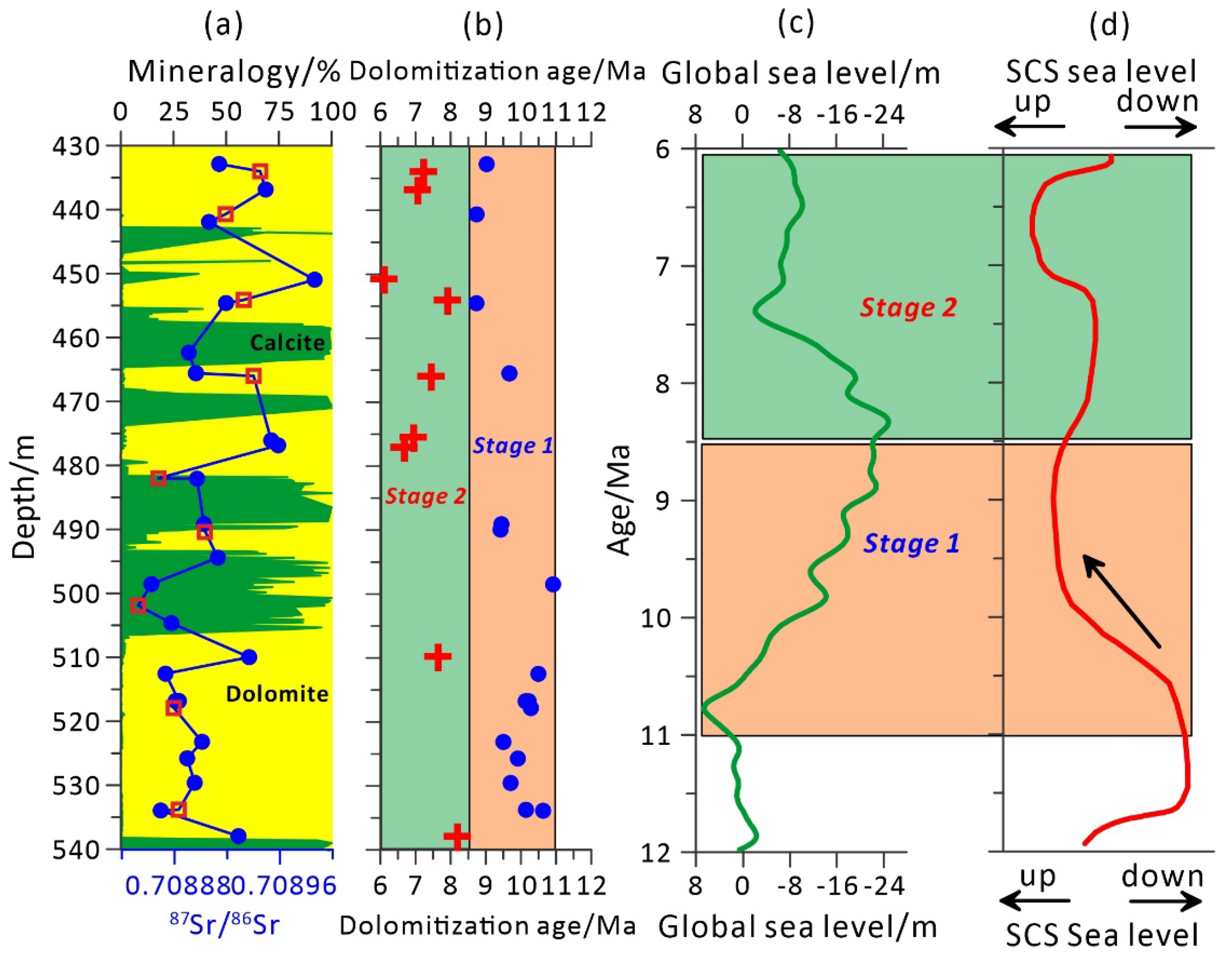
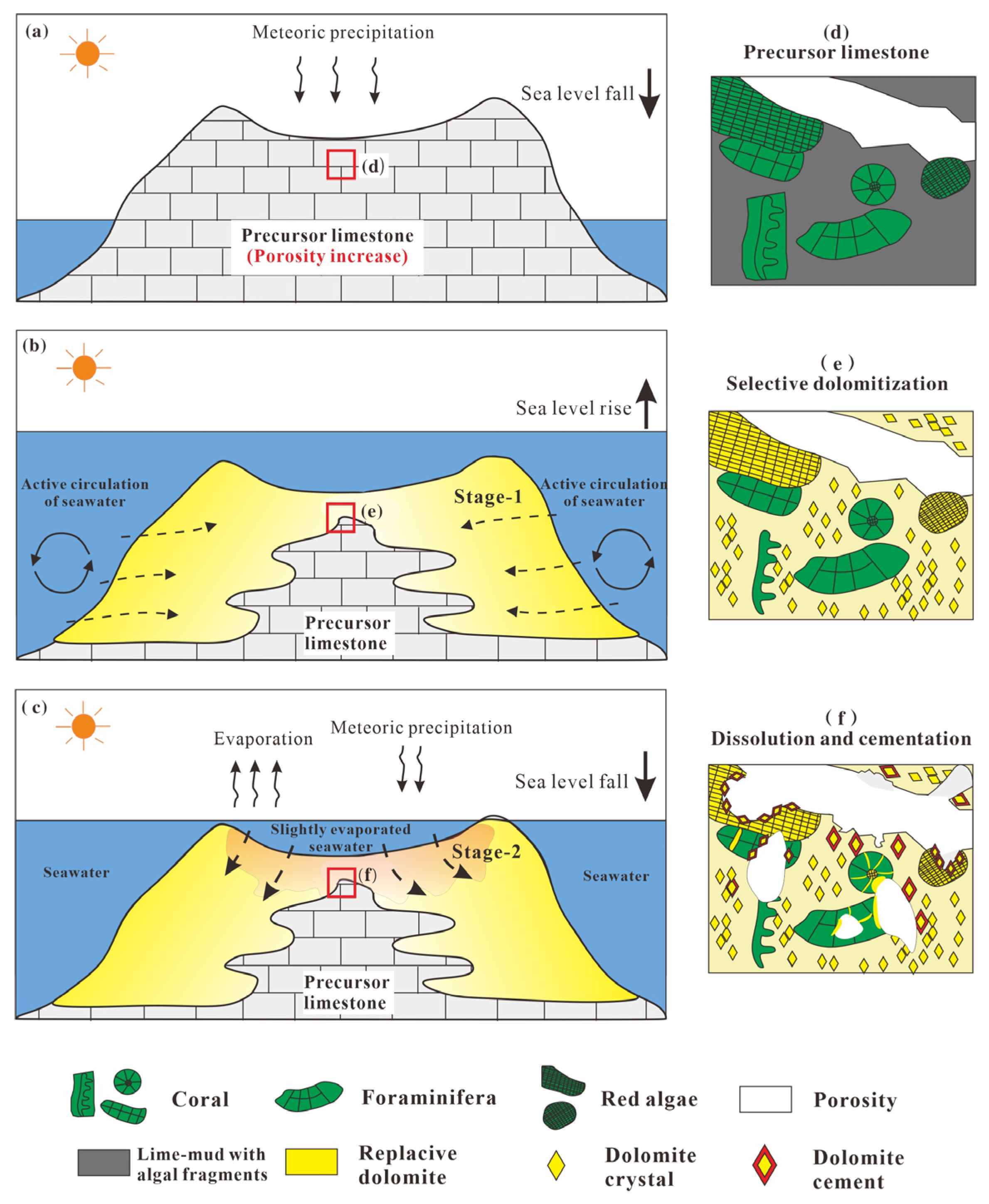
5.1. Ages and Stages of Dolomitization
5.2. Sources and Characteristics of the Dolomitizing Fluids
5.3. Multistage Dolomitization Models for Isolated Atolls
6. Conclusions
- (1)
- Undolomitized calcite, replacive dolomite, and dolomite cement coexisted in the lower Nanwan Formation in the Well NK-1. Replacive dolomite and dolomite cements are nonstoichiometric HCDs.
- (2)
- Fluids of replacive dolomite and dolomite cement in the lower Nanwan Formation are seawater. Higher Mg/Ca ratios are found in dolomite cements, which tended to be mediated by slightly evaporated seawater.
- (3)
- Strontium isotope ages and in situ U–Pb ages suggest that replacive dolomitization occurred soon after carbonate deposition. The age fluctuation of dolostones shows two-stage dolomitization occurred in the lower Nanwan Formation.
- (4)
- Coralline algae and lime mud with algal fragments is beneficial for the rapid nucleation of dolomite. Meteoric diagenesis before atoll dolomitization might have created important channels for diagenetic fluids.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budd, D.A. Cenozoic dolomites of carbonate islands: Their attributes and origin. Earth-Sci. Rev. 1997, 42, 1–47. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, B. Genesis of fabric-destructive dolostones: A case study of the Brac Formation (Oligocene), Cayman Brac, British West Indies. Sediment. Geol. 2012, 267–268, 36–54. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, B. Origin of “island dolostones”: A case study from the Cayman Formation (Miocene), Cayman Brac, British West Indies. Sediment. Geol. 2012, 243–244, 191–206. [Google Scholar] [CrossRef]
- Saller, A.H. Petrologic and geochemical constraints on the origin of subsurface dolomite, Enewetak Atoll: An example of dolomitization by normal seawater. Geology 1984, 12, 217–220. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, Y.; Yu, K.; Jones, B.; Wu, L.; Liang, F.; Yang, Y.; Chang, B. Temperature regimes during formation of Miocene island dolostones as determined by clumped isotope thermometry: Xisha Islands, South China Sea. Sediment. Geol. 2022, 429, 106079. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth-Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Ren, M.; Jones, B.; Pufahl, P. Genesis of island dolostones. Sedimentology 2018, 65, 2003–2033. [Google Scholar] [CrossRef]
- Land, L.S. The origin of massive dolomite. J. Geol. Educ. 1985, 33, 112–125. [Google Scholar] [CrossRef]
- Wallace, M.W. Origin of dolomitization on the Barbwire Terrace, Canning Basin, Western Australia. Sedimentology 1990, 37, 105–122. [Google Scholar] [CrossRef]
- Bi, D.J.; Zhai, S.K.; Zhang, D.J.; Liu, X.F.; Liu, X.Y.; Jiang, L.J.; Zhang, A.-B. Constraints of fluid inclusions and C, O isotopic compositions on the origin of the dolomites in the Xisha Islands, South China Sea. Chem. Geol. 2018, 493, 504–517. [Google Scholar] [CrossRef]
- Wang, R.; Yu, K.; Jones, B.; Wang, Y.; Zhao, J.; Feng, Y.; Bian, L.; Xu, S.; Fan, T.; Jiang, W.; et al. Evolution and development of Miocene “island dolostones” on Xisha Islands, South China Sea. Mar. Geol. 2018, 406, 142–158. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, H.; Wang, X.; Ning, M.; Wang, X.; Ge, Y.; Wang, H.; Tang, R.; Hou, M. Thermodynamic and Kinetic Studies of Dolomite Formation: A Review. Minerals 2023, 13, 1479. [Google Scholar] [CrossRef]
- Ren, M.; Jones, B. Spatial variations in the stoichiometry and geochemistry of Miocene dolomite from Grand Cayman: Implications for the origin of island dolostone. Sediment. Geol. 2017, 348, 69–93. [Google Scholar] [CrossRef]
- Swart Peter, K. The geochemistry of carbonate diagenesis: The past, present and future. Sedimentology 2015, 62, 1233–1304. [Google Scholar] [CrossRef]
- Liang, H.; Xu, F.; Xu, G.; Yuan, H.; Huang, S.; Wang, Y.; Wang, L.; Fu, D. Geochemical characteristics and origins of the diagenetic fluids of the Permian Changxing Formation calcites in the Southeastern Sichuan Basin: Evidence from petrography, inclusions and Sr, C and O isotopes. Mar. Pet. Geol. 2019, 103, 564–580. [Google Scholar] [CrossRef]
- Lohmann, K.C. Geochemical patterns of meteoric diagenetic systems and their application to studies of paleokarst. In Paleokarst; Springer: Berlin/Heidelberg, Germany, 1988; pp. 58–80. [Google Scholar]
- Guo, Y.; Deng, W.; Liu, X.; Kong, K.; Yan, W.; Wei, G. Clumped isotope geochemistry of island carbonates in the South China Sea: Implications for early diagenesis and dolomitization. Mar. Geol. 2021, 437, 106513. [Google Scholar] [CrossRef]
- Murray, S.T.; Swart, P.K. Evaluating formation fluid models and calibrations using clumped isotope paleothermometry on Bahamian dolomites. Geochim. Cosmochim. Acta 2017, 206, 73–93. [Google Scholar] [CrossRef]
- Ning, M.; Lang, X.; Huang, K.; Li, C.; Huang, T.; Yuan, H.; Xing, C.; Yang, R.; Shen, B. Towards understanding the origin of massive dolostones. Earth Planet. Sci. Lett. 2020, 545, 116403. [Google Scholar] [CrossRef]
- Shen, A.; Hu, A.; Ting, C.; Feng, L.; Pan, W.; Feng, Y.; Zhao, J. Laser ablation in situ U-Pb dating and its application to diagenesis-porosity evolution of carbonate reservoirs. Pet. Explor. Dev. 2019, 46, 1127–1140. [Google Scholar] [CrossRef]
- Li, G.; Xu, W.; Luo, Y.; Liu, J.; Zhao, J.; Feng, Y.; Cheng, J.; Sun, Z.; Xiang, R.; Xu, M.; et al. Strontium isotope stratigraphy and LA-ICP-MS U-Pb carbonate age constraints on the Cenozoic tectonic evolution of the southern South China Sea. GSA Bull. 2023, 135, 271–285. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Li, G.; Xu, W.; Yan, W.; Luo, Y.; Tian, Y.; Wang, M. Origin of large-scale variegated reef limestones in the southern South China Sea: Implications for Miocene regional and global geological evolution. J. Asian Earth Sci. 2022, 230, 105202. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Yang, Z.; Wu, T.; Gao, J.; Wang, D. Spatial and temporal evolution of Cenozoic carbonate platforms on the continental margins of the South China Sea: Response to opening of the ocean basin. Interpretation 2016, 4, SP1–SP19. [Google Scholar] [CrossRef]
- Steuer, S.; Franke, D.; Meresse, F.; Savva, D.; Pubellier, M.; Auxietre, J.-L. Oligocene–Miocene carbonates and their role for constraining the rifting and collision history of the Dangerous Grounds, South China Sea. Mar. Pet. Geol. 2014, 58 Pt B, 644–657. [Google Scholar] [CrossRef]
- Ding, W.; Li, J.; Dong, C.; Fang, Y. Oligocene–Miocene carbonates in the Reed Bank area, South China Sea, and their tectono-sedimentary evolution. Mar. Geophys. Res. 2015, 36, 149–165. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Wang, D.; Lü, F.; Lüdmann, T.; Fulthorpe, C.; Wang, B. Architecture, development and geological control of the Xisha carbonate platforms, northwestern South China Sea. Mar. Geol. 2014, 350, 71–83. [Google Scholar] [CrossRef]
- Miao, X.Q.; Huang, X.L.; Yan, W.; Yang, F.; Zhang, W.F.; Cai, Y.X.; Yu, Y.; He, P.L. Late Triassic dacites from Well NK-1 in the Nansha Block: Constraints on the Mesozoic tectonic evolution of the southern South China Sea margin. Lithos 2021, 398–399, 106337. [Google Scholar] [CrossRef]
- Shao, L.; Cui, Y.; Qiao, P.; Zhang, D.; Liu, X.; Zhang, C. Sea-level changes and carbonate platform evolution of the Xisha Islands (South China Sea) since the Early Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 485, 504–516. [Google Scholar] [CrossRef]
- Jones, B.; Luth, R.W.; Macneil, A.J. Powder X-ray diffraction analysis of homogeneous and heterogeneous sedimentary dolostones. J. Sediment. Res. 2001, 71, 790–799. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, K.; Fan, T.; Xu, S.; Wang, R.; Zhang, Y.; Yue, Y.; Zhao, J.X.; Feng, Y.X.; Wei, C.; et al. Coral reef carbonate record of the Pliocene-Pleistocene climate transition from an atoll in the South China Sea. Mar. Geol. 2019, 411, 88–97. [Google Scholar] [CrossRef]
- McArthur, J.M.; Howarth, R.J.; Shields, G.A.; Zhou, Y. Strontium Isotope Stratigraphy. In Geologic Time Scale 2020; Elsevier: Amsterdam, The Netherlands, 2020; pp. 211–238. [Google Scholar]
- Woodhead, J.D.; Hergt, J.M. Strontium, neodymium and lead isotope analyses of NIST glass certified reference materials: SRM 610, 612, 614. Geostand. Newsl. 2007, 25, 261–266. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Plümper, O.; Zhao, J.-X.; Feng, Y.; Defliese, W.F.; Müller, I.A.; Ziegler, M. Exhumation and carbonation of the Atlantis Bank core complex constrained by in situ U-Pb dating and Δ47 thermometry of calcite veins, SW Indian Ridge. Earth Planet. Sci. Lett. 2022, 584, 117474. [Google Scholar] [CrossRef]
- Luo, Y.; Li, G.; Xu, W.; Liu, J.; Cheng, J.; Zhao, J.; Yan, W. The effect of diagenesis on rare earth element geochemistry of the Quaternary carbonates at an isolated coral atoll in the South China Sea. Sediment. Geol. 2021, 420, 105933. [Google Scholar] [CrossRef]
- Alibo, D.S.; Nozaki, Y. Dissolved rare earth elements in the South China Sea: Geochemical characterization of the water masses. J. of Geo. Res. Oceans 2000, 105, 28771–28783. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. Geochem. Mineral. Rare Earth Elem. 1989, 21, 169–200. [Google Scholar]
- Fan, T.; Yu, K.; Zhao, J.; Jiang, W.; Xu, S.; Zhang, Y.; Wang, R.; Wang, Y.; Feng, Y.; Bian, L. Strontium isotope stratigraphy and paleomagnetic age constraints on the evolution history of coral reef islands, northern South China Sea. Geol. Soc. Am. Bull. 2020, 132, 803–816. [Google Scholar] [CrossRef]
- Elderfield, H. Strontium isotope stratigraphy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 57, 71–90. [Google Scholar] [CrossRef]
- McArthur, J.M.; Howarth, R.; Bailey, T. Strontium isotope stratigraphy: LOWESS version 3: Best fit to the marine Sr-isotope curve for 0–509 Ma and accompanying look-up table for deriving numerical age. J. Geol. 2001, 109, 155–170. [Google Scholar] [CrossRef]
- Uzelman, B.C. Sedimentology, Diagenesis, and Dolomitization of the Brac Formation (Lower Oligocene), Cayman Brac, British West Indies; University of Alberta: Canada, Alberta, 2009. [Google Scholar]
- Kaczmarek, S.E.; Sibley, D.F. On the evolution of dolomite stoichiometry and cation order during high-temperature synthesis experiments: An alternative model for the geochemical evolution of natural dolomites. Sediment. Geol. 2011, 240, 30–40. [Google Scholar] [CrossRef]
- Lumsden, D.N.; Chimahusky, J.S. Relationship between dolomite nonstoichiometry and carbonate facies parameters. Spec. Publ.-SEPM 1980, 28, 123–138. [Google Scholar]
- Folk, R.L.; Land, L.S. Mg/Ca ratio and salinity: Two controls over crystallization of dolomite. AAPG Bull. 1975, 59, 60–68. [Google Scholar]
- Kaczmarek, S.E.; Thornton, B.P. The effect of temperature on stoichiometry, cation ordering, and reaction rate in high-temperature dolomitization experiments. Chem. Geol. 2017, 468, 32–41. [Google Scholar] [CrossRef]
- Wang, R.; Yu, K.; Jones, B.; Jiang, W.; Xu, S.; Fan, T.; Zhang, Y. Dolomitization micro-conditions constraint on dolomite stoichiometry: A case study from the Miocene Huangliu Formation, Xisha Islands, South China Sea. Mar. Pet. Geol. 2021, 133, 105286. [Google Scholar] [CrossRef]
- Sperber, C.M.; Wilkinson, B.H.; Peacor, D.R. Rock composition, dolomite stoichiometry, and rock/water reactions in dolomitic carbonate rocks. J. Geol. 1984, 92, 609–622. [Google Scholar] [CrossRef]
- Wang, R.; Jones, B.; Yu, K. Island dolostones: Genesis by time-transgressive or event dolomitization. Sediment. Geol. 2019, 390, 15–30. [Google Scholar] [CrossRef]
- Ward, W.C.; Halley, R.B. Dolomitization in a mixing zone of near-seawater composition, late Pleistocene, northeastern Yucatan Peninsula. J. Sediment. Res. 1985, 55, 407–420. [Google Scholar]
- Wang, Z.; Huang, K.; Zhang, D.; You, L.; Liu, X.; Luo, W. Maturation of Neogene dolomite from Xuande Atoll of Xisha archipelago, the South China Sea. Mar. Pet. Geol. 2018, 92, 51–64. [Google Scholar] [CrossRef]
- Kaufman, A.J.; Jacobsen, S.B.; Knoll, A.H. The Vendian record of Sr and C isotopic variations in seawater: Implications for tectonics and paleoclimate. Earth Planet. Sci. Lett. 1993, 120, 409–430. [Google Scholar] [CrossRef]
- Jacobsen, S.B.; Kaufman, A.J. The Sr, C and O isotopic evolution of Neoproterozoic seawater. Chem. Geol. 1999, 161, 37–57. [Google Scholar] [CrossRef]
- Gregg, J.M.; Shelton, K.L. Dolomitization and dolomite neomorphism in the back reef facies of the Bonneterre and Davis formations (Cambrian), southeastern Missouri. J. Sediment. Res. 1990, 60, 549–562. [Google Scholar]
- Webb, G.E.; Nothdurft, L.D.; Kamber, B.S.; Kloprogge, J.T.; Zhao, J.X. Rare earth element geochemistry of scleractinian coral skeleton during meteoric diagenesis: A sequence through neomorphism of aragonite to calcite. Sedimentology 2009, 56, 1433–1463. [Google Scholar] [CrossRef]
- Banner, J.L.; Hanson, G.; Meyers, W. Rare earth element and Nd isotopic variations in regionally extensive dolomites from the Burlington-Keokuk Formation (Mississippian): Implications for REE mobility during carbonate diagenesis. J. Sediment. Res. 1988, 58, 415–432. [Google Scholar]
- Morrow, D. The influence of the Mg/Ca ratio and salinity on dolomitization in evaporite basins. Bull. Can. Pet. Geol. 1978, 26, 389–392. [Google Scholar]
- Budd, D.A.; Park, A.J.; Hollis, C. Bed-scale spatial patterns in dolomite abundance: Part II. Effect of varied fluid chemistry, flow rate, precursor mineralogy, temperature, textural heterogeneity, nucleation density and bed geometry. Sedimentology 2019, 66, 2721–2748. [Google Scholar] [CrossRef]
- Whitaker, F.F.; Smart, P.L.; Jones, G.D. Dolomitization: From conceptual to numerical models. Geol. Soc. Lond. Spec. Publ. 2004, 235, 99–139. [Google Scholar] [CrossRef]
- Lucia, F.; Major, R. Porosity evolution through hypersaline reflux dolomitization. Dolomites A Vol. Honour Dolomieu 1994, 21, 325–341. [Google Scholar]
- Liu, J.; Cao, L.; Xu, W.; Li, G.; Xiang, R.; Su, X.; Luo, Y.; Cheng, J.; Xu, X.; Zhao, Z.; et al. Formation and development of coral reefs in the South China Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 594, 110957. [Google Scholar] [CrossRef]
- Saunders, M.I.; Albert, S.; Roelfsema, C.M.; Leon, J.X.; Woodroffe, C.D.; Phinn, S.R.; Mumby, P.J. Tectonic subsidence provides insight into possible coral reef futures under rapid sea-level rise. Coral Reefs 2016, 35, 155–167. [Google Scholar] [CrossRef]
- Woodroffe, C.D.; Webster, J.M. Coral reefs and sea-level change. Mar. Geol. 2014, 352, 248–267. [Google Scholar] [CrossRef]
- Miller, K.G.; Browning, J.V.; Schmelz, W.J.; Kopp, R.E.; Mountain, G.S.; Wright, J.D. Cenozoic sea-level and cryospheric evolution from deep-sea geochemical and continental margin records. Sci. Adv. 2020, 6, eaaz1346. [Google Scholar] [CrossRef]
- Haq, B.U.; Hardenbol, J.; Vail, P.R. Mesozoic and Cenozoic chronostratigraphy and cycles of sea-level changes. Soc. Econ. Paleontol. Mineral. 1988, 42, 71–108. [Google Scholar]
- Amthor, J.E.; Friedman, G.M. Dolomite-rock textures and secondary porosity development in Ellenburger Group carbonates (Lower Ordovician), west Texas and southeastern New Mexico. Sedimentology 1991, 38, 343–362. [Google Scholar] [CrossRef]
- Sibley, D.F.; Dedoes, R.E.; Bartlett, T.R. Kinetics of dolomitization. Geology 1987, 15, 1112–1114. [Google Scholar] [CrossRef]
- Nash, M.C.; Opdyke, B.N.; Wu, Z.; Xu, H.; Trafford, J.M. Simple X-ray diffraction techniques to identify Mg calcite, dolomite, and magnesite in tropical coralline algae and assess peak asymmetry. J. Sediment. Res. 2013, 83, 1085–1099. [Google Scholar] [CrossRef]
- Nash, M.; Troitzsch, U.; Opdyke, B.; Trafford, J.; Russell, B.; Kline, D. First discovery of dolomite and magnesite in living coralline algae and its geobiological implications. Biogeosciences 2011, 8, 3331–3340. [Google Scholar] [CrossRef]
- Suzuki, Y.; Iryu, Y.; Inagaki, S.; Yamada, T.; Aizawa, S.; Budd, D.A. Origin of atoll dolomites distinguished by geochemistry and crystal chemistry: Kita-daito-jima, northern Philippine Sea. Sediment. Geol. 2006, 183, 181–202. [Google Scholar] [CrossRef]
- Ohde, S.; Elderfield, H. Strontium isotope stratigraphy of Kita-daito-jima Atoll, North Philippine Sea: Implications for Neogene sea-level change and tectonic history. Earth Planet. Sci. Lett. 1992, 113, 473–486. [Google Scholar] [CrossRef]
- Ohde, S.; Greaves, M.; Masuzawa, T.; Buckley, H.A.; Van Woesik, R.; Wilson, P.A.; Pirazzoli, P.A.; Elderfield, H. The chronology of Funafuti Atoll: Revisiting an old friend. Proc. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2002, 458, 2289–2306. [Google Scholar] [CrossRef]
- Vahrenkamp, V.C.; Swart, P.K.; Ruiz, J. Episodic dolomitization of late Cenozoic carbonates in the Bahamas; evidence from strontium isotopes. J. Sediment. Res. 1991, 61, 1002–1014. [Google Scholar]
- Betzler, C.; Eberli, G.P. Miocene start of modern carbonate platforms. Geology 2019, 47, 771–775. [Google Scholar] [CrossRef]
- Jian, Z.; Yu, Y.; Li, B.; Wang, J.; Zhang, X.; Zhou, Z. Phased evolution of the south–north hydrographic gradient in the South China Sea since the middle Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 230, 251–263. [Google Scholar] [CrossRef]
- Kennett, J.P.; Keller, G.; Srinivasan, M.S. Miocene planktonic foraminiferal biogeography and pale-oceanographic development of the Indo-Pacific region. In The Miocene Ocean: Paleoceanography and Biogeography; Geologic Society of America Memoir: Boulder, CO, USA, 1985; pp. 197–236. [Google Scholar]

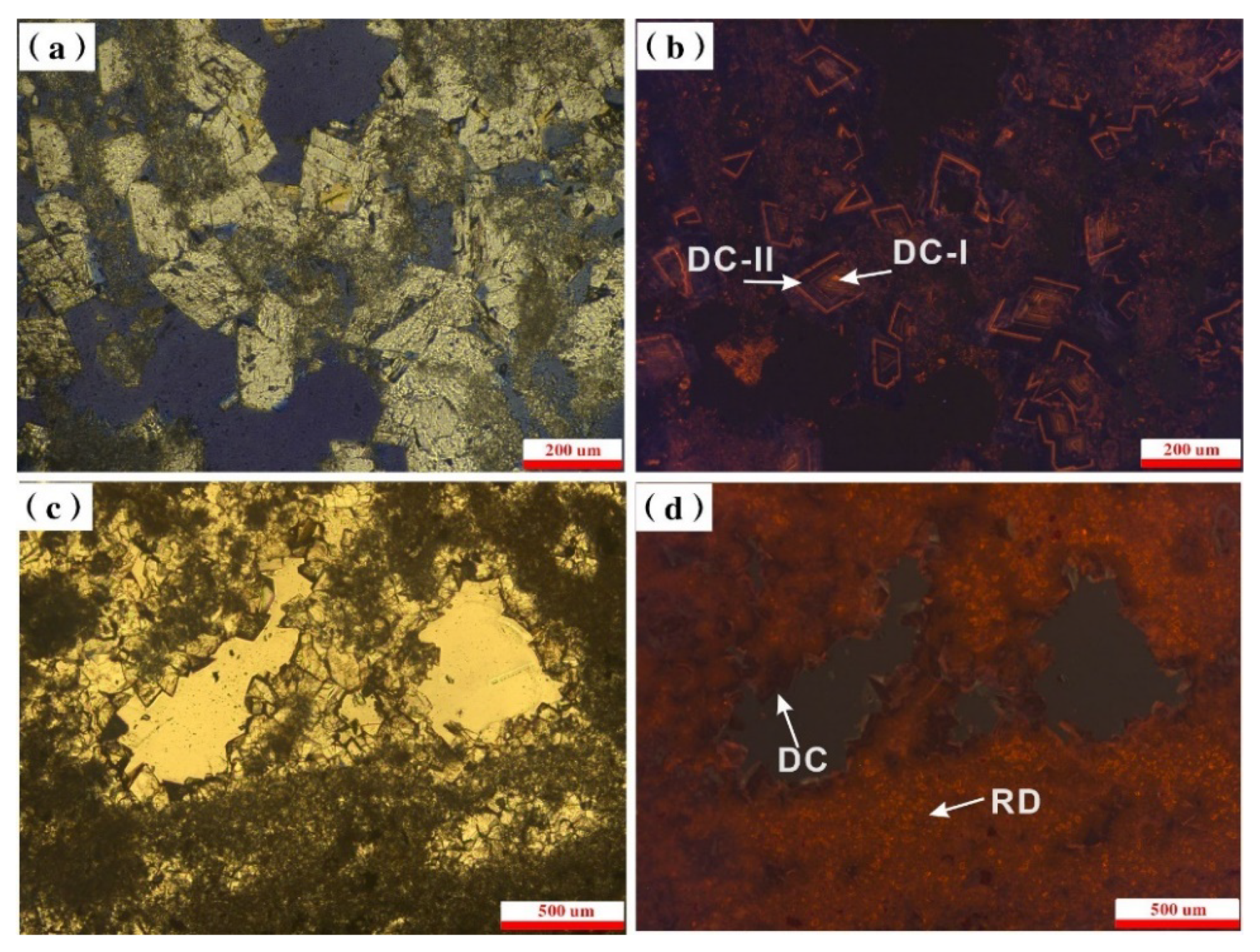


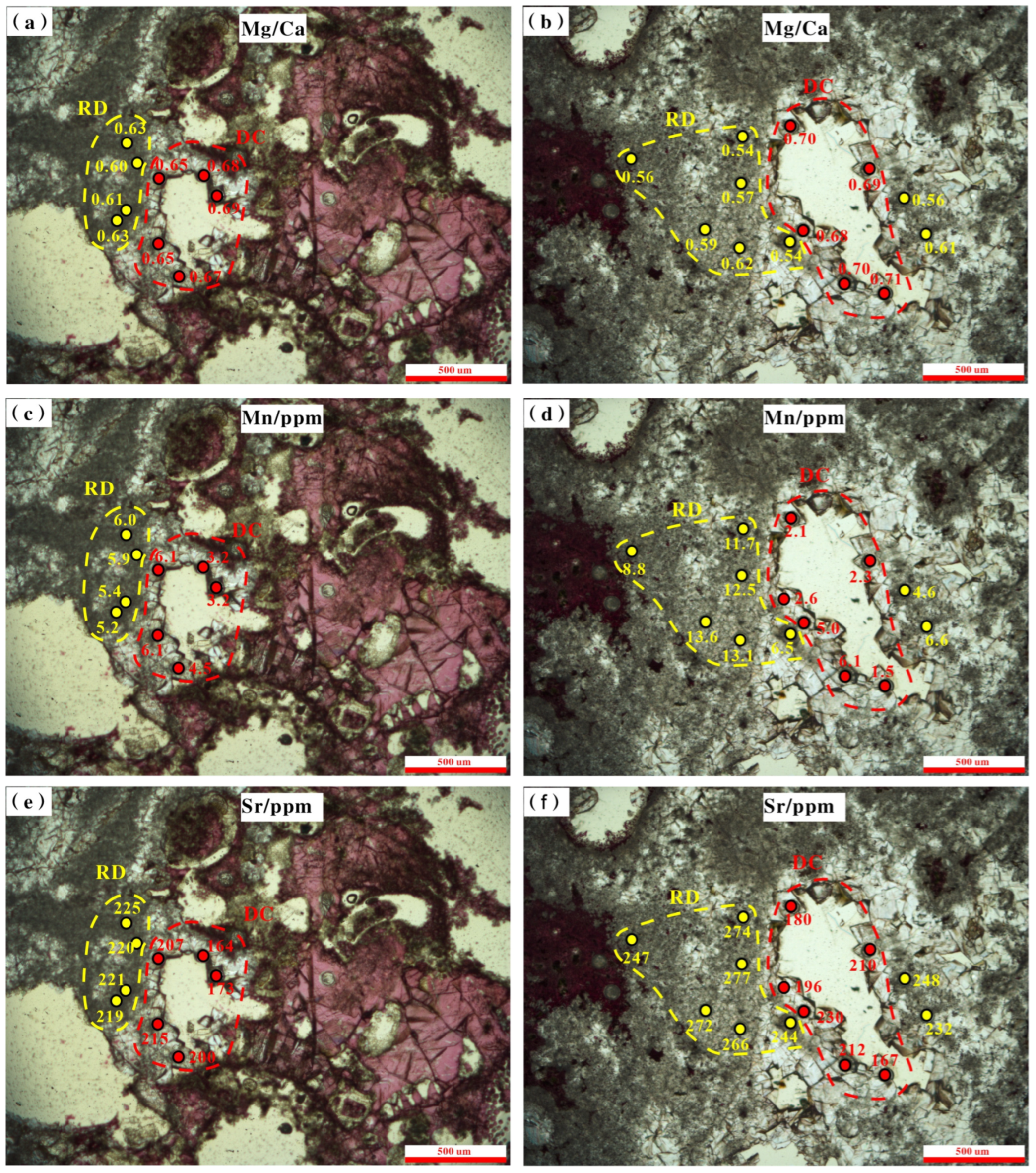
| Sample No. | Depth/m | Calcite/% | Dolomite/% | Cation Order of Dolomite | d(104) | CaCO3 (mol%) | Dolomite Type | Dolomite Cement (%) |
|---|---|---|---|---|---|---|---|---|
| S1 | 489.8 | 77.8 | 22.2 | 0.43 | 2.90806 | 58.2 | HCD | 40 |
| S2 | 495.5 | 71.9 | 28.1 | 0.43 | 2.90419 | 56.9 | HCD | 20 |
| S3 | 503.7 | 70.3 | 29.7 | 0.36 | 2.90529 | 57.3 | HCD | 10 |
| S4 | 494.3 | 48.5 | 51.5 | 0.36 | 2.90538 | 57.3 | HCD | 30 |
| S5 | 455.5 | 38.9 | 61.1 | 0.34 | 2.90197 | 56.2 | HCD | 10 |
| S6 | 468.6 | 20.4 | 79.6 | 0.33 | 2.90288 | 56.5 | HCD | 20 |
| S7 | 493.0 | 20.3 | 79.7 | 0.34 | 2.91119 | 59.3 | HCD | 20 |
| S8 | 492.0 | 18.9 | 81.1 | 0.38 | 2.91019 | 58.9 | HCD | 30 |
| No. | Depth/m | Mineralogy/% | 87Sr/86Sr | Mean Sr Age/Ma | In Situ U–Pb Age/Ma |
|---|---|---|---|---|---|
| S7-C | 493.0 | primary calcite, 100.0% | 0.708862 ± 5 | 10.9 | - |
| S7-D | replacive dolomite, 99.2% | 0.708865 ± 5 | 10.8 | 10.7 ± 0.8 | |
| S4-C | 494.3 | primary calcite, 100.0% | 0.708860 ± 5 | 11.0 | - |
| S4-D | replacive dolomite, 98.7% | 0.708861 ± 4 | 11.0 | 11.0 ± 0.3 |
| Sample No. | Depth/m | Mineral | Number of Points | Average Mg/Ca (mol/mol) | Average Mn/ppm | Average Fe/ppm | Average Sr/ppm | Average Mn/Sr |
|---|---|---|---|---|---|---|---|---|
| S1 | 489.8 | Primary calcite | 2 | 0.03 | 15.7 | 255 | 640 | 0.02 |
| Replacive dolomite | - | - | - | - | - | - | ||
| Dolomite cement | 9 | 0.53 | 6.7 | 118 | 173 | 0.04 | ||
| S2 | 495.5 | Primary calcite | 8 | 0.04 | 13.3 | 142 | 888 | 0.03 |
| Replacive dolomite | 9 | 0.48 | 10.3 | 130 | 251 | 0.04 | ||
| Dolomite cement | 4 | 0.62 | 5.2 | 102 | 204 | 0.02 | ||
| S3 | 503.7 | Primary calcite | 13 | 0.05 | 17.6 | 122 | 519 | 0.04 |
| Replacive dolomite | 15 | 0.53 | 19.1 | 110 | 222 | 0.09 | ||
| Dolomite cement | - | - | - | - | - | - | ||
| S5 | 455.5 | Primary calcite | 7 | 0.01 | 0.3 | 127 | 1523 | <0.01 |
| Replacive dolomite | 13 | 0.61 | 4.5 | 93 | 197 | 0.02 | ||
| Dolomite cement | 3 | 0.64 | 4.6 | 96 | 174 | 0.03 | ||
| S6 | 468.6 | Primary calcite | 3 | 0.01 | 0.9 | 118 | 494 | <0.01 |
| Replacive dolomite | 2 | 0.63 | 5.6 | 90 | 223 | 0.03 | ||
| Dolomite cement | 5 | 0.66 | 4.6 | 78 | 192 | 0.02 | ||
| S7 | 493.0 | Primary calcite | 12 | 0.03 | 22.6 | 134 | 239 | 0.11 |
| Replacive dolomite | 7 | 0.53 | 7.0 | 109 | 241 | 0.03 | ||
| Dolomite cement | 13 | 0.62 | 7.0 | 99 | 190 | 0.04 | ||
| S8 | 492.0 | Primary calcite | 2 | 0.02 | 4.6 | 127 | 1300 | <0.01 |
| Replacive dolomite | 7 | 0.58 | 10.1 | 99 | 259 | 0.04 | ||
| Dolomite cement | 6 | 0.67 | 3.9 | 89 | 212 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Li, G.; Zhang, X.; Xu, W.; Zhu, X.; Zhou, W.; Huang, H.; Yan, W.; Zhong, F. Multistage Diagenetic Fluid Shaping Miocene Island Dolostones on One Isolated Atoll in the South China Sea: Insights from LA-ICP-MS U–Pb Dating and Geochemical Characterization. Minerals 2024, 14, 157. https://doi.org/10.3390/min14020157
Luo Y, Li G, Zhang X, Xu W, Zhu X, Zhou W, Huang H, Yan W, Zhong F. Multistage Diagenetic Fluid Shaping Miocene Island Dolostones on One Isolated Atoll in the South China Sea: Insights from LA-ICP-MS U–Pb Dating and Geochemical Characterization. Minerals. 2024; 14(2):157. https://doi.org/10.3390/min14020157
Chicago/Turabian StyleLuo, Yun, Gang Li, Xiyang Zhang, Weihai Xu, Xiaowei Zhu, Wanqiu Zhou, Huiwen Huang, Wen Yan, and Fuchang Zhong. 2024. "Multistage Diagenetic Fluid Shaping Miocene Island Dolostones on One Isolated Atoll in the South China Sea: Insights from LA-ICP-MS U–Pb Dating and Geochemical Characterization" Minerals 14, no. 2: 157. https://doi.org/10.3390/min14020157






