Formation of Calcium Ferrite Containing Aluminum (CFA) in Sintering of Iron Ore Fines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactions of CFA Formation
2.2. Minerals Determination
2.3. Calculations of Formation Energy for Products
3. Results and Discussion
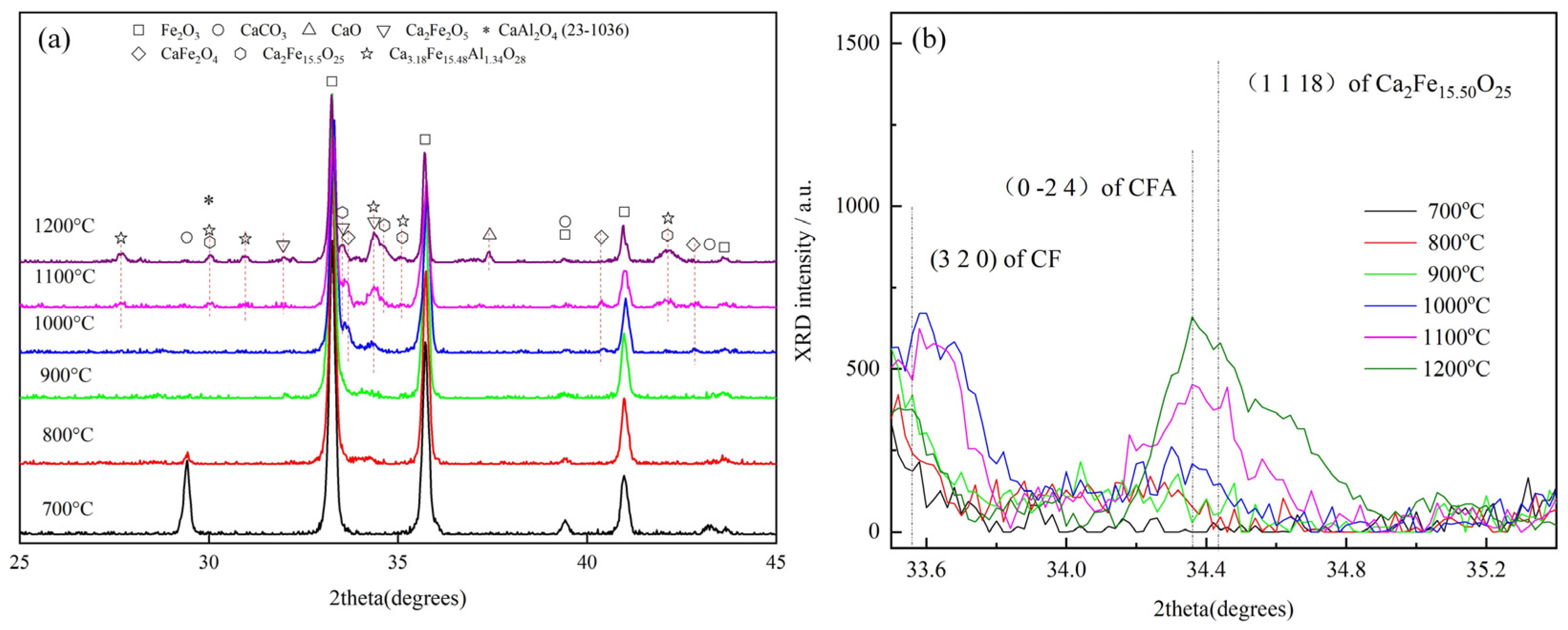
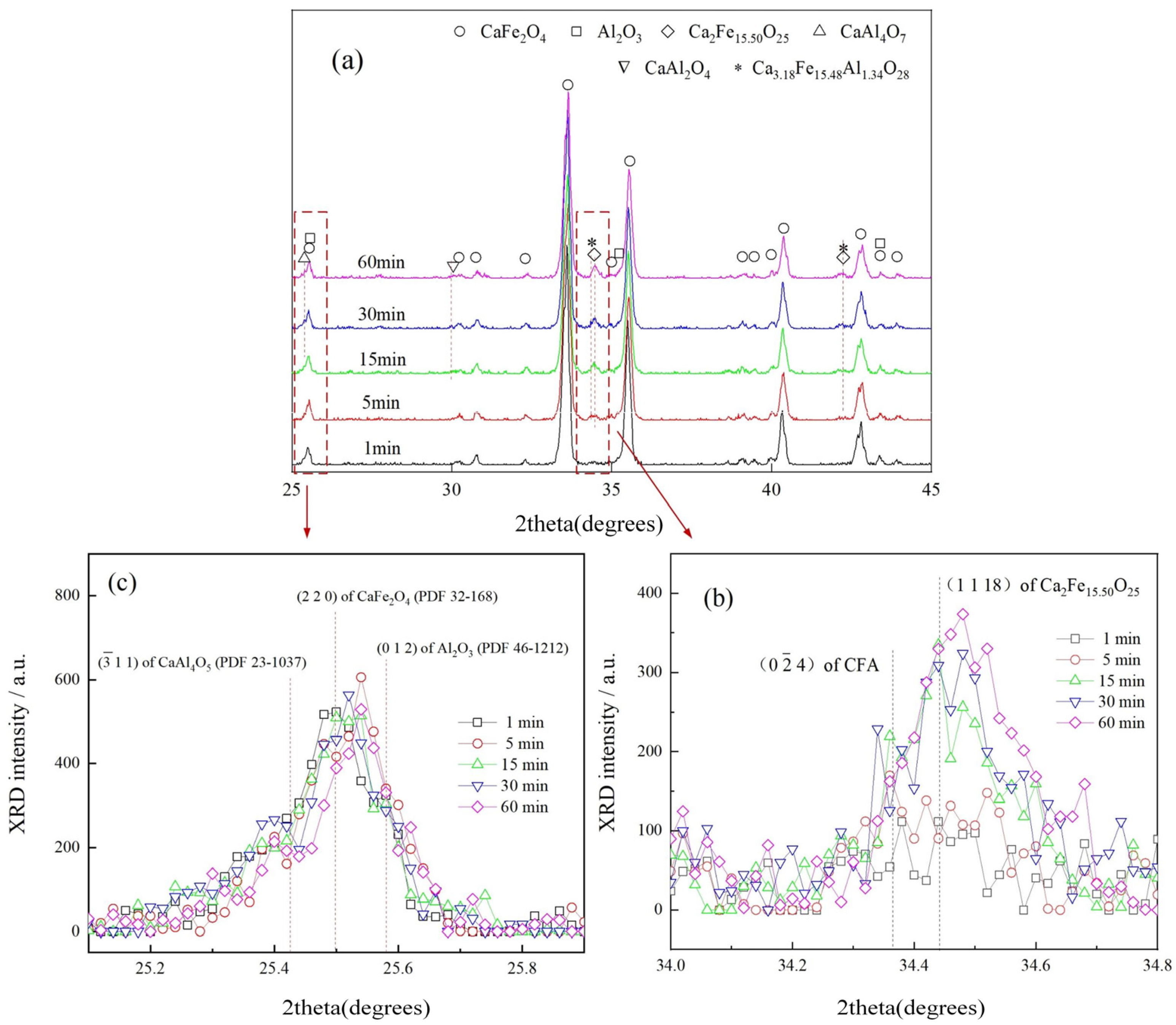
3.1. Effect of Temperature on CFA Formation
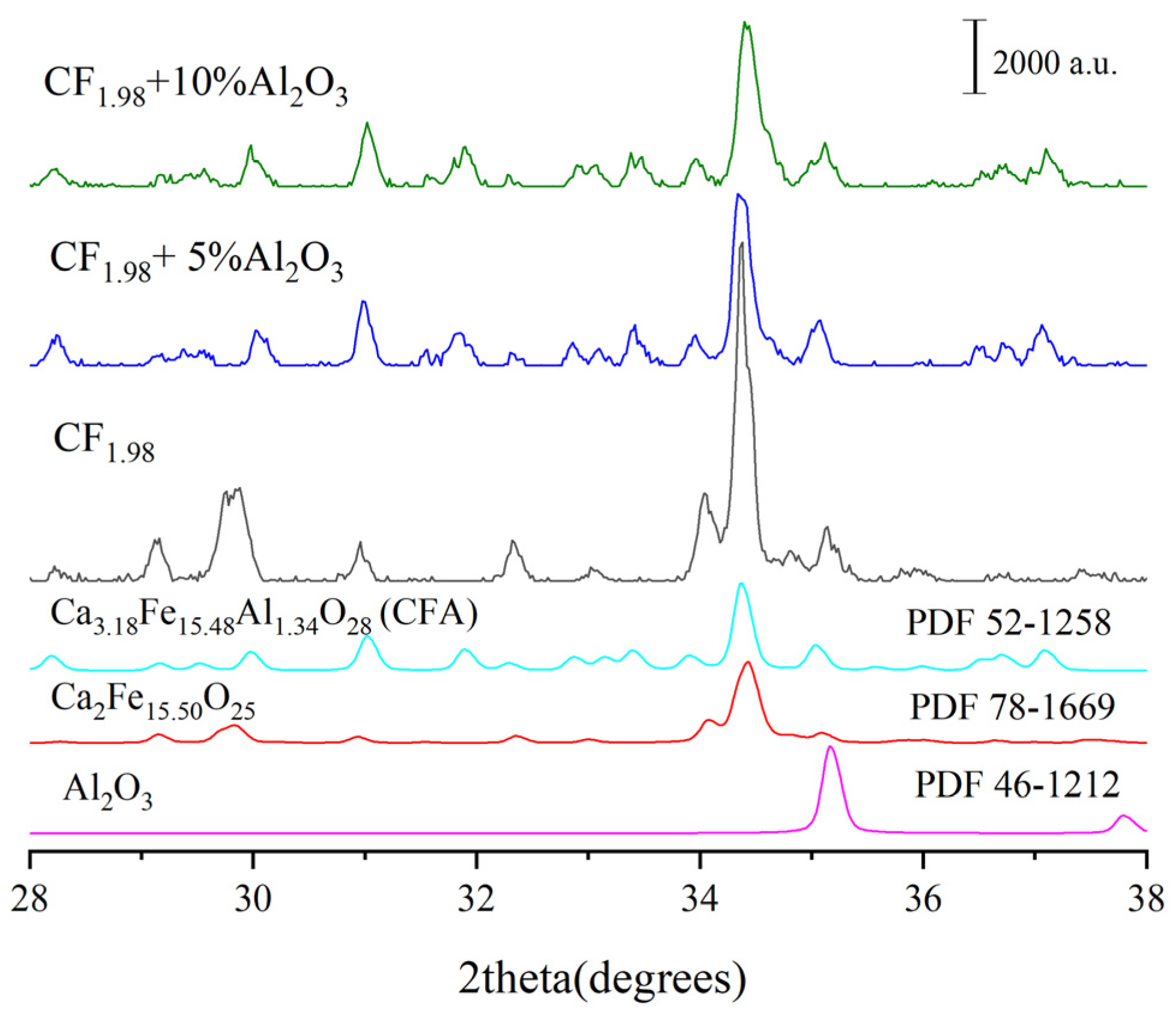
3.2. Effect of Different Types of Al-Containing Materials on CFA Formation
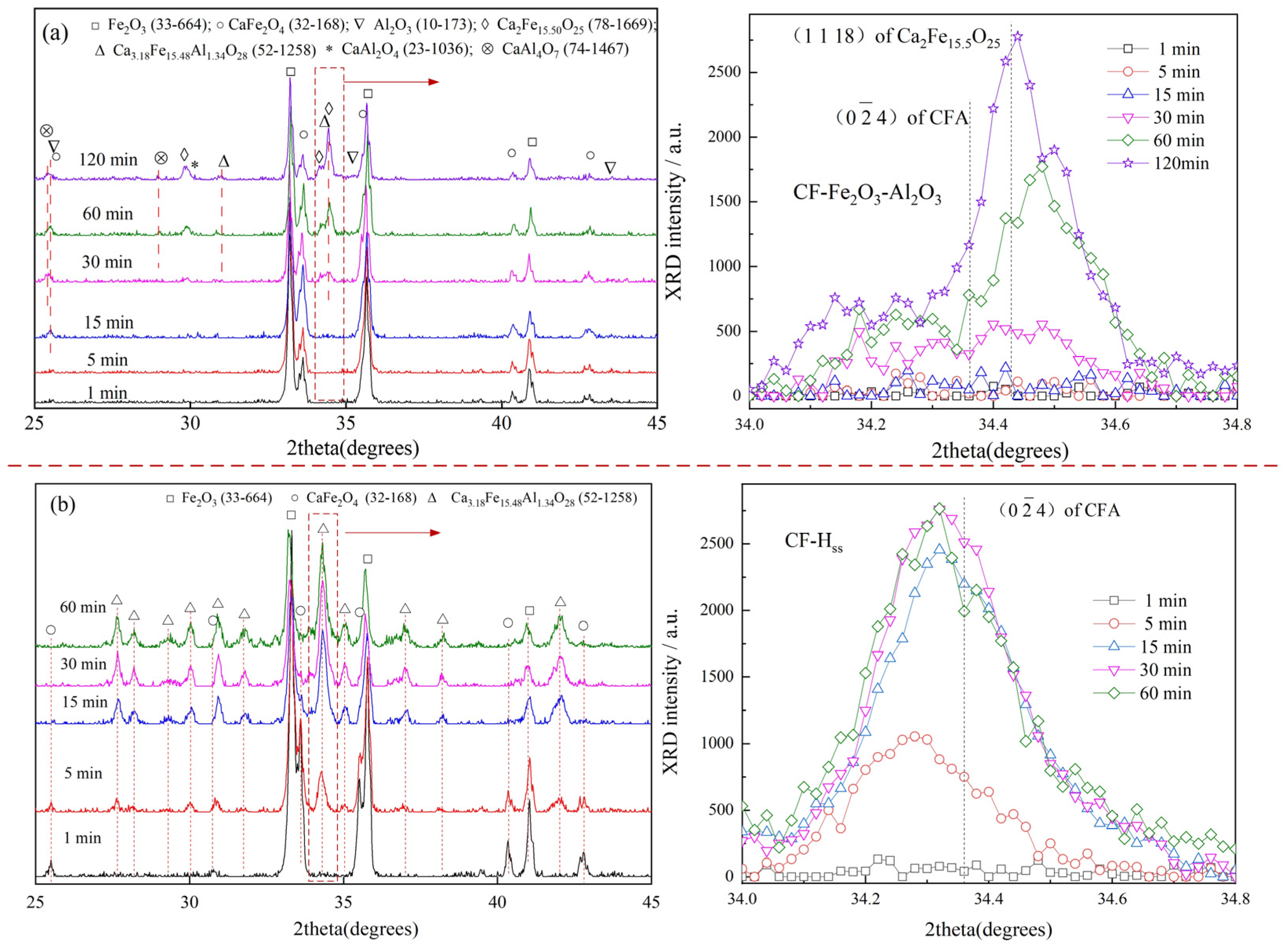
3.3. Reaction of CF with Fe2O3
3.4. Reaction of CF with Al2O3
3.5. Stability of Products Generated in Reactions
4. Conclusions
- (1)
- It was observed in the Fe2O3-CaO-Al2O3 sample sintered below 1200 °C that CF appeared at 1000 °C, while Ca2Fe15.5O25 and CFA appeared at 1100 °C.
- (2)
- It was found that CF and Ca2Fe15.5O25 are the precursors for CFA formation, and the chemical composition of Ca2Fe15.5O25 phase was determined to be CaFe3.96O6.94 (CF1.98), similar to CaFe4O7.
- (3)
- It was revealed that the appearance of CA or CA2 is a main reason to decrease the rate of CFA formation in Fe2O3-CaO-Al2O3 samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ohno, K.I.; Maeda, T.; Kunitomo, K.; Hara, M. Effect of FeO concentration in sinter iron ore on reduction behavior in a hydrogen-enriched blast furnace. Int. J. Miner. Metall. Mater. 2022, 29, 1820–1829. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, T.; Zhou, M.; Li, L.; Wen, J.; Wen, Y. Interdiffusion kinetics and solid-state reaction mechanism between Cr2O3 and calcium ferrite based on diffusion couple method. J. Alloys Compd. 2021, 865, 158754. [Google Scholar] [CrossRef]
- Honeyands, T.; Nguyen, T.B.T.; Pinson, D.; Connolly, P.R.; Pownceby, M.I.; Manuel, J.; Matthews, L.; Leedham, J.; Singh, T.; O’Dea, D.P. Variation in Iron Ore Sinter Mineralogy with Changes in Basicity. Minerals 2022, 12, 1249. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Sato, T.; Ishii, J.; Murai, R.; Watakabe, S. Agglomeration of return fines of sinter for blast furnace raw materials. ISIJ Int. 2020, 60, 1389–1394. [Google Scholar] [CrossRef]
- Tomas da Rocha, L.; Cho, S.; Kim, S.W.; Jung, S.M. Effects of High-Temperature Characteristics of Calcium Ferrites on the Sinter Strength. Metall. Mater. Trans. B 2022, 53, 3306–3321. [Google Scholar] [CrossRef]
- Li, T.; Sun, C.; Lan, D.; Song, J.; Song, S.; Wang, Q. Effect of mineral elements migration on softening–melting properties of Ti-bearing high basicity sinter. ISIJ Int. 2019, 59, 245–252. [Google Scholar] [CrossRef]
- Xin, R.F.; Guo, X.M. Effect of SiO2 on Crystallization of Calcium Ferrites in Fe2O3-CaO-SiO2-Al2O3 System in Cooling Process. Metall. Mater. Trans. B 2022, 53, 1904–1919. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, M.; Jiang, T.; Li, L. Observation of diffusion behavior between Cr2O3 and calcium ferrite based on diffusion couple method at 1373 K. J. Alloys Compd. 2019, 802, 103–111. [Google Scholar] [CrossRef]
- Vemdrame Flores, I.; Matos, O.; Lima da Silva, A.; Covcevich Bagatini, M. Microstructure and Porosity Evolution during the Reduction, Softening and Melting of Iron-Bearing Materials. Metall. Mater. Trans. B 2021, 52, 1716–1738. [Google Scholar] [CrossRef]
- Park, J.; Rajavaram, R.; Suh, I.K.; Jeon, J.; Son, S.; Lee, J. Effects of Basicity and Al2O3 Content on the Chemistry of Phases in Iron Ore Sinter Containing ZnO. Metall. Mater. Trans. B 2020, 51, 3016–3027. [Google Scholar] [CrossRef]
- Tomas da Rocha, L.; Cho, S.; Kim, S.W.; Jung, S.M. Effects of Recycling By-Products as Calcium Ferrites Added to the Sinter Mix on Sinter Quality and Emission of CO2, NO, and SO2. Metall. Mater. Trans. B 2022, 53, 3524–3542. [Google Scholar] [CrossRef]
- Ding, X.; Guo, X.M. Study of SiO2 involved in the formation process of silico-ferrite of calcium (SFC) by solid-state reactions. Int. J. Miner. Process 2016, 149, 69–77. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, S.; Shevchenko, M.; Hayes, P.C.; Jak, E. Investigation of the Thermodynamic Stability of C(A,F)3 Solid Solution in the FeO-Fe2O3-CaO-Al2O3 System and SFCA Phase in the FeO-Fe2O3-CaO-SiO2-Al2O3 System. Metall. Mater. Trans. B 2021, 52, 517–527. [Google Scholar] [CrossRef]
- Luo, G.P.; Wu, S.L.; Zhang, G.J.; Wang, Y.C. Effects of compound silicate gangue on formation of complex calcium ferrite during sintering process. J. Iron Steel Res. Int. 2013, 20, 18–23. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, S.; Chen, S.; Zhu, J.; Fan, J.; Su, B. Optimization of dolomite usage in iron ore sintering process. ISIJ Int. 2013, 53, 1515–1522. [Google Scholar] [CrossRef]
- Park, T.J.; Choi, J.S.; Min, D.J. Influence of Al2O3 Content and Cooling Rate on Crystallization in Fe2O3-CaO-SiO2-Al2O3 Systems. Met. Mater. Int. 2022, 28, 2033–2041. [Google Scholar] [CrossRef]
- Patrick, T.R.; Pownceby, M.I. Stability of silico-ferrite of calcium and aluminum (SFCA) in air-solid solution limits between 1240°C and 1390°C and phase relationships within the Fe2O3-CaO-Al2O3-SiO2 (FCAS) system. Metall. Mater. Trans. B 2002, 33, 79–89. [Google Scholar] [CrossRef]
- Scarlett, N.V.; Madsen, I.C.; Pownceby, M.I.; Christensen, A.N. In situ X-ray diffraction analysis of iron ore sinter phases. J. Appl. Crystallogr. 2004, 37, 362–368. [Google Scholar] [CrossRef]
- Webster, N.A.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Effect of oxygen partial pressure on the formation mechanisms of complex Ca-rich ferrites. ISIJ Int. 2013, 53, 774–781. [Google Scholar] [CrossRef]
- Yamauchi, T. A Study of the Celite Part (Part VII) The Systems CaO-Al2O3-Fe2O3 and CaO-Al2O3-Fe2O3-SiO2, whose Al2O3. J. Ceram. Soc. Jpn. 2000, 108, S7–S18. [Google Scholar] [CrossRef]
- Lister, D.H.; Glasser, F.P. Phase relations in the system CaO-Al2O3-iron oxide. Trans. Brit. Ceram. Soc. 1967, 66, 293–305. [Google Scholar]
- Mumme, W.G. The crystal structure of SFCA-II, Ca5.1A19.3Fe3+18.7Fe2+0.9O48 as a new homologue of the aenigmatite structure-type, and structure refinement of SFCA-type, Ca2Al5Fe7O20. Neues. Jb. Miner. Abh. 2003, 178, 307–335. [Google Scholar] [CrossRef]
- Webster, N.A.; Pownceby, M.I.; Madsen, I.C. In situ X-ray diffraction investigation of the formation mechanisms of silico-ferrite of calcium and aluminium-I-type (SFCA-I-type) complex calcium ferrites. ISIJ Int. 2013, 53, 1334–1340. [Google Scholar] [CrossRef]
- Webster, N.A.; O’dea, D.P.; Ellis, B.G.; Pownceby, M.I. Effects of gibbsite, kaolinite and Al-rich goethite as alumina sources on silico-ferrite of calcium and aluminium (SFCA) and SFCA-I iron ore sinter bonding phase formation. ISIJ Int. 2017, 57, 41–47. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X.M. Effect of aluminum dissolved in hematite on formation of Calcium Ferrites at 1473 K. Metall. Mater. Trans. B 2018, 49, 1974–1984. [Google Scholar] [CrossRef]
- Liao, F.; Guo, X.M. The effects of Al2O3 and SiO2 on the formation process of silico-ferrite of calcium and aluminum (SFCA) by solid-state reactions. Minerals 2019, 9, 101. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X.M. Effect of alumina on liquid phase formation in sintering process of iron ore fines. Steel Res. Int. 2019, 90, 1900138. [Google Scholar] [CrossRef]
- Semberg, P.; Andersson, C.; Björkman, B. Formation and decomposition of C4F7 type calcium ferrites in superfluxed magnetitebased pellets during oxidation and reduction. Ironmak. Steelmak. 2014, 41, 474–479. [Google Scholar] [CrossRef]
- Arakcheeva, A.V.; Karpinskii, O.G. Crystal structure of hexagonal ferrite, Ca2.95Fe14.85O25. Russ. Acad. Sci. 1983, 273, 1127–1129. [Google Scholar]
- Arakcheeva, A.V.; Karpinskii, O.G. Crystal structure of the ternary hexagonal Ca ferrite Ca3.0Fe14.82O25. Kristallografiya 1987, 32, 59–61. [Google Scholar]
- Karpinskii, O.G.; Arakcheeva, A.V. Crystal structure of ternary hexagonal ferrite phase Ca3.565Fe0.06Fe14.25O25. In Doklady Akademii Nauk. Russ. Acad. Sci. 1985, 282, 1139–1141. [Google Scholar]
- Ding, X.; Guo, X.M. The sintering characteristics of mixing SiO2 with calcium ferrite at 1473 K (1200 °C). Metall. Mater. Trans. B 2015, 46, 1742–1750. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Wang, X.; Yang, S.; Su, X. Stability and electronic structure of the Co-P compounds from first-principle calculations. J. Alloys Compd. 2011, 509, 165–171. [Google Scholar] [CrossRef]
- Ma, X.; Huang, X.; Zhang, H.; Hu, X.; Feng, T. Effect of calcium aluminates on the structure evolution of CaO during the calcium looping process: A DFT study. Chem. Eng. J. 2023, 452, 139552. [Google Scholar] [CrossRef]
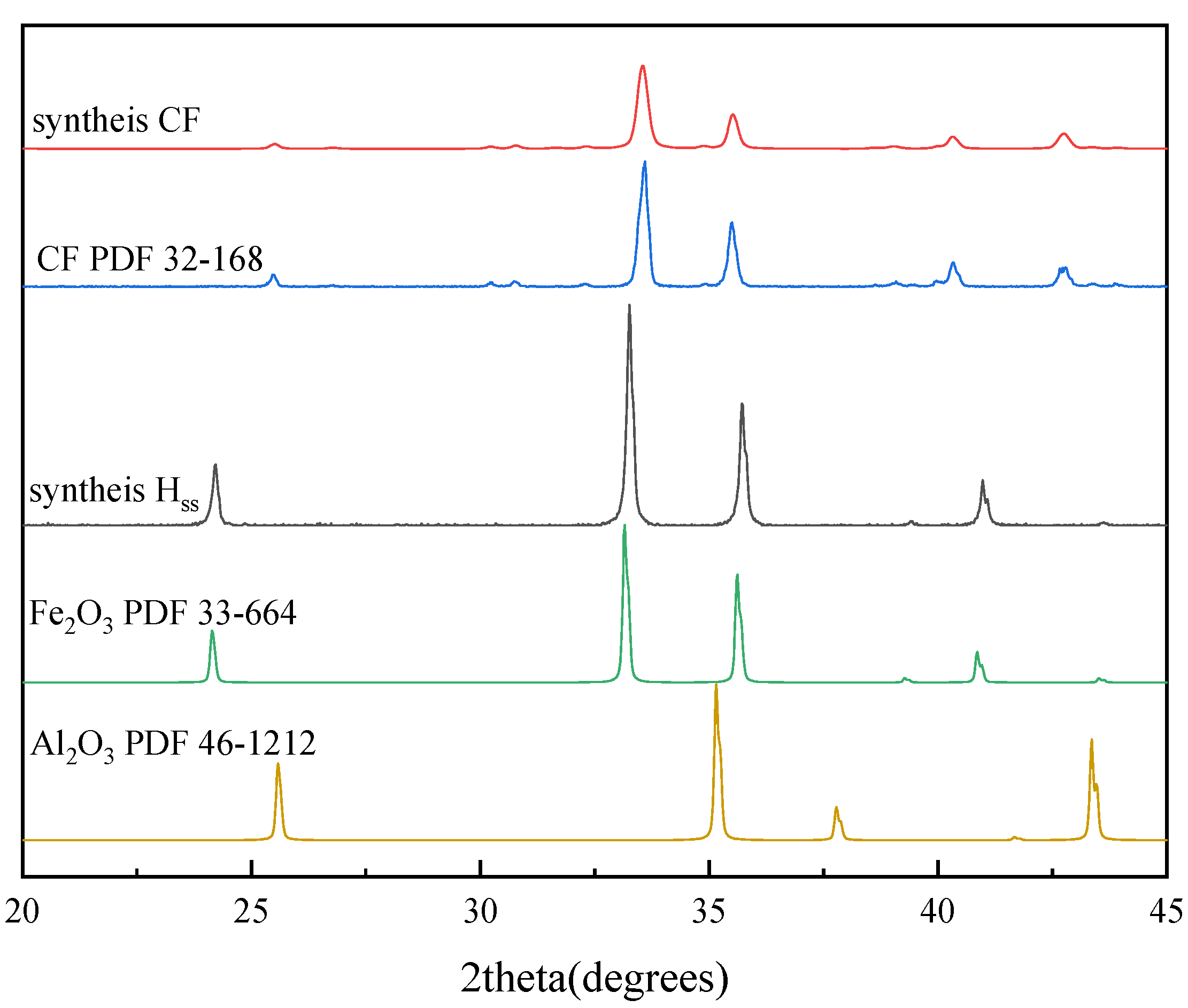

| Materials | Fe2O3 | CaCO3 | Al2O3 | Sintering Time | Sintering Temperature |
|---|---|---|---|---|---|
| CF | 61.5 | 38.5 | 480 min | 1200 °C | |
| Hss | 96.0 | 4.0 | 240 min | 1250 °C |
| Reaction | Fe2O3 | CaCO3 | Al2O3 | Hss | CF |
|---|---|---|---|---|---|
| Fe2O3-CaCO3-Al2O3 | 79.42 | 16.58 | 4 | ||
| CF-Fe2O3-Al2O3 | 57.3 | 4 | 38.7 | ||
| Hss-CF | 59.7 | 40.3 |
| Modules | Functional Used | Plane Wave Basis Set Cut-Off | k-Point | Relativistic Treatment | Pseudopotentials |
|---|---|---|---|---|---|
| CASTEP | Perdew–Burke–Ernzerhof | 489.8000 eV | 1 × 1 × 1 | Koelling–Harmon | OTFG ultrasoft |
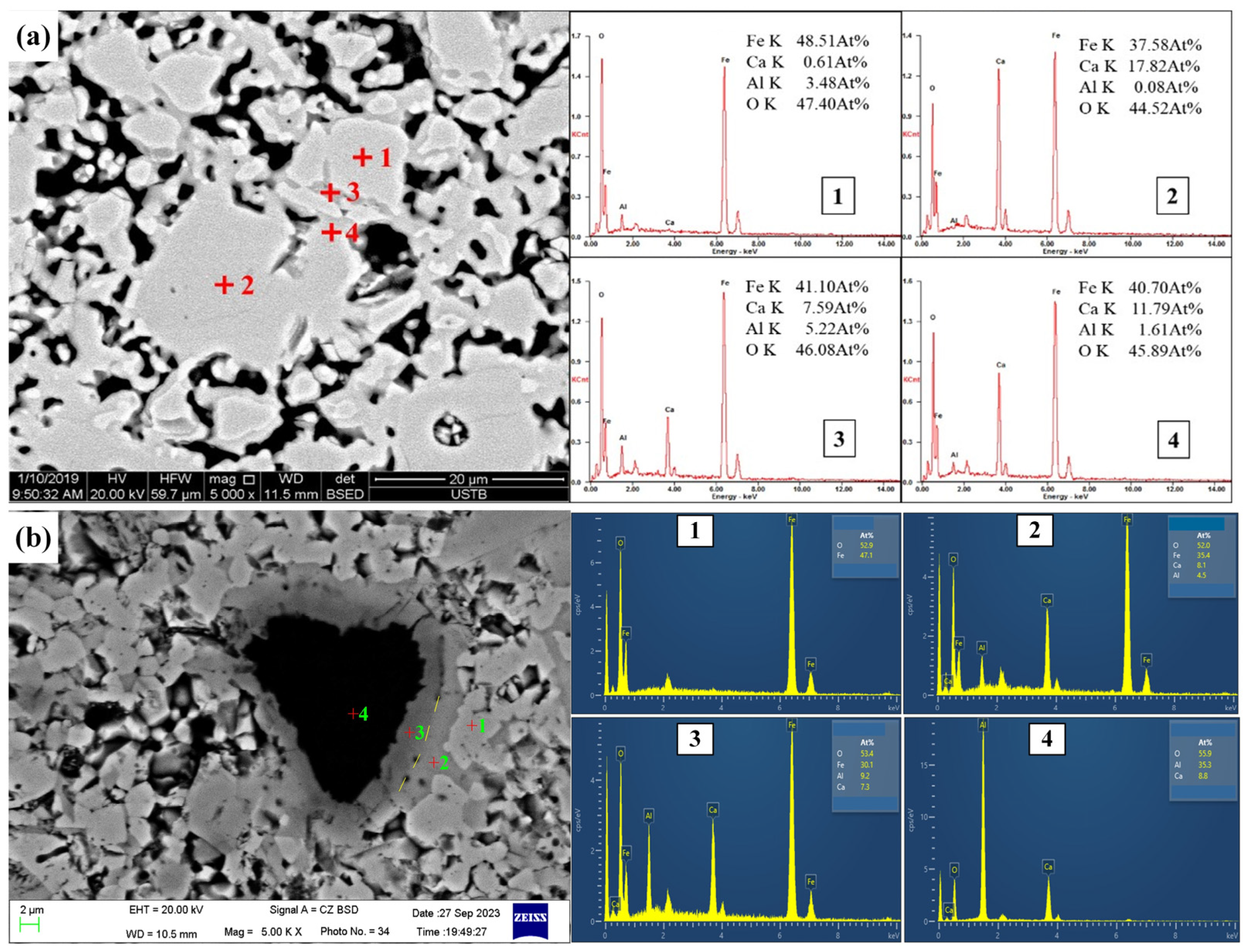
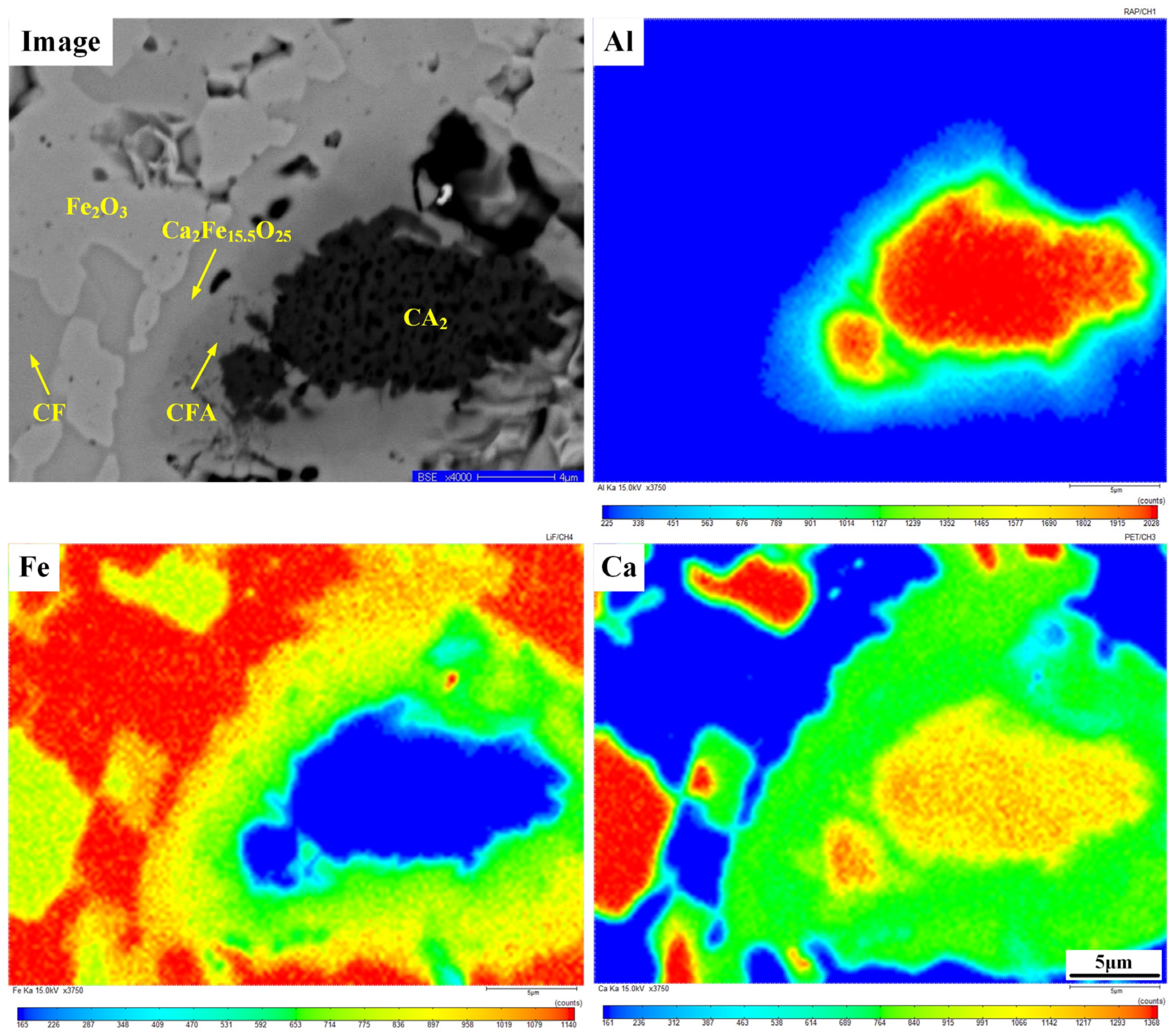
| Position | Fe | Ca | Al | O | Minerals |
|---|---|---|---|---|---|
| Figure 4(a1) | 48.51 | 0.61 | 3.48 | 47.40 | Hss |
| Figure 4(a2) | 37.58 | 17.82 | 0.08 | 44.52 | CF |
| Figure 4(a3) | 41.10 | 7.59 | 6.22 | 46.08 | CFA |
| Figure 4(a4) | 40.70 | 11.79 | 1.61 | 45.89 | CFA |
| Figure 4(b1) | 47.06 | 52.94 | Fe2O3 | ||
| Figure 4(b2) | 35.40 | 8.12 | 4.50 | 51.97 | Ca2Fe15.50O25 |
| Figure 4(b3) | 30.14 | 7.28 | 9.22 | 53.36 | CFA |
| Figure 4(b4) | 8.20 | 36.16 | 55.64 | CA2 |
| CF | CF1.98 | CFA | CA | C2F | CA2 |
|---|---|---|---|---|---|
| 53,777 | 52,281 | 49,546 | 35,963 | 23,753 | 9092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Guo, H.; Guo, X.-M. Formation of Calcium Ferrite Containing Aluminum (CFA) in Sintering of Iron Ore Fines. Minerals 2024, 14, 400. https://doi.org/10.3390/min14040400
Du Y, Guo H, Guo X-M. Formation of Calcium Ferrite Containing Aluminum (CFA) in Sintering of Iron Ore Fines. Minerals. 2024; 14(4):400. https://doi.org/10.3390/min14040400
Chicago/Turabian StyleDu, Yu, Hui Guo, and Xing-Min Guo. 2024. "Formation of Calcium Ferrite Containing Aluminum (CFA) in Sintering of Iron Ore Fines" Minerals 14, no. 4: 400. https://doi.org/10.3390/min14040400





