5.1. Abiotic and Biological Growth Models for Agate Geodes and Concretions
The following section describes some previously proposed models for the formation of agate geode and of concretions. First, some previously proposed crystallization mechanisms shall be considered alongside pattern formation. Oscillations over at least five orders of size dimension, from hundreds of nanometres to centimetre scales, have been described to form the self-similar fractal patterns in agate geodes [
24]. Within their radial patterns, the elongation direction of chalcedony fibres in agate geodes is typically down the [110] crystallographic direction as opposed to prismatic quartz crystals, which grow down the [001] direction [
25]. The acicular-fibrous chalcedony in radial quartz is also twisting as a screw dislocation about [110], which yields an oscillating fibre growth direction down the
c-axis [
24]. These geometric patterns underlie a specific process of mineralization. A model based on Ostwald’s rule, which is that when a substance changes from one state into another state, the favoured state it will adopt is the one nearest in stability to the original state [
26], can also be considered. Mineralisation sequence from colloidal silica to cristobalite, and then onto moganite and cryptocrystalline or chalcedonic quartz, is usually suggested based on detailed petrographic observations [
1]. It can then be understood that the favoured phase to form from a process resulting in crystallisation has been explained based on the irreversible thermodynamics of the crystal structure. In Palaeoproterozoic agates from the Ludikovi Group in northwest Russia, the mineralisation of quartz was demonstrated to be strongly influenced by the abundance of organic matter [
14]. The crystallisation of quartz has also been proposed to proceed from inside towards the outside edge, along with co-diffusing substances that eventually reach saturation and remain preserved as bands [
1].
The Liesegang diffusion phenomenon called “banding” is known to occur in gels and is proposed as a possible diffusion mechanism to explain patterns exhibited by natural agates from volcanic terrains [
2]. For instance, Liesegang experiments are known to produce periodic banding as well as dendritic patterns when a substance of a higher density and viscosity diffuses through another with a lower density and viscosity, which has been explained by the processes of viscous fingering and diffusion-limited aggregation [
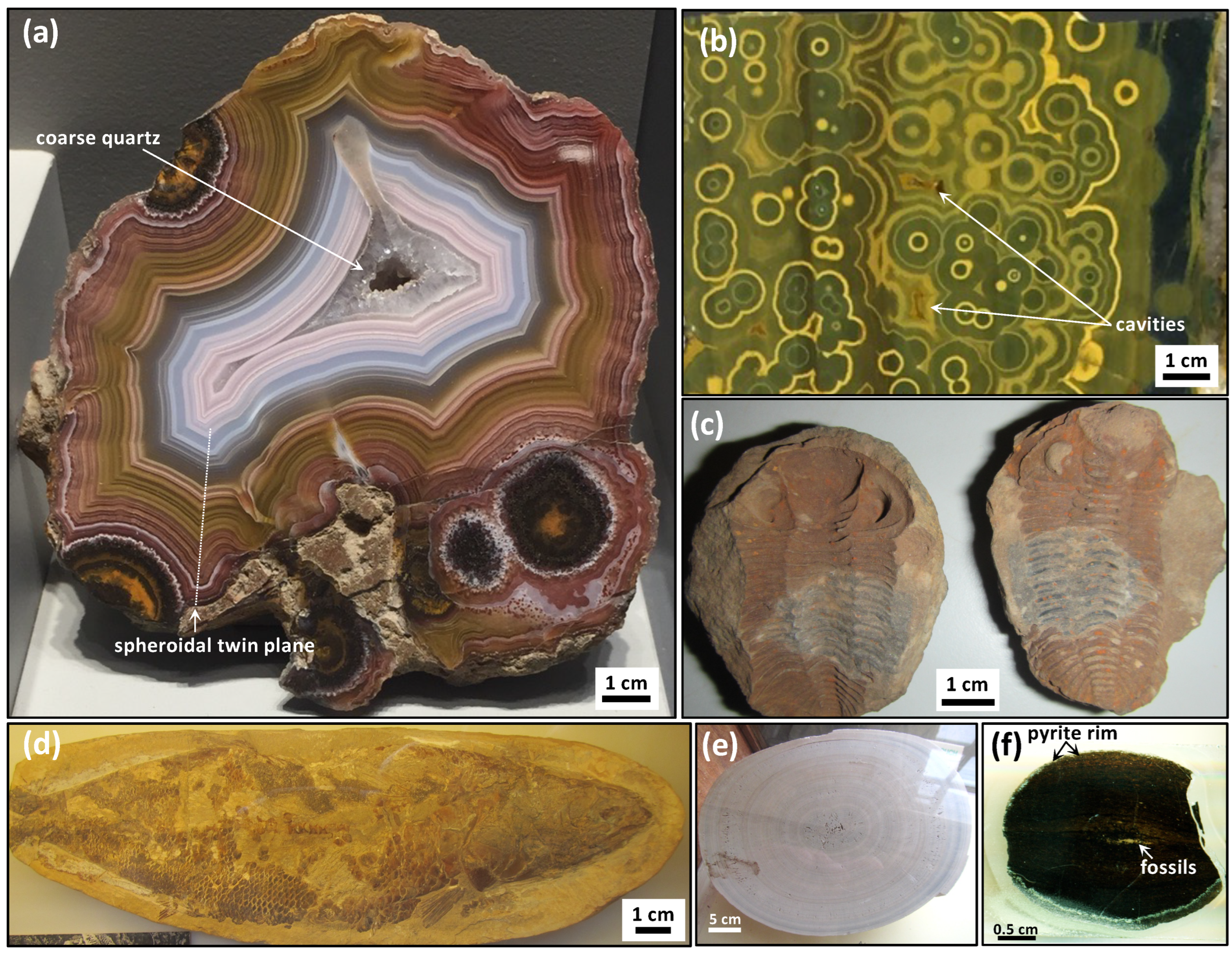
27]. However, the Liesegang diffusion phenomenon is not known to specifically involve reactions with carbon compounds, and these experiments are typically performed with metal salts, and consequently they are of limited relevance to the carbon cycle. Liesegang diffusion also does not specifically produce circularly concentric patterns that destructively interfere as circular twins, nor do they form cavity structures (
Figure 1a,b) [
28]. Hence, while the Liesegang phenomenon has also been proposed to explain the periodic banding in malachite [
29], the COR model explains much more elegantly the observed patterns and composition of botryoidal malachite [
4]. Similarly, while the Liesegang diffusion phenomenon has long been invoked to explain the formation of patterns in agate geodes, there is little support for the Liesegang phenomena in agate geodes, which often contain organic matter and lack evidence for the diffusion of metal ions in silica gels [
1].
Agate geodes are commonly believed to be formed through some interactions between heated fluids and volatiles associated with cavities in hot volcanic rocks [
30]. Thermogravimetric analyses have shown that various volatile compounds are present in agate geodes, including NO, SO, CO
32−, CH, and HF [
31]. However, this method cannot detect macromolecular carbon, halogenated hydrocarbons, sulphurated hydrocarbons, nor halogens in trace concentrations. Other techniques such as optical microscopy have been used to demonstrate that some agate geodes are rich in bitumen, and that masses of opaque organic matter can be concentrated in the geometric centres of radiating acicular quartz inside botryoids [
15]. Infrared spectroscopy has also been used to show the presence of alkanes, esters, ketones, and carboxylic acids organic matter from agate geodes, hinting at a biological origin [
15]. Agate geodes are now widely known to contain organic matter, various hydrocarbon compounds, and disordered graphitic carbons [
3]. Interestingly,
13C-depleted carbonate minerals are common in agate geodes [
1,
32], which may indicate an origin from oxidised biomass. Furthermore, minerals such as calcite (
Figure 3 and
Figure 4) and iron oxides or oxyhydroxides are almost ubiquitously found in agate geodes [
3], whereas hydroxylated or water-bearing minerals and fluorite are also common, especially in geodes from acidic volcanic rocks [
33]. Lastly, the oxygen isotope composition of agate is significantly more
18O-enriched than their host volcanic rocks, which can be explained by the isotopic exchange with hydrothermal fluids or magmatically heated water [
2]. However, biomass is also known to be
18O-enriched [
34], and carboxylic acids from decayed animals or plants could also have contributed to produce
18O-enrichements in agate geodes. Hence, agate geodes contain various substances that indicate C-cycling through the oxidation of organic matter (either from biomass or abiotic synthesis) in aqueous solutions involving Fe, halogens, and other volatiles. In this light, observations in agate geodes and in Petri dishes with COR experiments need to be compared for different types of substances and self-similar patterns (
Figure 5).
For concretions, there are two main growth processes proposed as models for the growth of carbonate concretions in the rock record: the cementation of diagenetic pore spaces by carbonate minerals and a displacive growth phenomenon, whereby diagenetic carbonate precipitation forces sedimentary layers apart [
35]. It has also been suggested that concentric growth starts with the formation of an early diagenetic core nucleus in the concretion followed by the addition and precipitation of successive layers around the core [
36]. Alternatively, an inverted growth model has further been proposed, whereby an outer rim of pyrite or carbonate first precipitates during diagenesis, followed by the radially inward growth of the concretion, with the concretion size controlled primarily by the availability of Fe [
6,
37]. While all these phenomena are not necessarily mutually exclusive, they do not explain nor predict the formation of specific geometric patterns commonly seen in concretions, including spheroidal, ellipsoidal, concentric, radial, irregularly rounded shapes, and the occurrence of fossils and organic matter within. Organic geochemical analyses have confirmed the presence of various residual molecular functional groups in kerogen from Toarcian organic shales and their limestone concretions, including carboxylic acids, aliphatics, ketones, phenols, and aromatics [
38], as well as organic biomarkers such as pristane, phytane, and methylhopanes [
11]. Organic matter in Lower Cambrian limestone concretions from the Niutitang Fm in south China have similar functional groups and have been considered in the COR model [
5]. Lastly, Ediacaran concretions with circularly concentric pyrite layers have a sulphur isotope range of 25‰ with positive values up to +40‰, which suggest both microbial and abiotic processes of isotope fractionation [
39]. Hence, it can be inferred that decarboxylation during the decomposition of biomass does not prevent the preservation of carboxylic acid functional groups in residual organic matter found associated with concretions.
Fossils and organic matter are commonly found in carbonate concretions (
Figure 1c,d) and such observations suggest that organic matter, or specific compounds in biomass, participate in the abiotic reactions that produce concretions, and that these involved oxidation–reduction reactions of carbon compounds. Evidence of microbial activity in the formation of concretions is suggested by their abundance and patterns in modern and Phanerozoic concretions, where metabolically diverse microbes play a role in the decomposition of biomass [
11]. Many concretions contain
13C-depleted carbonate, which is consistent with the idea that some carbonate carbon originated from biomass (e.g., [
6]). However, some concretions preserve evidence for methanogenesis during diagenesis, which has been inferred from
13C-enriched carbonate in fossiliferous concretions from the Carboniferous [
7,
8]. In fact, evidence from organic matter is usually attributed to organic decay, primarily by invoking heterotrophic microorganisms [
8]. Hence, microbial sulphate reduction most likely played a role in the production of sulphide minerals inside concretions, which has been based both on large ranges of
34S-depleted pyrite related to microbial sulphate reduction in non-limiting sulphate concentration and on large ranges of
34S-enriched pyrite related to microbial sulphate reduction under low concentrations of residual pore water sulphate [
7,
39]. These observations thus suggest that the mechanism of the formation of concretions is linked to the diagenetic cycles of C, S, and Fe and specifically during the decomposition of biomass. Yet again, however, these inferred microbial processes do not reliably predict why concretions should have any geometric patterns (
Figure 5, right column).
5.2. The Patterns and Substances of COR in Agate Geodes and Concretions
COR experiments show the same kinds of patterns and several overlapping compositional similarities with those in agate geodes and concretions. The similar patterns include spot proliferations, circular concentricity, twins, cavity structures, colour gradients, and radiations (
Figure 5, left column). Spot proliferation begins at the microscopic scale and can also be seen as millimetric spots in agate geodes and as centimetric to decimetric cherty limestone concretions. Circular concentricity is also represented at microscopic to decimetric scales in all these objects. Spheroidal twins uniquely characterize circularly concentric spots, and they form cavity structures over the same range of size magnitudes. Also, each chemical wave in COR exhibits a colour gradient that can vary in length, which is also observed at microscopic and macroscopic scales in agate geodes and concretions. The summative
Figure 5 remains incomplete, however, because there are other patterns produced by COR that are not represented here in any of the objects such as spirals, equidistant to branching lines, asymmetries, knobby or stromatolitic laminations, arborescences, and inversions. Hence, the COR model remains to be tested further on those patterns and on the substances involved in the reactions.
Now, the substances of agate geodes and concretions need to be compared with those of COR. The observations from agate geodes and concretions collectively suggest a role for redox reactions involving C and Fe in the formation of agate geodes and concretions and possibly also a role for halogens and S compounds. It is therefore natural to propose a significant role for the COR model in the formation of these objects, as it predicts that the underlying reactions should be abiotic, spontaneous, out-of-equilibrium, and produce fractal patterns. Observations of bubbles in the COR can be directly linked to the production of CO
2 during decarboxylation reactions, and in agate geodes, the unambiguous analogue is represented by micron-sized calcite inclusions in quartz, moganite, and cristobalite (
Figure 3 and
Figure 4). In limestone concretions, comparisons can be made with the carbonate minerals that compose these objects formed around fossils of carbonate–apatite (e.g.,
Figure 1d) or of pyrite–haematite (e.g.,
Figure 1c,f). These compositions (
Figure 5) are inferred based on Raman spectra as well as mineral colour, lustre, habit, and petrological context, and they are accompanied by zones of variable concentrations of organic matter. This is seen as brown to dark grey colour gradients in concretions and agate geodes, where these occur as concentrated disseminations of kerogen, in part, composing the circularly concentric waves described above. Fe-based catalysts in COR also analogously occur in agate geodes as ferruginous laminated cavity structures in red-coloured haematitic limestone, as haematitic fossils in limestone concretions, and as concentric pyrite rims in chert concretions. Hence, several substances representing the organic–carbonate and ferric–ferrous redox couples are directly linked through the substances of COR, agate geodes, and concretions.
For the immobilization of chemical waves, the variety of substances that can be involved in COR needs to be considered. Gels of N-isopropylacrylamide and polyacrylamide-silica gel composites have been used with success to immobilize chemical waves from the B–Z reaction [
40], which suggests that colloidal silica and carbonate micrite play the same role, although this awaits further investigation. However, pattern formation from acidic COR does take place in alkaline solutions with colloidal silica [
5]. Experiments have shown that COR can take place and produce patterns under a large range of reactant concentrations: 0.15–2.0 M H
2SO
4, 0.075–0.4 M NaBrO
3, and 0.05–0.8 M malonic acid [
16,
41,
42]. In fact, there are also various other organic acids that can be used for COR, including carbonic acid, mono- and di-carboxylic acids, and ketones [
16,
17]. Similarly, COR are also known to produce patterns with various kinds of other strong oxidizers such as chlorate, bromate, iodate, and hydrogen peroxide [
43]. Various strong acids have also been used like hypophosphite, arsenate, and sulfuric acid, and various metal catalysts have been used to successfully produce COR patterns, including Mn
2+, Ce
3+, Fe
2+, Ru
2+, Cr
2+, Co
2+, and Fe(phen)
32+ (ferroin), and Ru(bpy)
32+ (ruthenium) [
16,
17]. While no sufficiently detailed visually correlated geochemical analyses were performed on the agate geodes to detect halogens, specific metals, or volatile elements, micro-Raman analyses revealed the presence of anatase and an unknown phase with peaks at 632 and 574 cm
−1, a bandwidth usually for metal–oxide bond vibrations. It remains unclear whether these minerals could have played some catalytic role along with Fe. Halogen elements like Cl, Br, and I all have high reactivity with carboxylic groups in organic matter under standard conditions and, in nature, they can become concentrated in evaporitic, volcanic, or diagenetic environments. While redox-sensitive elements like Fe are common in both volcanic and sedimentary environments, organic matter is particularly concentrated by life and is more abundant in sediments compared to volcanic rocks, which is perhaps the reason why concretions are far more common than agate geodes in the rock record.
In experiments, chemical waves are varied due the chaotic distribution, period, amplitude of oxidation spots, and colour gradients [
4]. In fact, the accompanying oscillations of spots, zebra-stripes, fingerprint-like, grape-like, and turbinate columnar patterns remain unexplained. The oxidation spots develop as malonic acid, the only source of carbon in the experiment, which is oxidized to produce CO
2 (which accumulates in progressively larger bubbles), expected to produce halogenated organic acid intermediates. The ferroin redox indicator is red when reduced and blue when oxidized [
16], such that this compound or other metal-bearing catalysts participate in the electron-transfer chain and the blue chemical waves must represent the reaction products and intermediates that diffuse radially from oxidation spots. In agate geodes, gradients of Fe-oxides occur as circularly concentric waves and/or Fe-bearing minerals with variable Fe-oxidation states visible with colour gradients of brown–orange, orange–yellow, and yellow–green accessory minerals and phases (
Figure 1a,b). Twinned circular patterns of red haematite disseminations in chert from Paleoarchean botryoids [
22] and the common presence of dispersed iron oxide minerals in agate geodes [
1,
3] further support the proposed catalytic role for iron in the abiotic formation mechanism. They exhibit the same kind of self-similar patterns with a chaotic distribution and variably sized oxidations spots, from tens of micrometres to decimetres (e.g.,
Figure 1a and
Figure 3a).
The production of CO
2 bubbles during COR arises in part from the cleavage of carboxyl functional groups from malonate through nucleophilic attacks via bromide (Br
−). Halogen elements are known to strongly interact with organic molecules and especially react to decarboxylate carboxyl groups. As carbon dioxide can precipitate carbonates at equilibrium under various slightly alkaline pH, for as long as divalent cations are available in residual diagenetic pore water solutions, CO
2 can precipitate corresponding carbonate minerals. In the experiments shown in
Figure 2a–e, the circularly concentric patterns and the radially aligned quartz crystal converge towards a geometric centre where organic matter is concentrated, which is another key evidence that supports the interpretation of the diagenetic oxidation of organic matter. Not only are the compositions and patterns closely comparable between COR and agate geodes, but occurrences of micron-sized calcite in the Lyall geode (
Figure 3c) and of micron-sized dolomite and calcite in the agate geode (
Figure 4c,f) remarkably match with the random distribution of CO
2 bubbles in the COR experiments, all of which are also closely associated with the circularly concentric patterns (
Figure 2e (rightmost panel),
Figure 3c and
Figure 4b). Hence, the co-occurrence in agate geodes of the same kind of self-similar patterns composed of organic matter located in the geometric centre or forming gradients of dissemination in circularly concentric and twinning waves provide introconvertible evidence for the oxidation of organic matter during geode formation and for the relevance of COR and carbon cycling in the origin of their patterns.
5.3. Pattern-Forming COR during Prebiotic Carbon Cycling and for Exobiology
Biochemical metabolisms are driven by electron transfer between an electron donor and acceptor molecules. During respiration, many molecular intermediates in metabolism include carboxylic acids, and the carboxyl functional group is often the locus of chemical reactions. Bromine, iodine, and chlorine are all essential trace elements for animals and many microorganisms, although their exact biochemical functions in the nervous, endocrine, and immune systems are not fully elucidated. However, many patterns made by metazoans adopt morphologies geometrically like those observed in COR [
44]. Hence, there also exists a connection between COR and metabolic biochemistry, which has led to an abiotic model for pattern-forming abiotic carbon metabolism.
For instance, aerobic heterotrophy often involves glycolysis, which converts glucose to pyruvate. Pyruvate (an α-keto acid) can subsequently enter the tricarboxylic acid cycle (TCA cycle) during which decarboxylation occurs, which yields both energy (i.e., ATP and NADH) and CO
2 in a cyclic fashion that repeats until the reactants are depleted. This is analogous to COR because in the TCA cycle: 1—electron transfers with redox changes occur, 2—CO
2 is produced during the decarboxylation of organic acids, 3—self-similar patterns spontaneously and randomly appear on metazoans, and 4—Fe or other cationic catalysts are involved in the auto-catalysis of the reaction, such that both the TCA cycle and COR express different versions of the same oscillatory process [
44]. Hence, COR might explain the ‘vitality’ of life, whereby life-forms require electron donors and acceptors, and the spontaneous reactions work in concert to perpetuate characteristic cellular self-similar patterns until they run out of electron donors or electron acceptors. So, when the COR solution ultimately turns all blue at the end of every experiment, the system becomes starved of electron donors and/or of electron acceptors and terminates, dies, in a slurry of CO
2 bubbles and residual decayed organic acids.
A COR scenario for the origin of life in hydrothermal environments is consistent with the prebiotic-like environments inferred from agate geodes and concretions, with variable reactants derived from volcanism or evaporitic environmental conditions. The presence of abiotic or biological organic acids and the co-occurrence of redox-sensitive metalliferous minerals (especially those with iron) is also essential for prebiotic chemical synthesis and abiotic chemical reactions. The inference is that a combination of oscillatory reaction networks between Fe, C, S, and halogen-bearing compounds formed the agate geodes. The new observations show that different polymorphs of SiO
2, namely quartz, moganite, and cristobalite compose the matrix (
Figure 3 and
Figure 4). The precursor colloidal silica, as an alkaline hydrated-gel substance, was reorganised by the way in which the circularly concentric waves of reaction products and intermediates were radially diffusing. In volcanically heated rocks, moganite is a mineral almost exclusively associated with quartz replacements, botryoidal habits, or geodes. In comparison, low cristobalite can form at temperatures around 570 K, which can be conducive to the evaporation of aqueous solutions and the consequent naturally concentrated ions of Fe
2+, SO
42−, carboxylic acids, halogens, and many other possible reactants, as suggested by experiments. Hence, spontaneous COR in volcanogenic environments with evaporating aqueous solutions could have resulted in the pockets of abiotically produced carboxylic acids to form patterns in colloidal silica, now preserved in SiO
2 polymorphs, that preserve circularly concentric gradients and cavity structures along with organic matter and Fe-oxide minerals. If this interpretation is correct, then agate geodes could be completely abiotic in origin.
Biosignatures are ‘possible’ signs of life, as objects, patterns, and/or substances [
45]. Possible, because out of the large range of possible biosignatures, only a large number of independent observations can yield a solid biological interpretation. So, then, ‘abiotic biosignatures’ are those objects, patterns, or substances that arose from abiotic processes and that altered precursor-decomposed biomass. If the organic matter is biological in origin, then stable isotope compositions are predicted to record microbial metabolic fractionations. On the other hand, if the organic matter is demonstrably abiotic in origin, then the diagenetic spheroid objects should be considered an abiotic signature of carbon cycling, possibly of prebiotic-like origin [
43]. Hence, the expression ‘abiotic biosignature’ is not contradictory, and rather, it is expected to be widely applicable because diagenetic processes include both biological and abiotic reactions.
In summary, diagenetic spheroids are abiotic biosignatures or abiotic signatures of carbon cycling. It is important to emphasize that agate geodes and some types of concretions might be completely abiotic in origin if the organic acids used during diagenetic COR were abiotic in origin. This can happen in volcanic settings where abiotic carboxylic acids are synthesized from Fischer–Tropsch-type reactions, for instance during the hydrothermal circulation of carbonic fluids in mafic crust. For instance, the possibility of carboxylic acid oxidation by Fe-compounds could explain the highly similar botryoidal patterns in amygdules from Archean Beasley River Basalt [
46]. While these were argued to represent fossilised raindrop patterns, the similarity of their patterns with those of COR (
Figure 2a–e) rather suggest that organic matter and/or Fe-minerals should occur in those laminations. In any case, agate geodes most often come from volcanic environments, and, on Mars, haematite concretions are most likely abiotic in origin. This is because their formation on Mars would have involved volcanogenic and evaporitic concentrations of sulphate, halogens, and carboxylic acids, as have been detected in Martian soils [
47]. In summary, the COR model elegantly explains why metazoan fossils are preserved inside diagenetic spheroids and how these diagenetic spheroids formed abiotically and under a range of diagenetic conditions, during the decomposition and decarboxylation of organic acids.
For exobiology, agate geodes and concretions should be recognized as sedimentological evidence of abiotic carbon cycling and the spontaneous oxidation of carboxylic acids in diagenetic conditions with saline solutions with also iron and sulphate. This leads to the recognition of fossil-bearing diagenetic spheroids as abiotic biosignatures from decomposed biomass and agate geodes as prebiotic-like abiotic signatures. Fauture systematic documentation of δ13Corg and other biosignatures in agate geodes will reveal whether biological sources of organic molecules are common in these objects.