Heat Treatment at 1000 °C under Reducing Atmosphere of Commercial Vermiculites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Sample Characterizations
3. Results
3.1. Chemical Analyses
3.2. Loss Mass and Expansion with In Situ Heating at 1000 °C in Ambient and Reduced Atmosphere
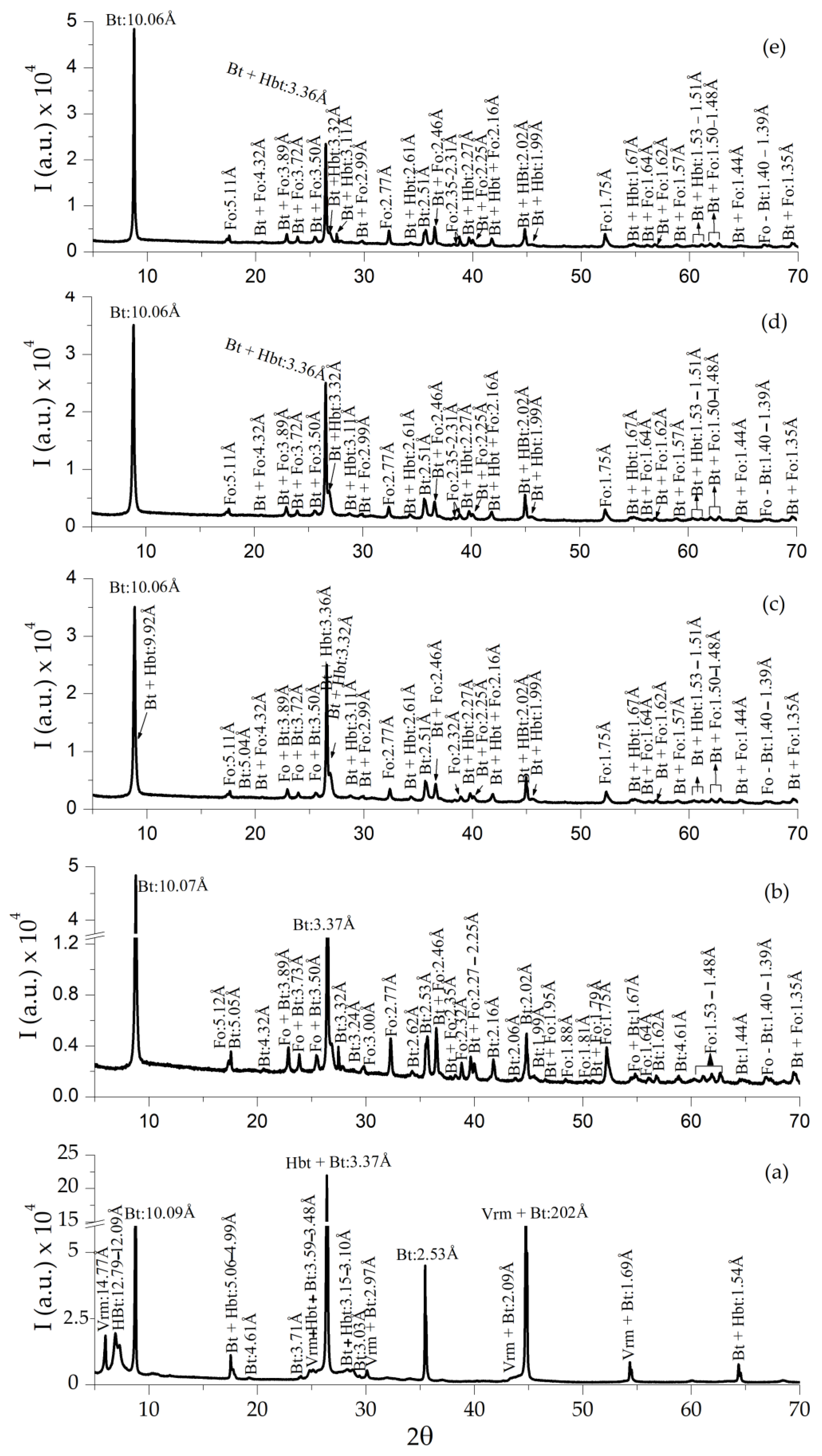
3.3. XRD Analyses
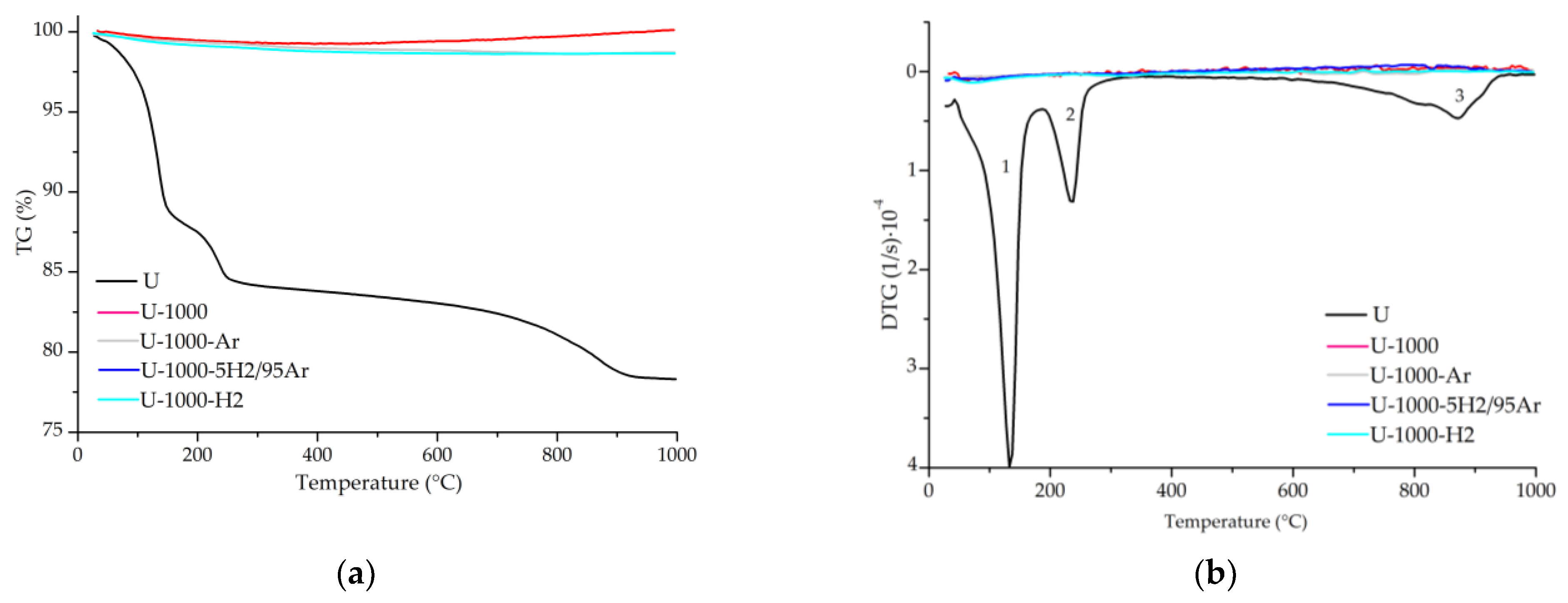
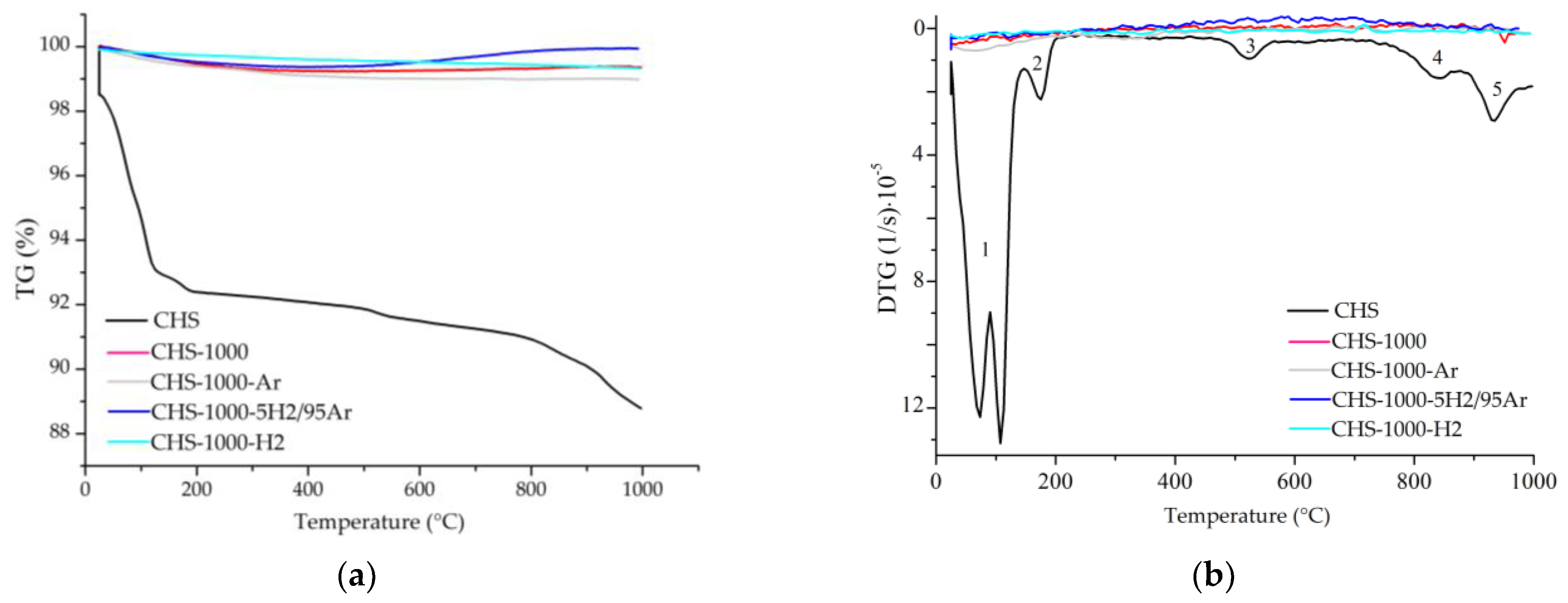
3.4. Thermogravimetric Analyses
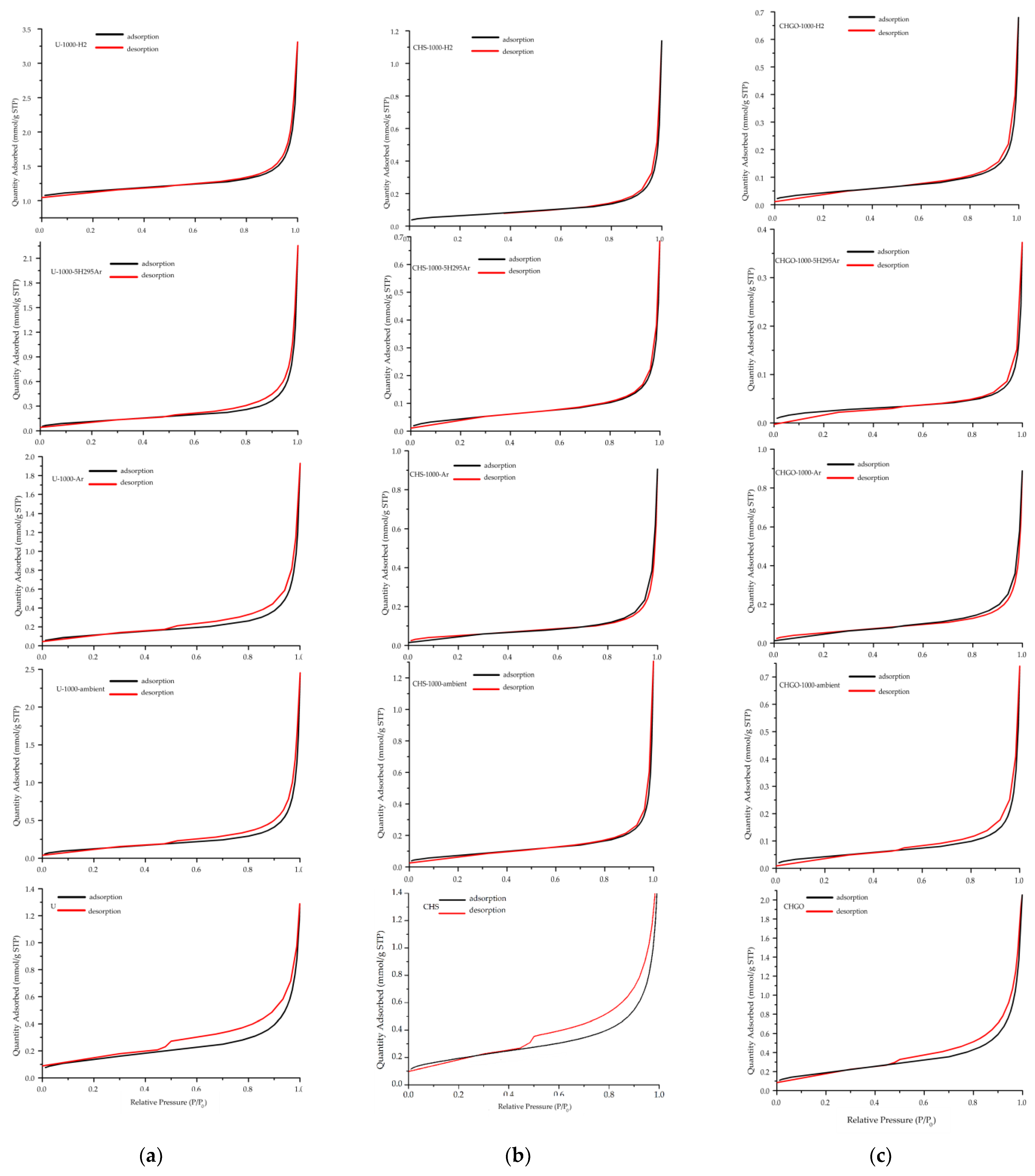
3.5. Textural Parameters Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathieson, A.M.; Walker, G.F. Crystal structure of magnesium-vermiculite. Am. Min. 1954, 39, 231–255. [Google Scholar]
- Vali, H.; Hesse, R. Identification of vermiculite by transmission electron microscopy and x-ray diffraction. Clay Miner. 1992, 27, 185–192. [Google Scholar] [CrossRef]
- Collins, D.R.; Fitch, A.N.; Catlow, R.A. Dehydration of vermiculites and montmorillonites: A time-resolved powder neutron diffraction study. J. Mater. Chem. 1992, 8, 865–873. [Google Scholar] [CrossRef]
- Reichenbach, H.G.; Beyer, J. Dehydration and rehydration of vermiculites: IV. Arrangements of interlayer components in the 1.43 nm and 1.38 nm hydrates of Mg-vermiculite. Clay Miner. 1994, 29, 327–340. [Google Scholar]
- Reichenbach, H.G.; Beyer, J. Dehydration and rehydration of vermiculites: II. Phlogopitic Ca-vermiculite. Clay Miner. 1995, 30, 273–286. [Google Scholar] [CrossRef]
- Reichenbach, H.G.; Beyer, J. Dehydration and rehydration of vermiculites: III. Phlogopitic Sr. and Ba-vermiculite. Clay Miner. 1997, 32, 573–586. [Google Scholar] [CrossRef]
- Marcos, C.; Argüelles, A.; Ruíz-Conde, A.; Sánchez-Soto, P.J.; Blanco, J.A. Study of the dehydration process of vermiculites by applying a vacuum pressure: Formation of interstratified phases. Mineral. Mag. 2003, 67, 1253–1268. [Google Scholar] [CrossRef]
- Marcos, C.; Arango, Y.C.; Rodríguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Valášková, M.; Martynková, G.S. Vermiculite: Structural properties and examples of the use. In Clay Minerals in Nature—Their Characterization, Modification and Application; Valášková, M., Martynková, G.S., Eds.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, I.S. Superparamagnetic behavior in a Ni vermiculite intercalation compound. Phys. Rev. B 2001, 64, 104418. [Google Scholar] [CrossRef]
- Hindman, J.R. Vermiculite. In Industrial Minerals and Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Krukowsky, S.T., Eds.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006; pp. 1015–1027. [Google Scholar]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. (Eds.) Developments in Clay Science. In Handbook of Clay Science, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; Volume 5. [Google Scholar]
- Klein, C.; Dutrow, B. Manual of Mineral Science, 23rd ed.; Wiley: New York, NY, USA, 2007; p. 716. [Google Scholar]
- Abollino, O.; Giacomino, A.; Malandrino, M.; Mentasti, E. Interaction of metal ions with montmorillonite and vermiculite. Appl. Clay Sci. 2008, 38, 227–236. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, J.; Wang, K.Y.; Cheng, L.; Wang, J.; Liu, B. Preparation and characterization of chitosan nanocomposites with vermiculite of different modification. Polym. Degrad. Stab. 2009, 94, 2121–2127. [Google Scholar] [CrossRef]
- Marcos, C.; Argüelles, A.; Khainakov, S.A.; Rodríguez- Fernández, J.; Blanco, J.A. Spin-glass freezing in Ni-vermiculite intercalation compound. J. Condens. Matter. Phys. 2012, 24, 346001–346010. [Google Scholar] [CrossRef]
- Marcos Pascual, C.; Argüelles, A.; Leoni, M.; Blanco, J.A. Location of Ni2+ in nickel-intercalated vermiculites. Appl. Clay Sci. 2014, 91–92, 79–86. [Google Scholar] [CrossRef]
- Midgley, H.G.; Midgley, C.M. The mineralogy of some commercial vermiculites. Clay Miner. 1960, 4, 142–150. [Google Scholar] [CrossRef]
- Couderc, P.; Douillet, P. Les Vermiculites industrielles: Exfoliation, caractéristiques mineralogiques et chimiques. Bull. Soc. Franc. Céram. 1973, 99, 51–59. [Google Scholar]
- Hillier, S.; Marwa, E.M.M.; Rice, C.M. On the mechanism of exfoliation of “Vermiculite”. Clay Miner. 2013, 48, 563–582. [Google Scholar] [CrossRef]
- Marcos, C. Structural changes in vermiculites induced by temperature, pressure, irradiation, and chemical treatments. In Clay Science and Technology; Nascimento, G.M.D., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Li, G.; Shi, C.; Wang, B.; Ling, Z. Exfoliated vermiculite nanosheets supporting tetraethylenepentamine for CO2 capture. Results Mater. 2020, 7, 100–102. [Google Scholar] [CrossRef]
- Pereira, M.H.S.; dos Santos, C.G.; de Lima, G.M.; Oliveira-Bruziquesi, C.G.; de Alvarenga-Oliveira, V. Capture of CO2 by vermiculite impregnated with CaO. Carbon Manag. 2022, 13, 117–126. [Google Scholar] [CrossRef]
- Abidi, S.; Nait-Ali, B.; Joliff, Y.; Favotto, C. Impact of perlite, vermiculite and cement on the thermal conductivity of a plaster composite material: Experimental and numerical approaches. Compos. B Eng. 2015, 68, 392–400. [Google Scholar] [CrossRef]
- Mo, K.H.; Lee, H.J.; Liu, M.Y.J.; Ling, T.-C. Incorporation of expanded vermiculite lightweight aggregate in cement mortar. Constr. Build Mater. 2018, 179, 302–306. [Google Scholar] [CrossRef]
- Alsaman, A.S.; Ibrahim, E.M.M.; Ahmed, M.S.; Askalany, A.A. Composite adsorbent materials for desalination and cooling applications: A state of the art. Int. J. Energy Res. 2022, 46, 10345–10371. [Google Scholar] [CrossRef]
- Cvejn, D.; Martausová, I.; Martaus, A.; Prech, J.; Vesely, O.; Cejka, J.; Lacny, Z.; Nedoma, J.; Martínek, R. Vermiculites catalyze unusual benzaldehyde and dioxane reactivity. Catal. Today 2021, 366, 218–226. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Loizidou, M. Performance of a membrane bioreactor used for the treatment of wastewater contaminated with heavy metals. Bioresour. Technol. 2011, 102, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Ge, L.; Liu, C.; Tang, Z.; Xiao, Y.; Chen, W.; Lei, Z.; Gao, W.; Blake, S.; De, D.; et al. Capturing functional two-dimensional nano sheets from sandwich-structure vermiculite for cancer theranostics. Nat. Commun. 2021, 12, 1124. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.; Rodríguez, I. Exfoliation of vermiculites with chemical treatment using hydrogen peroxide and thermal treatment using microwaves. Appl. Clay Sci. 2014, 87, 219–227. [Google Scholar] [CrossRef]
- Velde, B. High temperature or metamorphic vermiculites. Contrib. Miner. Petrol. 1978, 66, 319–323. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Inguanzo, M.; Menéndez, J.A.; Fuente, E.; Pis, J.J. Reactivity of pyrolyzed sewage sludge in air and CO2. J. Anal. Appl. Pyrolysis 2001, 58–59, 943–954. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Faass, G.S. Correlation of Gas Adsorption, Mercury Intrusion, and Electron Microscopy Pore Property Data for Porous Glasses; Georgia Institute of Technology: Atlanta, GA, USA, 1981; p. 260. [Google Scholar]
- Marcos, C.; Lahchich, A.; Álvarez-Lloret, P. Hydrothermally treated vermiculites: Ability to support products for CO2 adsorption and geological implications. Appl. Clay Sci. 2023, 232, 106791. [Google Scholar] [CrossRef]
- Stoch, L. Thermal analysis and thermochemistry of vitreous into crystalline state transition. J. Therm. Anal. Calorim. 2004, 77, 7–16. [Google Scholar] [CrossRef]
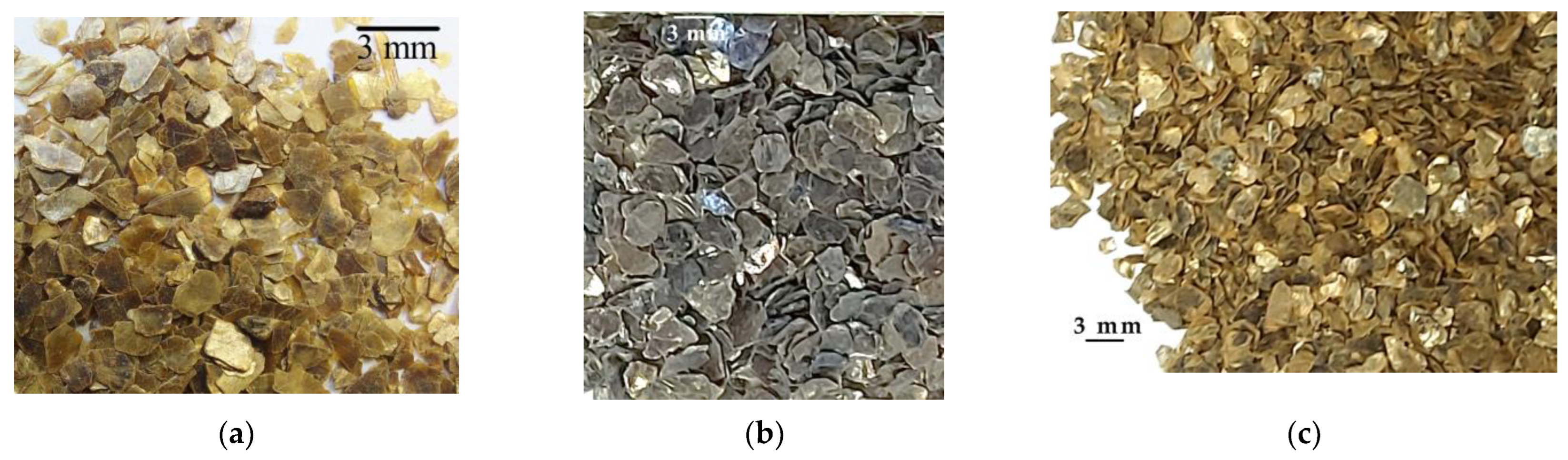
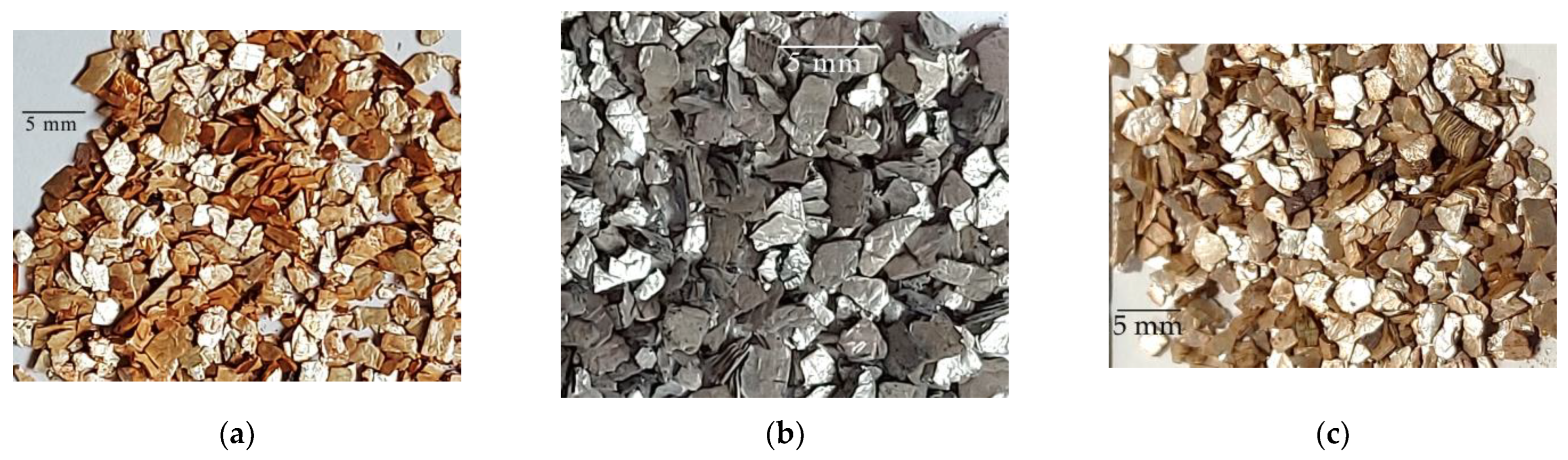
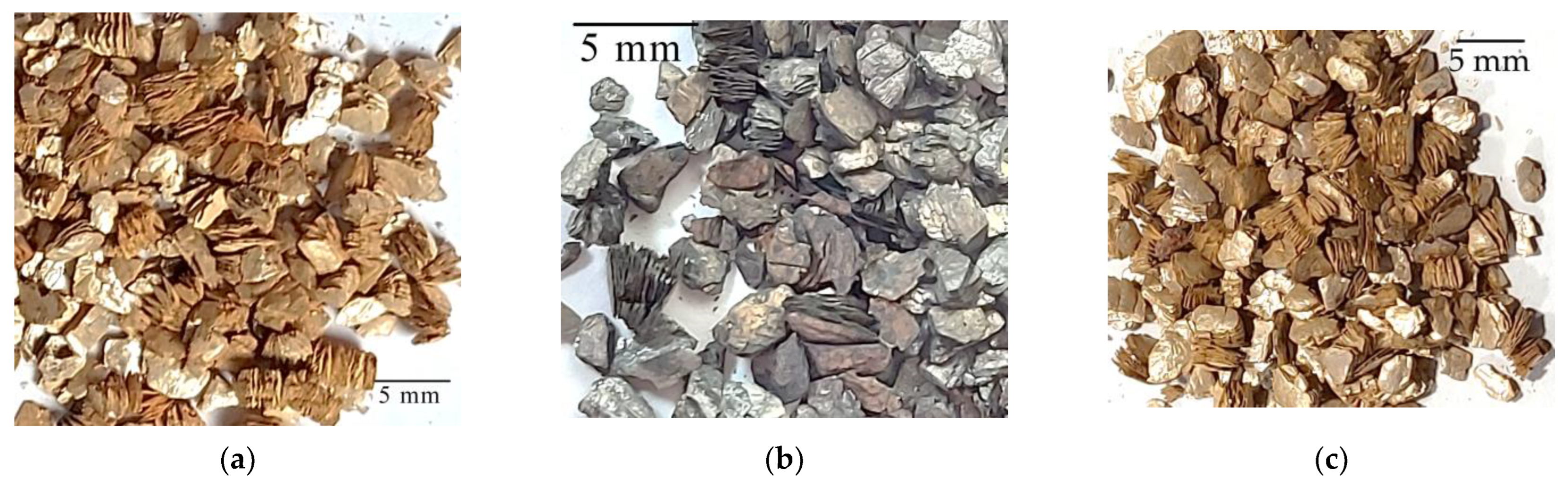
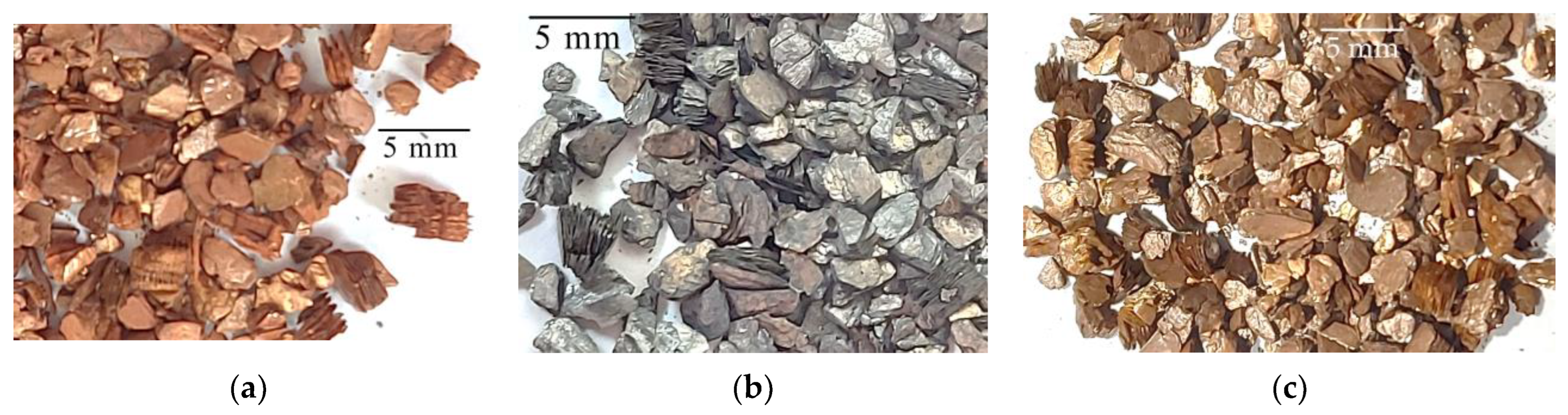
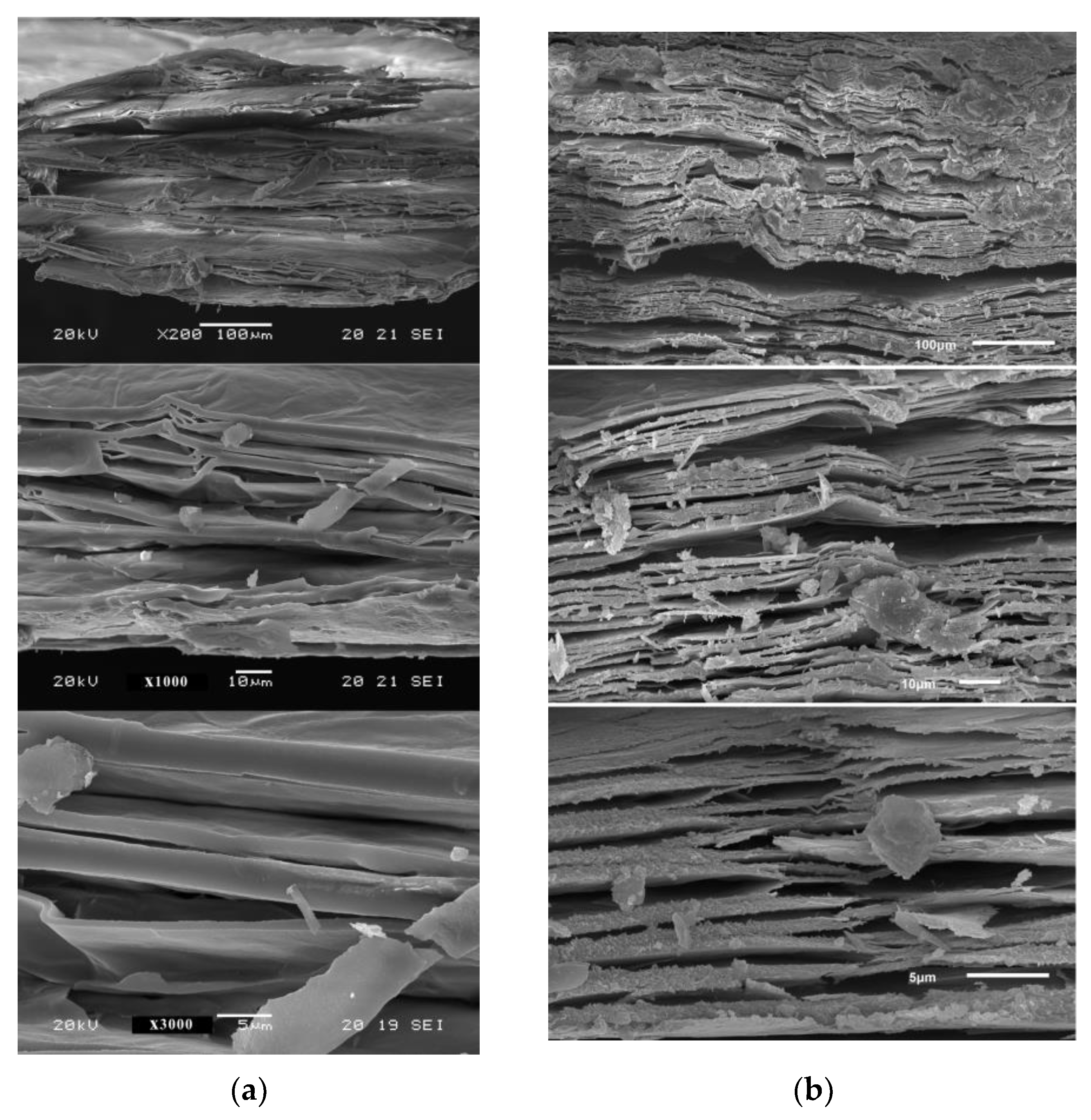
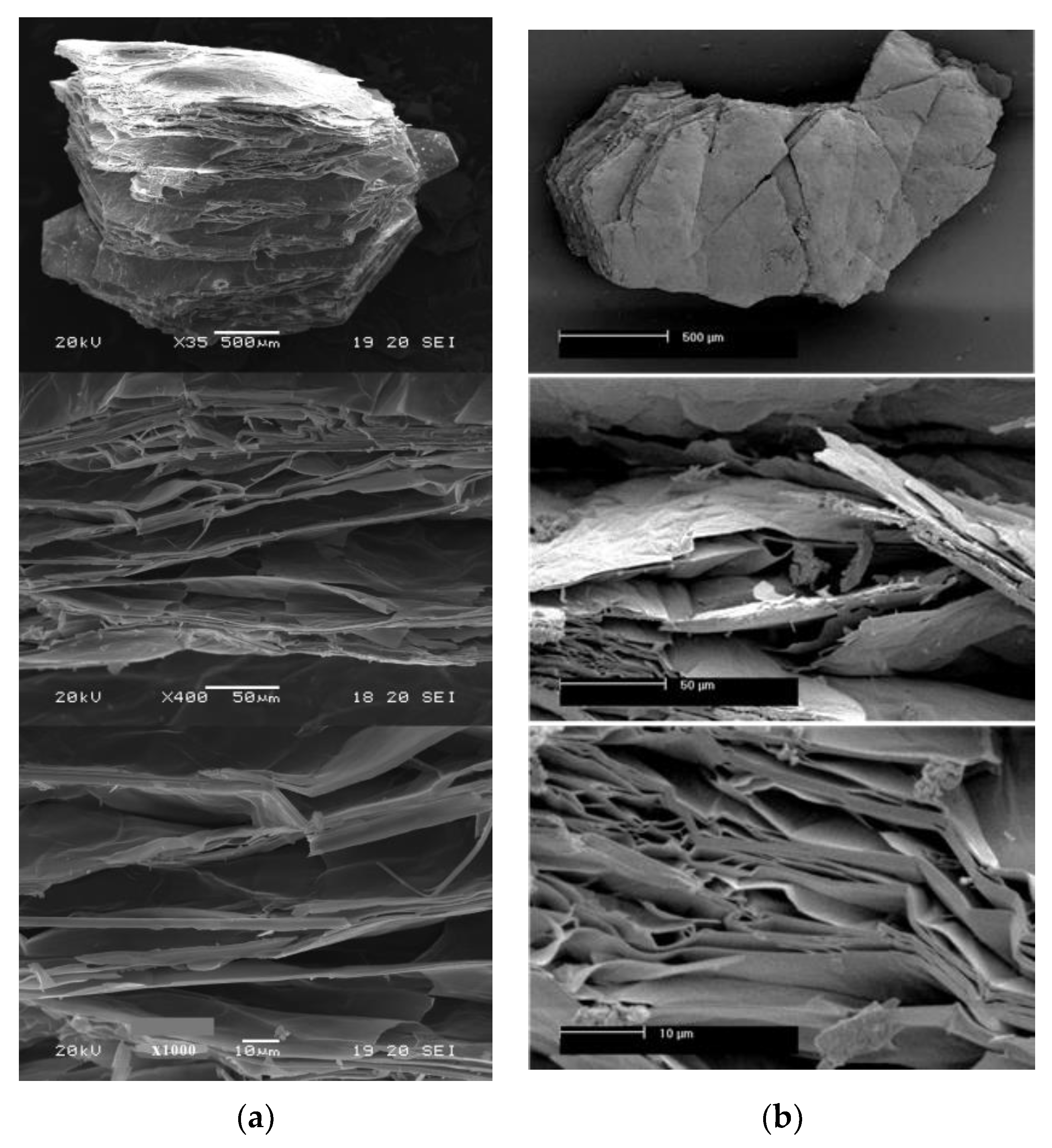
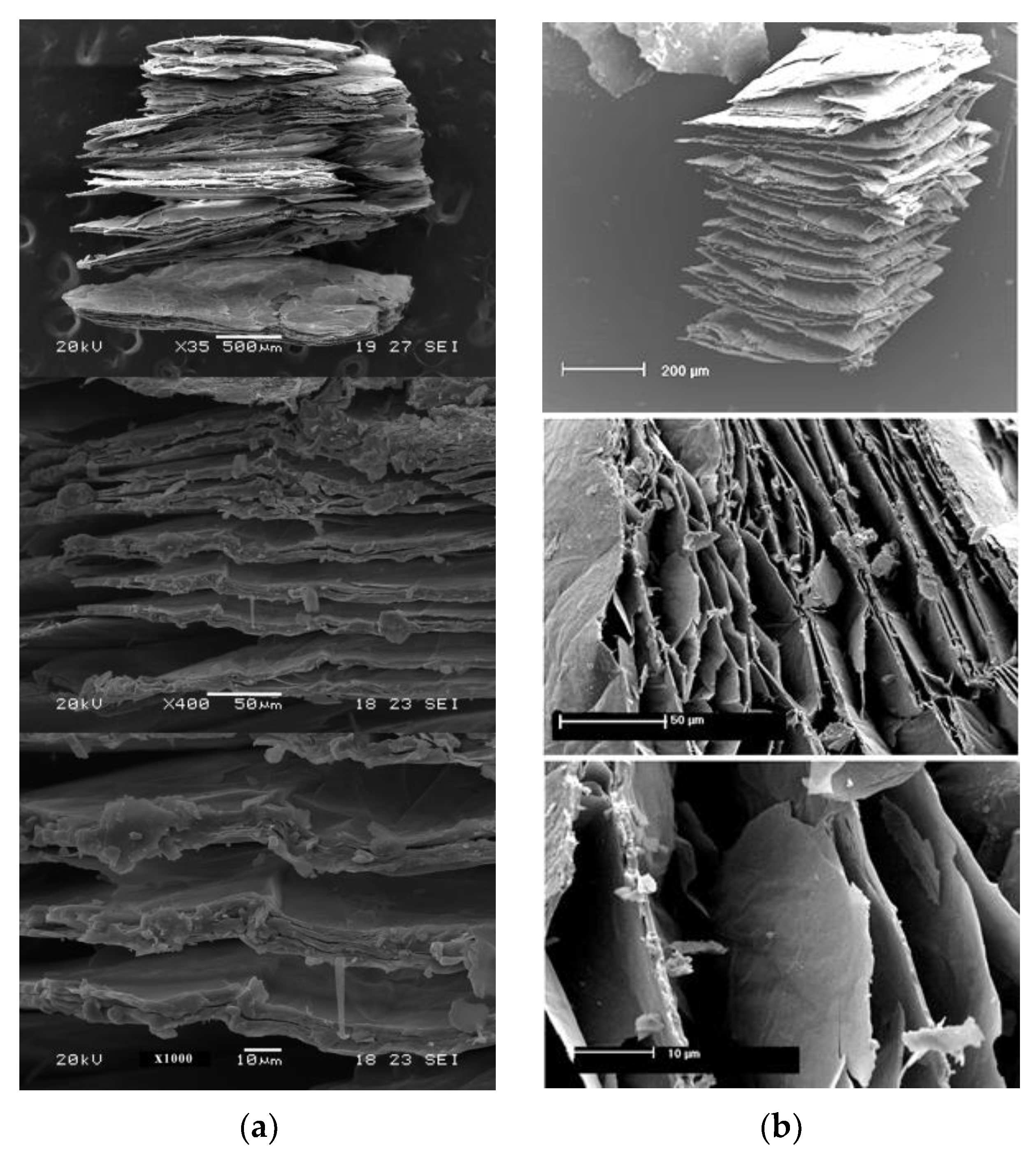



| High-Temperature Thermal Treatment | |||
|---|---|---|---|
| Ambient | 100% Ar | 5% H2/95% Ar | 100% H2 |
| U-1000 | U-1000-Ar | U-1000-5H2/95Ar | U-1000-H2 |
| CHS-1000 | CHS-1000-Ar | CHS-1000-5H2/95Ar | CHS-1000-H2 |
| CHGO-1000 | CHGO-1000-Ar | CHGO-1000-5H2/95Ar | CHGO-1000-H2 |
| Element Oxides | wt. % | ||
|---|---|---|---|
| U | CHS | CHGO | |
| SiO2 | 34.96 | 37.88 | 37.20 |
| Al2O3 | 12.05 | 12.08 | 13.62 |
| Fe2O3 | 8.38 | 5.29 | 18.39 |
| MnO | 0.11 | 0.04 | 0.16 |
| MgO | 21.52 | 23.15 | 10.04 |
| CaO | 0.36 | 1.16 | 1.39 |
| Na2O | 0.14 | 1.51 | 0.30 |
| K2O | 0.36 | 4.80 | 5.08 |
| TiO2 | 1.42 | 1.25 | 2.50 |
| P2O5 | 0.11 | 0.01 | 0.10 |
| L.O.I. | 20.52 | 11.87 | 11.11 |
| TOTAL | 99.92 | 99.05 | 99.88 |
| Samples | Mass Loss (wt %) |
|---|---|
| U-1000 | 23.5 |
| U-1000-Ar | 19.5 |
| U-1000-5H2/95Ar | 20.6 |
| U-1000-H2 | 18.3 |
| CHS-1000 | 12.7 |
| CHS-1000-Ar | 9.4 |
| CHS-1000-5H2/95Ar | 12.3 |
| CHS-1000-H2 | 10.4 |
| CHGO-1000 | 11.4 |
| CHGO-1000-Ar | 10.6 |
| CHGO-1000-5H2/95Ar | 13.2 |
| CHGO-1000-H2 | 10.5 |
| Mass Loss (%) | Gain Loss (%) | |
|---|---|---|
| U | 20.00 | 0.00 |
| U-1000 | 0.79 | 0.83 |
| U-1000-Ar | 0.53 | 0.08 |
| U-1000-5H2/95Ar | 0.83 | 1.08 |
| U-1000-H2 | 0.92 | 0.01 |
| CHS | 9.02 | 0.00 |
| CHS-1000 | 0.78 | 0.14 |
| CHS-1000-Ar | 0.60 | 0.01 |
| CHS-1000-5H2/95Ar | 0.64 | 0.57 |
| CHS-1000-H2 | 0.22 | 0.12 |
| CHGO | 9.20 | 0.00 |
| CHGO-1000 | 0.60 | 0.00 |
| CHGO-1000-Ar | 1.04 | 0.01 |
| CHGO-1000-5H2/95Ar | 0.35 | 2.77 |
| CHGO-1000-H2 | 0.20 | 3.17 |
| Sample | SBET (m2/g) | Qm (mmol/g STP) | Vp (mmol/g STP) | Pore Size (nm) | C | R2 |
|---|---|---|---|---|---|---|
| U | 11.70 ± 0.1 | 0.12 | 0.005 | 2.93 | 55.1 | 0.9999 |
| U-1000 | 10.63 ± 0.1 | 0.11 | 0.003 | 2.94 | 56.9 | 0.9995 |
| U-1000-Ar | 9.53 ± 0.1 | 0.10 | 0.002 | 2.90 | 58.4 | 0.9996 |
| U-1000-5H2/95Ar | 9.40 ± 0.1 | 0.10 | 0.003 | 2.95 | 69.7 | 0.9996 |
| U-1000-H2 | 11.48 ± 0.1 | 0.12 | 0.004 | 2.93 | 80.3 | 0.9997 |
| CHS | 15.40 ± 0.1 | 0.16 | 0.003 | 2.95 | 137.5 | 0.9998 |
| CHS-1000 | 5.98 ± 0.03 | 0.06 | 0.002 | 2.92 | 87.4 | 0.9997 |
| CHS-1000-Ar | 4.23 ± 0.02 | 0.04 | 0.001 | 2.92 | 80.4 | 0.9999 |
| CHS-1000-5H2/95Ar | 3.80 ± 0.03 | 0.04 | 0.001 | 2.52 | 45.2 | 0.9994 |
| CHS-1000-H2 | 4.94 ± 0.04 | 0.05 | 0.002 | 3.24 | 231.9 | 0.9998 |
| CHGO | 12.10 ± 0.1 | 0.16 | 0.007 | 2.52 | 155.6 | 0.9996 |
| CHGO-1000 | 3.59 ± 0.02 | 0.04 | 0.001 | 2.93 | 63.0 | 0.9997 |
| CHGO-1000-Ar | 4.54 ± 0.04 | 0.05 | 0.001 | 2.52 | 55.9 | 0.9994 |
| CHGO-1000-5H2/95Ar | 2.06 ± 0.02 | 0.02 | 0.001 | 2.95 | 44.0 | 0.9994 |
| CHGO-1000-H2 | 3.60 ± 0.03 | 0.04 | 0.001 | 2.93 | 68.8 | 0.9995 |
| Sample | SBET (m2/g) | Qm (mmol/g STP) | Vp (mmol/g STP) | Pore Size (nm) | C | R2 |
|---|---|---|---|---|---|---|
| U-1000 | 13.2 ± 0.0 | 0.14 | 0.06 | 2.52 | 184.7 | 0.9999 |
| CHS-1000 | 8.33 ± 0.1 | 0.09 | 0.006 | 2.51 | 86.2 | 0.9996 |
| CHGO-1000 | 9.04 ± 0.1 | 0.09 | 0.006 | 2.64 | 135.1 | 0.9998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahchich, A.; Álvarez-Lloret, P.; Leardini, F.; Marcos, C. Heat Treatment at 1000 °C under Reducing Atmosphere of Commercial Vermiculites. Minerals 2024, 14, 232. https://doi.org/10.3390/min14030232
Lahchich A, Álvarez-Lloret P, Leardini F, Marcos C. Heat Treatment at 1000 °C under Reducing Atmosphere of Commercial Vermiculites. Minerals. 2024; 14(3):232. https://doi.org/10.3390/min14030232
Chicago/Turabian StyleLahchich, Ayoub, Pedro Álvarez-Lloret, Fabrice Leardini, and Celia Marcos. 2024. "Heat Treatment at 1000 °C under Reducing Atmosphere of Commercial Vermiculites" Minerals 14, no. 3: 232. https://doi.org/10.3390/min14030232
APA StyleLahchich, A., Álvarez-Lloret, P., Leardini, F., & Marcos, C. (2024). Heat Treatment at 1000 °C under Reducing Atmosphere of Commercial Vermiculites. Minerals, 14(3), 232. https://doi.org/10.3390/min14030232







