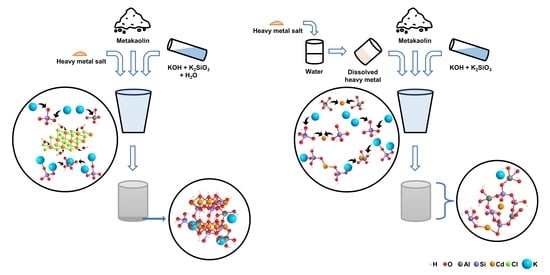
Immobilization Forms of Cadmium and Mercury in a Potassium-Activated Metakaolin-Based Geopolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
- i.
- Salt: The heavy metal was solid mixed with metakaolin in its original salt form. The mixture was then blended with the alkali activator before molding.
- ii.
- Ion: The heavy metal–geopolymer was prepared in a similar way to Salt mixing procedure, but the heavy metal salt was first dissolved in ultra-pure water. The solution was then poured onto metakaolin and later added to a mixture of potassium hydroxide and potassium silicate.
- iii.
- Salt-AA: The heavy metal–geopolymer was produced in reverse order to the Salt mixing procedure, as the salt was first mixed with the alkali activator before being combined with metakaolin.
2.2.2. Leaching Test
2.2.3. Liquid Sample Analysis
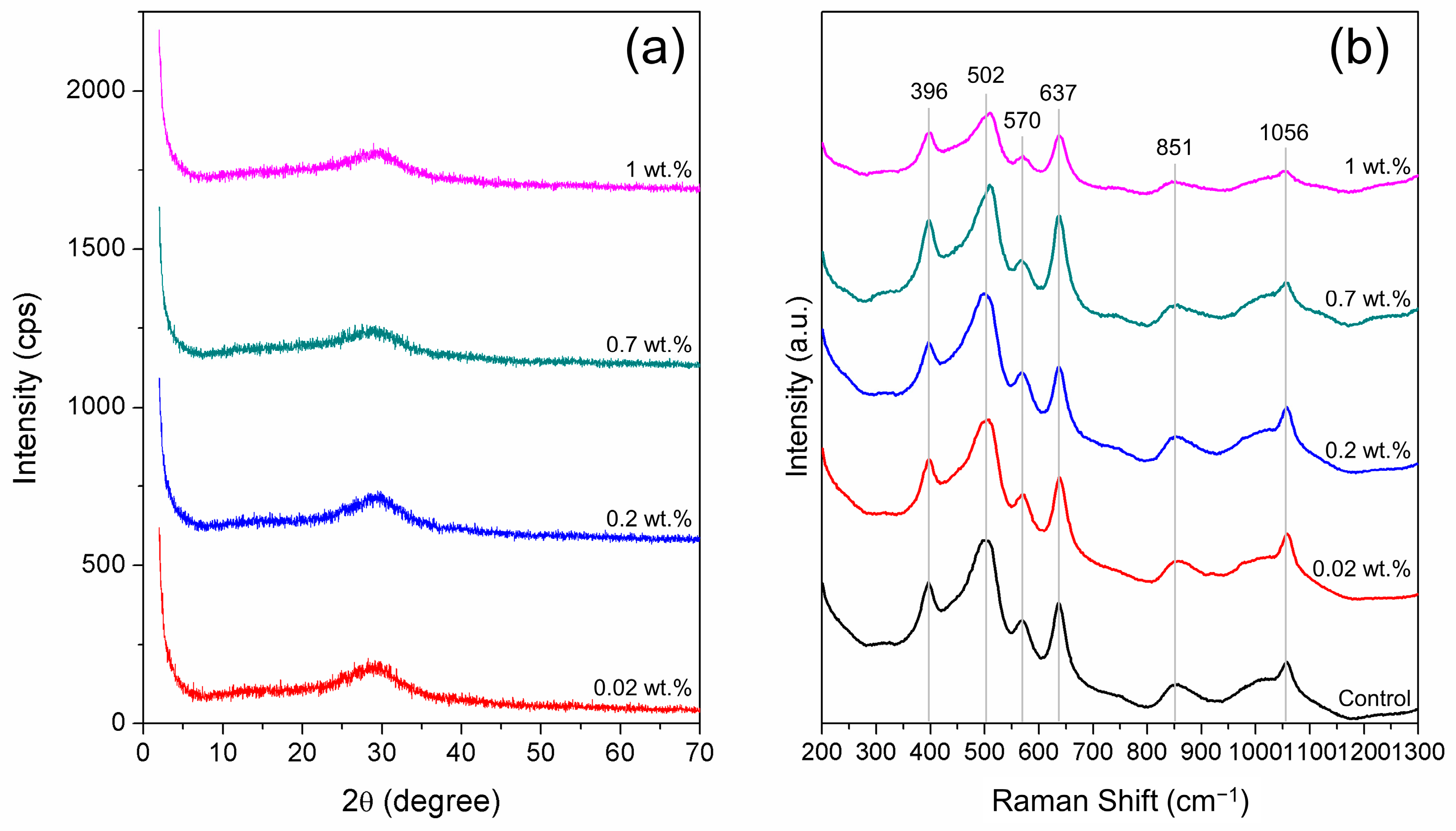
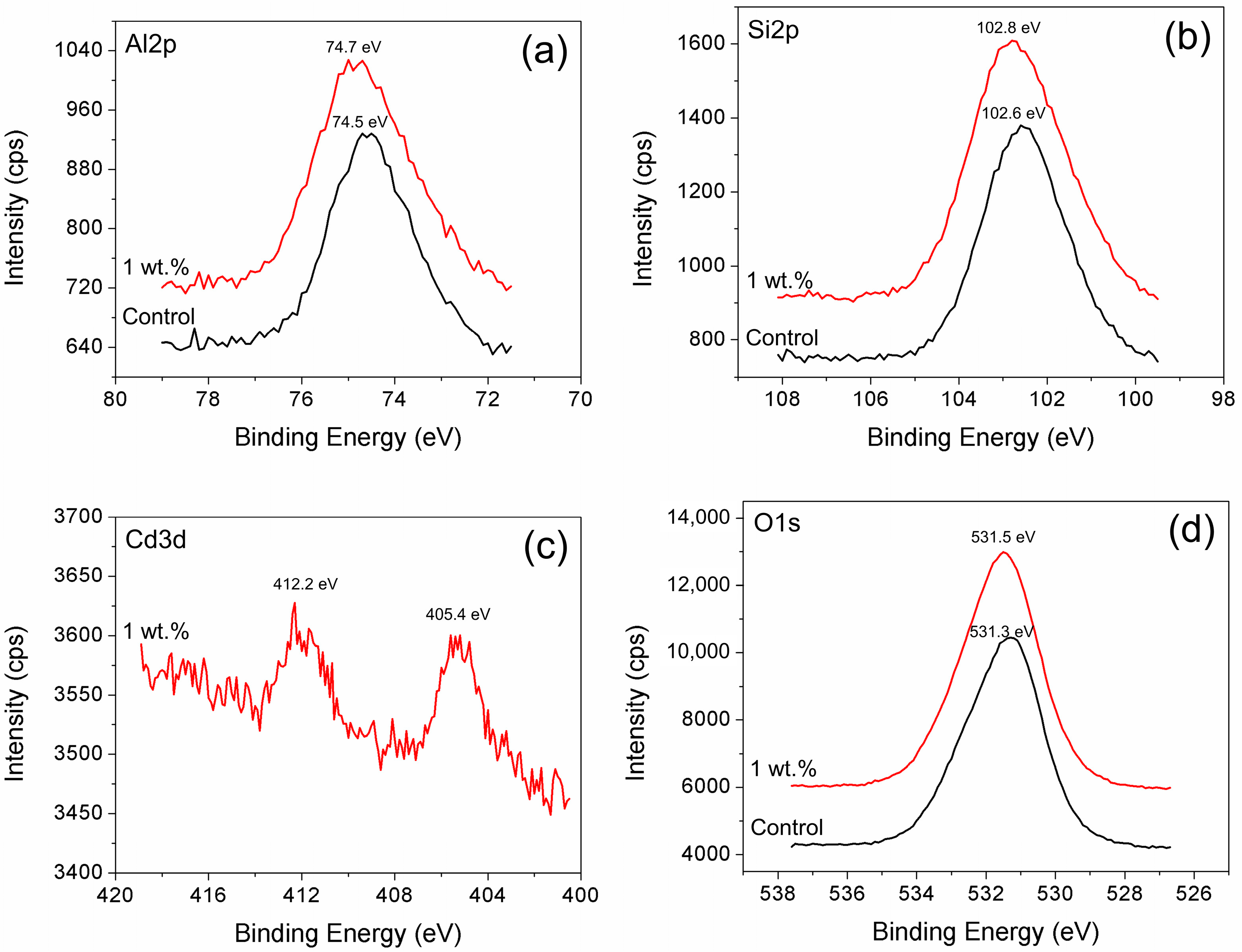
2.2.4. Solid Sample Analysis
3. Results and Discussion
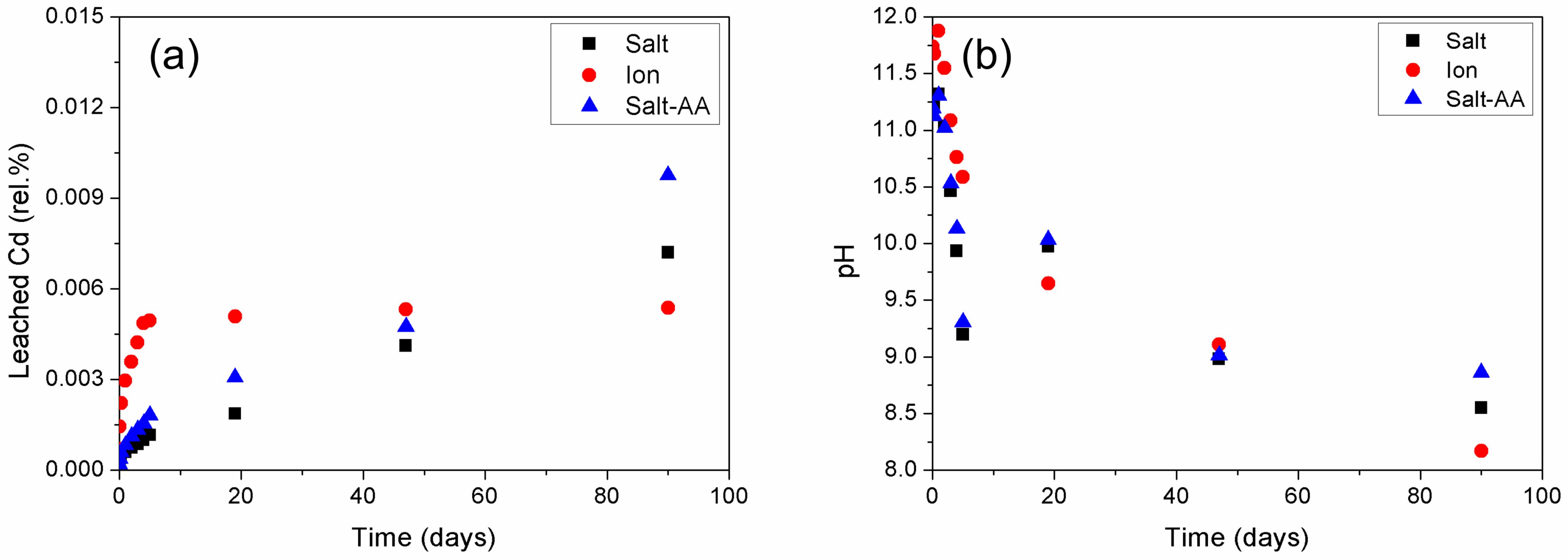
3.1. Effect of Mixing Procedure
3.1.1. Effect of Mixing Procedure on Cadmium Immobilization
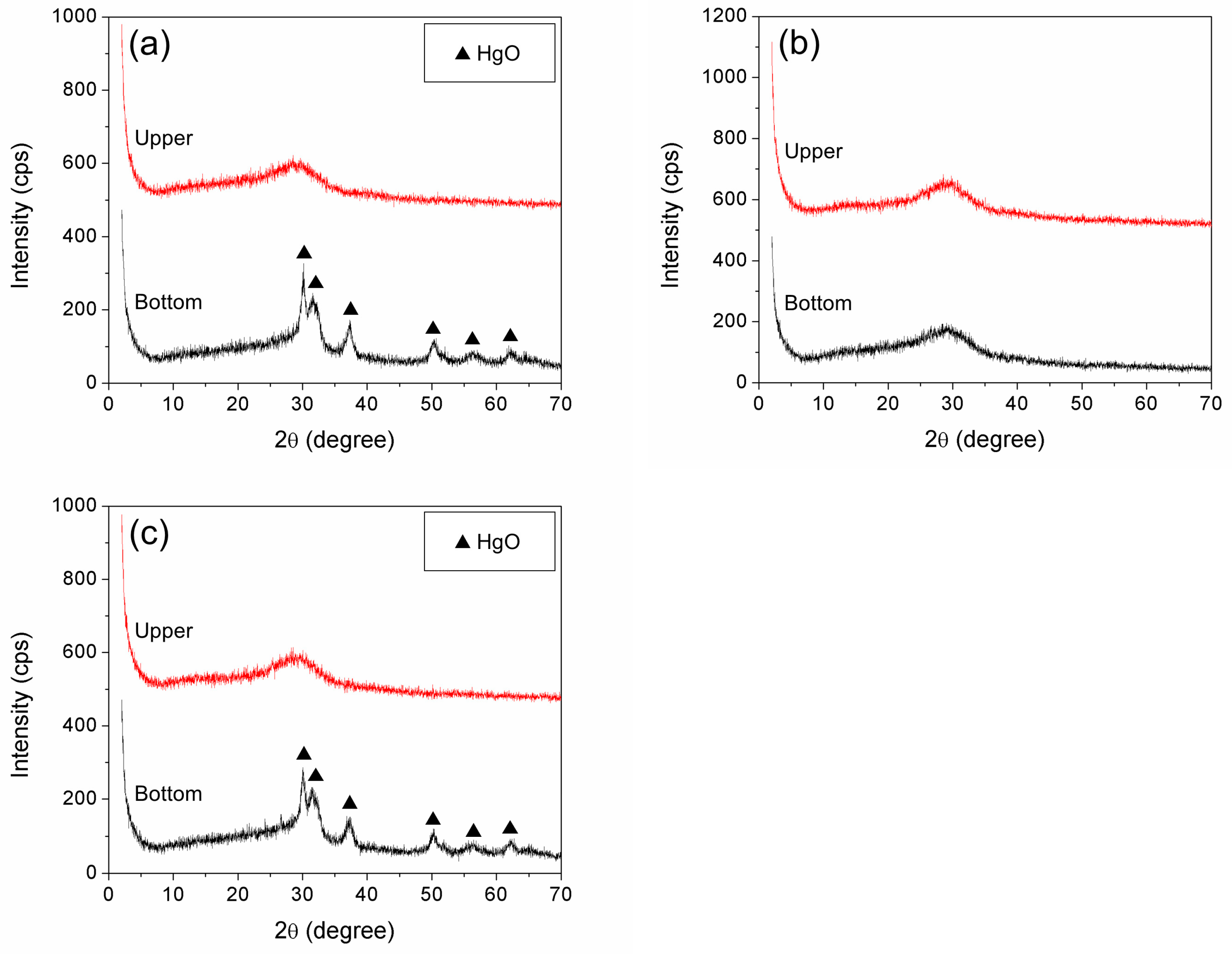
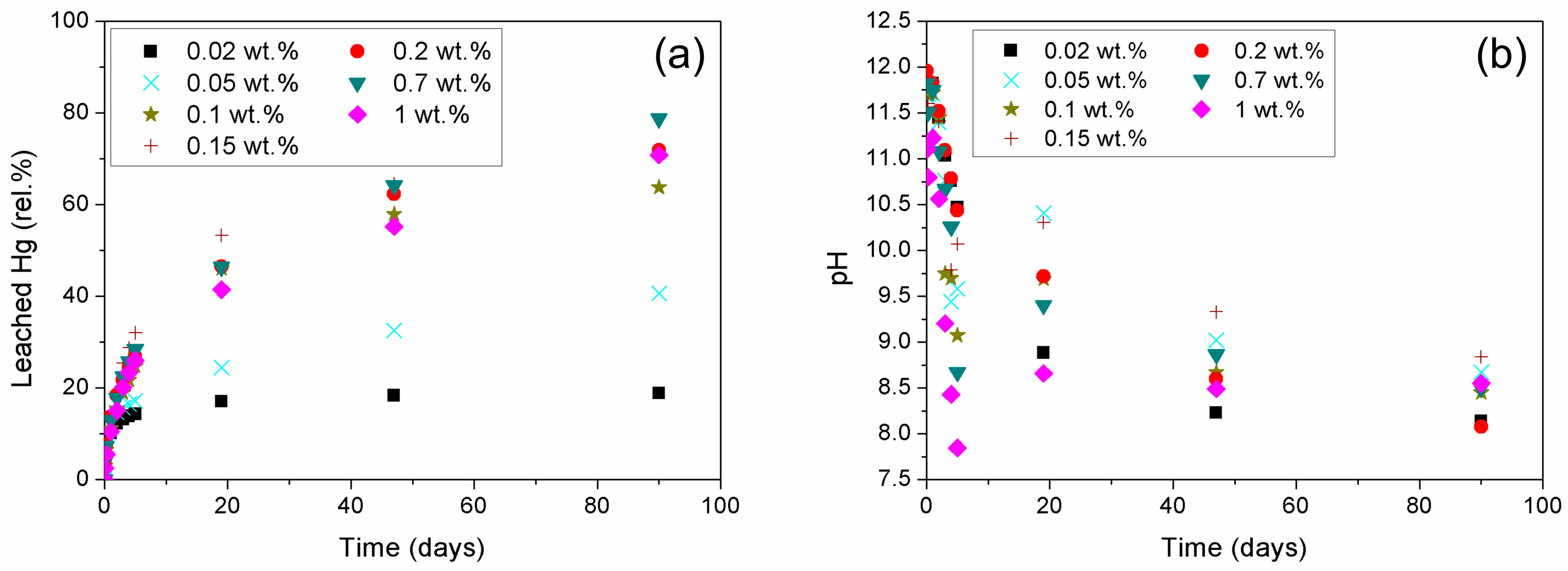
3.1.2. Effect of Mixing Procedure on Mercury Immobilization
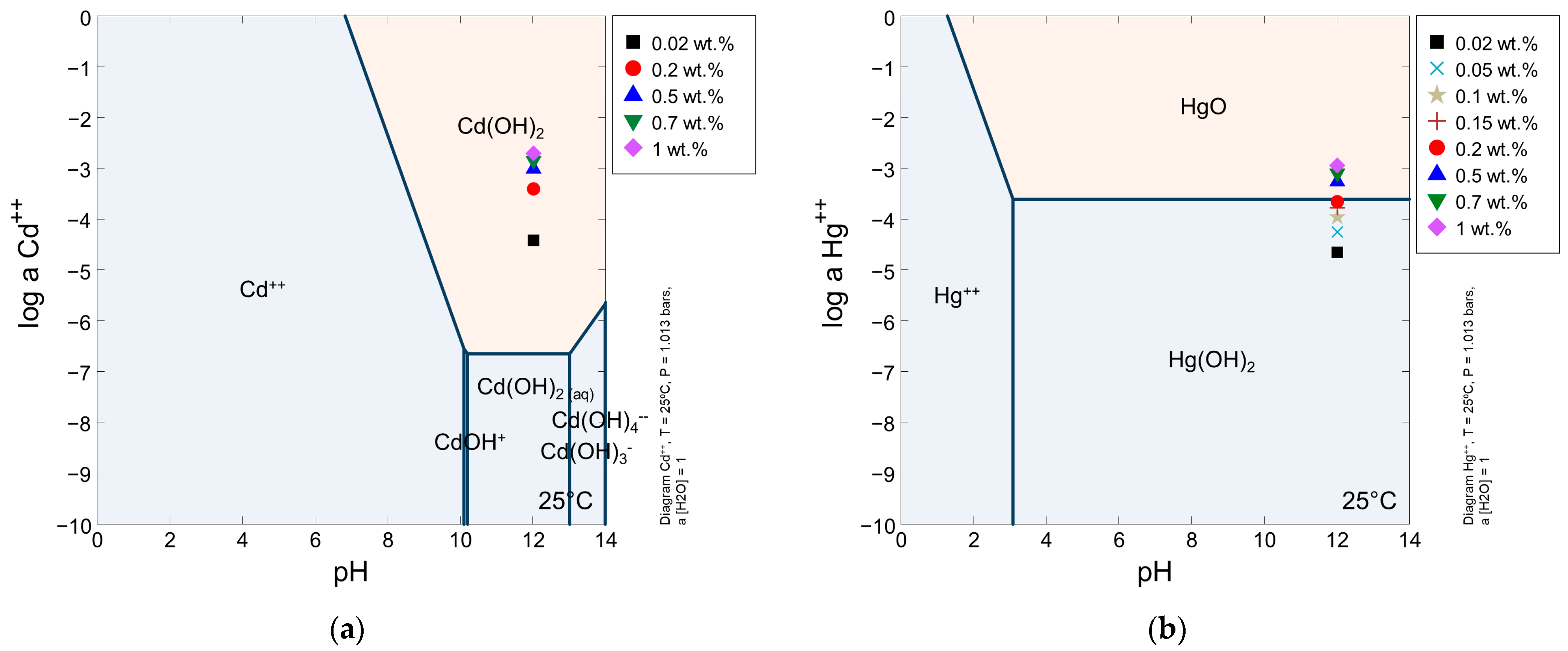
3.2. Effects of the Initial Heavy Metal Concentration
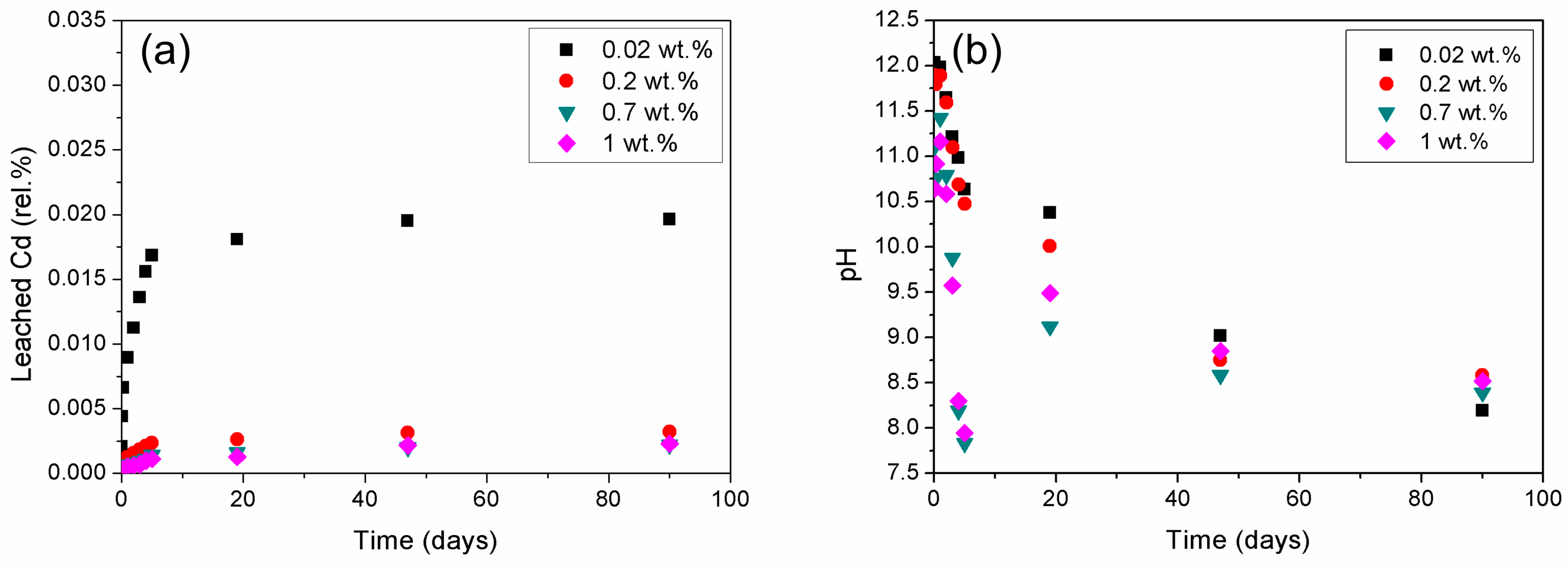
3.2.1. Effects of the Initial Heavy Metal Concentration on Cadmium Immobilization
3.2.2. Effects of Initial Heavy Metal Concentration on Mercury Immobilization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Environmental Protection Agency. Water-Related Environmental Fate of 129 Priority Pollutants Volume I: Introduction and Technical Background, Metals and Inorganics, Pesticides and PCBs; U.S. Environmental Protection Agency: Washington, DC, USA, 1979. [Google Scholar]
- U.S. Environmental Protection Agency. Mercury Study Report to Congress Volume V: Health Effects of Mercury and Mercury Compounds; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Kisku, G.C.; Yadav, S.; Sharma, R.K.; Negi, M.P.S. Potential Environmental Pollution Hazards by Coal Based Power Plant at Jhansi (UP) India. Environ. Earth Sci. 2012, 67, 2109–2120. [Google Scholar] [CrossRef]
- Zucha, W.; Weibel, G.; Wolffers, M.; Eggenberger, U. Inventory of MSWI Fly Ash in Switzerland: Heavy Metal Recovery Potential and Their Properties for Acid Leaching. Processes 2020, 8, 1668. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of Heavy Metals in Wastewater and Soil Samples from Open Drainage Channels in Nairobi, Kenya: Community Health Implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Ning, N.; Yang, Z. Chemical Stabilization of Heavy Metals in Municipal Solid Waste Incineration Fly Ash: A Review. Environ. Sci. Pollut. Res. 2022, 29, 40384–40402. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Sun, Y.; Fang, G.; Li, Y. Release of Soluble Ions and Heavy Metal during Fly Ash Washing by Deionized Water and Sodium Carbonate Solution. Chemosphere 2022, 307, 135860. [Google Scholar] [CrossRef]
- Dahlan, A.V.; Kitamura, H.; Sakanakura, H.; Shimaoka, T.; Yamamoto, T.; Takahashi, F. Possible Metal Speciation in the Fly Ash Produced from a Fluidized Bed of Municipal Solid Waste. In Proceedings of the 31st Annual Conference of JSMCWM, online, 16–18 September 2020; pp. 505–506. [Google Scholar]
- Liang, Y.; Tian, L.; Lu, Y.; Peng, L.; Wang, P.; Lin, J.; Cheng, T.; Dang, Z.; Shi, Z. Kinetics of Cd(II) Adsorption and Desorption on Ferrihydrite: Experiments and Modeling. Environ. Sci. Process. Impacts 2018, 20, 934–942. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Niu, X.; Elakneswaran, Y.; Islam, C.R.; Provis, J.L.; Sato, T. Adsorption Behaviour of Simulant Radionuclide Cations and Anions in Metakaolin-Based Geopolymer. J. Hazard. Mater. 2022, 429, 128373. [Google Scholar] [CrossRef] [PubMed]
- Soonthornwiphat, N.; Kobayashi, Y.; Toda, K.; Kuroda, K.; Islam, C.R.; Otake, T.; Elakneswaran, Y.; Provis, J.L.; Sato, T. Encapsulation of Sr-Loaded Titanate Spent Adsorbents in Potassium Aluminosilicate Geopolymer. J. Nucl. Sci. Technol. 2020, 57, 1181–1188. [Google Scholar] [CrossRef]
- Chaerun, R.I.; Soonthornwiphat, N.; Toda, K.; Kuroda, K.; Niu, X.; Kikuchi, R.; Otake, T.; Elakneswaran, Y.; Provis, J.L.; Sato, T. Retention Mechanism of Cesium in Chabazite Embedded into Metakaolin-Based Alkali Activated Materials. J. Hazard. Mater. 2022, 440, 129732. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-Zeolite Composites: A Review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Schwartzman, A. The Potential Use of Geopolymeric Materials to Immobilise Toxic Metals: Part II. Material and Leaching Characteristics. Min. Eng. 1999, 12, 75–91. [Google Scholar]
- da Silva Rocha, T.; Dias, D.P.; França, F.C.C.; de Salles Guerra, R.R.; da Costa de Oliveira Marques, L.R. Metakaolin-Based Geopolymer Mortars with Different Alkaline Activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Immobilization Efficiency and Mechanism of Metal Cations (Cd2+, Pb2+ and Zn2+) and Anions (AsO43− and Cr2O72−) in Wastes-Based Geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef] [PubMed]
- El-Eswed, B.I. Chemical Evaluation of Immobilization of Wastes Containing Pb, Cd, Cu and Zn in Alkali-Activated Materials: A Critical Review. J. Environ. Chem. Eng. 2020, 8, 104194. [Google Scholar] [CrossRef]
- Gomaa, E.; Sargon, S.; Kashosi, C.; Gheni, A.; ElGawady, M.A. Mechanical Properties of High Early Strength Class C Fly Ash-Based Alkali Activated Concrete. Transp. Res. Rec. 2020, 2674, 430–443. [Google Scholar] [CrossRef]
- Donatello, S.; Fernández-Jiménez, A.; Palomo, A. An Assessment of Mercury Immobilisation in Alkali Activated Fly Ash (AAFA) Cements. J. Hazard. Mater. 2012, 213–214, 207–215. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S.J. Geopolymers for Immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- El-Eswed, B.I.; Yousef, R.I.; Alshaaer, M.; Hamadneh, I.; Al-Gharabli, S.I.; Khalili, F. Stabilization/Solidification of Heavy Metals in Kaolin/Zeolite Based Geopolymers. Int. J. Min. Process. 2015, 137, 34–42. [Google Scholar] [CrossRef]
- Qian, G.; Sun, D.D.; Tay, J.H. Immobilization of Mercury and Zinc in an Alkali-Activated Slag Matrix. J. Hazard. Mater. 2003, 101, 65–77. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Geopolymers Produced from Drinking Water Treatment Residue and Bottom Ash for the Immobilization of Heavy Metals. Chemosphere 2019, 225, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, N.; Kljajević, L.; Nenadović, S.; Ivanović, M.; Čalija, B.; Gulicovski, J.; Trivunac, K. The Applications of New Inorganic Polymer for Adsorption Cadmium from Waste Water. J. Inorg. Organomet. Polym. Mater. 2020, 30, 554–563. [Google Scholar] [CrossRef]
- Ivanović, M.; Nenadović, S.; Pavlović, V.P.; Radović, I.; Kijevčanin, M.; Pavlović, V.B.; Kljajević, L. The Influence of Thermodynamic Parameters on Alkaline Activators of Geopolymers and the Structure of Geopolymers. Maced. J. Chem. Chem. Eng. 2021, 40, 99–109. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Liu, M.; Hu, X. Novel Sustainable Geopolymer Based Syntactic Foams: An Eco-Friendly Alternative to Polymer Based Syntactic Foams. Chem. Eng. J. 2017, 313, 74–82. [Google Scholar] [CrossRef]
- Vidal, L.; Gharzouni, A.; Joussein, E.; Colas, M.; Cornette, J.; Absi, J.; Rossignol, S. Determination of the Polymerization Degree of Various Alkaline Solutions: Raman Investigation. J. Sol-Gel Sci. Technol. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Depla, A.; Verheyen, E.; Veyfeyken, A.; Van Houteghem, M.; Houthoofd, K.; Van Speybroeck, V.; Waroquier, M.; Kirschhock, C.E.A.; Martens, J.A. UV-Raman and 29Si NMR Spectroscopy Investigation of the Nature of Silicate Oligomers Formed by Acid Catalyzed Hydrolysis and Polycondensation of Tetramethylorthosilicate. J. Phys. Chem. C 2011, 115, 11077–11088. [Google Scholar] [CrossRef]
- Lutz, H.D.; Mdller, H.; Schmidt, M. Lattice Vibration Spectra. Part LXXXII. Brucite-Type Hydroxides M(OH)2 (M = Ca, Mn, Co, Fe, Cd)-IR and Raman Spectra, Neutron Diffraction of Fe(OH)2. J. Mol. Struct. 1994, 328, 121–132. [Google Scholar] [CrossRef]
- Cooney, R.P.J.; Hall, J.R. Vibrational Spectra of Mercury(L) And Mercury(II) Acetate Compounds. J. Inorg. Nucl. Chem. 1972, 34, 1519–1527. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Wei, X.; Sun, Y.; Su, Y.; Shen, X.; Tang, Y.; Yan, F.; Zhang, Z. Structural Evolution of Geopolymers Incorporated with Heavy Metals: Solidification Mechanism of Pb2+ and Cd2+. J. Phys. Chem. C 2023, 127, 19563–19573. [Google Scholar] [CrossRef]
- Ji, Z.; Su, L.; Pei, Y. Synthesis and Toxic Metals (Cd, Pb, and Zn) Immobilization Properties of Drinking Water Treatment Residuals and Metakaolin-Based Geopolymers. Mater. Chem. Phys. 2020, 242, 122535. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of Geopolymerization and Factors Influencing Its Development: A Review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Kuenzel, C.; Cisneros, J.F.; Neville, T.P.; Vandeperre, L.J.; Simons, S.J.R.; Bensted, J.; Cheeseman, C.R. Encapsulation of Cs/Sr Contaminated Clinoptilolite in Geopolymers Produced from Metakaolin. J. Nucl. Mater. 2015, 466, 94–99. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Qiu, X.; Meng, Y.; Wang, H.; Zhou, S.; Qiao, Q.; Yan, C. Clinoptilolite Based Zeolite-Geopolymer Hybrid Foams: Potential Application as Low-Cost Sorbents for Heavy Metals. J. Environ. Manag. 2023, 330, 117167. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, U.; Das, A.K. Relativistic Effects on the Chemical Bonding Properties of the Heavier Elements and Their Compounds. Coord. Chem. Rev. 2023, 479, 215000. [Google Scholar] [CrossRef]
- Barysz, M.; Leszczyński, J.; Zielińska, B.; Bilewicz, A. Influence of Relativistic Effects on Hydrolysis of the Heavy Metal Cations. In Annual Report 2003; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2004; pp. 57–59. [Google Scholar]
- Hocsman, A.; Di Nezio, S.; Charlet, L.; Avena, M. On the Mechanisms of Dissolution of Montroydite [HgO(s)]: Dependence of the Dissolution Rate on PH, Temperature, and Stirring Rate. J. Colloid Interface Sci. 2006, 297, 696–704. [Google Scholar] [CrossRef]
- Cartledge, F.K.; Butler, L.G.; Chalasani, D.; Eaton, H.C.; Frey, F.P.; Herrera, E.; Tittlebaum, M.E.; Yang, S.-L. Immobilization Mechanisms in Solidification/Stabilization of Cd and Pb Salts Using Portland Cement Fixing Agents. Environ. Sci. Technol. 1990, 24, 867–873. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Tan, X.; Yu, P.; Xu, L. Review of the Interactions between Conventional Cementitious Materials and Heavy Metal Ions in Stabilization/Solidification Processing. Materials 2023, 16, 3444. [Google Scholar] [CrossRef]







| Researcher | Metal | Pozzolan | Metal Concentration (wt.%) | Mechanisms |
|---|---|---|---|---|
| Zhang et al. [21] | Cd | Fly ash + sand | 0.5 | Cd(OH)2 precipitate |
| El-Eswed et al. [22] | Cd | Zeolite + kaolinite | 0.001 and 0.006 | Cation exchange |
| Ji and Pei [24] | Cd | Bottom ash + drinking water treatment residue | 1, 2, and 4 | Covalent bonding |
| Qian et al. [23] | Hg | Blast furnace slag | 0.5 and 2 | Hg-silicate precipitate; C–S–H lattice incorporation; Physical encapsulation of HgO |
| Donatello et al. [20] | Hg | Fly ash | 0.5 and 5 | HgS; HgO/Hg-silicate; Hg absorbed onto N–A–S–H |
| Component | Percentage (wt.%) |
|---|---|
| SiO2 | 51.25 |
| Al2O3 | 45.82 |
| TiO2 | 1.19 |
| Fe2O3 | 0.58 |
| Na2O | 0.06 |
| K2O | 0.15 |
| CaO | 0.23 |
| MgO | 0.02 |
| P2O5 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prihutami, P.; Chaerun, R.I.; Ohya, Y.; Otake, T.; Kikuchi, R.; Sato, T. Immobilization Forms of Cadmium and Mercury in a Potassium-Activated Metakaolin-Based Geopolymer. Minerals 2024, 14, 311. https://doi.org/10.3390/min14030311
Prihutami P, Chaerun RI, Ohya Y, Otake T, Kikuchi R, Sato T. Immobilization Forms of Cadmium and Mercury in a Potassium-Activated Metakaolin-Based Geopolymer. Minerals. 2024; 14(3):311. https://doi.org/10.3390/min14030311
Chicago/Turabian StylePrihutami, Pramesti, Raudhatul Islam Chaerun, Yusuke Ohya, Tsubasa Otake, Ryosuke Kikuchi, and Tsutomu Sato. 2024. "Immobilization Forms of Cadmium and Mercury in a Potassium-Activated Metakaolin-Based Geopolymer" Minerals 14, no. 3: 311. https://doi.org/10.3390/min14030311
APA StylePrihutami, P., Chaerun, R. I., Ohya, Y., Otake, T., Kikuchi, R., & Sato, T. (2024). Immobilization Forms of Cadmium and Mercury in a Potassium-Activated Metakaolin-Based Geopolymer. Minerals, 14(3), 311. https://doi.org/10.3390/min14030311