Mineralization of Ni2+-Bearing Mn Oxide through Simultaneous Sequestration of Ni2+ and Mn2+ by Enzymatically Active Fungal Mn Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biogenic Manganese Oxide Production by Acremonium Strictum KR21-2
2.2. Repeated-Treatment Experiments of Biogenic Manganese Oxides
2.3. Two-Step Extraction Procedure for Biogenic Manganese Oxide
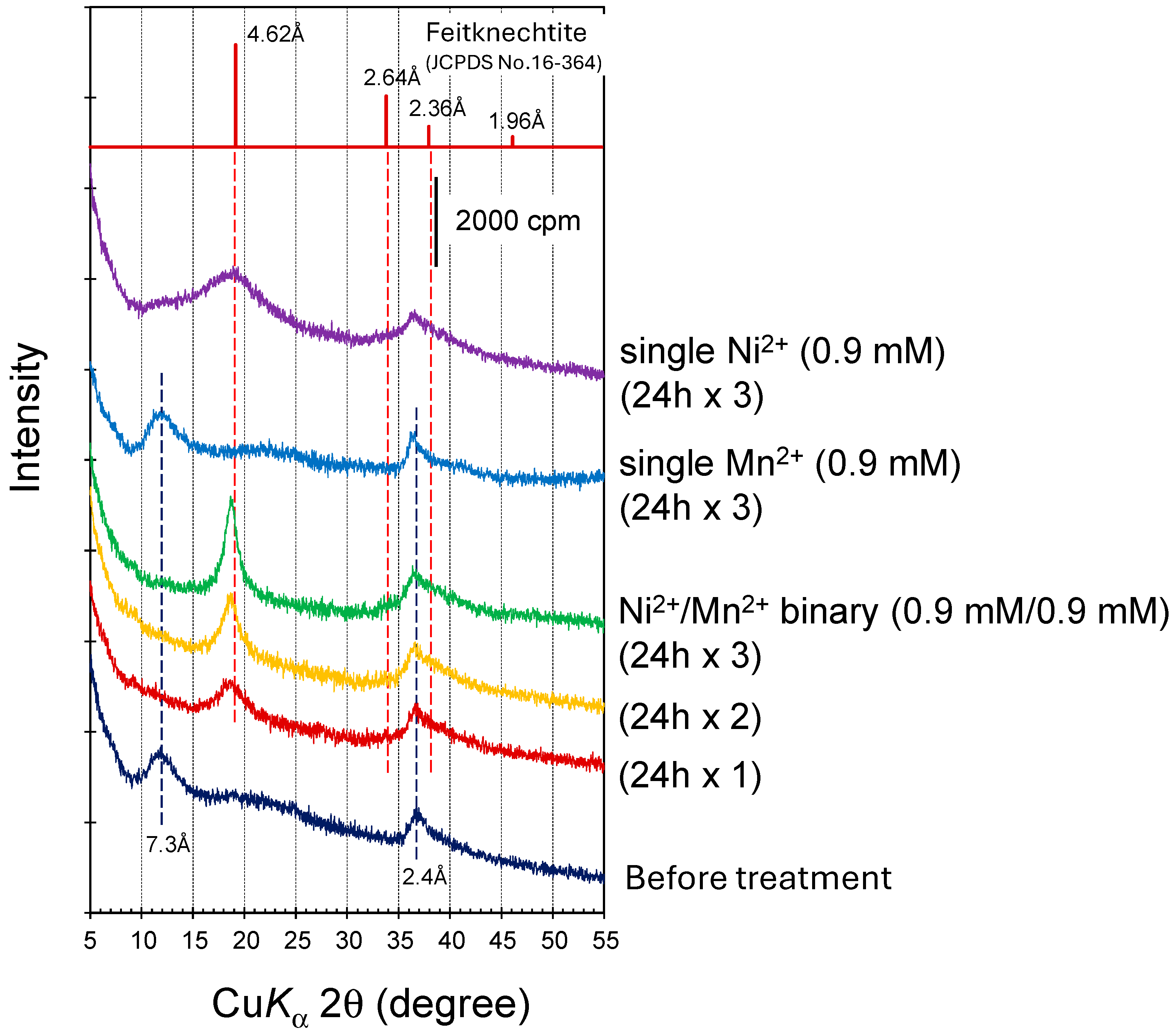
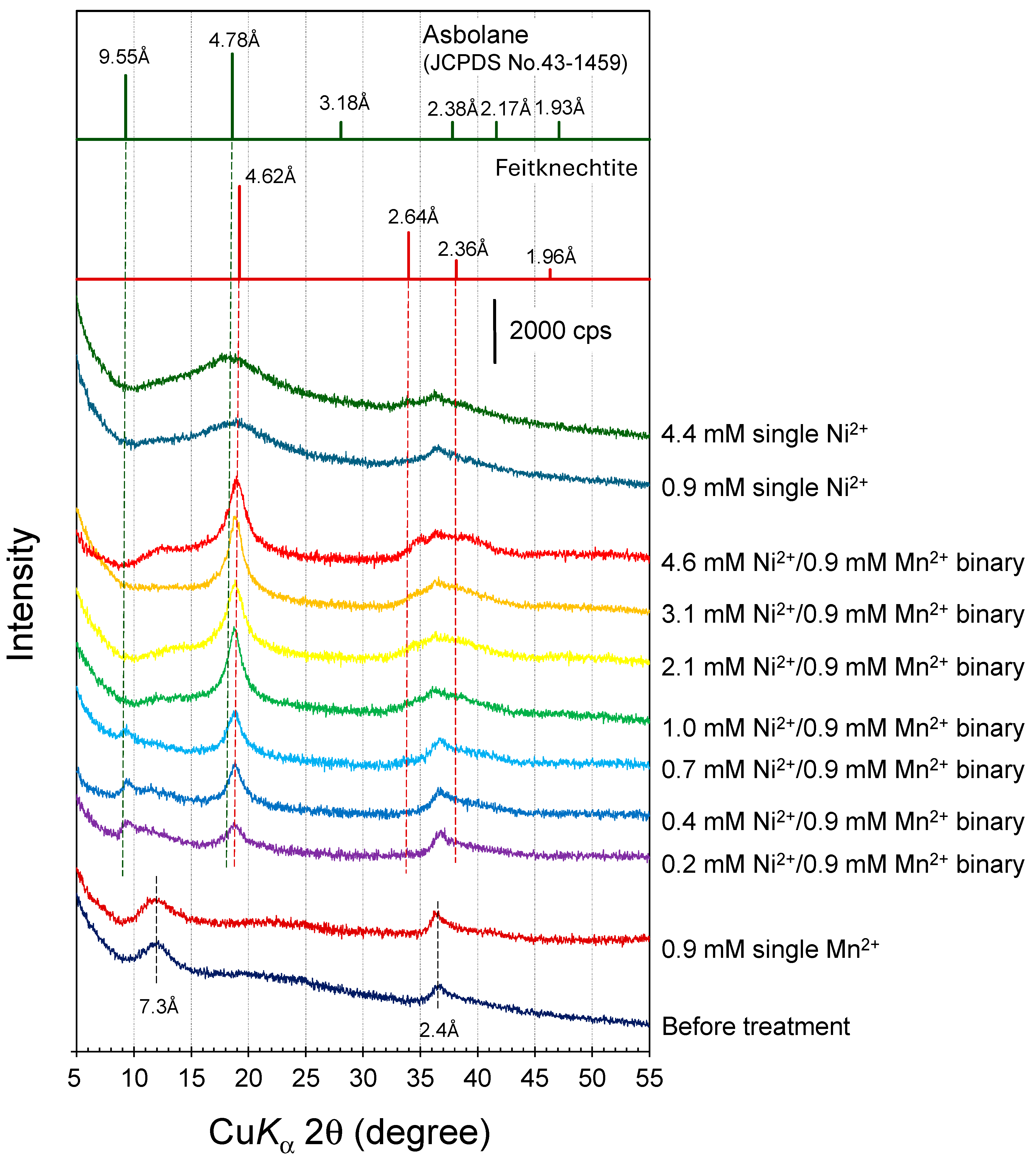
2.4. X-ray Diffraction Analysis
2.5. Single-Treatment Experiments of Enzymatically Inactivated Biogenic Manganese Oxides
3. Results and Discussion
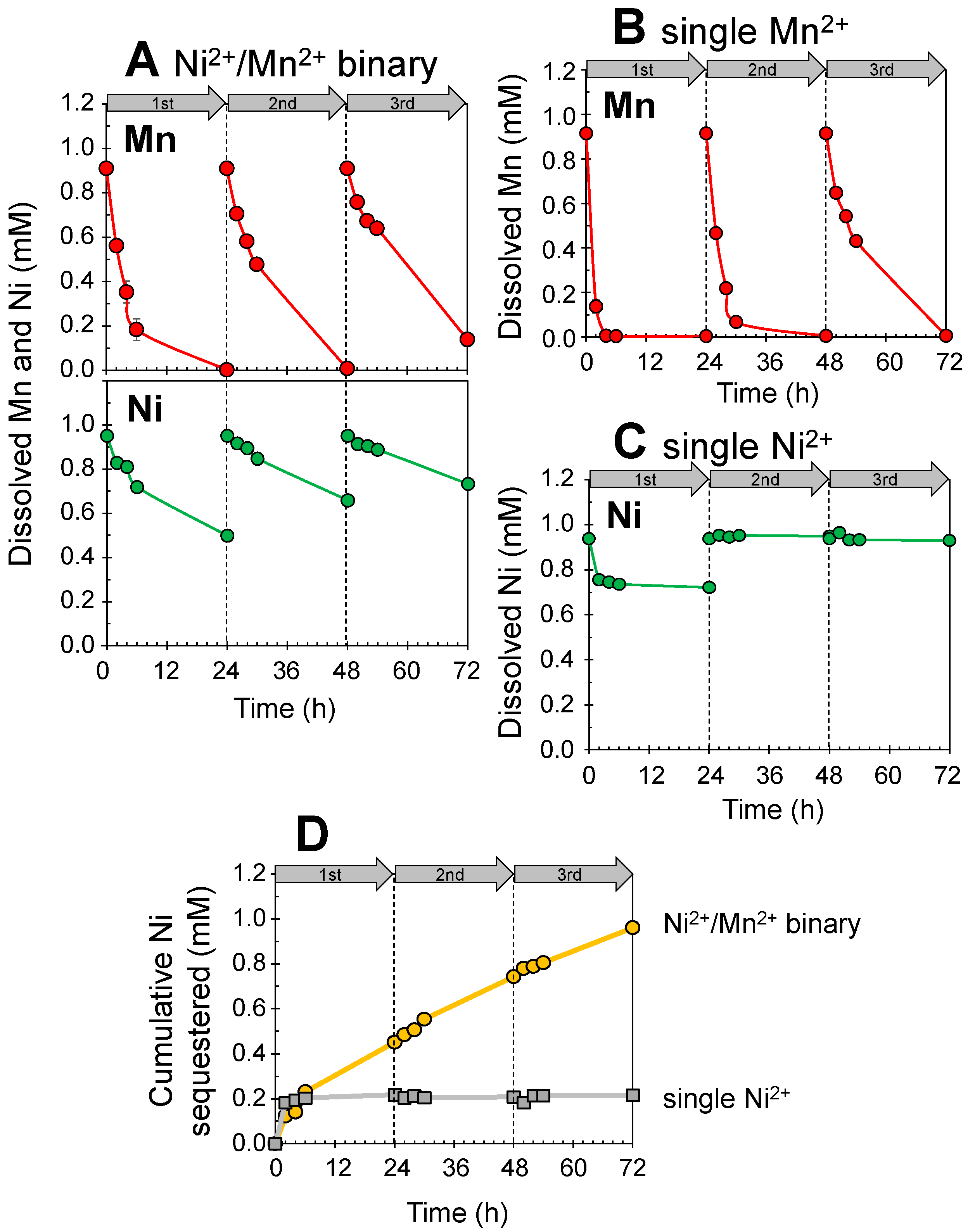
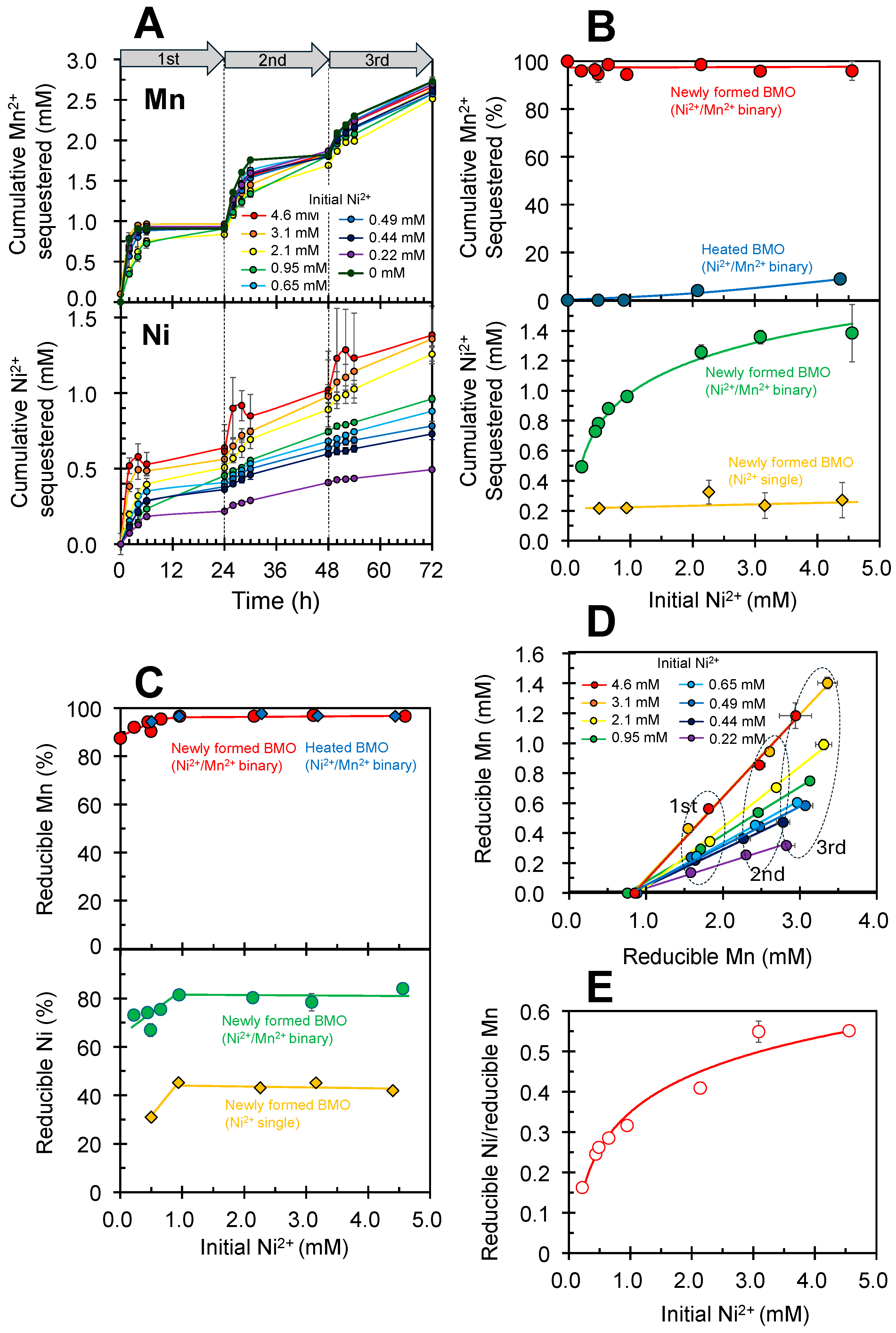
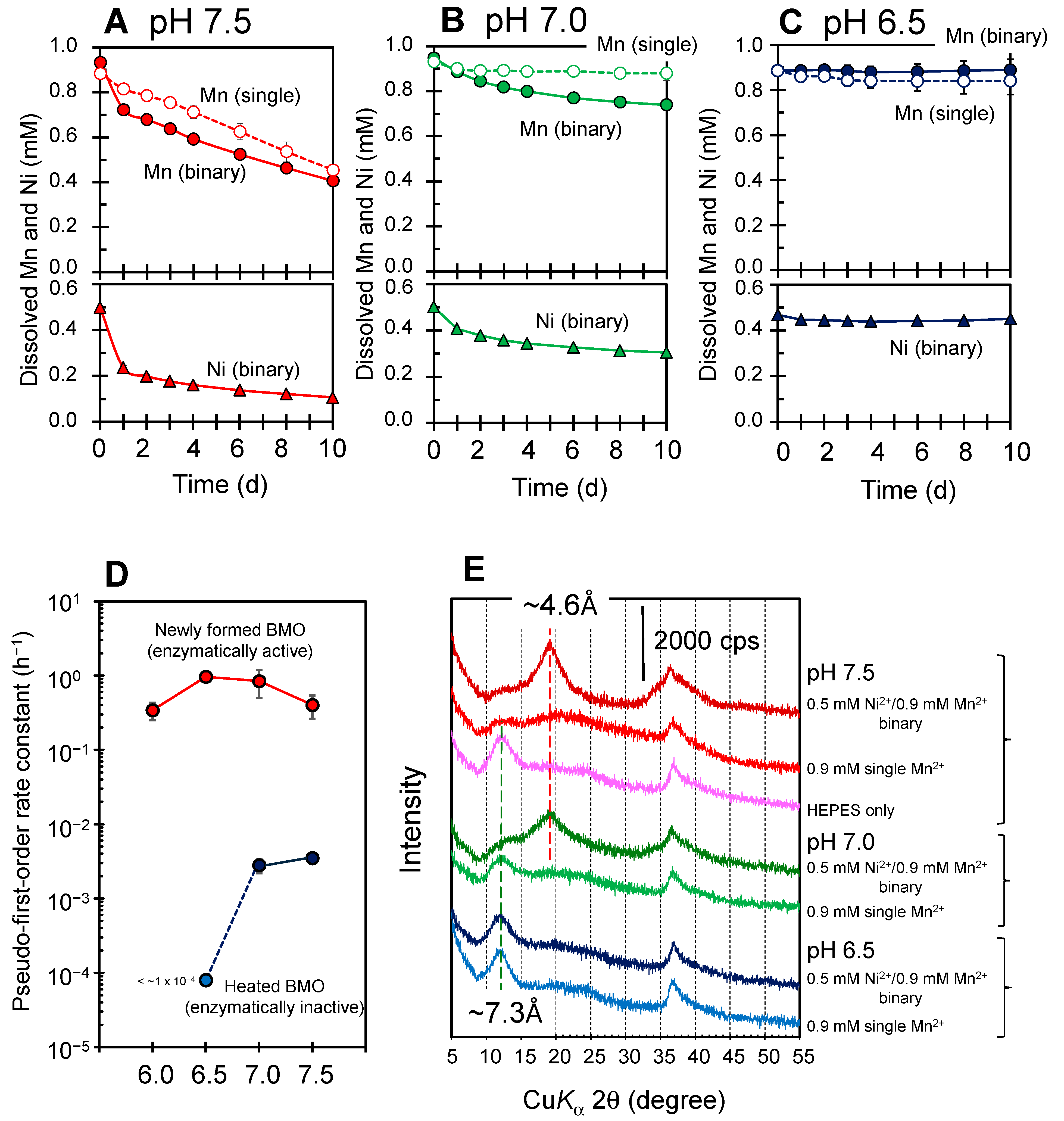
3.1. Simultaneous Sequestration of Mn2+ and Ni2+ by Newly Formed Biogenic Manganese Oxides
3.2. Mineralization of Ni(II)-Incorporated Manganese Oxide Phases in Ni2+/Mn2+ Binary Solution Systems
3.3. pH Dependence of Sequestration Efficiency in Ni2+/Mn2+ Binary Solution Systems
3.4. Abiotic Mn(II) Oxidation in Ni2+/Mn2+ Binary Solution Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reck, B.K.; Müller, D.B.; Rostkowski, K.; Graedel, T.E. Anthropogenic Nickel Cycle: Insights into Use, Trade, and Recycling. Environ. Sci. Technol. 2008, 42, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Yugo, M.; Soler, A. Outlook for battery raw materials (literature review). Concawe Rev. 2019, 28, 25–39. [Google Scholar]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Kumar, A.; Jigyasu, D.K.; Kumar, A.; Subrahmanyam, G.; Mondal, R.; Shabnam, A.A.; Cabral-Pinto, M.M.S.; Malyan, S.K.; Chaturvedi, A.K.; Gupta, D.K.; et al. Nickel in terrestrial biota: Comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere 2021, 275, 129996. [Google Scholar] [CrossRef] [PubMed]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.U.; Hussain, N.; Sumrin, A.; Shahbaz, A.; Noor, S.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Microbial bioremediation strategies with wastewater treatment potentialities—A review. Sci. Total Environ. 2022, 818, 151754. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Gabasawa, A.I.; Elenwo, C.E.; Agbeyegbe, O.O. Bioremediation techniques as affected by limiting factors in soil environment. Front. Soil Sci. 2022, 2, 937186. [Google Scholar] [CrossRef]
- Kour, D.; Khan, S.S.; Kour, H.; Kaur, T.; Devi, R.; Rai, P.K.; Judy, C.; McQuestion, C.; Bianchi, A.; Spells, S.; et al. Microbe-mediated bioremediation: Current research and future challenges. J. Appl. Biol. Biotechnol. 2022, 10, 6–24. [Google Scholar] [CrossRef]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H.; et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Li, X.; Feng, C.; Lei, M.; Luo, K.; Wang, L.; Liu, R.; Li, Y.; Hu, Y. Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review. Open Chem. 2022, 20, 793–807. [Google Scholar] [CrossRef]
- Dutta, D.; Rautela, R.; Gujjala, L.K.S.; Kundu, D.; Sharma, P.; Tembhare, M.; Kumar, S. A review on recovery processes of metals from E-waste: A green perspective. Sci. Total Environ. 2023, 859, 160391. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; De Gusseme, B.; Boon, N.; Verstraete, W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M.; Ali, M.; Abbasi, I.A.; Islam, T.; Ye, T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019, 230, 128–150. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Trace metal retention on biogenic manganese oxide nanoparticles. Elements 2005, 1, 223–226. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, S.; Bataillard, P.; Coussy, S. The nature of manganese oxides in soils and their role as scavengers of trace elements: Implication for soil remediation. In Environmental Soil Remediation and Rehabilitation; van Hullebusch, E.D., Huguenot, D., Pechaud, Y., Simonnot, M.-O., Colombano, S., Eds.; Springer Nature: Cham, Switzerland, 2020; Chapter 7; pp. 399–429. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, K.; Qiu, C.; Bi, Y.; Tian, B.; Bi, X. A Review of manganese-oxidizing bacteria (MnOB): Applications, future concerns, and challenges. Int. J. Environ. Res. Public Health 2023, 20, 1272. [Google Scholar] [CrossRef]
- Huang, Y.; Huangfu, X.; Ma, C.; Liu, Z. Sequestration and oxidation of heavy metals mediated by Mn(II) oxidizing microorganisms in the aquatic environment. Chemosphere 2023, 329, 138594. [Google Scholar] [CrossRef]
- Geszvain, K.; Butterfield, C.; Davis, R.E.; Madison, A.S.; Lee, S.-W.; Parker, D.L.; Soldatova, A.; Spiro, T.G.; Luther, G.W., III; Tebo, B.M. The molecular biogeochemistry of manganese(II) oxidation. Biochem. Soc. Trans. 2012, 40, 1244–1248. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Iwahori, K.; Soma, M. Enzymatic formation of manganese oxides by an Acremonium-like hyphomycete fungus, strain KR21-2. FEMS Microbiol. Ecol. 2004, 47, 101–109. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Maruo, K.; Tsuno, H.; Sakata, M.; Iwahori, K. Manganese(IV) oxide production by Acremonium sp. strain KR21-2 and extracellular Mn(II) oxidase activity. Appl. Environ. Microbiol. 2006, 72, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H. Fungal Mn oxides supporting Mn(II) oxidase activity as effective Mn(II) sequestering materials. Environ. Technol. 2013, 34, 2781–2787. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F.; Seyama, H. Zn(II) sequestration by fungal biogenic manganese oxide through enzymatic and abiotic processes. Chem. Geol. 2014, 383, 155–163. [Google Scholar] [CrossRef]
- Aoshima, M.; Tani, Y.; Fujita, R.; Tanaka, K.; Miyata, N.; Umezawa, K. Simultaneous sequestration of Co2+ and Mn2+ by fungal manganese oxide through asbolane formation. Minerals 2022, 12, 358. [Google Scholar] [CrossRef]
- Grangeon, S.; Lanson, B.; Miyata, N.; Tani, Y.; Manceau, A. Structure of nanocrystalline phyllomanganates produced by freshwater fungi. Am. Mineral. 2010, 95, 1608–1616. [Google Scholar] [CrossRef]
- Mattigod, S.V.; Rai, D.; Felmy, A.R.; Rao, L. Solubility and solubility product of crystalline Ni(OH)2. J. Solut. Chem. 1997, 26, 391–403. [Google Scholar] [CrossRef]
- Rosson, R.A.; Nealson, K.H. Manganese binding and oxidation by spores of a marine bacillus. J. Bacteriol. 1982, 151, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- De Vrind, J.P.; de Vrind-de Jong, E.W.; de Voogt, J.W.; Westbroek, P.; Boogerd, F.C.; Rosson, R.A. Manganese oxidation by spores and spore coats of a marine Bacillus species. Appl. Environ. Microbiol. 1986, 52, 1096–1100. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Sequestration of Cd(II) and Ni(II) ions on fungal manganese oxides associated with Mn(II) oxidase activity. Appl. Geochem. 2014, 47, 198–208. [Google Scholar] [CrossRef]
- Burlet, C.; Vanbrabant, Y. Study of the spectro-chemical signatures of cobalt–manganese layered oxides (asbolane–lithiophorite and their intermediates) by Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 941–952. [Google Scholar] [CrossRef]
- Grangeon, S.; Warmont, F.; Tournassat, C.; Lanson, B.; Lanson, M.; Elkaïm, E.; Claret, F. Nucleation and growth of feitknechtite from nanocrystalline vanadate precursor. Eur. J. Mineral. 2017, 29, 767–776. [Google Scholar] [CrossRef]
- Lefkowitz, L.P.; Elzinga, E.J. Structural alteration of hexagonal birnessite by aqueous Mn(II): Impacts on Ni(II) sorption. Chem. Geol. 2017, 466, 524–532. [Google Scholar] [CrossRef]
- Lefkowitz, J.P.; Rouff, A.A.; Elzinga, E.J. Influence of pH on the reductive transformation of birnessite by aqueous Mn(II). Environ. Sci. Technol. 2013, 47, 10364–10371. [Google Scholar] [CrossRef]
- Hem, J.D.; Lind, C.J.; Roberson, C.E. Coprecipitation and redox reactions of manganese oxides with copper and nickel. Geochim. Cosmochim. Acta 1989, 53, 2811–2822. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, Y.; Kumagai, H.; Tamari, M.; Umezawa, K.; Gotore, O.; Miyata, N. Mineralization of Ni2+-Bearing Mn Oxide through Simultaneous Sequestration of Ni2+ and Mn2+ by Enzymatically Active Fungal Mn Oxides. Minerals 2024, 14, 330. https://doi.org/10.3390/min14040330
Tani Y, Kumagai H, Tamari M, Umezawa K, Gotore O, Miyata N. Mineralization of Ni2+-Bearing Mn Oxide through Simultaneous Sequestration of Ni2+ and Mn2+ by Enzymatically Active Fungal Mn Oxides. Minerals. 2024; 14(4):330. https://doi.org/10.3390/min14040330
Chicago/Turabian StyleTani, Yukinori, Hanako Kumagai, Mako Tamari, Kazuhiro Umezawa, Obey Gotore, and Naoyuki Miyata. 2024. "Mineralization of Ni2+-Bearing Mn Oxide through Simultaneous Sequestration of Ni2+ and Mn2+ by Enzymatically Active Fungal Mn Oxides" Minerals 14, no. 4: 330. https://doi.org/10.3390/min14040330




