Leaching of Nickel and Cobalt from a Mixed Nickel-Cobalt Hydroxide Precipitate Using Organic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Procedure
3. Results and Discussion
3.1. Material Characterization
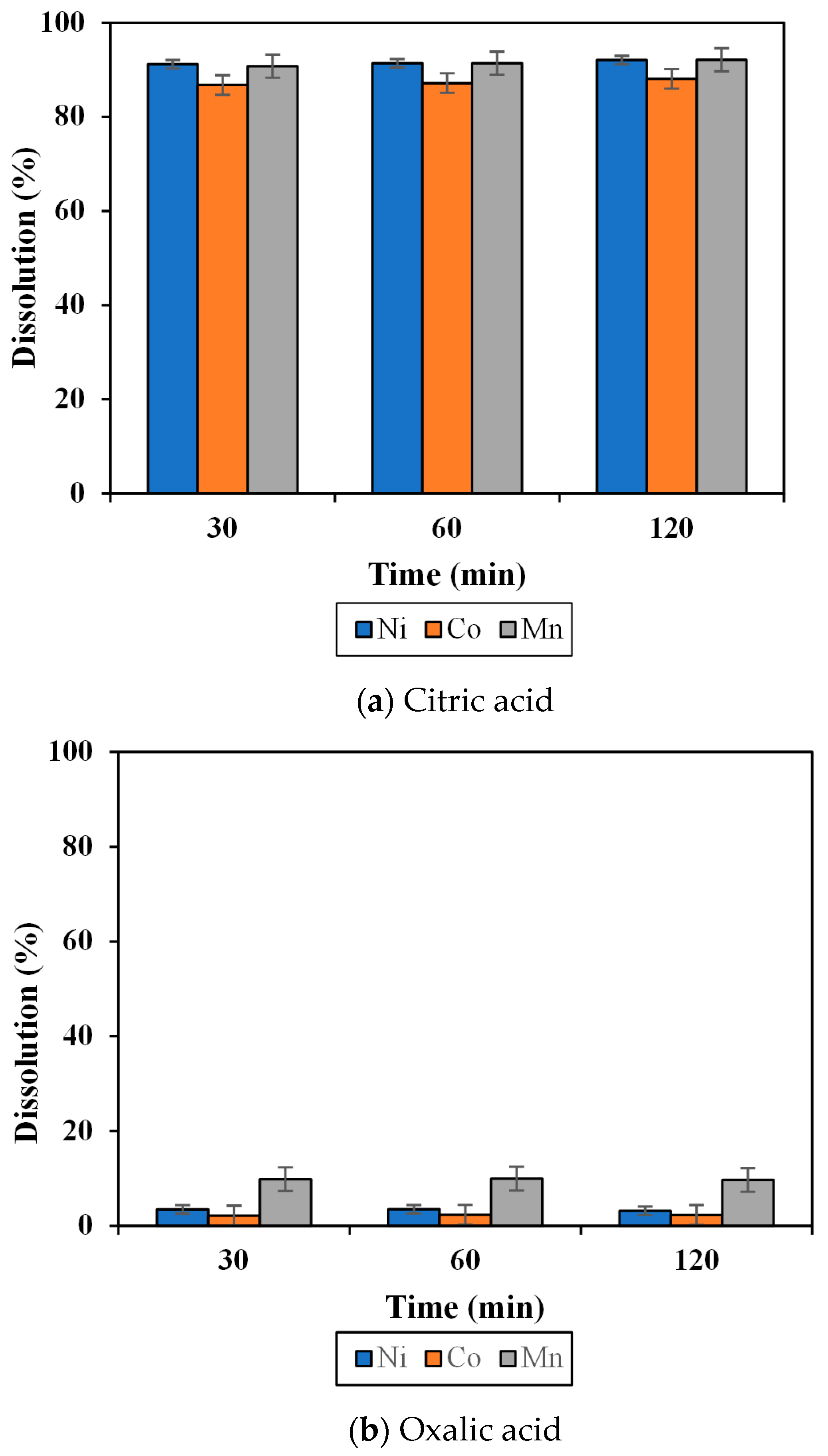
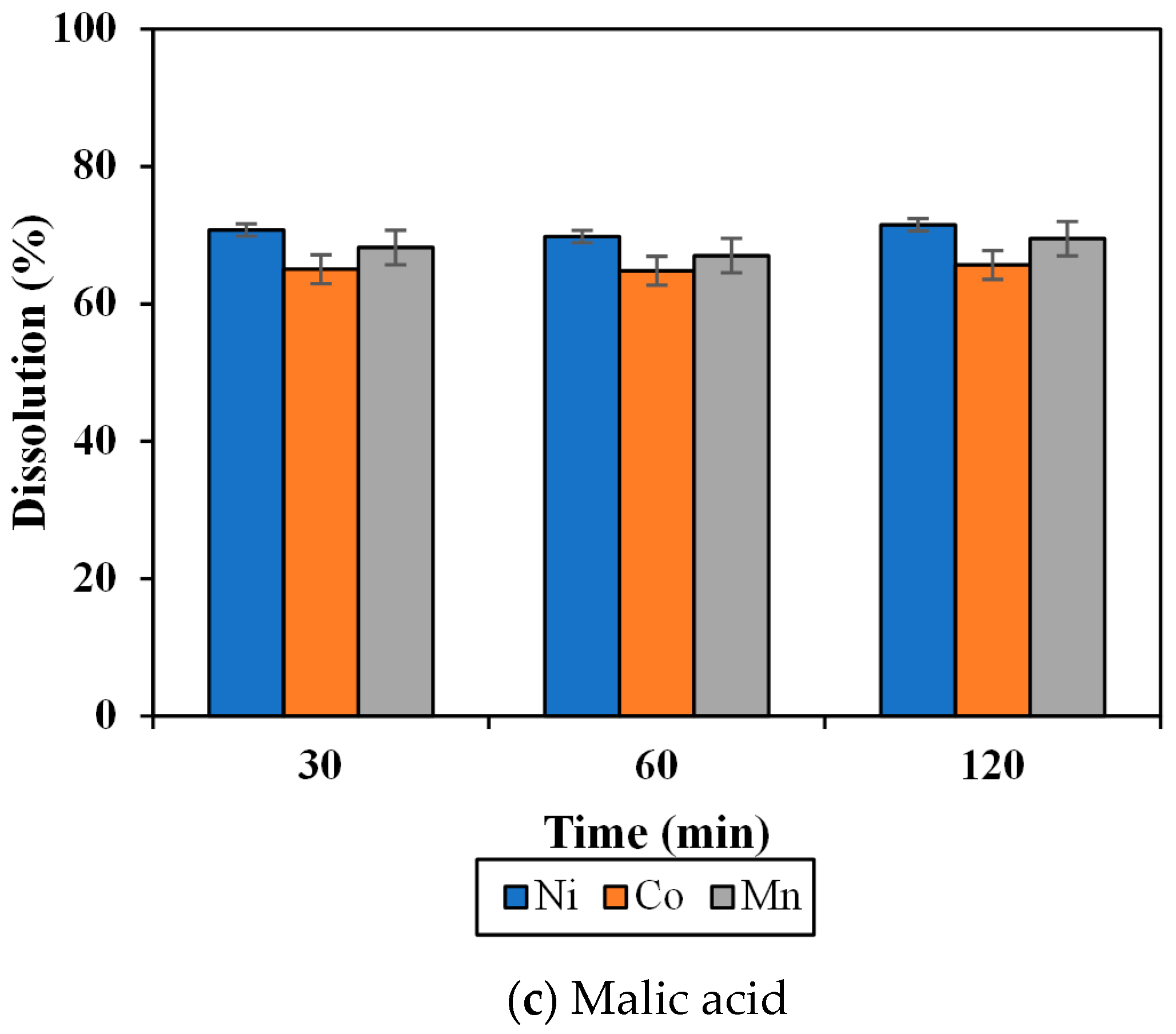
3.2. Effect of Organic Acid on Dissolution of the MHP Sample
3.3. Effect of Temperature on Dissolution of the MHP Sample
3.4. Effect of Citric Acid Concentration on the Dissolution of the MHP Sample
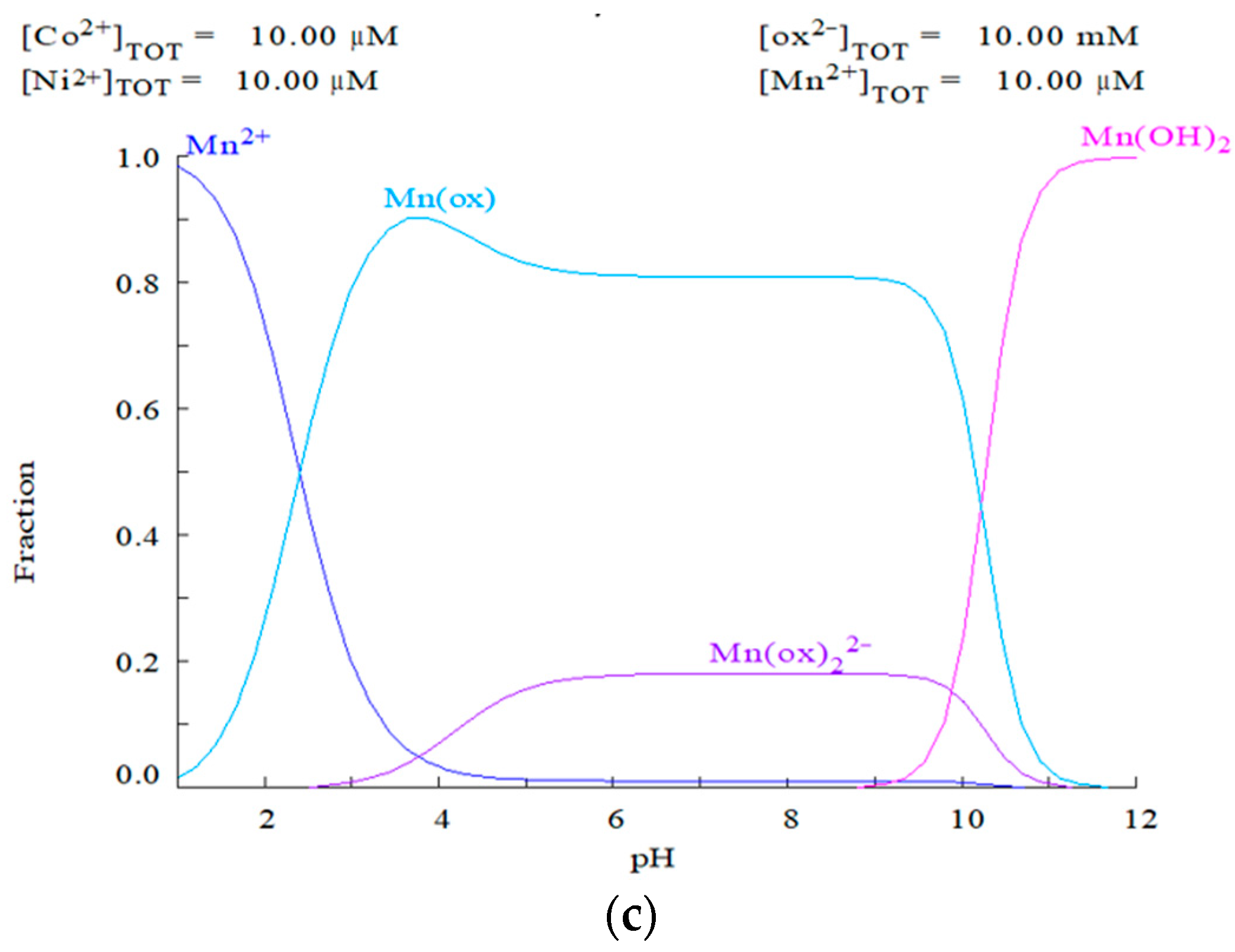
3.5. Synergistic Impact of Citric Acid and Oxalic Acid on the Dissolution of the MHP Sample
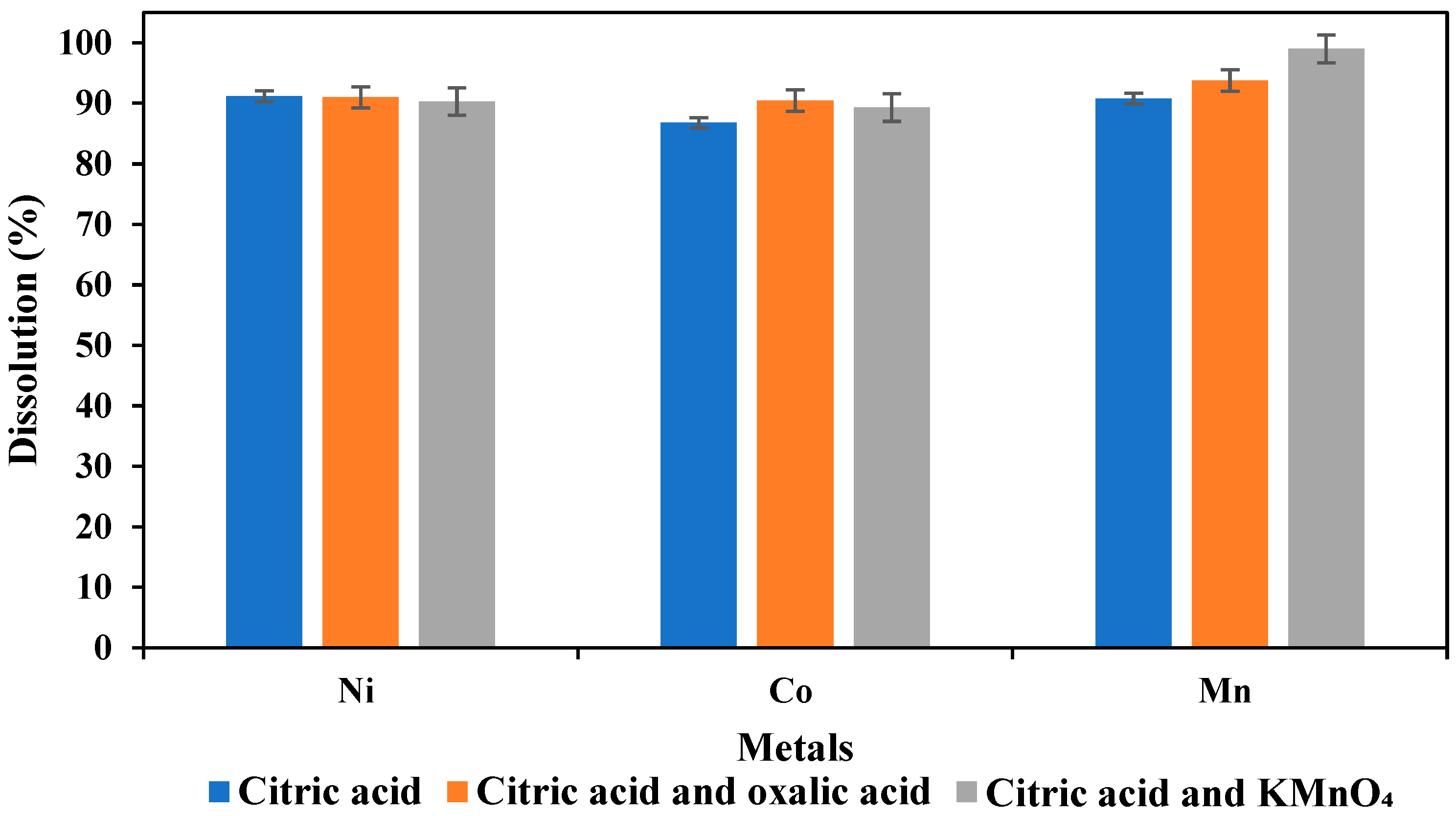
3.6. Effect of Oxidants on Metal Dissolution
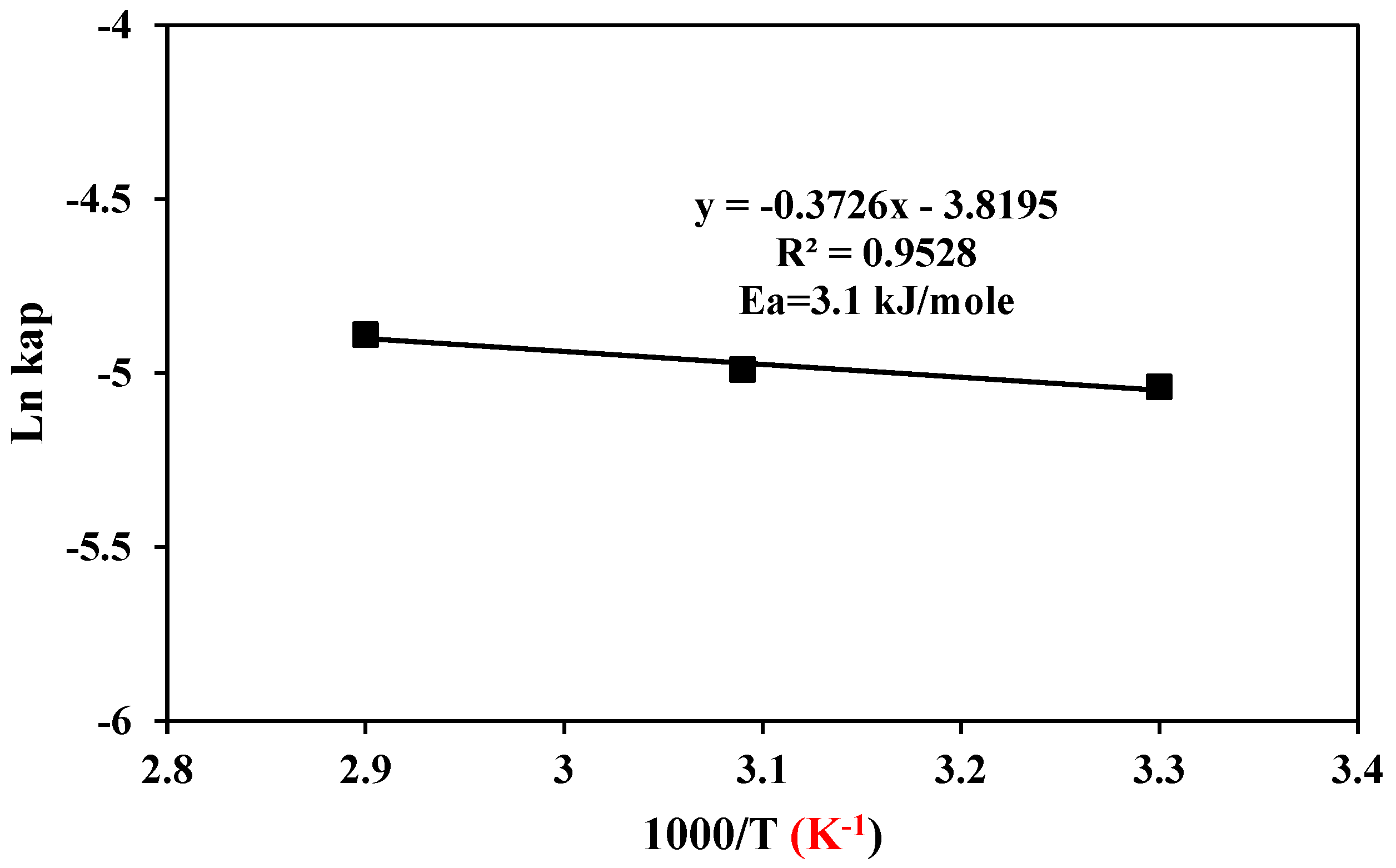
3.7. The Kinetic Studies of Nickel Dissolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Ma, B.; He, F.; Chen, Y.; Wang, C. Ammonia leaching process for selective extraction of nickel and cobalt from polymetallic mixed hydroxide precipitate. J. Environ. Chem. Eng. 2022, 10, 108936. [Google Scholar] [CrossRef]
- Hussaini, S.; Ichlas, Z.T.; Top, S.; Kursunoglu, S.; Kaya, M. Selective leaching of a mixed nickel-cobalt hydroxide precipitate in sulphuric acid solution with potassium permanganate as oxidant. Sep. Sci. Technol. 2020, 56, 2475–2484. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; Mubarok, M.Z.; Magnalita, A.; Vaughan, J.; Sugiarto, A.T. Processing mixed nickel-cobalt hydroxide precipitate by sulfuric acid leaching followed by selective oxidative precipitation of cobalt and manganese. Hydrometallurgy 2020, 191, 105185. [Google Scholar] [CrossRef]
- Astuti, W.; Nurjaman, F.; Rofiek Mufakhir, F.; Sumardi, S.; Avista, D.; Cleary Wanta, K.; Tri Bayu Murti Petrus, H. A novel method: Nickel and cobalt extraction from citric acid leaching solution of nickel laterite ores using oxalate precipitation. Miner. Eng. 2023, 191, 107982. [Google Scholar] [CrossRef]
- Harvey, R.; Hannah, R.; Vaughan, J. Selective precipitation of mixed nickel–cobalt hydroxide. Hydrometallurgy 2011, 105, 222–228. [Google Scholar] [CrossRef]
- Jones, A.N.; Welham, N.J. Properties of aged mixed nickel–cobalt hydroxide intermediates produced from acid leach solutions and subsequent metal recovery. Hydrometallurgy 2010, 103, 173–179. [Google Scholar] [CrossRef]
- Williams, C.; Hawker, W.; Vaughan, J.W. Selective leaching of nickel from mixed nickel cobalt hydroxide precipitate. Hydrometallurgy 2013, 138, 84–92. [Google Scholar] [CrossRef]
- Kaya, M.; Kursunoglu, S.; Hussaini, S.; Gül, E. Leaching of Turkish Oxidized Pb–Zn Flotation Tailings by Inorganic and Organic Acids. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: San Diego, USA, 2020; pp. 447–468. [Google Scholar]
- Mubarok, M.Z.; Astuti, W.; Chaerun, S.K. Leaching Behaviour of Nickel from Indonesian Laterite Ore in Some Organic Acids. In Proceedings of the International Mineral Processing Symposium, Kapadokya, Turkey, 6–8 October 2010. [Google Scholar]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Comparison of atmospheric citric acid leaching kinetics of nickel from different Indonesian saprolitic ores. Hydrometallurgy 2016, 161, 138–151. [Google Scholar] [CrossRef]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Comparison of effectiveness of citric acid and other acids in leaching of low-grade Indonesian saprolitic ores. Miner. Eng. 2016, 85, 1–16. [Google Scholar] [CrossRef]
- Wanta, K.C.; Astuti, W.; Petrus, H.T.B.M.; Perdana, I. Product Diffusion-Controlled Leaching of Nickel Laterite using Low Concentration Citric Acid Leachant at Atmospheric Condition. Int. J. Technol. 2022, 13, 410–421. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Kursunoglu, N.; Hussaini, S.; Kaya, M. Selection of an appropriate acid type for the recovery of zinc from a flotation tailing by the analytic hierarchy process. J. Clean. Prod. 2021, 283, 124659. [Google Scholar] [CrossRef]
- Behera, S.K.; Panda, P.P.; Singh, S.; Pradhan, N.; Sukla, L.B.; Mishra, B.K. Study on reaction mechanism of bioleaching of nickel and cobalt from lateritic chromite overburdens. Int. Biodeterior. Biodegrad. 2011, 65, 1035–1042. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Krishnamurty, K.V.; Harris, G.M. The Chemistry of the Metal Oxalato Complexes. Chem. Rev. 1961, 61, 213–246. [Google Scholar] [CrossRef]
- Li, P.; Zeng, G.M.; Xu, W.H.; Zhang, C.; Jiang, M. Effects of organic acids on zinc and lead leaching from contaminated sediments. Zhongguo Huanjing Kexue/China Environ. Sci. 2010, 30, 1235–1240. [Google Scholar]
- Yan, Y.; Gao, J.; Wu, J.; Li, B. Effects of Inorganic and Organic Acids on Heavy Metals Leaching in Contaminated Sediment. In An Interdisciplinary Response to Mine Water Challenges; Sui, W., Sun, W., Eds.; China University of Mining and Technology Press: Xuzhou, China, 2014. [Google Scholar]
- Kursunoglu, S. Synergistic effect of organic acid on the dissolution of mixed nickel-cobalt hydroxide precipitate in sulphuric acid solution. Metall. Res. Technol. 2019, 116, 319. [Google Scholar] [CrossRef]
- Byrne, K.; Hawker, W.; Vaughan, J. Effect of key parameters on the selective acid leach of nickel from mixed nickel-cobalt hydroxide. AIP Conf. Proc. 2017, 1805, 030001. [Google Scholar]
- Wang, R.-T.; Kong, L.-B.; Lang, J.-W.; Wang, X.-W.; Fan, S.-Q.; Luo, Y.-C.; Kang, L. Mesoporous Co3O4 materials obtained from cobalt–citrate complex and their high capacitance behavior. J. Power Sources 2012, 217, 358–363. [Google Scholar] [CrossRef]
- Ma, L.; Nie, Z.; Xi, X.; Han, X.g. Cobalt recovery from cobalt-bearing waste in sulphuric and citric acid systems. Hydrometallurgy 2013, 136, 1–7. [Google Scholar] [CrossRef]
- Ma, L.W.; Xi, X.L.; Zhang, Z.Z.; Huang, Z.Q.; Chen, J.P. Hydrometallurgical Treatment for Mixed Waste Battery Material. IOP Conf. Ser. Mater. Sci. Eng. 2017, 170, 012024. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Ichlas, Z.T.; Kaya, M. Solvent extraction process for the recovery of nickel and cobalt from Caldag laterite leach solution: The first bench scale study. Hydrometallurgy 2017, 169, 135–141. [Google Scholar] [CrossRef]
- Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Development of Novel Extractants with Amino Acid Structure for Efficient Separation of Nickel and Cobalt from Manganese Ions. Ind. Eng. Chem. Res. 2014, 53, 812–818. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Habashi, F. Principles of Extractive Metallurgy; Gordon&Breach: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Kinetics of nickel extraction from Indonesian saprolitic ore by citric acid leaching under atmospheric pressure. Min. Metall. Explor. 2015, 32, 176–185. [Google Scholar] [CrossRef]
- Vegliò, F.; Trifoni, M.; Pagnanelli, F.; Toro, L. Shrinking core model with variable activation energy: A kinetic model of manganiferous ore leaching with sulphuric acid and lactose. Hydrometallurgy 2001, 60, 167–179. [Google Scholar] [CrossRef]
- Habashi, F. Principles of Extractive Metallurgy, General Principles; Gordon and Breach: New York, NY, USA, 1969; Volume 1. [Google Scholar]
- Sohn, H.Y.; Wadsworth, M.E. Rate Process of Extractive Metallurgy; Plenum Press: New York, NY, USA, 1979. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussaini, S.; Tita, A.M.; Kursunoglu, S.; Kaya, M.; Chu, P. Leaching of Nickel and Cobalt from a Mixed Nickel-Cobalt Hydroxide Precipitate Using Organic Acids. Minerals 2024, 14, 314. https://doi.org/10.3390/min14030314
Hussaini S, Tita AM, Kursunoglu S, Kaya M, Chu P. Leaching of Nickel and Cobalt from a Mixed Nickel-Cobalt Hydroxide Precipitate Using Organic Acids. Minerals. 2024; 14(3):314. https://doi.org/10.3390/min14030314
Chicago/Turabian StyleHussaini, Shokrullah, Angela Manka Tita, Sait Kursunoglu, Muammer Kaya, and Pengbo Chu. 2024. "Leaching of Nickel and Cobalt from a Mixed Nickel-Cobalt Hydroxide Precipitate Using Organic Acids" Minerals 14, no. 3: 314. https://doi.org/10.3390/min14030314
APA StyleHussaini, S., Tita, A. M., Kursunoglu, S., Kaya, M., & Chu, P. (2024). Leaching of Nickel and Cobalt from a Mixed Nickel-Cobalt Hydroxide Precipitate Using Organic Acids. Minerals, 14(3), 314. https://doi.org/10.3390/min14030314






