Geochemistry, Sr-Nd Isotope Compositions, and U-Pb Chronology of Apatite from Kimberlite in Wafangdian, North China Craton: Constraints on the Late Magmatic Processes
Abstract
:1. Introduction
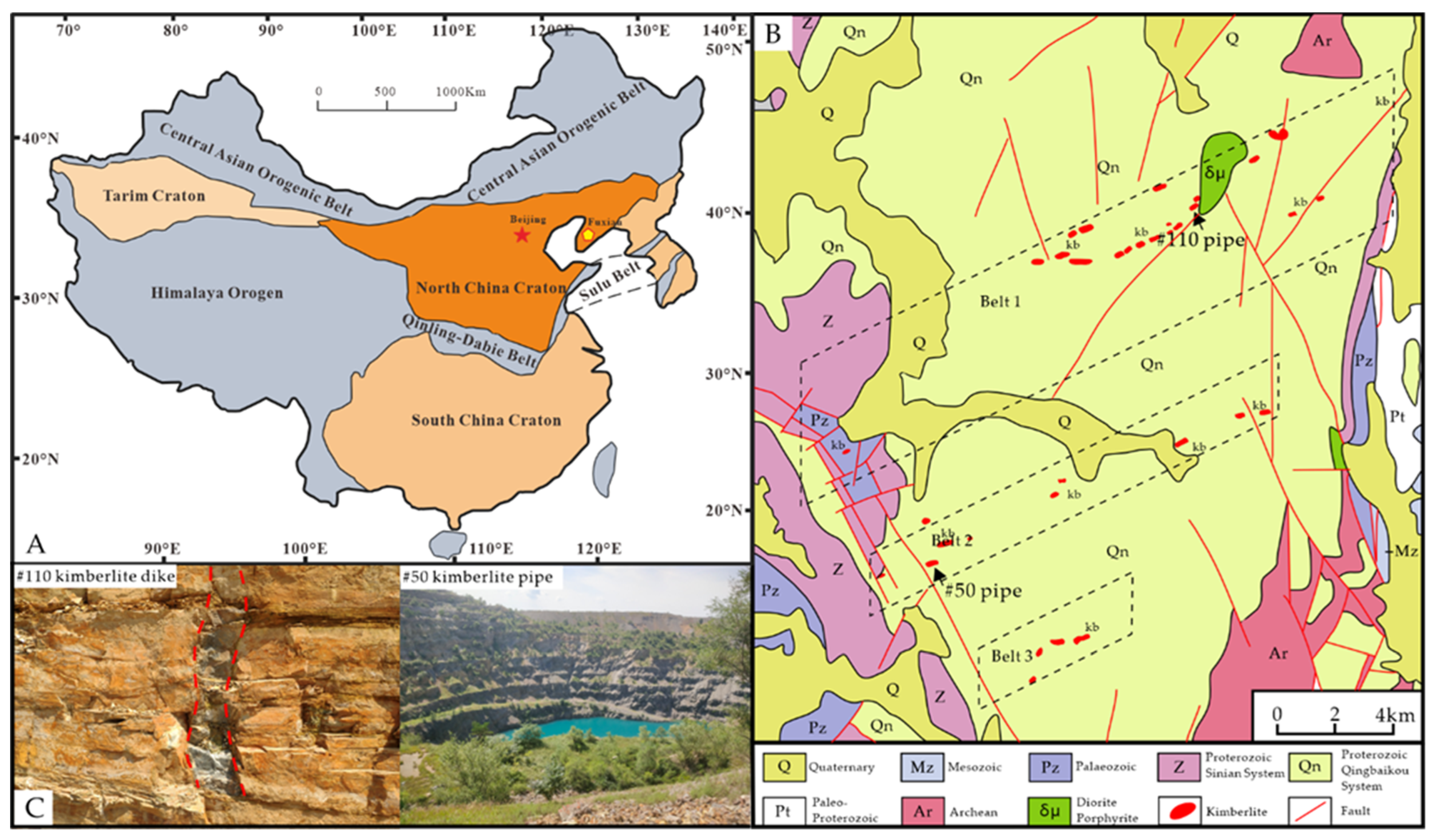
2. Geologic Setting
3. Petrography
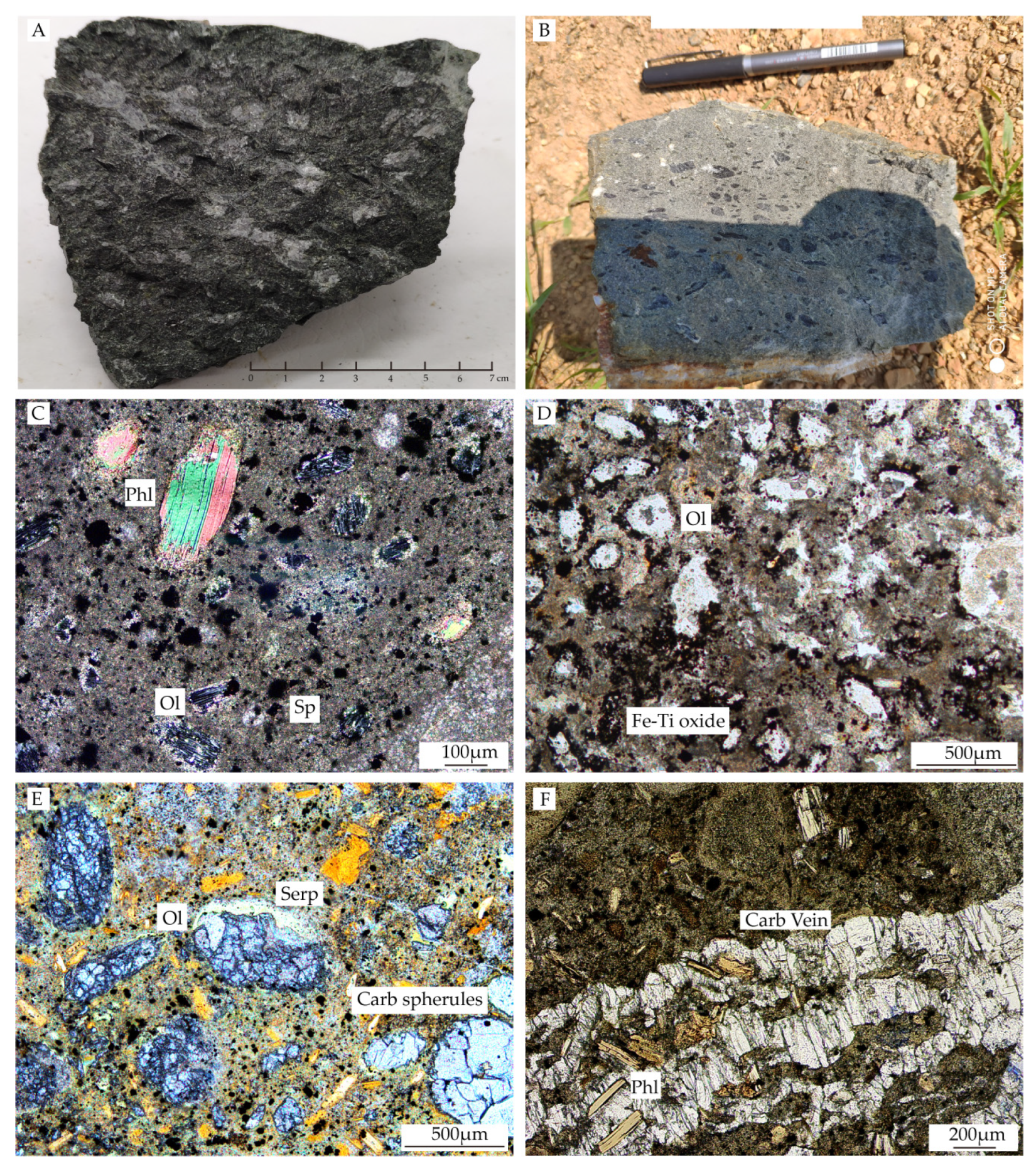
3.1. Kimberlite Petrography of Pipe No. 50 and 110
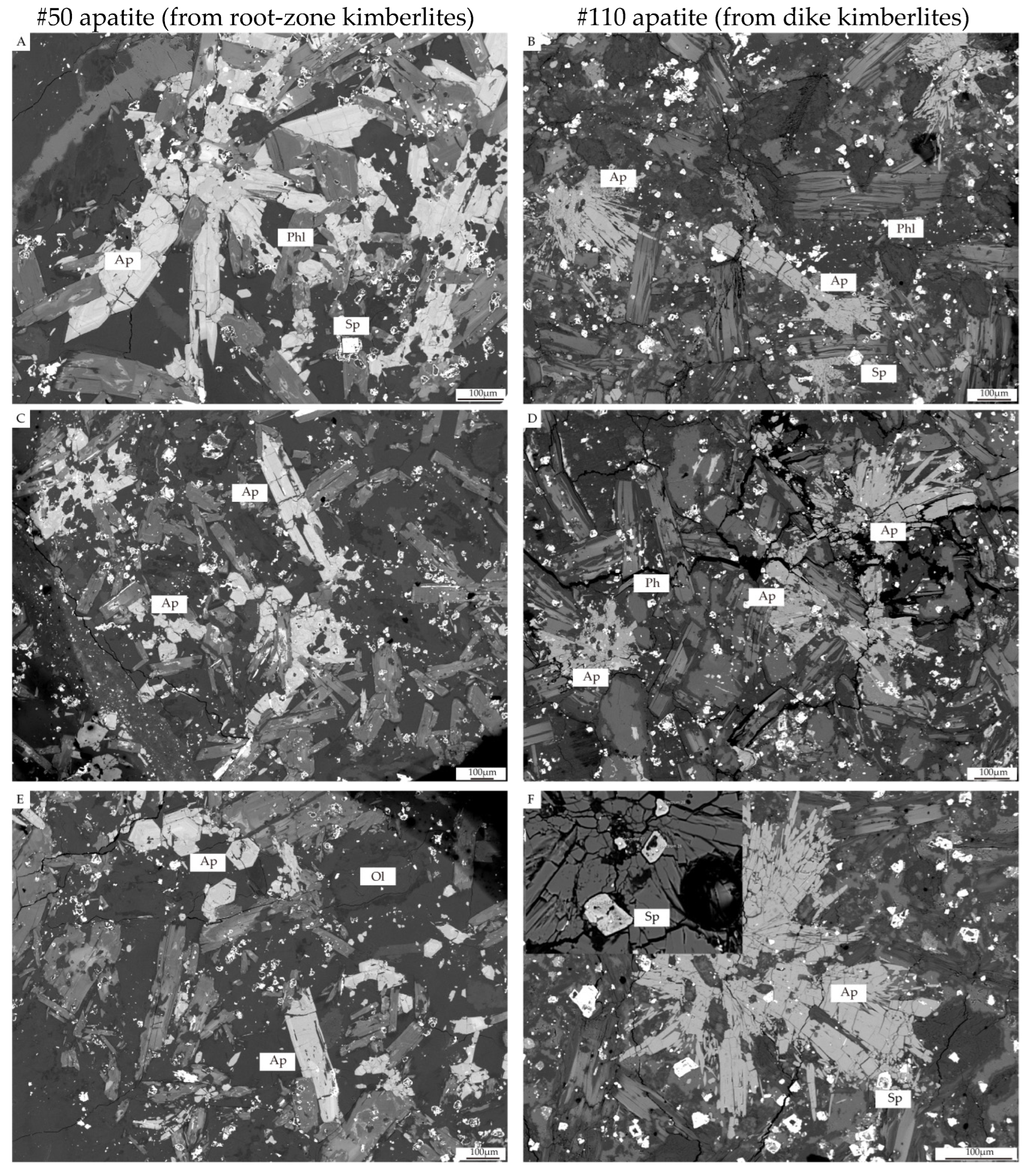
3.2. Apatite Petrography of Pipe No. 50 and 110
4. Analytical Methods
4.1. Electron Probe Micro-Analysis (EPMA) of Apatite
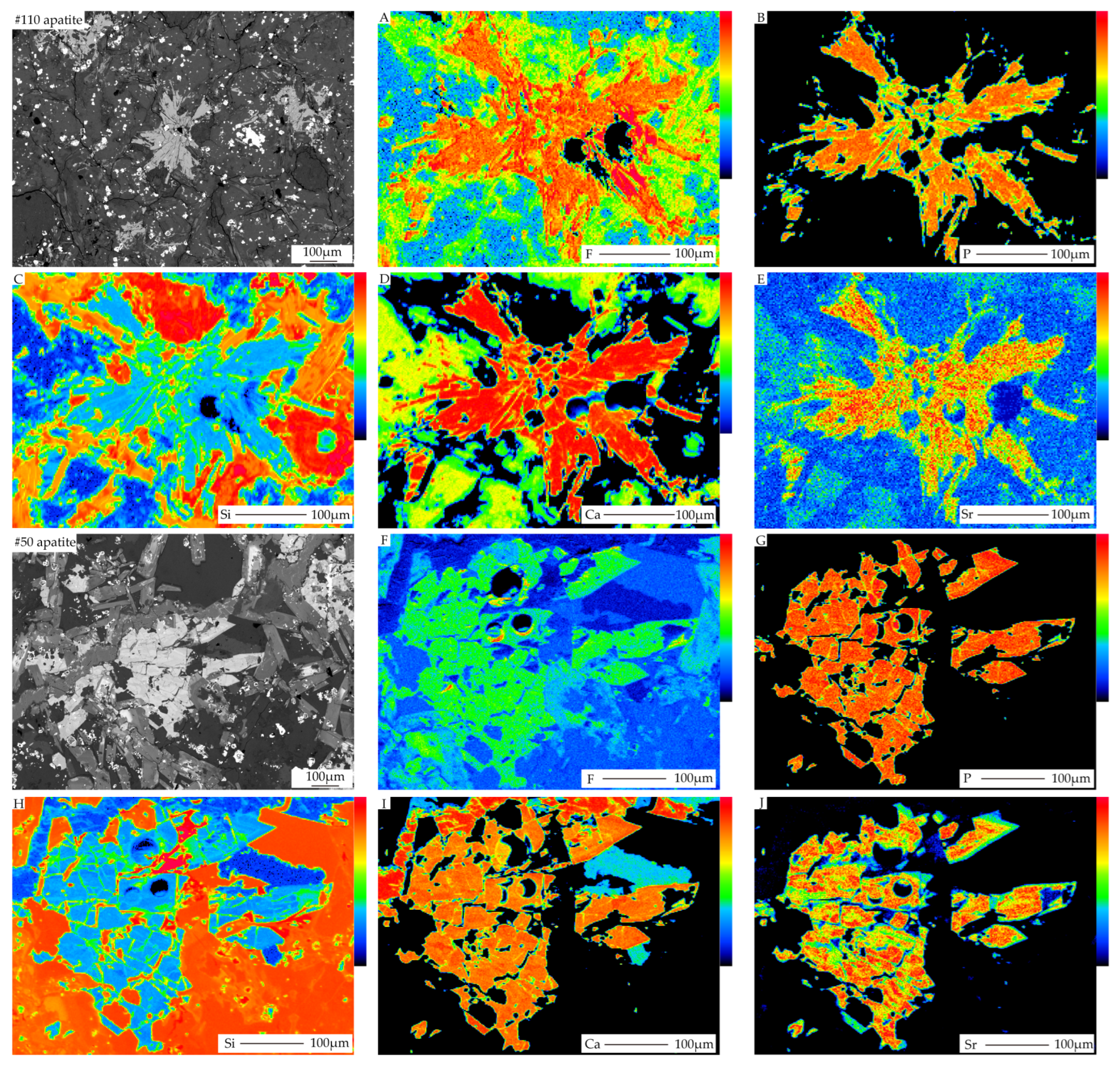
4.2. X-ray Mapping of Apatite
4.3. LA-ICP-MS Analysis of Apatite
4.4. In Situ LA-ICP-MS U-Pb Dating of Apatite
4.5. In Situ Sr-Nd Isotope Analysis of Apatite
4.5.1. In Situ Sr Isotope Analysis
4.5.2. In Situ Nd Isotope Analysis
5. Results
5.1. Major Element Characteristics
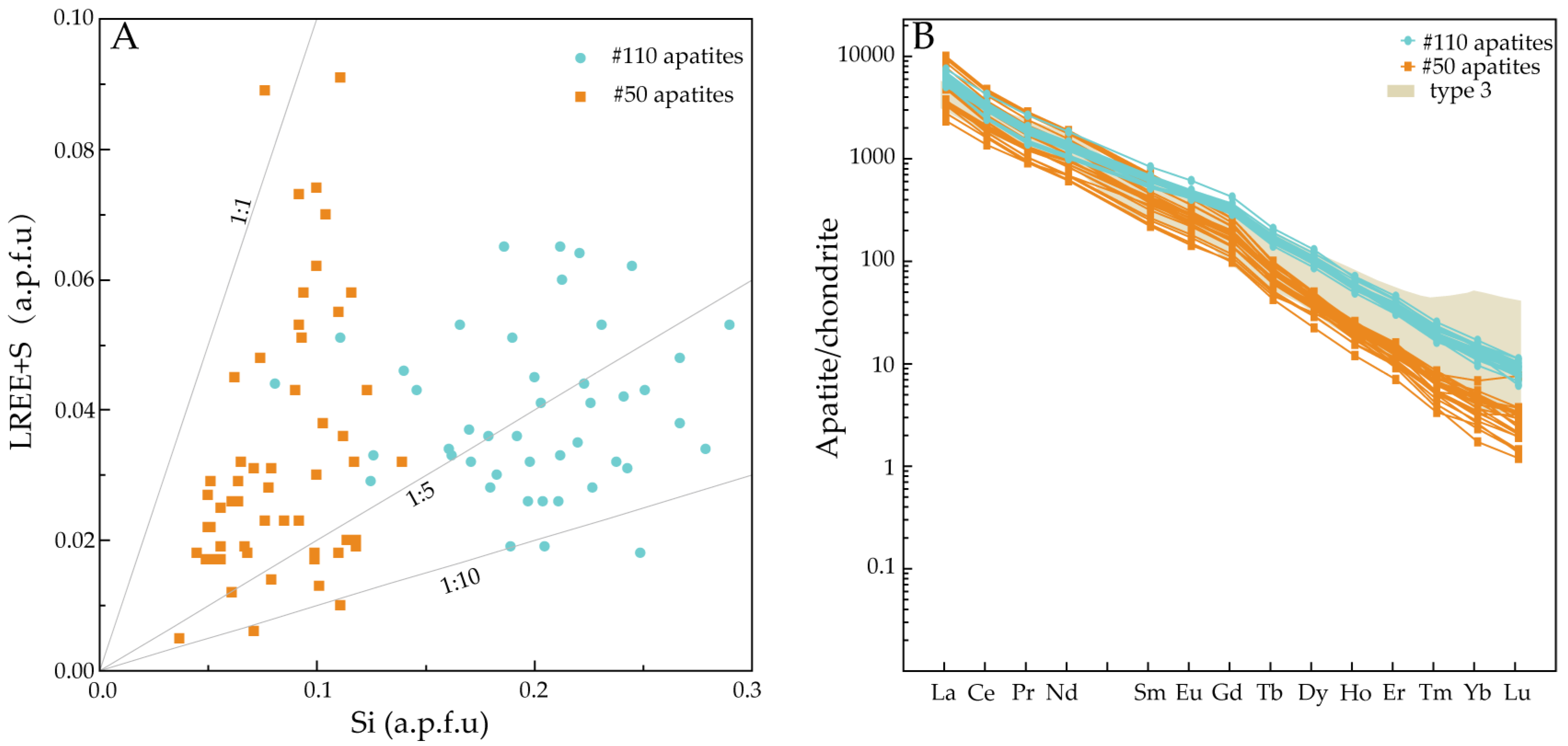
5.2. REE Characteristics
5.3. U-Th-Pb and U–Pb Isotopic Compositions of Apatite
5.3.1. U-Th-Pb Composition of Apatite
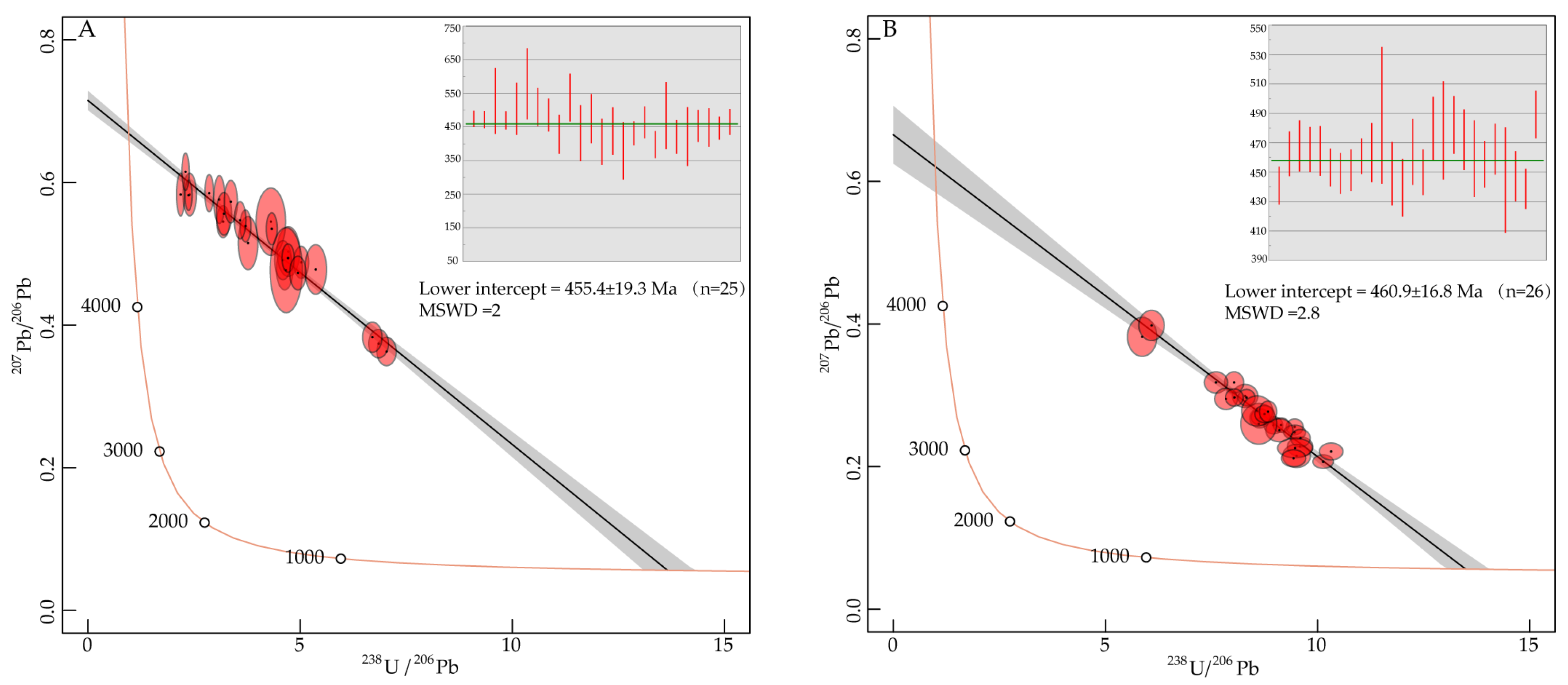
5.3.2. U–Pb Isotope Composition of Apatite
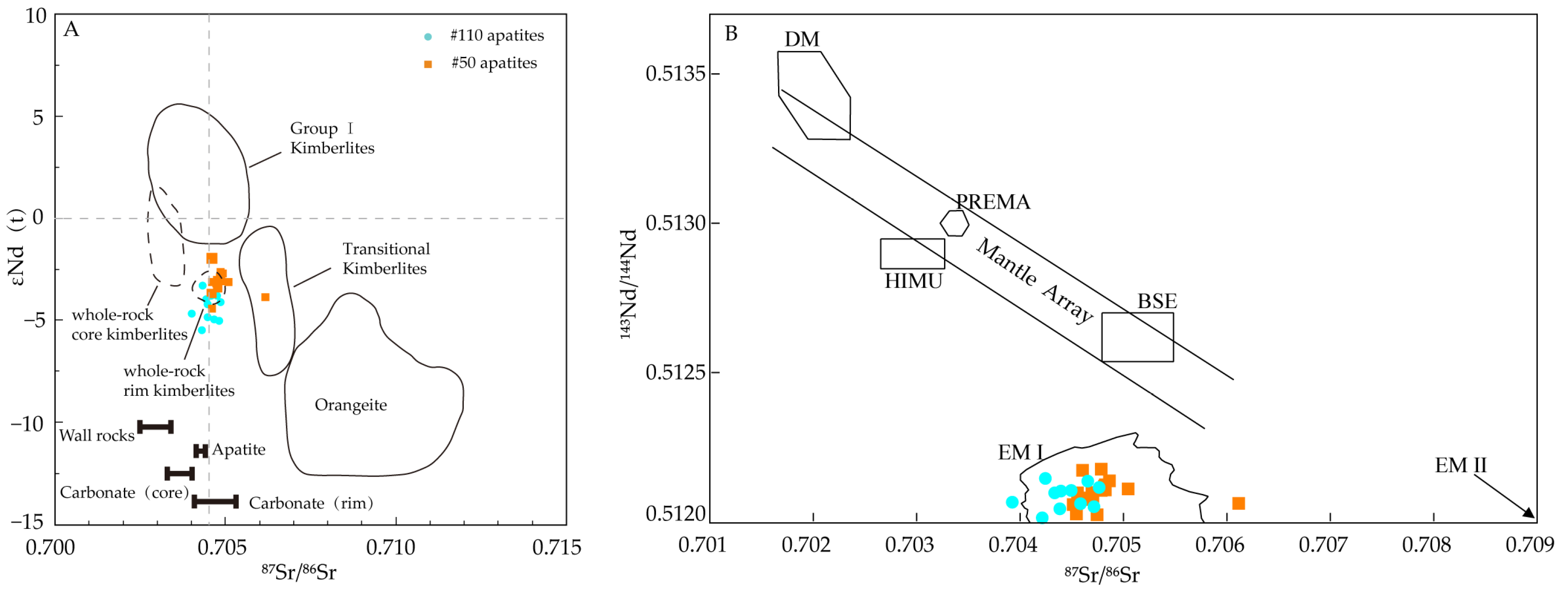
5.4. Sr-Nd Isotope Composition of Apatite
6. Discussion
6.1. Crystallization Age of the Wafangdian Kimberlite
6.2. Sr-Nd Isotope Constraints on Petrogenesis of the Wafangdian Kimberlites
6.3. Apatite Perspective: Late Magmatic Evolution of Kimberlite
6.4. Apatite Indication of Emplacement Pattern of Wafangdian Kimberlite
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giuliani, A.; Phillips, D.; Kamenetsky, V.S.; Goemann, K. Constraints on Kimberlite Ascent Mechanisms Revealed by Phlogopite Compositions in Kimberlites and Mantle Xenoliths. Lithos 2016, 240–243, 189–201. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A.; Creaser, R.A. The Timing of Kimberlite Magmatism in North America: Implications for Global Kimberlite Genesis and Diamond Exploration. Lithos 2003, 71, 153–184. [Google Scholar] [CrossRef]
- Castillo-Oliver, M.; Galí, S.; Melgarejo, J.C.; Griffin, W.L.; Belousova, E.; Pearson, N.J.; Watangua, M.; O’Reilly, S.Y. Trace-Element Geochemistry and U-Pb Dating of Perovskite in Kimberlites of the Lunda Norte Province (NE Angola): Petrogenetic and Tectonic Implications. Chem. Geol. 2016, 426, 118–134. [Google Scholar] [CrossRef]
- Soltys, A.; Giuliani, A.; Phillips, D. A New Approach to Reconstructing the Composition and Evolution of Kimberlite Melts: A Case Study of the Archetypal Bultfontein Kimberlite (Kimberley, South Africa). Lithos 2018, 304–307, 1–15. [Google Scholar] [CrossRef]
- Dalton, H.; Giuliani, A.; O’Brien, H.; Phillips, D.; Hergt, J. The Role of Lithospheric Heterogeneity on the Composition of Kimberlite Magmas from a Single Field: The Case of Kaavi-Kuopio, Finland. Lithos 2020, 354–355, 354–355. [Google Scholar] [CrossRef]
- Zhang, W.; Johnston, S.T.; Currie, C.A. Kimberlite Magmatism Induced by West-Dipping Subduction of the North American Plate. Geology 2019, 47, 395–398. [Google Scholar] [CrossRef]
- Dongre, A.; Viljoen, K.S.; Belyanin, G.; Le Roux, P.; Malandkar, M. Petrogenesis of the Diamondiferous Pipe-8 Ultramafic Intrusion from the Wajrakarur Kimberlite Field of Southern India and Its Relation to the Worldwide Mesoproterozoic (~1.1 Ga) Magmatism of Kimberlite and Related Rocks. Geosci. Front. 2020, 11, 793–805. [Google Scholar] [CrossRef]
- Le Roex, A.P.; Bell, D.R.; Davis, P. Petrogenesis of Group I Kimberlites from Kimberley, South Africa: Evidence from Bulk-Rock Geochemistry. J. Petrol. 2003, 44, 2261–2286. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Pankaj, P.; Pandit, D.; Chakrabarti, R.; Rao, N.V.C. Petrology and Sr-Nd Isotope Systematics of the Ahobil Kimberlite (Pipe-16) from the Wajrakarur Field, Eastern Dharwar Craton, Southern India. Geosci. Front. 2019, 10, 1167–1186. [Google Scholar] [CrossRef]
- Chalapathi Rao, N.V.; Lehmann, B.; Panwar, B.K.; Kumar, A.; Mainkar, D. Petrogenesis of the Crater-Facies Tokapal Kimberlite Pipe, Indra¯vati Basin, Central India. Geosci. Front. 2014, 5, 781–790. [Google Scholar] [CrossRef]
- Moussallam, Y.; Morizet, Y.; Gaillard, F. H2O–CO2 Solubility in Low SiO2-Melts and the Unique Mode of Kimberlite Degassing and Emplacement. Earth Planet. Sci. Lett. 2016, 447, 151–160. [Google Scholar] [CrossRef]
- Skinner, E.M.W.; Marsh, J.S. Distinct Kimberlite Pipe Classes with Contrasting Eruption Processes. Lithos 2004, 76, 183–200. [Google Scholar] [CrossRef]
- Sparks, R.S.J. Kimberlite Volcanism. Annu. Rev. Earth Planet. Sci. 2013, 41, 497–528. [Google Scholar] [CrossRef]
- Field, M.; Scott Smith, B.H. Contrasting Geology and Near-Surface Emplacement of Kimberlite Pipes in Southern Africa and Canada. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; Gurney, J.J., Gurney, J.L., Pascoe, M.D., Richardson, S.H., Eds.; Red Roof Design Cape Town 1; University of Cape Town: Cape Town, South Africa, 1999; pp. 214–237. [Google Scholar]
- Donnelly, C.L.; Griffin, W.L.; Yang, J.H.; O’Reilly, S.Y.; Li, Q.L.; Pearson, N.J.; Li, X.H. In Situ U-Pb Dating and Sr-Nd Isotopic Analysis of Perovskite: Constraints on the Age and Petrogenesis of the Kuruman Kimberlite Province, Kaapvaal Craton, South Africa. J. Petrol. 2012, 53, 2497–2522. [Google Scholar] [CrossRef]
- Malarkey, J.; Pearson, D.G.; Kjarsgaard, B.A.; Davidson, J.P.; Nowell, G.M.; Ottley, C.J.; Stammer, J. From Source to Crust: Tracing Magmatic Evolution in a Kimberlite and a Melilitite Using Microsample Geochemistry. Earth Planet. Sci. Lett. 2010, 299, 80–90. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Giuliani, A.; O’Brien, H. What Is a Kimberlite? Petrology and Mineralogy of Hypabyssal Kimberlites. Elements 2019, 15, 381–386. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in Igneous Systems. Rev. Miner. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Tollari, N.; Barnes, S.J.; Cox, R.A.; Nabil, H. Trace Element Concentrations in Apatites from the Sept-Îles Intrusive Suite, Canada—Implications for the Genesis of Nelsonites. Chem. Geol. 2008, 252, 180–190. [Google Scholar] [CrossRef]
- Watson, E.B. Apatite and Phosphorus in Mantle Source Regions: An Experimental Study of Apatite/Melt Equilibria at Pressures to 25 Kbar. Earth Planet. Sci. Lett. 1980, 51, 322–335. [Google Scholar] [CrossRef]
- Milligan, R.; Fedortchouk, Y.; Normandeau, P.X.; Fulop, A.; Robertson, M. Features of Apatite in Kimberlites from Ekati Diamond Mine and Snap Lake, Northwest Territories, Canada: Modelling of kimberlite Composition. In Proceedings of the 11th International Kimberlite Conference Extended Abstract, Gaborone, Botswana, 18–22 September 2017. [Google Scholar]
- Qian, L.; Wang, Y.; Xie, J.; Sun, W. The Late Mesozoic Granodiorite and Polymetallic Mineralization in Southern Anhui Province, China: A Perspective from Apatite Geochemistry. Solid. Earth Sci. 2019, 4, 178–189. [Google Scholar] [CrossRef]
- Yang, F.; Santosh, M.; Glorie, S.; Xue, F.; Zhang, S.; Zhang, X. Apatite Geochronology and Chemistry of Luanchuan Granitoids in the East Qinling Orogen, China: Implications for Petrogenesis, Metallogenesis and Exploration. Lithos 2020, 378–379, 105797. [Google Scholar] [CrossRef]
- Soltys, A.; Giuliani, A.; Zurich, E.; Phillips, D. Kimberlite Metasomatism of the Lithosphere and the Evolution of Olivine in Carbonate-Rich Melts-Evidence from the Kimberley Kimberlites (South Africa) Geochemical Investigations of LIP Magmatic Processes View Project. J. Petrol. 2020, 61, egaa062. [Google Scholar] [CrossRef]
- Xu, S. Apatite Geochemical Constraints on Petrogenesis and Metallogenesis of Mafic-Ultramafic Intrusions in the Emeishan Large Igneous Province. Master’s Thesis, Lanzhou University, Lanzhou, China, 2022. [Google Scholar]
- Yan, X.Y.; Yang, D.B.; Xu, W.L.; Quan, Y.K.; Wang, A.Q.; Hao, L.R.; Yang, H.T.; Wang, F. Apatite Geochemistry from Mafic Rocks in the Northeastern North China Craton: New Insights into Petrogenesis. Lithos 2023, 436–437, 106942. [Google Scholar] [CrossRef]
- Si, B. Petrography, Mineralogical and Geochemical Characteristics of Kimberlite in North China Platform. Adv. Geosci. 2023, 13, 292–303. [Google Scholar] [CrossRef]
- Yang, C.; Ba, Y.; Wang, Y.; He, K.; Bai, G. Ordovician Magmatism in the North China Craton-Evidence from U-Pb Age of the Inherited Zircon from Cretaceous Diorite in Lingtou Area, Anyang, Henan. Geol. Rev. 2023, 69, 1151–1160. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, D.; Nutman, A.; Zhou, H.; Dong, C.; Yin, X.; Ma, M. Multiple 3.8–3.1 Ga Tectono-Magmatic Events in a Newly Discovered Area of Ancient Rocks (the Shengousi Complex), Anshan, North China Craton. J. Asian Earth Sci. 2012, 54–55, 18–30. [Google Scholar] [CrossRef]
- Zhu, R.Z.; Ni, P.; Ding, J.Y.; Wang, D.Z.; Ju, Y.; Kang, N.; Wang, G.G. Petrography, Chemical Composition, and Raman Spectra of Chrome Spinel: Constraints on the Diamond Potential of the No. 30 Pipe Kimberlite in Wafangdian, North China Craton. Ore Geol. Rev. 2017, 91, 896–905. [Google Scholar] [CrossRef]
- Liu, L.; Wu, D.; Han, S.; Sun, H.; Li, H.; Xiong, Z. Geological Characteristics and Genesis of No.110 Kimberlite Pipe in Wafangdian Diamond Ore Field in Southern Liaoning. Miner. Explor. 2021, 12, 1339–1354. [Google Scholar]
- Li, Q.L.; Wu, F.Y.; Li, X.H.; Qiu, Z.L.; Liu, Y.; Yang, Y.H.; Tang, G.Q. Precisely Dating Paleozoic Kimberlites in the North China Craton and Hf Isotopic Constraints on the Evolution of the Subcontinental Lithospheric Mantle. Lithos 2011, 126, 127–134. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wu, F.Y.; Wilde, S.A.; Liu, X.M.; Zhang, Y.B.; Xie, L.W.; Yang, J.H. In Situ Perovskite Sr-Nd Isotopic Constraints on the Petrogenesis of the Ordovician Mengyin Kimberlites in the North China Craton. Chem. Geol. 2009, 264, 24–42. [Google Scholar] [CrossRef]
- Tian, R.; Wang, H.; Tian, J.; Shan, W.; Wang, X.; Chi, N.; Ma, X.; Chu, Z.; Li, S.; Lv, Q. Preliminary Investigation of the Eruption Time of Kimberlite in the Late Devonian in Mengyin, Shandong. Front. Earth Sci. 2023, 11, 1084673. [Google Scholar] [CrossRef]
- Li, D.; Wu, Z.; Sun, X.; Shuai, S.; Fu, Y.; Li, D.; Chen, H.; Lu, Y.; Hong, L. Emplacement Ages of Diamondiferous Kimberlites in the Wafangdian District, North China Craton: New Evidence from LA-ICP-MS U-Pb Geochronology of Andradite-Rich Garnet. Gondwana Res. 2022, 109, 493–517. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y. Emplacement Age and Sr-Nd-Hf Isotopic Characteristics of the Diamondiferous Kimberlites from the North China Craton. Acta Petrol. Sin. 2007, 23, 285–294. [Google Scholar]
- Wang, S.; Zheng, J.; Han, S.; Wang, J. Petrography and Petrogenesis of Porphyritic Kimberlite from South Liaoning. Acta Geol. Sin. 2020, 94, 2676–2686. [Google Scholar] [CrossRef]
- Thompson, J.M.; Meffre, S.; Danyushevsky, L. Impact of Air, Laser Pulse Width and Fluence on U-Pb Dating of Zircons by LA-ICPMS. J. Anal. Spectrom. 2018, 33, 221–230. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the Visualisation and Processing of Mass Spectrometric Data. J. Anal. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Paton, C.; Woodhead, J.D.; Hellstrom, J.C.; Hergt, J.M.; Greig, A.; Maas, R. Improved Laser Ablation U-Pb Zircon Geochronology through Robust Downhole Fractionation Correction. Geochem. Geophys. Geosystems 2010, 11, 1–36. [Google Scholar] [CrossRef]
- McDowell, F.W.; McIntosh, W.C.; Farley, K.A. A Precise 40Ar-39Ar Reference Age for the Durango Apatite (U-Th)/He and Fission-Track Dating Standard. Chem. Geol. 2005, 214, 249–263. [Google Scholar] [CrossRef]
- Thomson, S.N.; Gehrels, G.E.; Ruiz, J.; Buchwaldt, R. Routine Low-Damage Apatite U-Pb Dating Using Laser Ablation-Multicollector- ICPMS. Geochem. Geophys. Geosystems 2012, 13, 1–23. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L.; Zhao, Z.; Zhang, Z.; Zhao, H.; Li, X.; Qu, W. In-Situ Sr Isotopic Measurement of Scheelite Using Fs-LA-MC-ICPMS. J. Asian Earth Sci. 2018, 160, 38–47. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wu, F.Y.; Yang, J.H.; Chew, D.M.; Xie, L.W.; Chu, Z.Y.; Zhang, Y.B.; Huang, C. Sr and Nd Isotopic Compositions of Apatite Reference Materials Used in U-Th-Pb Geochronology. Chem. Geol. 2014, 385, 35–55. [Google Scholar] [CrossRef]
- Xing, K.; Shu, Q. Applications of Apatite in Study of Ore Deposits: A Review. Miner. Depos. 2021, 40, 189–205. [Google Scholar] [CrossRef]
- Kaur, G.; Mitchell, R.H. Mineralogy of the P2-West ‘Kimberlite’, Wajrakarur Kimberlite Field, Andhra Pradesh, India: Kimberlite or Lamproite? Miner. Mag. 2013, 77, 3175–3196. [Google Scholar] [CrossRef]
- Kaur, G.; Mitchell, R.H. Mineralogy of the P-12 K-Ti-Richterite Diopside Olivine Lamproite from Wajrakarur, Andhra Pradesh, India: Implications for Subduction-Related Magmatism in Eastern India. Miner. Pet. 2016, 110, 223–245. [Google Scholar] [CrossRef]
- Lei, X.; Yang, Z.; Xiang, Z. Mineralogical Characteristics of Apatites in the Mengyin Kimberlite in Shandong Province and Their Genetic Significances. Bull. Mineral. Petrol. Geochem. 2019, 38, 410–417. [Google Scholar] [CrossRef]
- Giuliani, A.; Soltys, A.; Phillips, D.; Kamenetsky, V.S.; Maas, R.; Goemann, K.; Woodhead, J.D.; Drysdale, R.N.; Griffin, W.L. The Final Stages of Kimberlite Petrogenesis: Petrography, Mineral Chemistry, Melt Inclusions and Sr-C-O Isotope Geochemistry of the Bultfontein Kimberlite (Kimberley, South Africa). Chem. Geol. 2017, 455, 342–356. [Google Scholar] [CrossRef]
- Konzett, J.; Krenn, K.; Rubatto, D.; Hauzenberger, C.; Stalder, R. The Formation of Saline Mantle Fluids by Open-System Crystallization of Hydrous Silicate-Rich Vein Assemblages—Evidence from Fluid Inclusions and Their Host Phases in MARID Xenoliths from the Central Kaapvaal Craton, South Africa. Geochim. Cosmochim. Acta 2014, 147, 1–25. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Litasov, K.D.; Shatskiy, A.; Golovin, A.V.; Ohtani, E.; Pokhilenko, N.P. Melting Phase Relations of the Udachnaya-East Group-I Kimberlite at 3.0–6.5 GPa: Experimental Evidence for Alkali-Carbonatite Composition of Primary Kimberlite Melts and Implications for Mantle Plumes. Gondwana Res. 2015, 28, 1391–1414. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.Z.; Tappe, S.; Kostrovitsky, S.I.; Wu, F.Y.; Yakovlev, D.; Yang, Y.H.; Yang, J.H. Repeated Kimberlite Magmatism beneath Yakutia and Its Relationship to Siberian Flood Volcanism: Insights from in Situ U-Pb and Sr-Nd Perovskite Isotope Analysis. Earth Planet. Sci. Lett. 2014, 404, 283–295. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlites: Mineralogy, Geochemistry, and Petrology; Springer Science and Business Media: New York, NY, USA, 1986. [Google Scholar]
- Wang, B. Characteristics and Genesis of Kimberlite Pipe in the Area of Dalian in Wafangdian Liaoning; Liaoning Technical University: Fuxin, China, 2015. [Google Scholar]
- Dong, Z. The Characteristics of Rare Earth Elements from Kimberlite in China. ACTA Petrol. Et Nineralogica 1992, 11, 126–134. [Google Scholar]
- Zhou, X.; Yang, J.; Huang, Y.; Qin, S. REE Geochemistry Characteristics of Kimberlite in Shandong and Liaoning. ACTA Petrol. Nineralogica 1990, 9, 302–308. [Google Scholar]
- Jones, A.P.; Wyllie, P.J. Minor Elements in Perovskite from Kimberlites and Distribution of the Rare Earth Elements” an Electron Probe Study. Earth Planet. Sci. Lett. 1984, 69, 128–140. [Google Scholar] [CrossRef]
- Batumike, J.M.; Griffin, W.L.; Belousova, E.A.; Pearson, N.J.; O’Reilly, S.Y.; Shee, S.R. LAM-ICPMS U-Pb Dating of Kimberlitic Perovskite: Eocene-Oligocene Kimberlites from the Kundelungu Plateau, D.R. Congo. Earth Planet. Sci. Lett. 2008, 267, 609–619. [Google Scholar] [CrossRef]
- Cox, R.A.; Wilton, D.H.C. U-Pb Dating of Perovskite by LA-ICP-MS: An Example from the Oka Carbonatite, Quebec, Canada. Chem. Geol. 2006, 235, 21–32. [Google Scholar] [CrossRef]
- Donnelly, C.L.; Griffin, W.L.; O’Reilly, S.Y.; Pearson, N.J.; Shee, S.R. The Kimberlites and Related Rocks of the Kuruman Kimberlite Province, Kaapvaal Craton, South Africa. Contrib. Mineral. Petrol. 2011, 161, 351–371. [Google Scholar] [CrossRef]
- Zindler, A.; Hart, S. CHEMICAL GEODYNAMICS. Annu. Rev. Earth Planet Sci. 1986, 14, 493–571. [Google Scholar] [CrossRef]
- Mitchell, R.H. Petrology of Hypabyssal Kimberlites: Relevance to Primary Magma Compositions. J. Volcanol. Geotherm. Res. 2008, 174, 1–8. [Google Scholar] [CrossRef]
- Sarkar, C.; Storey, C.D.; Hawkesworth, C.J. Using Perovskite to Determine the Pre-Shallow Level Contamination Magma Characteristics of Kimberlite. Chem. Geol. 2014, 363, 76–90. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Mitchell, R.H. Strontium-Apaiite: New Occurrences, and the Extent of Sr-for-Ca Substitution in Apatite-Group Minerals. Can. Mineral. 2002, 40, 121–136. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Huang, S.; Lei, X.; Li, X.; Zeng, X. Microfabrics of Perovskites in the Menyin Kimberlites and Their Geological Implications. Bull. Mineral. Petrol. Geochem. 2018, 37, 741–749. [Google Scholar]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Apatite as an Indicator Mineral for Mineral Exploration: Trace-Element Compositions and Their Relationship to Host Rock Type. J. Geochem. Explor. 2002, 76, 45–69. [Google Scholar] [CrossRef]
- Liu, L.; Wu, D. Geological Characteristics and Prospecting Predictions of No.50 Kimberlite Pipe in Wafangdian Diamond Deposit of Liaoning Province. Geol. Surv. China 2020, 7, 34–41. [Google Scholar] [CrossRef]
- Klemme, S. Dalpe Claude Trace-Element Partitioning between Apatite and Carbonatite Melt. Am. Mineral. 2003, 88, 639–646. [Google Scholar] [CrossRef]
- Watson, E.B.; Green, T.H. Apatite/Liquid Partition Coefficients for the Rare Earth Elements and Strontium. Earth Planet. Sci. Lett. 1981, 56, 405–421. [Google Scholar] [CrossRef]
- Prowatke, S.; Klemme, S. Trace Element Partitioning between Apatite and Silicate Melts. Geochim. Cosmochim. Acta 2006, 70, 4513–4527. [Google Scholar] [CrossRef]
- Ayers, J.C.; Watson, E.B. Apatite/Fluid Partitioning of Rare-Earth Elements and Strontium: Experimental Results at 1.0 GPa and 1000 °C and Application to Models of Fluid-Rock Interaction. Chem. Geol. 1993, 110, 299–314. [Google Scholar] [CrossRef]
- Howarth, G.H.; Giuliani, A. Contrasting Types of Micaceous Kimberlite-Lamproite Magmatism from the Man Craton (West Africa): New Insights from Petrography and Mineral Chemistry. Lithos 2020, 362–363, 105483. [Google Scholar] [CrossRef]
- Webster, J.D.; Kinzler, R.J.; Mathez, E.A. Chloride and Water Solubility in Basalt and Andesite Melts and Implications for Magmatic Degassing. Geochim. Cosmochim. Acta 1999, 63, 729–738. [Google Scholar] [CrossRef]
- Webster, J.D.; De Vivo, B. Experimental and Modeled Solubilities of Chlorine in Aluminosilicate Melts, consequences of Magma Evolution, and Implications for Exsolution of Hydrous Chloride Melt at Mt. Somma-Vesuvius. Am. Mineral. 2002, 87, 1046–1061. [Google Scholar] [CrossRef]
- Soltys, A.; Giuliani, A.; Phillips, D. Apatite Compositions and Groundmass Mineralogy Record Divergent Melt/Fluid Evolution Trajectories in Coherent Kimberlites Caused by Differing Emplacement Mechanisms. Contrib. Mineral. Petrol. 2020, 175, 49. [Google Scholar] [CrossRef]
- Sommerauer, J.; Katz-Lehnert, K. A New Partial Substitution Mechanism of CO3 2-/CO3OH3- and SiO4- for the PO3- Group in Hydroxyapatite from the Kaiserstuhl Alkaline Complex (SW-Germany). Contrib. Mineral. Petrol. 1985, 91, 360–368. [Google Scholar] [CrossRef]
- Kjarsgaard, B.A.; Pearson, D.G.; Tappe, S.; Nowell, G.M.; Dowall, D.P. Geochemistry of Hypabyssal Kimberlites from Lac de Gras, Canada: Comparisons to a Global Database and Applications to the Parent Magma Problem. Lithos 2009, 112, 236–248. [Google Scholar] [CrossRef]
- Kopylova, M.G.; Matveev, S.; Raudsepp, M. Searching for Parental Kimberlite Melt. Geochim. Cosmochim. Acta 2007, 71, 3616–3629. [Google Scholar] [CrossRef]
- Price, S.E.; Russell, J.K.; Kopylova, M.G. Primitive Magma From the Jericho Pipe, N.W.T., Canada: Constraints on Primary Kimberlite Melt Chemistry. J. Petrol. 2000, 41, 789–808. [Google Scholar] [CrossRef]
- Tovey, M.; Giuliani, A.; Phillips, D.; Moss, S. Controls on the Explosive Emplacement of Diamondiferous Kimberlites: New Insights from Hypabyssal and Pyroclastic Units in the Diavik Mine, Canada. Lithos 2020, 360–361, 105410. [Google Scholar] [CrossRef]
- Giuliani, A. Insights into Kimberlite Petrogenesis and Mantle Metasomatism from a Review of the Compositional Zoning of Olivine in Kimberlites Worldwide. Lithos 2018, 312–313, 322–342. [Google Scholar] [CrossRef]
- Lim, E.; Giuliani, A.; Phillips, D.; Goemann, K. Origin of Complex Zoning in Olivine from Diverse, Diamondiferous Kimberlites and Tectonic Settings: Ekati (Canada), Alto Paranaiba (Brazil) and Kaalvallei (South Africa). Miner. Pet. 2018, 112, 539–554. [Google Scholar] [CrossRef]
- Clement, C.R. A Comparative Geological Study of Some MajorKimberlite Pipes in the Northern Cape and Orange Free State; University of Cape Town: Cape Town, South Africa, 1982. [Google Scholar]
- Scott Smith, B.H.; Nowicki, J.K.; Russell, K.J.; Webb, R.H.; Mitchell, R.H.; Hetman, C.M.; Harder, M.; Skinner, E.M.W.; Robey, J.A. Kimberlite Terminology and Classification. In Proceedings of the 10th International Kimberlite Conference, Bangalore, India, 5–11 February 2012; Springer: New Delhi, India, 2013; Volume 2, pp. 1–17. [Google Scholar]
- Brown, R.J.; Manya, S.; Buisman, I.; Fontana, G.; Field, M.; Niocaill, C.M.; Sparks, R.S.J.; Stuart, F.M. Eruption of Kimberlite Magmas: Physical Volcanology, Geomorphology and Age of the Youngest Kimberlitic Volcanoes Known on Earth (the Upper Pleistocene/Holocene Igwisi Hills Volcanoes, Tanzania). Bull Volcanol 2012, 74, 1621–1643. [Google Scholar] [CrossRef]
- Brooker, R.A.; Sparks, R.S.J.; Kavanagh, J.L.; Field, M. The Volatile Content of Hypabyssal Kimberlite Magmas: Some Constraints from Experiments on Natural Rock Compositions. Bull Volcanol 2011, 73, 959–981. [Google Scholar] [CrossRef]
- Russell, J.K.; Porritt, L.A.; Lavallé, Y.; Dingwell, D.B. Kimberlite Ascent by Assimilation-Fuelled Buoyancy. Nature 2012, 481, 352–356. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Wang, E.; Fu, H.; Fu, J.; Men, Y.; You, X.; Song, K.; Wan, F.; Liu, L. Geochemistry, Sr-Nd Isotope Compositions, and U-Pb Chronology of Apatite from Kimberlite in Wafangdian, North China Craton: Constraints on the Late Magmatic Processes. Minerals 2024, 14, 284. https://doi.org/10.3390/min14030284
Ma S, Wang E, Fu H, Fu J, Men Y, You X, Song K, Wan F, Liu L. Geochemistry, Sr-Nd Isotope Compositions, and U-Pb Chronology of Apatite from Kimberlite in Wafangdian, North China Craton: Constraints on the Late Magmatic Processes. Minerals. 2024; 14(3):284. https://doi.org/10.3390/min14030284
Chicago/Turabian StyleMa, Sishun, Ende Wang, Haitao Fu, Jianfei Fu, Yekai Men, Xinwei You, Kun Song, Fanglai Wan, and Liguang Liu. 2024. "Geochemistry, Sr-Nd Isotope Compositions, and U-Pb Chronology of Apatite from Kimberlite in Wafangdian, North China Craton: Constraints on the Late Magmatic Processes" Minerals 14, no. 3: 284. https://doi.org/10.3390/min14030284




