Formation of Intergrowths of Platinum-Group Minerals and Gold from Magmatogenic Ores in Relation to Phase Changes in Pt-Pd-Fe-Cu-Au System
Abstract
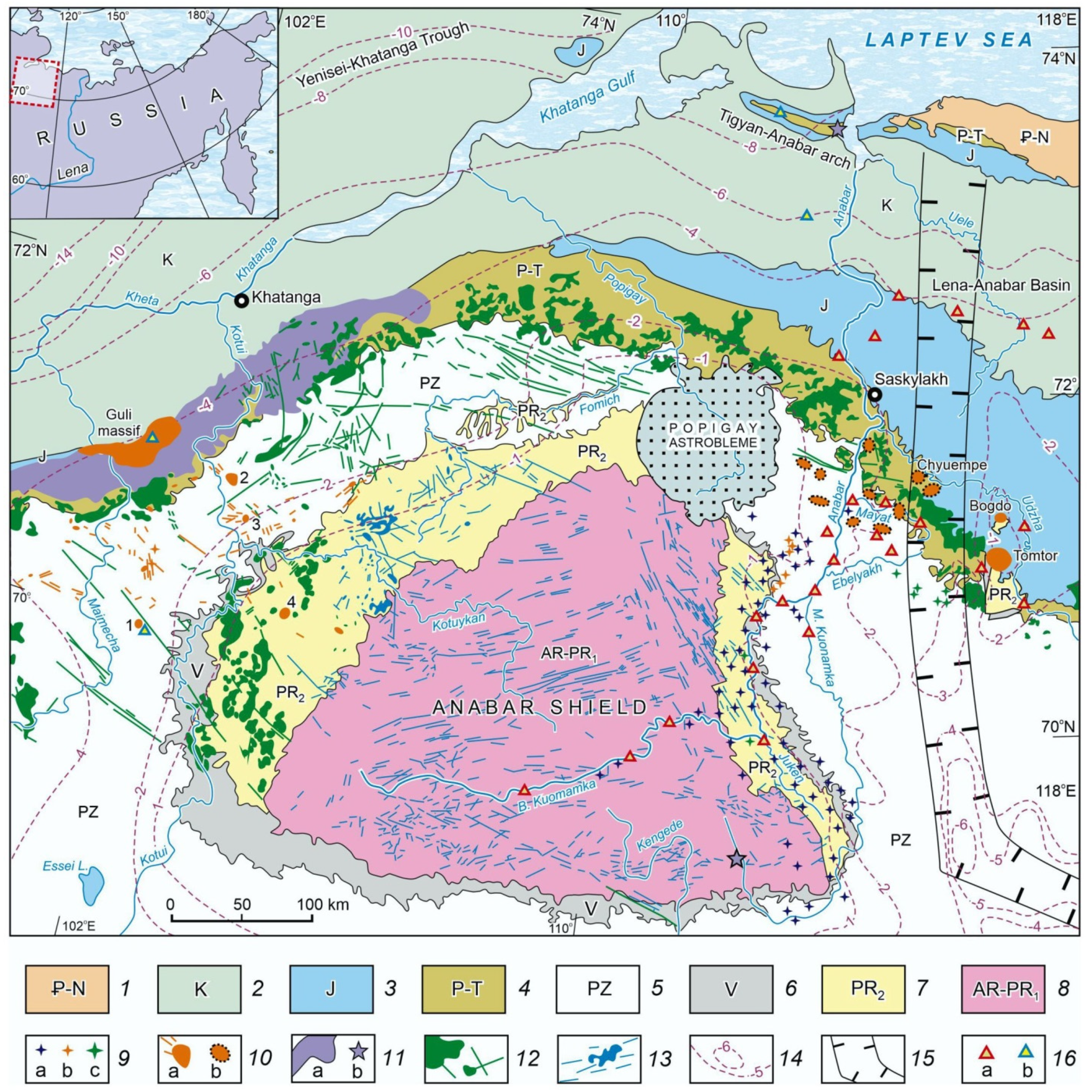
:1. Introduction
2. Materials and Methods
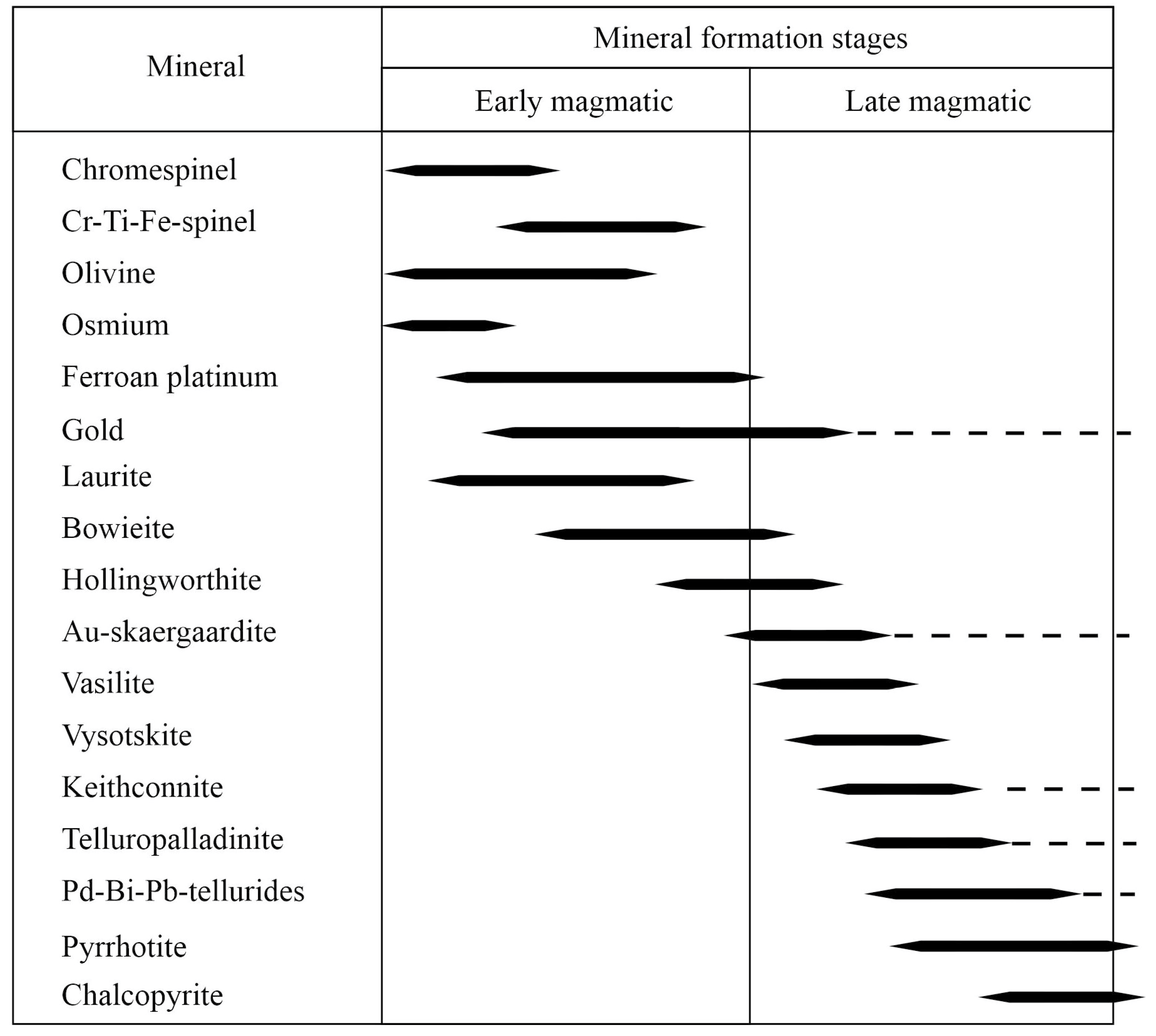
3. Forms of Intergrowths of PGM and Gold, Their Interpretation on Triangular Diagrams of the Phase State
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grakhanov, S.A.; Zinchuk, N.N.; Sobolev, N.V. The age of predictable primary diamond sources in the northeastern Siberian Platform. Dokl. Earth Sci. 2015, 465, 1297–1301. (In Russian) [Google Scholar] [CrossRef]
- Shpunt, B.R. Platinum minerals in Quaternary deposits of the Anabar-Olenek Uplift. Geol. Rudn. Mestorozhdenii 1970, 2, 123–126. (In Russian) [Google Scholar]
- Shpunt, B.R. Typomorphic features and genesis of placer gold in the north of the Siberian platform. Geol. Geophys. 1974, 9, 77–78. (In Russian) [Google Scholar]
- Okrugin, A.V. Mineralogy, Types, and Origin of Platinum-bearing placer deposits of the Siberian platform. Int. Geol. Rev. 1998, 8, 677–687. [Google Scholar] [CrossRef]
- Okrugin, A.V. Platinum-Bearing Placers of the Siberian Platform; Publishing House YaF SO RAN: Yakutsk, Russia, 2000. (In Russian) [Google Scholar]
- Gerasimov, B.B.; Nikiforova, Z.S.; Pavlov, V.I. Mineralogical and geochemical characteristics of placer gold in the Bolshaya Kuonamka river. Educ. Sci. 2014, 3, 74–78. (In Russian) [Google Scholar]
- Okrugin, A.; Gerasimov, B. Paragenetic Association of Platinum and Gold Minerals in Placers of the Anabar River in the Northeast of the Siberian Platform. Minerals 2023, 13, 96. [Google Scholar] [CrossRef]
- Porshnev, G.I.; Stepanov, L.L. Geologiya i minerageniya Udzhinskoi provintsii (severo-zapad Yakutskoi ASSR). Sov. Geol. 1981, 103–106. (In Russian) [Google Scholar]
- Okrugin, A.V.; Mazur, A.B.; Zemnuhov, A.L.; Popkov, P.A.; Sleptsov, S.V. The palladium gold-PGM association in the placers of the Anabar River basin, NE part of the Siberian platform, Russia. Otechestvennaya Geol. 2009, 5, 3–10. (In Russian) [Google Scholar]
- Geological Map of the Siberian Platform. Scale 1:1500000; VSEGEI Publishing House: Saint Petersburg, Russia, 1999. (In Russian)
- Parfenov, L.M. (Ed.) Geodynamic Map of Yakutia and Adjacent Territories. Scale 1:1 500 000; General Directorate of Geodesy and Cartography (GDGC): Yakutsk, Russia, 1994. (In Russian) [Google Scholar]
- Okrugin, A.V. Mineral Parageneses and the Origin of Isoferroplatinum Nuggets from the Ignali Placer Deposit (Siberian Platform). Geol. Ore Depos. 2001, 43, 239–250. [Google Scholar]
- Okrugin, A.V. Origin of platinum-group minerals in mafic-ultramafic rocks: From dispersed elements to nuggets. Can. Mineral. 2011, 49, 1397–1412. [Google Scholar] [CrossRef]
- Orlandea, E.; Vlad, Ș.N. A novel conceptual model of intrusion related gold bearing systems and exploration tools. Stud. UBB Geol. 2020, 63, 1–12. [Google Scholar] [CrossRef]
- Erlikh, E.N. New province of alkaline rocks in the northeast of the Siberian platform. Zap. WMO 1964, 90, 682–693. (In Russian) [Google Scholar]
- Koval’skii, V.V.; Nikishov, K.N.; Egorov, O.S. Kimberlite and Carbonatite Formations of the Anabar Anteclise; Nauka: Moscow, Russia, 1969. (In Russian) [Google Scholar]
- Milashev, V.A.; Tomanovskaya, Y.I. Manifestations of alkaline-ultramafic magmatism in the coastal part of the Laptev Sea. Kimberlite volcanism and prospects for the indigenous diamond content of the Siberian platform. Sci. Res. Inst. Arct. Geol. 1971, 127–133. (In Russian) [Google Scholar]
- Muzyka, G.M.; Chumirin, K.G. On the Issue of the Manifestation of Analogues of Meimechites on the Southern Outskirts of the Anabar Massif. In Geology, Petrography and Mineralogy of Magmatic Formations of the Northeastern Part of the Siberian Platform; Nauka: Moscow, Russia, 1970; pp. 183–190. (In Russian) [Google Scholar]
- Entin, A.R.; Zaitsev, A.I.; Nenashev, N.I.; Vasilenko, V.B.; Orlov, A.I.; Tyan, O.A.; Ol’khovik, Y.A.; Ol’shtynskii, S.I.; Tolstov, A.V. On the sequence of geological events associated with the intrusion of the Tomtor massif of ultrabasic alkaline rocks and carbonatites (Northwestern Yakutia). Geol. Geophys. 1990, 31, 42–51. (In Russian) [Google Scholar]
- Kravchenko, S.M.; Pokrovsky, B.G. The Tomtor alkaline ultrabasic massif and related REE-Nb deposits, northern Siberia. Econ. Geol. 1995, 71, 676–689. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Kotov, A.B.; Borisenko, A.S.; Yarmolyuk, V.V.; Pokhilenko, N.P.; Sal’nikova, E.B.; Travin, A.V.; Yakovleva, S.Z. Age boundaries of formation of the Tomtor alkaline-ultramafic pluton: U-Pb and 40Ar/39Ar geochronological studies. Dokl. Earth Sci. 2014, 454, 7–11. [Google Scholar] [CrossRef]
- Frolov, A.A.; Lapin, A.V.; Tolstov, A.V.; Zinchuk, N.N.; Belov, S.V.; Burgomistrov, A.A. Carbonatites and Kimberlites (Relationships, Minerageny, Forecast); NIA-Priroda: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Okrugin, A.V.; Tolstov, A.V. Petrogeochemical characteristics of the syenite—Alkali-ultrabasic silicate rock complex of the Tomtor massif (northeastern Siberian platform). Otechestvennaya Geol. 2017, 56–66. (In Russian) [Google Scholar]
- Okrugin, A.V.; Yakubovich, O.V.; Ernst, R.E.; Druzhinina, Z.Y. Platinum-bearing placers: Mineral associations and their 190Pt-4He and Re-Os ages, and potential links with large igneous provinces in the Siberian craton. Econ. Geol. 2020, 115, 1835–1853. [Google Scholar] [CrossRef]
- Hansen, M.; Anderko, K. Constitution of Binary Alloys; McGraw-Hill: New York, NY, USA, 1958. [Google Scholar]
- Raub, E.; Wörwag, G. Über Gold-Palladium-Kupfer-Legierungen. Zs. Metallkunde 1955, 46, 119–128. [Google Scholar] [CrossRef]
- Vol, A.E. Structure and Properties of Binary Metal Systems; State Scientific and Technical Publishing House: Moscow, Russia, 1962; Volume 2, p. 982. (In Russian) [Google Scholar]
- Vol, A.E.; Kagan, I.K. Structure and Properties of Binary Metal Systems; Nauka: Moscow, Russia, 1976; Volume 3, p. 814. (In Russian) [Google Scholar]
- Drits, M.E.; Bochvar, N.R.; Guzey, L.S.; Lysova, E.V.; Padeznova, E.M.; Rohlin, L.L.; Turkina, N.I. Binary and Multicomponent Copper-Based Systems; Handbook; Nauka: Moscow, Russia, 1979; p. 278. (In Russian) [Google Scholar]
- Savitsky, E.M. (Ed.) Precious Metals; Handbook; Metallurgy: Moscow, Russia, 1984; p. 592. (In Russian) [Google Scholar]
- Bryukvin, V.A.; Shekhter, L.N.; Reznichenko, V.A.; Blokhina, L.I.; Kukoyev, V.A. On phase equilibrium in the system Pt-PtS. Izvestiya Rossiiskoi Akademii Nauk. Metally. 1985, 191. (In Russian) [Google Scholar]
- Bannykh, O.A.; Drits, M.E. (Eds.) Diagrams of the State of Double and Multicomponent Systems Based on Iron; Reference Book; Moscow Metallurgy: Moscow, Russia, 1986; p. 440. (In Russian) [Google Scholar]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Rhines, F.N. Phase Diagrams in Metallurgy. Their Development and Application; McGraw–Hill Book Company. INC.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1956. [Google Scholar]
- Kurnakov, N.S. Introduction to Physical and Chemical Analysis; The Third Expanded Edition; United Scientific and Technical Publishing House: Leningrad, Russia, 1936; p. 193. (In Russian) [Google Scholar]
- Anosov, V.Y.; Pogodin, S.A. Basic Principles of Physical and Chemical Analysis; Moscow-Leningrad Publishing House of the USSR, AS.: Moscow, Russia, 1947; p. 876. (In Russian) [Google Scholar]
- Devereux, O.F. Topics in Metallurgical Thermodinamics; J. Wiley: New York, NY, USA, 1983. [Google Scholar]
- Shcheka, G.G.; Lehmann, B.; Gierth, E.; Gömann, K.; Wallianos, A. Macrocrystals of Pt–Fe alloy from the Kondyor PGE placer deposit, Khabarovskiy Kray, Russia: Trace-element content, mineral inclusions and reaction assemblages. Can. Mineral. 2004, 42, 601–617. [Google Scholar] [CrossRef]
- Sluzhenikin, S.; Mokhov, A. Gold and silver in PGE-Cu-Ni and PGE ores of the Noril’sk deposits, Russia. Miner. Depos. 2015, 50, 465–492. [Google Scholar] [CrossRef]
- Sinyakova, E.F.; Borisenko, A.S.; Karmanov, N.S.; Kosyakov, V.I. Behavior of noble metals during fractional crystallization of Cu–Fe–Ni–(Pt, Pd, Rh, Ir, Ru, Ag, Au, Te) sulfide melts. Russ. Geol. Geophys. 2019, 60, 642–661. [Google Scholar] [CrossRef]
- Nekrasov, I.Y.; Ivanov, V.V.; Lennikov, A.M.; Sapin, V.I.; Safronov, P.P.; Oktyabr’skii, R.A. Rare natural polycomponent alloys based on gold and copper from the platinum placer in the Konder alkaline-ultrabasic massif, southeastern Aldan shield, Russia. Geol. Ore Depos. 2001, 43, 406–417. [Google Scholar]
- Razin, L.V. Minerals—natural alloys of gold and copper in ores of copper-nickel deposits of the Norilsk type. Proc. Fersman Mineral. Museum 1975, 24, 93–106. (In Russian) [Google Scholar]
- Rudashevsky, N.S.; McDonald, A.M.; Cabri, L.J.; Nielsen, T.F.D.; Stanley, C.J.; Kretzer, Y.L.; Rudashevsky, V.N. Skaergaardite, PdCu, a new platinum-group intermetallic mineral from the Skaergaard intrusion, Greenland. Miner. Magaz. 2004, 68, 615–632. [Google Scholar] [CrossRef]
- Palyanova, G.A.; Zhegunov, P.S.; Beliaeva, T.V.; Murzin, V.V.; Borovikov, A.A.; Goryachev, N.A. Palladian Gold: Chemical Composition, Minerals in Association, and Physicochemical Conditions of Formation at Different Types of Gold Deposits. Minerals 2023, 13, 1019. [Google Scholar] [CrossRef]
- Naldrett, A.J.; Cabri, L.J. Ultramafic and Related Mafic Rocks: Their Classification and Genasis with Special Reference to the Consentration of Nickel Sulfides and Platinum-Group Elements. Econ. Geol. 1976, 71, 1131–1158. [Google Scholar] [CrossRef]
- Genkin, A.D.; Evstigneeva, T.L. Association of platinum-group minerals of the Noril’sk copper-nickel sulfide ores. Econ. Geol. 1986, 81, 1203–1212. [Google Scholar] [CrossRef]
- Distler, V.V.; Grokhovskaya, T.L.; Evstigneeva, T.L.; Sluzhenikin, S.F.; Filimonova, A.A.; Dyuzhikov, O.A.; Laputina, I.P. Petrology of Sulfide Magmatic Ore Formation; Nauka: Moscow, Russia, 1988. (In Russian) [Google Scholar]
- Nekrasov, I.Y.; Lennikov, A.M.; Zalishchak, B.L.; Oktyabrsky, R.A.; Ivanov, V.V.; Sapin, V.I.; Taskaev, V.I. Compositional variations in platinum-group minerals and gold, Konder alkaline-ultrabasic massif, Aldan shield, Russia. Can. Mineral. 2005, 43, 637–654. [Google Scholar] [CrossRef]
- Bezmen, N.L.; Asif, M.; Brugmann, G.E.; Romanenko, I.M.; Naldrett, A.J. Distribution of Pd, Rh, Ru, Ir, Os and Au between sulfide and silicate melts. Geochim. Cosmochim. Acta 1994, 58, 1251–1260. [Google Scholar] [CrossRef]
- Fleet, M.E.; Crocket, J.H.; Liu, M.; Stone, W.E. Laboratory partitioning of platinum-group elements (PGE) and gold with application to magmatic sulfide-PGE deposits. Lithos 1999, 47, 127–142. [Google Scholar] [CrossRef]
- Borisov, A.; Palme, H. Solubilities of noble metals in Fe-containing silicate melts as derived from experiments in Fe-free systems. Am. Mineral. 2000, 85, 1665–1673. [Google Scholar] [CrossRef]
- Crocket, J.H. Platimum-group element geochemistry of mafic and ultramafic rocks. The Geology, Geochemistry, Mineralogy and Mineral Beneficiation of Platinum-Group Elements. Can. Inst. Min. Metall. Pet. 2002, 54, 177–210. [Google Scholar]
- Cabri, L.J.; Laflamme, J.H.G. Platinum-group minerals from the Konder Massif, Russian Far East. Mineral. Record. 1997, 28, 97–106. [Google Scholar]
- Nekrasov, I.Y. Geochemistry, Mineralogy, and Genesis of Gold Deposits; Nauka: Moscow, Russia, 1991; p. 302. (In Russian) [Google Scholar]
- Malitch, K.N.; Auge, T.; Badanina IYu Goncharov, M.M.; Junk, S.A.; Pernicka, E. Os-rich nuggets from Au-PGE placers of the Maimecha-Kotui Province, Russia: A multi-disciplinary study. Miner. Petrol. 2002, 76, 121–148. [Google Scholar] [CrossRef]
- Okrugin, A.V. Chromite-Ulvöshpinel Series of Minerals from Alkaline Picrite-Basic Rocks of the North Siberian Platform and Their Oxythermobarometry. Zap. RMO 2023, 152, 80–94. (In Russian) [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.; Green, D. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: Implication for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Anikina, E.V.; Alekseev, A.V. Mineral-geochemical characteristics of gold–palladium mineralization in the Volkovsky gabbro-diorite massif (Platinum Belt of the Urals). Litosfera 2010, 5, 75–100. (In Russian) [Google Scholar]
- Mikhailov, V.V.; Stepanov SYu Kozlov, A.V.; Petrov, S.V.; Palamarchuk, R.S.; Shilovskikh, V.V.; Abramova, V.D. New copper–precious metal occurrence in gabbro of the Serebryansky Kamen massif, Ural platinum belt, Northern Urals. Geol. Ore Depos. 2021, 63, 528–555. [Google Scholar] [CrossRef]
- Naldrett, A.J.; Brugman, G.E. Models for the concentration of PGE in layered intrusions. Can. Mineral. 1990, 28, 389–408. [Google Scholar]
- Bird, D.K.; Brooks, C.K.; Gannicott, R.A.; Turner, P.A. A Gold-bearing horizon in the Skaergaard intrusion, East Greenland. Econ. Geol. 1991, 86, 1083–1092. [Google Scholar] [CrossRef]
- Rudashevsky, N.S.; Rudashevsky, V.N.; Nielsen, T.F.D. Intermetallides and alloys of copper and palladium in gold-palladium ores of Skaergaard massif (Greenland). Zap. RMO 2015, 144, 31–53. (In Russian) [Google Scholar]
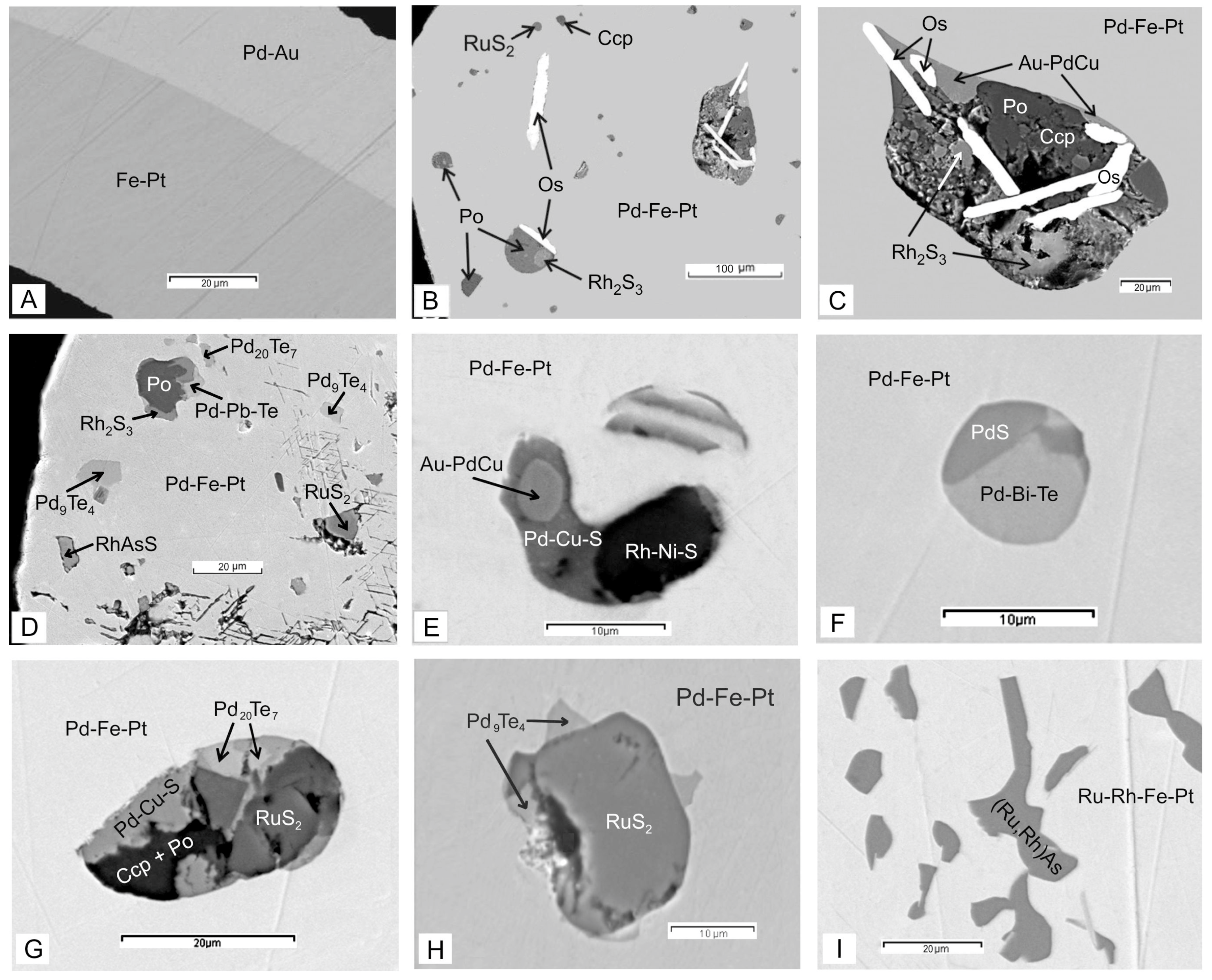


| Sample | Mineral | Pt | Ir | Os | Ru | Rh | Pd | Fe | Ni | Cu | Au, Pb, Bi | Te | S | As | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18-157 | Pd-Fe-Pt | 81.23 | 1.03 | 4.46 | 10.32 | 0.35 | 1.24 | 98.63 | |||||||
| Os-Ir-Ru | 0.21 | 10.03 | 79.55 | 10.14 | 99.93 | ||||||||||
| Os-Ir-Ru | 6.29 | 81.53 | 11.42 | 99.24 | |||||||||||
| RuS2 | 61.61 | 37.99 | 99.60 | ||||||||||||
| Au-PdCu | 4.17 | 60.62 | 5.57 | 25.71 | 1.35 (Au) | 2.37 | 99.79 | ||||||||
| Fe1-xS | 54.33 | 3.10 | 3.23 | 37.04 | 97.70 | ||||||||||
| CuFeS2 | 31.34 | 33.42 | 34.51 | 99.27 | |||||||||||
| (Rh,Fe)S | 34.73 | 19.34 | 9.08 | 5.50 | 29.26 | 97.91 | |||||||||
| 7-157 | Pd-Fe-Pt | 80.07 | 0.45 | 0.37 | 5.95 | 11.67 | 0.45 | 0.18 | 99.14 | ||||||
| RuS2 | 56.47 | 3.54 | 38.62 | 98.63 | |||||||||||
| RuS2 | 9.40 | 39.93 | 10.83 | 34.4 | 4.3 | 98.86 | |||||||||
| Rh2S3 | 12.24 | 38.03 | 9.77 | 1.36 | 6.73 | 30.54 | 98.67 | ||||||||
| Pd9Te4 | 63.63 | 34.37 | 98.00 | ||||||||||||
| Pb-Pd-Te | 48.42 | 10.44 (Pb) | 43.26 | 91.68 | |||||||||||
| RhAsS | 19.39 | 29.65 | 15.63 | 28.45 | 93.12 | ||||||||||
| Fe1-xS | 2.83 | 56.59 | 1.09 | 38.7 | 99.21 | ||||||||||
| 13-157 | Pd-Fe-Pt | 77.15 | 0.32 | 0.29 | 0.34 | 8.27 | 8.78 | 0.85 | 1.53 | 2.16 (Au) | 99.69 | ||||
| Au-PdCu | 6.90 | 54.20 | 1.45 | 27.46 | 4.99 (Au) | 3.18 | 98.18 | ||||||||
| Pd-Cu-S | 2.34 | 71.84 | 13.51 | 1.33 | 11.16 | 100.18 | |||||||||
| Rh-Ni-S | 3.57 | 27.73 | 4.87 | 21.31 | 7.64 | 29.98 | 95.10 | ||||||||
| 23-36 | Pd-Fe-Pt | 86.28 | 5.29 | 8.64 | 100.21 | ||||||||||
| PdBiTe | 71.88 | 14.03 (Bi) | 6.12 | 1.56 | 93.59 | ||||||||||
| PdS | 83.98 | 1.44 | 13.27 | 98.69 | |||||||||||
| 23-76 | Pd-Fe-Pt | 78.66 | 7.37 | 11.03 | 1.71 | 98.77 | |||||||||
| Pd-Cu-Pt | 75.71 | 10.30 | 12.73 | 98.74 | |||||||||||
| Pd9Te4 | 70.50 | 27.98 | 98.48 | ||||||||||||
| RuS2 | 60.99 | 36.52 | 97.51 | ||||||||||||
| 12-156 | Pd-Fe-Pt | 84.16 | 0.28 | 4.16 | 9.76 | 0.74 | 0.89 | 99.99 | |||||||
| Pd9Te4 | 68.82 | 0.81 | 30.19 | 99.82 | |||||||||||
| RuS2 | 60.19 | 38.83 | 99.02 | ||||||||||||
| 85-157 | Ru-Rh-Fe-Pt | 81.45 | 0.53 | 2.00 | 1.91 | 10.73 | 0.88 | 0.95 | 98.45 | ||||||
| (Ru,Rh)As | 1.23 | 1.07 | 22.45 | 34.20 | 40.62 | 99.57 | |||||||||
| MK-10 | Pd-Fe-Pt | 76.52 | 9.57 | 9.19 | 0.68 | 2.90 | 98.86 | ||||||||
| Cu-Pd-Au | 37.75 | 18.63 | 39.88 (Au) | 96.26 | |||||||||||
| Pd3Pb | 61.24 | 33.79 (Pb) | 95.03 | ||||||||||||
| Pd-Te | 68.44 | 8.35 (Pb) | 15.54 | 1.48 | 2.56 | 96.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrugin, A.; Gerasimov, B. Formation of Intergrowths of Platinum-Group Minerals and Gold from Magmatogenic Ores in Relation to Phase Changes in Pt-Pd-Fe-Cu-Au System. Minerals 2024, 14, 326. https://doi.org/10.3390/min14030326
Okrugin A, Gerasimov B. Formation of Intergrowths of Platinum-Group Minerals and Gold from Magmatogenic Ores in Relation to Phase Changes in Pt-Pd-Fe-Cu-Au System. Minerals. 2024; 14(3):326. https://doi.org/10.3390/min14030326
Chicago/Turabian StyleOkrugin, Alexander, and Boris Gerasimov. 2024. "Formation of Intergrowths of Platinum-Group Minerals and Gold from Magmatogenic Ores in Relation to Phase Changes in Pt-Pd-Fe-Cu-Au System" Minerals 14, no. 3: 326. https://doi.org/10.3390/min14030326






