Study on Process Mineralogy of the Combined Copper Oxide Ore in Tibet and Acid Leaching Behavior with Calcium Fluoride
Abstract
1. Introduction
2. Experiment
2.1. Material and Reagent
2.2. Experimental Method

2.3. Mineral Liberation Analysis (MLA) and Scanning Electron Microscopy (SEM)
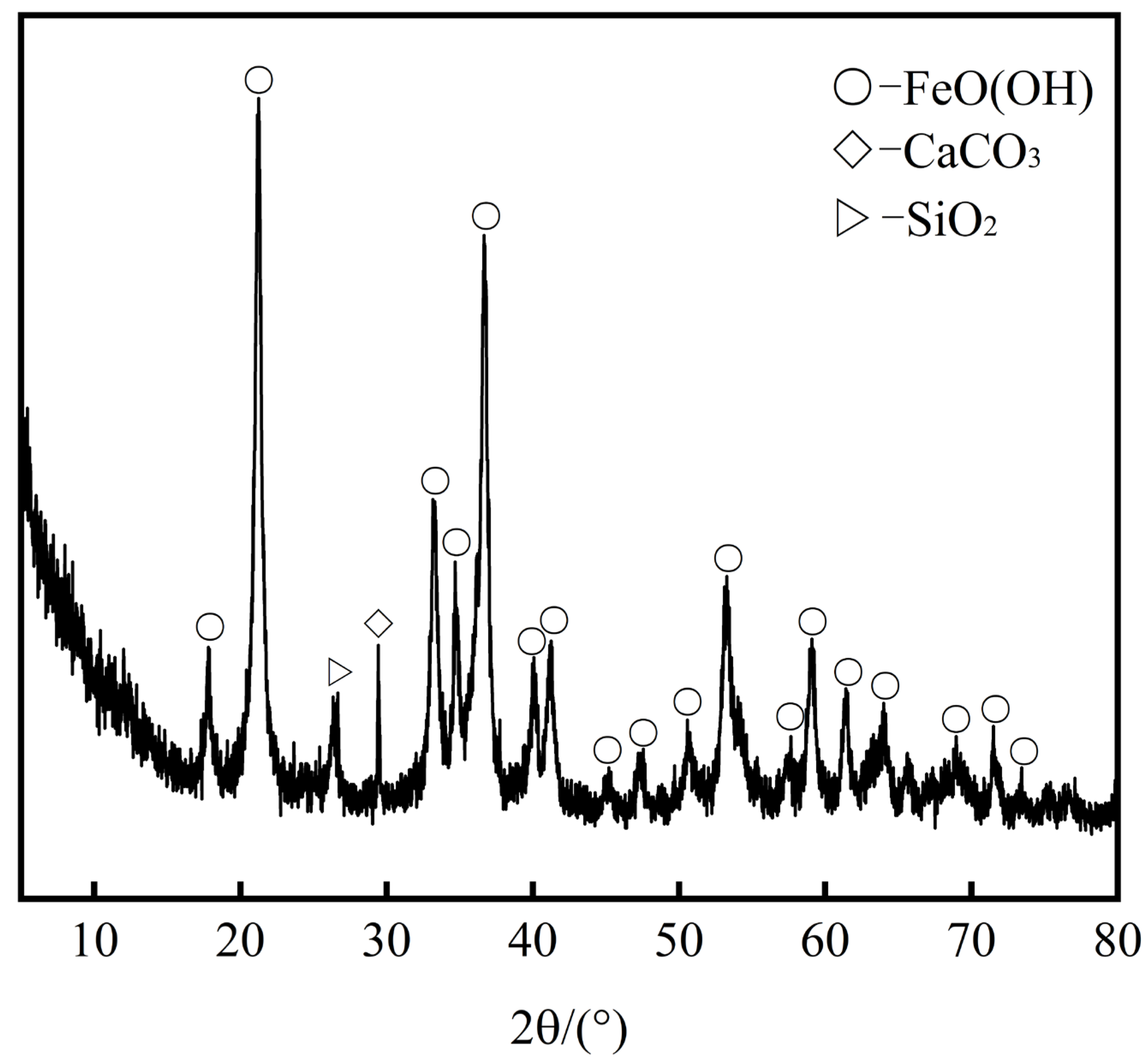
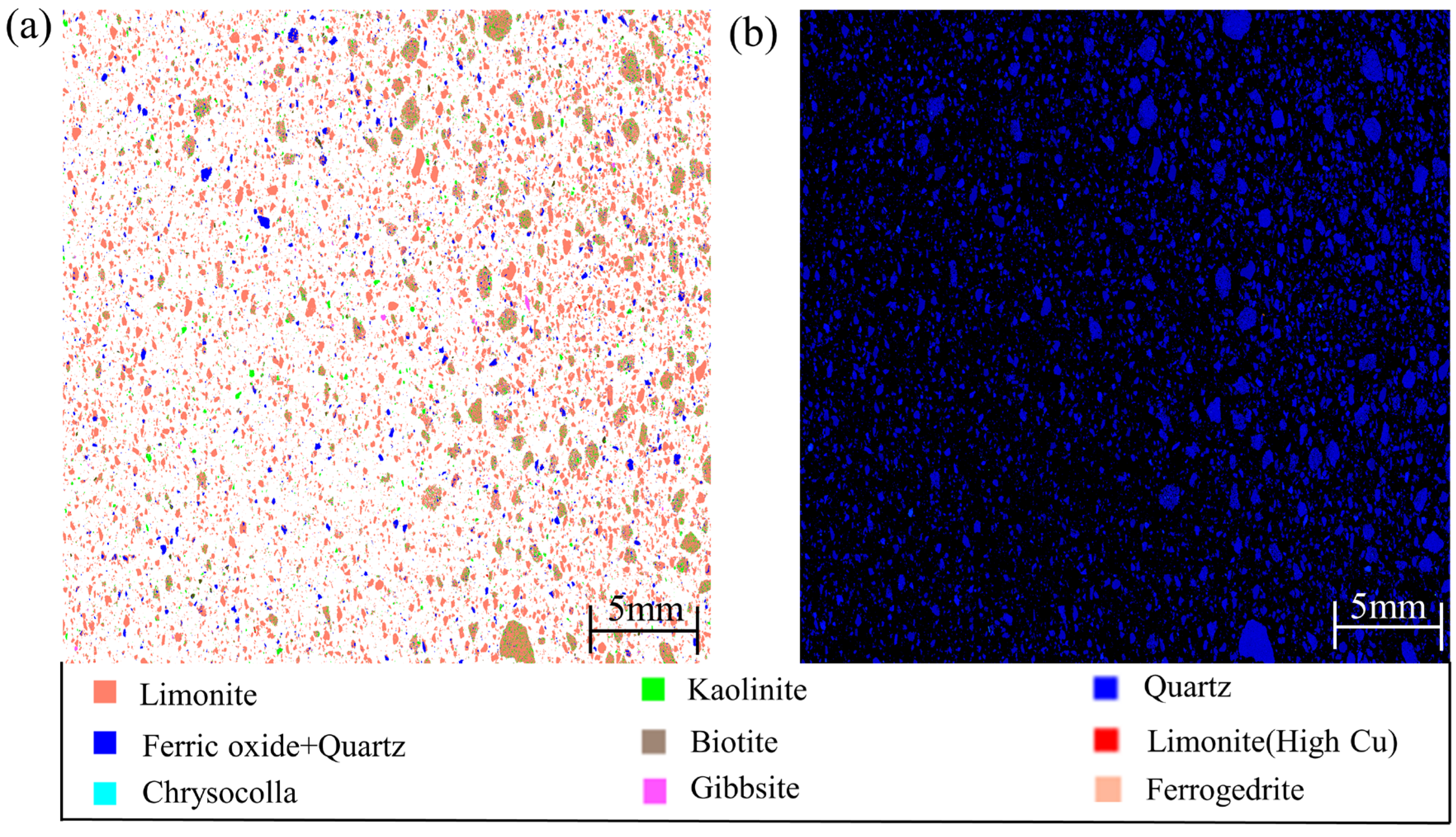
3. Process Mineralogy Analysis
4. Results and Discussion
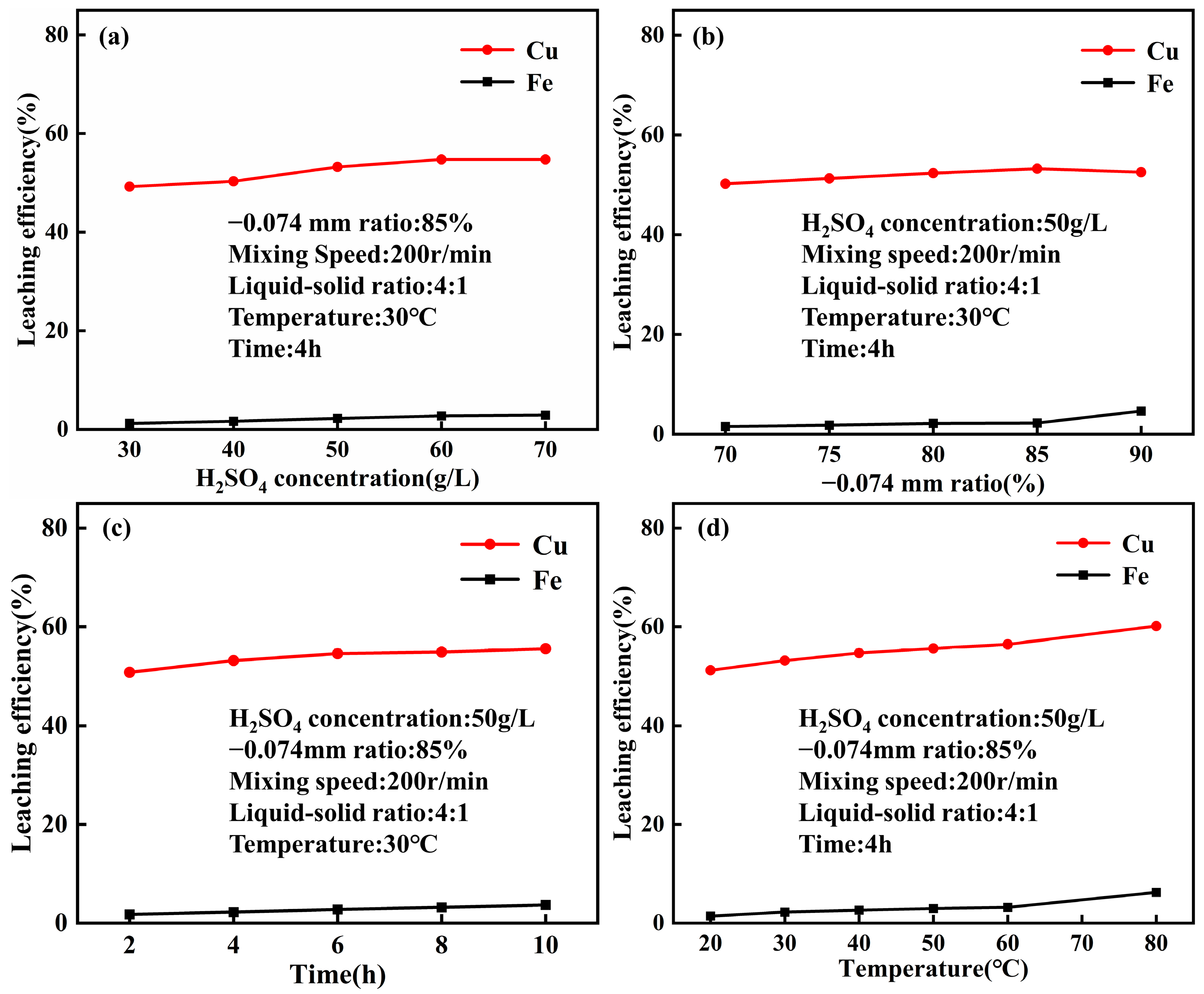
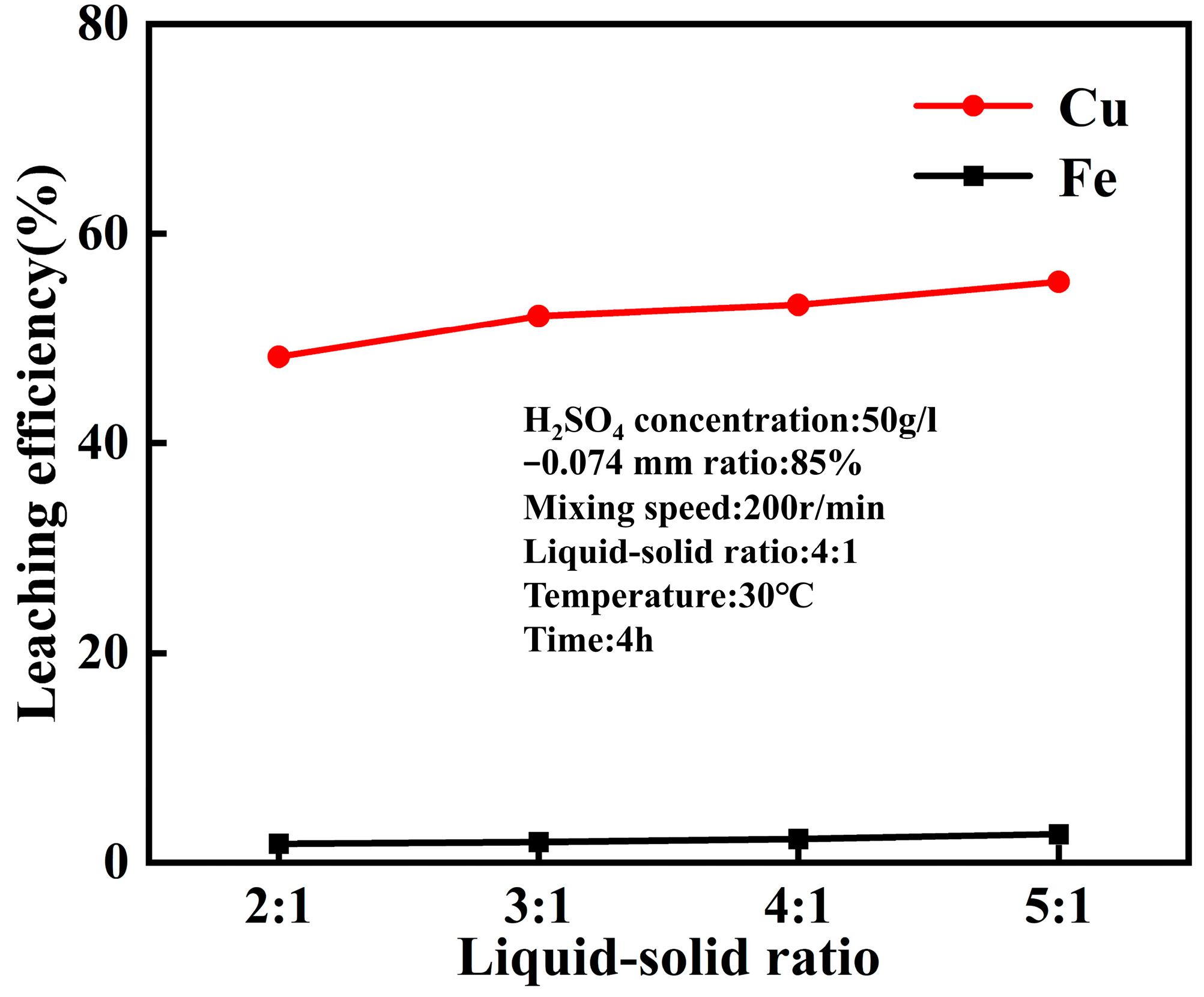
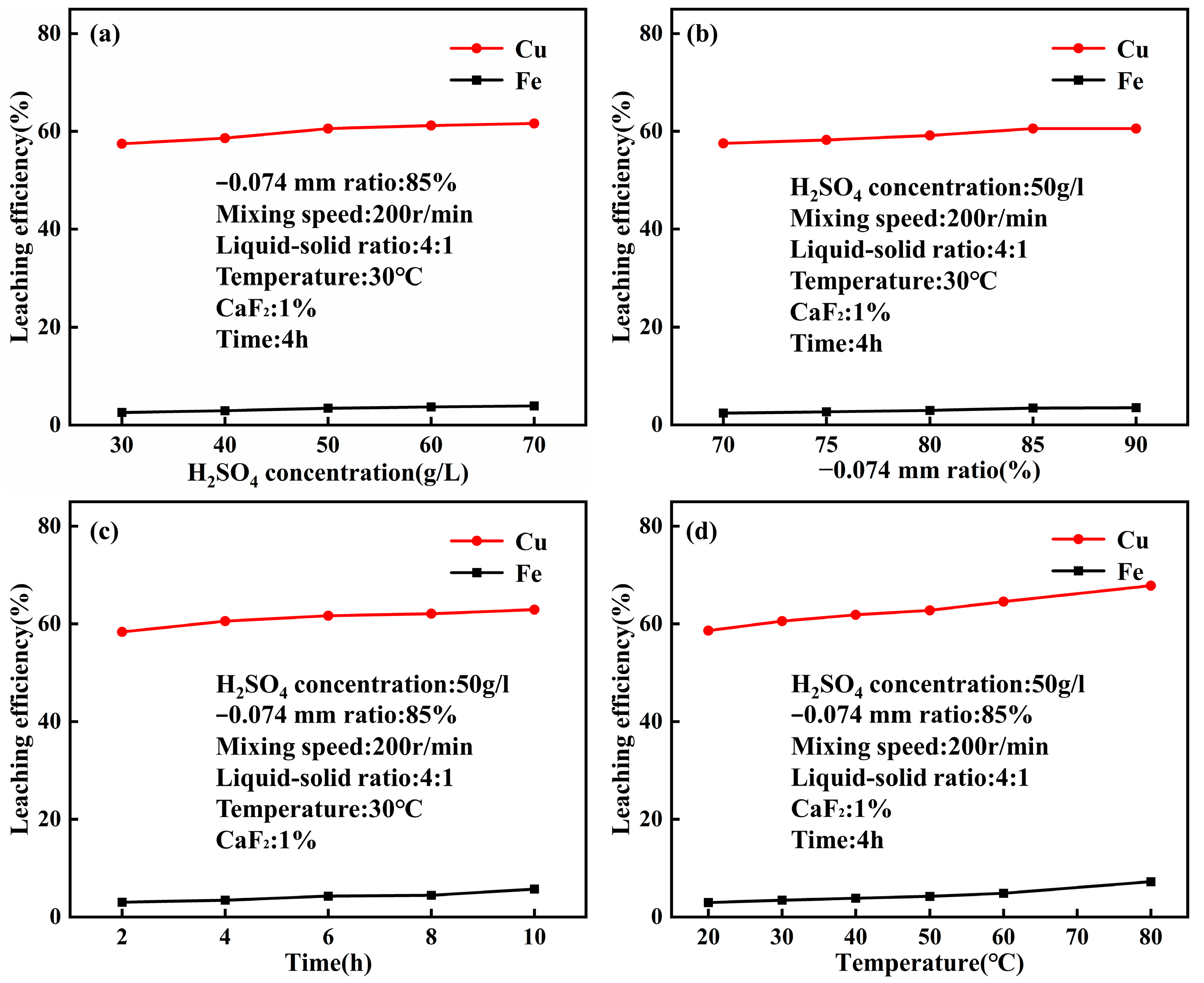
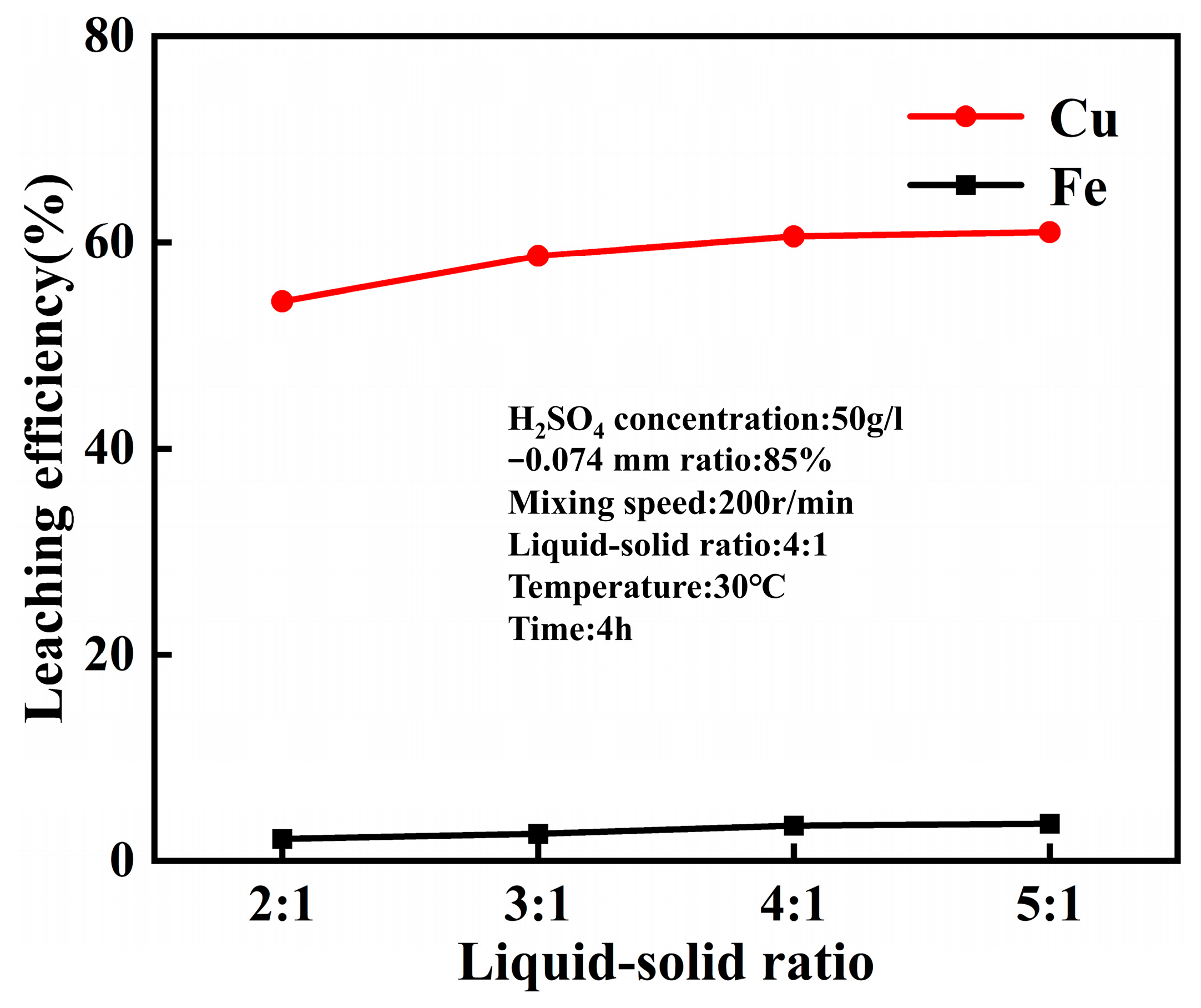
4.1. Leaching Behavior of Acid Leaching at Normal Pressure
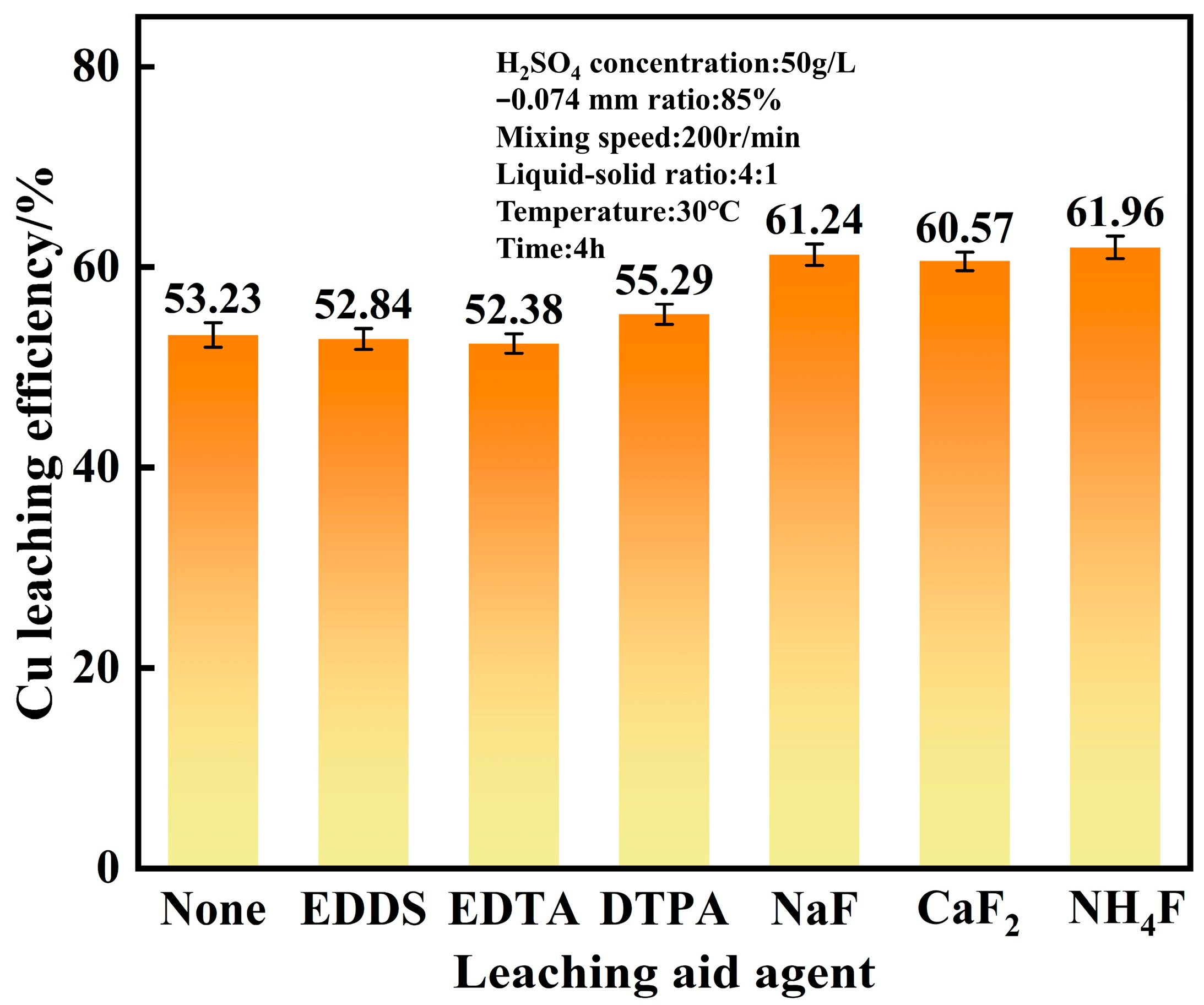
4.2. Effect of Type of Leaching Aid Agent on Copper Leaching Efficiency
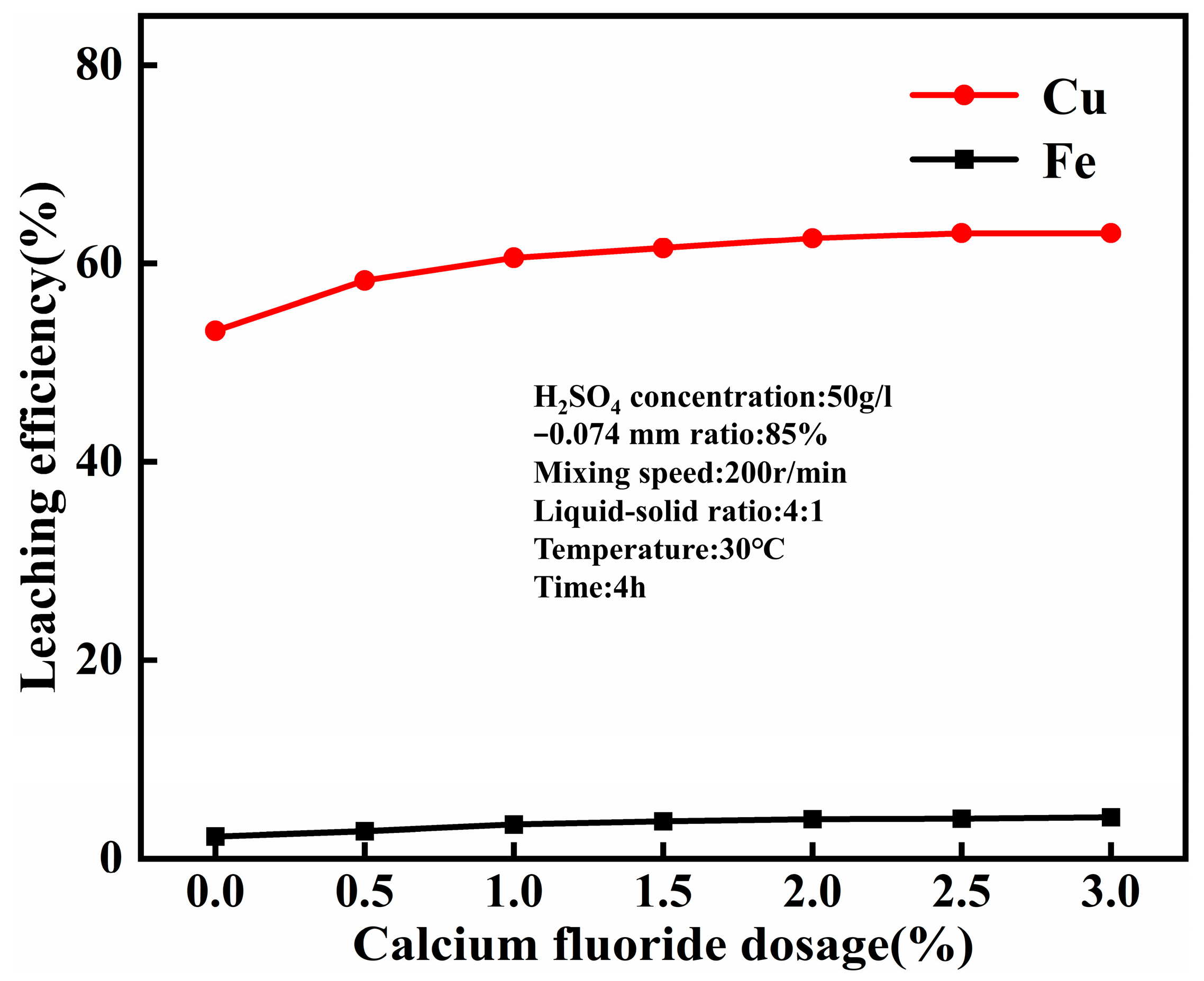
4.3. Effect of Calcium Fluoride Dosage on the Leaching Efficiency of Copper and Iron
4.4. Leaching Behavior of Calcium Fluoride in Enhanced Acid Leaching
5. Mechanism Analysis of Enhanced Leaching
6. Conclusions
- (1)
- The Yulong combined copper oxide ore sample was composed of 86.12% limonite, and most copper was distributed evenly within the limonite. The mineral components were complex and embedded in a fine grain size. Direct acid leaching requires the dissolution of iron, resulting in substantial acid consumption. Therefore, the direct atmospheric pressure acid leaching method is not suitable for the sample.
- (2)
- The optimal conditions for leaching combined copper oxide ore in Yulong, Tibet were established as follows: sulfuric acid concentration of 50 g/L, temperature at 30 °C, CaF2 dosage at 1% of the ore mass, leaching time of 4 h, liquid-solid ratio of 4:1, and rotation speed of 200 r/min. Under these conditions, the copper leaching efficiency reached 60.57%, representing a 7.34% improvement compared to acid leaching without calcium fluoride under normal pressure.
- (3)
- Fluorine ions can migrate and penetrate into limonite particles to erode limonite, which can improve the leaching of copper in combined copper oxide ore, and the erosion of fluorine is more intense in the periphery. In addition, the penetration of fluoride ions into limonite particles can also strengthen the replacement and desorption of hydrogen ions for combined copper oxide in limonite. Moreover, fluoride ions can strongly corrode silicate minerals such as kaolinite in the presence of sulfuric acid, promoting the release of adsorbed copper ions in kaolinite.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of Copper Oxide with Different Acid Solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Yang, J.; Yuan, Z.; Chen, Y. The Future of Copper in China—A Perspective Based on Analysis of Copper Flows and Stocks. Sci. Total Environ. 2015, 536, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Mai, Y.; Song, H.; Mai, J.; Jie, X. Heterogeneous Microstructure Enables a Synergy of Strength, Ductility and Electrical Conductivity in Copper Alloys. J. Alloys Compd. 2022, 902, 163646. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Y.; Xu, H.; Yang, Y.; Tian, L. Life Cycle Assessment of Valuable Metal Extraction from Copper Pyrometallurgical Solid Waste. Resour. Conserv. Recycl. 2023, 191, 106875. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Liu, W.; Chen, L.; Wen, Z.; Li, X. Prediction, Evaluation and Optimization of China’s Copper Resource Supply System under Carbon Constraints. Sustain. Prod. Consum. 2023, 39, 285–300. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Deng, J.; Wu, D. Extracting Copper from Copper Oxide Ore by a Zwitterionic Reagent and Dissolution Kinetics. Int. J. Min. Met. Mater. 2015, 22, 241–248. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Han, G.; He, Y.; Feng, Q. Adsorption Behavior and Mechanism of Copper Ions in the Sulfidization Flotation of Malachite. Int. J. Min. Sci. Technol. 2022, 32, 897–906. [Google Scholar] [CrossRef]
- Liu, C.; Song, S.; Li, H.; Ai, G. Sulfidization Flotation Performance of Malachite in the Presence of Calcite. Miner. Eng. 2019, 132, 293–296. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J.; Reuter, M.A. Flotation of Mixed Copper Oxide and Sulphide Minerals with Xanthate and Hydroxamate Collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Tan, G.; Liu, G.; Wu, B. Experimental Studies and Pilot Plant Tests for Acid Leaching of Low-Grade Copper Oxide Ores at the Tuwu Copper Mine. Hydrometallurgy 2016, 165, 227–232. [Google Scholar] [CrossRef]
- Razavizadeh, H.; Afshar, M.R. Leaching of Sarcheshmeh Copper Oxide Ore in Sulfuric Acid Solution. Min. Metall. Explor. 2008, 25, 85–90. [Google Scholar] [CrossRef]
- Sun, X.; Chen, B.; Yang, X.; Liu, Y. Technological Conditions and Kinetics of Leaching Copper from Complex Copper Oxide Ore. J. Cent. South Univ. Technol. 2009, 16, 936–941. [Google Scholar] [CrossRef]
- Bai, X.; Wen, S.; Feng, Q.; Liu, J.; Lin, Y. Utilization of High-Gradient Magnetic Separation–Secondary Grinding–Leaching to Improve the Copper Recovery from Refractory Copper Oxide Ores. Miner. Eng. 2019, 136, 77–80. [Google Scholar] [CrossRef]
- Han, J.; Xiao, J.; Qin, W.; Chen, D.; Liu, W. Copper Recovery from Yulong Complex Copper Oxide Ore by Flotation and Magnetic Separation. JOM 2017, 69, 1563–1569. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Wen, S.; Wang, H.; Zhao, W.; Han, G. Flotation of Copper Oxide Minerals: A Review. Int. J. Min. Sci. Technol. 2022, 32, 1351–1364. [Google Scholar] [CrossRef]
- Zheng, J.; Luo, A.; Jin, Y.; Feng, B.; Chen, J.; Zhang, X. Interface Adsorption Mechanism of 5-Nonyl Salicylaldehyde Oxime on Malachite Surface and Its Flotation Separation Performance with Calcite and Quartz. Sep. Purif. Technol. 2024, 332, 125758. [Google Scholar] [CrossRef]
- Barbaro, M.; Herrera Urbina, R.; Cozza, C.; Fuerstenau, D.; Marabini, A. Flotation of Oxidized Minerals of Copper Using a New Synthetic Chelating Reagent as Collector. Int. J. Miner. Process. 1997, 50, 275–287. [Google Scholar] [CrossRef]
- Senanayake, G. Review of Theory and Practice of Measuring Proton Activity and pH in Concentrated Chloride Solutions and Application to Oxide Leaching. Miner. Eng. 2007, 20, 634–645. [Google Scholar] [CrossRef]
- Han, J.; Liu, W.; Xue, K.; Qin, W.; Jiao, F.; Zhu, L. Influence of NH4HF2 Activation on Leaching of Low-Grade Complex Copper Ore in NH3-NH4Cl Solution. Sep. Purif. Technol. 2017, 181, 29–36. [Google Scholar] [CrossRef]
- Maurice, D.; Hawk, J.A. Ferric Chloride Leaching of a Mechanically Activated Pentlandite–Chalcopyrite Concentrate. Hydrometallurgy 1999, 52, 289–312. [Google Scholar] [CrossRef]
- Amer, A.M. Investigation of the Direct Hydrometallurgical Processing of Mechanically Activated Low-Grade Wolframite Concentrate. Hydrometallurgy 2000, 58, 251–259. [Google Scholar] [CrossRef]
- Wu, A.; Yin, S.; Yang, B.; Wang, J.; Qiu, G. Study on Preferential Flow in Dump Leaching of Low-Grade Ores. Hydrometallurgy 2007, 87, 124–132. [Google Scholar] [CrossRef]
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the Optimization of Heap Leaching. Miner. Eng. 2008, 21, 673–678. [Google Scholar] [CrossRef]
- Thenepalli, T.; Chilakala, R.; Habte, L.; Tuan, L.Q.; Kim, C.S. A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals. Sustainability 2019, 11, 3347. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Tong, L.; Jin, Z.; Chen, G.; Yang, H. Effect of Temperature on Leaching Behavior of Copper Minerals with Different Occurrence States in Complex Copper Oxide Ores. Trans. Nonferrous Met. Soc. China 2019, 29, 2192–2201. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Luo, W.; Ou, L. Study on Leaching Vanadium from Roasted Residue of Stone Coal. Min. Metall. Explor. 2008, 25, 181–184. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.-M.; Huang, J.; Liu, T.; Wang, Y.; Yang, X.; Zhao, J. Mechanisms of Aid-Leaching Reagent Calcium Fluoride in the Extracting Vanadium Processes from Stone Coal. Rare Met. 2013, 32, 57–62. [Google Scholar] [CrossRef]



| Element | O | Cu | Fe | Al | Si | Mn | Ca | Other |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 40.10 | 1.97 | 40.64 | 2.92 | 8.80 | 0.09 | 1.28 | 4.20 |
| Mineral | Content (%) |
|---|---|
| Limonite | 86.12 |
| Kaolinite | 8.29 |
| Chrysocolla | 0.41 |
| Calcite | 1.85 |
| Anorthose | 0.10 |
| Quartz | 1.41 |
| Potassium-feldspar | 0.19 |
| Jarosite | 0.22 |
| Malachite | 0.14 |
| Biotite | 0.51 |
| Pyroxene | 0.04 |
| Manganite | 0.21 |
| Fluorite | 0.00 |
| Amphibole | 0.04 |
| Diaspore | 0.05 |
| Chlorite | 0.11 |
| Other | 0.31 |
| Mineral | Limonite | Kaolinite | Malachite | Biotite | Chrysocolla | Other |
|---|---|---|---|---|---|---|
| Cu content (%) | 63.51 | 7.65 | 8.58 | 5.28 | 14.34 | 0.64 |
| Copper Phase | Content (%) | Proportion (%) |
|---|---|---|
| Free oxidized copper | 0.99 | 50.25 |
| Combined oxidized copper | 0.79 | 40.10 |
| Primary copper sulfide | 0.04 | 2.03 |
| Secondary copper sulfide | 0.15 | 7.62 |
| Total copper | 1.97 | 100 |
| Copper Phase | Raw Ore | Atmospheric Acid Leaching Slag | Calcium Fluoride Leaching Slag | |||
|---|---|---|---|---|---|---|
| Content (%) | Proportion (%) | Content (%) | Proportion (%) | Content (%) | Proportion (%) | |
| Free oxidized copper | 0.99 | 50.25 | 0.06 | 6.32 | 0.06 | 7.59 |
| Combined oxidized copper | 0.79 | 40.10 | 0.79 | 83.15 | 0.63 | 79.76 |
| Primary copper sulfide | 0.04 | 2.03 | 0.04 | 4.21 | 0.04 | 5.06 |
| Secondary copper sulfide | 0.15 | 7.62 | 0.06 | 6.32 | 0.06 | 7.59 |
| Total copper | 1.97 | 100 | 0.95 | 100 | 0.79 | 100.00 |
| Mineral | Limonite | Kaolinite | Malachite | Biotite | Chrysocolla | Other |
|---|---|---|---|---|---|---|
| Cu content (%) | 89.88 | 9.24 | 0.09 | 0.08 | 0.09 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Jian, C.; Peng, Z.; Fu, X.; He, R.; Yue, T.; Sun, W. Study on Process Mineralogy of the Combined Copper Oxide Ore in Tibet and Acid Leaching Behavior with Calcium Fluoride. Minerals 2024, 14, 352. https://doi.org/10.3390/min14040352
Pan Z, Jian C, Peng Z, Fu X, He R, Yue T, Sun W. Study on Process Mineralogy of the Combined Copper Oxide Ore in Tibet and Acid Leaching Behavior with Calcium Fluoride. Minerals. 2024; 14(4):352. https://doi.org/10.3390/min14040352
Chicago/Turabian StylePan, Zujiang, Cuo Jian, Zaihua Peng, Xinzhuang Fu, Rui He, Tong Yue, and Wei Sun. 2024. "Study on Process Mineralogy of the Combined Copper Oxide Ore in Tibet and Acid Leaching Behavior with Calcium Fluoride" Minerals 14, no. 4: 352. https://doi.org/10.3390/min14040352
APA StylePan, Z., Jian, C., Peng, Z., Fu, X., He, R., Yue, T., & Sun, W. (2024). Study on Process Mineralogy of the Combined Copper Oxide Ore in Tibet and Acid Leaching Behavior with Calcium Fluoride. Minerals, 14(4), 352. https://doi.org/10.3390/min14040352







