4.1. Processes Taking Place in Iron-Bearing Minerals during Formation of BCF
From the aspect of iron one of the principal net processes of diagenesis is the conversion of biotite group minerals and amphibole through a few intermediate stages finally to illite (and in part to chlorite). In the starting micas illite is not present at all, whereas chlorite may occur already. The other parallel main process is the formation and segregation of hematite during the alterations resulting in the addition of this mineral in the largest amount to the part that was present already in the weathered starting rhyolite.
The parent biotite group minerals belong to the more broad mica class with a general T-O-T-c structure (tetrahedral–octahedral–tetrahedral sheets with “
c” charge compensating cations, and iron occurs only in ferrous form in octahedral siting [
35]. The iron-containing phyllosilicates of BCF in the final stage are identified mainly as illite and chlorite from XRD and HRTEM analyses. The precursor aluminosilicate structure is preserved in illite, characterized by the (K,H
3O)(Al,Mg,Fe)
2(Si,Al)
4O
10(OH)
2 chemical formula [
36,
37]. However, an important difference is that the inclusion of three-valent ions (aluminum and ferric iron) is also allowed in octahedral sites in illite. Thus, iron and magnesium may be released in the biotite mineral => … => illite gross conversion process from the octahedral sites of biotite minerals in an amount corresponding to the excess charge provided by incorporation of the three valent aluminum and ferric iron ions in replacement of previous ferrous ones in the octahedral sheets. If the released cation is iron, iron oxyhydroxide or at higher temperatures (above ≈ 150 °C) hematite is formed in the presence of potassium-containing feldspar, as described in detail in
Section 4.2 in [
21]. Further, amphibole can also be transformed into illite [
21]. The third sheet silicate actor, chlorite has a T-O-T-O structure with corresponding (Mg,Fe)
3(Si,Al)
4O
10(OH)
2 ∙ (Mg,Fe)
3(OH)
6 stoichiometry. Similarly to illite, the triple T-O-T layer has an overall negative charge which, in the case of this mineral, is compensated with the positively charged second octahedral layer with (Mg,Fe)
3(OH)
6 composition [
38]
. Due to this, stoichiometry chlorite may also contain ferric iron, but usually to a lesser extent than is characteristic of illite. There are intermediate stages during the transformations, first probably swelling sheet silicates (smectite) form, then the charge compensating cations are replaced and more compact illite and chlorite are formed [
39]. Even microbially induced smectite to illite transformation can also take place at temperatures below 80 °C [
40]. It is worth repeating here that a significant amount of smectite and illite can be formed from the original glass and feldspar of rhyolite, besides the discussed transformations of biotite group minerals. Description of various further aspects of clay formation is discussed in detail in [
21].
As for the other main iron-bearing mineral, hematite, its presence probably originates at least from three sources. Hematite as a weathering product can already be present, originating from the starting rhyolite, granite and metamorphic rocks. Further, if the iron-containing chain silicate (e.g., amphibole) is partly decomposed, the released Fe
2+ ions may migrate in the aqueous phase rather far under reducing conditions, since Fe
2+ ions hydrolyze and precipitate between pH values 5 and 6. In a later stage, upon reverting to oxidation conditions at the playa lake the converted Fe
3+ ions immediately form hydroxide precipitate (since the threshold for hydrolysis for ferric iron is very low, pH > 2). The third source of the hematite is direct formation during illitization, as mentioned above [
21]. The processes described above are also reflected in Mössbauer spectra.
The
precursor state is characterized by the dominant presence of ferrous iron as shown in spectra of Üh-27 (204.6 m) and Bek-8 (54.9 m) in samples originating from the fresh Mórágy Metagranite in
Figure 3. The overwhelming part of ferrous iron in these samples is characterized by a doublet described with δ ≈ 1.1 mm s
−1 and Δ ≈ 2.6 mm s
−1 pair of data. This pair of data coincides well with parameters for the
cis-M2 sites (where the charge compensating –OH groups occupy
cis conformation in the octahedral sheet). The presence of this doublet is dominant and permanent over several biotite samples. The other iron site with
trans–OH arrangement is less expressed and the Δ parameter varies in between the 1.9–2.6 mm s
−1 range [
41]. The other possible starting iron-bearing mineral in granite is amphibole. The doublet characterized with the δ ≈ 1.1 mm s
−1 and Δ ≈ 2.6 mm s
−1 pair of data is present in the spectrum of amphibole with the simultaneous presence of another doublet in roughly the same intensity with Δ ≈ 2.9 mm s
−1 [
42]. This latter doublet is missing from our spectra, thus amphibole is probably a minor constituent in the examined starting Mórágy Metagranite, i.e., biotite minerals are the primary iron sources. The other starting iron-bearing rock can be the rhyolite. Its contribution to iron-bearing phyllosilicates is probably negligible since the total iron content is only c.a. one fifth that of the fresh granite and iron is mainly in the form of stable hematite which probably remains unaltered.
The present form of iron-bearing clay minerals in BAF is dominantly composed of illite and chlorite. During the diagenesis, the interlayer charge compensating cations may vary, as the XRD and HRTEM analyses attest. The Mössbauer spectroscopy can hardly distinguish among the various T-O-T structures since the primary information originated from iron located in the middle octahedral sheet.
The morphology of hematite is also worth mentioning. Hematite occurs in two specific forms: in regular crystallites (e.g., in
Figure 5a, and in
Figure 10, bottom left) or as interparticle inclusions between illite packets (
Figure 6b). The former regular large crystallites may probably develop from solutions containing Fe
2+ in the first step, just after leaving the biotite frame. Iron in this ferrous form may migrate in the solution since it hydrolyzes only at pH 5–6. Following oxidation to the ferric state, during the wet-dry cycles in a playa lake, iron oxyhydroxide may precipitate immediately, since ferric ions hydrolyze already at pH 2–3. In the following stage, the oxyhydroxide is transformed into regular hematite crystallites during burial and exposure to larger pressure and temperature. The other variant, the interpacket nanoscale hematite flakes, is probably precipitated during the illite formation.
In general, the principal T-O-T structural layers in sheet silicates are probably preserved in the process of evolution of BCF. However, a part of the ferrous ions may migrate out of them, and the oxidation state of a part of the remaining central ions in the octahedra can be altered to a ferric one. This statement is based on the permanent presence of the doublet with parameters of the δ ≈ 1.1 mm s−1 and Δ ≈ 2.6mm s−1 pair of data. This particular cis-M2 ferrous doublet is present to different extents in almost all spectra. The parameters of ferric ions in the octahedral sites are less specific, so structural deduction can hardly be based on them. However, an important observation should be mentioned, namely the δ isomer shift of the Fe3+ component never drops below 0.3 mm s−1, i.e., ferric ions always occupy octahedral sites, and never are inserted into tetrahedral sheet. In a rough approximation, considering the change of proportions of iron among the different species, the extent of disintegration of the biotite (and amphibole) can also be estimated from the increase in hematite proportion, c.a. ½ portion of the original ferrous iron is converted to hematite.
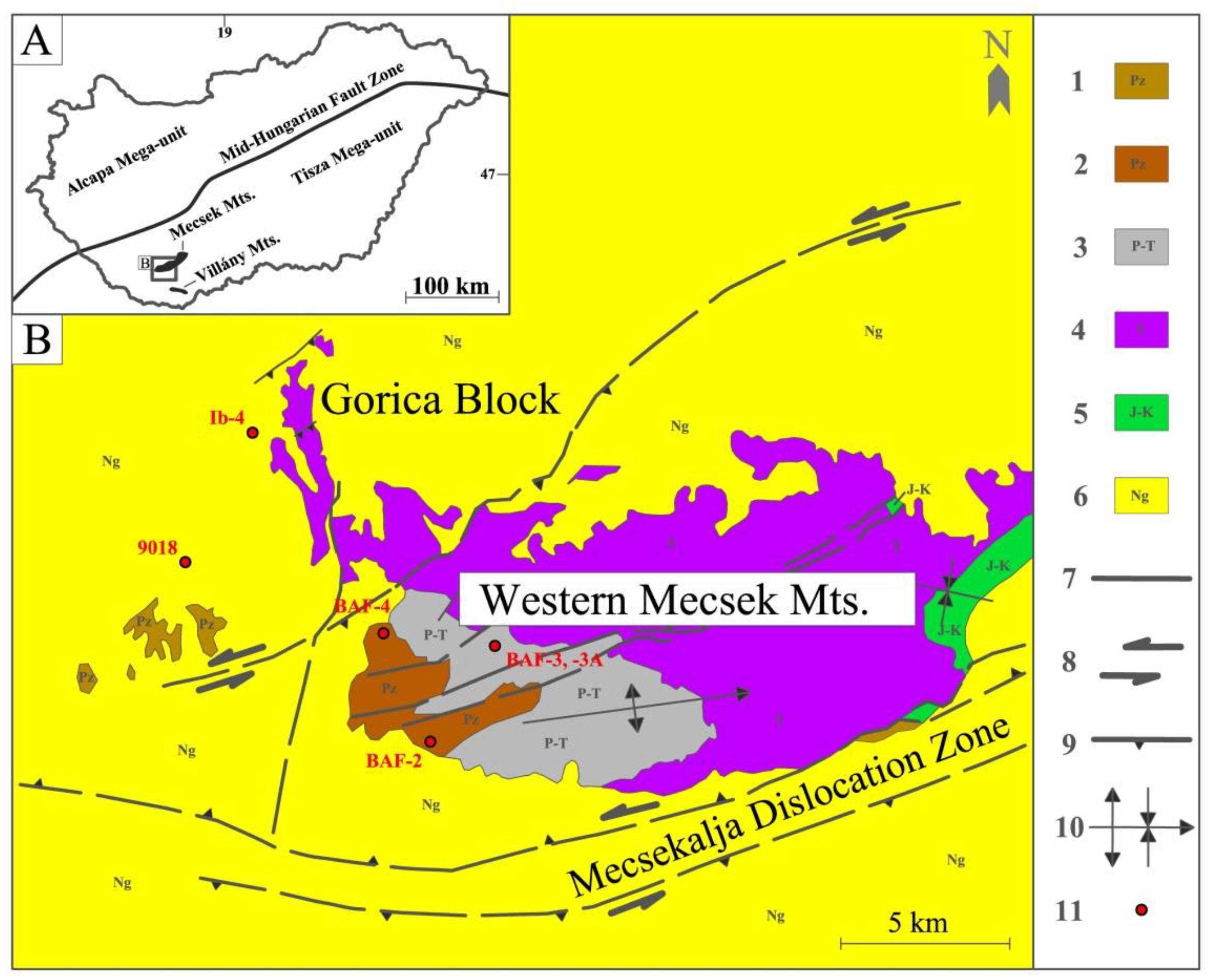
4.2. Comparison of Gorica Block and WMA Samples
A distinct difference can be observed in the comparison of the proportions of Fe
2+ and Fe
3+ contributions of layered silicates in the Ib-4 (Gorica Block) and BAF-2, BAF-3A (WMA) samples. The Fe
3+ contribution in Mössbauer spectra can be correlated with the illite proportion derived from XRD studies (
Table 4,
Table 8 and
Table 9). The proportion of iron located in hematite can also be derived from Mössbauer spectra. The distribution of iron among the Fe
2+, Fe
3+ (doublets from layered silicates) and hematite (magnetic sextet) contributions are represented in the top panels in
Figure 11. Illite, chlorite and hematite mineral proportions extracted from XRD data are also displayed at the bottom of the panels. Comparison of the top and bottom parts of the panels provides an excellent illustration of the significant difference in detection sensitivities of the Mössbauer and XRD techniques with regard to the iron-bearing phases due to the different relative iron contents of corresponding minerals, as was already mentioned in connection with biotitic minerals and hematite in interpreting spectra in
Figure 3. For illite, similar considerations are valid, as illite contains 13 wt% iron in a nominal [K(Mg,Fe)
2(Si,Al)
4O
10(OH)
2] composition, compared to 67% in hematite. Thus, the partial contribution of hematite is ca. 5 times less in XRD tables than percentages obtained from Mössbauer spectra. Further, the average iron contents of illite and chlorite are also different. Illite usually contains 2–3 times less iron than chlorite, since the former usually consists of a T-O-T dioctahedral structure, whereas the latter prefers to exist in a more iron-rich trioctahedral T-O-T-O structure. The comparison of the corresponding plots attests that the Fe
3+ contributions in Ib-4 samples can be correlated with the illite content, and the 50–30% illite presence in minerals (from XRD) can be correlated with 55–30% Fe
3+ iron in sheet silicates (from Mössbauer). In BAF-2 and BAF-3A 15–33% illite content is correlated only with 20–30% Fe
3+. In reverse, Fe
2+ is in good agreement with chlorite in BAF-2 and BAF-3A, the 10–15% amount of chlorite corresponds to 30–50% Fe
2+ in sheet silicates. On the other side, the 20–40% illite content corresponds only to 20–30% Fe
3+ in layered silicates of the latter two borecores. These correlations confirm the presumption that illite contains mostly Fe
3+ and, in reverse, the dominant form of iron in chlorite is Fe
2+.
Samples from the Gorica Block (Ib-4) and the two WMA (BAF-2 and BAF-3A) sources can be compared from further aspects. Fe
3+ is predominant in Ib-4 in comparison to Fe
2+, and in reverse, Fe
2+ is predominant over Fe
3+ in BAF-2 and BAF-3A. The conversion of ferrous iron to ferric one in sheet silicates was less expressed in the WMA region or more probably, the reducing environment sustained longer for the WMA section, the burial took place to larger depths and the process was longer. In contrast, the percentage of iron located in hematite is similar for both regions. Thus, once stable hematite was formed, its state was preserved. On the other side, iron in layered silicates was probably more sensible to changes in the local environment. This can be in correspondence with other observations. Namely, structural iron in smectite is reduced than in pure oxides [
43], i.e., the similar hematite content can be accompanied by different Fe
2+/Fe
3+ occurrences in illite/chlorite. Further, the reduction of structural Fe
3+ may proceed with interfacial electron transfer as the Mössbauer studies demonstrated [
44].